- 1Division of Hematology-Oncology, Department of Internal Medicine, Chang Gung Memorial Hospital at Linkou, Taoyuan, Taiwan
- 2College of Medicine, Chang Gung University, Taoyuan, Taiwan
- 3Department of Public Health, College of Medicine, Chang Gung University, Taoyuan, Taiwan
- 4Division of Cardiology, Department of Internal Medicine, Chang Gung Memorial Hospital at Linkou, Taoyuan, Taiwan
- 5Division of Hematology-Oncology, Department of Internal Medicine, Chang Gung Memorial Hospital at Keelung, Keelung, Taiwan
- 6Division of Rheumatology, Allergy and Immunology, Department of Internal Medicine, Chang Gung Memorial Hospital at Linkou, Taoyuan, Taiwan
- 7Biostatistics Core Laboratory, Molecular Medicine Research Center, Chang Gung University, Taoyuan, Taiwan
Background: Patients with colorectal cancer (CRC) are more likely to develop cardiovascular disease (CVD) than those without cancer. Little is known regarding their CV risk after operative chemotherapy. We aimed to compare the risk of CV disease among different fluoropyrimidine derivatives.
Methods: We assembled a nationwide cohort of patients with newly diagnosed CRC between 2004 and 2015 who received fluoropyrimidine-based adjuvant chemotherapy for resected CRC by linking the Taiwan Cancer Registry (TCR), National Health Insurance Research Database (NHIRD), and Taiwan Death Registry (TDR). All eligible patients were followed from CRC diagnosis (index date) until a CV event, death, loss to follow-up, or December 31st 2018, whichever came first. CV outcomes included acute myocardial infarction (AMI), life-threatening arrhythmia (LTA), congestive heart failure (CHF), and ischemic stroke (IS). We used stabilized inverse probability of treatment weighting using propensity score (SIPTW) to balance all covariates among the three chemotherapy groups: tegafur-uracil (UFT), non-UFT, and mixed. In addition, survival analysis was conducted to examine the association between study outcomes and chemotherapy groups.
Results: From 2004 to 2015, 10,615 (32.8%) patients received UFT alone, 14,511 (44.8%) patients received non-UFT, and 7,224 (22.3%) patients received mixed chemotherapy. After SIPTW, the UFT group had significantly lower all-cause mortality and cancer-related death rates than the other two chemotherapy groups. However, the UFT group had significantly higher rates of cancer death, ischemic stroke, and heart failure than those of the other two chemotherapy groups. The UFT group also had a significantly higher AMI rate than the mixed group. There was no significant difference in LTA among the three groups. Similar findings were observed in the subgroup analysis (stage II and age <70 years, stage II and age ≥70 years, stage III and age <70 years, stage III and age ≥70 years) as the overall population was observed.
Conclusion: Higher heart failure and ischemic stroke rates were found in the UFT group than in the other two chemotherapy groups, especially those with stage III CRC and ≥70 years of age. Careful monitoring of this subset of patients when prescribing UFT is warranted.
Introduction
Over the past two decades, early-stage cancer detection and treatment improvements have significantly improved the prognosis of several major cancers, such as colorectal cancer (CRC), prostate cancer, and breast cancer (1). It has been postulated that the population of cancer survivors in the USA will increase to eighteen million by 2022 (2). Within the population of cancer survivors, the awareness of health problems that can occur after cancer survival is increasing. Among these, treatment-related cardiovascular diseases are a major concern (3). The US Surveillance, Epidemiology, and End Results (SEER) study showed that cardiovascular (CV) death was the most common cause of non-cancer deaths among cancer patients in 1973–2012 (4). One cohort study, with 36,232 2-year survivors from 2000 to 2007 who were followed up until 2012, found that cancer survivors had significantly more CV adverse events than non-cancer controls (5).
Patients with colorectal cancer are more likely to develop cardiovascular disease (CVD) than those without cancer (6). Kenzik et al. conducted a US population-based study to determine the long-term risk of cardiovascular disease (including stroke and myocardial infarction) and congestive heart failure (CHF) in stage I–III CRC survivors aged 65 years. The 10-year cumulative incidence of new-onset cardiovascular disease and CHF was 57.4 and 54.5% for patients with stage I–III CRC compared with 22 and 18% for the matched cohort without cancer, respectively (p < 0.001) (7). Correspondingly, a Korean cohort study reported 141 (4.9%) patients who developed new-onset CVD among postoperative CRC patients (8). These studies raised the increasing concern of CV-related adverse events among CRC survivors.
Postoperative chemotherapy is associated with an increased risk of CVD (7, 8). Fluoropyrimidine is the backbone of chemotherapy in the adjuvant setting in patients with CRC. In addition to intravenous 5- fluorouracil (FU), oral fluoropyrimidine including UFT, TS-1 and capecitabine were commonly used in Asian countries (9, 10). While these oral prodrugs were finally metabolized to 5-FU, their adverse events were somewhat different, which may be due to the components of prodrugs (Figure 1). For example, gimeracil (the compoenent of TS-1) and uracil (the component of UFT) inhibit dihydropyrimidine dehydrogenase, which degrades 5-FU, leading to enhance cytotoxic effects.
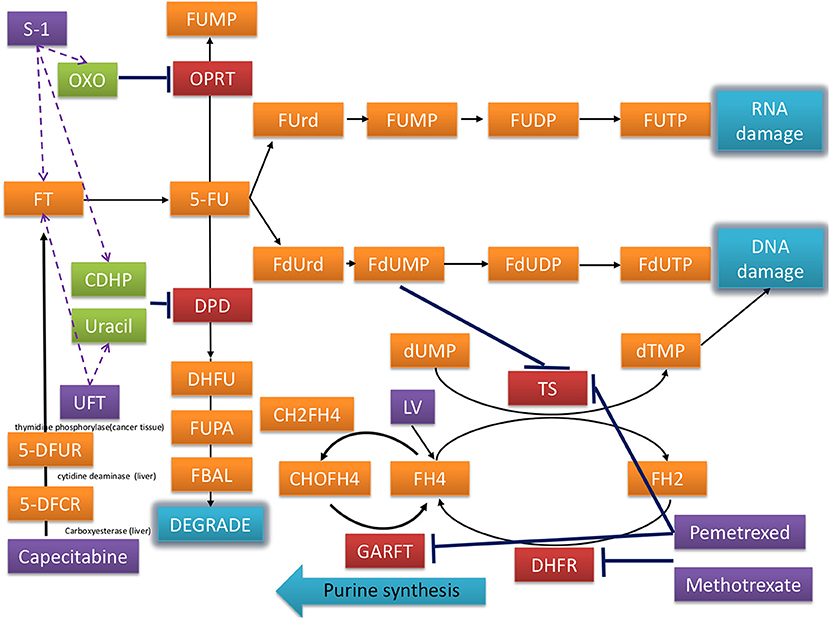
Figure 1. Mechanisms of fluorouracil-related medications including UFT, capecitabine, S-1, and others in the treatment of CRC.
Notably, exposure to fluoropyrimidine increases the risk of CV in patients with cancer (11). However, whether fluoropyrimidine derivatives including intravenous 5-FU, capecitabine, and tegafur-uracil (UFT) have a different effect on cardiotoxicity remains unclear. UFT is an oral agent in which uracil competes with dihydropyrimidine dehydrogenase, which reduces the catabolism of 5-FU and its cardiotoxic metabolites. No study has compared UFT with other fluoropyrimidine derivatives concerning their subsequent CVD risk. The Taiwan Cancer Registry (TCR), National Death Registry (NDR), and National Health Insurance Research Database (NHIRD) provide comprehensive and accurate information on the diagnosis, staging, treatment, and survival of cancer patients in Taiwan. Here, we linked the above databases to evaluate the cardiotoxicity of different 5-FU derivatives in the adjuvant setting for patients with CRC.
Methods
Data sources
The data sources for this study included the TCR, NHIRD, and TDR. These three databases are linked with encrypted personal identification numbers and are available at the Health and Welfare Data Center (HWDC). In addition, we obtained approval from the IRB of the Chang Gung Medical Foundation, Taiwan (201901844B0). The need for informed consent was waived because the personal ID had already been encrypted.
Study design
We established a nationwide cohort of patients with newly diagnosed stage II–III CRC in 2004–2015 and received FU-based adjuvant chemotherapy for resection. All eligible patients were followed up from 6 months after the first diagnosis of CRC (index date) until the occurrence of cardiotoxicity (AMI, LTA, CHF, IS, independently), death, and loss to follow-up on December 31st 2018, whichever came first. The index date was set 6 months after CRC first diagnosis because (1) the majority of patients with stage II–III CRC had their tumor removed surgically and started FU-based adjuvant chemotherapy within 6 months after the initial diagnosis of CRC, and (2) all eligible patients were followed equally with the same initial time point to reduce immortal time bias (Figure 2) (12).
The cohort was divided into three adjuvant chemotherapy groups: UFT, non-UFT, and mixed. Patients were excluded if they (1) had non-adenocarcinomatous CRC; (2) did not receive surgical resection; (3) had a positive resection margin; (4) did not receive any adjuvant chemotherapy; (5) did not start chemotherapy before the index date (6 months after initial CRC diagnosis); (6) had AMI, LTA, CHF, or IS before the index date; (7) missing sex and birth year data; and (8) implausible data, such as death before CRC diagnosis or inconsistent initial date of adjuvant chemotherapy in TCR, NHIRD, and TDR (Figure 2).
Outcomes
The study outcomes were as follows: (1) mortality, all-cause mortality, cancer mortality, CV mortality, and non-CV mortality; and (2) CV events including acute myocardial infarction (AMI), life-threatening arrhythmia (LTA), congestive heart failure (CHF), and ischemic stroke (IS). Furthermore, to reduce misclassification, all CV outcomes had to be the principal diagnosis of hospitalization admission or the first diagnosis through the emergency department based on ICD-9 (until 2015) or ICD-10 (since 2016) (Supplementary Table 1).
Covariates
For demographic characteristics, we obtained age, sex, income level, and enrollment category from the NHIRD. In addition, we obtained the calendar year of CRC diagnosis, primary site, stage, tumor grade, and tumor treatment modality from the TCR for cancer-related characteristics. For comorbidities, we obtained data on hypertension, dyslipidemia, diabetes, coronary artery disease, chronic obstructive pulmonary disease (COPD), peripheral arterial disease, chronic kidney disease, atrial fibrillation, and moderate or severe liver disease from the NHIRD. All comorbidities had to occur 1 year before the first diagnosis of CRC. Regarding CV-related medication, we were confined to metformin, aspirin, and statins, which were prescribed 1 year before the first diagnosis of CRC from the NHIRD (Supplementary Table 1).
Statistical analysis
We first balanced all covariates among the three chemotherapy groups by stabilizing the inverse probability of treatment weighting using the propensity score (SIPTW) (13). The advantage of using SIPTW is obtaining an appropriate estimation of the variance of the main effect (average treatment effect for the population, ATE) and maintaining an adequate type I error by preserving the sample size of the original data. In SIPTW, we used a generalized boosted model (GBM) (14) to compute the propensity score because GBM gives the best performance in various scenarios (additivity and linearity, mild non-additivity and non-linearity, and moderate non-additivity and non-linearity in different weight trimming percentiles) (15), and can be extended to more than two treatment groups (14). The covariates in Table 1 were included in the GBM. Next, the absolute standardized mean difference (ASMD) was used to assess the balance of covariates at baseline (index date) among the three chemotherapy groups. A maximum value of ASMD ≤ 0.1 indicated an insignificant difference in covariates among the three chemotherapy groups (16).
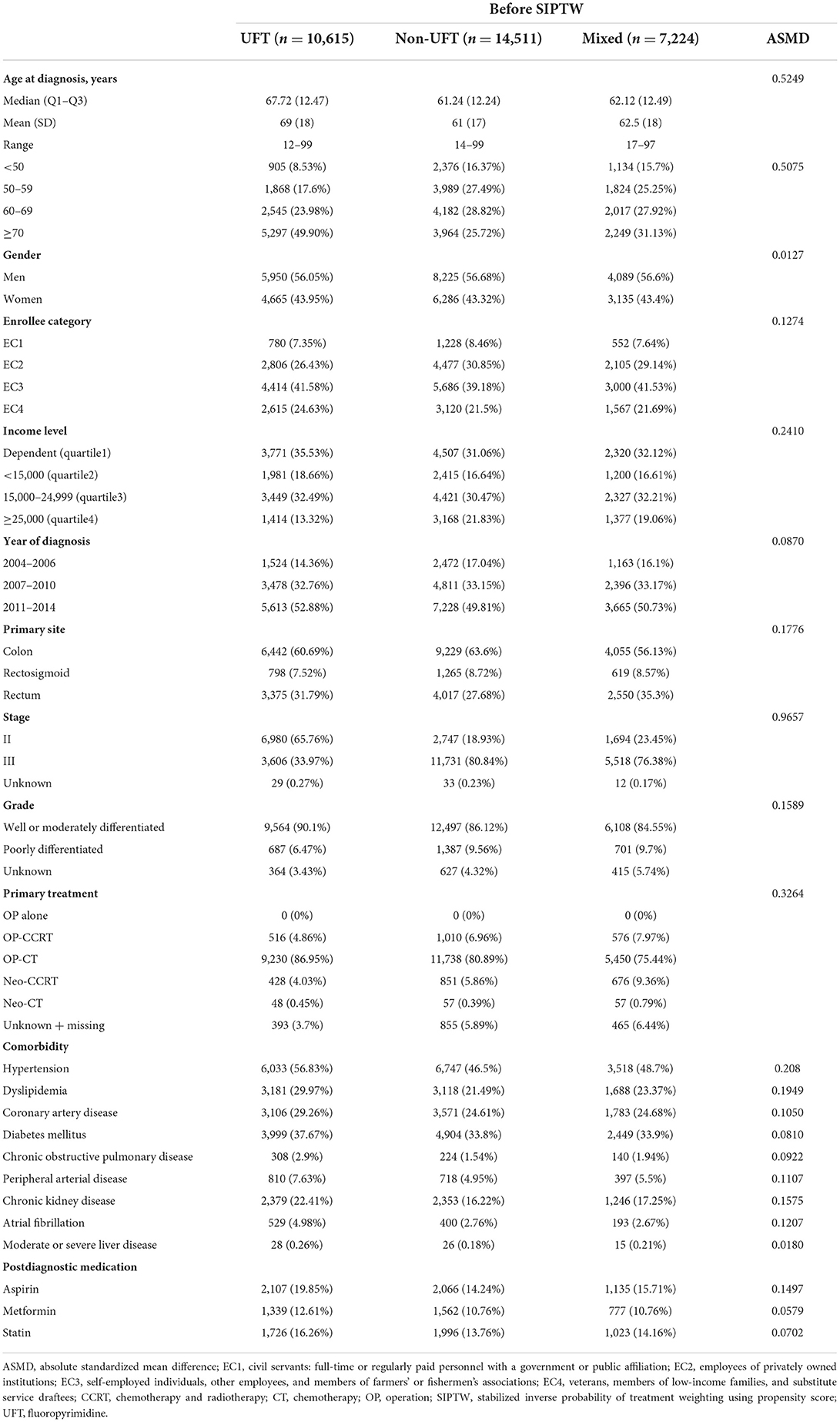
Table 1. Demographic, cancer, comorbidity, and medication characteristics among patients with stage II–III colorectal cancer before SIPTW.
Next, we performed survival analysis [log-rank test in univariate analysis, Cox's proportional hazard model (17) and cause-specific hazard model (18) in the multivariate analysis] to examine the association between study outcomes and chemotherapy groups. We treated death as a competing risk event in the cause-specific hazard model. In either Cox's or the cause-specific hazard models, chemotherapy (CT) grouping was the only covariate because the three chemotherapy groups were balanced after SIPTW (19). We plotted log {-log[S(t)]} vs. log(t) to check the proportional hazards assumption in the Cox and cause-specific hazard models, where S(t) is the cumulative survival over time t. The lines of the three CT grouping within each plot of log {-log [S(t)]} vs. log(t) were parallel, indicating no violation of proportional hazards (20).
We also performed subgroup analysis to examine whether the differences in study outcomes between the three CT groups were maintained in specific subgroups: stage II and age <70 years, stage II and age ≥70 years, stage III and age <70 years, and stage III and age ≥70 years. For each subgroup analysis, we re-estimated the SIPTW to ensure a balance of covariates across groups.
Results
From 2004 to 2015, 78,105 patients were newly diagnosed with stage II–III CRC. Based on the exclusion criteria, 32,350 patients were eligible for this study. Among these 32,350 patients, 10,615 (32.8%) received UFT alone, 14,511 (44.8%) received non-UFT, and 7,224 (22.3%) received mixed chemotherapy (Figure 2). Before SIPTW, the UFT group was older, had a lower income; had better tumor grade; had a higher proportion of receiving OP-CT; had a higher prevalence of hypertension, coronary artery disease, diabetes, peripheral arterial disease, and COPD; and a higher aspirin prescription rate than those of the other two CT groups (Table 1). After SIPTW, the three CT groups were well-balanced in demographic characteristics, cancer-related characteristics, comorbidities, and CV-related medication (Table 2).
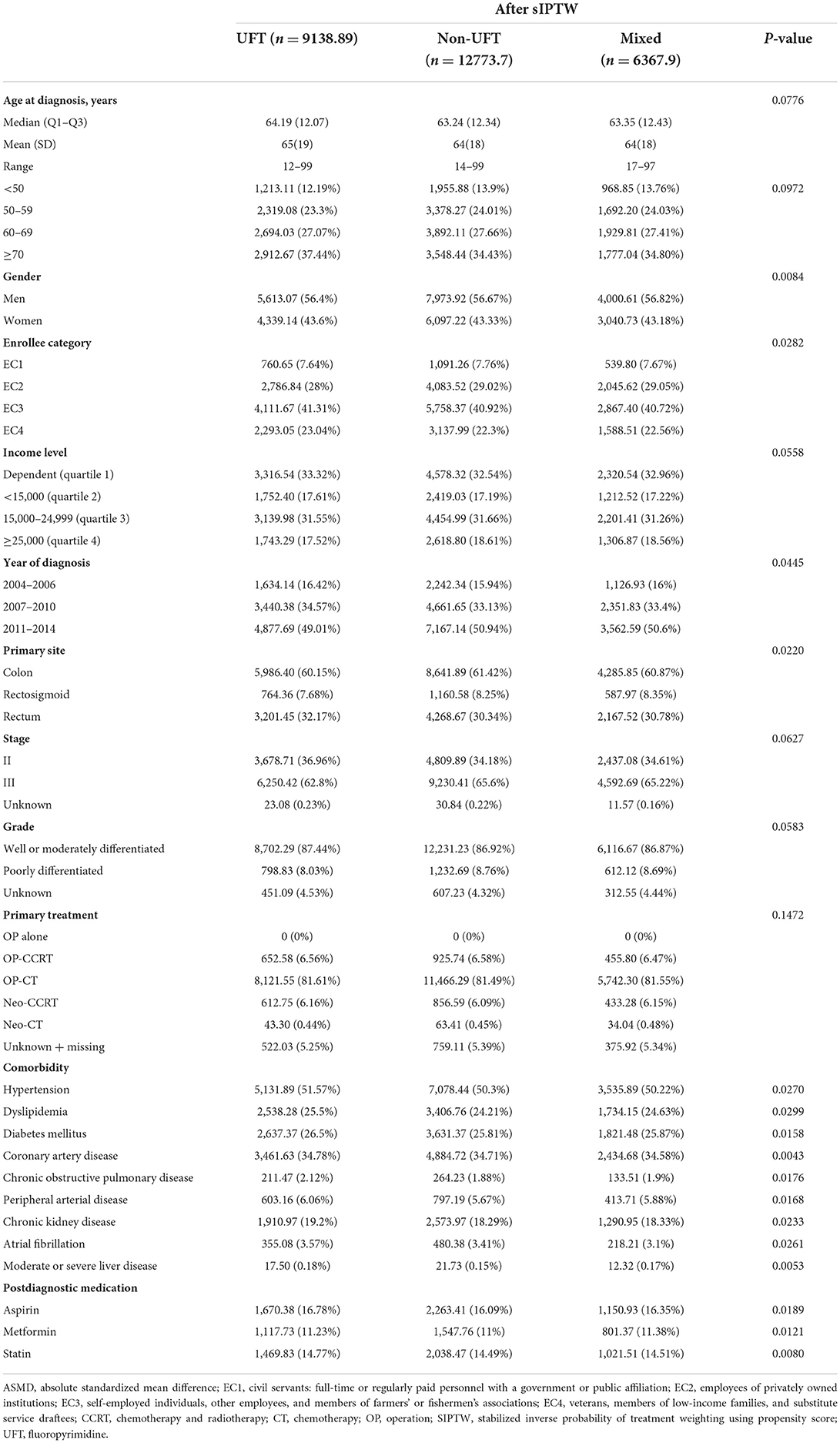
Table 2. Demographic, cancer, comorbidity, and medication characteristics among patients with stage II–III colorectal cancer after SIPTW.
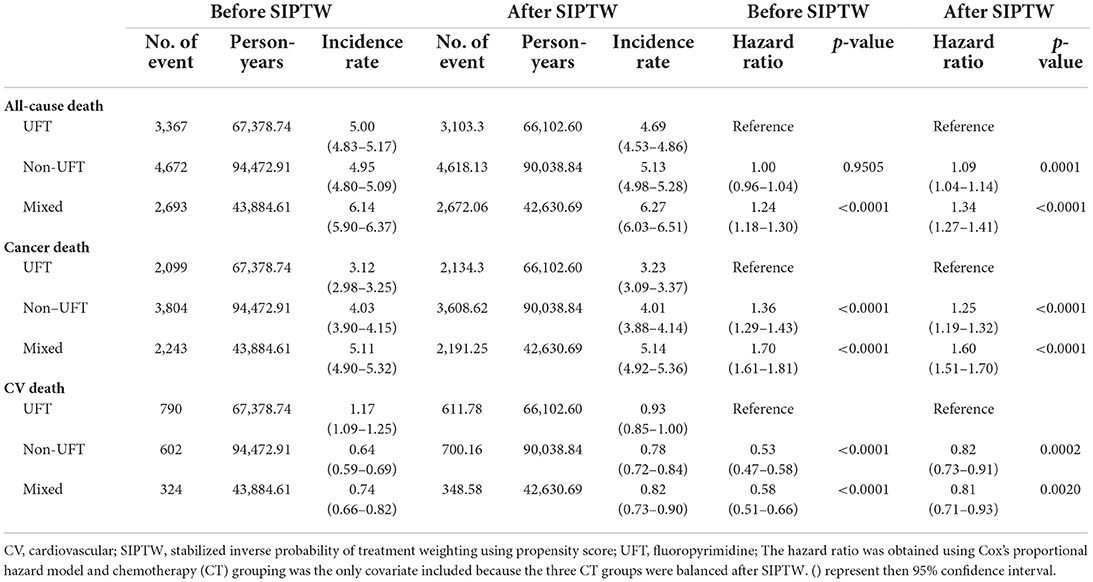
Before SIPTW, there were 3,367, 4,672, and 2,693 all-cause deaths in the UFT, non-UFT, and mixed groups, respectively, equivalent to all-cause mortality of 5.00, 4.95, and 6.14 per 100 person-years. The mixed group had a significantly higher risk of all-cause mortality than the UFT group (HR = 1.24, 95% CI = 1.18–1.30). Cancer was still the leading cause of death, and the cancer death rates per 100 person-years were 3.12, 4.03, 5.11 for the UFT, non-UFT, and mixed groups, respectively. The non-UFT group (HR = 1.36, 95% CI = 1.29–1.43) and the mixed group (HR = 1.70, 95% CI = 1.61–1.81) had significantly more cancer deaths than the UFT group. CV events accounted for 23.5, 12.9, and 12.0% of all-cause deaths, and the CV death rates per 100 person-years were 1.17, 0.64, and 0.74 in the UFT, non-UFT, and mixed groups, respectively. The non-UFT (HR = 0.53, 95% CI = 0.47–0.58) and mixed groups (HR = 0.58, 95% CI = 0.51–0.66) had significantly lower CV death rates than the UFT group (Supplementary Figure 1). After SIPTW, the differences in all-cause mortality, cancer death rate, and CV death rates between the three CT groups were similar to those before SIPTW, except that a significantly higher all-cause mortality was observed in the non-UFT group than in the UFT group (HR = 1.09, 95% CI = 1.04–1.14) (Figure 3; Table 3).
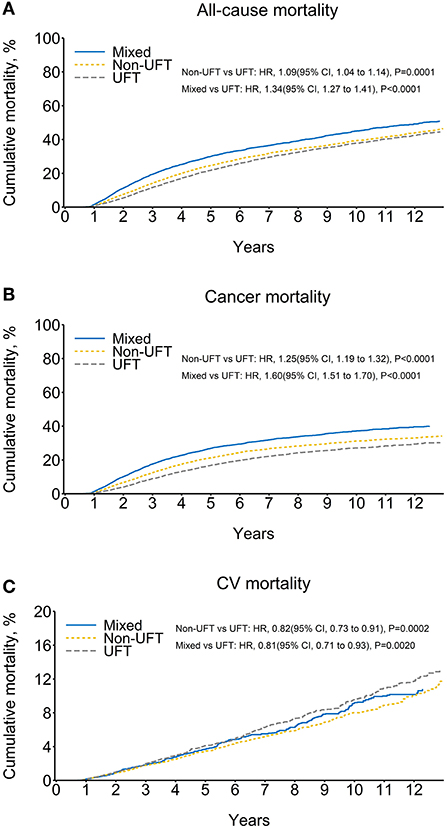
Figure 3. Cumulative mortality among patients with stage II–III colorectal cancer, after SIPTW (SIPTW, stabilized inverse probability of treatment weighting using propensity score; UFT, fluoropyrimidine).
The ischemic stroke had the highest CV outcomes, followed by heart failure, AMI, and life-threatening arrhythmia. The non-UFT and mixed groups had significantly lower ischemic stroke and heart failure rates than those in the UFT group before and after SIPTW. There was no significant difference in life-threatening arrhythmias between the three CT groups before or after SIPTW. The non-UFT group and the mixed group had a significantly lower AMI rate than the UFT group before SIPTW (Supplementary Figure 2), but the difference was non-significant between the non-UFT and UFT groups after SIPTW (HR = 0.96, 95% CI = 0.76–1.21) (Figure 4; Table 4).
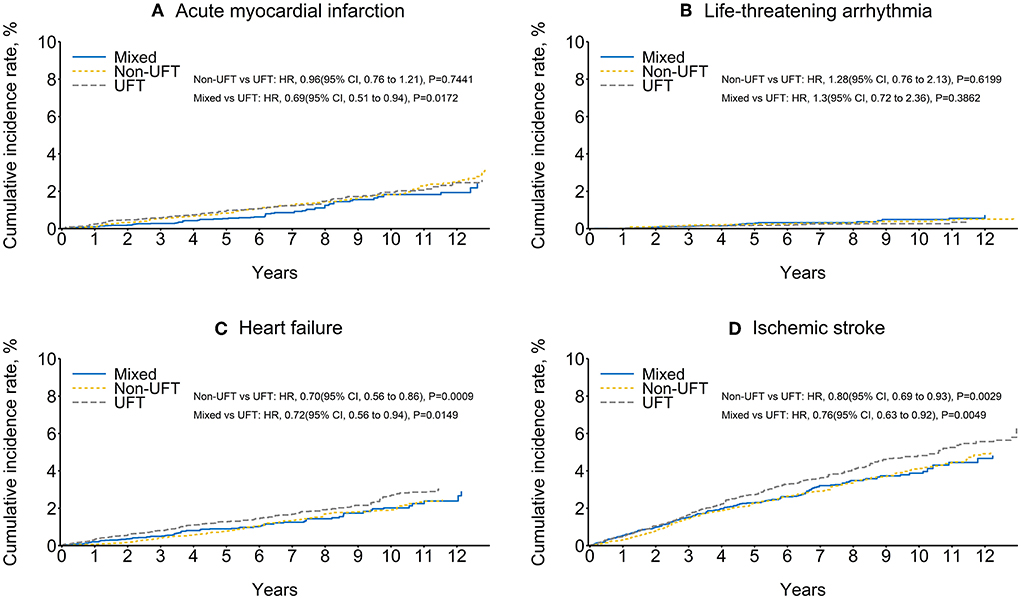
Figure 4. Cumulative rate of cardiovascular outcomes among patients with stage II–III colorectal cancer after SIPTW (SIPTW, stabilized inverse probability of treatment weighting using propensity score; UFT, fluoropyrimidine).
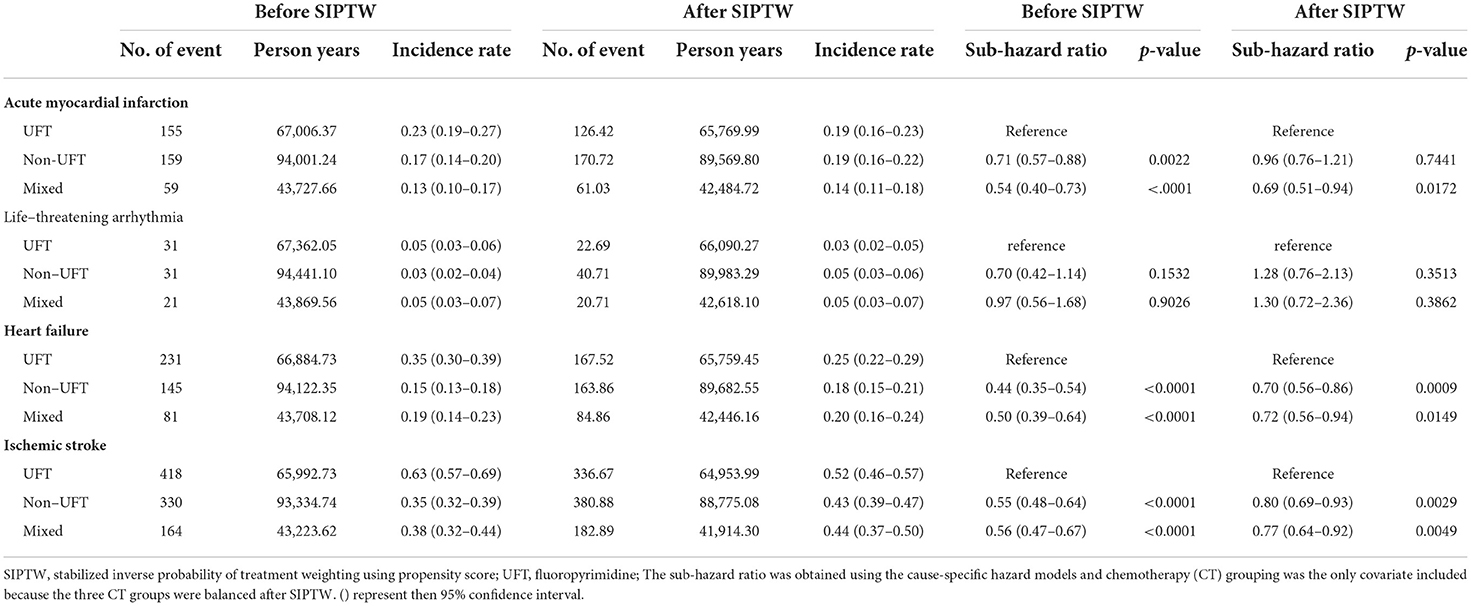
Table 4. Cardiovascular outcomes among patients with stage II–III colorectal cancer before and after SIPTW.
Figure 5 presents the mortality results of subgroup analysis for stage II and age <70 years, stage II and age ≥70 years, stage III and age <70 years, and stage III and age ≥70 years. Again, the UFT group had significantly lower all-cause and cancer mortality rates than the other two CT groups, except for stage III and age ≥70 years and the non-UFT users in the group of stage II and age ≥70 years (p = 0.0761). In contrast, the UFT group had higher CV mortality than the non-UFT group and reached significance in the stage II and age <70 years subgroup.
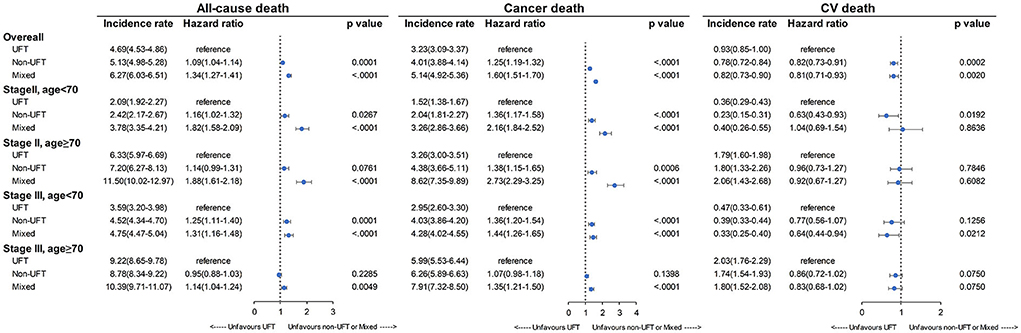
Figure 5. Mortality rate among patients with stage II–III colorectal cancer after SIPTW subgroup analysis (SIPTW, stabilized inverse probability of treatment weighting using propensity score; UFT, fluoropyrimidine).
Figure 6 presents the CV outcomes of subgroup analysis for stage II and age <70 years, stage II and age ≥70 years, stage III and age <70 years, and stage III and age ≥70 years. There was no significant difference among the three CT groups for the four subgroup analyses for AMI, LTA, heart failure, and ischemic stroke, except for the following: the UFT group had a significantly higher heart failure rate than the non-UFT groups in those with stage III and age ≥70 years (p = 0.0263). However, for ischemic stroke, a marginally higher rate in the UFT group than in the non-UFT group was seen only in those with stage III disease and aged ≥70 years (p = 0.0661).
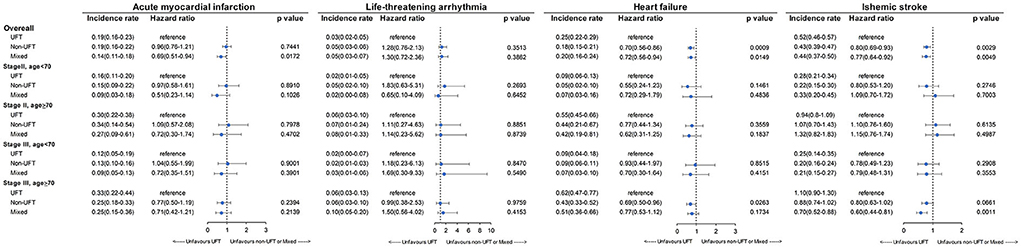
Figure 6. Incidence rate of cardiovascular outcomes among patients with stage II–III colorectal cancer after SIPTW subgroup analysis (SIPTW, stabilized inverse probability of treatment weighting using propensity score; UFT, fluoropyrimidine).
Discussion
Although the survival benefit of adjuvant chemotherapy in patients with high-risk stage II or III CRC is generally accepted (21–23), cardiotoxicity from 5-FU derivatives may compromise the quality of life and overall survival. Therefore, this study explored the association between cardiotoxicity and fluoropyrimidine-based adjuvant chemotherapy in CRC patients. Mechanistically, two hypotheses of 5-FU-related cardiotoxicity included (1) dihydropyrimidine dehydrogenase (DPD) downstream metabolites and (2) 5-FU direct injury to endothelial cells (24).
First, oral fluoropyrimidines, including TS-1 and UFT, may affect the risk of cardiotoxicity by regulating DPD enzymes. DPD enzyme activity related to cardiotoxicity has been reported previously (25). The downstream metabolites are fluoroacetate and F-citrate, which have been related to potent cardiotoxicity in previous studies (26, 27). Second, according to our hypothesis, UFT, an adjuvant chemotherapeutic agent, inhibits DPD with uracil and may decrease CV events. However, our results did not support this hypothesis.
Second, 5-FU can directly damage endothelial cells, causing vasoconstriction and thromboembolism. Previous studies have reported that continuous intravenous injection (2–18%) is more likely to induce cardiotoxicity than a bolus regimen (1.6–3.0%) (28, 29). Another oral prodrug, capecitabine, is 5.9% in cardiotoxicity (30). Therefore, prodrug or continuous intravenous injection may prolong 5-FU toxic exposure, inducing more endothelial cell damage. UFT was a prodrug that increased 5-FU toxicity exposure time in our study. Our results revealed a continuing need for a mechanism of cardiotoxicity induced by metabolites in this pathway. Much more also needs to be known about the role of uracil and DPD in cardiotoxicity. Future work will hopefully clarify the mechanism of 5-FU-induced cardiotoxicity.
Several studies have examined the association between 5-FU-based adjuvant chemotherapy and cardiotoxicity. However, there are no retrospective studies on a large number of patients with CRC comparing different 5-FU-based adjuvant chemotherapy regimens. Our study took steps to compare different regimens in a nationwide cohort. UFT did not decrease the possibility of induced cardiotoxicity. Oral UFT, the most common and clinically prescribed drug in outpatients, may be associated with a higher likelihood of CV events. Further surveys of heart function should be considered before UFT use, especially in older adults and advanced-stage disease.
The first limitation of our study is selection bias. Oncologists may consider performance status and, therefore, prescribe different 5-FU derivatives. In addition, higher comorbidities were noted in the UFT group. Patients in poor condition may prefer oral UFT. Another limitation is that potential confounders were not recorded in the Taiwan's National Health Insurance Research Database. Individual demographic and lifestyle factors, including performance status, BMI, smoking, and exercise activity, may be associated with the development of CV events. Lastly, we did not have data of UFT or other FU dose for further analysis of dose-event relationship.
Conclusion
In conclusion, UFT use was associated with a higher CV events and deaths rate than adjuvant chemotherapy-induced cardiotoxicity. Specifically, the subgroup analysis revealed higher CV events in the UFT group, older patients, and stage III patients. The present study provides the first comparative analysis of cardiotoxicity between UFT and other 5-FU derivatives. Our results suggest that caregivers should be alert to UFT use in older patients with multiple comorbidities. Further research is needed to develop a risk prediction tool to stratify patients and guide the choice of 5-FU regimens accordingly.
Data availability statement
We used the Taiwan Cancer Registry, National Health Insurance Research Database Taiwan, Taiwan Death Registry, which are only available in the Health and Welfare Data Science Center, Taiwan. We cannot make our research data available, accessible, discoverable, and usable.
Ethics statement
The study was approved by the Institutional Review Board of the Chang Gung Medical Foundation (201901844B0). Informed consent was waived because all personal identification information was encrypted.
Author contributions
W-KH, W-PH, and L-CS contributed to the conceptualization and drafted the manuscript. W-KH, W-PH, H-CH, and L-CS designed this study. S-HC and L-CS performed data curation. S-HC, W-KH, and L-CS performed formal analyses. W-KH, H-CH, W-CC, and S-HC are involved in the resources and software development. D-YC, W-CC, P-HC, T-SY, and J-SC contributed to project administration. L-CS was responsible for the funding acquisition. W-KH, W-PH, H-CH, S-HC, D-YC, W-CC, P-HC, J-SC, T-SY, and L-CS contributed to the review and editing of this manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by grants NMRPD1K0771 and CMRPD1K0022 from the Chang Gung Medical Foundation and grant MOST 109-2314-B-182-035 from the Ministry of Science and Technology, Taiwan.
Acknowledgments
This study was based in part on the National Health Insurance Research Database provided by the Health and Welfare Data Center (HWDC) from the Ministry of Health and Welfare (MOHW), Taiwan. The interpretation and conclusions contained herein do not represent the National Health Insurance Administration, Ministry of Health and Welfare, Taiwan.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.880956/full#supplementary-material
References
1. Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. (2016) 66:271–89. doi: 10.3322/caac.21349
2. de Moor JS, Mariotto AB, Parry C, Alfano CM, Padgett L, Kent EE, et al. Cancer survivors in the United States: prevalence across the survivorship trajectory and implications for care. Cancer Epidemiol Biomarkers Prev. (2013) 22:561–70. doi: 10.1158/1055-9965.EPI-12-1356
3. Keats MR, Cui Y, Grandy SA, Parker L. Cardiovascular disease and physical activity in adult cancer survivors: a nested, retrospective study from the Atlantic PATH cohort. J Cancer Surviv. (2017) 11:264–73. doi: 10.1007/s11764-016-0584-x
4. Zaorsky N, Churilla T, Egleston B, Fisher S, Ridge J, Horwitz E, et al. Causes of death among cancer patients. Ann Oncol. (2016) 28:400–7. doi: 10.1093/annonc/mdw604
5. Armenian SH, Xu L, Ky B, Sun C, Farol LT, Pal SK, et al. Cardiovascular disease among survivors of adult-onset cancer: a community-based retrospective cohort study. J Clin Oncol. (2016) 34:1122–30. doi: 10.1200/JCO.2015.64.0409
6. Passarelli MN. Obesity and the importance of cardiovascular disease surveillance after colorectal cancer. JAMA Oncol. (2019) 5:973–4. doi: 10.1001/jamaoncol.2019.0676
7. Kenzik KM, Balentine C, Richman J, Kilgore M, Bhatia S, Williams G, et al. New-onset cardiovascular morbidity in older adults with stage I to III colorectal cancer. J Clin Oncol. (2018) 36:609–16. doi: 10.1200/JCO.2017.74.9739
8. Kim H, Park IJ, Han Y, Kwon TW, Cho YP. Cardiovascular morbidities in postoperative colorectal cancer patients. Sci Rep. (2021) 11:21359. doi: 10.1038/s41598-021-00735-3
9. Kawamura H, Morishima T, Sato A, Honda M, Miyashiro I. Effect of adjuvant chemotherapy on survival benefit in stage III colon cancer patients stratified by age: a Japanese real-world cohort study. BMC Cancer. (2020) 20:19. doi: 10.1186/s12885-019-6508-1
10. Zhang J, Yen YC, Qin L, Chang CL, Yuan KS, Wu ATH, et al. Outcomes of adjuvant oral versus intravenous fluoropyrimidines for high-risk stage II or stage III colon adenocarcinoma: a propensity score-matched, nationwide, population-based cohort study. J Cancer. (2020) 11:4157–65. doi: 10.7150/jca.42404
11. Li C, Ngorsuraches S, Chou C, Chen L, Qian J. Risk factors of fluoropyrimidine induced cardiotoxicity among cancer patients: a systematic review and meta-analysis. Crit Rev Oncol Hematol. (2021) 162:103346. doi: 10.1016/j.critrevonc.2021.103346
12. Gleiss A, Oberbauer R, Heinze G. An unjustified benefit: immortal time bias in the analysis of time-dependent events. Transpl Int. (2018) 31:125–30. doi: 10.1111/tri.13081
13. Xu S, Ross C, Raebel MA, Shetterly S, Blanchette C, Smith D. Use of stabilized inverse propensity scores as weights to directly estimate relative risk and its confidence intervals. Value Health. (2010) 13:273–7. doi: 10.1111/j.1524-4733.2009.00671.x
14. McCaffrey DF, Griffin BA, Almirall D, Slaughter ME, Ramchand R, Burgette LF, et al. A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Stat Med. (2013) 32:3388–414. doi: 10.1002/sim.5753
15. Lee BK, Lessler J, Stuart EA. Weight trimming and propensity score weighting. PLoS ONE. (2011) 6:e18174. doi: 10.1371/journal.pone.0018174
16. Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comp. (2009) 38:1228–34. doi: 10.1080/03610910902859574
17. Cox DR. Regression models and life-tables. J Royal Stat Soc Series B. (1972) 34:187–202. doi: 10.1111/j.2517-6161.1972.tb00899.x
18. Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol. (2009) 170:244–56. doi: 10.1093/aje/kwp107
19. Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med. (2014) 33:1242–58. doi: 10.1002/sim.5984
20. Hess KR. Graphical methods for assessing violations of the proportional hazards assumption in Cox regression. Stat Med. (1995) 14:1707–23. doi: 10.1002/sim.4780141510
21. Wu X, Zhang J, He X, Wang C, Lian L, Liu H, et al. Postoperative adjuvant chemotherapy for stage II colorectal cancer: a systematic review of 12 randomized controlled trials. J Gastrointest Surg. (2012) 16:646–55. doi: 10.1007/s11605-011-1682-8
22. National Comprehensive Cancer Network (NCCN). NCCN Guidelines for Patients: Rectal Cancer, 2021. (2021). Available online at: https://www.nccn.org/patientresources/patient-resources/guidelines-for-patients/guidelines-for-patients-details?patientGuidelineId=36 (accessed July 21, 2022).
23. National Comprehensive Cancer Network (NCCN). NCCN Guidelines for Patients: Rectal Cancer, 2021. (2021). Available online at: https://www.nccn.org/patientresources/patient-resources/guidelines-for-patients/guidelines-for-patients-details?patientGuidelineId=8 (accessed July 21, 2022).
24. Polk A, Vistisen K, Vaage-Nilsen M, Nielsen DL. A systematic review of the pathophysiology of 5-fluorouracil-induced cardiotoxicity. BMC Pharmacol Toxicol. (2014) 15:47. doi: 10.1186/2050-6511-15-47
25. Milano G, Etienne MC, Pierrefite V, Barberi-Heyob M, Deporte-Fety R, Renee N. Dihydropyrimidine dehydrogenase deficiency and fluorouracil-related toxicity. Br J Cancer. (1999) 79:627–30. doi: 10.1038/sj.bjc.6690098
26. Muneoka K, Shirai Y, Yokoyama N, Wakai T, Hatakeyama K. 5-Fluorouracil cardiotoxicity induced by alpha-fluoro-beta-alanine. Int J Clin Oncol. (2005) 10:441–3. doi: 10.1007/s10147-005-0516-7
27. Goncharov NV, Jenkins RO, Radilov AS. Toxicology of fluoroacetate: a review, with possible directions for therapy research. J Appl Toxicol. (2006) 26:148–61. doi: 10.1002/jat.1118
28. Meydan N, Kundak I, Yavuzsen T, Oztop I, Barutca S, Yilmaz U, et al. Cardiotoxicity of de Gramont's regimen: incidence, clinical characteristics and long-term follow-up. Jpn J Clin Oncol. (2005) 35:265–70. doi: 10.1093/jjco/hyi071
29. Kosmas C, Kallistratos MS, Kopterides P, Syrios J, Skopelitis H, Mylonakis N, et al. Cardiotoxicity of fluoropyrimidines in different schedules of administration: a prospective study. J Cancer Res Clin Oncol. (2008) 134:75–82. doi: 10.1007/s00432-007-0250-9
30. Kwakman JJ, Simkens LH, Mol L, Kok WE, Koopman M, Punt CJ. Incidence of capecitabine-related cardiotoxicity in different treatment schedules of metastatic colorectal cancer: a retrospective analysis of the CAIRO studies of the Dutch colorectal cancer group. Eur J Cancer. (2017) 76:93–9. doi: 10.1016/j.ejca.2017.02.009
Keywords: cardiovascular disease, fluoropyrimidine, colorectal cancer, mortality, adjuvant chemotherapy
Citation: Huang W-K, Ho W-P, Hsu H-C, Chang S-H, Chen D-Y, Chou W-C, Chang P-H, Chen J-S, Yang T-S and See L-C (2022) Risk of cardiovascular disease among different fluoropyrimidine-based chemotherapy regimens as adjuvant treatment for resected colorectal cancer. Front. Cardiovasc. Med. 9:880956. doi: 10.3389/fcvm.2022.880956
Received: 22 February 2022; Accepted: 11 July 2022;
Published: 03 August 2022.
Edited by:
Gian Marco Rosa, San Martino Hospital (IRCCS), ItalyReviewed by:
Chung-Han Ho, Chi Mei Medical Center, TaiwanHao Chen, Guangdong Academy of Medical Sciences, China
Copyright © 2022 Huang, Ho, Hsu, Chang, Chen, Chou, Chang, Chen, Yang and See. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lai-Chu See, bGljaHVAbWFpbC5jZ3UuZWR1LnR3
†These authors have contributed equally to this work
 Wen-Kuan Huang
Wen-Kuan Huang Wei-Pang Ho1,2†
Wei-Pang Ho1,2† Hung-Chih Hsu
Hung-Chih Hsu Dong-Yi Chen
Dong-Yi Chen Pei-Hung Chang
Pei-Hung Chang Jen-Shi Chen
Jen-Shi Chen Lai-Chu See
Lai-Chu See