- 1Department of Anesthesiology and Pain Medicine, Samsung Medical Center, School of Medicine, Sungkyunkwan University, Seoul, South Korea
- 2Department of Anesthesiology and Pain Medicine, Kangwon National University Hospital, Chuncheon, South Korea
- 3Division of Cardiology, Department of Internal Medicine, Kangbuk Samsung Hospital, School of Medicine, Sungkyunkwan University, Seoul, South Korea
- 4Department of Biomedical Sciences, Ajou University Graduate School of Medicine, Suwon, South Korea
- 5Center for Health Promotion, Samsung Medical Center, School of Medicine, Sungkyunkwan University, Seoul, South Korea
- 6Department of Emergency Medicine, Samsung Medical Center, School of Medicine, Sungkyunkwan University, Seoul, South Korea
- 7Statistics and Data Center, Research Institute for Future Medicine, Samsung Medical Center, Seoul, South Korea
- 8Rehabilitation and Prevention Center, Heart Vascular Stroke Institute, Samsung Medical Center, School of Medicine, Sungkyunkwan University, Seoul, South Korea
- 9College of Medicine, Kyung Hee University, Seoul, South Korea
- 10Department of Biomedical Engineering, Seoul National University College of Medicine, Seoul, South Korea
Background: Postoperative atrial fibrillation (POAF) is related to mortality after non-cardiac surgery. Left atrial volume index (LAVI) is known to be associated with prognosis and development of atrial fibrillation, but it has not been fully investigated in patients undergoing non-cardiac surgery.
Materials and methods: A total of 203,787 consecutive adult patients underwent non-cardiac surgery at our institution between January 2011 and June 2019. After identifying those with available LAVI estimated during preoperative echocardiography, we divided them into those with LAVI higher and lower than 34 mL/m2. The primary outcome was incidence of POAF.
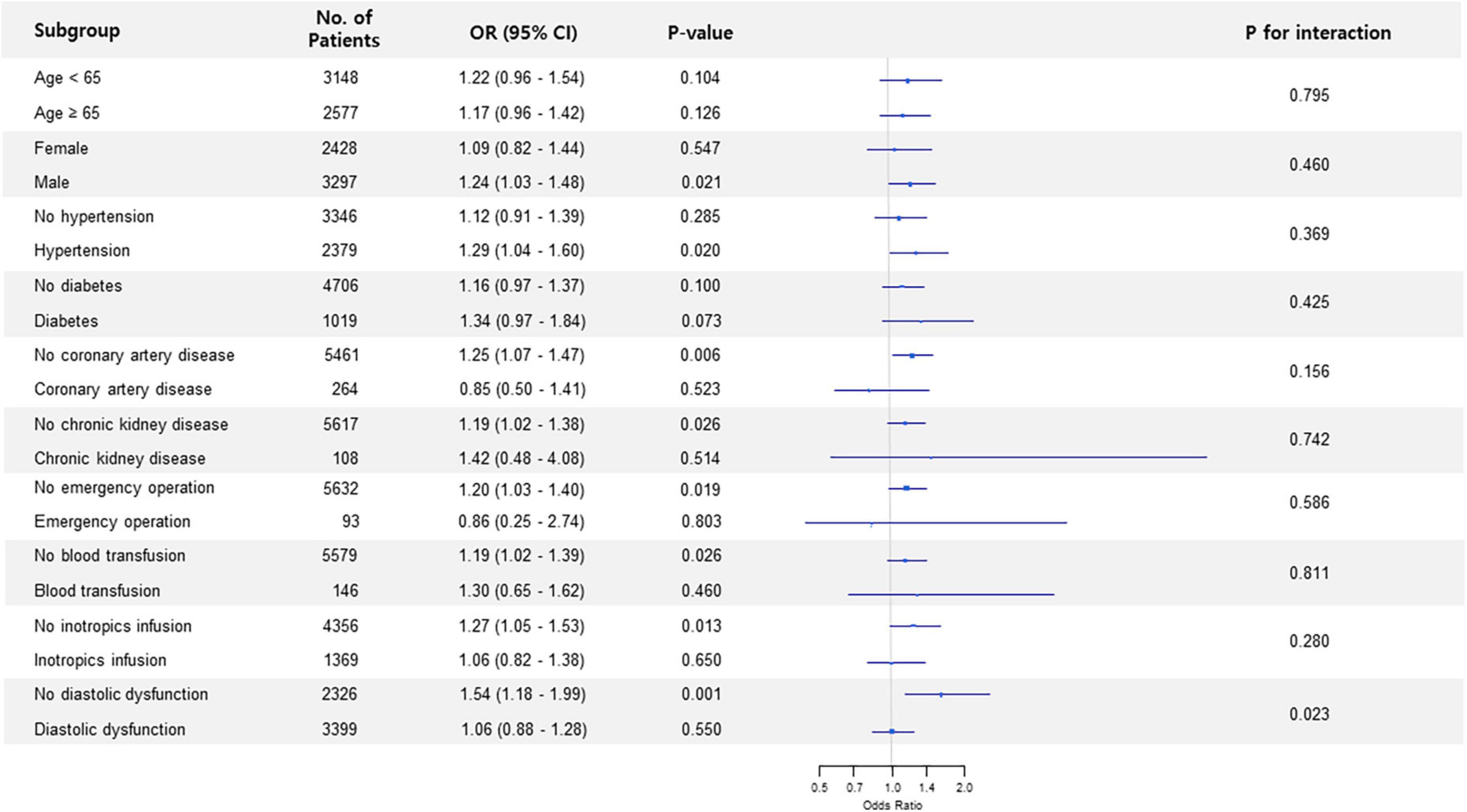
Results: A total of 83,097 patients were enrolled in this study. The study patients were divided into the low (57,838 [69.6%]) and high (25,259 [30.4%]) LAVI groups. After an adjustment, higher LAVI was associated with increased incidence of POAF (5.1% vs. 8.1%; odds ratio [OR], 1.33; 95% confidence interval [CI], 1.25–1.41; p < 0.001). In 24,549 pairs of propensity-score-matched population, the result was similar (6.2% vs. 7.9%; OR, 1.30; 95% CI, 1.21–1.39; p < 0.001). The estimated threshold of LAVI associated with POAF was 36.4 mL/m2 with an area under the curve of 0.571. Subgroup analysis in non-thoracic and thoracic surgery showed that the association between preoperative LAVI and POAF significantly interacted with diastolic dysfunction (p for interaction < 0.001), and the observed association was valid in patients without diastolic dysfunction.
Conclusion: Preoperative LAVI was shown to be associated with POAF in non-cardiac surgery. Our result needs verification in further studies.
Introduction
As average age and risk of surgery increase, postoperative complications have become a major health issue (1). Cardiac complications are closely associated with perioperative mortality (2), and new-onset atrial fibrillation following surgery, commonly referred to as postoperative atrial fibrillation (POAF), has been reported to frequently occur even in non-cardiac surgeries (3). POAF in non-cardiac surgery is now widely accepted to potentially lead to long-term consequences such as increased morbidity, mortality, hospital length of stay, and long-term risk of stroke (4, 5).
Left atrial volume index (LAVI) on echocardiography has been shown to be associated with atrial fibrillation in various clinical situations. In addition, LAVI was shown a predictive marker for the occurrence of atrial fibrillation and recurrence after treatment (6–8). In preoperative echocardiographic evaluation of cardiac surgery, LAVI was associated with POAF in various procedures (9, 10). However, this association remains unclear for non-cardiac surgery and may be related to a wide variety of non-cardiac surgeries or clinical events during surgical procedures because the development of POAF can largely vary depending on these factors. In the present study, consecutive adult patients who underwent non-cardiac surgery in a tertiary center between January 2011 and June 2019 were enrolled and divided into two groups based on preoperative LAVI and the incidence of POAF was compared. In addition, whether the significance of this association differed based on subgroup was evaluated.
Materials and methods
The approval and requirement for written informed consent for this study were waived by the Institutional Review Board of Samsung Medical Center (SMC 2021-06-078) because the registry for this study was curated in de-identified form. The present study was conducted according to the Declaration of Helsinki and reported following the Strengthening the Reporting of Observational Studies in Epidemiology guidelines.
Data curation, study population, and definitions
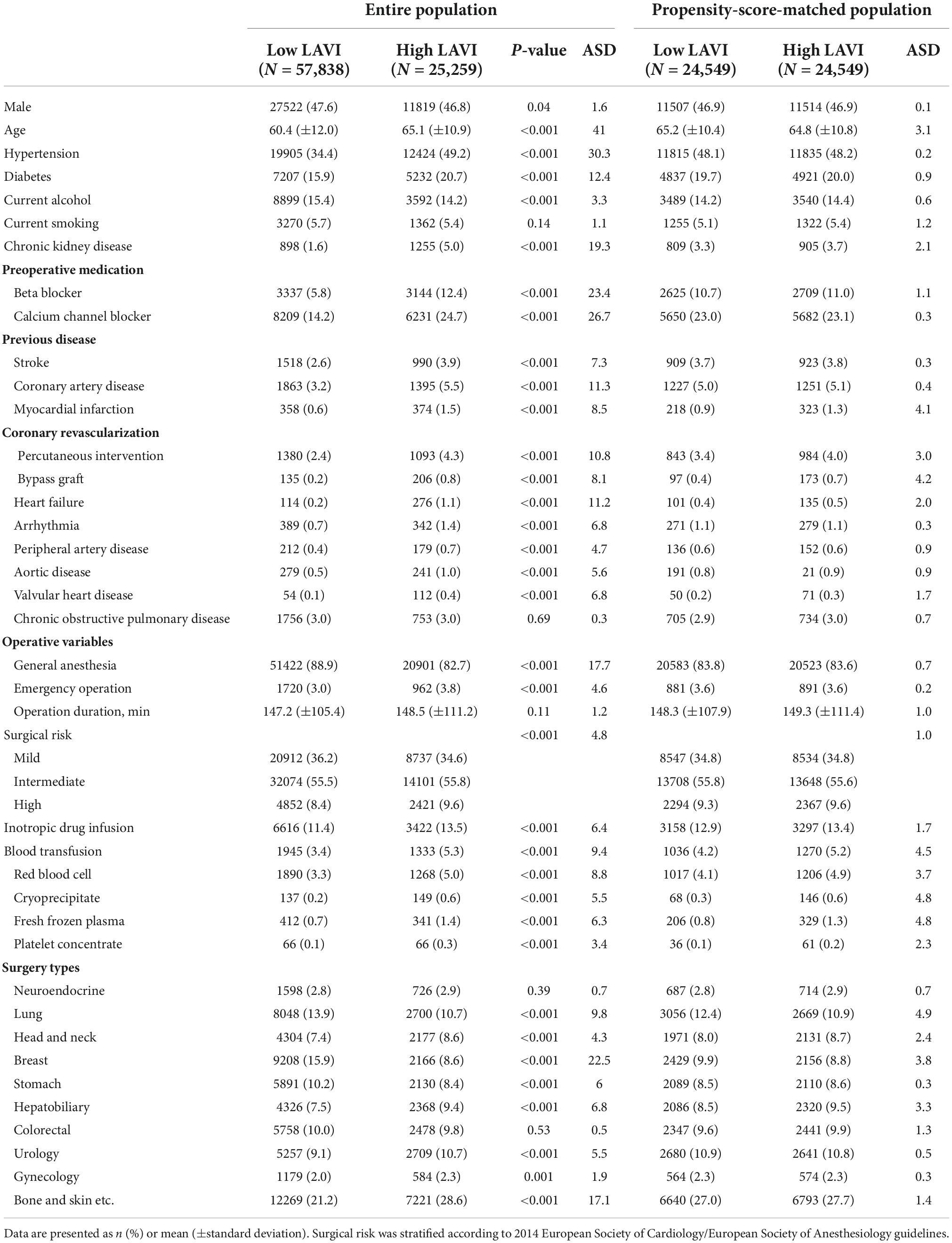
The Samsung Medical Center-Non-Cardiac operation, KCT 0006363 (SMC-NoCop) registry was used to identify subjects. SMC-NoCop is a large, single-center, de-identified cohort which consists of 203,787 consecutive adult patients who underwent non-cardiac surgery at Samsung Medical Center, Seoul, South Korea between January 2011 and June 2019. The Clinical Data Warehouse Darwin-C was used to extract data from our institutional electronic archive system which allows investigators to search and retrieve de-identified medical information from electronic hospital records of more than 4 million patients with more than 900 million laboratory findings and 200 million prescriptions. In this system, the mortality outside our institution is verified by a unique personal identification number and consistently updated according to the National Population Registry of the Korea National Statistical Office. From the entire population, we excluded the patients with preoperative atrial fibrillation or those without LAVI estimated from preoperative echocardiography. The patients were divided into high LAVI (≥34 mL/m2) and low LAVI (<34 mL/m2) groups according to current updated cut-off value (11).
Preoperative variables such as demographic data, preoperative medication, underlying disease, and blood laboratory tests were recorded based on preoperative evaluation by independent investigators. The International Classification of Diseases-10 codes were also used to estimate preoperative Charlson comorbidity index (12). The risk of surgical procedure was stratified according to the European Society of Cardiology/European Society of Anesthesiology guidelines on non-cardiac surgery (13). The diagnosis of heart failure was made by our cardiologists using Framingham criteria and other relevant clinical information (14). These included a history of dyspnea and symptomatic exercise intolerance with signs of pulmonary congestion or peripheral edema, the presence of moist rales on auscultation, or left ventricular enlargement or dysfunction by chest X-ray or echocardiography (15). The patients with heart failure were categorized into heart failure with preserved ejection fraction (HFpEF; ejection fraction > 40%) and heart failure with reduced ejection fraction (HFrEF; ejection fraction ≤ 40%) groups (16).
In our institution, preoperative transthoracic echocardiography including Doppler imaging was selectively obtained for non-cardiac surgery patients. An echocardiographic evaluation is recommended for moderate- to high-risk surgery or for patients with at least one major cardiovascular risk factor such as history of ischemic heart disease, heart failure, stroke, including transient ischemic attack, diabetes mellitus patients on insulin therapy, or chronic kidney disease, based on current guidelines (13). In patients with minor risk factors, transthoracic echocardiography was obtained at the discretion of the attending clinician based on older age or recent presentation of cardiovascular symptoms. Echocardiographic parameters were calculated according to current guidelines (17, 18). Left atrial volume was assessed by the biplane area-length method from apical 4- and 2-chamber views (19). Measurements were obtained in end systole from the frame preceding mitral valve opening, and the volume was indexed for body surface area. The normal value of indexed left atrial volume has been reported to be 20 ± 6 mL/m2 (20). The left atrium is directly exposed to left ventricle pressure during diastole, so left atrium size reflects the duration and severity of diastolic dysfunction (21).
Study endpoints
The primary endpoint was the occurrence of POAF during hospitalization. We followed-up the patients during this period, considering that the risk for POAF peaks at around 48 h after surgery (22). We used postoperative diagnosis based on the International Classification of Diseases-10 codes and reviewed electrocardiogram reading, in-hospital progress notes, nursing charts, discharge notes, and replies for cardiologist consultation. The secondary outcome was POAF that required interventional treatment such as intravenous administration of antiarrhythmic agents such as propafenone, flecainide, amiodarone, diltiazem, or verapamil or patients who required electrical cardioversion for rhythm or rate control.
Statistical analysis
Baseline characteristics of study patients are presented by number and percentage for categorical variables and the mean ± standard deviation (SD) or median with interquartile range (IQR) for continuous variables. Difference between groups was compared using the chi-square, Fisher’s exact test, t-test, or the Mann–Whitney test, as applicable. The incidence of POAF was compared using the logistic regression analysis and reported with odds ratio (OR) and 95% confidence interval (CI). The multivariable adjustment included age, hypertension, diabetes, chronic kidney disease, coronary aery disease, heart failure, general anesthesia, and preoperative use of beta blocker and calcium channel blocker. In addition, a propensity score matching to generate 1:1 individually matched populations without replacement was performed. Patients were matched on all variables, and propensity score using a nearest-neighbor matching technique. The propensity score was calculated using the logistic regression model with patients with high LAVI as the dependent variable. A greedy matching technique was employed to match patients using the logit of the propensity-score matching with a caliper width of 0.1 difference of the logit of the propensity score. After propensity score matching, 24,549 pairs were obtained with an absolute standard mean deviation (ASD) of <10% which was deemed a successful balance between the groups. Based on the sample size, the power of the analysis was 0.79 when OR was 1.1, and 0.99 when OR was >1.2 (23). In addition, the effects of unmeasured confounding factors were calculated. In this method, we evaluated the significance of the observed association between LAVI and POAF assuming the prevalence of unmeasured confounding factor was 40% (24). Subgroup analysis was performed in non-thoracic and thoracic surgery to evaluate whether the observed association interacted with relevant variables such as age, sex, hypertension, diabetes, coronary artery disease, chronic kidney disease, general anesthesia, emergency operation, intraoperative blood transfusion, intraoperative infusion of inotropic drugs, and diastolic dysfunction. The receiver operating characteristic (ROC) curve was constructed to estimate the threshold of LAVI associated with POAF and compute its specificity and sensitivity. The difference between two areas under the ROC curves was tested with the z test. All analyses were performed using R 4.1.11 (Vienna, Austria).
Results
Baseline characteristics
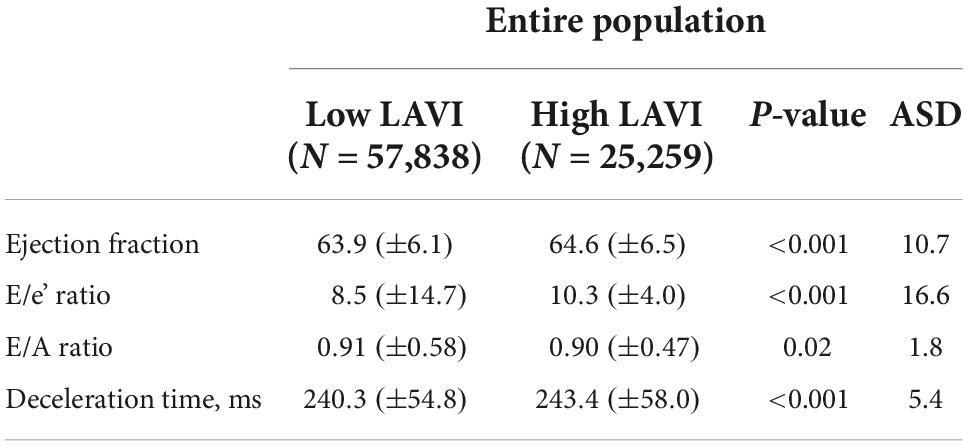
A total of 203,787 patients are in the SMC-NoCop registry, and 1,923 (0.9%) patients with preoperatively detected atrial fibrillation and 118,767 (58.3%) patients without estimated LAVI during preoperative evaluation were excluded from the present study. The remaining 83,097 patients were enrolled in the study and divided into two groups based on LAVI: low LAVI [<34 mL/min2, n = 57,838 (69.6%)] and high LAVI [≥ 34 mL/min2, n = 25,259 (30.4%)] groups (11). The baseline characteristics of the two groups are summarized in Table 1. Subjects in the high LAVI group were more frequently male, older, and had higher incidence of comorbidities. Other parameters on preoperative echocardiography are summarized in Table 2.
Postoperative atrial fibrillation
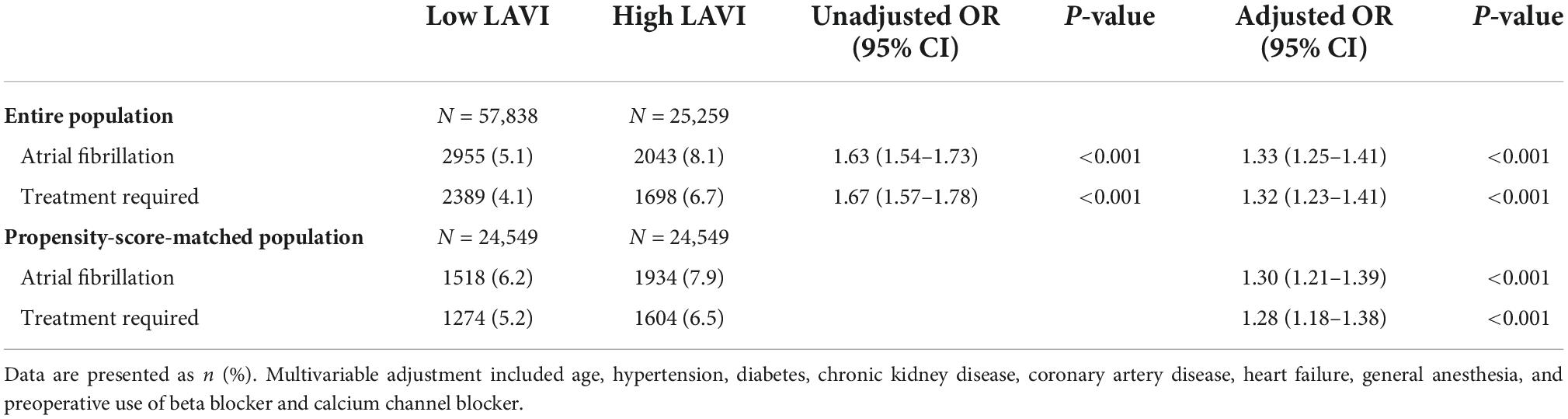
The overall incidence of POAF was 6.0% (4,998/83,097) and 4.9% (4,087/83,097) needed interventional treatment. Subjects in the high LAVI group showed increased incidence of POAF (5.1 vs. 8.1%; OR, 1.63; 95% CI, 1.54–1.73; p < 0.001; Table 3). After adjustment, the high LAVI group consistently showed an increased incidence of POAF (OR, 1.33; 95% CI, 1.25–1.41; p < 0.001). The risk of POAF that required interventional treatment was also increased in the high LAVI group (4.1 vs. 6.7%; OR, 1.32; 95% CI, 1.23–1.41; p < 0.001; Table 3).
After propensity score matching, 24,549 pairs of patients with well-balanced variables were generated. The high LAVI group was consistently associated with increased incidence of POAF (6.2 vs. 7.9%; OR, 1.30; 95% CI, 1.21–1.39; p < 0.001 for overall POAF and 5.2 vs. 6.5%; OR, 1.28; 95% CI, 1.18–1.38; p < 0.001 for treatment required; Table 3). The observed association between LAVI and POAF remained significant under any circumstance regardless of unmeasured confounding factors (Supplementary Table 1).
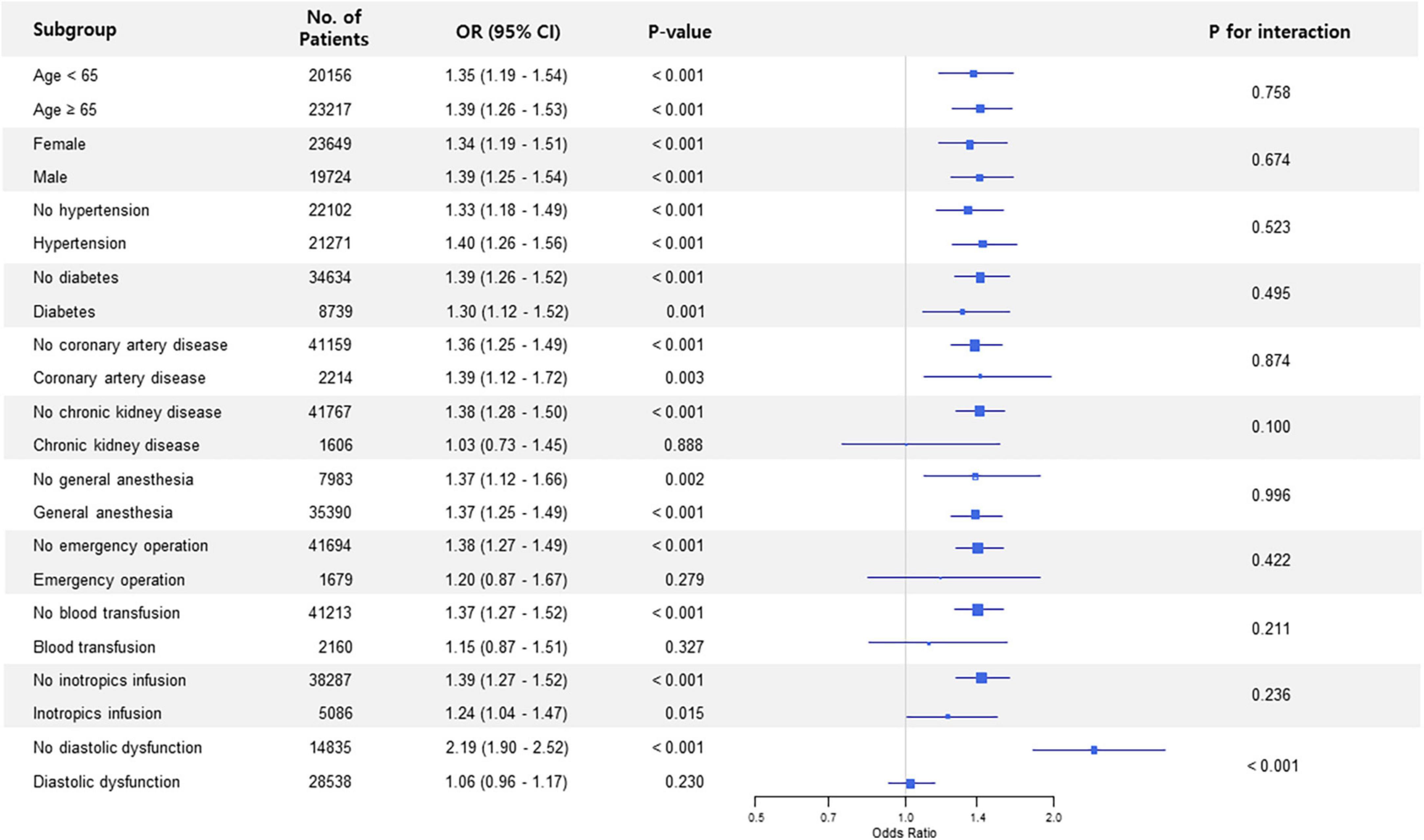
Subgroup analysis in non-thoracic surgery showed that the association between preoperative LAVI and POAF significantly interacted with diastolic dysfunction (p for interaction < 0.001) (Figure 1). The observed association was valid in patients without diastolic dysfunction but was non-significant in subjects who had diastolic dysfunction. This trend in diastolic dysfunction was also retained in subgroup analysis for thoracic surgery (p for interaction = 0.023) (Figure 2).
In the ROC analysis, the estimated threshold of LAVI associated with POAF in entire cohort was 36.4 mL/min2 with an area under the curve (AUC) of 0.571. Using this threshold, the sensitivity and specificity were 33.9 and 77.6%, respectively (Figure 3). We also evaluated the predictive performance of LAVI in different subgroups. In patients with heart failure, the ROC analysis showed that AUC value was 0.611 for HFpEF (cut-off 46.3 mL/min2, sensitivity 59.3%, specificity 63.0%) and 0.606 for HFrEF (cut-off 36.9 mL/min2, sensitivity 100%, specificity 37.8%) (Supplementary Figure 1). The AUC value in non-thoracic and thoracic surgery was 0.590 (cut-off 37.3 mL/min2, sensitivity 34.7%, specificity 79.4%) and 0.543 (cut-off 36.6 mL/min2, sensitivity 24.2%, specificity 83.4%), respectively (Supplementary Figure 2). The AUC calculated for LAVI in non-thoracic surgery was significantly higher than that in thoracic surgery (P < 0.001).
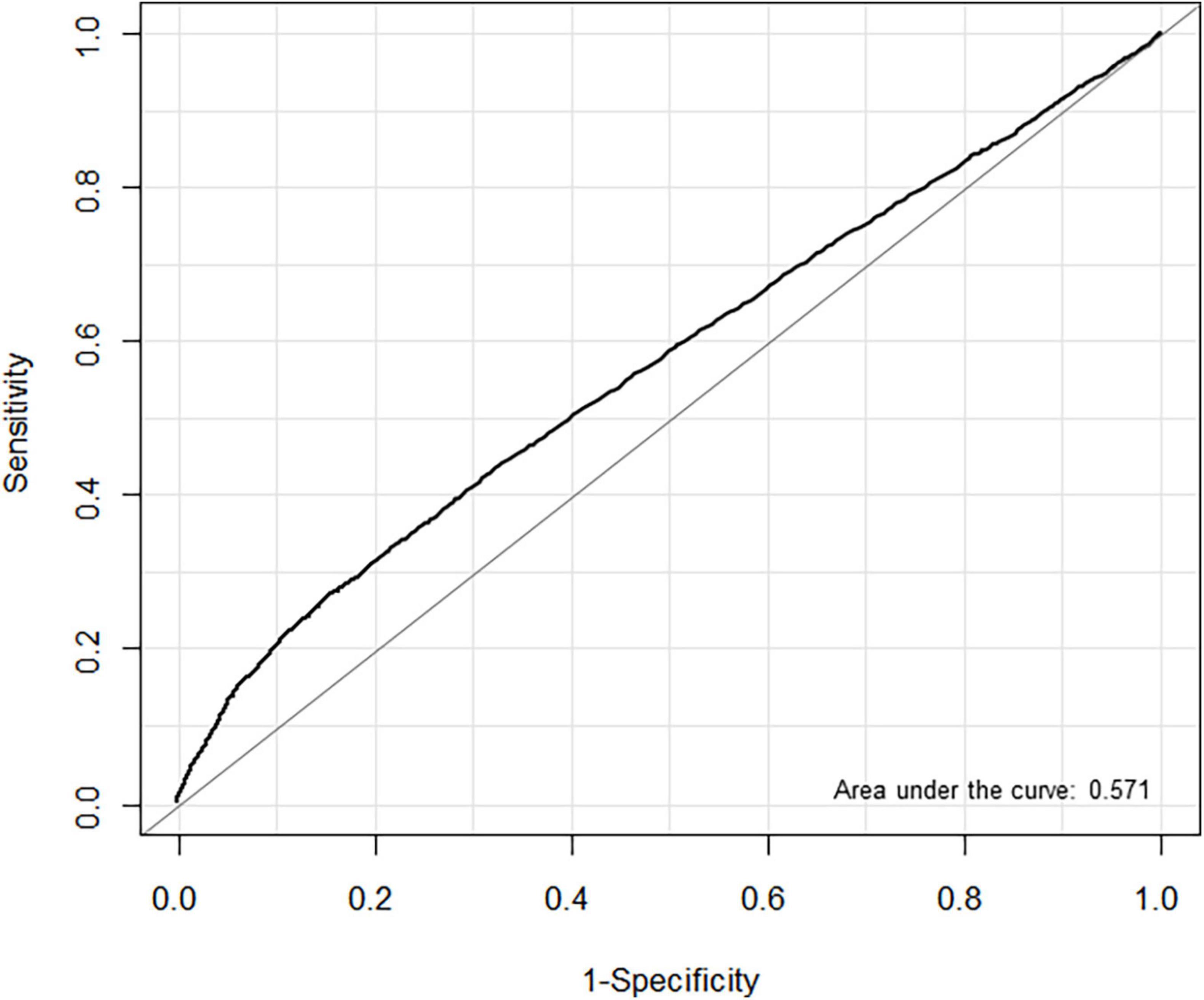
Figure 3. Receiver operating characteristic (ROC) curves for left atrial volume index (LAVI) associated with postoperative atrial fibrillation (POAF) in non-cardiac surgery.
Discussion
In the present study, high preoperative LAVI showed association with POAF in patients undergoing non-cardiac surgery. The overall incidence of POAF was 6.0% and 4.9% needed interventional treatment. Subgroup analysis based on perioperative variables showed this association was valid only in patients without diastolic dysfunction in non-thoracic and thoracic surgery.
Left atrial volume index is a reliable and readily measurable indicator for the burden of cardiovascular disease. In cardiac surgery, LAVI was shown the strongest predictor of POAF (25). Although an exact mechanism how left atrial enlargement affects development of atrial fibrillation remains unclear, a possible explanation may be associated with atrial stretch which activates atrial fibrosis and increases the atrial effective refractory period leading to atrial arrhythmia (26, 27). In non-cardiac surgery, the association between LAVI and POAF has been evaluated in several studies; however, those studies were limited by surgery type and showed conflicting results (28, 29). Furthermore, most of the previous studies predate recently accepted upper normal value of LAVI (34 mL/m2) and analysis performed based on a significantly lower value (11). In the present study, LAVI was assessed with a revised reference value and preoperatively increased LAVI was associated with POAF in a broad non-cardiac surgical population.
Preexisting conditions such as older age, male sex, and history of cardiovascular disease have been previously reported association with POAF (30, 31). However, these are rather general risk factors for cardiac complications and are difficult to apply in a clinical practice to predict POAF. Clinically, LAVI is a value that can be measured objectively and reflects pathophysiologic change directly associated with atrial fibrillation. Furthermore, in the present study, LAVI showed association with the development of POAF that requires an intervention. POAF that requires urgent treatment is a commonly found clinical situation for patients with significant heart disease. Clinically, the results of this study indicate the possibility that LAVI can be used as a relatively more specific marker for POAF prediction compared with other general cardiac risk factors.
The estimated LAVI threshold associated with POAF was 36.4 mL/min2, close to the revised reference limit of LAVI (11). However, the overall predictive accuracy was low (AUC = 0.571) compared with the results from cardiac surgeries (32, 33). Also, the predictive performance of LAVI in HFpEF (AUC = 0.611) and HFrEF (AUC = 0.606) were similar, although HFpEF and HFrEF represent two distinct disease entities in the heart failure spectrum (34, 35). These results are possibly because development of POAF involves multifactorial mechanisms in non-cardiac surgeries and indicate the limitation of predicting POAF solely by preoperative echocardiographic findings.
To evaluate the usefulness of single value of LAVI in non-cardiac surgery, we divided the surgery type into non-thoracic and thoracic surgery and investigated the cut-off value of LAVI associated with POAF. The non-thoracic group showed a larger cut-off value with significantly higher AUC than the thoracic group. This discrepancy may be attributed to the fact that the two types of surgeries have different incidence and risk factors for POAF (36, 37). Therefore, LAVI may have a different threshold and predictive accuracy in each subgroup. Nevertheless, the optional cut-off value of LAVI for predicting POAF in non-cardiac surgery has yet to be evaluated well, so further multicenter and larger cohort studies are required to verify our results.
In addition, subgroup analysis was conducted in non-thoracic and thoracic surgery based on perioperative factors. The association between LAVI and POAF was significantly affected by diastolic dysfunction, and the association was not observed in patients with diastolic dysfunction in both types of surgeries. Diastolic dysfunction is associated with an increasing stretch in pulmonary veins due to increased left atrial pressure (38), which elevates the arrhythmogenic activity of the pulmonary veins (39). These effects may have outweighed the effect of preoperative left atrial volume, so the observed association might have been significant only in patients without diastolic dysfunction. The subgroup analysis results need further verification in prospectively collected data.
Left atrial volume index is not the only echocardiographic parameter associated with diastolic dysfunction and higher incidence of POAF (40). Therefore, other parameters were compared between the two groups, and the indicators of diastolic dysfunction such as E/e’ ratio and deceleration were significantly higher in the high LAVI group. In previous studies, LAVI was also reportedly not an independent predictor of POAF in patients with well-preserved left ventricular ejection fraction (29–41). In the present study, the association between LAVI and POAF was significant although the ejection fraction tended to be preserved in both groups. Therefore, other echocardiographic parameters also need further investigation regarding the development of POAF in non-cardiac surgery.
The present study had several limitations that should be considered when interpreting the results. First, because this was a retrospective study, unmeasured confounding factors may have affected the results despite rigorous statistical adjustment using propensity score matching. Despite our effort to exclude patients with preoperative atrial fibrillation, the patients with paroxysmal atrial fibrillation not specified in medical records may have been included in this study. Second, only patients who had echocardiography before surgery were included. Therefore, the study cohort may have had more cardiovascular disease which may have caused selection bias and overestimation of the incidence of POAF in non-cardiac surgery. Third, echocardiography was not performed after the surgery; thus, there is no information regarding postoperative LAVI, which may have contributed to the development of POAF. Fourth, we only observed POAF that occurred during a short period of hospitalization after surgery. However, surgical stress can persist even after patients are discharged and induce POAF for a long-term period. This short-term follow-up may have underestimated the risk of POAF. Despite these limitations, an association was found between preoperative LAVI and POAF using large real-world data. Preoperative echocardiographic measures could be used to identify patients who are at a high risk for developing POAF and may provide useful prognostic information for individual patients. Also, for these high-risk populations, proven perioperative interventions aimed at reducing POAF might be very cost-effective in non-cardiac surgery.
Conclusion
The present study results showed the increased preoperative LAVI on echocardiography was associated with the development of POAF in non-cardiac surgery. Preoperative LAVI may be helpful in predicting POAF; however, further verification is needed.
Data availability statement
The data we used for this study was curated using Clinical Data Warehouse (CDW) which psuedonomynize the data from our institutional electronic medical records. So, our data is de-identified by eliminating all identifiable variables such as name, social security number, hospital number, and etc. However, it is illegal to open this data to the public without restriction. Regarding the availability of our data, please contact jong-hwan.park@samsung.com, the head of our institutional data security department.
Author contributions
JP and SL: study conception and design. DC, KY, JA, and BC: data acquisition and analysis. AO and SL: writing of the manuscript. J-HL, J-HC, and JS: study supervision and data interpretation. All authors approved the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.1008718/full#supplementary-material
Supplementary Figure 1 | Receiver operating characteristic (ROC) curves for left atrial volume index (LAVI) associated with postoperative atrial fibrillation (POAF) in (A) HFpEF and (B) HFrEF subgroups.
Supplementary Figure 2 | Receiver operating characteristic (ROC) curves for left atrial volume index (LAVI) associated with postoperative atrial fibrillation (POAF) in (A) non-thoracic and (B) thoracic surgery.
Footnotes
References
1. Siddiqui NF, Coca SG, Devereaux PJ, Jain AK, Li L, Luo J, et al. Secular trends in acute dialysis after elective major surgery–1995 to 2009. CMAJ. (2012) 184:1237–45. doi: 10.1503/cmaj.110895
2. Bartels K, Karhausen J, Clambey ET, Grenz A, Eltzschig HK. Perioperative organ injury. Anesthesiology. (2013) 119:1474–89. doi: 10.1097/ALN.0000000000000022
3. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. (2016) 37:2893–962. doi: 10.1093/eurheartj/ehw210
4. Hravnak M, Hoffman LA, Saul MI, Zullo TG, Whitman GR, Griffith BP. Predictors and impact of atrial fibrillation after isolated coronary artery bypass grafting. Crit Care Med. (2002) 30:330–7. doi: 10.1097/00003246-200202000-00011
5. Mariscalco G, Klersy C, Zanobini M, Banach M, Ferrarese S, Borsani P, et al. Atrial fibrillation after isolated coronary surgery affects late survival. Circulation. (2008) 118:1612–8. doi: 10.1161/CIRCULATIONAHA.108.777789
6. Njoku A, Kannabhiran M, Arora R, Reddy P, Gopinathannair R, Lakkireddy D, et al. Left atrial volume predicts atrial fibrillation recurrence after radiofrequency ablation: a meta-analysis. Europace. (2018) 20:33–42. doi: 10.1093/europace/eux013
7. Toufan M, Kazemi B, Molazadeh N. The significance of the left atrial volume index in prediction of atrial fibrillation recurrence after electrical cardioversion. J Cardiovasc Thorac Res. (2017) 9:54–9. doi: 10.15171/jcvtr.2017.08
8. Vaziri SM, Larson MG, Benjamin EJ, Levy D. Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham heart study. Circulation. (1994) 89:724–30. doi: 10.1161/01.cir.89.2.724
9. Naito Y, Yamazaki K. Preoperative left atrial volume index predicts postoperative atrial fibrillation in patients with severe aortic valve stenosis. J Anesth. (2013) 27:699–704. doi: 10.1007/s00540-013-1594-8
10. Mahmood E, Khabbaz KR, Bose R, Mitchell J, Zhang Q, Chaudhary O, et al. Immediate preoperative transthoracic echocardiography for the prediction of postoperative atrial fibrillation in high-risk cardiac surgery. J Cardiothorac Vasc Anesth. (2020) 34:719–25. doi: 10.1053/j.jvca.2019.09.026
11. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. (2015) 16:233–70. doi: 10.1093/ehjci/jev014
12. Sundararajan V, Henderson T, Perry C, Muggivan A, Quan H, Ghali WA. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol. (2004) 57:1288–94. doi: 10.1016/j.jclinepi.2004.03.012
13. Kristensen SD, Knuuti J. New ESC/ESA guidelines on non-cardiac surgery: cardiovascular assessment and management. Eur Heart J. (2014) 35:2344–5. doi: 10.1093/eurheartj/ehu285
14. McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. (1971) 285:1441–6. doi: 10.1056/NEJM197112232852601
15. King M, Kingery J, Casey B. Diagnosis and evaluation of heart failure. Am Fam Physician. (2012) 85:1161–8.
16. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American college of cardiology foundation/American heart association task force on practice guidelines. J Am Coll Cardiol. (2013) 62:e147–239. doi: 10.1016/j.jacc.2013.05.019
17. Reeves ST, Finley AC, Skubas NJ, Swaminathan M, Whitley WS, Glas KE, et al. Basic perioperative transesophageal echocardiography examination: a consensus statement of the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr. (2013) 26:443–56. doi: 10.1016/j.echo.2013.02.015
18. Mitchell C, Rahko PS, Blauwet LA, Canaday B, Finstuen JA, Foster MC, et al. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: recommendations from the American society of echocardiography. J Am Soc Echocardiogr. (2019) 32:1–64. doi: 10.1016/j.echo.2018.06.004
19. Basnight MA, Gonzalez MS, Kershenovich SC, Appleton CP. Pulmonary venous flow velocity: relation to hemodynamics, mitral flow velocity and left atrial volume, and ejection fraction. J Am Soc Echocardiogr. (1991) 4:547–58. doi: 10.1016/s0894-7317(14)80213-7
20. Wang Y, Gutman JM, Heilbron D, Wahr D, Schiller NB. Atrial volume in a normal adult population by two-dimensional echocardiography. Chest. (1984) 86:595–601. doi: 10.1378/chest.86.4.595
21. Simek CL, Feldman MD, Haber HL, Wu CC, Jayaweera AR, Kaul S. Relationship between left ventricular wall thickness and left atrial size: comparison with other measures of diastolic function. J Am Soc Echocardiogr. (1995) 8:37–47. doi: 10.1016/s0894-7317(05)80356-6
22. Hyun J, Cho MS, Nam GB, Kim M, Do U, Kim J, et al. Natural course of new-onset postoperative atrial fibrillation after noncardiac surgery. J Am Heart Assoc. (2021) 10:e018548. doi: 10.1161/JAHA.120.018548
23. Latouche A, Porcher R, Chevret S. Sample size formula for proportional hazards modelling of competing risks. Stat Med. (2004) 23:3263–74. doi: 10.1002/sim.1915
24. Groenwold RH, Nelson DB, Nichol KL, Hoes AW, Hak E. Sensitivity analyses to estimate the potential impact of unmeasured confounding in causal research. Int J Epidemiol. (2010) 39:107–17. doi: 10.1093/ije/dyp332
25. Osranek M, Fatema K, Qaddoura F, Al-Saileek A, Barnes ME, Bailey KR, et al. Left atrial volume predicts the risk of atrial fibrillation after cardiac surgery: a prospective study. J Am Coll Cardiol. (2006) 48:779–86. doi: 10.1016/j.jacc.2006.03.054
26. Boixel C, Fontaine V, Rucker-Martin C, Milliez P, Louedec L, Michel JB, et al. Fibrosis of the left atria during progression of heart failure is associated with increased matrix metalloproteinases in the rat. J Am Coll Cardiol. (2003) 42:336–44. doi: 10.1016/s0735-1097(03)00578-3
27. Satoh T, Zipes DP. Unequal atrial stretch in dogs increases dispersion of refractoriness conducive to developing atrial fibrillation. J Cardiovasc Electrophysiol. (1996) 7:833–42. doi: 10.1111/j.1540-8167.1996.tb00596.x
28. Brecher O, Gulati H, Roistacher N, Zhang H, Shi W, Thaler HT, et al. Preoperative echocardiographic indices of diastolic dysfunction and brain natriuretic peptide in predicting postoperative atrial fibrillation after noncardiac surgery. Anesth Analg. (2017) 124:1099–104. doi: 10.1213/ANE.0000000000001471
29. Ai D, Lasala J, Mehran JR, Xu G, Banchs J, Cata JP. Preoperative echocardiographic parameters of diastolic dysfunction did not provide a predictive value for postoperative atrial fibrillation in lung and esophageal cancer surgery. J Cardiothorac Vasc Anesth. (2015) 29:1127–30. doi: 10.1053/j.jvca.2015.01.012
30. Bhave PD, Goldman LE, Vittinghoff E, Maselli J, Auerbach A. Incidence, predictors, and outcomes associated with postoperative atrial fibrillation after major noncardiac surgery. Am Heart J. (2012) 164:918–24. doi: 10.1016/j.ahj.2012.09.004
31. Bessissow A, Khan J, Devereaux PJ, Alvarez-Garcia J, Alonso-Coello P. Postoperative atrial fibrillation in non-cardiac and cardiac surgery: an overview. J Thromb Haemost. (2015) 13:S304–12. doi: 10.1111/jth.12974
32. Tayyareci Y, Yildirimturk O, Aytekin V, Memic K, Behramoglu F, Demiroglu IC, et al. Preoperative left atrial mechanical dysfunction predicts postoperative atrial fibrillation after coronary artery bypass graft operation - a velocity vector imaging-based study. Circ J. (2010) 74:2109–17. doi: 10.1253/circj.cj-10-0197
33. Madhu Reddy Y, Satpathy R, Shen X, Holmberg M, Hunter C, Mooss A, et al. Left atrial volume and post-operative atrial fibrillation after aortic valve replacement. J Atr Fibrillation. (2010) 3:338. doi: 10.4022/jafib.338
34. Borlaug BA, Redfield MM. Diastolic and systolic heart failure are distinct phenotypes within the heart failure spectrum. Circulation. (2011) 123:2006–14. doi: 10.1161/CIRCULATIONAHA.110.954388
35. Ho JE, Lyass A, Lee DS, Vasan RS, Kannel WB, Larson MG, et al. Predictors of new-onset heart failure: differences in preserved versus reduced ejection fraction. Circ Heart Fail. (2013) 6:279–86. doi: 10.1161/CIRCHEARTFAILURE.112.972828
36. Joshi KK, Tiru M, Chin T, Fox MT, Stefan MS. Postoperative atrial fibrillation in patients undergoing non-cardiac non-thoracic surgery: a practical approach for the hospitalist. Hosp Pract. (2015) 43:235–44. doi: 10.1080/21548331.2015.1096181
37. Smith H, Yeung C, Gowing S, Sadek M, Maziak D, Gilbert S, et al. A review and analysis of strategies for prediction, prevention and management of post-operative atrial fibrillation after non-cardiac thoracic surgery. J Thorac Dis. (2018) 10:S3799–808. doi: 10.21037/jtd.2018.09.144
38. Kalifa J, Jalife J, Zaitsev AV, Bagwe S, Warren M, Moreno J, et al. Intra-atrial pressure increases rate and organization of waves emanating from the superior pulmonary veins during atrial fibrillation. Circulation. (2003) 108:668–71. doi: 10.1161/01.CIR.0000086979.39843.7B
39. Melduni RM, Suri RM, Seward JB, Bailey KR, Ammash NM, Oh JK, et al. Diastolic dysfunction in patients undergoing cardiac surgery: a pathophysiological mechanism underlying the initiation of new-onset post-operative atrial fibrillation. J Am Coll Cardiol. (2011) 58:953–61. doi: 10.1016/j.jacc.2011.05.021
40. Nagarakanti R, Ezekowitz M. Diastolic dysfunction and atrial fibrillation. J Interv Card Electrophysiol. (2008) 22:111–8. doi: 10.1007/s10840-008-9203-8
41. Hu J, Peng L, Qian H, Li YJ, Meng W, Xiao ZH, et al. Transoesophageal echocardiography for prediction of postoperative atrial fibrillation after isolated aortic valve replacement: two-dimensional speckle tracking for intraoperative assessment of left ventricular longitudinal strain. Eur J Cardiothorac Surg. (2015) 47:833–9. doi: 10.1093/ejcts/ezu234
Keywords: non-cardiac surgery, atrial fibrillation, left atrial volume index, echocardiography, postoperative cardiac complications
Citation: Oh AR, Lee SH, Park J, Lee J-H, Cha D, Yang K, Choi J-H, Ahn J, Sung JD, Choi B and Lee S-H (2022) Preoperative left atrial volume index may be associated with postoperative atrial fibrillation in non-cardiac surgery. Front. Cardiovasc. Med. 9:1008718. doi: 10.3389/fcvm.2022.1008718
Received: 22 August 2022; Accepted: 17 October 2022;
Published: 03 November 2022.
Edited by:
Sandro Gelsomino, Maastricht University, NetherlandsReviewed by:
Sula Mazimba, University of Virginia, United StatesYousef Rezaei, Iran University of Medical Sciences, Iran
Copyright © 2022 Oh, Lee, Park, Lee, Cha, Yang, Choi, Ahn, Sung, Choi and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jungchan Park, jc83.park@samsung.com; Seung-Hwa Lee, shua9999@gmail.com
†These authors have contributed equally to this work
 Ah Ran Oh1,2†
Ah Ran Oh1,2†