Corrigendum: Cardiovascular risk according to body mass index in reproductive-aged women with polycystic ovary syndrome: a systematic review and meta-analysis
Commentary: Cardiovascular risk according to body mass index in women of reproductive age with polycystic ovary syndrome: A systematic review and meta-analysis
- 1Hypertension Center, Lanzhou University Second Hospital, Lanzhou, China
- 2School of Public Health, Lanzhou University, Lanzhou, China
Background: Polycystic ovary syndrome (PCOS) is a heterogeneous condition that affects women of reproductive age. The association between PCOS and cardiovascular risk according to body mass index (BMI) categories is unclear.
Objective: We evaluated the association between cardiovascular risk according to BMI categories and PCOS in women of reproductive age.
Methods: A literature search was conducted in the EMBASE, MEDLINE, Cochrane Library, and PubMed databases from their inception to 9 September, 2021. Observational cross-sectional, retrospective, and prospective controlled studies were included. The main analyses examined the relationship between cardiovascular risks (i.e., blood pressure and lipid levels) and BMI in women of reproductive age with PCOS.
Results: Thirty-eight studies, with a total of 6,078 subjects, were included in this metaanalysis. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were higher in women of reproductive age with PCOS. Lower high-density lipoprotein (HDL)-cholesterol [SMD (95% CI): −0.21 (−0.35, −0.08), p = 0.002], higher triglycerides [SMD (95% CI): 0.38 (0.29, 0.48), p < 0.001], higher low-density lipoprotein (LDL)-cholesterol [SMD (95% CI): 0.29 (0.20, 0.39), p < 0.001], higher nonHDL-cholesterol [SMD (95% CI): 0.42 (0.31, 0.52), p < 0.001] and waist-to-hip ratio (WHR) [MD (95% CI): 0.03 (0.02, 0.04), p < 0.001] were seen in women of reproductive age with PCOS. In addition, the subgroup analysis revealed that systolic BP and HDL-cholesterol increased at BMI < 25 kg/m2 and BMI 25–30 kg/m2. Diastolic BP increased at BMI 25–30 kg/m2. Triglycerides, LDL-cholesterol, nonHDL-cholesterol, and WHR increased in all BMI categories.
Conclusions: PCOS is associated with cardiovascular risk. Lipid levels and BP increased in women of reproductive age with PCOS, regardless of BMI.
Systematic Review Registration: Open Science Framework (10.17605/OSF.IO/92NBY).
Introduction
Polycystic ovary syndrome (PCOS) is one of the most common female endocrinopathies and is a highly prevalent disorder that affects ~7–14% of women of reproductive age (1, 2). The clinical manifestations of PCOS are heterogeneous; however, the hallmarks of the syndrome are anovulation, insulin resistance, and androgen excess. Furthermore, PCOS leads to adverse metabolic sequelae, including high blood pressure, dyslipidemia, and obesity, all of which individually confer a cardiovascular risk. Each of these features promotes cardiovascular risk in this population. The American Society for Reproductive Medicine Practice Committee reported that cardiovascular risk was increased in women with PCOS (3). Recent studies have shown that cardiovascular risk factors are more frequent among women with PCOS than among women without PCOS (4, 5). Apart from the deleterious effects of PCOS per se, individual risk markers including blood pressure and lipid profile are important mediators of further cardiovascular outcomes. Therefore, PCOS may represent an important key to lipid and blood pressure alterations starting at the age of reproduction.
The effect of PCOS on obesity and that of obesity on PCOS is complex. However, it is agreed that the prevalence of increased body mass index (BMI) is higher in women with PCOS (6, 7). A previous metaanalysis showed that obesity was more prevalent in women with PCOS than in women without PCOS (8). PCOS occurs both in obese and lean women. However, studies of cardiovascular risk and PCOS have not distinguished the effects of BMI from those of PCOS. In addition, lipid profiles may differ in their association with blood pressure and PCOS. However, there is little consensus that the possible increase in cardiovascular risk among women with PCOS is merely related to obesity. Based on BMI, our metaanalysis classified patients as underweight (<18.5 kg/m2), normal weight (18.5–25 kg/m2), overweight (25–30 kg/m2), and obese (≥30 kg/m2) (9). These standard categories have been increasingly used in published studies of BMI and PCOS, but the literature has not been systematically reviewed.
The present metaanalysis examined the relationship between cardiovascular risk (i.e., blood pressure and lipids) and BMI in women of reproductive age with PCOS. To reduce biases that may appear in all metaanalyses, only BMI-matched studies with a sufficient number of subjects were included.
Methods
The present study was approved by the Ethics Committee Board of Lanzhou University Second Hospital (D2019-098) and conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (10). The protocol was prospectively registered with Open Science Framework (10.17605/OSF.IO/92NBY).
Search Strategy
Two independent authors (CC Zhuang and XF Luo) searched the Cochrane Library, EMBASE, and MEDLINE databases from inception to 9 September 2021 for full-text articles without any language restriction. The medical subject headings (MeSHs) and keywords included polycystic ovary syndrome, Stein Leventhal, cardiovascular risk, blood pressure, hypertension, lipoprotein, dyslipidemia, and hyperlipidemia (see Table 1 for the detailed search strategies). For all identified studies, a manual search was conducted of their references and review articles to locate additional relevant studies.
Study Selection and Criteria
Two reviewers (CC Zhuang and XF Luo) independently selected the articles by title, abstract and then full-text. The selection criteria for the retrieved articles in our metaanalysis were as follows: (1) the studies were observational cross-sectional, retrospective, and prospective controlled; (2) the subjects were women of reproductive age with PCOS (18–49 years old), diagnosed by the Rotterdam criteria or that of the National Institutes of Health; (3) the subjects did not have comorbidities; (4) there was no evidence of androgen-secreting tumors, congenital adrenal hyperplasia, or medication that altered androgen metabolism or lipids; and (5) each article had to conduct BMI matching or the equal BMI (mean). Each study had to have evaluated ≥30 adult women with PCOS that were ≥18 years old but ≤ 45 years old (to avoid the perimenopausal transition). Control subjects without PCOS of the same age range had to be included. The exclusion criteria of the metaanalysis were as follows: (1) studies that were case-only; (2) studies with incomplete data; (3) articles that were metaanalyses, letters, reviews, or editorial articles. Non-patient community control studies were excluded, as PCOS occurs infrequently and some phenotypic elements of PCOS can occur in regularly menstruating women. The selected data were screened based on the search terms and the inclusion and exclusion criteria, and a PRISMA flow diagram was used to describe the stepwise article selection process in detail.
Data Extraction
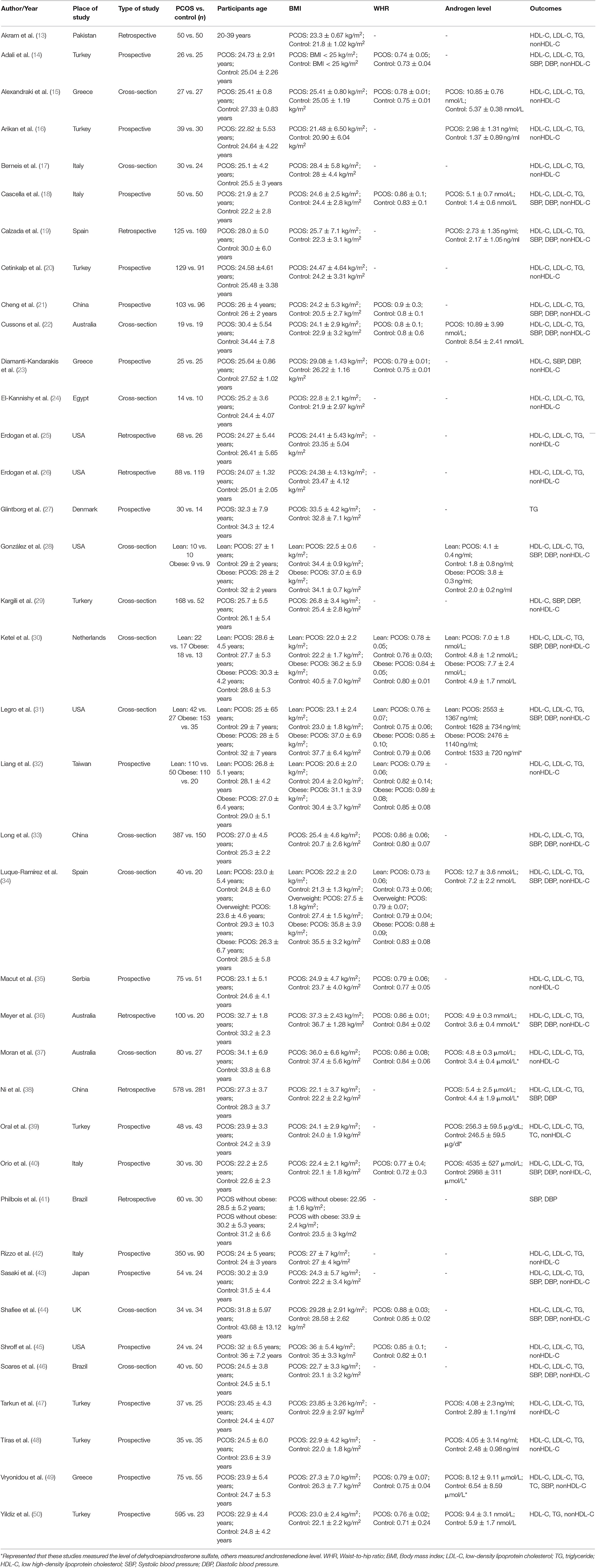
Authors of studies eligible for inclusion in the present metaanalysis were invited to join our study and share their data. The following data were extracted from the included studies: (1) name of the first author, (2) publication year, (3) type of study, (4) number of participants, (5) patient characteristics, (6) WHR, (7) androgen levels, and (8) patient outcomes. The outcomes included BMI, LDL-C or triglycerides (TG), low HDL-C, high SBP, DBP), and WHR. When not reported in the individual study, nonHDL-C was calculated as the total cholesterol minus HDL-C. Data extraction was independently performed by three investigators (CC Zhuang, XF Luo, and WJ wang). Disagreements were resolved through discussion.
Quality of the Included Studies
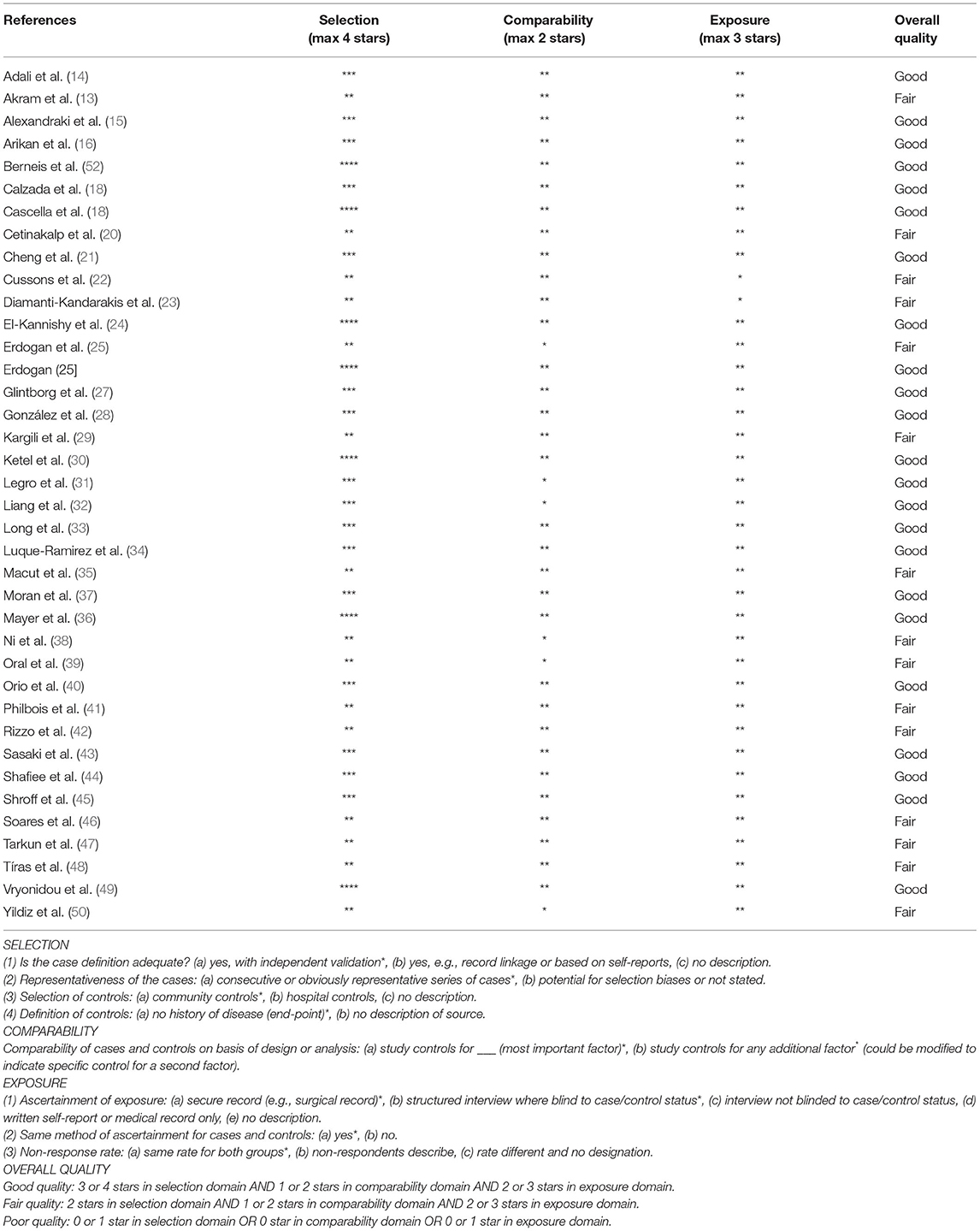
The quality of the included studies was assessed by the Newcastle–Ottawa Scale (NOS), a validated scale for metaanalyses of observational studies (11, 12). The proposed scale was further adapted to the outcome of interest of this metaanalysis, and the items were divided into three domains [selection (representativeness of the sample, sample size, and non-responders), comparability (confounding factors) and outcome (blinding and calibration of the examiners); details are provided in Table 2]. The scale was also used to analyze cohorts and case-controls. We scored (maximum, nine points) the following items relevant to the risk of bias in non-randomized cohort studies: representativeness of the exposed cohort, adequate selection of controls, adequate definition of the outcome, adequacy of follow-up, and comparability of exposed and non-exposed women (two points). Studies with a group of comparisons were arbitrarily defined as having a high risk of bias if they scored between zero and three, moderate risk if they scored between four and six, and low risk if they scored between seven and nine.
Statistical Analysis
Continuous normally distributed data were summarized with means and standard deviations (SDs), and non-normally distributed variables were summarized with medians and interquartile ranges (IQRs). If the outcome was measured on the same scale, we used the weighted mean difference (MD) and 95% CI. Otherwise, standardized mean difference (SMD) and 95% CI were calculated. Random-effects models were used to calculate estimates. A p-value < 0.05 was considered statistically significant for all analyses except heterogeneity tests.
We assessed the possibility of publication bias by constructing a funnel plot of each trial's effect size against its standard error. We assessed funnel plot asymmetry using the Egger's regression test and defined significant publication bias as a p-value < 0.1. The trim-and-fill computation was used to estimate the effect of publication bias on the interpretation of results (51). Heterogeneity between studies was assessed using I2 tests (I2> 50% was considered substantial heterogeneity). Analyses were performed by the Stata statistical software version 14.0 (StataCorp, College Station, TX, USA).
Results
Study Design and Analysis Characteristics
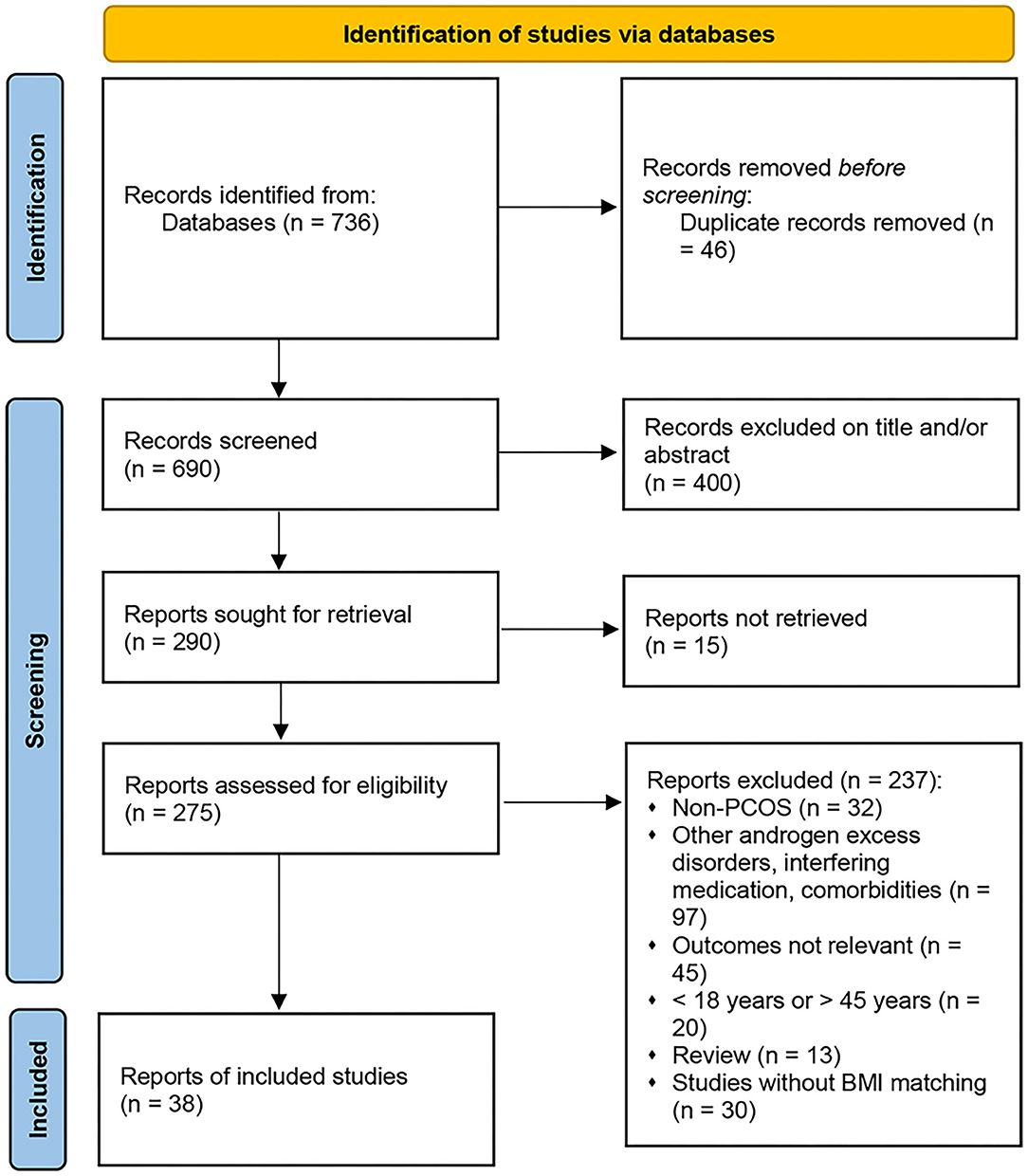
The search yielded 736 potential reports, as outlined in the PRISMA flow diagram (Figure 1). After the removal of duplicates, 690 records remained. Initial screening of the titles and abstracts resulted in the exclusion of 400 reports, and 275 studies proceeded for detailed evaluation. After further examination, 13 cross-sectional studies and 25 cohort studies met the inclusion criteria and were included in the metaanalysis (13–16, 18–50, 52). The basic characteristics of each study are summarized in Table 2. A total of 38 studies and 6,078 subjects were included in the present metaanalysis which assessed HDL-C, TGs, nonHDL-C and LDL-C, SBP, DBP, and WHR according to BMI categories.
Risk of Bias and Quality Assessment
The 38 studies included in our metaanalysis were reviewed with the NOS tool. One study scored seven out of nine points, and one study scored six points, both indicating high quality. Three studies scored four points or less. The details are provided in Table 3.
Lipid Profiles
Figures 2–5 are forest plots summarizing the comparisons of HDL-C, TG, nonHDL-C, and LDL-C, respectively.
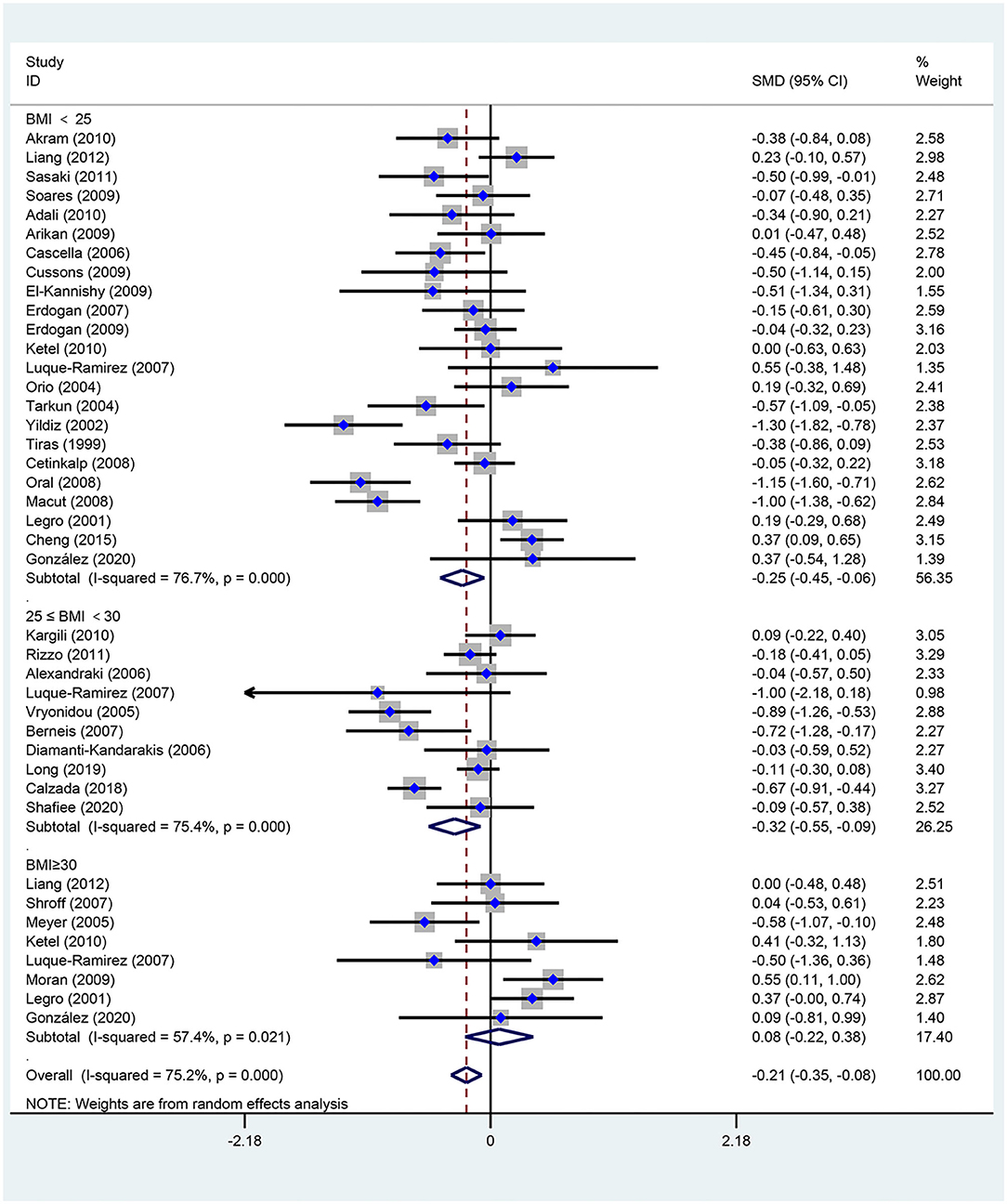
Figure 2. Forest plot for comparison of high-density lipoprotein-cholesterol in subjects with polycystic ovary syndrome (PCOS) vs. control subjects. Studies are classified by different body mass index (BMI) categories (BMI <25 kg/m2, BMI ≥ 30 kg/m2, and BMI 25–30 kg/m2).
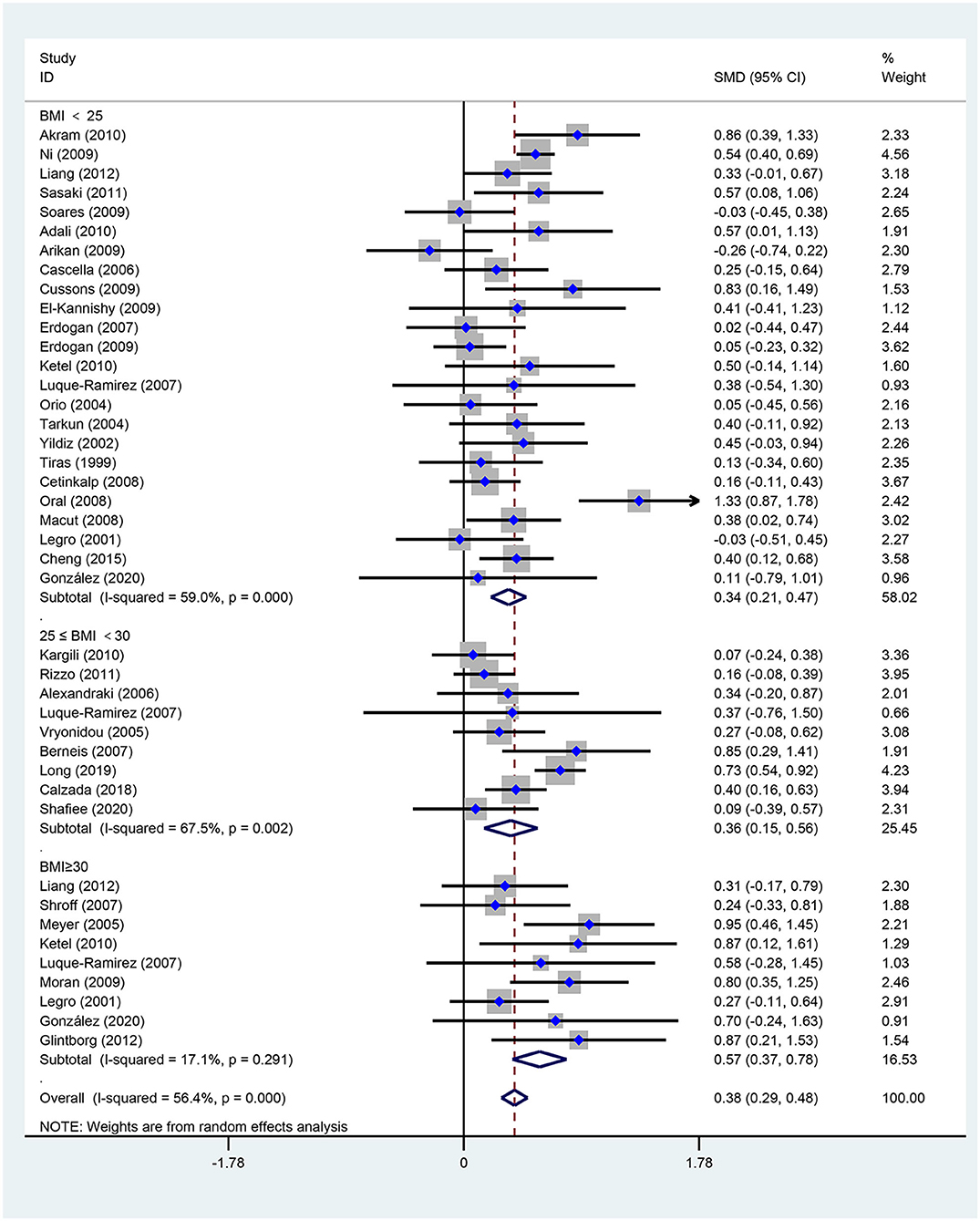
Figure 3. Forest plot for comparison of triglycerides in polycystic ovary syndrome vs. control subjects. Studies are classified by different body mass index (BMI) categories (BMI <25 kg/m2, BMI ≥ 30 kg/m2, and BMI 25–30 kg/m2).
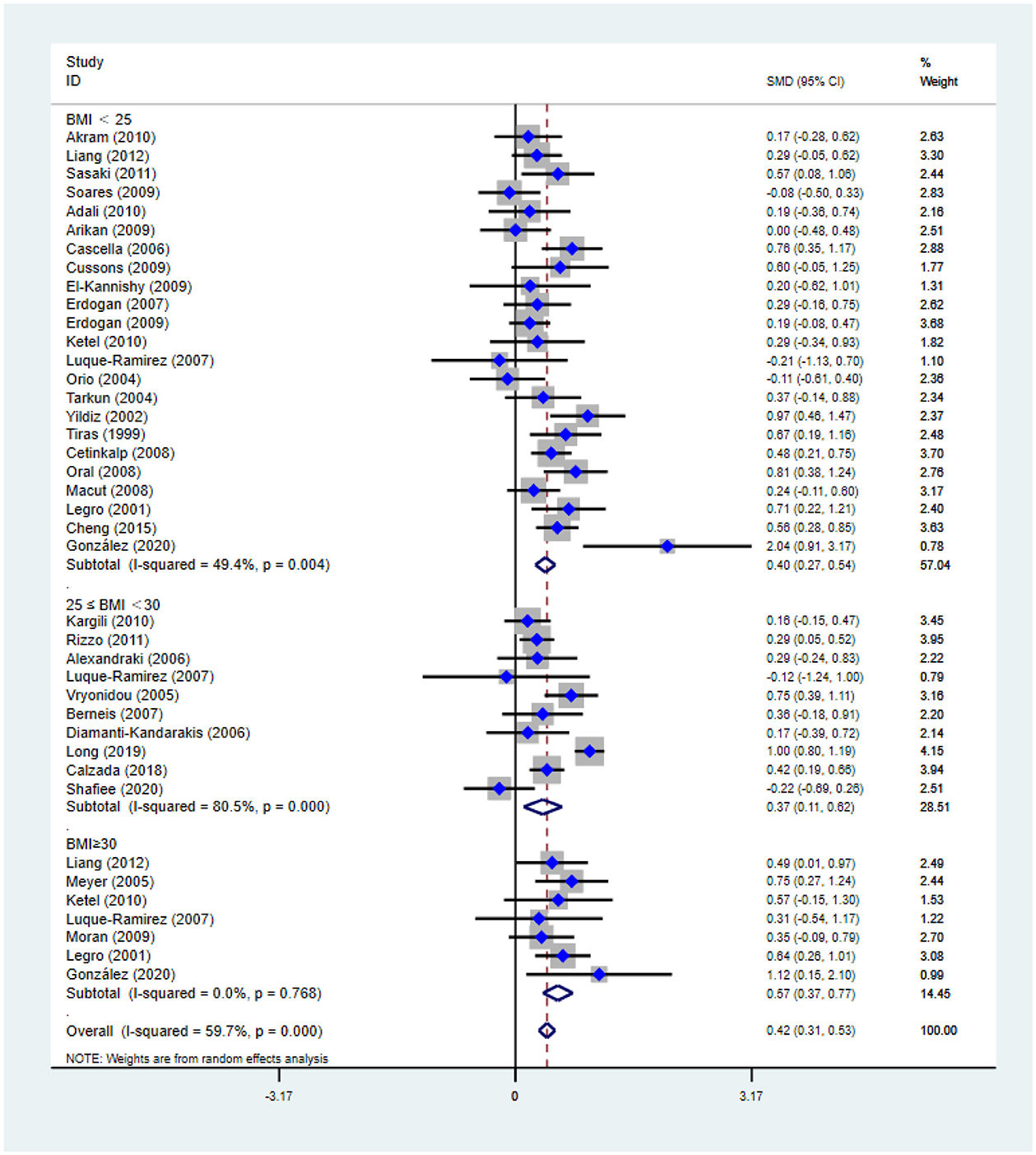
Figure 4. Forest plot for comparison of non-high-density lipoprotein-cholesterol in polycystic ovary syndrome vs. control subjects. Studies are classified by different body mass index (BMI) categories (BMI < 25 kg/m2, BMI ≥ 30 kg/m2, and BMI 25–30 kg/m2).
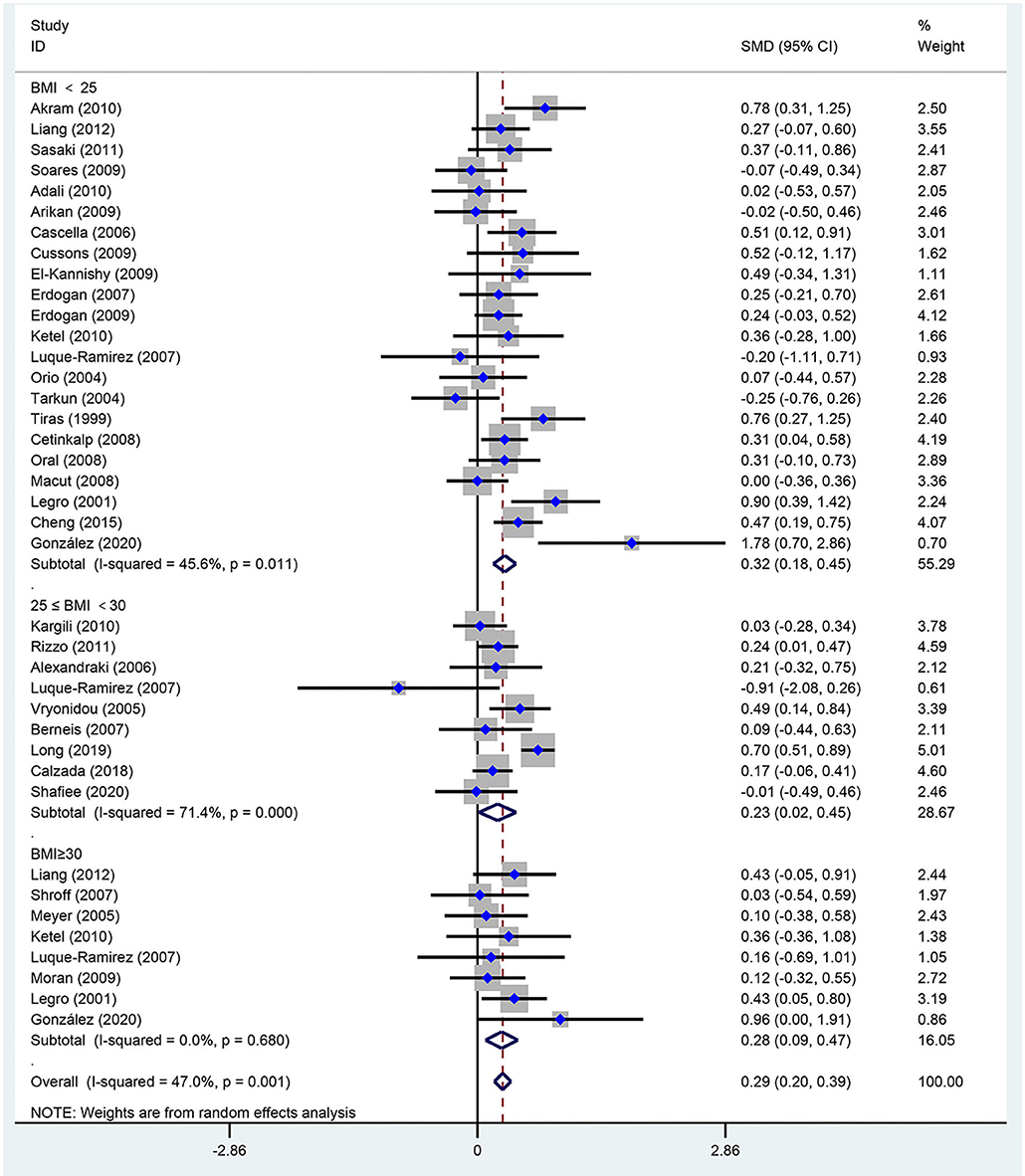
Figure 5. Forest plot for comparison of low-density lipoprotein-cholesterol in polycystic ovary syndrome vs. control subjects. Studies are classified by different body mass index (BMI) categories (BMI <25 kg/m2, BMI ≥ 30 kg/m2, and BMI 25–30 kg/m2).
As shown in Figure 2, women of reproductive age with PCOS had a significant decrease in HDL-C [SMD (95% CI): −0.21 (−0.35, −0.08), p = 0.002], with statistically significant between-study heterogeneity. The subgroup analysis showed that HDL-C significantly decreased in BMI <25 kg/m2 [SMD (95% CI): −0.25 (−0.45, −0.06), p = 0.012], and BMI 25–30 kg/m2 [MD (95% CI): −0.32 (−0.55, −0.09), p = 0.006]. However, HDL-C did not significantly differ at BMI ≥ 30 kg/m2 [MD (95% CI): 0.08 (−0.22, 0.38), p = 0.618]. There was no significant publication bias in this analysis (asymmetry test P = 0.680). Further sensitivity analysis comparing PCOS patients and controls showed a mean reduction in HDL-C in PCOS patients.
As illustrated in Figure 3, women of reproductive age with PCOS had a significant difference in TGs [SMD (95% CI): 0.38 (0.29, 0.48), p < 0.001], with significant between-study heterogeneity. The subgroup analysis of TGs revealed that TGs were increased in women of reproductive age with PCOS at BMI < 25 kg/m2 [SMD (95% CI): 0.34 (0.21, 0.47), p < 0.001], BMI ≥ 30 kg/m2 [MD (95% CI): 0.57 (0.37, 0.78), p < 0.001], and BMI 25–30 kg/m2 [MD (95% CI): 0.36 (0.15, 0.56), p = 0.001]. In this analysis, there was no publication bias (asymmetry test P = 0.882). In the sensitivity analysis comparing PCOS patients and controls, we observed an increase in TGs in women with PCOS.
As shown in Figure 4, non-HDL-C [SMD (95% CI): 0.42 (0.31, 0.53), p < 0.001] increased in reproductive-aged women with PCOS, with significant between-study heterogeneity. The subgroup analysis showed that non-HDL-C increased in women with PCOS at the three BMI levels, including BMI < 25 kg/m2 [SMD (95% CI): 0.40 (0.27, 0.54), p < 0.001], BMI ≥ 30 kg/m2 [MD (95% CI): 0.57 (0.37, 0.77), p < 0.001], and BMI 25–30 kg/m2 [MD (95% CI): 0.37 (0.11, 0.62), p = 0.005]. In this analysis, no publication bias was evident (asymmetry test P = 0.291). In the sensitivity analysis, women of reproductive age with PCOS had an increase in nonHDL-C compared with that of controls.
As seen in Figure 5, women of reproductive age with PCOS had an increased level of LDL-C [SMD (95% CI): 0.29 (0.20, 0.39), p < 0.001], with no significant between-study heterogeneity. In addition, LDL-C increased in women with PCOS at BMI <25 kg/m2 [SMD (95% CI): 0.32 (0.18, 0.45), p < 0.001], BMI ≥ 30 kg/m2 [MD (95% CI): 0.28 (0.09, 0.47), p = 0.003] and BMI 25–30 kg/m2 [MD (95% CI): 0.23 (0.02, 0.45), p = 0.034]. There was no publication bias (asymmetry test P = 0.414). In the sensitivity analysis, women of reproductive age with PCOS showed an increase in LDL-C compared with that of controls.
Blood Pressure
Figures 6, 7 are forest plots summarizing comparisons of SBP and DBP, respectively.
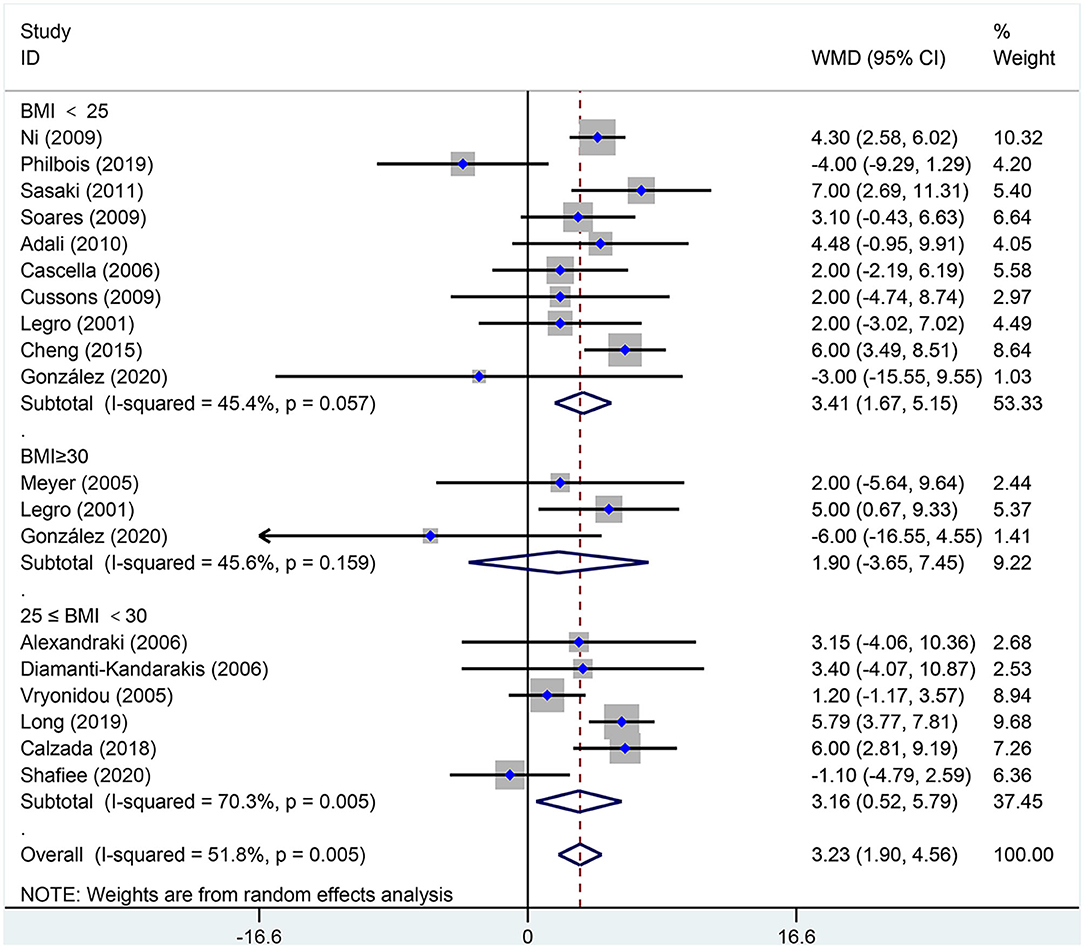
Figure 6. Forest plot for comparison of systolic blood pressure in polycystic ovary syndrome vs. control subjects. Studies are classified by different body mass index (BMI) categories (BMI <25 kg/m2, BMI ≥ 30 kg/m2, and BMI 25–30 kg/m2).
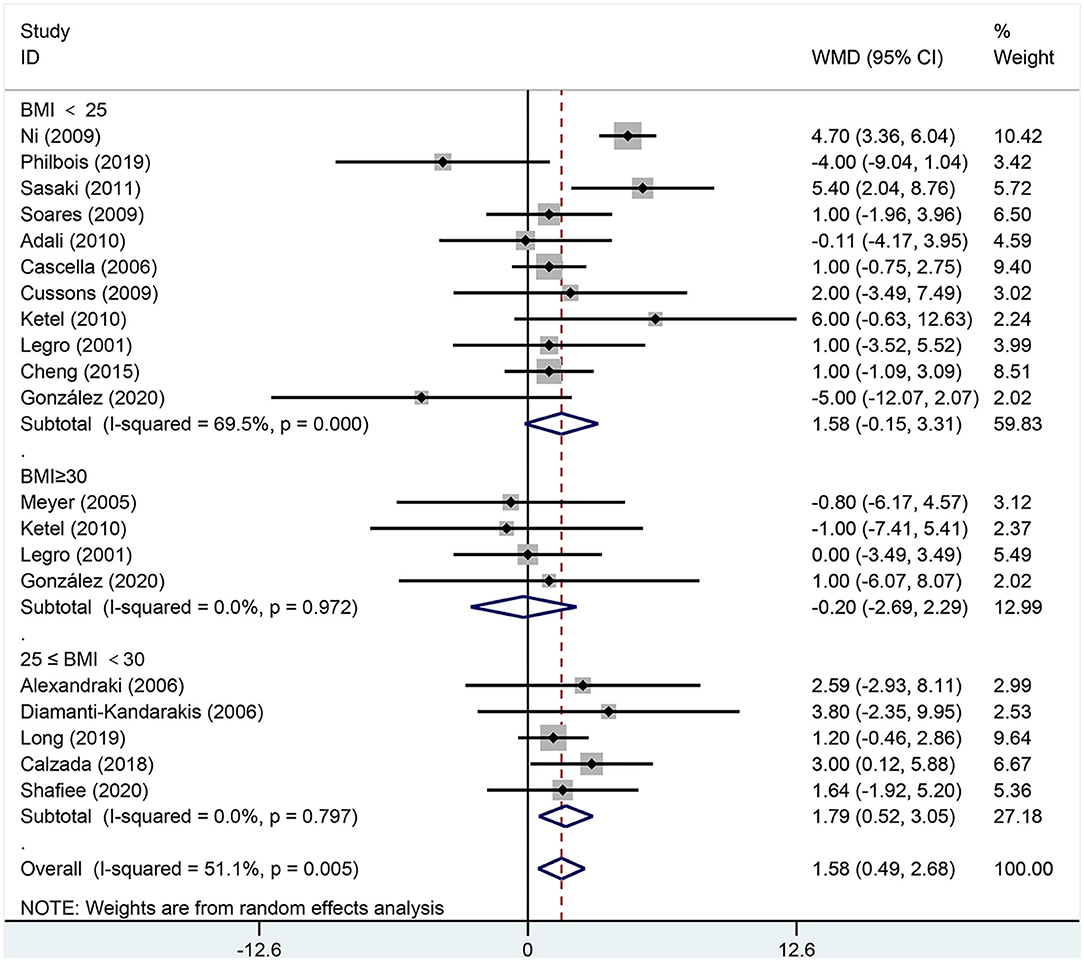
Figure 7. Forest plot for comparison of diastolic blood pressure in polycystic ovary syndrome vs. control subjects. Studies are classified by different body mass index (BMI) categories (BMI <25 kg/m2, BMI ≥ 30 kg/m2, and BMI 25–30 kg/m2).
As shown in Figure 6, SBP [MD (95% CI): 3.23 mmHg (1.90, 4.56), p < 0.001] increased in women of reproductive age with PCOS. In the subgroup analysis of SBP, SBP increased in women with PCOS at BMI <25 kg/m2 [MD (95% CI): 3.41 mmHg (1.67, 5.15), p < 0.001] and BMI 25–30 kg/m2 [MD (95% CI): 3.16 mmHg (0.52, 5.79), p = 0.019]; however, women with PCOS at BMI ≥ 30 kg/m2 [MD (95% CI): 1.90 mmHg (−3.65, 7.45), p = 0.502] did not differ in SBP. In this analysis, the Egger's test detected publication bias (asymmetry test p = 0.062). However, further analysis with a trim-and-fill test indicated that this publication bias did not impact the estimates (i.e., no trimming was done because the data were unchanged). Further sensitivity analysis comparing PCOS patients and controls showed an increase in SBP in PCOS patients.
As shown in Figure 7, DBP [MD (95% CI): 1.58 mmHg (0.49, 2.68), p = 0.005] increased in women of reproductive age with PCOS. In the subgroup analysis of DBP in women with PCOS, BMI 25–30 kg/m2 [MD (95% CI): 1.79 mmHg (0.52, 3.05), p = 0.006] showed positive results, while DBP did not increase at BMI <25 kg/m2 [MD (95% CI): 1.58 mmHg (−0.15, 3.31), p = 0.073] or BMI ≥ 30 kg/m2 [MD (95% CI): −0.20 mmHg (−2.69, 2.29), p = 0.876]. In this analysis, there was publication bias as detected by the Egger's test (asymmetry test P = 0.098). However, further analysis with a trim-and-fill test indicated that this publication bias did not impact the estimates.
Waist-to-Hip Ratio
As shown in Figure 8, WHR [MD (95% CI): 0.03 (0.02, 0.04), p < 0.001] increased in women of reproductive age with PCOS. In the subgroup analysis of WHR in women with PCOS, BMI <25 kg/m2 [MD (95% CI): 0.02 (0.00, 0.03), p = 0.022], BMI 25–30 kg/m2 [MD (95% CI): 0.04 (0.03, 0.05), p < 0.001] and BMI ≥ 30 kg/m2 [MD (95% CI): 0.03 (0.02, 0.05), p < 0.001] showed positive results. In this analysis, there was no publication bias on Egger's test (asymmetry test P = 0.547).
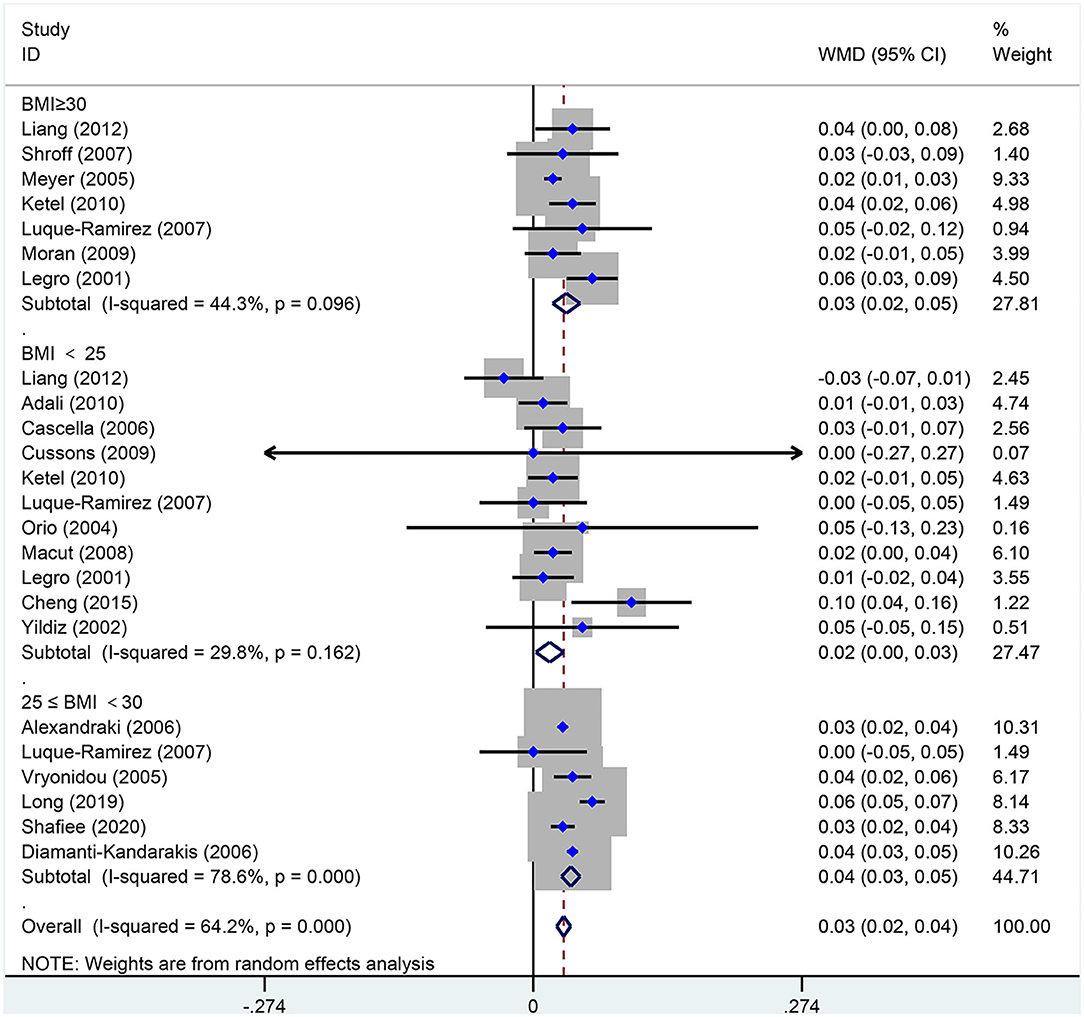
Figure 8. Forest plot for comparison of waist-to-hip ratio in polycystic ovary syndrome vs. control subjects. Studies are classified by different body mass index (BMI) categories (BMI <25 kg/m2, BMI ≥ 30 kg/m2, and BMI 25 <30 kg/m2).
Discussion
This systematic review of 38 observational studies reports the correlation between cardiovascular risk and BMI for women of reproductive age with PCOS. To the best of our knowledge, this is the first time that BMI categories were evaluated as cardiovascular risk factors in women of reproductive age with PCOS. Dyslipidemia and high baseline blood pressure are common in women of reproductive age with PCOS, and earlier focus had been on alterations in TGs and HDL-C. This metaanalysis showed that LDL-C and nonHDL-C increased across all BMI categories for women with PCOS. LDL-C and nonHDL-C, which are critical targets for preventing atherosclerosis, may be more important for the prevention of cardiovascular risk. In addition, blood pressure should also be considered.
In the present metaanalysis, increased baseline blood pressure was seen in women of reproductive age with PCOS. SBP and DBP were considerably higher in PCOS subjects whose BMI was between 25 and 30 kg/m2. Moreover, SBP alone increased in PCOS subjects whose the BMI was <25 kg/m2. However, there was no difference in SBP and DBP in PCOS subjects whose BMI was more than 30 kg/m2. One cross-sectional Brazilian study on 233 women with PCOS and 70 controls found a higher prevalence of hypertension among the PCOS group (53). The prevalence of hypertension was 65% among women with PCOS (mean age: 25-26.5 years) and 41% among control women without PCOS (mean age: 29 years) in that study (53). Zimmermann et al. reported that BMI did not reveal an association between women of reproductive age with PCOS and hypertension (54). A study from Australia reported that hypertension in women of reproductive age with PCOS was not associated with BMI, further indicating that cardiometabolic abnormalities may be independent of weight in women with PCOS (55). Because of the small sample size, we did not evaluate the prevalence of hypertension. However, the present metaanalysis found that baseline blood pressure was higher in women of reproductive age with PCOS than in age- and BMI-matched controls. Schmidt et al. showed that high blood pressure and hypertriglyceridemia were the only cardiovascular risk factors that persisted in postmenopausal women with PCOS. The main factors for increased blood pressure in women with PCOS, include endothelial dysfunction and decreased vascular compliance (56). The increased risk of hypertension can be explained by insulin resistance and hyperinsulinemia, which result in hypertrophy of the vascular muscle wall and reduce vascular compliance by interfering with endothelium-dependent vasodilatation mechanisms (57). Hyperinsulinemia promotes endothelin-1 (ET-1) production and influences its hypertrophic effect on vascular endothelial and smooth muscle cells (56). In addition, ET-1, which regulates endothelial function, is commonly chronically increased in women with PCOS.
The present meta-analysis showed dyslipidemia in women of reproductive age with PCOS, as increased LDL-C, nonHDL-C and TGs were found across all BMI categories. These findings are consistent with those of previous studies (58, 59). Women with PCOS are known to have increased levels of atherogenic apoB-containing particles, with a predominance of LDL particles that are smaller and have higher density (17). Small, dense LDL particles are the most atherogenic LDL particles and are strongly associated with cardiovascular risk due to their enhanced susceptibility to oxidation, reduced affinity to LDL receptor and greater arterial entry and retention (60). Women with PCOS younger than 40 appear to have dyslipidemia (59). Therefore, PCOS per se increases lipid levels, although the absolute value of the resultant lipid levels and the related cardiovascular risk may differ among individual patients. This lipid pattern assessment is an important finding for decreasing cardiovascular risk in women of reproductive age with PCOS women across all BMI categories. Subgroup analyses from a previous metaanalysis showed that higher LDL-C was found in women with higher BMI categories (61). However, our finding that women of reproductive age with PCOS showed that increased LDL-C was seen across all BMI categories. Because the measurement of LDL-C can be influenced by increased TGs in cardiovascular risk assessment (62), an appropriate way to estimate the amount of apo B-containing lipoproteins is to determine nonHDL-C (63). In addition, nonHDL-C has a comparable prognostic relevance to LDL-C (64, 65). We found that nonHDL-C increased in all BMI categories. It is important to evaluate nonHDL-C levels in women of reproductive age with PCOS. Increase in the levels of nonHDL-C, particularly at young ages, predicts long-term cardiovascular risks. This suggests that obesity (or being overweight) is not the largest cardiovascular risk factor in women of reproductive age with PCOS. Because the atherosclerotic process starts early in life, this confirms the need to assess and eventually treat altered lipid profiles in young women with PCOS (66).
Although obesity is an important confound in the relationship between PCOS diagnosis and cardiovascular risk, non-obese subjects also have a high dyslipidemia risk (56, 67). In addition to the reproductive and psychological symptoms of PCOS, the metabolic aberrations of PCOS worsen with obesity (68). Notably, non-obese women with PCOS are also at an increased risk of similar cardiometabolic aberrations. Women with PCOS have enlarged adipocytes in subcutaneous adipose tissue (SAT), lower circulating levels of adiponectin and increased abdominal adiposity independent of BMI (69–71). Although it was long postulated that SAT was less relevant than visceral adipose tissue (VAT) for metabolic dysfunction, SAT has recently been linked with metabolic alterations as well (72). Enlarged adipocytes in the adipose tissue of women with PCOS are associated with decreased adiponectin production and increased insulin resistance (IR), which indicates alterations in the function and morphology of adipose tissue as a PCOS process (70). In addition, women with PCOS had increased accumulations of adipose tissue in abdominal depots, predisposing them to the development of metabolic diseases, such as IR and diabetes, or to a higher risk for metabolic sequelae (73, 74). Adipose tissue dysfunction further correlates with an adverse metabolic profile. The close relationship between PCOS and cardiovascular risk is independent of BMI but may be due to SAT and VAT. In this metaanalysis, we found WHR increased in all BMI categories. Therefore, it is necessary to screen lipid levels, especially nonHDL, for women of reproductive age with PCOS of all BMI categories. We have shown that PCOS is associated with an increased cardiovascular risk. Although increased BMI was not the sole cause of the increased risk for cardiovascular events, the role of BMI should be examined in more detail in future studies. Dyslipidemia is common in women of reproductive age with PCOS. Furthermore, nonHDL-C plays an important role in cardiovascular risk in women with PCOS.
Another feature of PCOS, hyperandrogenism, results in excessive androgen. Excessive androgen can independently aggravate the development of cardiovascular risk in women with PCOS (75). Excessive androgen increases carotid intima–media thickness and calcification in the coronary and aortic arteries in women with PCOS, which may reflect dyslipidemia induced by androgen excess (34, 76). Reports have shown that high blood pressure is positively correlated with androgen excess in women (75). Moreover, lipid-induced inflammation may stimulate androgen excess in women with PCOS (28). Androgen can induce inflammation and oxidative stress in the vascular endothelium and increase renal reabsorption of sodium and water, which indirectly decreases circulating HDL-C levels and LDL-C clearance (77). Thus, excess androgen plays a role in the development of hypertension and the progression of atherosclerosis (78).
Several limitations of this metaanalysis should be noted. First, PCOS is a heterogeneous disease, and not all phenotypes were examined in this metaanalysis. Second, there was significant clinical and statistical heterogeneity in the pooled analysis. This could be due to confounding effects related to factors such as age, BMI, study quality, ethnicity, PCOS phenotypes, or other clinical features. In addition, we used country regions as a proxy for ethnicity in this metaanalysis due to a lack of participant ethnic compositions in most studies. Further studies should include the ethnicity of participants and different phenotypes of PCOS to provide a better understanding of the differential effects of these factors on PCOS or metabolic syndromes.
In conclusion, high baseline blood pressure and dyslipidemia are common in women of reproductive age with PCOS and are characterized by high SBP and DBP, low HDL-C, and increased TGs, nonHDL-C and LDL-C. These lipid parameters and BPs are worse in women of reproductive age with PCOS than in controls, regardless of BMI. Lipid profiles (i.e., TGs, LDL-C and nonHDL-C) should receive increased attention in all women of reproductive age with PCOS.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
CZ and JY designed this work. CZ and XL are responsible for the acquisition and analysis of data and also drafted the work. WW, RS, and MQ are responsible for acquiring data as well. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the grants of National Natural Science Foundation of China (Nos. 81960086 and 81670385), Innovation Ability Promotion Project of Colleges and Universities in Gansu Province, China (No. 2019B-017), and Cuiying Science and Technology Innovation Project of Lanzhou University Second Hospital, China (No. CY2018-QN05).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to acknowledge Prof. Yuanhang Cheng (City University of Hong Kong) for constructive suggestions. Extremely careful and thoughtful reviews by Associate Editor and Reviewer improved this manuscript greatly.
References
1. Cooney LG, Dokras A. Beyond fertility: polycystic ovary syndrome and long-term health. Fertility Sterility. (2018) 110:794–809. doi: 10.1016/j.fertnstert.2018.08.021
2. Bozdag G, Mumusoglu S, Zengin D, Karabulut E, Yildiz BO. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and metaanalysis. Hum Reproduct. (2016) 31:2841–55. doi: 10.1093/humrep/dew218
3. Obesity and reproduction: an educational bulletin. Fertility Sterility. (2008) 90:S21-9. doi: 10.1016/j.fertnstert.2008.08.005
4. Behboudi-Gandevani S, Ramezani Tehrani F, Hosseinpanah F, Khalili D, Cheraghi L, Kazemijaliseh H, et al. Cardiometabolic risks in polycystic ovary syndrome: long-term population-based follow-up study. Fertility Sterility. (2018) 110:1377–86. doi: 10.1016/j.fertnstert.2018.08.046
5. Sirmans SM, Pate KA. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clinical Epidemiol. (2013) 6:1–13. doi: 10.2147/CLEP.S37559
6. Glueck CJ, Goldenberg N. Characteristics of obesity in polycystic ovary syndrome: etiology, treatment, and genetics. Metabolism Clin Experiment. (2019) 92:108–20. doi: 10.1016/j.metabol.2018.11.002
7. Jeanes YM, Reeves S. Metabolic consequences of obesity and insulin resistance in polycystic ovary syndrome: diagnostic and methodological challenges. Nutr Res Rev. (2017) 30:97–105. doi: 10.1017/S0954422416000287
8. Lim SS, Davies MJ, Norman RJ, Moran LJ. Overweight, obesity and central obesity in women with polycystic ovary syndrome: a systematic review and metaanalysis. Hum Reproduct Update. (2012) 18:618–37. doi: 10.1093/humupd/dms030
9. Clinical Clinical guidelines on the identification evaluation and and treatment of overweight and obesity in adults: executive summary. Expert panel on the identification, evaluation, and treatment of overweight in adults. Am J Clin Nutr. (1998) 68:899–917. doi: 10.1093/ajcn/68.4.899
10. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
11. Wells G, Shea, B, O'Connell, J,. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Ottawa, ON: Health Research Institute Web site (2014). Available online at: http://www.ohri.ca/programs/clinical_epidemiology/nos_manual.pdf
12. Silva M, Lyra MCA, Almeida HCR, Alencar Filho AV, Heimer MV, Rosenblatt A. Caries experience in children and adolescents with Down Syndrome: a systematic review and metaanalysis. Arch Oral Biol. (2020) 115:104715. doi: 10.1016/j.archoralbio.2020.104715
13. Akram T, Hasan S, Imran M, Karim A, Arslan M. Association of polycystic ovary syndrome with cardiovascular risk factors. Gynecol Endocrinol. (2010) 26:47–53. doi: 10.3109/09513590903159565
14. Adali E, Yildizhan R, Kurdoglu M, Bugdayci G, Kolusari A, Sahin HG. Increased plasma thrombin-activatable fibrinolysis inhibitor levels in young obese women with polycystic ovary syndrome. Fertility Sterility. (2010) 94:666–72. doi: 10.1016/j.fertnstert.2009.03.037
15. Alexandraki K, Protogerou AD, Papaioannou TG, Piperi C, Mastorakos G, Lekakis J, et al. Early microvascular and macrovascular dysfunction is not accompanied by structural arterial injury in polycystic ovary syndrome. Hormones. (2006) 5:126–36. doi: 10.14310/horm.2002.11176
16. Arikan S, Akay H, Bahceci M, Tuzcu A, Gokalp D. The evaluation of endothelial function with flow-mediated dilatation and carotid intima media thickness in young nonobese polycystic ovary syndrome patients; existence of insulin resistance alone may not represent an adequate condition for deterioration of endothelial function. Fertility Sterility. (2009) 91:450–5. doi: 10.1016/j.fertnstert.2007.11.057
17. Berneis K, Rizzo M, Hersberger M, Rini GB, Di Fede G, Pepe I, et al. Atherogenic forms of dyslipidaemia in women with polycystic ovary syndrome. Int J Clin Pract. (2009) 63:56–62. doi: 10.1111/j.1742-1241.2008.01897.x
18. Cascella T, Palomba S, Tauchmanovà L, Manguso F, Di Biase S, Labella D, et al. Serum aldosterone concentration and cardiovascular risk in women with polycystic ovarian syndrome. J Clin Endocrinol Metab. (2006) 91:4395–400. doi: 10.1210/jc.2006-0399
19. Calzada M, López N, Noguera JA, Mendiola J, Torres AM. Elevation of isoprostanes in polycystic ovary syndrome and its relationship with cardiovascular risk factors. J Endocrinol Invest. (2019) 42:75–83. doi: 10.1007/s40618-018-0888-y
20. Cetinkalp S, Karadeniz M, Erdogan M, Zengi A, Cetintas V, Tetik A, et al. Apolipoprotein E gene polymorphism and polycystic ovary syndrome patients in Western Anatolia, Turkey. J Assist Reproduct Genet. (2009) 26:1–6. doi: 10.1007/s10815-008-9280-8
21. Cheng F, Zhao L, Wu Y, Huang T, Yang G, Zhang Z, et al. Serum vascular endothelial growth factor B is elevated in women with polycystic ovary syndrome and can be decreased with metformin treatment. Clin Endocrinol. (2016) 84:386–93. doi: 10.1111/cen.12950
22. Cussons AJ, Watts GF, Stuckey BG. Dissociation of endothelial function and arterial stiffness in nonobese women with polycystic ovary syndrome (PCOS). Clin Endocrinol. (2009) 71:808–14. doi: 10.1111/j.1365-2265.2009.03598.x
23. Diamanti-Kandarakis E, Alexandraki K, Piperi C, Protogerou A, Katsikis I, Paterakis T, et al. Inflammatory and endothelial markers in women with polycystic ovary syndrome. Eur J Clin Invest. (2006) 36:691–7. doi: 10.1111/j.1365-2362.2006.01712.x
24. El-Kannishy G, Kamal S, Mousa A, Saleh O, Badrawy AE, Farahaty RE, et al. Endothelial function in young women with polycystic ovary syndrome (PCOS): implications of body mass index (BMI) and insulin resistance. Obes Res Clin Pract. (2010) 4:e1-82. doi: 10.1016/j.orcp.2009.08.001
25. Erdogan M, Karadeniz M, Alper GE, Tamsel S, Uluer H, Caglayan O, et al. Thrombin-activatable fibrinolysis inhibitor and cardiovascular risk factors in polycystic ovary syndrome. Exp Clin Endocrinol Diab. (2008) 116:143–7. doi: 10.1055/s-2007-992118
26. Erdogan M, Karadeniz M, Berdeli A, Tamsel S, Yilmaz C. The relationship of the interleukin-6−174 G>C gene polymorphism with cardiovascular risk factors in Turkish polycystic ovary syndrome patients. Int J Immunogenet. (2009) 36:283–8. doi: 10.1111/j.1744-313X.2009.00867.x
27. Glintborg D, Hermann AP, Rasmussen LM, Andersen M. Plasma osteoprotegerin is associated with testosterone levels but unaffected by pioglitazone treatment in patients with polycystic ovary syndrome. J Endocrinol Invest. (2013) 36:460–5. doi: 10.3275/8767
28. González F, Considine RV, Abdelhadi OA, Acton AJ. Lipid-induced mononuclear cell cytokine secretion in the development of metabolic aberration and androgen excess in polycystic ovary syndrome. Hum Reproduct. (2020) 35:1168–77. doi: 10.1093/humrep/deaa056
29. Kargili A, Karakurt F, Kasapoglu B, Derbent A, Koca C, Selcoki Y. Association of polycystic ovary syndrome and a non-dipping blood pressure pattern in young women. Clinics. (2010) 65:475–9. doi: 10.1590/S1807-59322010000500004
30. Ketel IJ, Stehouwer CD, Henry RM, Serné EH, Hompes P, Homburg R, et al. Greater arterial stiffness in polycystic ovary syndrome (PCOS) is an obesity–but not a PCOS-associated phenomenon. J Clin Endocrinol Metab. (2010) 95:4566–75. doi: 10.1210/jc.2010-0868
31. Legro RS, Kunselman AR, Dunaif A. Prevalence and predictors of dyslipidemia in women with polycystic ovary syndrome. Am J Med. (2001) 111:607–13. doi: 10.1016/S0002-9343(01)00948-2
32. Liang SJ, Liou TH, Lin HW, Hsu CS, Tzeng CR, Hsu MI. Obesity is the predominant predictor of impaired glucose tolerance and metabolic disturbance in polycystic ovary syndrome. Act Obstetr Gynecol Scand. (2012) 91:1167–72. doi: 10.1111/j.1600-0412.2012.01417.x
33. Long J, Li L, Wang F, Yang G, Cheng W, Wei J, et al. Screening for a simple and effective indicator of insulin resistance in Chinese women of reproductive age, with the insulin clamp technique as a reference. Metab Syndr Relat Disord. (2019) 17:423–9. doi: 10.1089/met.2019.0019
34. Luque-Ramírez M, Mendieta-Azcona C, Alvarez-Blasco F, Escobar-Morreale HF. Androgen excess is associated with the increased carotid intima-media thickness observed in young women with polycystic ovary syndrome. Hum Reproduct. (2007) 22:3197–203. doi: 10.1093/humrep/dem324
35. Macut D, Panidis D, Glisić B, Spanos N, Petakov M, Bjekić J, et al. Lipid and lipoprotein profile in women with polycystic ovary syndrome. Can J Physiol Pharmacol. (2008) 86:199–204. doi: 10.1139/Y08-014
36. Meyer C, McGrath BP, Teede HJ. Overweight women with polycystic ovary syndrome have evidence of subclinical cardiovascular disease. J Clin Endocrinol Metab. (2005) 90:5711–6. doi: 10.1210/jc.2005-0011
37. Moran LJ, Hutchison SK, Meyer C, Zoungas S, Teede HJ. A comprehensive assessment of endothelial function in overweight women with and without polycystic ovary syndrome. Clin Sci. (2009) 116:761–70. doi: 10.1042/CS20080218
38. Ni RM, Mo Y, Chen X, Zhong J, Liu W, Yang D. Low prevalence of the metabolic syndrome but high occurrence of various metabolic disorders in Chinese women with polycystic ovary syndrome. Eur J Endocrinol. (2009) 161:411–8. doi: 10.1530/EJE-09-0298
39. Oral B, Mermi B, Dilek M, Alanoglu G, Sütçü R. Thrombin activatable fibrinolysis inhibitor and other hemostatic parameters in patients with polycystic ovary syndrome. Gynecol Endocrinol. (2009) 25:110–6. doi: 10.1080/09513590802549874
40. Orio F Jr., Palomba S, Cascella T, De Simone B, Di Biase S, Russo T, et al. Early impairment of endothelial structure and function in young normal-weight women with polycystic ovary syndrome. J Clin Endocrinol Metab. (2004) 89:4588–93. doi: 10.1210/jc.2003-031867
41. Philbois SV, Gastaldi AC, Facioli TP, Felix ACS, Reis RMD, Fares TH, et al. Women with polycystic ovarian syndrome exhibit reduced baroreflex sensitivity that may be associated with increased body fat. Arquivos brasileiros de cardiologia. (2019) 112:424–9. doi: 10.5935/abc.20190031
42. Rizzo M, Longo RA, Guastella E, Rini GB, Carmina E. Assessing cardiovascular risk in Mediterranean women with polycystic ovary syndrome. J Endocrinol Invest. (2019) 34:422–6. doi: 10.1007/BF03346706
43. Sasaki A, Emi Y, Matsuda M, Kamada Y, Chekir C, et al. Increased arterial stiffness in mildly-hypertensive women with polycystic ovary syndrome. J Obstet Gynaecol Res. (2011) 37:402–11. doi: 10.1111/j.1447-0756.2010.01375.x
44. Shafiee MN, Ortori CA, Barrett DA, Mongan NP, Abu J, Atiomo W. Lipidomic biomarkers in polycystic ovary syndrome and endometrial cancer. Int J Mol Sci. (2020) 21:4753. doi: 10.3390/ijms21134753
45. Shroff R, Kerchner A, Maifeld M, Van Beek EJ, Jagasia D, Dokras A. Young obese women with polycystic ovary syndrome have evidence of early coronary atherosclerosis. J Clin Endocrinol Metab. (2007) 92:4609–14. doi: 10.1210/jc.2007-1343
46. Soares GM, Vieira CS, Martins WP, Franceschini SA, dos Reis RM, Silva de Sá MF, et al. Increased arterial stiffness in nonobese women with polycystic ovary syndrome (PCOS) without comorbidities: one more characteristic inherent to the syndrome? Clin Endocrinol. (2009) 71:406–11. doi: 10.1111/j.1365-2265.2008.03506.x
47. Tarkun I, Arslan BC, Cantürk Z, Türemen E, Sahin T, Duman C. Endothelial dysfunction in young women with polycystic ovary syndrome: relationship with insulin resistance and low-grade chronic inflammation. J Clin Endocrinol Metab. (2004) 89:5592–6. doi: 10.1210/jc.2004-0751
48. Tíras MB, Yalcìn R, Noyan V, Maral I, Yìldìrìm M, Dörtlemez O, et al. Alterations in cardiac flow parameters in patients with polycystic ovarian syndrome. Hum Reproduct. (1999) 14:1949–52. doi: 10.1093/humrep/14.8.1949
49. Vryonidou A, Papatheodorou A, Tavridou A, Terzi T, Loi V, Vatalas IA, et al. Association of hyperandrogenemic and metabolic phenotype with carotid intima-media thickness in young women with polycystic ovary syndrome. J Clin Endocrinol Metab. (2005) 90:2740–6. doi: 10.1210/jc.2004-2363
50. Yildiz BO, Haznedaroglu IC, Kirazli S, Bayraktar M. Global fibrinolytic capacity is decreased in polycystic ovary syndrome, suggesting a prothrombotic state. J Clin Endocrinol Metab. (2002) 87:3871–5. doi: 10.1210/jcem.87.8.8716
51. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in metaanalysis. Biometrics. (2000) 56:455–63. doi: 10.1111/j.0006-341X.2000.00455.x
52. Berneis K, Rizzo M, Lazzarini V, Fruzzetti F, Carmina E. Atherogenic lipoprotein phenotype and low-density lipoproteins size and subclasses in women with polycystic ovary syndrome. J Clin Endocrinol Metab. (2007) 92:186–9. doi: 10.1210/jc.2006-1705
53. Marchesan LB, Spritzer PM. ACC/AHA 2017 definition of high blood pressure: implications for women with polycystic ovary syndrome. Fertility Sterility. (2019) 111:579–87.e1. doi: 10.1016/j.fertnstert.2018.11.034
54. Zimmermann S, Phillips RA, Dunaif A, Finegood DT, Wilkenfeld C, Ardeljan M, et al. Polycystic ovary syndrome: lack of hypertension despite profound insulin resistance. J Clin Endocrinol Metab. (1992) 75:508–13. doi: 10.1210/jcem.75.2.1639952
55. Joham AE, Boyle JA. Zoungas, Teede HJ. Hypertension in women of reproductive age with polycystic ovary syndrome and association with obesity. Am J Hypertens. (2015) 28:847–51. doi: 10.1093/ajh/hpu251
56. Lim SS, Kakoly NS, Tan JWJ, Fitzgerald G, Bahri Khomami M, Joham AE, et al. Metabolic syndrome in polycystic ovary syndrome: a systematic review, metaanalysis and meta-regression. Obes Rev. (2019) 20:339–52. doi: 10.1111/obr.12762
57. Executive Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection Evaluation And And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. (2001) 285:2486–97. doi: 10.1001/jama.285.19.2486
58. Wild RA, Painter PC, Coulson PB, Carruth KB, Ranney GB. Lipoprotein lipid concentrations and cardiovascular risk in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 61:946–51. doi: 10.1210/jcem-61-5-946
59. Talbott E, Clerici A, Berga SL, Kuller L, Guzick D, Detre K, et al. Adverse lipid and coronary heart disease risk profiles in young women with polycystic ovary syndrome: results of a case-control study. J Clin Epidemiol. (1998) 51:415–22. doi: 10.1016/S0895-4356(98)00010-9
60. Rizzo M, Berneis K. Who needs to care about small, dense low-density lipoproteins? Int J Clin Pract. (2007) 61:1949–56. doi: 10.1111/j.1742-1241.2007.01596.x
61. Wild RA, Rizzo M, Clifton S, Carmina E. Lipid levels in polycystic ovary syndrome: systematic review and metaanalysis. Fertility Sterility. (2011) 95:1073–9.e1-11. doi: 10.1016/j.fertnstert.2010.12.027
62. Martin SS, Blaha MJ, Elshazly MB, Brinton EA, Toth PP, McEvoy JW, et al. Friedewald-estimated versus directly measured low-density lipoprotein cholesterol and treatment implications. J Am Coll Cardiol. (2013) 62:732–9. doi: 10.1016/j.jacc.2013.01.079
63. Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. (2009) 302:1993–2000. doi: 10.1001/jama.2009.1619
64. Palazón-Bru A, Carbayo-Herencia JA, Simarro-Rueda M, Artigao-Ródenas LM, Divisón-Garrote JA, Molina-Escribano F, et al. Comparison between non-high-density lipoprotein cholesterol and low-density lipoprotein cholesterol to estimate cardiovascular risk using a multivariate model. J Cardiovasc Nurs. (2018) 33:E17–23. doi: 10.1097/JCN.0000000000000534
65. Palazón-Bru A, Folgado-de la Rosa DM, Cortés-Castell E, López-Cascales MT, Gil-Guillén VF. Sample size calculation to externally validate scoring systems based on logistic regression models. PLoS ONE. (2017) 12:e0176726. doi: 10.1371/journal.pone.0176726
66. Wild RA, Carmina E, Diamanti-Kandarakis E, Dokras A, Escobar-Morreale HF, Futterweit W, et al. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. J Clin Endocrinol Metab. (2010) 95:2038–49. doi: 10.1210/jc.2009-2724
67. Ma X, Hayes E, Prizant H, Srivastava RK, Hammes SR, Sen A. Leptin-Induced CART (Cocaine- and Amphetamine-Regulated Transcript) is a novel intraovarian mediator of obesity-related infertility in females. Endocrinology. (2016) 157:1248–57. doi: 10.1210/en.2015-1750
68. Liou TH, Yang JH, Hsieh CH, Lee CY, Hsu CS, Hsu MI. Clinical and biochemical presentations of polycystic ovary syndrome among obese and nonobese women. Fertility Sterility. (2009) 92:1960–5. doi: 10.1016/j.fertnstert.2008.09.003
69. Echiburú B, Pérez-Bravo F, Galgani JE, Sandoval D, Saldías C, Crisosto N, et al. Enlarged adipocytes in subcutaneous adipose tissue associated to hyperandrogenism and visceral adipose tissue volume in women with polycystic ovary syndrome. Steroids. (2018) 130:15–21. doi: 10.1016/j.steroids.2017.12.009
70. Mannerås-Holm L, Leonhardt H, Kullberg J, Jennische E, Odén A, Holm G, et al. Adipose tissue has aberrant morphology and function in PCOS: enlarged adipocytes and low serum adiponectin, but not circulating sex steroids, are strongly associated with insulin resistance. J Clin Endocrinol Metab. (2011) 96:E304-11. doi: 10.1210/jc.2010-1290
71. Maliqueo M, Galgani JE, Pérez-Bravo F, Echiburú B, de Guevara AL, Crisosto N, et al. Relationship of serum adipocyte-derived proteins with insulin sensitivity and reproductive features in pre-pubertal and pubertal daughters of polycystic ovary syndrome women. Eur J Obstet Gynecol Reprod Biol. (2012) 161:56–61. doi: 10.1016/j.ejogrb.2011.12.012
72. Cortón M, Botella-Carretero JI, Benguría A, Villuendas G, Zaballos A, San Millán JL, et al. Differential gene expression profile in omental adipose tissue in women with polycystic ovary syndrome. J Clin Endocrinol Metab. (2007) 92:328–37. doi: 10.1210/jc.2006-1665
73. Escobar-Morreale HF, San Millán JL. Abdominal adiposity and the polycystic ovary syndrome. Trends Endocrinol Metab. (2007) 18:266–72. doi: 10.1016/j.tem.2007.07.003
74. Carmina E, Bucchieri S, Esposito A, Del Puente A, Mansueto P, Orio F, et al. Abdominal fat quantity and distribution in women with polycystic ovary syndrome and extent of its relation to insulin resistance. J Clin Endocrinol Metab. (2007) 92:2500–5. doi: 10.1210/jc.2006-2725
75. Escobar-Morreale HF, Alvarez-Blasco F, Botella-Carretero JI, Luque-Ramírez M. The striking similarities in the metabolic associations of female androgen excess and male androgen deficiency. Hum Reprod. (2014) 29:2083–91. doi: 10.1093/humrep/deu198
76. Yilmaz SA, Kebapcilar A, Koplay M, Kerimoglu OS, Pekin AT, Gencoglu B, et al. Association of clinical androgen excess with radial artery intima media thickness in women with polycystic ovary syndrome. Gynecol Endocrinol. (2015) 31:477–82. doi: 10.3109/09513590.2015.1014783
77. Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. (2012) 33:981–1030. doi: 10.1210/er.2011-1034
Keywords: polycystic ovary syndrome, cardiovascular risk, reproductive-age, meta-analysis, body mass index
Citation: Zhuang C, Luo X, Wang W, Sun R, Qi M and Yu J (2022) Cardiovascular Risk According to Body Mass Index in Women of Reproductive Age With Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 9:822079. doi: 10.3389/fcvm.2022.822079
Received: 26 November 2021; Accepted: 17 January 2022;
Published: 16 February 2022.
Edited by:
Davide Lauro, University of Rome Tor Vergata, ItalyReviewed by:
Manfredi Rizzo, University of Palermo, ItalyTao Tao, Shanghai Jiao Tong University, China
Copyright © 2022 Zhuang, Luo, Wang, Sun, Qi and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Yu, ZXJ5X2p5dUBsenUuZWR1LmNu
†These authors have contributed equally to this work
 Chenchen Zhuang
Chenchen Zhuang Xufei Luo
Xufei Luo Wenjuan Wang1
Wenjuan Wang1 Jing Yu
Jing Yu