
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Cardiovasc. Med., 23 September 2022
Sec. Cardiovascular Therapeutics
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.944902
Background: The cardiovascular protection effect of metformin on patients with type 2 diabetes mellitus (T2DM) remains inconclusive. This systemic review and meta-analysis were to estimate the effect of metformin on mortality and cardiovascular events among patients with T2DM.
Methods: A search of the Pubmed and EMBASE databases up to December 2021 was performed. Adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) were pooled by a random-effects model with an inverse variance method.
Results: A total of 39 studies involving 2473009 T2DM patients were adopted. Compared to non-metformin therapy, the use of metformin was not significantly associated with a reduced risk of major adverse cardiovascular event (MACE) (HR = 1.06, 95%CI 0.91–1.22; I2 = 82%), hospitalization (HR = 0.85, 95%CI 0.64–1.13; I2 = 98%), heart failure (HR = 0.86, 95%CI 0.60–1.25; I2 = 99%), stroke (HR = 1.16, 95%CI 0.88–1.53; I2 = 84%), and risk of AMI (HR = 0.88, 95%CI 0.69–1.14; I2 = 88%) in T2DM patients. Metformin was also not associated with significantly lowered risk of MACE compared to dipeptidyl peptidase-4 inhibitor (DPP-4i) in T2DM patients (HR = 0.95, 95%CI 0.73–1.23; I2 = 84%).
Conclusions: The effect of metformin on some cardiovascular outcomes was not significantly better than the non-metformin therapy or DPP-4i in T2DM patients based on observational studies.
Cardiovascular disease (CVD) is the predominant cause of death globally, resulting in a great economic burden and a tremendous threat to public health. Approximately 19.05 million deaths are estimated to CVD globally (1). Moreover, the incidence of CVD has been increasing or stagnating among younger individuals (aged 18–50 years) over the past few decades (2). Study shows that T2DM significantly increase the risk of CVD and aggressive glycemic control can reduce both macrovascular and microvascular events in T2DM patients (3).
Metformin, a biguanide derivative, has been used as a first-line hypoglycemic treatment for type 2 diabetes mellitus (T2DM) patients since 1957 when it was recommended by the European Association for the Study of Diabetes (EASD) and the American Diabetes Association (ADA) (4). Apart from its hypoglycemic effect, metformin has been found to confer protection against breast cancer (5), polycystic ovary syndrome (6), and neural recovery in patients with brain tumors (7). Metformin is also associated with a lower risk of major adverse cardiovascular events (MACEs) and all-cause mortality (8, 9). The UK Prospective Diabetes Study (UKPDS) shows that metformin has an effect of lowering the risk of cardiovascular morbidity and mortality over 20 years (10). Metformin can reduce the risk of heart failure, hospitalization, and stroke in patients with T2DM (10, 11). In T2DM patients with chronic kidney disease or heart failure, metformin may also show a cardiovascular protection effect (12, 13). Compared with other classic hypoglycemic agents (e.g., sulphonylurea), metformin reduces the risks of all-cause or cardiovascular mortality, stroke, and heart failure (14). When compared with new antidiabetic drugs such as sodium-glucose cotransporter-2 inhibitors, metformin is associated with a low rate of genital infection and ketoacidosis (15). However, recent conflicting reports have shown that metformin could not reduce all-cause and cardiovascular mortality (16, 17). Moreover, the combination of metformin and other hypoglycemic drugs may even impose higher death risks (17).
Han et al. (18) found Metformin could reduce cardiovascular mortality, all-cause mortality, and cardiovascular events in coronary artery disease (CAD) patients. However, Han's study only targeted CAD patients rather than the common population. Since more observational studies showed up and current observational evidence on the effect of metformin on CVD risk was still inconclusive, we carried out this meta-analysis on a synthesis of published data to estimate adverse cardiovascular outcomes following metformin treatment in patients with T2DM.
Overall, the corresponding authors designed the research criteria, and two reviewers independently performed the literature search, study selection, data abstraction, quality assessment, and data analysis. Disagreements were resolved by discussion between two reviewers, or consultation with the corresponding authors. Ethical approval was not necessary for this study because only the published studies were included.
We included observational retrospective or prospective cohort studies, in which the adverse outcomes were compared between patients with T2DM treated with metformin monotherapy and those treated with any other single drug or diet/lifestyle modification. The adverse outcomes of interest included all-cause mortality, MACE, hospitalization, heart failure, cardiovascular mortality, stroke, and AMI. The primary outcome was MACE, whereas others were secondary outcomes. The definitions of the studied outcomes were applied that were reported in the originally included studies.
We excluded studies focusing on patients with type 1 diabetes mellitus or patients without T2DM but with metformin treatment. Studies in which patients with T2DM were treated with two or more antidiabetic drugs or with one antidiabetic drug combined with insulin were also excluded. Certain publication types were excluded (e.g., reviews, comments, case reports, case series, letters, editorials, and meeting abstracts) due to insufficient data.
A prior meta-analysis by Han et al. (18) has studied the effect of metformin on adverse outcomes in patients with T2DM, and the end date of the literature search in this study was October 2019. Therefore, we abstracted the included studies for the meta-analysis by Han et al., and then systemically searched the PubMed and Embase databases from January 2019 to December 2021 to identify studies about the effect of metformin monotherapy vs. other treatments on adverse outcomes in patients with T2DM. The search terms combined with “AND” were applied as follows: (1) “metformin,” (2) “diabetes mellitus” OR “diabetes.” No linguistic restrictions were applied in the literature search.
We first screened the titles and abstracts of the retrieved studies from the PubMed and Embase databases, and subsequently, read the full texts of the potential studies. Those studies included in the prior meta-analysis by Han et al. (18) were also checked. Eligible studies would be chosen based on the pre-defined inclusion criteria. The following information of the included studies was collected: first author, publication year, country, study design, patient characteristics (study population, sample size, age, sex), follow-up time, type of treatment compared to metformin, sample size, and the number of events in the metformin and control groups, and adverse outcomes.
The Newcastle-Ottawa Scale (NOS) tool was used to assess the quality of cohort studies. The NOS tool had three domains with a total of nine points: the selection of population (0–4 points), the comparability between experimental groups and control groups in the study (0–2 points), and the assessment of the outcome (0–3 points). In this meta-analysis, the study with a NOS score of less than 6 points was defined as low quality (19). This assessment method was used previously (20).
The statistical heterogeneity across the included studies was assessed using the P-value of the Cochrane Q test and the I2 statistic, where a P < 0.10 in the Cochrane Q test or an I2 > 50% suggested significant heterogeneity. The adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) were considered as the effect sizes, and we converted them to the natural logarithms and standard errors, which were pooled by a random-effects model with an inverse variance method. The data analyses were performed based on the type of treatment and complications of patients. In the sensitivity analysis, we re-performed the above-mentioned analyses by deleting studies in which the sample size was smaller than 1,000 in either the control group or the experimental group. The publication bias for the reported effect estimates was assessed by funnel plots, egger and begg tests, and trim and fill analyses.
All the statistical analyses of this meta-analysis were performed using the Review Manager version 5.4 software (the Cochrane Collaboration 2014, Nordic Cochrane Centre Copenhagen, Denmark; https://community.cochrane.org/). In this study, a P-value of less than 0.05 was considered statistically significant.
The flow chart of the literature retrieval is presented in Supplementary Figure 1. A total of 10,244 studies were retrieved from the PubMed and Embase databases for the title and abstract screenings, after which 32 potential studies from databases were pooled with the other 34 studies included in the prior meta-analysis by Han et al. (18) to receive full-text screenings. Then we excluded 27 studies for the following reasons: (1) 21 studies did not focus on the adverse outcomes we set in the inclusion criteria (21–39); (2) 3 studies included patients without type 2 diabetes mellitus (40–42); (3) 1 ongoing study without outcomes (43); (4) 1 research investigated methodology of estimating the effect of metformin (44); (5) 1 study used metformin as the baseline drug in combination therapy (45). Finally, 39 studies [8 prospective cohort studies (15, 46–52) and 31 retrospective cohort studies (11, 13, 19, 26, 53–79)] were included in this meta-analysis.
Table 1 shows the baseline characteristics of the included studies. Twenty-seven studies investigated the effect of metformin on all-cause mortality (13, 19, 46–53, 55–59, 61, 62, 64–66, 68, 72–74, 77–79), 16 studies investigated the effect of metformin on risk of MACE (26, 49, 52, 55, 60, 62, 64, 65, 69, 72, 74–79), 18 studies investigated the effect of metformin on risk of hospitalization (11, 13, 15, 47, 49, 50, 52, 56–60, 63, 67, 69, 71–73), 14 studies investigated the effect of metformin on risk of heart failure (11, 15, 49, 50, 52, 53, 56, 57, 59, 67–69, 71, 73), 14 studies investigated the effect of metformin on cardiovascular mortality (19, 26, 46, 47, 49, 50, 56, 59, 60, 62, 64, 71, 74, 75), 8 studies investigated the effect of metformin on risk of stroke (15, 26, 49, 57, 65, 68, 69, 74), and 6 studies investigated the effect of metformin on the risk of AMI (15, 26, 49, 54, 69, 74). Only 1 retrospective cohort study by Liu et al. (19) had a low quality with 4 points assessed by the NOS tool.
As shown in Figures 1A–C, the use of metformin was not associated with a decrease in the risk of MACE when compared to non-metformin (HR = 1.06, 95%CI 0.91–1.22; I2 = 82%). Specifically, metformin was associated with a decreased risk of MACE when compared to sulphonylurea (HR = 0.83, 95%CI 0.77–0.90; I2 = 48%). What's more, the use of metformin did not alter the risk of MACE significantly compared to dipeptidyl peptidase-4 inhibitor (DPP-4i) in T2DM patients (HR = 0.95, 95%CI 0.73–1.23; I2 = 84%).
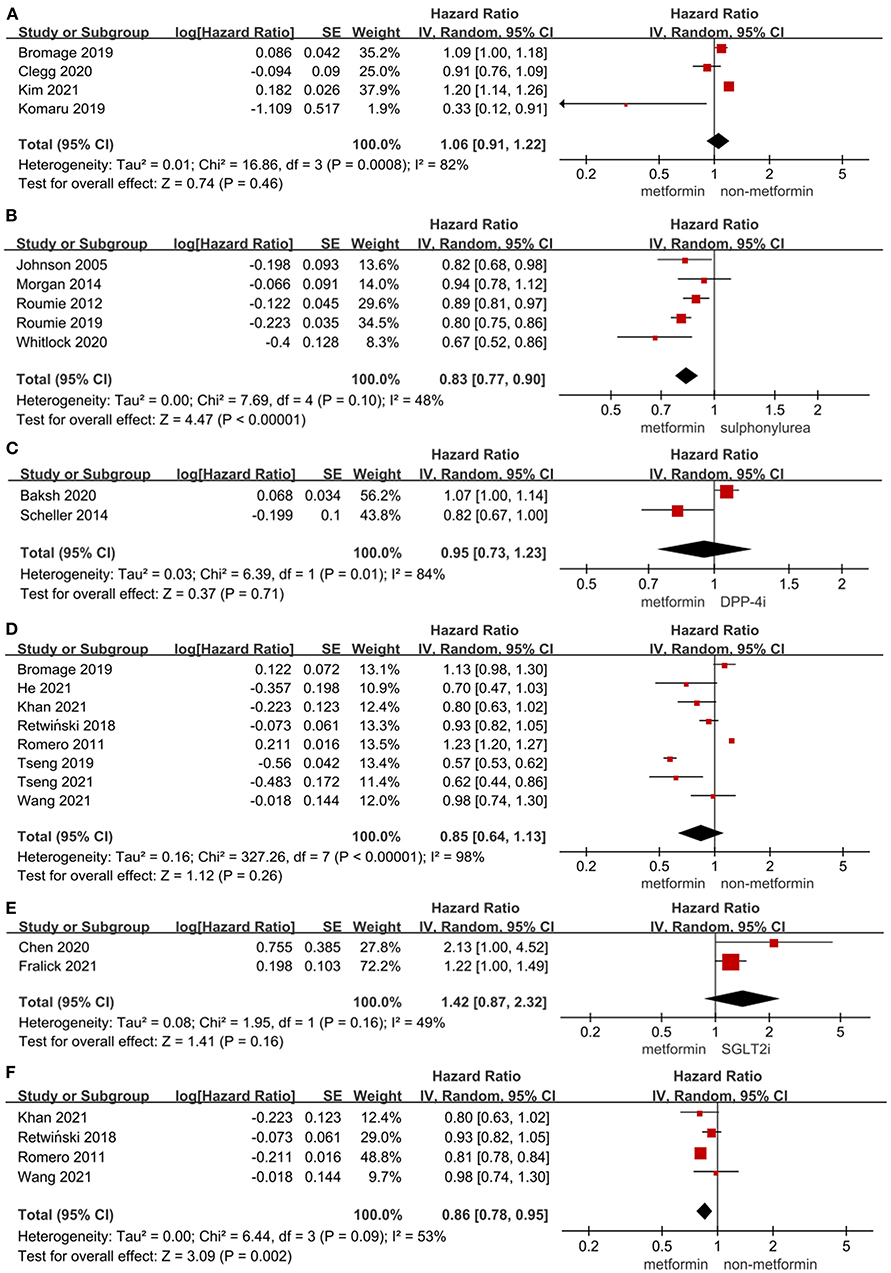
Figure 1. (A) Forest plot of hazard ratio of MACE among patients with metformin therapy vs non-metformin therapy. (B) Forest plot of hazard ratio of MACE among patients with metformin therapy vs. sulphonylurea therapy. (C) Forest plot of hazard ratio of MACE among patients with metformin therapy vs. DPP-4i therapy. (D) Forest plot of hazard ratio of hospitalization among patients with metformin therapy vs non-metformin therapy. (E) Forest plot of hazard ratio of hospitalization among patients with metformin therapy vs. SGLT2i. (F) Forest plot of hazard ratio of hospitalization among heart failure patients with metformin therapy vs non-metformin therapy. CI, confidence interval; SE, standard error; IV, inverse of the variance.
The results of the effect of metformin on all-cause mortality in T2DM patients were presented in Figures 2B–F, showing that the use of metformin was associated with a significantly lower all-cause mortality in T2DM patients compared to non-metformin therapy (HR = 0.82, 95%CI 0.77–0.88; I2 = 73%), sulphonylurea (HR = 0.58, 95%CI 0.49–0.68; I2 = 74%), and diet therapy (HR = 0.76, 95%CI 0.64–0.90; I2 = 0%). Also, there was a significant reduction in all-cause mortality in T2DM patients with heart failure (HR = 0.84, 95%CI 0.84–0.88; I2 = 40%) or CKD (HR = 0.79, 95%CI 0.75–0.82; I2 = 0%) using metformin vs. non-metformin therapy. In addition, two studies by Scheller et al. (78) and Chen et al. (57), respectively, compared the effect of metformin on all-cause mortality in T2DM patients with DPP-4i (HR = 0.8, 95%CI 0.58–1.09) and sodium-dependent glucose transporter-2 inhibitor (SGLT-2i) (HR = 2.04, 95%CI 1.82–2.27).

Figure 2. (A) Forest plot of hazard ratio of hospitalization among patients with metformin therapy vs. sulphonylurea therapy. (B) Forest plot of hazard ratio of all-cause mortality among patients with metformin therapy vs. non-metformin therapy. (C) Forest plot of hazard ratio of all-cause mortality among patients with metformin therapy vs. sulphonylurea therapy. (D) Forest plot of hazard ratio of all-cause mortality among patients with metformin vs. diet therapy. (E) Forest plot of hazard ratio of all-cause mortality among heart failure patients with metformin therapy vs. non-metformin therapy. (F) Forest plot of hazard ratio of all-cause mortality among patients with CKD treated with metformin therapy vs non-metformin therapy. CI, confidence interval; SE, standard error; IV, inverse of the variance.
Figures 1D–F, 2A presented the effect of metformin on hospitalization in T2DM patients. Metformin was not associated with a significant lower risk of hospitalization in T2DM patients compared to non-metformin therapy (HR = 0.85, 95%CI 0.64–1.13; I2 = 98%) and SGLT2i (HR = 1.42, 95%CI 0.87–2.32; I2 = 49%), but it significantly lowered the risk of hospitalization compared to non-metformin therapy (HR = 0.86, 95%CI 0.78–0.95; I2 = 53%) in T2DM patients with heart failure and sulphonylurea (HR = 0.83, 95%CI 0.78–0.88; I2 = 40%) in T2DM patients. And as reported by Baksh (69) the use of metformin was not associated with a significantly lower risk of hospitalization compared with DPP-4i in T2DM patients (HR = 1.04, 95%CI 0.77–1.40).
As shown in Figure 3, the use of metformin was not associated with a significantly lower risk of heart failure in T2DM patients compared to non-metformin therapy (HR = 0.86, 95%CI 0.60–1.25; I2 = 99%). However, metformin significantly lowered the risk of heart failure compared to sulphonylurea (HR = 0.80, 95%CI 0.76–0.85; I2 = 0%) and the risk of recurrent incidents of heart failure compared to non-metformin therapy (HR = 0.82, 95%CI 0.76–0.87; I2 = 7%) in T2DM patients. And metformin was not significantly associated with reduced risk of heart failure compared to diet therapy (HR = 0.688, 95%CI 0.435–1.086) in T2DM patients in the study by Fung et al. (68) and compared to rosiglitazone (HR = 0.86, 95%CI 0.58–1.28) in T2DM patients in the study by Pantalone et al. (53).

Figure 3. (A) Forest plot of hazard ratio of heart failure among patients with metformin therapy vs non-metformin therapy. (B) Forest plot of hazard ratio of heart failure among patients with metformin therapy vs. sulphonylurea therapy. (C) Forest plot of hazard ratio of the recurrent incident of heart failure among heart failure patients with metformin therapy vs. non-metformin therapy. CI, confidence interval; SE, standard error; IV, inverse of the variance.
Figures 4A–C showed the effect of metformin on cardiovascular mortality in T2DM patients. The use of metformin was not only significantly associated with lower cardiovascular mortality in T2DM patients (HR = 0.83, 95%CI 0.70–0.98; I2 = 85%) and in T2DM patients with heart failure (HR = 0.78, 95%CI 0.74–0.82; I2 = 0%) compared to non-metformin therapy, but also significantly lowering cardiovascular mortality compared to sulphonylurea in T2DM patients (HR = 0.70, 95%CI 0.58–0.84; I2 = 0%). Liu et al. (19) reported that metformin vs. diet therapy was not associated with significantly lower cardiovascular mortality (HR = 0.87, 95%CI 0.45–1.68).

Figure 4. (A) Forest plot of hazard ratio of cardiovascular mortality among patients with metformin therapy vs non-metformin therapy. (B) Forest plot of hazard ratio of cardiovascular mortality among heart failure patients with metformin therapy vs. non-metformin therapy. (C) Forest plot of hazard ratio of cardiovascular mortality among patients with metformin therapy vs. sulphonylurea. (D) Forest plot of hazard ratio of stroke among patients with metformin therapy vs. non-metformin therapy. (E) Forest plot of hazard ratio of stroke among patients with metformin therapy vs. SGLT2i. (F) Forest plot of hazard ratio of AMI among patients with metformin therapy vs. non-metformin therapy. CI, confidence interval; SE, standard error; IV, inverse of the variance.
The effect of metformin on the risk of stroke and AMI in T2DM patients was shown in Figures 4D–F. The use of metformin was not associated with a significant decrease in the risk of stroke compared to non-metformin therapy (HR = 1.16, 95%CI 0.88–1.53; I2 = 84%) and SGLT2i (HR = 1.03, 95%CI 0.65–1.63; I2 = 87%) in T2DM patients. The use of metformin significantly lowered the risk of stroke compared to diet therapy in T2DM patients (HR = 0.698, 95%CI 0.511–0.954) in the study by Fung et al. (68) while the alteration of risk of stroke was not significant compared to DPP-4i (HR = 0.81, 95%CI 0.6–1.09) in T2DM patients in the study by Baksh et al. (69). And the risk of AMI did not decrease significantly in T2DM patients with metformin therapy vs. non-metformin therapy (HR = 0.88, 95%CI 0.69–1.14; I2 = 88%). What's more, the use of metformin was not associated with a significantly lower risk of AMI in T2DM patients compared to DPP-4i (HR = 0.95, 95%CI 0.72–1.27) and SGLT2i (HR = 1.10, 95%CI 0.66–1.85) in the study by Baksh et al. (69) and Fralick et al. (15), respectively.
Supplementary Table 2 showed the results of sensitivity analysis for the outcomes. The majority of the re-analyses showed similar results as the analysis before deleting studies with a sample size smaller than 1,000 in either the control group or the experimental group. However, only 1 study by Roussel et al. (64) remained after deleting the data in the analyses studying the effect of metformin on all-cause mortality and cardiovascular mortality vs. non-metformin therapy in T2DM patients with heart failure. And 0 study was left in the analyses studying the effect of metformin on the risk of hospitalization and the risk of heart failure vs. non-metformin therapy in T2DM patients with heart failure. Besides, the effect of metformin on cardiovascular mortality vs. non-metformin therapy in T2DM patients altered significantly (HR = 0.85, 95%CI 0.69–1.06; I2 = 78%) in the re-analysis compared to the one before deleting the data (HR = 0.83, 95%CI 0.70–0.98; I2 = 85%). This might mainly result from the long follow-up period (9 years) in the study by Romero et al. (50), suggesting that a longer follow-up period might better demonstrate the efficacy of metformin.
The funnel plots and the results of egger and begg tests and trim and fill analyses in Supplementary Figures 2–22 indicated no potential publication biases for the adverse outcomes.
We included 39 subjects in this study involving 2473009 T2DM patients. We found:
(1) Metformin couldn't remarkably reduce the risk of MACEs compared to non-metformin therapy but could remarkably reduce the risk when compared with sulphonylurea in T2DM patients. (2) Metformin could significantly reduce cardiovascular mortality compared to non-metformin therapy in T2DM patients with or without heart failure or when compared with sulphonylurea in T2DM patients. (3) Metformin could significantly reduce all-cause mortality compared to non-metformin therapy, sulphonylurea, and diet therapy, and it could also significantly reduce all-cause mortality compared to non-metformin therapy in T2DM patients with heart failure or CKD. (4) Compared with non-metformin therapy, metformin was not effective in reducing the risk of heart failure in patients with T2DM, but it did reduce the risk of heart failure recurrence. Metformin was effective in reducing the risk of heart failure when compared with sulphonylurea in T2DM patients. (5) Metformin couldn't significantly reduce the risk of hospitalization compared to non-metformin therapy but could remarkably reduce the risk when compared with sulphonylurea in T2DM patients. Particularly, in patients with T2DM and heart failure, metformin can significantly reduce the risk of hospitalization. (6) Metformin couldn't remarkably reduce the risk of stroke compared to non-metformin therapy and SGLT2i in T2DM patients. (7) Metformin couldn't remarkably reduce the risk of AMI compared to non-metformin therapy in T2DM patients.
Major adverse cardiovascular events (MACEs) have different definitions in different studies. In this meta-analysis, some of the events were counted together as follows: AMI, stroke, heart failure, cardiovascular death, cardiac arrest, hospitalization, coronary angioplasty, transient ischemic attack, and unstable angina. T2DM is always related to cardiovascular complications. Since 1988, the study showed that in patients with T2DM, lowering blood glucose could reduce microvascular complications (80). There are usually exit raised levels of inflammatory cytokines among patients with T2DM. hyperglycemia and these inflammatory cytokines would harm the vascular endothelial cells, which would result in atherosclerosis in T2DM patients (81). At the same time, this condition decreases pro-angiogenic factors especially vascular endothelial growth factors and other collateral vessel growth-related parameters, which would impede collateral vessel growth (82). All of these are associated with CVD in T2DM patients to a great extent. Metformin has been thought to be protective of the cardiovascular system in the human body and here are some possible mechanisms: (I) Metformin was found to decrease cardiovascular inflammation and/or oxidative stress through activation of (AMP sensitive protein kinase) AMPK phosphorylation (83). (II)Metformin would attenuate atherosclerosis by the Inhibition of Drp1-mediated mitochondrial fission (84). However, in this meta-analysis, we found that metformin could not significantly reduce the risk of MACEs in T2DM patients. It might indicate that SGLT2i and DPP-4i have relatively the same cardiovascular protection ability as metformin. Considering the cardiovascular protection mechanism we mentioned above, it seemed to be unreasonable. And these results are opposite to some previous studies. A meta-analysis of randomized controlled trials found that metformin is significantly associated with lower risks of MACEs compared to placebo or other anti-hyperglycemic drugs among T2DM patients (85). A retrospective cohort analysis in China showed that metformin monotherapy could reduce the risk of heart failure in T2DM patients when compared with no metformin medications (59). A recent retrospective cohort analysis in Korea showed that metformin would significantly decrease the risk of AMI in all patients (54). But there also exist some studies showing the same results. Chang-Qian Wang found that metformin wasn't associated with a reduced risk of MACEs (76). A meta-analysis of 13 randomized trials showed that metformin couldn't remarkably reduce AMI and stroke (86), so there remains uncertainty about whether metformin reduces the risk of MACEs, AMI, stroke, and heart failure in patients with T2DM or not, and our included studies were limited (3 AMI studies, 3 stroke studies, and 4 MACEs studies) in this meta-analysis. More studies need to be implemented to test them.
All-cause mortality and cardiovascular mortality, which are vital living indicators in CVD patients, were found to be reduced significantly in T2DM patients with the use of metformin in this study. These results are similar to lots of previous studies (9, 85). Research showed that heart disease, cancer, stroke, and diabetes are the major causes of death in the US population (87). Metformin, as one kind of classic first-line hypoglycemic agent, has cardiovascular protection, and it could also protect against many kinds of cancers (e.g., breast, colorectal, and prostate cancer) (88). These might lead to reduced risks of all-cause and cardiovascular mortality. However, Liu et al. (19) reported that metformin vs. diet therapy was not associated with significantly lower cardiovascular mortality in T2DM patients. Dietary therapy always refers to a low carbohydrate diet or/and a low-fat diet, which is also called a low-calorie diet (89). A low-calorie diet could reduce the risk of CVD by reducing the body weight, body mass index, fat mass, and low-density lipoprotein cholesterol levels (90, 91), which may contribute to lowing cardiovascular mortality. In T2DM patients, a low-calorie diet also could normalize insulin sensitivity and improve pancreatic beta-cell function by reducing pancreatic fat content (92, 93), which may show similar function as metformin.
The risk of hospitalization can be used to evaluate the quality of life among patients with T2DM. Studies show different opinions on whether metformin is associated with reducing the risk of hospitalization. A propensity-matched study in the community showed that metformin would remarkably reduce the hospitalization rate (50). While a 2021 retrospective cohort study found that metformin could not significantly reduce the risk of hospitalization (73). In this meta-analysis, we found metformin could not significantly reduce the risk of hospitalization in T2DM patients compared to the control group. We included 8 studies without differentiating the reasons for hospitalization, which may exist some biases. More research is needed to analyze the relationship between metformin management and the risk of hospitalization divided by different diseases.
Sulphonylurea, which has been existing for approximately 70 years, is recommended as a second-line treatment in the management of type 2 diabetes (94). Many studies have testified that compared with metformin, sulphonylurea was associated with higher risks of MACEs, heart failure, hospitalization rate, all-cause, and cardiovascular mortality (14, 71, 72). In our meta-analysis, these results were proved. Therefore, it is reasonable to recommend metformin considering its benefits above. SGLT2i and DPP-4i are relatively new hypoglycemic agents. In recent studies, these two kinds of drugs show cardiovascular benefits beyond glycemic control through anti-inflammatory pathways (95, 96). In this meta-analysis, we found there existed no significantly different effect of SGLT2i or DPP-4i vs. metformin on reducing the risk of MACEs, hospitalization, stroke, and AMI in different studies. It suggests that SGLT2i and DPP-4i may have cardiovascular protective capacity comparable to metformin. However, due to the data limitation, we couldn't make related assessments and we hope more studies could be included to find some results. In this meta-analysis, we also found metformin could reduce the risk of heart failure, and hospitalization compared with non-metformin therapy in T2DM patients with heart failure, while the difference was not significant in T2DM patients, which indicated that metformin might have higher cardiovascular protection among patients with heart failure. Related research needs to be implemented to find some mechanisms.
Our results support that metformin should be recommended as a first-line hypoglycemic drug to all T2DM patients, including those with heart failure or CKD. Because it can reduce all-cause mortality and cardiovascular mortality. Dietary management is supposed to popularize among all T2DM patients since its effect on reducing all-cause mortality has no significant difference with metformin. Metformin significantly reduced MACE, heart failure, in-hospital all-cause mortality, and cardiovascular mortality compared with sulfonylureas. Thus, we have more reasons to recommend metformin as an antidiabetic drug between these two drugs. Metformin could reduce cardiovascular mortality while it couldn't reduce the risk of MACEs, heart failure, and AMI. Perhaps metformin can reduce the severity of cardiovascular events and more studies need to testify to it. The studies on the effect of metformin monotherapy on the risk of AMI, stroke, heart failure, and hospitalization are insufficient, more multicenter studies should be implemented to guide us in the use of metformin on T2DM patients better.
Our meta-analysis still had several limitations. First, although we included observational studies in this study, we didn't include RCTs in this study and therefore more data from large RCTs were still needed to bring clarity to the effect of metformin on adverse outcomes in T2DM patients. Second, the comparison between metformin and SGLT2i or DPP-4i needs to be further explored because of limited data in our study in which only 2 studies were pooled for analysis, and there was still limited data focusing on comparing the long-term effect of metformin and SGLT2i or DPP-4i in T2DM patients, remaining an empty field for meta-analysis in the future. Third, significant heterogeneity with I2 > 50% was found in a major part of our data analyses with a random-effects model, of which the results should be explained cautiously.
The effect of metformin on some of the adverse outcomes was not significantly better than the non-metformin therapy or DPP-4i in T2DM patients based on observational studies.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared a shared affiliation with the authors HZ, CW, and JJ at the time of review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.944902/full#supplementary-material
1. Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, et al. Heart disease and stroke statistics-2022 update: a report from the American Heart Association. Circulation. (2022) 145:e153–e639. doi: 10.1161/CIR.0000000000001052
2. American Diabetes Association. Improving care and promoting health in populations: standards of medical care in diabetes-2020. Diabetes Care. (2020) 43 (Suppl. 1):S7–S13. doi: 10.2337/dc20-S001
3. Zarich SW. Antidiabetic agents and cardiovascular risk in type 2 diabetes. Nat Rev Endocrinol. (2009). 5:500–6. doi: 10.1038/nrendo.2009.150
4. Andersson C, Vasan RS. Epidemiology of cardiovascular disease in young individuals. Nat Rev Cardiol. (2018) 15:230–40. doi: 10.1038/nrcardio.2017.154
5. Jiralerspong S, Palla SL, Giordano SH, Meric-Bernstam F, Liedtke C, Barnett CM, et al. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J Clin Oncol. (2009) 27:3297–302. doi: 10.1200/JCO.2009.19.6410
6. Løvvik TS, Carlsen SM, Salvesen Ø, Steffensen B, Bixo M, Gómez-Real F, et al. Use of metformin to treat pregnant women with polycystic ovary syndrome (PregMet2): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. (2019) 7:256–66. doi: 10.1016/S2213-8587(19)30002-6
7. Ayoub R, Ruddy RM, Cox E, Oyefiade A, Derkach D, Laughlin S, et al. Assessment of cognitive and neural recovery in survivors of pediatric brain tumors in a pilot clinical trial using metformin. Nat Med. (2020) 26:1285–94. doi: 10.1038/s41591-020-0985-2
8. Zhang K, Yang W, Dai H, Deng Z. Cardiovascular risk following metformin treatment in patients with type 2 diabetes mellitus: results from meta-analysis. Diabetes Res Clin Pract. (2020) 160:108001. doi: 10.1016/j.diabres.2020.108001
9. Li T, Providencia R, Jiang W, Liu M, Yu L, Gu C, et al. Association of metformin with the mortality and incidence of cardiovascular events in patients with pre-existing cardiovascular diseases. Drugs. (2022) 82:311–22. doi: 10.1007/s40265-021-01665-0
10. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. (1998) 352:854–65. doi: 10.1016/S0140-6736(98)07037-8
11. Tseng CH. Metformin use is associated with a lower risk of hospitalization for heart failure in patients with type 2 diabetes mellitus: a retrospective cohort analysis. J Am Heart Assoc. (2019) 8:e011640. doi: 10.1161/JAHA.118.011640
12. Charytan DM, Solomon SD, Ivanovich P, Remuzzi G, Cooper ME, McGill JB, et al. Metformin use and cardiovascular events in patients with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab. (2019) 21:1199–208. doi: 10.1111/dom.13642
13. Retwiński A, Kosmalski M, Crespo-Leiro M, Maggioni A, Opolski G, Ponikowski P, et al. The influence of metformin and the presence of type 2 diabetes mellitus on mortality and hospitalisation in patients with heart failure. Kardiol Pol. (2018) 76:1336–43. doi: 10.5603/KP.a2018.0127
14. Filion KB, Douros A, Azoulay L, Yin H, Yu OH, Suissa S. Sulfonylureas as initial treatment for type 2 diabetes and the risk of adverse cardiovascular events: a population-based cohort study. Br J Clin Pharmacol. (2019) 85:2378–89. doi: 10.1111/bcp.14056
15. Fralick M, Schneeweiss S, Redelmeier DA, Razak F, Gomes T, Patorno E. Comparative effectiveness and safety of sodium-glucose cotransporter-2 inhibitors versus metformin in patients with type 2 diabetes: an observational study using data from routine care. Diabetes Obes Metab. (2021) 23:2320–8. doi: 10.1111/dom.14474
16. Lee CG, Heckman-Stoddard B, Dabelea D, Gadde KM, Ehrmann D, Ford L, et al. Effect of metformin and lifestyle interventions on mortality in the diabetes prevention program and diabetes prevention program outcomes study. Diabetes Care. (2021) 44:2775–82. doi: 10.2337/figshare.16652587.v1
17. Li T, Providencia R, Mu N, Yin Y, Chen M, Wang Y, et al. Association of metformin monotherapy or combined therapy with cardiovascular risks in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. (2021) 20:30. doi: 10.1186/s12933-020-01202-5
18. Han Y, Xie H, Liu Y, Gao P, Yang X, Shen Z. Effect of metformin on all-cause and cardiovascular mortality in patients with coronary artery diseases: a systematic review and an updated meta-analysis. Cardiovasc Diabetol. (2019) 18:96. doi: 10.1186/s12933-019-0900-7
19. Liu L, Simon B, Shi J, Mallhi AK, Eisen HJ. Impact of diabetes mellitus on risk of cardiovascular disease and all-cause mortality: evidence on health outcomes and antidiabetic treatment in United States adults. World J Diabetes. (2016) 7:449–61. doi: 10.4239/wjd.v7.i18.449
20. Zhu W, Ye Z, Chen S, Wu D, He J, Dong Y, et al. Comparative effectiveness and safety of non-vitamin k antagonist oral anticoagulants in atrial fibrillation patients. Stroke. (2021) 52:1225–33. doi: 10.1161/STROKEAHA.120.031007
21. Kitao N, Miyoshi H, Furumoto T, Ono K, Nomoto H, Miya A, et al. The effects of vildagliptin compared with metformin on vascular endothelial function and metabolic parameters: a randomized, controlled trial (Sapporo Athero-Incretin Study 3). Cardiovasc Diabetol. (2017) 16:125. doi: 10.1186/s12933-017-0607-6
22. Wong AK, Symon R, AlZadjali MA, Ang DS, Ogston S, Choy A, et al. The effect of metformin on insulin resistance and exercise parameters in patients with heart failure. Eur J Heart Fail. (2012) 14:1303–10. doi: 10.1093/eurjhf/hfs106
23. Al Ali L, Hartman MT, Lexis CP, Hummel YM, Lipsic E, van Melle JP, et al. The effect of metformin on diastolic function in patients presenting with ST-elevation myocardial infarction. PLoS ONE. (2016) 11:e0168340. doi: 10.1371/journal.pone.0168340
24. Lexis CP, van der Horst-Schrivers AN, Lipsic E, Valente MA, Muller Kobold AC, de Boer RA, et al. The effect of metformin on cardiovascular risk profile in patients without diabetes presenting with acute myocardial infarction: data from the glycometabolic intervention as adjunct to primary coronary intervention in ST elevation myocardial infarction (GIPS-III) trial. BMJ Open Diabetes Res Care. (2015) 3:e000090. doi: 10.1136/bmjdrc-2015-000090
25. Eroglu TE, Jia L, Blom MT, Verkerk AO, Devalla HD, Boink GJJ, et al. Sulfonylurea antidiabetics are associated with lower risk of out-of-hospital cardiac arrest: real-world data from a population-based study. Br J Clin Pharmacol. (2021) 87:3588–98. doi: 10.1111/bcp.14774
26. Komaru Y, Takeuchi T, Suzuki L, Asano T, Urayama KY. Recurrent cardiovascular events in patients with newly diagnosed acute coronary syndrome: influence of diabetes and its management with medication. J Diabetes Complications. (2020) 34:107511. doi: 10.1016/j.jdiacomp.2019.107511
27. Lexis CP, van der Horst IC, Lipsic E, van der Harst P, van der Horst-Schrivers AN, Wolffenbuttel BH, et al. Metformin in non-diabetic patients presenting with ST elevation myocardial infarction: rationale and design of the glycometabolic intervention as adjunct to primary percutaneous intervention in ST elevation myocardial infarction (GIPS)-III trial. Cardiovasc Drugs Ther. (2012) 26:417–26. doi: 10.1007/s10557-012-6413-1
28. Preiss D, Lloyd SM, Ford I, McMurray JJ, Holman RR, Welsh P, et al. Metformin for non-diabetic patients with coronary heart disease (the CAMERA study): a randomised controlled trial. Lancet Diabetes Endocrinol. (2014) 2:116–24. doi: 10.1016/S2213-8587(13)70152-9
29. Basnet S, Kozikowski A, Makaryus AN, Pekmezaris R, Zeltser R, Akerman M, et al. Metformin and myocardial injury in patients with diabetes and st-segment elevation myocardial infarction: a propensity score matched analysis. J Am Heart Assoc. (2015) 4:e002314. doi: 10.1161/JAHA.115.002314
30. Zeller M, Labalette-Bart M, Juliard JM, Potier L, Feldman LJ, Steg PG, et al. Metformin and contrast-induced acute kidney injury in diabetic patients treated with primary percutaneous coronary intervention for ST segment elevation myocardial infarction: amulticenter study. Int J Cardiol. (2016) 220:137–42. doi: 10.1016/j.ijcard.2016.06.076
31. Kooy A, de Jager J, Lehert P, Bets D, Wulffelé MG, Donker AJ, et al. Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch Intern Med. (2009) 169:616–25. doi: 10.1001/archinternmed.2009.20
32. Liu Y, Jiang X, Chen X. Liraglutide and Metformin alone or combined therapy for type 2 diabetes patients complicated with coronary artery disease. Lipids Health Dis. (2017) 16:227. doi: 10.1186/s12944-017-0609-0
33. Fauchier G, Bisson A, Bodin A, Herbert J, Angoulvant D, Ducluzeau PH, et al. Glucose-lowering drug use and new-onset atrial fibrillation in patients with diabetes mellitus. Diabetologia. (2021) 64:2602–5. doi: 10.1007/s00125-021-05551-y
34. Halabi A, Yang H, Wright L, Potter E, Huynh Q, Negishi K, et al. Evolution of myocardial dysfunction in asymptomatic patients at risk of heart failure. JACC Cardiovasc Imaging. (2021) 14:350–61. doi: 10.1016/j.jcmg.2020.09.032
35. Ono K, Wada H, Satoh-Asahara N, Inoue H, Uehara K, Funada J, et al. Effects of metformin on left ventricular size and function in hypertensive patients with type 2 diabetes mellitus: results of a randomized, controlled, multicenter, phase IV trial. Am J Cardiovasc Drugs. (2020) 20:283–293. doi: 10.1007/s40256-019-00381-1
36. Deshmukh A, Ghannam M, Liang J, Saeed M, Cunnane R, Ghanbari H, et al. Effect of metformin on outcomes of catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol. (2021) 32:1232–9. doi: 10.1111/jce.14954
37. Kruszelnicka O, Chyrchel B, Golay A, Surdacki A. Differential associations of circulating asymmetric dimethylarginine and cell adhesion molecules with metformin use in patients with type 2 diabetes mellitus and stable coronary artery disease. Amino Acids. (2015) 47:1951–9. doi: 10.1007/s00726-015-1976-3
38. Lexis CP, Wieringa WG, Hiemstra B, van Deursen VM, Lipsic E, van der Harst P, et al. Chronic metformin treatment is associated with reduced myocardial infarct size in diabetic patients with ST-segment elevation myocardial infarction. Cardiovasc Drugs Ther. (2014) 28:163–71. doi: 10.1007/s10557-013-6504-7
39. Lu Y, Wang Y, Weng T, Chen Z, Sun X, Wei J, et al. Association between metformin use and coronary artery calcification in type 2 diabetic patients. J Diabetes Res. (2019) 2019:9484717. doi: 10.1155/2019/9484717
40. Mohan M, Al-Talabany S, McKinnie A, Mordi IR, Singh JSS, Gandy SJ, et al. A randomized controlled trial of metformin on left ventricular hypertrophy in patients with coronary artery disease without diabetes: the MET-REMODEL trial. Eur Heart J. (2019) 40:3409–3417. doi: 10.1093/eurheartj/ehz203
41. Lexis CP, van der Horst IC, Lipsic E, Wieringa WG, de Boer RA, van den Heuvel AF, et al. Effect of metformin on left ventricular function after acute myocardial infarction in patients without diabetes: the GIPS-III randomized clinical trial. Jama. (2014) 311:1526–35. doi: 10.1001/jama.2014.3315
42. Hartman MHT, Prins JKB, Schurer RAJ, Lipsic E, Lexis CPH, van der Horst-Schrivers ANA, et al. Two-year follow-up of 4 months metformin treatment vs. placebo in ST-elevation myocardial infarction: data from the GIPS-III RCT. Clin Res Cardiol. (2017) 106:939–46. doi: 10.1007/s00392-017-1140-z
43. Sundström J, Kristófi R, Östlund O, Bennet L, Eliasson B, Jansson S, et al. A registry-based randomised trial comparing an SGLT2 inhibitor and metformin as standard treatment of early stage type 2 diabetes (SMARTEST): rationale, design and protocol. J Diabetes Complications. (2021) 35:107996. doi: 10.1016/j.jdiacomp.2021.107996
44. Dong YH, Wang SV, Gagne JJ, Wu LC, Chang CH. Comparison of different case-crossover variants in handling exposure-time trend or persistent-user bias: using dipeptidyl peptidase-4 inhibitors and the risk of heart failure as an example. Value Health. (2020) 23:217–26. doi: 10.1016/j.jval.2019.09.2746
45. Crowley MJ, McGuire DK, Alexopoulos AS, Jensen TJ, Rasmussen S, Saevereid HA, et al. Effects of liraglutide on cardiovascular outcomes in type 2 diabetes patients with and without baseline metformin use: post hoc analyses of the LEADER trial. Diabetes Care. (2020) 43:e108–110. doi: 10.2337/dc20-0437
46. Sillars B, Davis WA, Hirsch IB, Davis TM. Sulphonylurea-metformin combination therapy, cardiovascular disease and all-cause mortality: the Fremantle Diabetes Study. Diabetes Obes Metab. (2010) 12:757–65. doi: 10.1111/j.1463-1326.2010.01230.x
47. Evans JM, Ogston SA, Emslie-Smith A, Morris AD. Risk of mortality and adverse cardiovascular outcomes in type 2 diabetes: a comparison of patients treated with sulfonylureas and metformin. Diabetologia. (2006) 49:930–6. doi: 10.1007/s00125-006-0176-9
48. Jong CB, Chen KY, Hsieh MY, Su FY, Wu CC, Voon WC, et al. Metformin was associated with lower all-cause mortality in type 2 diabetes with acute coronary syndrome: a Nationwide registry with propensity score-matched analysis. Int J Cardiol. (2019) 291:152–57. doi: 10.1016/j.ijcard.2019.03.021
49. Bromage DI, Godec TR, Pujades-Rodriguez M, Gonzalez-Izquierdo A, Denaxas S, Hemingway H, et al. Metformin use and cardiovascular outcomes after acute myocardial infarction in patients with type 2 diabetes: a cohort study. Cardiovasc Diabetol. (2019) 18:168. doi: 10.1186/s12933-019-0972-4
50. Romero SP, Andrey JL, Garcia-Egido A, Escobar MA, Perez V, Corzo R, et al. Metformin therapy and prognosis of patients with heart failure and new-onset diabetes mellitus. A propensity-matched study in the community. Int J Cardiol. (2013) 166:404–12. doi: 10.1016/j.ijcard.2011.10.141
51. Fácila L, Fabregat-Andrés Ó, Bertomeu V, Navarro JP, Miñana G, García-Blas S, et al. Metformin and risk of long-term mortality following an admission for acute heart failure. J Cardiovasc Med (Hagerstown). (2017) 18:69–73. doi: 10.2459/JCM.0000000000000420
52. Ritsinger V, Lagerqvist B, Lundman P, Hagström E, Norhammar A. Diabetes, metformin and glucose lowering therapies after myocardial infarction: insights from the SWEDEHEART registry. Diab Vasc Dis Res. (2020) 17:1479164120973676. doi: 10.1177/1479164120973676
53. Pantalone KM, Kattan MW, Yu C, Wells BJ, Arrigain S, Jain A, et al. The risk of developing coronary artery disease or congestive heart failure, overall mortality. In type 2 diabetic patients receiving rosiglitazone, pioglitazone, metformin, or sulfonylureas: a retrospective analysis. Acta Diabetol. (2009) 46:145–54. doi: 10.1007/s00592-008-0090-3
54. Jung I, Kwon H, Park SE, Han KD, Park YG, Rhee EJ, et al. The effects of glucose lowering agents on the secondary prevention of coronary artery disease in patients with type 2 diabetes. Endocrinol Metab (Seoul). (2021) 36:977–87. doi: 10.3803/EnM.2021.1046
55. Abualsuod A, Rutland JJ, Watts TE, Pandat S, Delongchamp R, Mehta JL. The effect of metformin use on left ventricular ejection fraction and mortality post-myocardial infarction. Cardiovasc Drugs Ther. (2015) 29:265–75. doi: 10.1007/s10557-015-6601-x
56. Wang J, Lu Y, Min X, Yuan T, Wei J, Cai Z. The association between metformin treatment and outcomes in type 2 diabetes mellitus patients with heart failure with preserved ejection fraction: a retrospective study. Front Cardiovasc Med. (2021) 8:648212. doi: 10.3389/fcvm.2021.648212
57. Chen TH, Li YR, Chen SW, Lin YS, Sun CC, Chen DY, et al. Sodium-glucose cotransporter 2 inhibitor versus metformin as first-line therapy in patients with type 2 diabetes mellitus: a multi-institution database study. Cardiovasc Diabetol. (2020) 19:189. doi: 10.1186/s12933-020-01169-3
58. Chen CB, Lin M, Eurich DT, Johnson JA. Safety of concomitant metformin and proton pump inhibitor use: a population retrospective cohort study. Clin Ther. (2016) 38:1392–400. doi: 10.1016/j.clinthera.2016.03.024
59. He S, Qian X, Chen Y, Shen X, Zhang B, Chen X, et al. Risk of death and heart failure among patients with type 2 diabetes treated by metformin and nonmetformin monotherapy: a real-world study. J Diabetes Res. (2021) 2021:5534387. doi: 10.1155/2021/5534387
60. Johnson JA, Simpson SH, Toth EL, Majumdar SR. Reduced cardiovascular morbidity and mortality associated with metformin use in subjects with Type 2 diabetes. Diabet Med. (2005) 22:497–502. doi: 10.1111/j.1464-5491.2005.01448.x
61. Duncan AI, Koch CG, Xu M, Manlapaz M, Batdorf B, Pitas G, et al. Recent metformin ingestion does not increase in-hospital morbidity or mortality after cardiac surgery. Anesth Analg. (2007) 104:42–50. doi: 10.1213/01.ane.0000242532.42656.e7
62. Schramm TK, Gislason GH, Vaag A, Rasmussen JN, Folke F, Hansen ML, et al. Mortality and cardiovascular risk associated with different insulin secretagogues compared with metformin in type 2 diabetes, with or without a previous myocardial infarction: a nationwide study. Eur Heart J. (2011) 32:1900–8. doi: 10.1093/eurheartj/ehr077
63. Tseng CH. Metformin use is associated with a lower incidence of hospitalization for atrial fibrillation in patients with type 2 diabetes mellitus. Front Med (Lausanne). (2020) 7:592901. doi: 10.3389/fmed.2020.592901
64. Roussel R, Travert F, Pasquet B, Wilson PW, Smith Jr SC, Goto S, et al. Metformin use and mortality among patients with diabetes and atherothrombosis. Arch Intern Med. (2010) 170:1892–9. doi: 10.1001/archinternmed.2010.409
65. Kim MH, Oh HJ, Kwon SH, Jeon JS, Noh H, Han DC, et al. Metformin use and cardiovascular outcomes in patients with diabetes and chronic kidney disease: a nationwide cohort study. Kidney Res Clin Pract. (2021) 40:660–72. doi: 10.23876/j.krcp.20.222
66. Shah DD, Fonarow GC, Horwich TB. Metformin therapy and outcomes in patients with advanced systolic heart failure and diabetes. J Card Fail. (2010) 16:200–6. doi: 10.1016/j.cardfail.2009.10.022
67. Richardson TL Jr., Hackstadt AJ, Hung AM, Greevy RA, Grijalva CG, et al. Hospitalization for heart failure among patients with diabetes mellitus and reduced kidney function treated with metformin versus sulfonylureas: a retrospective cohort study. J Am Heart Assoc. (2021) 10:e019211. doi: 10.1161/JAHA.120.019211
68. Fung CS, Wan EY, Wong CK, Jiao F, Chan AK. Effect of metformin monotherapy on cardiovascular diseases and mortality: a retrospective cohort study on Chinese type 2 diabetes mellitus patients. Cardiovasc Diabetol. (2015) 14:137. doi: 10.1186/s12933-015-0304-2
69. Baksh SN, Segal JB, McAdams-DeMarco M, Kalyani RR, Alexander GC, Ehrhardt S. Dipeptidyl peptidase-4 inhibitors and cardiovascular events in patients with type 2 diabetes, without cardiovascular or renal disease. PLoS One. (2020) 15:e0240141. doi: 10.1371/journal.pone.0240141
70. Wang CP, Lorenzo C, Habib SL, Jo B, Espinoza SE. Differential effects of metformin on age related comorbidities in older men with type 2 diabetes. J Diabetes Complications. (2017) 31:679–86. doi: 10.1016/j.jdiacomp.2017.01.013
71. Roumie CL, Min JY, D'Agostino McGowan L, Presley C, Grijalva CG, Hackstadt AJ, et al. Comparative safety of sulfonylurea and metformin monotherapy on the risk of heart failure: a cohort study. J Am Heart Assoc. (2017) 6:5379. doi: 10.1161/JAHA.116.005379
72. Roumie CL, Hung AM, Greevy RA, Grijalva CG, Liu X, Murff HJ, et al. Comparative effectiveness of sulfonylurea and metformin monotherapy on cardiovascular events in type 2 diabetes mellitus: a cohort study. Ann Intern Med. (2012) 157:601–10. doi: 10.7326/0003-4819-157-9-201211060-00003
73. Khan MS, Solomon N, DeVore AD, Sharma A, Felker GM, Hernandez AF, et al. Clinical outcomes with metformin and sulfonylurea therapies among patients with heart failure and diabetes. JACC Heart Fail. (2022) 10:198–210. doi: 10.1016/j.jchf.2021.11.001
74. Clegg LE, Jing Y, Penland RC, Boulton DW, Hernandez AF, Holman RR, et al. Cardiovascular and renal safety of metformin in patients with diabetes and moderate or severe chronic kidney disease: Observations from the EXSCEL and SAVOR-TIMI 53 cardiovascular outcomes trials. Diabetes Obes Metab. (2021) 23:1101–10. doi: 10.1111/dom.14313
75. Roumie CL, Chipman J, Min JY, Hackstadt AJ, Hung AM, Greevy Jr RA, et al. Association of treatment with metformin vs sulfonylurea with major adverse cardiovascular events among patients with diabetes and reduced kidney function. Jama. (2019) 322:1167–77. doi: 10.1001/jama.2019.13206
76. Gu J, Yin ZF, Zhang JF, Wang CQ. Association between long-term prescription of metformin and the progression of heart failure with preserved ejection fraction in patients with type 2 diabetes mellitus and hypertension. Int J Cardiol. (2020) 306:140–5. doi: 10.1016/j.ijcard.2019.11.087
77. Morgan CL, Mukherjee J, Jenkins-Jones S, Holden SE, Currie CJ. Association between first-line monotherapy with sulphonylurea versus metformin and risk of all-cause mortality and cardiovascular events: a retrospective, observational study. Diabetes Obes Metab. (2014) 16:957–62. doi: 10.1111/dom.12302
78. Scheller NM, Mogensen UM, Andersson C, Vaag A, Torp-Pedersen C. All-cause mortality and cardiovascular effects associated with the DPP-IV inhibitor sitagliptin compared with metformin, a retrospective cohort study on the Danish population. Diabetes Obes Metab. (2014) 16:231–6. doi: 10.1111/dom.12197
79. Whitlock RH, Hougen I, Komenda P, Rigatto C, Clemens KK, Tangri N. A safety comparison of metformin vs sulfonylurea initiation in patients with type 2 diabetes and chronic kidney disease: a retrospective cohort study. Mayo Clin Proc. (2020) 95:90–100. doi: 10.1016/j.mayocp.2019.07.017
80. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. (1998) 352:837–53. doi: 10.1016/S0140-6736(98)07019-6
81. Sposito AC, Breder I, Soares AAS, Kimura-Medorima ST, Munhoz DB, Cintra RMR, et al. Dapagliflozin effect on endothelial dysfunction in diabetic patients with atherosclerotic disease: a randomized active-controlled trial. Cardiovasc Diabetol. (2021) 20:74. doi: 10.1186/s12933-021-01264-z
82. Shen Y, Ding FH, Dai Y, Wang XQ, Zhang RY, Lu L, et al. Reduced coronary collateralization in type 2 diabetic patients with chronic total occlusion. Cardiovasc Diabetol. (2018) 17:26. doi: 10.1186/s12933-018-0671-6
83. Gao J, Yuan J, Wang Q, Lei T, Shen X, Cui B, et al. Metformin protects against PM (2.5)-induced lung injury and cardiac dysfunction independent of AMP-activated protein kinase α2. Redox Biol. (2020) 28:101345. doi: 10.1016/j.redox.2019.101345
84. Wang Q, Zhang M, Torres G, Wu S, Ouyang C, Xie Z, et al. Metformin suppresses diabetes-accelerated atherosclerosis via the inhibition of drp1-mediated mitochondrial fission. Diabetes. (2017) 66:193–205. doi: 10.2337/db16-0915
85. Monami M, Candido R, Pintaudi B, Targher G, Mannucci E. Effect of metformin on all-cause mortality and major adverse cardiovascular events: an updated meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. (2021) 31:699–704. doi: 10.1016/j.numecd.2020.11.031
86. Griffin SJ, Leaver JK, Irving GJ. Impact of metformin on cardiovascular disease: a meta-analysis of randomised trials among people with type 2 diabetes. Diabetologia. (2017) 60:1620–9. doi: 10.1007/s00125-017-4337-9
87. Ma J, Ward EM, Siegel RL, Jemal A. Temporal trends in mortality in the United States, 1969-2013. Jama. (2015) 314:1731–9. doi: 10.1001/jama.2015.12319
88. Coyle C, Cafferty FH, Vale C, Langley RE. Metformin as an adjuvant treatment for cancer: a systematic review and meta-analysis. Ann Oncol. (2016) 27:2184–95. doi: 10.1093/annonc/mdw410
89. Forouhi NG, Misra A, Mohan V, Taylor R, Yancy W. Dietary and nutritional approaches for prevention and management of type 2 diabetes. Bmj. (2018) 361:k2234. doi: 10.1136/bmj.k2234
90. Sofi F, Dinu M, Pagliai G, Cesari F, Gori AM, Sereni A, et al. Low-calorie vegetarian versus mediterranean diets for reducing body weight and improving cardiovascular risk profile: CARDIVEG study (cardiovascular prevention with vegetarian diet). Circulation. (2018) 137:1103–13. doi: 10.1161/CIRCULATIONAHA.117.030088
91. Srour B, Fezeu LK, Kesse-Guyot E, Allès B, Méjean C, Andrianasolo RM, et al. Ultra-processed food intake and risk of cardiovascular disease: prospective cohort study (NutriNet-Santé). Bmj. (2019) 365:l1451. doi: 10.1136/bmj.l1451
92. Lim EL, Hollingsworth KG, Aribisala BS, Chen MJ, Mathers JC, Taylor R. Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia. (2011) 54:2506–14. doi: 10.1007/s00125-011-2204-7
93. White MG, Shaw JA, Taylor R. Type 2 diabetes: the pathologic basis of reversible β-Cell dysfunction. Diabetes Care. (2016) 39:2080–8. doi: 10.2337/dc16-0619
94. Groop LC, Sulfonylureas in NIDDM. Diabetes Care. (1992) 15:737–54. doi: 10.2337/diacare.15.6.737
95. Tomovic K, Lazarevic J, Kocic G, Deljanin-Ilic M, Anderluh M, Smelcerovic A. Mechanisms and pathways of anti-inflammatory activity of DPP-4 inhibitors in cardiovascular and renal protection. Med Res Rev. (2019) 39:404–22. doi: 10.1002/med.21513
Keywords: metformin, type 2 diabetes mellitus, adverse outcomes, meta-analysis, cardiovascular
Citation: Xu Z, Zhang H, Wu C, Zheng Y and Jiang J (2022) Effect of metformin on adverse outcomes in T2DM patients: Systemic review and meta-analysis of observational studies. Front. Cardiovasc. Med. 9:944902. doi: 10.3389/fcvm.2022.944902
Received: 16 May 2022; Accepted: 08 July 2022;
Published: 23 September 2022.
Edited by:
Yuling Zhang, Sun Yat-sen Memorial Hospital, ChinaReviewed by:
Zhijian He, The First Affiliated Hospital of Guangdong Pharmaceutical University, ChinaCopyright © 2022 Xu, Zhang, Wu, Zheng and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhicheng Xu, 532141796@qq.com; Haidong Zhang, 1013722016@qq.com; Jingzhou Jiang, 178009867@qq.com
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.