- 1Department of Internal Medicine, University of Medicine and Pharmacy at Ho Chi Minh City, Ho Chi Minh City, Vietnam
- 2Department of Cardiology, Cho Ray Hospital, Ho Chi Minh City, Vietnam
- 3Cardiovascular Center, University Medical Center Ho Chi Minh City, Ho Chi Minh City, Vietnam
- 4Center for Molecular Biomedicine, University of Medicine and Pharmacy at Ho Chi Minh City, Ho Chi Minh City, Vietnam
- 5Faculty of Public Health, University of Medicine and Pharmacy at Ho Chi Minh City, Ho Chi Minh City, Vietnam
Background: The severity of coronary artery disease is a prognostic factor for major adverse cardiovascular events in patients diagnosed with acute myocardial infarction. ACE I/D polymorphism is one of the genetic factors that may affect the severity of coronary artery disease. This study aimed to investigate the association between ACE I/D genotypes and the severity of coronary artery disease in patients with acute myocardial infarction.
Materials and methods: A single-center, prospective, observational study was conducted at the Department of Cardiology and Department of Interventional Cardiology, Cho Ray Hospital, Ho Chi Minh City, Vietnam from January 2020 to June 2021. All participants diagnosed with acute myocardial infarction underwent contrast-enhanced coronary angiography. The severity of coronary artery disease was determined by Gensini score. ACE I/D genotypes were identified in all subjects by using the polymerase chain reaction method.
Results: A total of 522 patients diagnosed with first acute myocardial infarction were recruited. The patients' median Gensini score was 34.3. The II, ID, and DD genotype rates of ACE I/D polymorphism were 48.9%, 36.4%, and 14.7%, respectively. After adjusting for confounding factors, multivariable linear regression analysis showed that the ACE DD genotype was independently associated with a higher Gensini score compared with the II or ID genotypes.
Conclusion: The DD genotype of the ACE I/D polymorphism was associated with the severity of coronary artery disease in Vietnamese patients diagnosed with first acute myocardial infarction.
Introduction
Despite major advances in diagnosis and management, acute myocardial infarction (AMI) remains a serious healthcare burden worldwide, significantly increasing patients’ morbidity and mortality (1). The severity of coronary artery disease (CAD) is a prognostic factor for major adverse cardiovascular outcomes in patients with AMI (2–4). Several scoring systems are available for the quantitative evaluation of coronary artery lesions, and among them, the Gensini score is the most frequently used in clinical settings. The Gensini score assesses the quantity, location, and degree of stenosis of epicardial coronary artery lesions, providing a scientific evaluation standard for CAD severity (5).
In addition to environmental factors, genetic components have been revealed to be associated with the severity of CAD in AMI patients. Recent research has shown that CAD severity is influenced by variations in the angiotensin-converting enzyme (ACE) gene (6, 7). ACE, an important component of the renin-angiotensin-aldosterone system, converts angiotensin I to angiotensin II and inactivates bradykinin via the kallikrein-kininogen system (8). The ACE gene is located on the long arm of chromosome 17 (17q23), has a length of 21 kilobases (kb), and contains 26 exons and 25 introns. Insertion (I) and deletion (D) polymorphisms in the intron 16 of the ACE gene are defined by the presence or absence of a 287 bp Alu repeat (9). High serum ACE levels vary in the order homozygote deletion (DD) > heterozygote (ID) > homozygote insertion (II) (9). Cardiac ACE activity is also higher in DD-carrying subjects (10). High ACE levels are associated with an increased angiotensin II concentration, with harmful effects including vasoconstriction, aldosterone secretion, cellular proliferation, vascular remodeling, and oxidative stress; these effects give rise to endothelial dysfunction and atherosclerosis (8).
Since it was first described in the 1990s, the ACE I/D genetic polymorphism has been shown to be a potential risk factor for AMI. Cambien and colleagues were the first to report an association between the ACE I/D genetic variant and the risk of AMI; the study sample from ECTIM (Etude Cas-Temoin de l'Infarctus du Myocarde) included 610 men aged 25–64 years who had survived for 3–9 months after AMI and 733 participants with no history of CAD (11). The association between ACE I/D polymorphism and the risk of AMI has been studied further, but the findings are inconsistent (12–14). A meta-analysis by Chen et al. concluded that the D allele of the ACE I/D genetic polymorphism is a potential risk factor for AMI in both Asians and Caucasians (15).
Although ACE I/D genetic variants have been extensively studied in relation to AMI, only a few studies have investigated the clinical significance of ACE I/D genotypes to the severity of CAD. In addition, the severity of CAD in the previous studies was mostly based on the number of stenosed coronary arteries, rather than the Gensini score. Thus, there is a lack of data on the association between ACE I/D genetic polymorphism and CAD severity evaluated using the Gensini score. The identification of factors affecting the severity of CAD can contribute to strategies for the primary and secondary prevention of AMI. Therefore, this study aimed to investigate the association between ACE I/D genotypes and the severity of CAD assessed using the Gensini score for Vietnamese patients with AMI.
Materials and methods
Study design and population
This was a single-center, prospective, observational study conducted at the Department of Cardiology and Department of Interventional Cardiology, Cho Ray Hospital, Ho Chi Minh City, Vietnam from January 2020 to June 2021. This research was approved by the Ethics Committee in Biomedical Research of the University of Medicine and Pharmacy at Ho Chi Minh City (HEC/IRB number 550/UMP-BOARD). Eligible patients were ≥18 years old with a confirmed diagnosis of AMI according to the fourth universal definition from the European Heart Association, the American College of Cardiology, the American Heart Association, and the World Heart Federation published in 2018 (16). Patients who had any of the following criteria were excluded: (1) a history of myocardial infarction, percutaneous coronary intervention, or coronary artery bypass surgery; (2) no coronary angiography performed; (3) no stenosis or less than 50% luminal diameter stenosis of all major epicardial coronary arteries; (4) clinical presentations of connective tissue diseases such as systemic lupus erythematosus, rheumatoid arthritis, scleroderma, systemic vasculitis, antiphospholipid syndrome, amyloidosis, thyroid diseases, and active cancer; (5) refused to provide the informed consent or did not want to take part in this study.
Demographic data and data on cardiovascular risk factors, prior use of medications, clinical type of AMI, Killip class, lipid profile, and estimated glomerular filtration rate (eGFR) were collected. Obesity was defined as a body mass index (BMI) ≥ 25 kg/m2 for the Asia-Pacific population (17). BMI was calculated as weight (kg) divided by the square of height (m) (18); body surface area was estimated by the square root of the height (cm) multiplied by the weight (kg) divided by 3,600 (19). Hypertension was defined as either a previous diagnosis of hypertension or newly-diagnosed hypertension with at least two times of blood pressure measurements ≥140/90 mmHg according to the 2018 European Society of Hypertension and the European Heart Association guidelines (20). A prior or new diagnosis of diabetes was defined according to the diagnostic criteria of American Diabetes Association (21). Smoking was defined as a patient who was currently smoking or had stopped smoking within the last 12 months (22). Dyslipidemia was defined as having one of the following abnormalities: total cholesterol ≥200 mg/dl, high-density lipoprotein (HDL) cholesterol <40 mg/dl, low-density lipoprotein (LDL) cholesterol ≥130 mg/dl, triglyceride ≥150 mg/dl, or a previous diagnosis of dyslipidemia (23). A family history of premature CAD was defined when there were any first-degree male relative <55 years old or a female relative <65 years old having CAD (24). ST-segment elevation myocardial infarction (STEMI) was confirmed if AMI patients had new ST-elevation at the J-point in two contiguous leads with a cut-off point of ≥1 mm in all leads other than leads V2-V3, where the following cut-off points applied: ≥ 2 mm in men ≥40 years, ≥ 2.5 mm in men <40 years, and ≥1.5 mm in women regardless of age (16). Non-ST-segment elevation myocardial infarction (NSTEMI) was confirmed if AMI patients had new horizontal or downsloping ST-depression of ≥0.5 mm in two contiguous leads and/or T inversion >1 mm in two contiguous leads with a prominent R wave or an R/S ratio >1 (16).
Assessment of coronary angiography
All participants underwent contrast-enhanced coronary angiography, and at least two interventional physicians assessed the results. Significant lesions of coronary arteries were defined as ≥50% stenosed diameter on coronary angiography. The Gensini score was applied by two experienced cardiologists to determine the severity of CAD (5). When Gensini scores were not consistent between the two assessors, they discussed with each other and decided the final exact result. The degree of stenosis and the location of the coronary artery lesions were scored using the following scale: 1 point for less than 25% stenosis, 2 points for 26%–50% stenosis, 4 points for 51%–75% stenosis, 8 points for 76%–90% stenosis, 16 points for 91%–99% stenosis, and 32 points for total occlusion (5).
Each lesion score was then multiplied by a factor that took into consideration the importance of the lesion's site in the coronary tree (5 for the left main coronary artery, 2.5 for the proximal segment of the left anterior descending artery, 2.5 for the proximal segment of the circumflex artery, 1.5 for the mid-segment of the left anterior descending artery, 1.0 for the right coronary artery, the distal segment of the left anterior descending artery, the posterolateral artery, and the obtuse marginal artery, and 0.5 for other segments). The sum of the scores from each coronary segment was used to calculate the Gensini score (5).
Genetic analysis
The ACE I/D genotypes were identified by the polymerase chain reaction (PCR) method at the Center for Molecular Biomedicine, University of Medicine and Pharmacy at Ho Chi Minh City, Vietnam. A sample of 2 milliliters (ml) of venous blood was taken from each patient, placed in a tube anticoagulated with EDTA, and gently shaken. Genomic DNA from white blood cells was extracted within 24 h using the GeneJet TM Whole Blood Genomic DNA Purification Mini Kit (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer's instructions. Primers for PCR were designed using CLC Main Workbench software based on the human ACE gene sequence (Genebank NG_011648).
The conditions for PCR amplification to create sequence regions were that each PCR tube had a volume of 15 microliters (µl) containing the following components: 1.5 μl PCR buffer 10X; 1.5 μl dNTP 2.5 mM; 0.75 μl each forward and reverse primer (10 nM/μl), 0.1 μl TaKaRa TaqTM HotStart Polymerase (Takara, Japan), 2 μl genomic DNA (20–50 ng/μl), and 8.4 μl double distilled deionized water. PCR responses were accompanied by a negative control that did not contain DNA for external infection control and positive controls that were variants of the ACE I/D previously identified by the Sanger DNA sequencing. Thermal cycles for PCR were performed using a Mastercycler@Pro S (Eppendorf, Hamburg, Germany). The thermal cycles consisted of one cycle at 98°C for 3 min and 40 cycles of denaturation at 98°C for 10 s, annealing at 60°C for 20 s, extension at 72°C for 30 s, and final extension at 72°C for 2 min. PCR products were stored at 4°C and identified by electrophoresis on 2% agarose agar, stained with Gel Red, and observed using a Geldoc-ItTM electrophoresis imaging system (UVP, USA). A single band of 510 bp was observed for the II genotype and 206 bp for the DD genotype; both bands were observed for the heterozygous ID genotype. The protocol for ACE I/D genotyping is described in Supplementary Material, Table S1. To assure the genotyping accuracy, 10% of the samples were randomly chosen and directly sequenced using the previously described protocol with appropriate primers (25, 26).
Data analysis
Data were analyzed using SPSS 22.0 for Windows software (SPSS, Inc). Continuous variables are presented as mean (standard deviation) or median (interquartile range) when depending on data distribution. Categorical variables were presented in the form of frequency and percentages. Differences in characteristics between three ACE I/D genotypes were assessed using Chi-squared or Fisher's exact tests for categorical variables and ANOVA or Kruskal Wallis tests for continuous variables. Differences in Gensini scores between ACE I/D genotypes in the genetic models (the recessive model: DD vs. II + ID, the dominant model: II vs. ID + DD, the homozygous model: DD vs. II, and the heterozygous model: ID vs. II) were compared using the Student's t-tests or the Mann-Whitney U tests.
The association between participant's characteristic and the severity of CAD in AMI were investigated first by univariate linear regression analysis. Multivariable linear regression model was then fitted using all significant variables in univariate analysis. A p-value of < 0.05 was considered statistically significant.
Results
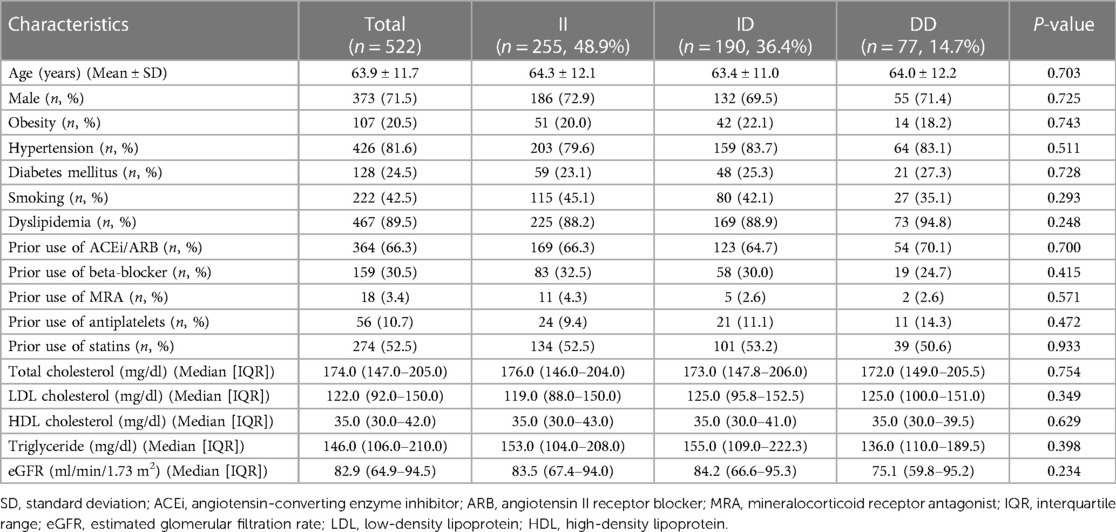
During the study period, 522 AMI patients were recruited. The baseline characteristics of participants are presented in Table 1. The mean age of subjects was 63.9 ± 11.7. Males were predominant (71.5%). Dyslipidemia and hypertension were the most common cardiovascular risk factors. The percentages of ACE II, ID, and DD genotypes were 48.9%, 36.4%, and 14.7%, respectively. There was no significant difference in clinical and laboratory parameters between ACE I/D genetic polymorphism categories.
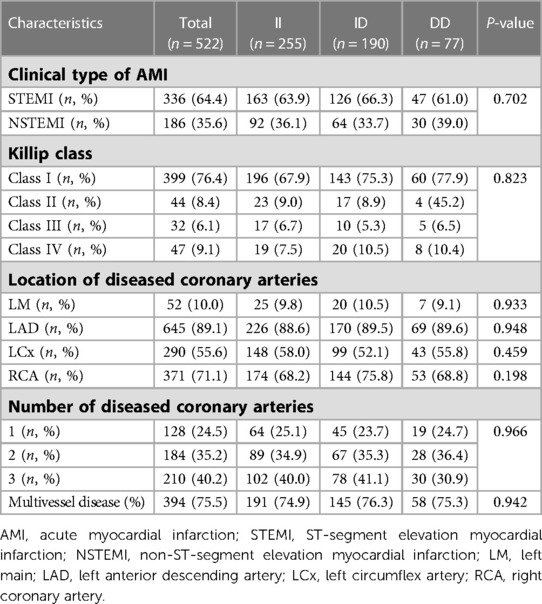
The clinical type of AMI, Killip class and coronary angiographic features are shown in Table 2. STEMI occurred in 64.4% of patients, the majority of whom exhibited Killip class I (76.4%). The percentage of multivessel disease was 75.5%. There was no significant difference in the location and number of diseased coronary arteries between ACE I/D genotypes.
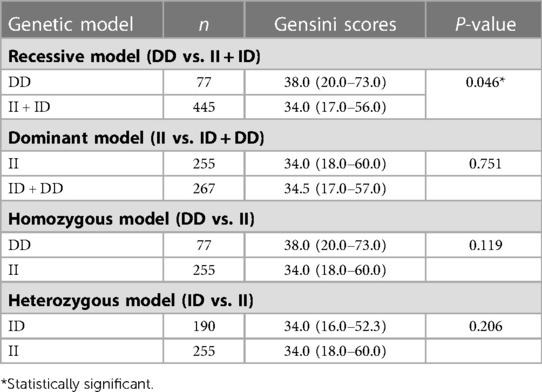
The median Gensini score was 34.3 (IQR = 17.0–58.3). The ACE DD genotype was associated with the severity of CAD evaluated by the Gensini score in the recessive model (DD vs. II + ID) (Table 3). Patients with DD genotype had higher Gensini scores than those with ID or II genotypes (p = 0.046). This association was not found in the other genetic models including the dominant, homozygous, and heterozygous models.
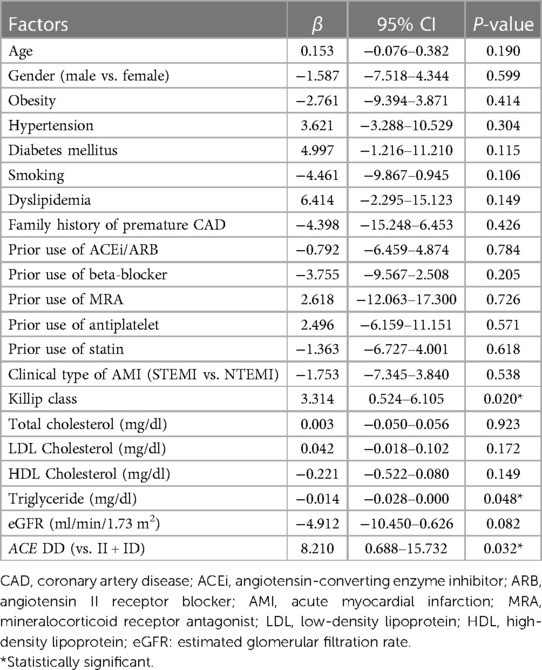
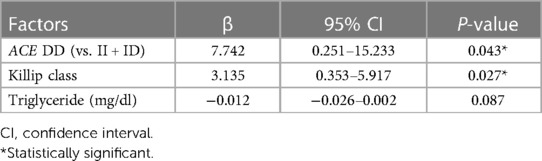
In terms of factors affecting the severity of CAD in AMI patients, univariate linear regression analysis showed that the ACE I/D genetic variant, Killip class, and triglyceride concentration were statistically associated with the Gensini score (Table 4). In multivariable linear regression, ACE I/D genetic polymorphism remained statistically associated with the Gensini score. DD-carrying participants presented higher Gensini scores compared with II or ID-carrying subjects (Table 5).
Discussion
In this study, patients with AMI had the lowest percentage of the DD genotype (14.7%) compared to the II (48.9%) and ID (36.4%) genotypes. Studies in other Asian countries also found the ACE DD genotype to account for the lowest proportion in Japanese, Chinese, and Indian (27–29). In contrast, studies of Western and African populations demonstrated that the II genotype has the lowest proportion (11, 30, 31). Despite differences in the distribution of ACE I/D genotypes, several studies indicated that the D allele and DD genotype were associated with the risk of AMI and CAD in both Asians and Caucasians (15, 32).
The Gensini score is frequently used to determine the severity of CAD based on coronary angiography (4, 5, 33). In our study, ACE I/D and Killip class were found to be associated with the severity of CAD assessed by Gensini score in patients diagnosed with their first AMI. Factors that were previously reported as associated factors of the severity of CAD (age, gender, cardiovascular risk factors, clinical type of AMI and prior use of ACE inhibitors, angiotensin II receptor blockers, mineralocorticoid receptor antagonists, beta-blockers, antiplatelets, and statins) were not statistically different between ACE I/D genotypes. The Gensini score was found to be significantly higher in DD compared with II or ID-carrying participants after adjustment for confounding factors, such as serum triglyceride concentration and Killip class. Chen et al. showed that acute coronary syndrome patients with the DD genotype have a 3.87-fold increased risk of stenosis in three coronary vessels, a 3.08-fold higher risk of stenosis of the left anterior descending artery, and a 3.07-fold higher risk of anterior wall infarction (6). Another study also indicated that the D allele was associated with the number of stenosed coronary arteries, especially its interaction with cardiovascular risk factors, such as sex (male), age (>65 years old), hypertension, diabetes, obesity, and smoking (7). In a group of 647 patients who underwent elective coronary angiography, Borzyszkowska et al. found a significant association between the ACE DD genotype and the Gensini score in men with high total cholesterol, high LDL cholesterol, and low HDL cholesterol levels (34). These consistent data emphasize the role of ACE I/D as a potential marker for the severity of coronary lesions in patients diagnosed with AMI beside traditional cardiovascular risk factors.
Like other Asian populations, the mechanism of coronary artery disease, especially acute myocardial infarction, in Vietnamese patients might be caused by both traditional cardiovascular risks and genetic factors (35, 36). The observed associations of ACE I/D with the severity of coronary artery lesions might be explained by the fact that ACE I/D polymorphism is responsible for 20%-50% of the variability in serum ACE level in the following order: DD > ID > II (37). A high concentration of ACE leads to increased synthesis of angiotensin II and the inactivation of bradykinin, which results in increased vascular resistance and hypertension (38). In addition, angiotensin II regulates the proliferation, migration, and hypertrophy of vascular smooth muscle cells and therefore plays a crucial role in the pathophysiology of coronary atherosclerosis (39).
There were several limitations to this study. First, our study is from a single center, and thus may not represent the characteristics of AMI and ACE I/D genetic polymorphism in the wider Vietnamese population. Second, the Gensini score was chosen to evaluate the severity of CAD in AMI, which did not consider bifurcation, calcification and distortion of coronary artery lesions compared to the SYNTAX score. Nevertheless, the Gensini score has been widely used in studies where significantly positive association between the Gensini score with the GRACE score and prognosis in AMI patients were reported (4, 33). Third, the serum ACE concentration was not measured and determined its association with different ACE I/D genotypes and the severity of CAD. Finally, as AMI is a polygenic disease, ACE I/D may not comprehensively explain the difference in CAD severity between genotype groups. Thus, further research with complete genetic analysis, such as genome-wide association study, is required to better understand the association between genetic factors and the severity of CAD in Vietnamese patients with AMI.
In conclusion, the ACE DD genotype was found to be associated with the severity of CAD compared with the II or ID genotypes in Vietnamese patients with their first AMI. To the best of our knowledge, this work is the first study in Vietnam describing the association between a genetic polymorphism and the severity of coronary artery lesion, and it contributes to the literature of the role of ACE I/D polymorphism in the pathophysiology of AMI in Asian populations. Therefore, ACE I/D should be considered to improve the comprehensive assessment and aggressive treatment of AMI patients.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary materials, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee for Biomedical Research of the University of Medicine and Pharmacy at Ho Chi Minh City, Vietnam. The patients/participants provided their written informed consent to participate in this study.
Author contributions
DT, TT, SH, BT, and MD designed the study. DT, SH and LL recruited the patients for the study. LL, MD, and DT performed the genotyping procedure. DT, TT, SH, BT, and MD analyzed the data. DT, MD, and TT wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported partially by the University of Medicine and Pharmacy at Ho Chi Minh City, Vietnam. Duy Cong Tran was funded by Vingroup JSC and supported by the Masters and PhD Scholarship Programme of Vingroup Innovation Foundation (VINIF), Institute of Big Data, code VINIF.2022.TS027.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1091612/full#supplementary-material.
References
1. Anderson JL, Morrow DA. Acute myocardial infarction. N Engl J Med. (2017) 376:2053–64. doi: 10.1056/NEJMra1606915
2. Ye Q, Zhang J, Ma L. Predictors of all-cause one-year mortality in myocardial infarction patients. Medicine (Baltimore). (2020) 99(29):e21288. doi: 10.1097/MD.0000000000021288
3. Li XT, Fang H, Li D, Xu FQ, Yang B, Yang R, et al. Association of platelet to lymphocyte ratio with in-hospital major adverse cardiovascular events and the severity of coronary artery disease assessed by the Gensini score in patients with acute myocardial infarction. Chin Med J. (2020) 133:415–23. doi: 10.1097/CM9.0000000000000650
4. Hoang SV, Nguyen KM, Nguyen AH, Huynh KLA, Tran HPN. The value of the Global Registry of Acute Coronary Events and Gensini scores in predicting long-term outcomes of Vietnamese patients with non-ST-elevation acute coronary syndrome. Biomed Res Ther. (2021) 8:4233–41. doi: 10.15419/bmrat.v8i2.662
5. Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. (1983) 51:606. doi: 10.1016/S0002-9149(83)80105-2
6. Chen YH, Liu JM, Hsu RJ, Hu SC, Harn HJ, Chen SP, et al. Angiotensin converting enzyme DD genotype is associated with acute coronary syndrome severity and sudden cardiac dealth in Taiwan: a case-control emergency room study. BMC Cardiovasc Disord. (2012) 12:6–15. doi: 10.1186/1471-2261-12-6
7. Vladeanu MC, Bojan IB, Bojan A, Iliescu D, Badescu MC, Badulescu OV, et al. Angiotensin-converting enzyme gene D-allele and the severity of coronary artery disease. Exp Ther Med. (2020) 20:3407–11. doi: 10.3892/etm.2020.8978
8. Danser AHJ, Schunkert H. Renin–angiotensin system gene polymorphisms: potential mechanisms for their association with cardiovascular diseases. Eur J Pharmacol. (2000) 410:303–16. doi: 10.1016/s0014-2999(00)00823-2
9. Rigat B, Hubert C, Alhenc-Gelas F, Cambien F, Corvol P, Soubrier F. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest. (1990) 86:1343–6. doi: 10.1172/JCI114844
10. Danser AHJ, Schalekamp MADH, Bax WA, Van den-Brink AM, Saxena PR, Riegger GAJ, et al. Angiotensin-converting enzyme in the human heart: effect of the deletion/insertion polymorphism. Circulation. (1995) 92:1387–8. doi: 10.1161/01.cir.92.6.1387
11. Cambien F, Poirier O, Lecerf L, Evans A, Cambou JP, Arveiler D, et al. Deletion polymorphism in the gene for angiotensin-converting enzyme is a potent risk factor for myocardial infarction. Nature. (1992) 359:641–4. doi: 10.1038/359641a0
12. Keavney B, McKenzie C, Parish S, Palmer A, Clark S, Youngman L, et al. Large-scale test of hypothesised associations between the angiotensin-converting-enzyme insertion/deletion polymorphism and myocardial infarction in about 5000 cases and 6000 controls. International studies of infarct survival (ISIS) collaborators. Lancet. (2000) 355:434–42. doi: 10.1016/s0140-6736(00)82009-7
13. Pulla Reddy B, Srikanth Babu BM, Venkata Karunakar K, Jasovanthi A, Munshi A, Sampath Kumar A, et al. Angiotensinconverting enzyme gene variant and its levels: risk factors for myocardial infarction in a South Indian population. Singapore Med J. (2010) 51:576–81.20730398
14. Andrikopoulos GK, Richter DJ, Needham EW, Tzeis SE, Zairis MN, Gialafos EJ, et al. The paradoxical association of common polymorphisms of the renin–angiotensin system genes with risk of myocardial infarction. Eur J Cardiovasc Prev Rehabil. (2004) 11:477–83. doi: 10.1097/00149831-200412000-00006
15. Chen Y, Dong S, He M, Qi T, Zhu W. Angiotensin-converting enzyme insertion/deletion polymorphism and risk of myocardial infarction in a updated meta-analysis based on 34993 participants. Gene. (2013) 522:196–205. doi: 10.1016/j.gene.2013.03.076
16. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al.; executive group on behalf of the joint European society of cardiology (ESC)/American college of cardiology (ACC)/American heart association (AHA)/world heart federation (WHF) task force for the universal definition of myocardial infarction. Fourth universal definition of myocardial infarction. Circulation. (2018) 138:e618–51. doi: 10.1161/CIR.0000000000000617
17. World Health Organization. Regional office for the Western Pacific. The Asia-pacific perspective: Redefining obesity and its treatment. Sydney: Health Communications Australia. (2000). Available online at: https://apps.who.int/iris/handle/10665/206936 (accessed October 24, 2022).
18. Executive summary of the clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. Arch Intern Med. (1998) 158:1855–67. doi: 10.1001/archinte.158.17.185530
19. Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. (1987) 317:1098. doi: 10.1056/NEJM198710223171717
20. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. ESC Scientific document group. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. (2018) 39:3021–104. doi: 10.1093/eurheartj/ehy339
21. American Diabetes Association. Classification and diagnosis of diabetes: standards of medical care in diabetes. Diabetes Care. (2018) 41(Suppl 1):S13–27. doi: 10.2337/dc18-S002
22. Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, et al. Heart disease and stroke statistics—2022 update: a report from the American heart association. Circulation. (2022) 145:e153–639. doi: 10.1161/CIR.0000000000001052
23. National cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. (2002) 106:3143–421. doi: 10.1161/circ.106.25.3143
24. Isordia-Salasa I, Alvarado-Morenoa JA, Jimenez-Alvaradob RM, Hernandez-Juareza J, Santiago-German D, Leanos-Mirandad A, et al. Association of renin–angiotensin system genes polymorphisms and risk of premature ST elevation myocardial infarction in young Mexican population. Blood Coagul Fibrinolysis. (2018) 29:267–74. doi: 10.1097/MBC.0000000000000714
25. Do MD, Pham DV, Le LP, Le LHG, Tran LBM, Huynh MDD, et al. Recurrent PROC and novel PROS1 mutations in Vietnamese patients diagnosed with idiopathic deep venous thrombosis. Int J Lab Hematol. (2021) 43(2):266–72. doi: 10.1111/ijlh.13345
26. Mai PT, Le DT, Nguyen TT, Gia HLL, Le THN, Le M, et al. Novel GDAP1 mutation in a Vietnamese family with Charcot-Marie-Tooth disease. Biomed Res Int. (2019) 2019:7132494. doi: 10.1155/2019/7132494
27. Yoshida M, Iwai N, Ohmichi N, Izumi M, Nakamura Y, Kinoshita M. D allele of the angiotensin-converting enzyme gene is a risk factor for secondary cardiac events after myocardial infarction. Int J Cardiol. (1999) 70:119–25. doi: 10.1016/s0167-5273(99)00064-9
28. Zhao W, Ma ST, Cui LQ. Meta-analysis of angiotensin-converting enzyme insertion/deletion polymorphism and myocardial infarction in Han Chinese. Genet Mol Res. (2015) 14:8068–76. doi: 10.4238/2015.July.17.15
29. Baruah S, Chaliha MS, Borah PS, Rajkakati R, Borua PK, Mahanta J. Insertion/insertion genotype of angiotensin I converting-enzyme gene predicts risk of myocardial infarction in North East India. Biochem Genet. (2016) 54:34–46. doi: 10.1007/s10528-015-9706-9
30. Mehri S, Baudin B, Mahjoub S, Zaroui A, Bénéteau-Burnat B, Mechmeche R, et al. Angiotensin-converting enzyme insertion/deletion gene polymorphism in a Tunisian healthy and acute myocardial infarction population. Genet Test Mol Biomarkers. (2010) 14:85–91. doi: 10.1089/gtmb.2009.0105
31. Martinez-Quintana E, Chirino R, Nieto-Lago V, Pérez-Jiménez P, López-Ríos L, Rodríguez-González F. Prognostic value of ACE I/D, AT1R A1166C, PAI-I 4G/5G and GPIIIa a1/a2 polymorphisms in myocardial infarction. Cardiol J. (2014) 21:229–37. doi: 10.5603/CJ.a2013.0107
32. Staessen JA, Wang JG, Ginocchio G, Petrov V, Saavedra AP, Soubrier F, et al. The deletion/insertion polymorphism of the angiotensin converting enzyme gene and cardiovascular-renal risk. J Hypertens. (1997) 15:1579–92. doi: 10.1097/00004872-199715120-00059
33. Wang K-Y, Zheng Y-Y, Wu T-T, Ma Y-T, Xie X. Predictive value of Gensini score in the long-term outcomes of patients with coronary artery disease who underwent PCI. Front Cardiovasc Med. (2022) 8:778615. doi: 10.3389/fcvm.2021.778615
34. Borzyszkowska J, Stanislawska-Sachadyn A, Wirtwein M, Sobiczewski W, Ciecwierz D, Targonski R, et al. Angiotensin converting enzyme gene polymorphism is associated with severity of coronary artery disease in men with high total cholesterol levels. J Appl Genetics. (2012) 53:175–82. doi: 10.1007/s13353-012-0083-3
35. Sucato V, Coppola G, Testa G, Amata F, Martello M, Siddique R, et al. Coronary artery disease in South Asian patients: cardiovascular risk factors, pathogenesis and treatments. Curr Probl Cardiol. (2022):1–17. doi: 10.1016/j.cpcardiol.2022.101228. [Epub ahead of print]
36. Sucato V, Coppola G, Testa G, Amata F, Martello M, Siddique R, et al. Evaluation of remnant cholesterol levels and monocyte-to-HDLcholesterol ratio in South Asian patients with acute coronary syndrome. Nutr Metab Cardiovasc Dis. (2021) 31(7):2144–50. doi: 10.1016/j.numecd.2021.04.007
37. Cambien F, Costerousse O, Tiret L, Poirier O, Lecerf L, Gonzales MF, et al. Plasma level and gene polymorphism of angiotensin-converting enzyme in relation to myocardial infarction. Circulation. (1994) 90:669–76. doi: 10.1161/01.cir.90.2.669
38. Murphey LJ, Gainer JV, Vaughan DE, Brown NJ. Angiotensin-converting enzyme insertion/deletion polymorphism modulates the human in vivo metabolism of bradykinin. Circulation. (2000) 102:829–32. doi: 10.1161/01.cir.102.8.829
Keywords: genetic polymorphism, coronary artery disease, Gensini score, acute myocardial infarction, Vietnamese, ACE I/D
Citation: Tran DC, Le LHG, Thai TT, Hoang SV, Do MD and Truong BQ (2023) Association between ACE I/D genetic polymorphism and the severity of coronary artery disease in Vietnamese patients with acute myocardial infarction. Front. Cardiovasc. Med. 10:1091612. doi: 10.3389/fcvm.2023.1091612
Received: 7 November 2022; Accepted: 20 April 2023;
Published: 3 May 2023.
Edited by:
Olivier M. Vanakker, Ghent University, BelgiumReviewed by:
Vincenzo Sucato, University of Palermo, ItalyMasoud Sadeghi, Islamic Azad University, Iran
Yaser Jenab, Tehran University of Medical Sciences, Iran
© 2023 Tran, Le, Thai, Hoang, Do and Truong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Minh Duc Do ZHVjbWluaEB1bXAuZWR1LnZu Binh Quang Truong YmluaC50cUB1bWMuZWR1LnZu
 Duy Cong Tran
Duy Cong Tran Linh Hoang Gia Le4
Linh Hoang Gia Le4 Truc Thanh Thai
Truc Thanh Thai Minh Duc Do
Minh Duc Do