
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 25 October 2022
Sec. Heart Valve Disease
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.897657
This article is part of the Research Topic Heart Valve Diseases: From Molecular Mechanisms to Clinical Implications View all 6 articles
 Olivier Schussler1,2*
Olivier Schussler1,2* Luc Maroteaux3,4,5
Luc Maroteaux3,4,5 Ramadan Jashari6
Ramadan Jashari6 Pierre Falcoz7
Pierre Falcoz7 Marco Alifano8
Marco Alifano8 Yves Lecarpentier9
Yves Lecarpentier9 Jean-Marie Launay10
Jean-Marie Launay10Objectives: Although critical in animal and human development and pathology, a measurement of the quantitative expression of 5-HTR serotonin receptors on animal or human valvular tissues has never been performed.
Methods: Quantification of the most frequent 5-HTRs reported as being present in human peripheral tissue was performed using radiolabeled agonists/antagonists. A membrane protein extract from normal human valves (aortic/mitral/tricuspid and some pulmonary) and associated diseased left myocardium, all unusable in clinics, were obtained from the Homograft bank.
Results: We analyzed 5-HT1AR/5-HT1B/DR/5-HT2AR/5-HT2BR/5-HT 2CR/5-HT4R/5-HT7R from 28 hearts. We confirmed the presence of tissue and measured the quantitative content for respective proteins in femtomol/mg of protein extracts: for 5-HT2AR (35.9+/−0.7), 5-HT2BR (28.8+/−1.3) but also a newly observed and robust expression for 5-HT4R (38+/−4.2). We identified one, 5-HT1ARs (4.9+/−0.3), and the possible expression, but at a very low level, of previously reported 5-HT1B/DRs (1.3+/−0.5) as well as the new 5-HT7Rs (3.5+/0.1) and 5-HT2CRs (1.2+/−0.1). Interestingly, by using univariate analysis, we were able to observe many correlations between the different 5-HTR levels of expression especially between 5-HT1AR/5-HT1B/DR and also between 5-HT4R/5-HT7R, but none were observed between 5-HT2AR and 5-HT2BR. Using multivariate analyses for a specific 5-HTR level of expression, after adjustment for implantation sites and other 5-HTRs, we found that 5-HT1AR was correlated with 5-HT1B/DR;5-HT4R with 5-HT7R and 5-HT1AR;5-HT2BR with 5-HT2AR only. For 5-HT2C, no correlation was observed.
Conclusion: 5-HT2AR/5-HT2BR and 5-HT4R were all observed to have a high and equal level of expression on human valves, but that of 5-HT1AR was more limited. Since these non-5-HT2Rs are coupled with different G-proteins, with specific signaling, theoretically they may control the main 5-HT2R signaling (i.e., PLC/DAG-PKC-ERK/Ras/Src signaling) involved in valvular fibrosis and degeneration.
The role of increased serotonin (5-HT) signaling in heart valvular development and disease is of growing importance and interest (1–4). The implication of 5-HT was first described in patients with carcinoid diseases presenting valvular injuries secondary to increased circulation of 5-HT. A secondary effect observed was the valvular toxicity of serotoninergic receptor drug agonists (3). More recently, its role has been reported in myxoid valvular degeneration and mitral prolapse (MVP) (2, 3, 5). The common macroscopic and histological characteristics shared by drug-induced valvulopathy and acute rheumatic fever make it difficult to determine the involvement of 5-HT agonists in heart valve diseases (HVD) and to suggest the possibility of a similar serotoninergic mechanism. In carcinoid tumors, increased circulation of 5-HT leads to the formation of “carcinoid plaques” at the valve surface and corresponds to activation of valvular interstitial cells (VIC) and the deposition of glycosaminoglycans (GAG) within the extracellular matrix (ECM). These lesions are very similar to those observed in patients under serotonin receptor drug agonist treatment. Valvulopathies associated with various serotoninergic drugs have been shown to share a common feature in the form of activated 5-HT2BR receptors (5-HT2BR) (6–8). MVP is the most frequent heart valve disease affecting 2–3% of the population older than 65 years (9) and therefore millions of individuals in the world (9, 10). The pathophysiology of MVP involves “myxomatous degeneration”, defined as the accumulation of mucopolysaccharides and other ECM components and the activation of VICs that are responsible for the thickening and proliferative aspect of the valve tissue (3). It has been shown that 5-HT is locally secreted in the valvular cusps. One isoform of the enzyme involved in its synthesis, tryptophan hydroxylase 1 (TPH1), is enhanced by mechanical stimulation and in degenerative human myxomatous heart valves (11). The remodeling induced by these factors could be prevented by 5-HT2BR, 5-HT2AR, or TPH1 antagonists (11, 12).
Up to now, only the expression of the transcription factor for 5-HTRs has been investigated and not the true protein expression of the receptors (2, 3, 13). In humans with myxoid mitral valve regurgitation, observations have revealed an up-regulation by qPCR on mitral prolapse tissue for 5-HT2AR, 5-HT2BR, and TPH1, but a decrease in the serotonin transporter (SERT). However, at the same time, on histological sections, only 5-HT2BR staining is enhanced (2), but not the 5-HT2AR staining. This highlights the fact that qPCRs are not accurate in defining protein expression.
While some authors of the present article have considerable experience in the quantitative dosage of 5-HTRs in tissues (14–16), these dosages have never been performed simultaneously in any animal or on human tissues. With respect to cardiac valvular cusps, the quantitative dosage for any 5-HTR has never been performed on animal or human tissues.
In this study, we not only confirmed the presence of 5-HT2AR and 5-HT2BR on human heart valves that are critical in valvular pathology but also demonstrated that these two receptors are expressed at the same level. In addition, we reported, for the first time, the presence and abundance of 5-HT4R, which was observed to be at the same level as 5-HT2AR and 5-HT2BR. Beside these three main receptors, many 5-HTRs were expressed at low levels, such as 5-HT1AR and 5-HT7R, or very low levels, such as 5-HT2CR or the 5-HT1B/DR reported earlier (17). Most interestingly, we found very strong correlations between the quantitative expressions of the following pairs of non-5-HT2R serotonin receptors: 1) 5-HT1AR and 5-HT1B/DR; 2) 5-HT4R and 5-HT7R. Unlike 5-HT2AR and 5-HT2BR that are coupled with a specific G-protein, Gq/G11 (18), and thus cannot control cAMP levels. 5-HT1AR and 5-HT1B/DR are coupled with G-protein, Gi/Go, and can thus potentially decrease cAMP levels (18). 5-HT4R and 5-HT7R are coupled with another G-protein, Gs (18), and can thus increase cAMP. By controlling the level of non-5-HT2R expression and possible subsequent signaling, it may therefore be possible to control the main 5-HT2R signaling activity involved in valvular pathology.
All experimental procedures were carried out in accordance with the ethical standards of the responsible institutional and national committees on human experimentation, thereby respecting the Helsinki Declaration (1975). Normal human heart valves were obtained from the European homograft program in Belgium (19, 20). Patients or members of the patients' families gave their written consent. In this program, pulmonary valves and some aortic valves are generally used. In Brussels, for example, 50% of the pulmonary or aortic valves could not be used, mainly because of functional incompetence, morphological alteration, surgical cuts, or bacterial contamination (19, 21). Donors were younger than 55 years (20). A cardiac surgeon involved in the program collected only heart valve cusps and their associated left ventricles to be used as controls. Valvular cusps were put directly into three separate tubes for each valve and a piece of the associated left myocardium into three additional tubes and labeled. The project was approved by the institutional review boards of the University Hospital of Geneva, Switzerland [Approbation number CER: 12-150 (NAC 12-056)] and by a local committee at the European Homograft Bank in Brussels. After collection, the samples were preserved in N2 liquid vapor until transfer.
Methods for membrane preparation have been previously reported (14) as well as the methods for membrane radiolabeling for specific 5-HTRs in other non-cardiac tissues (14, 15, 22).
The specific ligands used in the study, purchased from Perkin-Elmer Life Sciences, were as follows: for 5-HT1AR: agonist 8-0H-DPAT; for 5-HT1B/DR: agonist GTI (Serotonin-5-O-carboxymethyl-Glycil-iodo-tyrosamine); for 5-HT2AR: antagonist MDL 100.97; for 5-HT2BR: antagonist LY26.6097; for 5-HT2CR: antagonist mesulergine; for 5-HT4R: antagonist GR113808; for 5-HT7R: antagonist Ly269970. The specificity of the different agonists or antagonists for each 5-HTR used in the study and the different results of membrane radioligand binding assays were discussed in an extensive review of the pharmacological consortium for 5-HTRs, in which one of the co-authors of the present study (Luc Maroteaux) was involved (23). In the radio-binding assay for each 5-HTR, the zero of fixation corresponded to the level of fixation obtained in the presence of the labeled 5-HTR agonist or antagonist and of very high amounts (i.e., 1 μM) of its unlabeled agonist or antagonist. In the radioligand binding assay, a positive signal above 5 fmol of radioligand binding per milligram of protein from the extract is considered to be true (23). Values of 0–5 fmol of this radio in femtomol/mg of protein extracts ligand binding per milligram may indicate that the binding is not in fact present.
To prepare crude membranes for binding assays, the membrane cusps were washed twice with cold PBS and then harvested with a rubber policeman in 1.5 ml of PBS containing 1 μg/ml pepstatin, 1 μg/ml antipain, 15 μg/ml benzamidine, and 0.1 mM phenylmethylsulfonyl fluoride, as described earlier (14). After centrifugation, the resulting pellet was frozen at −70°C before homogenization. The frozen pellet was thawed at 37°C, resuspended in 10 ml of cold EDTA, 1 mM EGTA, 0.1 mM phenylmethylsulfonyl fluoride, and a 10 mM imidazole buffer pH 7.30, then centrifuged for 10 min at 5,000 × g. The supernatant obtained from this centrifugation was collected, poured onto a 20% sucrose cushion, and then centrifuged for 90 min. at 100,000 × g. The pellet containing the membrane was then resuspended in 75 mM KCl 5 mM MgCl2 and a 1 mM EGTA 10 mM imidazole buffer pH 7.3 for use in binding assays. Protein contents were determined using the protein assay kit.
The different radiolabeled agonists or antagonists were incubated with a membrane protein extract of fresh human valvular cusp membranes as reported in earlier publications for other tissues (14, 15, 22). The different radiolabeled agonists or antagonists for the 5-HTRs were as follows: for 5-HT1AR: partial agonist [3H] 80H-DPAT; for 5-HT1B/DR: [125I] GTI, for 5-HT2AR: antagonist [3H] MDL 100.97; for 5-HT2BR: antagonist [3H] LY26.6097; for 5-HT2CR: antagonist [3H] Mesulergine; for 5-HT4R: antagonist [3H] GR113808; for 5-HT7R: antagonist [3H] Ly269970.
Binding experiments were performed at room temperature and involved tissue shaking. Binding was initiated by the addition of 50 μl of 50 mM Tris Buffer, pH 7.40, containing 0.1–10 nM radiolabeled agonist or antagonist to 5-HTRs, or appropriate competing ligands to 50 μl of membrane (representing 20 μg of protein from heart valve extract). The preparations were incubated for 30 min at RT followed by the addition of 3 ml of ice-cold 50 mM Tris Buffer with a pH 7.40. Samples were filtered using polyethyleneimine-treated filters and counted. The specific binding was defined as the binding inhibited by 1 μM levels of unlabeled agonist/antagonist for each 5-HTR sub-type. All the experiments were performed in triplicate.
Mean comparisons between groups were performed using ANOVA. Correlations between quantitative variables were made using Spearman's Rank Correlation. Multivariate regression models were built to assess the independent relationships (each variable in relation to the others). Two types of models were used. In one type, we compared specific 5-HTR receptor levels with explicative variables being another specific single 5-HTR and the sites of implantation (i.e., aortic/mitral or tricuspid). In the second type, we analyzed specific 5-HTR receptor levels with the explicative variables being all the other 5-HTRs and the sites of implantation (i.e., aortic/mitral or tricuspid). A P-value < 0.05 was considered as statistically significant. Data processing and analysis were performed using the statistical software system SEM (SILEX Development, Mirefleurs, France).
Heart valve leaflets were harvested from donor heart recipients and comprised valves that were not suitable for transplantation. For all 28 hearts, we have at the same time tricuspid and mitral valves. For 14 of the 28 hearts, we also had the aortic valve and, for 3 of the 28 hearts, we had the four valves including the pulmonary valves. For all the 28 hearts, we also checked the expression of 5-HTRs in associated diseased left myocardium (i.e., mostly those having chronic cardiomyopathies, but with normal heart valves, or having an acute heart transplant dysfunction). All samples were collected from hearts obtained from patients younger than 55 years in the homograft program.
As shown in Figure 1, 5-HT1AR, 5-HT1B/DR, 5-HT2AR, 5-HT2BR, 5-HT2CR, 5-HT4R, and 5-HT7R are detected on human heart valve cusps. Among the different 5-HTRs tested, 5-HT2AR, 5-HT2BR, and 5-HT4R were quantitatively the most abundant (see Table 1 for mean values and SD). In this study, we confirmed the presence of 5-HT2AR and 5-HT2BR on human heart valves but also demonstrated that they were expressed in the same quantity. 5-HT4R has been reported in the myocardium but not on heart valves. The levels for 5-HT2AR/5-HT2BR/5-HT4R were around 30 femtomol/mg of proteins and thus very high (23).
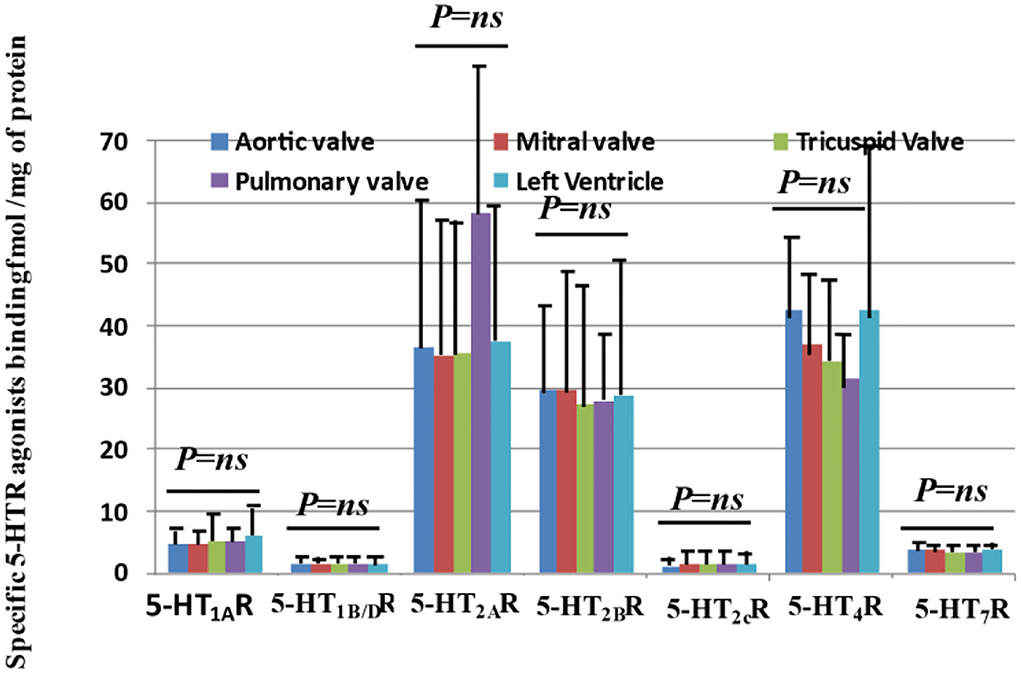
Figure 1. Quantitative expressions of 5-HTR receptors on human “normal” heart valves by radioligand binding affinity with the human left ventricle (i.e., diseased left cardiomyopathy) as a control. This figure shows the quantitative measurements of receptor expression for aortic, mitral, tricuspid, and pulmonary valves with the left ventricle as a control. Specific quantification of the protein expression for 5-HTRs (5-HT1AR, 5-HT1B/DR, 5-HT2AR, 5-HT2BR, 5-HT2CR, 5-HT4R, and 5-HT7R) on valvular tissue protein extract was performed with specific radioligands. 5-HT1AR and 5-HT1B/DR are known to be associated with a Gi/Go protein and thus decrease cAMP levels. On the other hand, 5-HT4R and 5-HT7R are associated with a Gs protein and thus increase cAMP levels. 5-HT2Rs are associated with another G-type protein (Gq/G11) and thus activate PLC, Src, and Ras. 5-HTR7R was the most recent receptor to be identified. Up until now, no quantification of 5-HTRs has been conducted on human heart valves. We had access to a total of 28 transplant hearts and had mitral and tricuspid sites for all of them. For 14 hearts, we had the three main sites (aortic/mitral/tricuspid), and for three hearts, we had four sites at the same time (including pulmonary site). All measurements of expression levels were performed in triplicate. As shown in this figure, all types of receptors we tested, known to be expressed in peripheral tissues, are expressed in valvular tissues, with the main receptors being quantitatively 5-HT2AR, 5HT2BR, and the unreported 5-HT4R. Interestingly, we did not observe any difference in terms of the level of expression between the different sides and sites investigated. Each bar diagram in the figure is based on 28 samples for tricuspid and mitral valves, with left ventricle as control, and 14 samples for the aortic position. P-values correspond to the results of ANOVA tests. No particular difference was observed between the right sides (i.e., tricuspid, pulmonary) and left sides (i.e., aortic, mitral) (ANOVA test). The pulmonary positions (i.e., only 3 samples available) tended to have the same level of expression as the other sites.
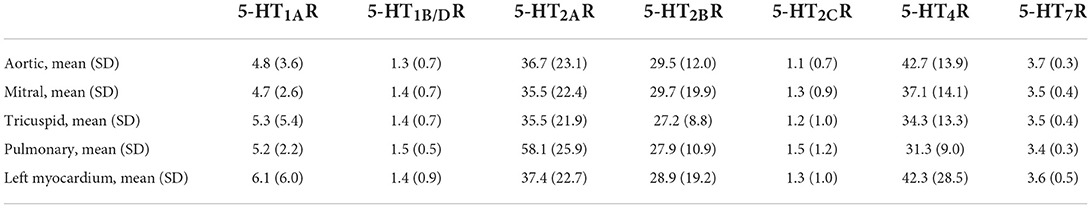
Table 1. Quantitative expression of the different 5-HTRs (i.e., 5-HT1AR, 5-HT1B/DR, 5-HT2AR, 5-HT2BR, 5-HT2CR, 5-HT4R, 5-HT7R) on human valvular cusps (i.e., aortic n = 14, mitral n = 28, tricuspid n = 28 or pulmonary n = 3) or on human left myocardium as a control (n = 28) (see also Figure 1).
For the first time, we reported the presence of one 5-HT1R receptor, the 5-HT1AR, at a low level (around 5 fmol/mg of proteins, or slightly above) which is nevertheless sufficient for specificity (23). We also detected a small amount of 5-HT1B/DR but at a very low level of around 1.3 femtomol/mg of proteins. Up to now, only 5-HT1B/DR (17) and not 5-HT1AR are present and functional on human heart valves (17).
Finally, we possibly detected a very low amount of 5-HT7R, around 3.5 femtomol/mg of proteins, and of 5-HT2cR around 1.5 femtomol/mg of proteins (23). Following univariate analyses, we did not observe any significant difference in the mean quantitative expression of specific 5-HTR receptors between the right and left side valves (Figure 1). When considering a particular patient and a specific 5-HTR, we only found a statistically positive correlation between the different locations for 5-HT2BRs, but not for the other 5-HTRs. For 5-HT2BRs, there were correlations between aortic and tricuspid valves (correlation 0.75 [0.17; 1.34]; P = 0.0042; n = 14; Spearman's Rank correlation) but not between aortic and mitral valves (correlation 0.077 [−0.38; 0.54]; P = 0.71; n = 28; Spearman's Rank correlation; Supplementary Figure S1). Surprisingly, the expressions of 5-HTRs in valvular cusps were very similar to those observed for the left myocardium ventricle that we used as a control (i.e., mostly obtained from patients with cardiomyopathies but with normal valves).
As shown in Table 2, following a univariate analysis that included all the valves (except pulmonary; n = 70 = 14 + 28 + 28), we found numerous correlations between 5-HTR quantitative levels (i.e., Spearman's Rank correlation analysis). The highest correlations were found between 5-HT1AR and 5-HT1B/DR levels (r = +0.86; P < 0.0000001; n = 70; Figure 2A1) and between 5-HT4R and 5-HT7R levels (r = +0.97; P < 0.0000001; n = 70; Figure 2A2). 5-HT2CR was the only receptor to be negatively correlated with all the other 5-HTRs, including 5-HT2AR (r = −0.27; P = 0.024; n = 70) and 5-HT2BR (r = −0.23; P = 0.052; n = 70; Figures 2B1–B4). Using univariate analysis (Spearman's Rank Correlation; n = 70 samples for each analysis), we were unable to find any correlation between 5-HT2AR and 5-HT2BR levels (Figure 2D) or between 5-HT2AR or 5-HT2BR levels and other 5-HTRs, except with 5-HT2CR. However, we found a strong negative correlation between 5-HT1AR levels and 5-HT4R levels (r = +0.37; P = 0.0009; n = 70; Figure 2C1) and between 5-HT1AR levels and 5-HT7R levels (r = +0.38; P = 0.0013; n = 70; Figure 2C2; see also Table 2).
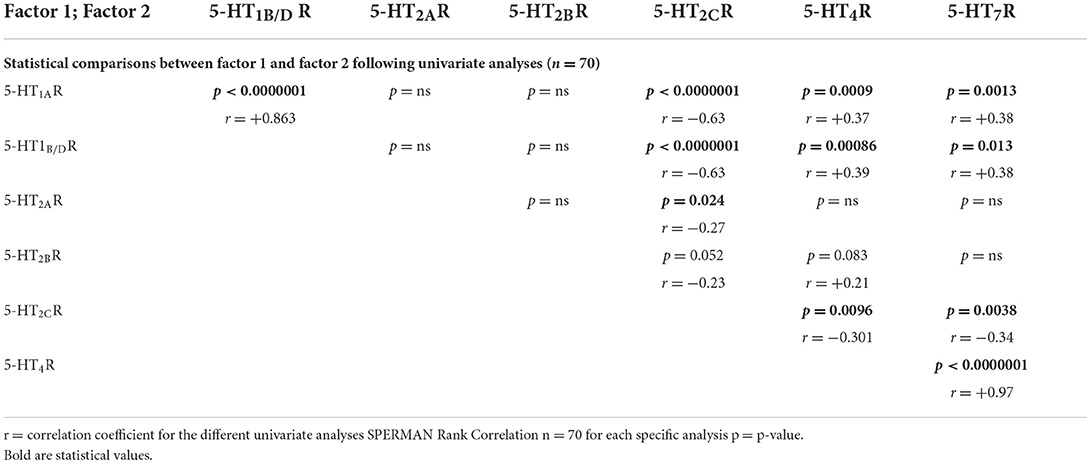
Table 2. Statistical correlations between the different 5-HTR receptors following univariate analysis.
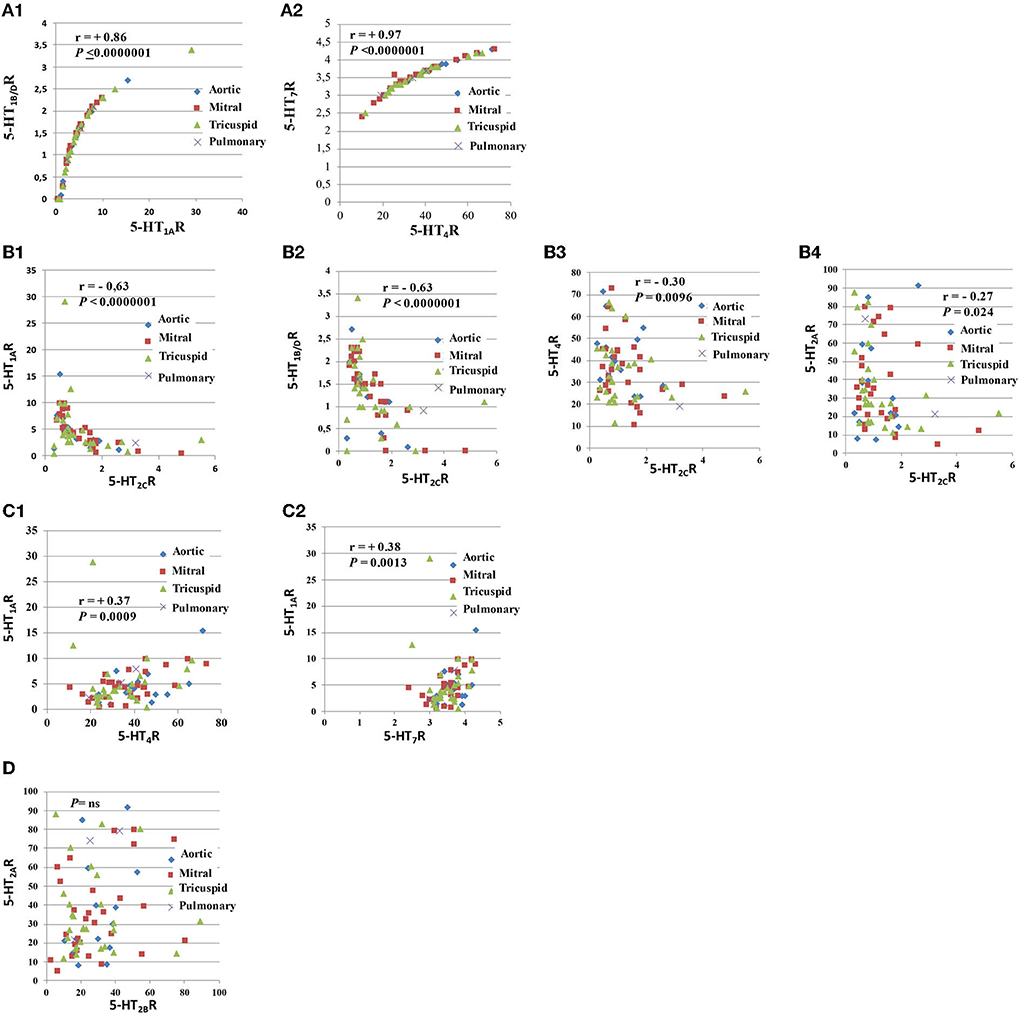
Figure 2. Comparison of the levels of expression of different pairs of 5-HTRs. This figure corresponds to this figure. As shown in this figure, there were strong correlations between several 5-HTRs (A1–D). The strongest correlations were found between the 5-HT1AR and 5-HT1B/DR quantitative protein levels (A1) and between 5-HT4R and 5-HT7R levels (A2). 5-HT1AR and 5-HT1B/DR are coupled with the same G protein (i.e., Gi/Go), while 5-HT4R and 5-HT7R are coupled with G protein Gs. We observed that 5-HT2CR levels were negatively correlated with all other 5-HTRs (C). The negative correlation between 5-HT2CR levels and other 5-HT2Rs (i.e., 5-HT2AR or 5-HT2BR) was less marked (B4). We also observed positive correlations between receptors coupled with different G proteins: 5-HT4R levels were negatively correlated with 5-H1AR levels (C1) and 5-HT1AR levels with 5-HT7R levels (C2). There was no correlation between 5-HT2AR levels and 5-HT2BR levels after univariate analysis (D). Respective correlations following univariate analyses are shown in different panels. All correlations between 5-HTRs following univariate analysis are presented in Table 2. Spearman's Rank Correlation analysis (n = 70 samples for each analysis).
Using multivariate regression analysis, we first compared the expression of a specific 5-HTR receptor and examined, as possible explicative variables, other specific 5-HTR levels and implantation sites (i.e., aortic, mitral, tricuspid; Table 3 and Supplementary Figure S2). We performed several different multivariate analyses and found the same type of significant correlations as revealed by univariate analysis. Each multivariate regression analysis was performed with 70 samples. Using multivariate regression analyses, after adjustment for implantation sites, 5-HT1AR levels were significantly correlated with 5-H1B/DR levels (coef. +0.54 [+0.49; +0.54]; P < 0.0000001). Similarly, 5-HT4R levels were found to be correlated with 5-HT7R levels (coef. +0.56 [+0.50; +0.62]; P < 0.0000001).
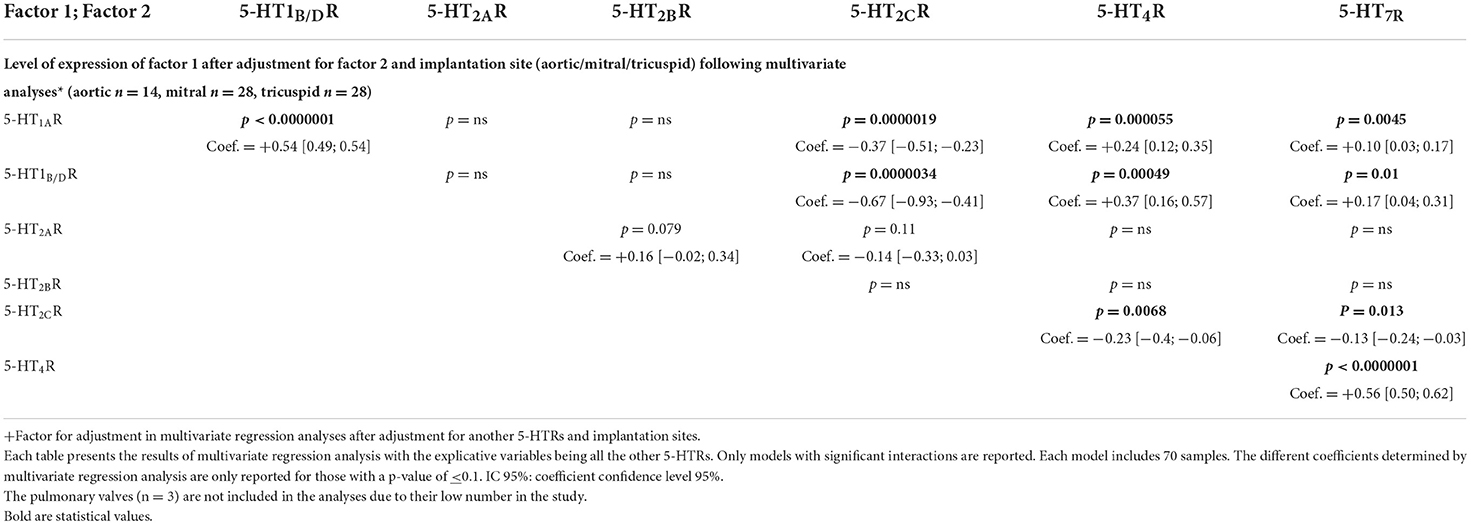
Table 3. Multivariate analysis after adjustment for another 5-HTRs for implantation sites (see also of Supplementary Figure S3).
In the second type of multivariate regression analyses (see Table 4 and Figure 3), we also compared the quantitative expression level for a specific 5-HTR, with the explicative variables being all the other 5-HTRs and the different implantation sites (i.e., aortic, mitral, tricuspid). Figure 3 shows only the significant interactions in our study, i.e., those with a P-value < 0.1 for the different multivariate analyses. The subsequent signaling (in yellow boxes) is only speculative. Each multivariate regression analysis included 70 samples. Correlation coefficients are reported only for P-values < 0.1. As in Table 4, following multivariate regression analysis, 5-HT1AR levels were independently correlated only with 5-HT1B/DR levels (coef. +1.53, [1.35; 1.7], P < 0.0000001). 5-HT4R levels were independently correlated with 5-HT7R levels (coef. +1.43 [+1.28; +1.58], P < 0.0000001) and 5-HT1AR levels (coef. +0.07 [+0.02; +0.12], P = 0.0087). 5-HT2BR levels were independently correlated with 5-HT2ARs level only (coef. +0.20 [+0.01; +0.39]; P = 0.037).
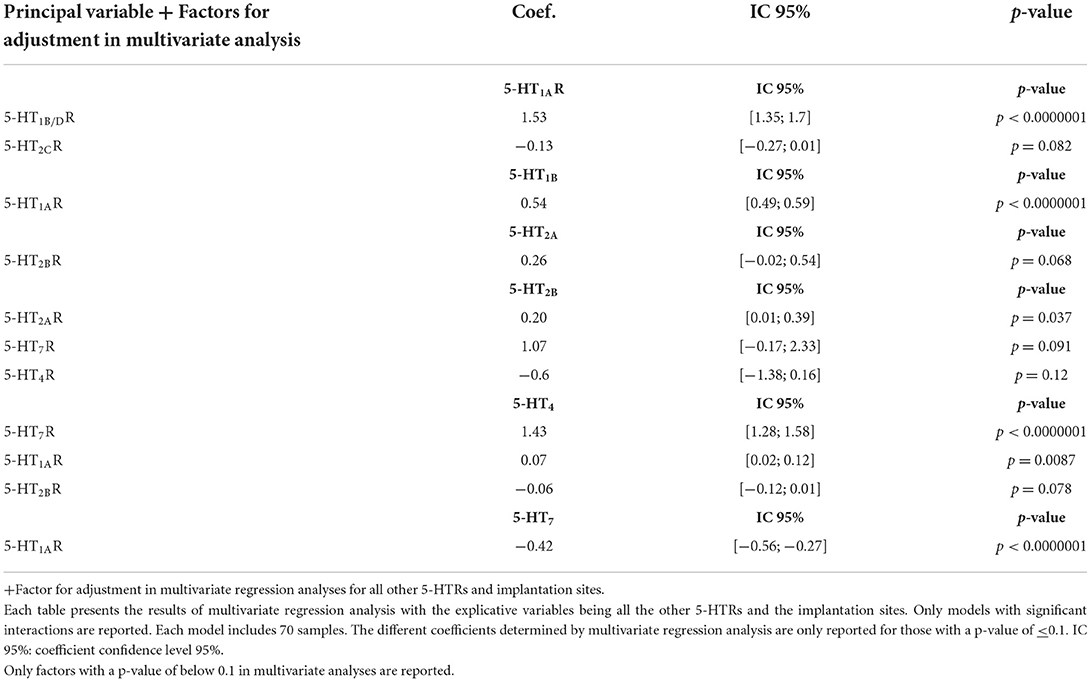
Table 4. Factors controlling the expression of specific 5-HTR based on multivariate analysis after adjustment for all other 5-HTRs and implantation sites (see also Figure 4).
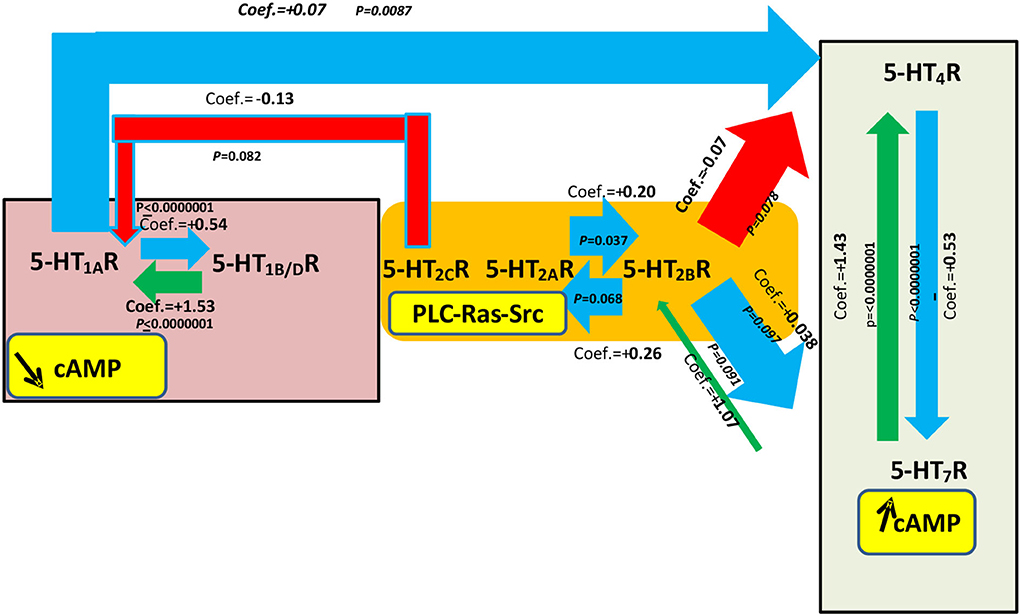
Figure 3. Most significant statistical associations based on multivariate regression analyses for a specific 5-HTR level of expression with explicative variables being all other 5-HTR receptors and the implantation sites. This figure is based on Table 4. Seventy samples were included in each statistical analysis. See also the corresponding Supplementary Figure S2 adapted from Table 3 corresponding to the most significant statistical associations after multivariate regression analysis between 5-HTR levels with the explicative variables being another 5-HTR and the site of implantation. The yellow boxes show the main classical signal transduction pathways that are possibly activated after stimulation from specific 5-HTRs: 5-HT1AR, 5-HT1B/DR, 5-HT2CR, 5-HT2AR, 5-HT2BR, 5-HT4R, and 5-HT7R especially with respect to the main signaling cAMP, but other signaling may also be present. The width of the arrows is proportional to the level of statistical correlation found following multivariate regression analysis. Only those comparisons between 5-HTRs with a P-value < 0.1 following multivariate regression analysis are shown in this figure. Blue arrows correspond to a correlation between 5-HTRs where the coefficient has an absolute value between 0 and 1 and is positive. Red arrows correspond to a correlation where the coefficient has an absolute value between 0 and 1 and is negative. In summary, as shown in this figure, statistically significant correlations were observed between levels for 5-HT1AR and 5-HT1B/DR and levels for 5-HT4R and 5-HT7R. For 5-HT4R levels, there was also a negative correlation with 5-HT1AR levels. Interestingly, we observed a negative correlation between 5-HT2AR levels and 5-HT2BR levels (coef. = +0.20 [−0.02; 0.54]; P = 0.037). The yellow boxes show the main signaling pathways for each 5-HTR if their specific G protein is involved. These signaling pathways are just hypothetical in heart valves and need confirmation.
One limitation of the study is that we just did check for correlations and but we did not check for causality. If the correlations are a result of direct causality and some of the 5-HTRs can be used to control for others, it might then be possible to explain the correlations through physical interaction between the different 5-HTRs in valvular tissue. Even if the different receptors are not present in the same cells, they can still interfere with other 5-HTR signaling. Regulation of the membrane expression of 5-HTRs may be a way of controlling their specific signaling. Putative mechanisms linking the level of expression of different 5-HTRs on heart leaflets and possible secondary signaling, especially with regard to their respective known associated G proteins, are presented in Supplementary Figure S3.
In this study, we confirmed the expressions of 5-HT2AR and 5-HT2BR on human heart valve leaflets and, interestingly, also found that they were quantitatively very similar. We also reported, for the first time, the presence of 5-HT4R and at a very high level and in the same quantity as for 5-HT2AR and 5-HT2BR. 5-HT2AR and 5-HT2BR are known to be coupled with a specific G protein, Gq/G11, and thus cannot directly control the cAMP levels, while 5-HT4R are coupled with another G protein, Gs, (s = that stimulates adenylate cyclase activation), resulting in an increase in the intracellular cAMP concentration. Furthermore, we found a lower amount of 5-HT1AR and very low amounts of 5-HT7R, 5-H1B/DR and 5-H2CR. 5-H1Rs are coupled with another G protein: Gi/G0 (“i” for inhibiting the adenylate cyclase adenylyl cyclase) and thus potentially decrease the cAMP levels, while 5-HT7Rs, like 5-HT4Rs, are coupled with Gs, and thus increase cAMP levels (18). To date, apart from 5-HT2AR and 5-HT2BR, only 5-H1B/DR (which has a very low level of expression in our study) has been shown to be present and functional on heart valves (17).
5-HT2AR, 5-HT2BR (2, 6), and possibly 5-HT 1B/DR (17, 24) have been shown to play a role in human valvular pathology. In this study, we confirmed the presence of 5-HT2AR and 5-HT2BR in human valvular leaflets but also observed that both were present at a very high level and in the same quantities, suggesting that both receptors may also play an important role in humans. Evidence of the involvement of serotonin in valvular heart diseases, such as in degenerative myxoid heart valves, is increasing (3). As with mice, recent studies using microarray technology highlight the contribution of 5-HT2BRs in pathological conditions, such as human myxomatous mitral disease (25) (for summary, see Figure 4). Recently, using qPCR, 5-HT2BRs and 5-HT2ARs have been reported as being over-expressed in human mitral valve prolapse, but only the 5-HT2BR protein was found to be increased in histological sections (2). Over-expression of mRNA for 5-HT2AR (x12) and 5-HT2BR (x28) has been observed on human heart valves in prolapses (2). However, another study using qPCR has not confirmed an increase in 5-HT2BR in human valvular prolapse (5). 5-HT2BR may also play a crucial role in the propensity of valvular tissue to develop calcification. Antagonists for 5-HT2BR have been shown to prevent aortic valve calcification by inhibiting VIC through physically arresting Src-tyrosin-kinase (26). In humans, as with mice, TPH1, the enzyme involved in its peripheral synthesis of serotonin (5-HT), is locally secreted in valvular leaflets and may play a crucial role in pathology. In vitro, its transcription is increased by mechanical stimulation only (12). In vitro, specific antagonists for 5-HT2AR, 5-HT2BR, and TPH1 also block the valvular remodeling induced by mechanical stimulation (12). In vivo, transcriptions for TPH1, 5-HT2AR, and 5-HT2BR are increased during valvular pathology (12).
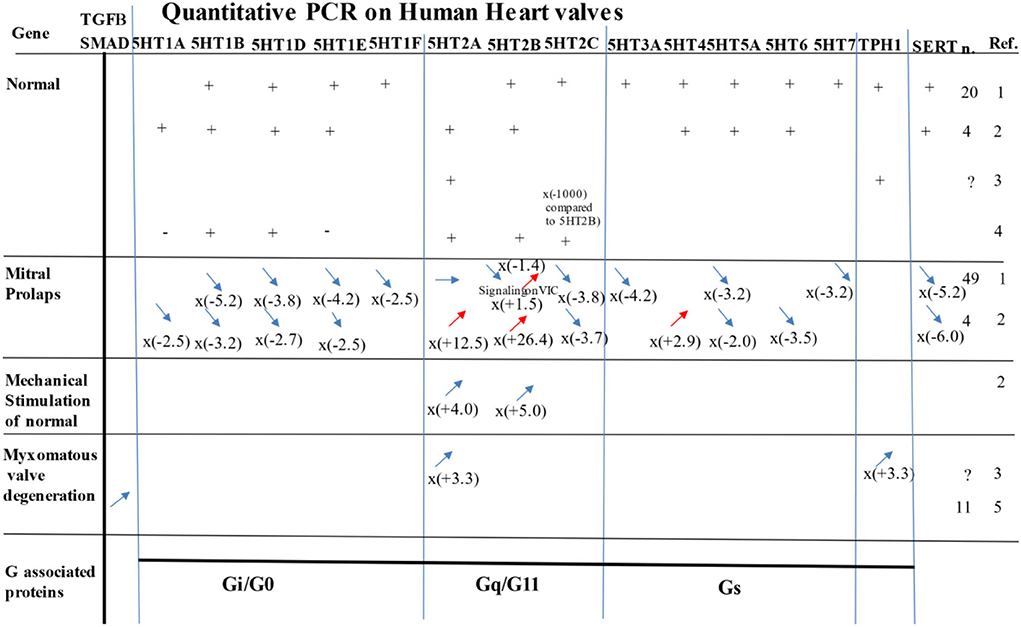
Figure 4. Most significant study results evaluating expression of 5-HTRS, TPH1, or SCC6 (i.e., the gene for SERT) by PCR on human samples in normal, prolapse, myxomatous degenerative valves, or under mechanical stimulation. n = number of samples analyzed. For PCR quantification for mitral prolapse and myxomatous valve degeneration, the values are compared to the normal. Ref. references for this figure/ 1) Levy et al. (5); 2) Driesbaugh et al. (2); 3) Hulin et al. (25); 4) Roy et al. (27); 5) Sainger et al. (28). See also for review: Ayme-Dietrich et al. (3) and Barnes et al. (11, 23).
In this study, we found a very high level of 5-HT4R as well as some 5-HT1R (i.e., 5-HR1AR) that had never been reported before. Recently, qPCR has been used in human adult leaflets, taking into account the limits of the technique because of possible alternative splicing (3, 13), to observe mRNA expression for 5-HT1AR, 5-HT1B/DR, 5-HT2AR, 5-HT2BR, 5-HT2CR, 5-HT4R, 5-HT5R, 5-HT6R, and SERT (2) (see Figure 4).
In the present study, we also reported a robust high quantitative protein expression of 5-HT4R on human leaflets. Until now, 5-HT4R has only been reported in the human myocardium and in association with atrial arrhythmias (29–32).
We reported the presence of several 5-HT1Rs (i.e., 5-HT1B/DR and 5-HT1AR) with a higher amount of 5-HT1AR than 5-HT1B/DR. Up to now, only the 5-HT1B/DRs (3, 13) have been reported in human valve leaflets, but not the 5-HT1ARs. 5-HT1B/DRs have also been shown to play a role in valvular pathology (17, 24). In our study, we reported a very low level of expression for other 5-HT2Rs (i.e., 5-HT2CR). Earlier studies using the qPCR technique found a very low level of transcription for 5-HT2C (compared with 5-HT2AR or 5-HT2BR) in valvular tissue (−300x) (27).
Carcinoid tumors are known to be more often associated with valvular heart diseases on the right side. Most (90%) of the 5-HT synthesized in the body comes from the periphery, where it is mainly produced by gut enterochromaffin cells from the essential amino acid, tryptophan, and the limiting enzyme, tryptophan hydroxylase-1 (TPH1), and then taken by SERT and stored in platelet dense granula (33). After release, the 5-HT will thus predominantly react with the right-side valves before being degraded in the pulmonary circulation (33). This is also in agreement with observations in humans with the unusual involvement of valvular diseases of the left side, when an anormal patent foramen is present, enabling blood from the right side to directly interact with the left heart valves. In animals, intravenous administration of 5-HT has been shown to be associated with right and left side diseases, while in humans some serotonin drug agonists have been shown to be associated with diseases on both sides. In our experiments, we did not find any differential expression for various 5-HTRs between the left and right sides. This study confirmed that it was rather the spatial distribution of the agonist 5-HT and not the spatial distribution of 5-HTRs between leaflets that explained the specific leaflet involvement. Our data are also in agreement with valvular development, in that during development, 5-HTRs are mainly expressed by neural crest cells, while SERT is expressed by secondary-heart-field cardiomyocytes. Both these cell types will be redistributed equally on the right and left side leaflets and thus 5-HTRs should be equally distributed between the different leaflets (34).
We reported strong correlations between the quantitative expressions of non-5-HT2R serotonin receptors on normal human heart valves.
We also observed very strong correlations between the quantitative expressions of 5-HT1AR and 5-HT1B/DR, and between 5-HT4R and 5-HT7R. We found that many non-5-HT2Rs had levels of membrane expression that were closely correlated with the level of other non-5-HT2Rs. Such a complex regulatory relationship has not been shown so far in any tissue. The highest correlations were observed between the quantitative expressions of proteins of 5-HT1AR and 5-HT1B/DR and between the quantitative expressions of proteins of 5-HT4R and 5-HT7R. While we had expected to find some correlations between 5-HT2AR and 5-HT2BR, we found none. Thus, it seems most probable that 5-HT2Rs, which are crucial for terminal valvular functionality and pathology, do not regulate each other.
The strongest correlations between 5-HTRs were found between 5-HT1AR and 5-HT1B/DR, both of which are associated with the same G protein (Gi/G0) and can thus decrease the cAMP levels, and between 5-HT4R and 5-HT7R, which are associated with another G protein, Gs, and can thus increase the cAMP levels (18).
Thanks to the quantitative assays we performed, revealing significant differences between the levels of 5-HTR expression on human heart valve tissues, it would seem that the regulatory mechanisms of 5-HTR expression are probably not unique. As we discussed, several cell types are present in valvular leaflet preparation (i.e., endothelial cells, mesenchymal cells/fibroblasts/myofibroblasts, valvular interstitial cells). The control of the regulation of 5-HTRs between each other may not necessarily require physical contact between the different 5-HTRs. If the different receptors are on the same cells, they might form homodimers or interfere with other 5-HTR signaling or levels of expression. Heterodimerization can theoretically explain the tight correlations between the levels of 5-HTRs with the same levels of expression (i.e., with a high or a low level) such as for 5-HT1AR and 5-HT1B/DR that have the same low levels in our study. At the same time, heterodimerization cannot explain the strong correlations observed between 5-HT4R and 5-HT7R, which had levels of quantitative protein expression that were very different. Thus other mechanisms are most likely involved.
Possible heterodimerization between non-5-HT2Rs for the regulation of their respective quantitative levels of expression has been reported in the literature for some tissues.
Heterodimerization requires that 5-HTRs are on the same cell and that there is some physical contact between 5-HTR monomers.
5-HTRs are present at the cell surface in a dynamic equilibrium with constant formation and dissociation of new receptor complexes (35). 5-HT2AR/5-HT2CR can function as stable homodimers, but 5-HT2BR homodimers have not yet been found (36). Dimers have also been documented for 5-HT1AR/5-HT1B/DR/5-HT4R/5-HT7R (36).
In cells in non-cardiac tissue, heterodimerization has been shown between 5-HT1BR and 5-HT1DR, 5-HT1AR and 5-HT7R, 5-HT1AR and 5-HT2AR, 5-HT2AR and 5-HT2BR, and, for 5-HT2CR, only with 5-HT2AR or 5-HT2BR (37).
A physical dimerization has been shown (38) between 5-HT1BR and 5-HT1DR, but not between 5-H1AR and 5-HT 1B/DR. Heterodimerization between Gi/0- and Gs-coupled receptors has also been reported, while heterodimerization between 5-HT1AR and 5-HT7R, has been demonstrated with inhibition of 5-HT1AR signaling (37). Heterodimerizations between Gi/0 and Gq have also been reported between 5-HT1AR and 5-HT2AR (37). 5-HT2BR heterodimerization with 5-HT1B/DR has been shown to increase 5-HT1B/DR internalization after 5-HT2BR stimulation with a specific agonist (37).
In cardiac fibroblasts, heterodimerization between 5-HT2BR and non-5-HTRs (i.e., angiotensin II) has been reported. In this experiment, in which one of the authors of the present study was involved, stimulation by angiotensin II promoted hypertrophy (39).
Besides homodimerization, in non-cardiac tissues, other complex signaling regulatory networks have been shown to allow, for a specific 5-HTR, the control of other 5-HTR expression levels.
On the same cell, but without the physical contact that is required for “heterodimerization”, the 5-HTRs can still theoretically control their respective levels of expression or signaling.
A general feature of GPGRs (i.e., “G-protein-coupled receptor families”) (35) such as 5-HTRs is the existence of complex intracellular regulatory mechanisms that modulate the receptor responsiveness. Receptor desensitization and down-regulation are well documented and are important for homeostatic mechanisms. Homologous desensitization occurs when a receptor decreases its response to an agonist at high concentrations. Heterologous desensitization involves desensitization after stimulation of another receptor. The serotonin agonist itself has been shown to provoke internalization of 5-HT2AR, 5-HT2BR, and 5-HT2CR. Stimulation of 5-HT1BR or 5-HT2BR without physical interaction has been shown to affect the internalization dynamics in a heterologous manner, especially for 5-HT2BR (15). It has been shown that the co-expression of 5-HT2BR with 5-HT1BR induces a marked acceleration of 5-HT1BR internalization, without direct physical interaction (15). Thus, 5-HT2BR can control and diminish 5-HT1B/DR membrane expression levels by inducing their internalization.
Serotonin signaling is known to be one of the few key pathways involved in human myxoid valve degeneration.
Heart valves are dynamic multilayered structures that are actively remodeled by activation of the main cellular type, the valve interstitial cells (VICs). In adults, VICs are quiescent but become activated by mechanical stimuli.
In vitro, on human mitral valvular interstitial cells, it has been shown that mechanical stress induces an early and transient TGF-β2, αSMA, and CTGF (i.e., profibrotic growth factor). The signaling pathways are RhoC/ROCK/MRTF-A and ERK1/2 (40). The 5-HT2BR antagonists do not inhibit the canonical TGF-β/smad3 phosphorylation but prevent the non-canonical p38 MAPK phosphorylation by physically arresting Src (26). With the progression of valvular disease, there is a progressive shift from initial canonical TGF-β pathways (Smad 2/3/4) to TGF-β (Smad 5/6/7), BMP, and the canonical Wnt signaling (41). The myxoid heart valve has been related to serotonin (2, 42), angiotensin II (43), and activation of TGF-β pathways (44). In humans, several studies have shown a possible link between serotonin 5-HT dysregulation and the development of MVR. It has been shown that 5-HT is locally secreted in valvular cusps and the enzyme involved in its synthesis, the TPH1 is enhanced in the degenerative human myxomatous heart valve (11). In humans, recent observations have revealed an up-regulation of RNAs for 5-HT2AR and 5-HT2BR (2). In vitro, tissue leaflet remodeling can be prevented by an antagonist for 5-HT2AR or 5-HT2BR only, or an inhibitor of TPH1 (12). Today, it is increasingly recognized that mechanical stress is a major etiological factor underlying soft connective tissue remodeling, including pathological MVP. Various studies in animal and human hearts with aortic and mitral valve leaflets have shown that mechanical stimulation is associated with VICs activation toward SMC phenotypes, increased synthesis of PGs, GAGs, and collagen as well as an increased expression of proteolytic enzymes.
In humans with myxomatous degenerative mitral heart valves (n = 11), on the regurgitating leaflet, an up-regulation of RNA expression was observed on mitral prolapse tissue for 5-HT2AR (12x) and 5-HT2BR (28x), and a decrease in 5-HT1AR (−2.5x), 5-HT1B/DR (−2.7x), and 5-HT2CR (−3.7x). There was also an increase in 5-HT4R (2.9x) and TGF-β2 (3x) and a decrease in SERT (−6x; see Figure 4). Another recent study, using also qPCR in patients (n = 44) with MV prolapse, has shown no up-regulation of 5-HT2AR, while 5-HT2BR was even down-regulated: (−1.4x), 5-HT2CR (−3.9x), 5-HT1BR (−5.5x), 5-HT1DR (−3.1), 5-HT7R (−3.2x), and SERT (−5.2x). In this study, TPH1 has been shown to be down-regulated (−2.3x) but 5-HT1AR and 5-HT4R were not significantly changed (5) (see Figure 4). TGF-β2 was again increased (2.3x), especially BMP4 (+2.0x), but not BMP3 (−2.7x) or BMP5 (−4.9x). The blockers of SERT in vitro increased the 5-HT2BR on VIC (5).
Regulation of the level of specific non-5-HT2Rs and subsequent signaling, especially regarding cAMP signaling, might theoretically be a way of interfering with valvular pathological progression.
The 5-HT2AR and 5-HT2BR signaling is essential in human valvulopathy and involves activation of Gq/G11 proteins and subsequent PLC/DAG-PKC-ERK/Ras/Src signaling. 5-HT2AR and 5-HT2BR, in general tissue in animals and humans, are associated with increased fibrosis. In animal and human heart valves, 5-HT2AR and 5-HT2BR are also associated with valvular fibrosis (3). Among the different 5-HTRs, only HT2AR and 5-HT2BR have been shown, in both animals and humans, to be associated with valvular pathology (3). None of the other 5-HTRs have been shown to have such a capacity (3). In mice and humans, 5-HT2AR and 5-HT2BR, and subsequent PLC/DAG-PKC-ERK/Ras/Src signaling, have been reported to be associated with valvular fibrosis and, among different 5-HTRs, are the key 5-HTRs involved in the degeneration process (2, 5, 11, 12, 23, 42). In animal and human myxomatous degenerative valves, the local secretion of serotonin is also increased with increased TPH1 (11). We hypothesize that this signaling might be controlled by the regulation of non-5-HT2Rs in particular (i.e., 5-HT1AR, 5-HT1B/DR, 5-HT4R, and 5-HT7R), which can regulate the cAMP levels. The role of cAMP is to inhibit ERK1/2 and Smad 2/3/4 and to stimulate Notch and PKA, a role that has been reviewed recently (45). By acting on cAMP levels, the non-5-HT2Rs may promote or reduce fibrosis.
One limitation of the study is that the levels of expression for 5-HTRs, other than 5-HT2AR, 5-HT2BR and 5-HT4R, were very low, below the standard detection level for pharmacology assays that is around 5 fmol of ligand binding per mg of protein extracts (23). With respect to 5-HT1ARs, their levels of expression were 5.3 (±5.4) fmol/mg of protein in tricuspid position and 4.7 (+/2.6) fmol/mg of protein in mitral position (23).
In our study, 5-HT1B/DRs presented one of the lowest levels of expression, with a level of around 1.4 (±0.7) fmol/mg of protein for the different valvular positions and thus far below the 5 fmol/mg level of proteins. However, its true presence and functionality have been proven by other groups in mice (24) and human fibroblasts derived from human heart valves (17).
In recent studies comparing normal human valvular leaflets and regurgitating leaflets, RNA expression was observed on normal valve and mitral prolapse tissues for most 5-HTRs that we identified in this study: 5-HT1B/DR, 5-HT1AR, 5-HT2AR, 5-HT2BR, 5-HT2CR, and R 5-HT4R (2, 4).
The best correlations we found were between 5-HT1AR and 5-HT1B/DR and between 5-HT4R and 5-HT7R. Since we are below the theoretical level of 5 fmol/mg of proteins for 5-HT1AR, 5-HT1B/DR, and 5-HT7R, we cannot exclude that for these receptors the interactions with the specific agonist may not be real. In a recent study on human mitral prolapse compared to human normal valve, the variations for 5-HT1AR and 5-HT1B/DR are almost the same [i.e., (−2.5x) and (−2.7x)], suggesting that a possible close regulatory relationship between these receptors in human pathology may exist (2, 4).
Another important limitation of the study is that we performed the analysis on an extract taken from the valvular tissues and not on a specific cellular population subtype. Heterodimerization between serotonin receptors requires that 5-HTR receptors be on the same cell. We cannot prove this. At the same time, in human valvular tissues, the expression of 5-HTR has so far been shown to be limited to a few cell types in valves (i.e., endothelial cells, fibroblasts/myofibroblasts, and interstitial valvular cells) (2, 17). The overall idea of having possible crosstalk between different cell types to regulate the level of expression of serotonin 5-HT receptors is still valid at the level of the overall preparation.
In this study, we did not specifically investigate signaling proteins in the preparation for cAMP or other signaling and all the mechanisms evoked were merely putative. Considering the 5-HTRs, it is known that their main signaling mechanism is due to their associated G proteins (18). At the same time, signal transduction following 5-HTR stimulation by agonists is not limited to the signal associated with their G protein, and each 5-HTR has a specific signal (18). 5-HT1Rs inhibit the adenylate cyclase (AC) and thus decrease cAMP. Both 5-HT1AR and 5-HT1B/DR stimulate ERK. In addition, 5-HT1AR frequently activates the K+ channel and inhibits the Ca2+ conductance. There are other pathways such as PLC and NOS (18). 5-HT7R activates AC and PKA as well as ERK (18). With respect to 5-HT2AR and 5HT2BR, they have the same main signaling: PLC, ERK, and PLA2. However, 5-HT2AR also activates PKC, and is responsible for the activation or inhibition of AC, and the activation of Jak2, Stat3, and Ca2+ channels. 5-HT2B can also activate the cell cycle and iNOS. Like other 5-HT2Rs, 5-HT2B can also activate ERK1/2. Finally, 5-HT4R can activate AC and various channels.
Another limitation of our study is that we only identified correlations between 5-HTRs and did not check for possible causality. This should be evaluated in future studies.
In conclusion, our study reveals that many 5-HTR proteins are present in the extract of “normal” human valvular leaflets, especially 5-HT2AR, 5-HT2BR and the newly reported 5-HT4R, all three of which were observed at similar levels. There is also a smaller amount of 5-HT1AR and a possible expression of 5-HT1B/D R and 5-HT2CR, but at even lower levels. All these 5-HTRs are known to be linked to specific G proteins: Gi/G0, Gq/G11, or Gs. Interestingly, very strong correlations were found between non-5-HT2BR levels, especially between 5-HT1AR and 5-HT1B/DR and between 5-HT4R and 5-HT7R. 5-HT1AR and 5-HT1B/DR are associated with the same G-protein (Gs) and can thus increase the cAMP in valvular tissue, while 5-HT1AR and 5-HT1B/DR are coupled to another G protein (Gi/G0) and can thus theoretically decrease cAMP. All these signaling mechanisms, and particularly their associated proteins, need to be assessed, If other groups can show causality, rather than just the presence of correlations between expressions of 5-HTRs, as we have done in this study, the regulation of the level of expression of non-5-HT2Rs, and subsequent signaling, might be a way to control the signaling activity of 5-H2BR and 5-HT2AR, which are the main serotonin receptors involved in animal and human pathology (i.e., PLC, ERK1/2, IP3, Ca2+, Src, Ras, and TGF-β).
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Human Specimens. All experimental procedures were done in accordance with the ethical standards of the responsible institutional and national committee on human experimentation, adhering to the Helsinki Declaration (1975). Patient or patient's family gave their written consent to the program of homograft. The project was approved by the Institutional Review Boards of the University Hospital of Geneva, Switzerland [Approbation number CER: 12-150 (NAC 12-056)] and by a local committee at the European Homograft Bank in Brussels, Belgium. The patients/participants provided their written informed consent to participate in this study.
OS, LM, and YL: design. RJ: samples collection. J-ML: quantitative dosages. OS and MA: statistics. All authors participate in the drafting of manuscript, read, and approved the submission of the manuscript.
The authors would like to thank Brian Keogh for rereading and improving the English in the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.897657/full#supplementary-material
1. Ayme-Dietrich E, Lawson R, Cote F, de Tapia C, Da Silva S, Ebel C, et al. The role of 5-HT2B receptors in mitral valvulopathy: bone marrow mobilization of endothelial progenitors. Br J Pharmacol. (2017) 174:4123–39. doi: 10.1111/bph.13981
2. Driesbaugh KH, Branchetti E, Grau JB, Keeney SJ, Glass K, Oyama MA, et al. Serotonin receptor 2B signaling with interstitial cell activation and leaflet remodeling in degenerative mitral regurgitation. J Mol Cell Cardiol. (2018) 115:94–103. doi: 10.1016/j.yjmcc.2017.12.014
3. Ayme-Dietrich E, Lawson R, Da-Silva S, Mazzucotelli JP, Monassier L. Serotonin contribution to cardiac valve degeneration: new insights for novel therapies? Pharmacol Res. (2019) 140:33–42. doi: 10.1016/j.phrs.2018.09.009
4. Thalji NM, Hagler MA, Zhang H, Casaclang-Verzosa G, Nair AA, Suri RM, et al. Nonbiased molecular screening identifies novel molecular regulators of fibrogenic and proliferative signaling in myxomatous mitral valve disease. Circ Cardiovasc Genet. (2015) 8:516–28. doi: 10.1161/CIRCGENETICS.114.000921
5. Levy RJ, Fitzpatrick E, Castillero E, Shukla HJ, Inamdar VV, Aghali AE, et al. Inhibition and down regulation of the serotonin transporter contribute to the progression of degenerative mitral regurgitation. bioRxiv. (2020) doi: 10.1101/2020.03.10.985382
6. Hutcheson JD, Setola V, Roth BL, Merryman WD. Serotonin receptors and heart valve disease: it was meant 2B. Pharmacol Ther. (2011) 132:146–57. doi: 10.1016/j.pharmthera.2011.03.008
7. Rothman RB, Baumann MH, Savage JE, Rauser L, McBride A, Hufeisen SJ, et al. Evidence for possible involvement of 5-HT(2B) receptors in the cardiac valvulopathy associated with fenfluramine and other serotonergic medications. Circulation. (2000) 102:2836–41. doi: 10.1161/01.CIR.102.23.2836
8. Dawson P, Moffatt JD. Cardiovascular toxicity of novel psychoactive drugs: lessons from the past. Prog Neuropsychopharmacol Biol Psychiatry. (2012) 39:244–52. doi: 10.1016/j.pnpbp.2012.05.003
9. Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, et al. Heart disease and stroke statistics-2021 update: a report from the American heart association. Circulation. (2021) 143:e254–743. doi: 10.1161/CIR.0000000000000950
10. Guy TS, Hill AC. Mitral valve prolapse. Annu Rev Med. (2012) 63:277–92. doi: 10.1146/annurev-med-022811-091602
11. Disatian S, Lacerda C, Orton EC. Tryptophan hydroxylase 1 expression is increased in phenotype-altered canine and human degenerative myxomatous mitral valves. J Heart Valve Dis. (2010) 19:71–8.
12. Lacerda CM, Maclea HB, Kisiday JD, Orton EC. Static and cyclic tensile strain induce myxomatous effector proteins and serotonin in canine mitral valves. J Vet Cardiol. (2012) 14:223–30. doi: 10.1016/j.jvc.2011.12.002
13. Roy A, Brand NJ, Yacoub MH. Expression of 5-hydroxytryptamine receptor subtype messenger RNA in interstitial cells from human heart valves. J Heart Valve Dis. (2000) 9:256–60; discussion 260–1.
14. Loric S, Maroteaux L, Kellermann O, Launay JM. Functional serotonin-2B receptors are expressed by a teratocarcinoma-derived cell line during serotoninergic differentiation. Mol Pharmacol. (1995) 47:458–66.
15. Janoshazi A, Deraet M, Callebert J, Setola V, Guenther S, Saubamea B, et al. Modified receptor internalization upon coexpression of 5-HT1B receptor and 5-HT2B receptors. Mol Pharmacol. (2007) 71:1463–74. doi: 10.1124/mol.106.032656
16. Belmer A, Doly S, Setola V, Banas SM, Moutkine I, Boutourlinsky K, et al. Role of the N-terminal region in G protein-coupled receptor functions: negative modulation revealed by 5-HT2B receptor polymorphisms. Mol Pharmacol. (2013) 85:127–38. doi: 10.1124/mol.113.089086
17. Seuwen K, Magnaldo I, Pouyssegur J. Serotonin stimulates DNA synthesis in fibroblasts acting through 5-HT1B receptors coupled to a Gi-protein. Nature. (1988) 335:254–6. doi: 10.1038/335254a0
18. Raymond JR, Mukhin YV, Gelasco A, Turner J, Collinsworth G, Gettys TW, et al. Multiplicity of mechanisms of serotonin receptor signal transduction. Pharmacol Ther. (2001) 92:179–212. doi: 10.1016/S0163-7258(01)00169-3
19. Jashari R. Transplantation of cryopreserved human heart valves in Europe: 30 years of banking in Brussels and future perspectives. Cell Tissue Bank. (2021) 22:519–37. doi: 10.1007/s10561-021-09902-2
20. Jashari R, Van Hoeck B, Tabaku M, Vanderkelen A. Banking of the human heart valves and the arteries at the European homograft bank (EHB): overview of a 14-year activity in this international association in brussels. Cell Tissue Bank. (2004) 5:239–51. doi: 10.1007/s10561-004-1441-0
21. Leeten K, Ditkowski B, Jashari R, Mela P, Jones EAV, Heying R. An in vitro model to study endothelialization of cardiac graft tissues under flow. Tissue Eng C Methods. (2021) 27:233–41. doi: 10.1089/ten.tec.2020.0359
22. Loric S, Launay JM, Colas JF, Maroteaux L. New mouse 5-HT2-like receptor. Expression in brain, heart and intestine. FEBS Lett. (1992) 312:203–7. doi: 10.1016/0014-5793(92)80936-B
23. Barnes NM, Ahern GP, Becamel C, Bockaert J, Camilleri M, Chaumont-Dubel S, et al. International union of basic and clinical pharmacology. CX Classification of receptors for 5-hydroxytryptamine; pharmacology and function. Pharmacol Rev. (2021) 73:310–520. doi: 10.1124/pr.118.015552
24. Mekontso-Dessap A, Brouri F, Pascal O, Lechat P, Hanoun N, Lanfumey L, et al. Deficiency of the 5-hydroxytryptamine transporter gene leads to cardiac fibrosis and valvulopathy in mice. Circulation. (2006) 113:81–9. doi: 10.1161/CIRCULATIONAHA.105.554667
25. Hulin A, Deroanne C, Lambert C, Defraigne JO, Nusgens B, Radermecker M, et al. Emerging pathogenic mechanisms in human myxomatous mitral valve: lessons from past and novel data. Cardiovasc Pathol. (2012) 22:245–50. doi: 10.1016/j.carpath.2012.11.001
26. Hutcheson JD, Ryzhova LM, Setola V, Merryman WD. 5-HT(2B) antagonism arrests non-canonical TGF-beta1-induced valvular myofibroblast differentiation. J Mol Cell Cardiol. (2012) 53:707–14. doi: 10.1016/j.yjmcc.2012.08.012
27. Fitzgerald LW, Burn TC, Brown BS, Patterson JP, Corjay MH, Valentine PA, et al. Possible role of valvular serotonin 5-HT(2B) receptors in the cardiopathy associated with fenfluramine. Mol Pharmacol. (2000) 57:75–81.
28. Sainger R, Grau JB, Branchetti E, Poggio P, Seefried WF, Field BC, et al. Human myxomatous mitral valve prolapse: role of bone morphogenetic protein 4 in valvular interstitial cell activation. J Cell Physiol. (2011) 227:2595–604. doi: 10.1002/jcp.22999
29. Bach T, Syversveen T, Kvingedal AM, Krobert KA, Brattelid T, Kaumann AJ, et al. 5HT4(a) and 5-HT4(b) receptors have nearly identical pharmacology and are both expressed in human atrium and ventricle. Naunyn Schmiedebergs Arch Pharmacol. (2001) 363:146–60. doi: 10.1007/s002100000299
30. Brattelid T, Qvigstad E, Lynham JA, Molenaar P, Aass H, Geiran O, et al. Functional serotonin 5-HT4 receptors in porcine and human ventricular myocardium with increased 5-HT4 mRNA in heart failure. Naunyn Schmiedebergs Arch Pharmacol. (2004) 370:157–66. doi: 10.1007/s00210-004-0963-0
31. Brattelid T, Kvingedal AM, Krobert KA, Andressen KW, Bach T, Hystad ME, et al. Cloning, pharmacological characterisation and tissue distribution of a novel 5-HT4 receptor splice variant, 5-HT4(i). Naunyn Schmiedebergs Arch Pharmacol. (2004) 369:616–28. doi: 10.1007/s00210-004-0919-4
32. Gergs U, Baumann M, Bockler A, Buchwalow IB, Ebelt H, Fabritz L, et al. Cardiac overexpression of the human 5-HT4 receptor in mice. Am J Physiol Heart Circ Physiol. (2010) 299:H788–98. doi: 10.1152/ajpheart.00691.2009
33. Ayme-Dietrich E, Aubertin-Kirch G, Maroteaux L, Monassier L. Cardiovascular remodeling and the peripheral serotonergic system. Arch Cardiovasc Dis. (2016) 110:51–9. doi: 10.1016/j.acvd.2016.08.002
34. Schussler O, Gharibeh L, Mootoosamy P, Murith N, Tien V, Rougemont AL, et al. Cardiac neural crest cells: their rhombomeric specification, migration, and association with heart and great vessel anomalies. Cell Mol Neurobiol. (2020) 41:403–29. doi: 10.1007/s10571-020-00863-w
35. Ferre S, Baler R, Bouvier M, Caron MG, Devi LA, Durroux T, et al. Building a new conceptual framework for receptor heteromers. Nat Chem Biol. (2009) 5:131–4. doi: 10.1038/nchembio0309-131
36. Moutkine I, Quentin E, Guiard BP, Maroteaux L, Doly S. Heterodimers of serotonin receptor subtypes 2 are driven by 5-HT2C protomers. J Biol Chem. (2017) 292:6352–68. doi: 10.1074/jbc.M117.779041
37. Maroteaux L, Bechade C, Roumier A. Dimers of serotonin receptors: impact on ligand affinity and signaling. Biochimie. (2019) 161:23–33. doi: 10.1016/j.biochi.2019.01.009
38. Xie Z, Lee SP, O'Dowd BF, George SR. Serotonin 5-HT1B and 5-HT1D receptors form homodimers when expressed alone and heterodimers when co-expressed. FEBS Lett. (1999) 456:63–7. doi: 10.1016/S0014-5793(99)00918-7
39. Jaffre F, Bonnin P, Callebert J, Debbabi H, Setola V, Doly S, et al. Serotonin and angiotensin receptors in cardiac fibroblasts coregulate adrenergic-dependent cardiac hypertrophy. Circ Res. (2009) 104:113–23. doi: 10.1161/CIRCRESAHA.108.180976
40. Blomme B, Deroanne C, Hulin A, Lambert C, Defraigne JO, Nusgens B, et al. Mechanical strain induces a pro-fibrotic phenotype in human mitral valvular interstitial cells through RhoC/ROCK/MRTF-A and Erk1/2 signaling pathways. J Mol Cell Cardiol. (2019) 135:149–59. doi: 10.1016/j.yjmcc.2019.08.008
41. Chopra S, Al-Sammarraie N, Lai Y, Azhar M. Increased canonical WNT/beta-catenin signalling and myxomatous valve disease. Cardiovasc Res. (2017) 113:6–9. doi: 10.1093/cvr/cvw236
42. Perrucci GL, Zanobini M, Gripari P, Songia P, Alshaikh B, Tremoli E, et al. Pathophysiology of aortic stenosis and mitral regurgitation. Compr Physiol. (2017) 7:799–818. doi: 10.1002/cphy.c160020
43. Geirsson A, Singh M, Ali R, Abbas H, Li W, Sanchez JA, Hashim S, Tellides G. Modulation of transforming growth factor-beta signaling and extracellular matrix production in myxomatous mitral valves by angiotensin II receptor blockers. Circulation. (2012) 126(11 Suppl 1):S189–97. doi: 10.1161/CIRCULATIONAHA.111.082610
44. Hulin A, Deroanne CF, Lambert CA, Dumont B, Castronovo V, Defraigne JO, et al. Metallothionein-dependent up-regulation of TGF-beta2 participates in the remodelling of the myxomatous mitral valve. Cardiovasc Res. (2011) 93:480–9. doi: 10.1093/cvr/cvr337
Keywords: serotonin receptor 5-HTRs, human heart valves, serotonin 5-HT, quantitative expression, valvular degeneration, heart valve disease
Citation: Schussler O, Maroteaux L, Jashari R, Falcoz P, Alifano M, Lecarpentier Y and Launay J-M (2022) First quantitative dosages: Strong correlations between non-5-HT2Rs serotonin receptors on normal human heart valves. Front. Cardiovasc. Med. 9:897657. doi: 10.3389/fcvm.2022.897657
Received: 16 March 2022; Accepted: 06 September 2022;
Published: 25 October 2022.
Edited by:
Joshua D. Hutcheson, Florida International University, United StatesReviewed by:
Philippe De Deurwaerdere, Université de Bordeaux, FranceCopyright © 2022 Schussler, Maroteaux, Jashari, Falcoz, Alifano, Lecarpentier and Launay. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Olivier Schussler, b2xpdmllci5zY2h1c3NsZXJAZ21haWwuY29t; b2xpdmllci5zY2h1c3NsZXJAYXBocC5mcg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.