- 1Medical Oncology Department, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy
- 2Department of Oncology and Hemato-Oncology, University of Milan, Milan, Italy
- 3First Pathology Division, Department of Pathology and Laboratory Medicine, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy
- 4Institute for Maternal and Child Health, IRCCS Burlo Garofalo, Trieste, Italy
Neuroendocrine tumors (NETs) are classified based on morphology and are graded based on their proliferation rate as either well-differentiated low-grade (G1) to intermediate (G2–G3) or poorly differentiated high-grade neuroendocrine carcinomas (NEC G3). Recently, in gastroenteropancreatic (GEP) NETs, a new subgroup of well-differentiated high-grade tumors (NET G3) has been divided from NEC by WHO due to its different clinical–pathologic features. Although several mutational analyses have been performed, a molecular classification of NET is an unmet need in particular for G3, which tends to be more aggressive and have less benefit to the available therapies. Specifically, new possible prognostic and, above all, predictive factors are highly awaited, giving the basis for new treatments. Alteration of KRAS, TP53, and RB1 is mainly reported, but also druggable alterations, including BRAF and high microsatellite instability (MSI-H), have been documented in subsets of patients. In addition, PD-L1 demonstrated to be highly expressed in G3 NETs, probably becoming a new biomarker for G3 neuroendocrine neoplasm (NEN) discrimination and a predictive one for immunotherapy response. In this review, we describe the current knowledge available on a high-grade NET molecular landscape with a specific focus on those harboring potentially therapeutic targets in the advanced setting.
Introduction
Neuroendocrine neoplasms (NENs) are a heterogeneous group of rare malignant cancers that arise from diffuse neuroendocrine cells. In recent years, the incidence and prevalence of NENs have steadily risen, with a 6.4-fold increase in age-adjusted incidence rate from 1.09 cases per 100,000 in 1973 to 6.98 per 100,000 in 2012 in the United States (1). About 62%–67% of all NEN cases are of gastroenteropancreatic (GEP) origin, 22%–27% of cases have a thoracic origin (lung and thymus NEN), and 10% of the primary tumor remains unknown (2–4). According to the 2019 WHO classification, GEP NENs are classified into well-differentiated neuroendocrine tumors (NETs) and poorly differentiated neuroendocrine carcinomas (NECs) based on both morphological features and proliferation rate (Ki-67 and/or mitotic index) (5). Recently, the NET category G3 was distinguished from the others. It is characterized by well-differentiated neoplasms but with a Ki-67 proliferative index >20%, which is typical of NECs. The need to recognize this new subgroup arose from the observation of a more favorable clinical trend and a different response to medical therapies of this subgroup of patients compared with patients with poorly differentiated tumors. Specifically, as we recently demonstrated, well-differentiated morphology constitutes an independent prognostic factor for GEP NEN with Ki-67 of between 20% and 55% (NET G3 and NEC with Ki-67 20%–55%), while the 55% cutoff of Ki-67 is an independent prognostic factor for poorly differentiated GEP NENs (6). Ki-67 of the neuroendocrine component appears to be the main prognostic factor also for mixed neuroendocrine non-NENs (MiNEN), and lung large cell NECs (LCNECs) (7, 8). Different from NETs, GEP NECs encompass poorly differentiated G3 neoplasms with Ki-67 proliferation index >20% and/or mitotic index >20 per 10 high-power fields (5). They are characterized by a proliferation of tumor cells with irregular nuclei and high mitotic features, with limited immunohistochemical staining for neuroendocrine markers, often displaying faint or focal staining for chromogranin A and diffuse synaptophysin expression (9). Of note, up to 40% of NECs may contain elements of non-neuroendocrine histology (9, 10). While well-differentiated NETs tend to have a relatively indolent behavior, with an excellent prognosis for NETs G1 (Ki-67 < 3%) and good to intermediate for NETs G2 (Ki-67 3–20%), NETs G3 and NECs display an aggressive disease course leading to poor survival outcomes with median overall survival (OS) ranging from 7.5 to 15 months (6, 11). NENs of the lung, on the other hand, according to the latest WHO classification of thoracic tumors (5th edition 2021), remain classified into four histological variants according to necrosis amount and mitotic count: typical and atypical carcinoid, LCNEC, and small cell lung cancer (SCLC) (12). According to the unifying nomenclature proposed by the International Agency for Research on Cancer (IARC) and the WHO Classification of Tumours Group, carcinoids are NETs with low mitoses number and absent or focal necrosis, contrary to LCNECs and SCLCs, which are NECs with extensive necrosis and high mitosis number. Therefore, high-grade NENs of the lung and thymus include SCLC and LCNECs by definition (12). Although several next-generation sequencing (NGS) analyses have been performed, one of the main unmet needs is the lack of a molecular classification of NETs, in particular for high-grade tumors, which tend to be more aggressive and have less benefit from the scantily available therapies. Chemotherapy with platinum compounds plus etoposide still represents the gold standard of first-line treatment, whereas the use of other chemotherapeutic agents [such as irinotecan, fluoropyrimidines, and temozolomide (TMZ)] in further lines of treatment is mostly supported by non-randomized or retrospective evidences (13). Nevertheless, recent progress in tumor genomic profiling has shed some light on the complex molecular scenario of high-grade NETs, identifying a wide range of genomic alterations (mutations, translocations, or amplifications) that could play both a prognostic role, conferring a much aggressive behavior to the tumor, and a predictive one, identifying tumors that may be suitable to biologic agents, allowing a deeper treatment personalization. In this review, we will describe all the available data on the landscape of molecular alteration in NENs with high-grade features (NETs G3 and NECs) particularly focusing on their future clinical and therapeutic role.
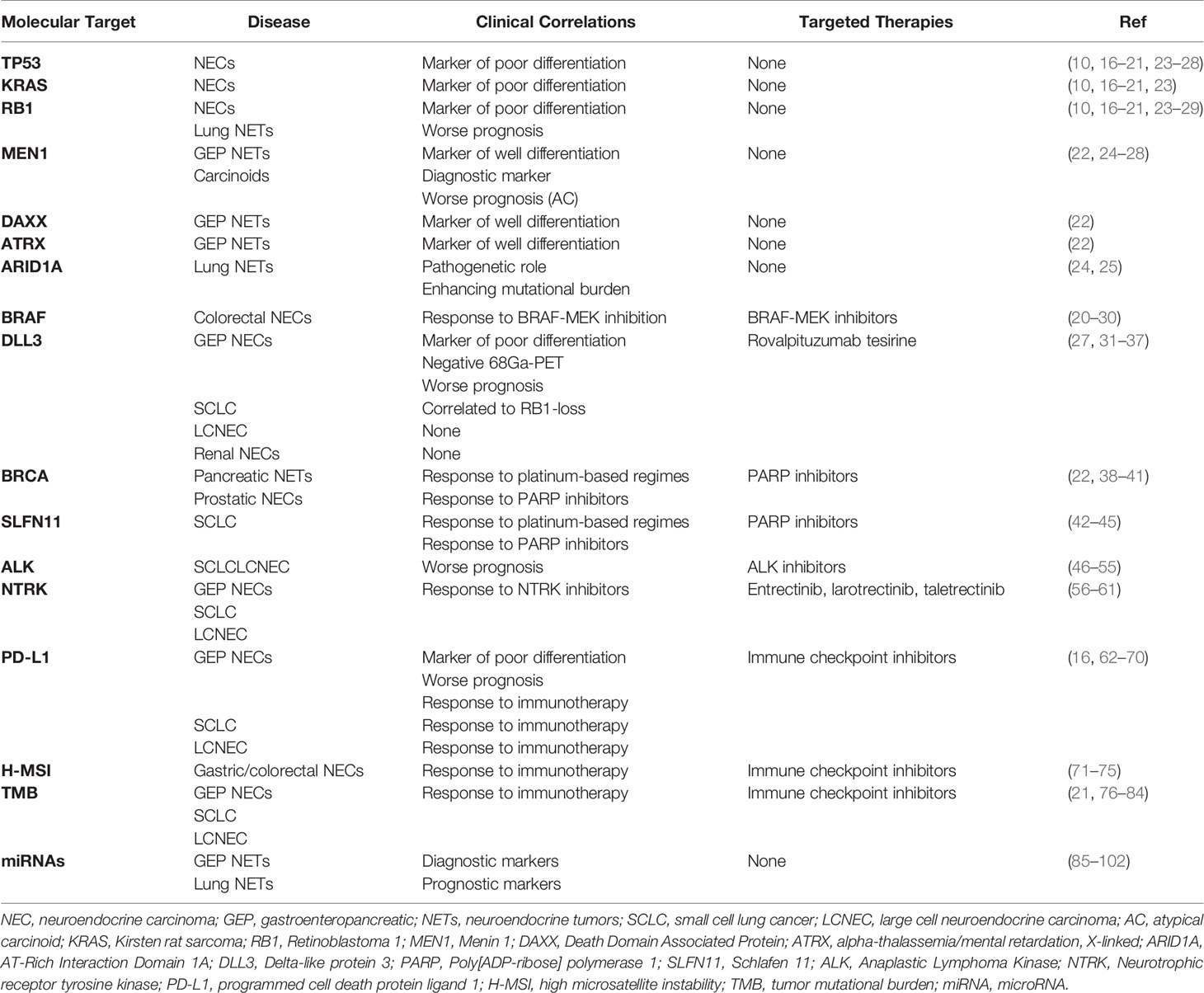
Genomic Alterations
Personalized oncology, defined as the use of molecular profiling to drive treatment strategies for a single patient, is currently a reality in many cancers. In the last decades, the discovery of several oncogenic drive mutations in different malignancies, i.e., Epidermal Growth Factor Receptor, and BRAF mutations, led to the development of a huge number of targeted drugs with a totally different mechanism of action compared with chemotherapy, which is still, however, commonly used. As far as NENs are concerned, excluding well-established hereditary genetic syndromes caused by germline mutations and commonly associated with well-differentiated NETs, only a few data exist on tissue somatic gene alterations as markers of prognosis or predictive of treatment benefit in high-grade NETs. However, NGS data are expected to emerge rapidly in this field. In the first reports, all the genomic abnormalities observed seemed to be similar to those of the corresponding exocrine neoplasm of the same site (14, 15). Nonetheless, additional mutations specifically related to NETs were also described. Several studies showed that TP53, Kirsten rat sarcoma (KRAS), and Retinoblastoma 1 (RB1) mutations were highly represented in NECs and represent markers of poor differentiation (16–21). On the contrary, several gene mutations may characterize well-differentiated NETs, as observed with Menin 1 (MEN1), Death Domain Associated Protein (DAXX), and alpha-thalassemia/mental retardation, X-linked (ATRX) mutations in well-differentiated pancreatic NETs (22). Based on this, along with morphological differentiation and proliferation rate, NETs and NECs can be classified and differentiated according to their molecular profile (10). In GEP NETs, the presence of TP53, KRAS, and RB1 mutations may also help in differentiating pancreatic NECs from NETs G3 and in predicting the response to platinum-based chemotherapy in the first ones (23). Molecular classification can be also hypothesized in lung NENs according to their genomic alterations (24). Mutations in TP53 and RB1 are present in all classes of lungs NENs (typical and atypical carcinoids, SCLCs, and LCNECs) but significantly enriched in NECs (24). Specifically, when mutations and copy number changes were combined, MEN1 alterations were almost exclusive to carcinoids, whereas alterations of the TP53 and RB1 cell cycle regulation genes and Phosphatidylinositol-4,5-Bisphosphate 3-Kinase (PI3K)/AKT/Mechanistic Target of Rapamycin Kinase (mTOR) pathway genes were significantly enriched in carcinomas (25). Recently, Simbolo et al., based on transcriptomic and genomic data, separated atypical carcinoids and LCNECs into three different and clinically relevant molecular diseases (26). Furthermore, in LCNECs, two mutually exclusive genomic subtypes have been identified: one profile shows concurrent TP53 and RB1 mutations similarly to SCLC, whereas the other subtype is predominantly RB1 wild-type and displays concurrent biallelic TP53 and Serine/Threonine Kinase 11 (STK11)/Kelch Like ECH Associated Protein 1 (KEAP1) alterations, similarly to non-SCLC instead (27, 28). Besides a potentially new molecular classification, deep sequencing would be helpful also to predict patient outcomes. Indeed, RB1 mutation and Telomerase Reverse Transcriptase (TERT) gain are shown to be independent unfavorable prognostic markers in all lung NENs, MEN1 mutation was associated with poor prognosis in atypical carcinoids, and Histone-Lysine N-Methyltransferase 2D mutation was associated with longer survival in SCLCs (25, 26). Likewise, to those genes described before, chromatin-modifying genes, in particular, AT-Rich Interaction Domain 1A (ARID1A), could also play a major role in atypical carcinoids and LCNECs (24, 25).
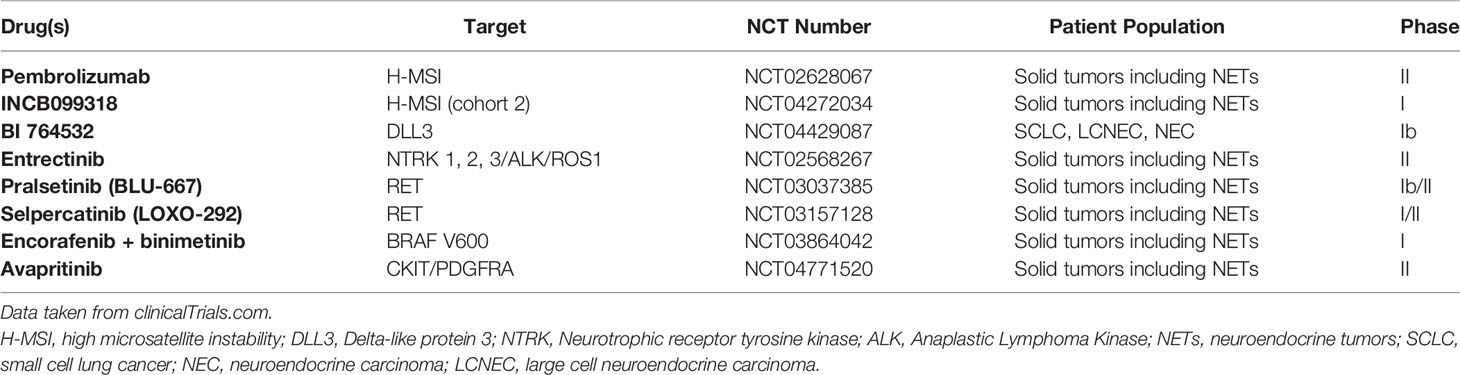
In addition to those previously described, mutations of other genes have been also described in NECs (Table 1) (16, 29). In a recent NGS dataset analysis, Chen et al. found that about 20.8% of patients with colorectal NECs harbored BRAF V600E mutation (20). This may represent a potential target for tyrosine kinase inhibitors (TKIs), such as dabrafenib and trametinib, as it happens in colorectal and lung cancers (29, 30, 103). Another novel potential therapeutic target is Delta-like protein 3 (DLL3) (31), an inhibitory ligand of the Notch receptor pathway, which is highly expressed in lung NECs (about 80% of SCLCs and 65% of LCNECs) (32, 33), GEP NECs (34), and renal NECs (35). In a recent retrospective analysis, Liverani et al. demonstrated that Dll3 expression assessed via immunohistochemistry (IHC) was present in GEP NEC and absent in GEP NET G3, representing a valuable histological marker, for the diagnosis of NECs. In addition, Dll3 expression was also correlated with RB1-loss (p < 0.001), negative 68Ga-PET/CT scan (p = 0.001), and a worse OS (34). A correlation between Dll3 expression and RB1-loss was also observed in SCLC but not in LCNEC (27, 31). Dll3 has been recently studied as a potential target for a novel antibody-drug conjugate called rovalpituzumab tesirine. Despite early-phase trials showing encouraging single-agent antitumor activity, rovalpituzumab tesirine failed, unfortunately, to demonstrate OS superiority in SCLC over placebo as maintenance after platinum-based therapy (36) and over topotecan in second-line setting (37) in phase III trials. Nonetheless, there were several trials investigating the role of novel Dll3 inhibitors in SCLCs, LCNECs, and NECs (Table 2).
Furthermore, the role of the homologous recombination repair of the double-stranded DNA pathway in the pathogenesis of NENs has been also recently suggested (42). Recent studies have shown, indeed, that pancreatic NENs can be associated with germline pathogenic variants in genes involved in DNA damage repair, such as MutY DNA Glycosylase, Checkpoint Kinase 2, and above all BRCA (22, 38, 39). Two case reports described patients with prostate NEC, a highly aggressive histologic subtype of prostate cancer, one with germline and the second with somatic BRCA mutation, confirming platinum and Poly[ADP-ribose] polymerase 1 (PARP) inhibitor sensibility similar to that of malignancies that frequently present this type of alteration (40, 41). Interestingly, in one of these cases, a novel reversion mutation that restores Brca 1/2 function was described, which might be the reason for primary resistance to PAPR inhibitors (41). In addition, the role of Schlafen (SLFN) 11 was also recently explored in SCLC. Besides its known antiviral properties, several preclinical and clinical studies have been shown its ability to sensitize cancer cells to DNA damaging agents such as chemotherapy and PARP inhibitors (42–45). In the MA 11.07 trial, 100 SCLC patients with 1–2 prior lines of therapy were treated with TMZ with either veliparib or placebo. Although the primary endpoint was not met in this trial, patients receiving the combination of TMZ plus veliparib had an almost 3-fold higher response rate as compared with the temozolomide plus placebo arm (39% vs. 19%). Median OS was 8.2 months in the temozolomide plus veliparib arm and 7.0 months in the temozolomide plus placebo arm (p = 0.50). However, a significantly longer progression-free survival (PFS) and OS were observed in patients receiving TMZ/veliparib combination who had detectable Slfn11 by IHC (44).
Gene Rearrangements
The advances in the genomic profiling of solid tumors shed a light on the contribution of gene translocations, fusions, and amplifications in cancer initiation and progression. In addition to this, recently, gene rearrangements demonstrated also their potential role as prognostic and predictive markers or, most important, as therapeutic targets with the aim of personalizing the treatment algorithm (104). Nevertheless, the frequency of likely oncogenic recurrent gene fusions across the different cancer types is globally low, about 2%–3%, thus limiting the investigation on the singular genomic alterations (105, 106). In the setting of high-grade NENs, the deeper understanding of the molecular scenario recently provided interesting insights into their genomic landscape. With the limitation of the high clinical and molecular heterogeneity of NET G3/NEC, concerning gene fusions or amplifications, a few potential targets have been identified, with frequent tissue-specific features, and are under study (22, 107–110).
Anaplastic Lymphoma Kinase
Anaplastic lymphoma kinase (ALK) gene encodes for the Alk protein, which is a receptor tyrosine kinase belonging to the insulin receptor superfamily that activates a downstream signaling pathway involved in cell survival, proliferation, and oncogenesis. A gene rearrangement involving the fusion of ALK with another gene, generating a novel driver oncogene, was first identified in anaplastic large cell lymphoma and afterward in other tumors, i.e., lung cancer (in about 5% of cases), and it represents nowadays a key biomarker for targeted treatments, with a much improved clinical outcome (46, 111). In the setting of lung NENs, including typical and atypical carcinoids, SCLCs, and LCNECs, the occurrence of ALK fusions is extremely rare, with few cases reported (Table 1) (47–52). With the available literature data, the incidence of ALK fusions in high-grade lung NENs appeared lower than in NSCLC, <3% versus 3%–5%. In a dataset of 108 patients with lung NENs, ALK fusions were reported in 0.9% of cases (53). In these cases, no associations with a particular histological type were observed, and the main fusion partner was EMAP Like 4, as in NSCLC. Rarer partners have been reported, such as Kinesin Family Member 5B with no impact on the clinical and therapeutic outcomes (48, 54). Interestingly, most NENs with ALK translocation were characterized by high-grade and advanced stage with disseminated lesions, even to the brain, with features that closely correlate with a poor prognosis. Therefore, the rearrangement of ALK in lung NEC may represent a specific molecular subtype endowed with more aggressive behavior (47). The diagnostic assessment should include either fluorescence in situ hybridization (FISH), reverse transcription PCR, or NGS to confirm the evidence of Alk expression by IHC, especially in cases with focal or heterogeneous expression. In fact, in a high-sensitivity Alk immunostaining on 227 lung NEC tissue microarrays dataset, it was shown that focal positivity with heterogeneous intensity did not correlate with ALK rearrangement/amplification in FISH or somatic mutation. Therefore, the aberrant expression of Alk could represent a potential pitfall in the molecular diagnosis of lung NECs, and its relevance relies particularly on the potential therapeutic implication of targeted treatment with Alk inhibitors (55). Due to their practice-changing results on NSCLC, crizotinib, ceritinib, and alectinib were investigated also in lung NECs harboring ALK fusion, showing significant disease responses with manageable tolerability in several cases (about 7 partial responses on the 13 cases collected in a literature-based case series review) (47, 49, 51, 54). Nevertheless, the low level of evidence, due to the rarity of the disease and the low frequency of this alteration, limits the clinical implication of ALK rearrangement in lung NECs. The greatest burden of data on ALK fusions has been collected for lung NECs, given the relevant role in the therapeutic management of NSCLC patients, whereas for non-lung NECs, the evidence of ALK fusions/amplifications is scarce, with reports of complete lack of expression in pancreatic NETs (0/46 cases) (46).
Neurotrophic Receptor Tyrosine Kinase
The neurotrophic receptor tyrosine kinase (NTRK) is a tyrosine kinase receptor family including NTRK 1, 2, and 3, which encode the tropomyosin receptor kinase receptors, Trka, Trkb, and Trkc, respectively, involved in normal development, survival, and functionality of the nervous system. The Trk receptor, thanks to the binding with its ligand, homodimerizes and activates a downstream signaling cascade that modulates the activity of several key pathways including RAS/MAPK and mTOR/AKT. In solid tumors, NTRK translocations may occur, resulting in constitutively active protein fusions that display an oncogenic action (112). NTRK fusions are a rare finding in the most frequent tumors, although they are enriched in selected low-frequency cancers, such as secretory breast carcinoma, mammary analog secretory carcinoma, and congenital infantile fibrosarcoma, where NTRK fusion represents a defining diagnostic parameter (113, 114). In a large dataset of 2,417 NET patients, a total number of 6 cases (0.3%) of NTRK fusions were identified, including both intra- and inter-chromosomal translocations, and frequent or unique fusion partners, with no specific characteristics for organ of origin (lung, pancreas, uterus, and unknown primary) although with a peculiar selection for high-grade tumors as NECs or LCNECs (56). The relevance of NTRK fusions, aside from their low prevalence in solid tumors, is the potential therapeutic implication since NTRK rearrangements have emerged as a powerful actionable driver for targeted therapy. Recently, the selective inhibitors entrectinib and larotrectinib showed practice-changing results in the treatment of tumors with NTRK fusions, leading to the agnostic approval of the Food and Drug Administration (FDA) in advanced adult or pediatric tumors bearing this alteration (57–59). For NETs, the evidence collected on this topic is limited. In detail, a patient with metastatic well-differentiated NET, likely originating from the small intestine, bearing an ETS Variant Transcription Factor 6-NTRK3 fusion, was treated with entrectinib in the STARTRK2 trial with a rapid and meaningful tumor response preceded by initial tumor growth and necrosis (60). Moreover, 12 patients with NENs were treated with taletrectinib, a ROS1/NTRK inhibitor in a phase I study, reporting 1 partial response and 7 stable diseases according to Response Evaluation Criteria in Solid Tumors (RECIST) criteria, with a manageable toxicity profile (61). Although limited, these results appear promising for further investigations besides being impaired by the double rarity of the cases, that is, NETs that represent rare cancers and NTRK fusions that are a low-frequency molecular alteration (Table 1).
Human Epidermal Growth Factor Receptor 2
Human epidermal growth factor receptor 2 (HER2 or ERBB2) is a member of the epidermal growth factor receptor family, involved in the regulation of tumor cell proliferation, apoptosis, adhesion, migration, and differentiation (115). HER2 plays a central role in several tumors with evidence of amplification or overexpression in 7%–34% of all cancers, namely, breast, colon, bladder, ovarian, endometrial, lung, uterine cervix, head and neck, esophageal, and gastric cancers (116). It also represents a key target for the definition of the therapeutic algorithm in many cancer diseases, with numerous approved targeted agents that are able to provide a significant advantage on the clinical outcome (117, 118).
In NENs, the prognostic and predictive role of the amplification/overexpression of HER2 has not been defined due to its rarity. Most data have been provided in NECs of breast and gastric primitivity, in concordance with the non-NENs (Table 1). In particular, breast NEC is a rare subset of breast cancer, accounting for 2%–5% of cases, even though neuroendocrine differentiation is observed in up to 20% of breast tumors, and it belongs mainly to the luminal subtype, with a low rate of Her2 positivity (119). The real impact of the amplification/overexpression of HER2 on the prognosis of breast NENs is not clear, but an anti-Her2-targeted approach could be considered, even though solid evidence has not been collected (120). Concerning gastric cancer, case series studies have been performed on this topic, 51 gastric NECs (15 pure and 36 associated with adenocarcinoma and/or dysplasia) were analyzed, and HER2 amplification was reported in 3 NECs (6%) and 7 (19%) mixed tumors. However, none of them displayed Her2 expression in IHC (121). Consistently, in the other three studies, Her2 expression in IHC was found to be negative, or HER2 copy number analysis did not show amplification in 31 primitive gastric NECs overall (122–124). Therefore, the available evidence suggests that HER2 may not represent a valid therapeutic target, although this could be influenced by intratumoral heterogeneity, and further studies should be warranted on this topic. Finally, a study encompassing an expression profiling analysis in LCNECs reported that two cases displayed overexpression of Her2 at IHC, suggesting a potential role as a treatment target to be further investigated (125).
Immune Response Biomarkers
Recently, the introduction of immunotherapy dramatically changed the natural history of several cancer subtypes, like melanoma, lung cancer, and kidney cancer. Nonetheless, in some cases, the benefit of this treatment is confined only to a small portion of patients who show predictive biomarkers such as programmed cell death protein 1/ligand 1 (PD-1/PD-L1) or deficient mismatch repair (dMMR)/high microsatellite instability (MSI-H) status. In NENs, an increasing number of clinical trials with immunotherapy have been conducted (62). In March 2017, based on the results of the JAVELIN Merkel 200 trials, avelumab became the first FDA-approved agent for the treatment of metastatic Merkel cell carcinoma, a rare but aggressive NEC of the skin, and represented a new therapeutic option to improve patients’ survival (126, 127). Two years later, following the results of the IMpower133 trial, atezolizumab combined with chemotherapy was approved by the FDA for first-line treatment of extensive-stage SCLC. In this trial, the combination of chemotherapy and immunotherapy improved PFS and OS, with median PFS 5.2 versus 4.3 months (hazard ratio [HR] 0.77; 95% CI: 0.62–0.96; p = 0.02) and median OS 12.3 versus 10.3 months (HR 0.70; 95% CI: 0.54–0.91; p = 0.007), compared with chemotherapy alone (128). More recently, in phase II studies, the significant activity of spartalizumab in thoracic NENs (129) and also with the combination of ipilimumab plus nivolumab (objective response rate (ORR) 44%) in patients with non-pancreatic high-grade NENs (130).
With the exception of these few cases, unfortunately, there is a relatively low efficacy of immunotherapy in the unselected population of NENs, especially in GEP-NET. Therefore, one of the biggest challenges is to find those biomarkers that will allow to select those patients who will have a higher probability to benefit from this kind of treatment. Due to the heterogeneity of NENs and their rarity, as well as the fact that different primary tumor sites have different microenvironments, exploration in this field is indeed quite difficult. However, there is increasing evidence of the role that PD-1/PD-L1, tumor mutational burden (TMB), and dMMR/H-MSI status may also have in NENs (Table 1).
Targeting PD-1/PDL-1 Pathway
PD-L1, an immune inhibitory protein, is often upregulated in tumor cells by interferon-gamma secreted from effector T cells when tumor antigens are recognized. By interacting with PD-1, PD-L1 can suppress many immune cell functions, especially T-cell activation favoring tumor cell immune escape. Expression levels of PD-L1 assessment via IHC on tumor cells are one of the predictive factors for patients treated with immunotherapy. Several retrospective studies demonstrated that PD-L1 expression is a frequent occurrence in high-grade GEP-NENs (62). Kim et al. firstly reported a 21.9% (7/32) PD-L1 expression rate in patients with metastatic GEP-NET, which was significantly associated (p = 0.008) with high-grade classification (63). Similar to this, PD-L1 positivity was found by Cavalcanti et al. in approximately 28% (16/57) of cases, and again, PD-L1 expression in both tumor and infiltrating immune cells was significantly higher in poorly differentiated NENs (p = 0.001), and its expression rates increased with the tumor aggressiveness. These findings may be related to possibly acquired resistance to immune surveillance by the upregulation of PD-L1 and the inhibition of peritumoral and intratumoral infiltrating lymphocytes limiting T cell-mediated tumor aggression (64). This may explain the higher PD-L1 expression rates observed in later case series restricted to high-grade GEP NETs. PD-L1 positivity of 48.8% was observed by Yang et al. in 43 gastric NECs (65), while 24.1% was described by Busico et al. in tumor-infiltrating lymphocytes (TILs) of 54 GEP high-grade NENs (16). In both studies, the high expression of PD-L1 was associated with poor OS. An increase of PD-L1 expression along the GEP-NENs grading stages was also reported in a retrospective study performed in our institution (66). In addition, we demonstrated that the transition from G1/G2 NETs to G3 NETs and G3 NECs is associated with profound changes in the tumor and stromal profile for inflammatory and immune-related markers and point to more frequent activation of adaptive immunity in NECs and a strong immune escape mechanism. Moreover, a subset of NECs has microenvironment features consistent with spontaneous activation of adaptive immunity (co-expression of CD3, CD4, CD8, PD-1, and PD-L1). Recently, we further evaluated the tumor microenvironment of high-grade NENs, by expanding the immune profiling to myeloid markers and identifying two prognostic subpopulations of tumors likely compatible with the “hot/cold tumor” idea: high-grade NENs characterized by a prevalent immune infiltrate cells had better survival (67). According to this, it was suggested that microenvironment-related immune and inflammatory markers can improve prognostic prediction in GEP-NENs when combined with the known prognostic factors, and they may predict potential responsiveness to immunotherapy of GEP NECs (66, 67). Furthermore, Bosch et al. demonstrated that high TILs and PD-1 expression are significantly associated with shorter survival and higher grading in GEP NENs. In addition, high expression of PD-L1 in tumor cells was associated with high rates of PD-1-positive lymphocytes and a significantly higher number of TILs. According to this, the authors suggested that in high TIL tumors, a higher number of PD-1-positive lymphocytes is present; thereby, tumor cells with the higher PD-L1 expression may be more able to escape from the immune response by upregulation of this pathway (68).
In summary, according to previous data, PD-L1 expression may be a useful biomarker first to discriminate GEP high-grade NENs, and then, it may potentially be a prognostic and, above all, predictive biomarker for response to immune checkpoint inhibitors (ICIs).
When considering high-grade lung NENs, PD-L1 positive rates tend to vary immensely across different studies. A reason for this wide range may be related to the use of different clones of anti-PD-L1 antibody for IHC along with variable cutoffs. But in those studies in which FDA-approved anti-PD-L1 antibodies and their relative cutoffs were used, expression rates tend to be low (69, 70). Interestingly, substantial PD-L1 expression occurs on stroma cells, including TILs, in SCLCs with favorable clinical outcomes. Overall, this relatively low PD-L1 expression along with the deficient expression of major histocompatibility complex class I molecules, which prevents tumor cells from presenting neoantigens to CD8+ T cells in the lymph nodes and inhibiting cytotoxic T lymphocytes, may be one of the main reasons why the efficacy of ICIs in SCLCs is not as good as that in NSCLCs (69).
High Microsatellite Instability
H-MSI phenotype is another well-known biomarker that is under investigation in many neoplastic diseases. MMR proteins represent a complex system involved in DNA repair mechanisms, which ensure genomic integrity and remove DNA errors. Deficiency in MMR proteins (MLH1, MSH2, MSH6, and PMS2), commonly assessed by IHC, leads to an accumulation of DNA replication errors and mutations as well as expansion or contraction of microsatellite regions (131). The resulting hyper-mutated phenotype strongly enhances the formation of neo-antigens, making cancer cells more recognizable by the host immune system. Additionally, dMMR/H-MSI tumors have prominent lymphocyte infiltrates (132) and are more likely to express PD-L1 (133), which may predict response and durable clinical benefit to PD-1 blockade. For all these reasons, dMMR/H-MSI tumors are responsive to immunotherapy. Recently, FDA approval was granted for use of the anti-PD-1 antibody pembrolizumab for the treatment of metastatic non-hematologic cancers that are characterized by this alteration. Usually, dMMR is related to Lynch syndrome, which is caused by germline mutations of MMR proteins, leading to a 50%–70% lifetime risk of colorectal cancer, 40%–60% risk of endometrial cancer, and increased risks of several other malignancies (134). Despite this, dMMR/H-MSI can be also observed in sporadic cancer. Data on H-MSI in NENs are limited. Recent studies demonstrated that the presence of H-MSI phenotype on subsets of gastrointestinal (GI) NECs and MiNEN of the stomach and colorectum with an incidence rate up to 15%; it was mostly subsequent to MHL1 promoter methylation and with a more favorable prognosis (71, 72). In contrast, defects in DNA MMR proteins are rare in pancreatic NETs, small intestinal NETs (73, 74), and NECs of the endometrium (75) and cervix (75). These data suggest the prevalence of H-MSI in relatively low NETs; it is site-dependent and closely related to those organ sites in which H-MSI status is usually observed in the exocrine neoplastic counterparts, such as colorectal, gastric, and endometrial adenocarcinomas. Nevertheless, given the potential prognostic role and the clinical benefit of immunotherapy, dMMR/H-MSI testing must be encouraged as well as testing of other malignancies like colorectal cancer.
Tumor Mutational Burden
In addition to the previous two TMBs is another recently discovered biomarker. It is broadly defined as the number of somatic mutations per megabase of interrogated genomic sequence. TMB is believed to be a key driver in the generation of immunogenic neopeptides displayed on major histocompatibility complexes on the tumor cell surface that influences patient response to ICIs (76). In a phase II study in patients with previously treated, unresectable, or metastatic solid tumors (KEYNOTE-158), TMB-high status (≥10 mut/Mb) was associated with a clinically meaningful improvement in the efficacy of pembrolizumab (77). According to this, the FDA approved pembrolizumab monotherapy for the subgroup of solid-tumor patients with TMB ≥10 mut/Mb who are treatment-refractory and lack satisfactory alternative treatment options.
TMB of NETs has not been fully studied yet. In a study of 4,125 patients with various GI cancer types, TMB levels have been analyzed. Among those, pancreatic NETs were found to have one of the lowest TMB (5.8 mut/Mb) (78). More recently, in another retrospective study, Shao et al. assessed TMB in 2,559 patients with different tumors. SCLC was found to have the highest median TMB (8.6 mut/Mb) and the highest rate of TMB-high (cutoff ≥10 mut/Mb, 40%), which is, interestingly, followed by the NETs (29.3%). However, this remarkable rate was driven by the patients with LCNEC in which TMB high rate was 45.6%. On the contrary, in the small bowel, colon, and rectal NETs grouped with LCNECs, the rate was lower (5.9%, 11.8%, and 0%, respectively). Despite this, no differences in OS were seen between TMB high and low tumors (79). High TMB and elevated TMB-high rates in SCLC were described in several other studies (80–83). Furthermore, the role of TMB as a predictive biomarker in extensive-stage SCLC was also explored in patients who were treated with nivolumab alone or combined with ipilimumab after the failure of at least one prior chemotherapy regimen (CheckMate032 trial). In these populations, ORR by treatment arm increased in patients whose tumors showed high versus medium versus low TMB levels. In addition, in patients with high TMB tumors, dual ICI treatment was associated with an impressive ORR of 46.2% and an estimated 1-year OS rate of 62.4% (84). Lastly, in another recent report, Hoffman-Censits et al. demonstrated that over 26% of small cell bladder cancer had high TMB, in particular TMB > 10 mutations/Mb, and 3% had TMB > 20 mut/Mb, with a median of 6.2 mut/Mb (21).
MicroRNA
MicroRNAs (miRNAs) are small, non-coding RNAs with a length of 21–25 nucleotides and participate in gene regulation on the post-transcriptional level (135, 136). The role of miRNAs in cancerogenesis is now well-established, and several studies demonstrated the correlation between specific miRNA and different cancer subtypes (85). According to this, miRNA expression profiles are potentially exploited as practical supportive markers for differential NEN diagnosis and prognosis and provide adequate information on proper patient care and management (85–90). When considering pancreatic NETs, the expression of specific miRNAs is able to discriminate them from normal pancreas and other pathologic conditions such as pancreatic ductal adenocarcinoma and acinar pancreatic tumors (87, 91, 92). Specifically, the expressions of miR-144/451 cluster, miRNA-21, and MiR-193b were observed in insulinomas compared with normal pancreatic tissue, while miR-103 and miR-107 overexpression and miR-155 underexpression distinguish pancreatic NETs from acinar cell carcinomas (87, 91, 93). In addition to this, different miRNA expressions discriminate different clinical behaviors and prognoses of pancreatic NETs (88). Indeed, the overexpression of miR-21, miR-642, and miR-196a was found to be positively correlated with the Ki-67 proliferation index, whereas miR-210 correlated with the presence of liver metastases (93, 94). Additionally, miR-196a expression was significantly associated with stage, mitotic count, and decreased OS and disease-free survival (95). The pattern of miRNA expression was also explored in small bowel NETs (91, 96). MiR-7-5p, miR-182, miR-183, and miR-96-5p were found to be upregulated in NETs of the small bowel compared with normal tissue (91). In addition, the last three, along with the downregulation of miR-129-5p and miR-133a, were found to be overexpressed in the metastatic lesions compared with primary tumors (91, 97). Considering the prognostic role, high levels of circulating miR-21-5p and miR-22-3p and low levels of miR-150-5p were associated with shorter OS (98). Specific miRNA expressions were also reported in other GEP-NENs such as gastrin-induced miR-222 overexpression in hypergastrinemic patients and type 1 gastric NETs, which may be associated with tumor development by decreasing p27 expression (99); low levels of miR-96 and high levels of miR-133a expression in appendiceal carcinoids (91); underexpression of miR-186 in colorectal NETs (100); and overexpression of miR-885-5p in rectal carcinoids (101). Lastly, in a recent study, Cavalcanti et al. reported that 8 miRNAs were expressed in all GEP-NETs grades (miR-10b-5p, miR-130b-3p, miR-192-5p, miR-194-5p, miR-210-3p, miR-214-3p, miR-7-5p, and miR-96-5p), but their expression level was different between differentiation grades. Among these, miR-96-5p were found to have increased expression levels from G1 to G3, and this may be probably related to the downregulation of FoxO1 gene by this miRNA (85).
The role of miRNAs as a diagnostic, prognostic, and chemoresistance tool was also explored in lung NENs. Recently, Yoshimoto et al. collected formalin-fixed paraffin-embedded samples of lung and GEP NETs, lung and GI adenocarcinomas, olfactory neuroblastomas, schwannomas, and related normal tissue for the analysis of their miRNA expression. After a very complex hierarchical clustering analysis, they found that lung and GI-NETs had a similar pattern of miRNA expression, suggesting a common origin between them, which was different from adenocarcinomas, SCLCs, and normal tissue. They also showed a distinct miRNA expression profile of SCLCs from lung carcinoids (89), and this may be useful to distinguish between low- and high-grade lung NENs. In addition, Rapa et al. showed that lung carcinoids have distinct miRNA expression profiles as compared with high-grade NECs, explaining that specific miRNAs might have potential implications as diagnostic tools or clinical biomarkers (102). As described for GEP-NENs, specific miRNA expression may also be used as prognostic markers (88). Specifically, overexpression of miR-92a2* and miR-7 and low levels of miR-150, miR-886-3p, miR-192, miR-200c, and miR-205 were described to be correlated to OS and PFS of SCLCs. MiR-92a2* and miR-7, along with mir-147 and miR-574-5p, were found to be associated with chemoresistance too (88, 90). A correlation with survival was also observed in typical and atypical carcinoids and LCNECs with upregulation of mir let-7d, miR-19, miR576-5p, miR-340*, and miR-1286, while overexpression miR-21 and low levels of miR-409-3p, miR-409-5p, and miR-431-5p correlated with the presence of lymph node metastases (88).
Conclusions
Emerging evidence suggests an important role for biomarker identification and also NENs, in particular those with high-grade features. High-grade NENs can express different biomarkers (PD-L1, H-MSI status, miRNA expression patterns, and other alterations). A comprehensive exploration of biomarkers is still lacking as well as a molecular-driven clinical trial involving patients with NENs apart from the phase I/II multi-disease trial (Table 2). So considering that many of those biomarkers can be the target for new generations of drugs, with a subsequent significant clinical benefit, greater effort should be focused on spreading routine molecular analysis also in this setting of patients, like what usually happens with other malignancies. This may be important firstly for the patients themselves, giving the chance to obtain additional treatments with expanded access programs or nominal use, and secondly, because it may the basis for future clinical trials specific for this group of patients that may significantly change the currently untailored chemotherapy-based treatment strategies.
Author Contributions
Conceptualization: MP, MMi, and SP. Methodology: MP, FM, NP, MN, and SC. Investigation: MP, AM, AR, GG, and FC. Resources: GR, FP, EC, and MMa. Data curation: MP, AM, AR, and FC. Writing—original draft preparation: MP, AM, AR, and FC. Writing—review and editing: MP, FM, NP, MN, SC, SP, GC, GS, AM, GC, and MB. Visualization: GR, FP, EC, and MMa. Supervision: MB, FB, MMi, and SP. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by the Italian Ministry of Health (ERP-2017-23671129 “PMTR-pNET”) to MM and by Fondazione IRCCS Istituto Nazionale Tumori Milano 5x1000 Funds—grant “Integrative molecular analysis of pure and combined lung large cell neuroendocrine carcinoma (LCNEC)” to MM. GC was supported by a FIRC-AIRC fellowship for Italy.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, et al. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol (2017) 3(10):1335–42. doi: 10.1001/jamaoncol.2017.0589
2. Taal BG, Visser O. Epidemiology of Neuroendocrine Tumours. Neuroendocrinology (2004) 80:3–7. doi: 10.1159/000080731
3. Polish A, Vergo MT, Agulnik M. Management of Neuroendocrine Tumors of Unknown Origin. J Natl Compr Cancer Netw (2011) 9(12):1397–402. doi: 10.6004/jnccn.2011.0118
4. Perren A, Couvelard A, Scoazec JY, Costa F, Borbath I, Delle Fave G, et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Pathology: Diagnosis and Prognostic Stratification. Neuroendocrinology (2017) 105(3):196–200. doi: 10.1159/000457956
5. Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, et al. The 2019 WHO Classification of Tumours of the Digestive System. Histopathology (2020) 76(2):182–8. doi: 10.1111/his.13975
6. Milione M, Maisonneuve P, Spada F, Pellegrinelli A, Spaggiari P, Albarello L, et al. The Clinicopathologic Heterogeneity of Grade 3 Gastroenteropancreatic Neuroendocrine Neoplasms: Morphological Differentiation and Proliferation Identify Different Prognostic Categories. Neuroendocrinology (2017) 104(1):85–93. doi: 10.1159/000445165
7. Milione M, Maisonneuve P, Grillo F, Mangogna A, Centonze G, Prinzi N, et al. Ki-67 Index of 55% Distinguishes Two Groups of Bronchopulmonary Pure and Composite Large Cell Neuroendocrine Carcinomas With Distinct Prognosis. Neuroendocrinology (2021) 111(5):475–89. doi: 10.1159/000508376
8. Milione M, Maisonneuve P, Pellegrinelli A, Grillo F, Albarello L, Spaggiari P, et al. Ki67 Proliferative Index of the Neuroendocrine Component Drives MANEC Prognosis. Endocrine-Relat Cancer (2018) 25(5):583–93. doi: 10.1530/erc-17-0557
9. Oronsky B, Ma PC, Morgensztern D, Carter CA. Nothing But NET: A Review of Neuroendocrine Tumors and Carcinomas. Neoplasia (2017) 19(12):991–1002. doi: 10.1016/j.neo.2017.09.002
10. Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S. The Pathologic Classification of Neuroendocrine Tumors: A Review of Nomenclature, Grading, and Staging Systems. Pancreas (2010) 39(6):707–12. doi: 10.1097/MPA.0b013e3181ec124e
11. Elvebakken H, Perren A, Scoazec JY, Tang LH, Federspiel B, Klimstra DS, et al. A Consensus Developed Morphological Re-Evaluation of 196 High-Grade Gastroenteropancreatic Neuroendocrine Neoplasms and its Clinical Correlations. Neuroendocrinology (2020) 111(9):883–94. doi: 10.1159/000511905.Citedin:Pubmed
12. International Agency for Research on Cancer. International Academy of Pathology: Lyon F. Who Classification of Tumours of the Lung, Pleura, Thymus and Heart Lyon, France: International Agency for Research on Cancer (IARC). Vol. 5. (2021).
13. Thomas KEH, Voros BA, Boudreaux JP, Thiagarajan R, Woltering EA, Ramirez RA. Current Treatment Options in Gastroenteropancreatic Neuroendocrine Carcinoma. Oncologist (2019) 24(8):1076–88. doi: 10.1634/theoncologist.2018-0604
14. Girardi DM, Silva ACB, Rêgo JFM, Coudry RA, Riechelmann RP. Unraveling Molecular Pathways of Poorly Differentiated Neuroendocrine Carcinomas of the Gastroenteropancreatic System: A Systematic Review. Cancer Treat Rev (2017) 56:28–35. doi: 10.1016/j.ctrv.2017.04.002
15. Karkouche R, Bachet JB, Sandrini J, Mitry E, Penna C, Côté JF, et al. Colorectal Neuroendocrine Carcinomas and Adenocarcinomas Share Oncogenic Pathways. A Clinico-Pathologic Study of 12 Cases. Eur J Gastroenterol Hepatol (2012) 24(12):1430–7. doi: 10.1097/MEG.0b013e3283583c87
16. Busico A, Maisonneuve P, Prinzi N, Pusceddu S, Centonze G, Garzone G, et al. Gastroenteropancreatic High-Grade Neuroendocrine Neoplasms: Histology and Molecular Analysis, Two Sides of the Same Coin. Neuroendocrinology (2020) 110(7-8):616–29. doi: 10.1159/000503722
17. Konukiewitz B, Schlitter AM, Jesinghaus M, Pfister D, Steiger K, Segler A, et al. Somatostatin Receptor Expression Related to TP53 and RB1 Alterations in Pancreatic and Extrapancreatic Neuroendocrine Neoplasms With a Ki67-Index Above 20. Modern Pathol (2017) 30(4):587–98. doi: 10.1038/modpathol.2016.217
18. Kim ST, Lee SJ, Park SH, Park JO, Lim HY, Kang WK, et al. Genomic Profiling of Metastatic Gastroenteropancreatic Neuroendocrine Tumor (GEP-NET) Patients in the Personalized-Medicine Era [Research Paper]. J Cancer (2016) 7(9):1044–8. doi: 10.7150/jca.14815
19. Bergsland EK, Roy R, Stephens P, Ross JS, Bailey M, Olshen A. Genomic Profiling to Distinguish Poorly Differentiated Neuroendocrine Carcinomas Arising in Different Sites. J Clin Oncol (2016) 34(15_suppl):4020–0. doi: 10.1200/JCO.2016.34.15_suppl.4020
20. Chen L, Liu M, Zhang Y, Guo Y, Chen MH, Chen J. Genetic Characteristics of Colorectal Neuroendocrine Carcinoma: More Similar to Colorectal Adenocarcinoma. Clin Colorectal Cancer (2020) 20(2):177–85.e13. doi: 10.1016/j.clcc.2020.09.001
21. Hoffman-Censits J, Choi W, Pal S, Trabulsi E, Kelly WK, Hahn NM, et al. Urothelial Cancers With Small Cell Variant Histology Have Confirmed High Tumor Mutational Burden, Frequent TP53 and RB Mutations, and a Unique Gene Expression Profile. Eur Urol Oncol (2021) 4(2):297–300. doi: 10.1016/j.euo.2019.12.002
22. Scarpa A, Chang DK, Nones K, Corbo V, Patch AM, Bailey P, et al. Whole-Genome Landscape of Pancreatic Neuroendocrine Tumours. Nature (2017) 543(7643):65–71. doi: 10.1038/nature21063
23. Hijioka S, Hosoda W, Matsuo K, Ueno M, Furukawa M, Yoshitomi H, et al. Rb Loss and KRAS Mutation Are Predictors of the Response to Platinum-Based Chemotherapy in Pancreatic Neuroendocrine Neoplasm With Grade 3: A Japanese Multicenter Pancreatic NEN-G3 Study. Clin Cancer Res (2017) 23(16):4625–32. doi: 10.1158/1078-0432.Ccr-16-3135
24. Centonze G, Biganzoli D, Prinzi N, Pusceddu S, Mangogna A, Tamborini E, et al. Beyond Traditional Morphological Characterization of Lung Neuroendocrine Neoplasms: In Silico Study of Next-Generation Sequencing Mutations Analysis Across the Four World Health Organization Defined Groups. Cancers (Basel) (2020) 12(10):2753. doi: 10.3390/cancers12102753
25. Simbolo M, Mafficini A, Sikora KO, Fassan M, Barbi S, Corbo V, et al. Lung Neuroendocrine Tumours: Deep Sequencing of the Four World Health Organization Histotypes Reveals Chromatin-Remodelling Genes as Major Players and a Prognostic Role for TERT, RB1, MEN1 and KMT2D. J Pathol (2017) 241(4):488–500. doi: 10.1002/path.4853
26. Simbolo M, Barbi S, Fassan M, Mafficini A, Ali G, Vicentini C, et al. Gene Expression Profiling of Lung Atypical Carcinoids and Large Cell Neuroendocrine Carcinomas Identifies Three Transcriptomic Subtypes With Specific Genomic Alterations. J Thorac Oncol (2019) 14(9):1651–61. doi: 10.1016/j.jtho.2019.05.003
27. George J, Walter V, Peifer M, Alexandrov LB, Seidel D, Leenders F, et al. Integrative Genomic Profiling of Large-Cell Neuroendocrine Carcinomas Reveals Distinct Subtypes of High-Grade Neuroendocrine Lung Tumors. Nat Commun (2018) 9(1):1048. doi: 10.1038/s41467-018-03099-x
28. Rekhtman N, Pietanza MC, Hellmann MD, Naidoo J, Arora A, Won H, et al. Next-Generation Sequencing of Pulmonary Large Cell Neuroendocrine Carcinoma Reveals Small Cell Carcinoma-Like and Non-Small Cell Carcinoma-Like Subsets. Clin Cancer Res (2016) 22(14):3618–29. doi: 10.1158/1078-0432.ccr-15-2946
29. Klempner SJ, Gershenhorn B, Tran P, Lee TK, Erlander MG, Gowen K, et al. BRAFV600E Mutations in High-Grade Colorectal Neuroendocrine Tumors May Predict Responsiveness to BRAF-MEK Combination Therapy. Cancer Discov (2016) 6(6):594–600. doi: 10.1158/2159-8290.Cd-15-1192
30. Burkart J, Owen D, Shah MH, Abdel-Misih SRZ, Roychowdhury S, Wesolowski R, et al. Targeting BRAF Mutations in High-Grade Neuroendocrine Carcinoma of the Colon. J Natl Compr Cancer Netw (2018) 16(9):1035–40. doi: 10.6004/jnccn.2018.7043
31. Brcic L, Kuchler C, Eidenhammer S, Pabst D, Quehenberger F, Gazdar AF, et al. Comparison of Four DLL3 Antibodies Performance in High Grade Neuroendocrine Lung Tumor Samples and Cell Cultures. Diagn Pathol (2019) 14(1):47. doi: 10.1186/s13000-019-0827-z
32. Rudin CM, Pietanza MC, Bauer TM, Ready N, Morgensztern D, Glisson BS, et al. Rovalpituzumab Tesirine, a DLL3-Targeted Antibody-Drug Conjugate, in Recurrent Small-Cell Lung Cancer: A First-in-Human, First-in-Class, Open-Label, Phase 1 Study. Lancet Oncol (2017) 18(1):42–51. doi: 10.1016/S1470-2045(16)30565-4
33. Saunders LR, Bankovich AJ, Anderson WC, Aujay MA, Bheddah S, Black K, et al. Dylla SJ. A DLL3-Targeted Antibody-Drug Conjugate Eradicates High-Grade Pulmonary Neuroendocrine Tumor-Initiating Cells In Vivo. Sci Transl Med (2015) 7(302):302ra136. doi: 10.1126/scitranslmed.aac9459
34. Liverani C, Bongiovanni A, Mercatali L, Pieri F, Spadazzi C, Miserocchi G, et al. Diagnostic and Predictive Role of DLL3 Expression in Gastroenteropancreatic Neuroendocrine Neoplasms. Endocr Pathol (2021) 32(2):309–17. doi: 10.1007/s12022-020-09657-8
35. Amin M, Trikalinos N, Chatterjee D. Single Institutional Experience on Primary Neuroendocrine Neoplasms of the Kidney- A Rare Distinct Entity. Hum Pathol (2021) 114:36–43. doi: 10.1016/j.humpath.2021.04.006
36. Johnson ML, Zvirbule Z, Laktionov K, Helland A, Cho BC, Gutierrez V, et al. Rovalpituzumab Tesirine as a Maintenance Therapy After First-Line Platinum-Based Chemotherapy in Patients With Extensive-Stage-SCLC: Results From the Phase 3 MERU Study. J Thorac Oncol (2021) 16(9):1570–81. doi: 10.1016/j.jtho.2021.03.012
37. Blackhall F, Jao K, Greillier L, Cho BC, Penkov K, Reguart N, et al. Efficacy and Safety of Rovalpituzumab Tesirine Compared With Topotecan as Second-Line Therapy in DLL3-High SCLC: Results From the Phase 3 TAHOE Study. J Thorac Oncol (2021) 16(9):1547–58. doi: 10.1016/j.jtho.2021.02.009
38. Di Domenico A, Wiedmer T, Marinoni I, Perren A. Genetic and Epigenetic Drivers of Neuroendocrine Tumours (NET). Endocrine-Relat Cancer (2017) 24(9):R315–r334. doi: 10.1530/erc-17-0012
39. Szybowska M, Mete O, Weber E, Silver J, Kim RH. Neuroendocrine Neoplasms Associated With Germline Pathogenic Variants in the Homologous Recombination Pathway. Endocr Pathol (2019) 30(3):237–45. doi: 10.1007/s12022-019-9569-4
40. Wu Y, Gao Y, Dou X, Yue J. Metastatic Castration-Resistant Prostate Cancer With Neuroendocrine Transformation and BRCA 1 Germ-Line Mutation: A Case Report and Literature Review. Onco Targets Ther (2020) 13:8049–54. doi: 10.2147/OTT.S264347
41. Pandya D, Shah M, Kaplan F, Martino C, Levy G, Kazanjian M, et al. Treatment-Emergent Neuroendocrine Prostate Cancer With a Germline BRCA2 Mutation: Identification of a Candidate Reversion Mutation Associated With Platinum/PARP-Inhibitor Resistance. Cold Spring Harbor Mol Case Stud (2021) 7(1):487–95. doi: 10.1101/mcs.a005801
42. Sen T, Gay CM, Byers LA. Targeting DNA Damage Repair in Small Cell Lung Cancer and the Biomarker Landscape. Transl Lung Cancer Res (2018) 7(1):50–68. doi: 10.21037/tlcr.2018.02.03
43. Lok BH, Gardner EE, Schneeberger VE, Ni A, Desmeules P, Rekhtman N, et al. PARP Inhibitor Activity Correlates With SLFN11 Expression and Demonstrates Synergy With Temozolomide in Small Cell Lung Cancer. Clin Cancer Res (2017) 23(2):523–35. doi: 10.1158/1078-0432.CCR-16-1040
44. Byers LA, Krug L, Waqar S, Dowlati A, Hann C, Chiappori A, et al. MA11.07 Improved Small Cell Lung Cancer (SCLC) Response Rates With Veliparib and Temozolomide: Results From a Phase II Trial. J Thorac Oncol (2017) 12(1):S406–7. doi: 10.1016/j.jtho.2016.11.466
45. Mu Y, Lou J, Srivastava M, Zhao B, X-h F, Liu T, et al. SLFN11 Inhibits Checkpoint Maintenance and Homologous Recombination Repair. EMBO Rep (2016) 17(1):94–109. doi: 10.15252/embr.201540964
46. Graham RP, Oliveira AM, Zhang L. Rare ALK Expression But No ALK Rearrangement in Pancreatic Ductal Adenocarcinoma and Neuroendocrine Tumors. Pancreas (2013) 42(6):949–51. doi: 10.1097/MPA.0b013e3182847bd0
47. Zheng Q, Zheng M, Jin Y, Shen X, Shan L, Shen L, et al. ALK-Rearrangement Neuroendocrine Carcinoma of the Lung: A Comprehensive Study of a Rare Case Series and Review of Literature. Onco Targets Ther (2018) 11:4991–8. doi: 10.2147/OTT.S172124
48. Fukuizumi A, Akagi K, Sakai H. A Case of Atypical Carcinoid: Harboring Variant 3a/B EML4-ALK Rearrangement. J Thorac Oncol (2015) 10(10):e104–6. doi: 10.1097/jto.0000000000000635
49. Nakajima M, Uchiyama N, Shigemasa R, Matsumura T, Matsuoka R, Nomura A. Atypical Carcinoid Tumor With Anaplastic Lymphoma Kinase (ALK) Rearrangement Successfully Treated by an ALK Inhibitor. Intern Med (Tokyo Jpn) (2016) 55(21):3151–3. doi: 10.2169/internalmedicine.55.6738
50. Hoton D, Humblet Y, Libbrecht L. Phenotypic Variation of an ALK-Positive Large-Cell Neuroendocrine Lung Carcinoma With Carcinoid Morphology During Treatment With ALK Inhibitors. Histopathology (2018) 72(4):707–10. doi: 10.1111/his.13388
51. Hayashi N, Fujita A, Saikai T, Takabatake H, Sotoshiro M, Sekine K, et al. Large Cell Neuroendocrine Carcinoma Harboring an Anaplastic Lymphoma Kinase (ALK) Rearrangement With Response to Alectinib. Intern Med (Tokyo Jpn) (2018) 57(5):713–6. doi: 10.2169/internalmedicine.9368-17
52. Wang VE, Young L, Ali S, Miller VA, Urisman A, Wolfe J, et al. A Case of Metastatic Atypical Neuroendocrine Tumor With ALK Translocation and Diffuse Brain Metastases. Oncologist (2017) 22(7):768–73. doi: 10.1634/theoncologist.2017-0054
53. Lou G, Yu X, Song Z. Molecular Profiling and Survival of Completely Resected Primary Pulmonary Neuroendocrine Carcinoma. Clin Lung Cancer (2017) 18(3):e197–201. doi: 10.1016/j.cllc.2016.11.014
54. Shimizu N, Akashi Y, Fujii T, Shiono H, Yane K, Kitahara T, et al. Use of ALK Immunohistochemistry for Optimal Therapeutic Strategy of Pulmonary Large-Cell Neuroendocrine Carcinoma and Identification of a Novel KIF5B-ALK Fusion Oncokinase. Anticancer Res (2019) 39(1):413–20. doi: 10.21873/anticanres.13127
55. Nakamura H, Tsuta K, Yoshida A, Shibata T, Wakai S, Asamura H, et al. Aberrant Anaplastic Lymphoma Kinase Expression in High-Grade Pulmonary Neuroendocrine Carcinoma. J Clin Pathol (2013) 66(8):705–7. doi: 10.1136/jclinpath-2012-201329
56. Sigal DS, Bhangoo MS, Hermel JA, Pavlick DC, Frampton G, Miller VA, et al. Comprehensive Genomic Profiling Identifies Novel NTRK Fusions in Neuroendocrine Tumors. Oncotarget (2018) 9(88):35809–12. doi: 10.18632/oncotarget.26260
57. Drilon A, Siena S, Ou SI, Patel M, Ahn MJ, Lee J, et al. Safety and Antitumor Activity of the Multitargeted Pan-TRK, ROS1, and ALK Inhibitor Entrectinib: Combined Results From Two Phase I Trials (ALKA-372-001 and STARTRK-1). Cancer Discov (2017) 7(4):400–9. doi: 10.1158/2159-8290.Cd-16-1237
58. Doebele RC, Drilon A, Paz-Ares L, Siena S, Shaw AT, Farago AF, et al. Entrectinib in Patients With Advanced or Metastatic NTRK Fusion-Positive Solid Tumours: Integrated Analysis of Three Phase 1-2 Trials. Lancet Oncol (2020) 21(2):271–82. doi: 10.1016/s1470-2045(19)30691-6
59. Hong DS, DuBois SG, Kummar S, Farago AF, Albert CM, Rohrberg KS, et al. Larotrectinib in Patients With TRK Fusion-Positive Solid Tumours: A Pooled Analysis of Three Phase 1/2 Clinical Trials. Lancet Oncol (2020) 21(4):531–40. doi: 10.1016/s1470-2045(19)30856-3
60. Sigal D, Tartar M, Xavier M, Bao F, Foley P, Luo D, et al. Activity of Entrectinib in a Patient With the First Reported NTRK Fusion in Neuroendocrine Cancer. J Natl Compr Cancer Netw (2017) 15(11):1317–22. doi: 10.6004/jnccn.2017.7029
61. Papadopoulos KP, Borazanci E, Shaw AT, Katayama R, Shimizu Y, Zhu VW, et al. Phase I First-In-Human Study of Taletrectinib (DS-6051b/AB-106), a ROS1/TRK Inhibitor, in Patients With Advanced Solid Tumors. Clin Cancer Res (2020) 26(18):4785–94. doi: 10.1158/1078-0432.Ccr-20-1630
62. Xu G, Wang Y, Zhang H, She X, Yang J. Immunotherapy and Potential Predictive Biomarkers in the Treatment of Neuroendocrine Neoplasia. Future Oncol (2021) 17(9):1069–81. doi: 10.2217/fon-2020-0703
63. Kim ST, Ha SY, Lee S, Ahn S, Lee J, Park SH, et al. The Impact of PD-L1 Expression in Patients With Metastatic GEP-NETs. J Cancer (2016) 7(5):484–9. doi: 10.7150/jca.13711
64. Cavalcanti E, Armentano R, Valentini AM, Chieppa M, Caruso ML. Role of PD-L1 Expression as a Biomarker for GEP Neuroendocrine Neoplasm Grading. Cell Death Dis (2017) 8(8):e3004. doi: 10.1038/cddis.2017.401
65. Yang M-W, Fu X-L, Jiang Y-S, Chen X-J, Tao L-Y, Yang J-Y, et al. Clinical Significance of Programmed Death 1/Programmed Death Ligand 1 Pathway in Gastric Neuroendocrine Carcinomas. World J Gastroenterol (2019) 25(14):1684–96. doi: 10.3748/wjg.v25.i14.1684
66. Milione M, Miceli R, Barretta F, Pellegrinelli A, Spaggiari P, Tagliabue G, et al. Microenvironment and Tumor Inflammatory Features Improve Prognostic Prediction in Gastro-Entero-Pancreatic Neuroendocrine Neoplasms. J Pathol Clin Res (2019) 5(4):217–26. doi: 10.1002/cjp2.135
67. Centonze G, Lagano V, Sabella G, Mangogna A, Garzone G, Filugelli M, et al. Myeloid and T-Cell Microenvironment Immune Features Identify Two Prognostic Sub-Groups in High-Grade Gastroenteropancreatic Neuroendocrine Neoplasms. J Clin Med (2021) 10(8):1741. doi: 10.3390/jcm10081741
68. Bösch F, Brüwer K, Altendorf-Hofmann A, Auernhammer CJ, Spitzweg C, Westphalen CB, et al. Immune Checkpoint Markers in Gastroenteropancreatic Neuroendocrine Neoplasia. Endocrine-Relat Cancer (2019) 26(3):293–301. doi: 10.1530/erc-18-0494
69. Tian Y, Zhai X, Han A, Zhu H, Yu J. Potential Immune Escape Mechanisms Underlying the Distinct Clinical Outcome of Immune Checkpoint Blockades in Small Cell Lung Cancer. J Hematol Oncol (2019) 12(1):67. doi: 10.1186/s13045-019-0753-2
70. Guleria P, Kumar S, Malik PS, Jain D. PD-L1 Expression in Small Cell and Large Cell Neuroendocrine Carcinomas of Lung: An Immunohistochemical Study With Review of Literature. Pathol Oncol Res (2020) 26(4):2363–70. doi: 10.1007/s12253-020-00832-0
71. Sahnane N, Furlan D, Monti M, Romualdi C, Vanoli A, Vicari E, et al. Microsatellite Unstable Gastrointestinal Neuroendocrine Carcinomas: A New Clinicopathologic Entity. Endocrine-Relat Cancer (2015) 22(1):35–45. doi: 10.1530/erc-14-0410
72. Furlan D, Sahnane N, Mazzoni M, Pastorino R, Carnevali I, Stefanoli M, et al. Diagnostic Utility of MS-MLPA in DNA Methylation Profiling of Adenocarcinomas and Neuroendocrine Carcinomas of the Colon–Rectum. Virchows Archiv (2013) 462(1):47–56. doi: 10.1007/s00428-012-1348-2
73. Fraune C, Simon R, Hube-Magg C, Makrypidi-Fraune G, Kluth M, Büscheck F, et al. Homogeneous MMR Deficiency Throughout the Entire Tumor Mass Occurs in a Subset of Colorectal Neuroendocrine Carcinomas. Endocr Pathol (2020) 31(2):182–9. doi: 10.1007/s12022-020-09612-7
74. Arnason T, Sapp HL, Rayson D, Barnes PJ, Drewniak M, Nassar BA, et al. Loss of Expression of DNA Mismatch Repair Proteins Is Rare in Pancreatic and Small Intestinal Neuroendocrine Tumors. Arch Pathol Lab Med (2011) 135(12):1539–44. doi: 10.5858/arpa.2010-0560-OA
75. Pei X, Xiang L, Chen W, Jiang W, Yin L, Shen X, et al. The Next Generation Sequencing of Cancer-Related Genes in Small Cell Neuroendocrine Carcinoma of the Cervix. Gynecol Oncol (2021) 161(3):779–86. doi: 10.1016/j.ygyno.2021.04.019
76. Sha D, Jin Z, Budczies J, Kluck K, Stenzinger A, Sinicrope FA. Tumor Mutational Burden as a Predictive Biomarker in Solid Tumors. Cancer Discovery (2020) 10(12):1808–25. doi: 10.1158/2159-8290.Cd-20-0522
77. Marabelle A, Fakih M, Lopez J, Shah M, Shapira-Frommer R, Nakagawa K, et al. Association of Tumour Mutational Burden With Outcomes in Patients With Advanced Solid Tumours Treated With Pembrolizumab: Prospective Biomarker Analysis of the Multicohort, Open-Label, Phase 2 KEYNOTE-158 Study. Lancet Oncol (2020) 21(10):1353–65. doi: 10.1016/S1470-2045(20)30445-9
78. Salem ME, Puccini A, Grothey A, Raghavan D, Goldberg RM, Xiu J, et al. Landscape of Tumor Mutation Load, Mismatch Repair Deficiency, and PD-L1 Expression in a Large Patient Cohort of Gastrointestinal Cancers. Mol Cancer Res (2018) 16(5):805–12. doi: 10.1158/1541-7786.MCR-17-0735
79. Shao C, Li G, Huang L, Pruitt S, Castellanos E, Frampton G, et al. Prevalence of High Tumor Mutational Burden and Association With Survival in Patients With Less Common Solid Tumors. JAMA Netw Open (2020) 3(10):e2025109. doi: 10.1001/jamanetworkopen.2020.25109
80. Spigel DR, Schrock AB, Fabrizio D, Frampton GM, Sun J, He J, et al. Total Mutation Burden (TMB) in Lung Cancer (LC) and Relationship With Response to PD-1/PD-L1 Targeted Therapies. J Clin Oncol (2016) 34(15_suppl):9017–7. doi: 10.1200/JCO.2016.34.15_suppl.9017
81. Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SAJR, Behjati S, Biankin AV, et al. Signatures of Mutational Processes in Human Cancer. Nature (2013) 500(7463):415–21. doi: 10.1038/nature12477
82. George J, Lim JS, Jang SJ, Cun Y, Ozretić L, Kong G, et al. Comprehensive Genomic Profiles of Small Cell Lung Cancer. Nature (2015) 524(7563):47–53. doi: 10.1038/nature14664
83. Peifer M, Fernández-Cuesta L, Sos ML, George J, Seidel D, Kasper LH, et al. Integrative Genome Analyses Identify Key Somatic Driver Mutations of Small-Cell Lung Cancer. Nat Genet (2012) 44(10):1104–10. doi: 10.1038/ng.2396
84. Hellmann MD, Callahan MK, Awad MM, Calvo E, Ascierto PA, Atmaca A, et al. Tumor Mutational Burden and Efficacy of Nivolumab Monotherapy and in Combination With Ipilimumab in Small-Cell Lung Cancer. Cancer Cell (2018) 33(5):853–61.e4. doi: 10.1016/j.ccell.2018.04.001
85. Cavalcanti E, Galleggiante V, Coletta S, Stasi E, Chieppa M, Armentano R, et al. Altered miRNAs Expression Correlates With Gastroenteropancreatic Neuroendocrine Tumors Grades. Front Oncol (2020) 10:1187. doi: 10.3389/fonc.2020.01187
87. Zimmermann N, Knief J, Kacprowski T, Lazar-Karsten P, Keck T, Billmann F, et al. MicroRNA Analysis of Gastroenteropancreatic Neuroendocrine Tumors and Metastases. Oncotarget (2018) 9(47):28379–90. doi: 10.18632/oncotarget.25357
88. Zatelli MC, Grossrubatscher EM, Guadagno E, Sciammarella C, Faggiano A, Colao A. Circulating Tumor Cells and miRNAs as Prognostic Markers in Neuroendocrine Neoplasms. Endocrine-Relat Cancer (2017) 24(6):R223–37. doi: 10.1530/erc-17-0091
89. Yoshimoto T, Motoi N, Yamamoto N, Nagano H, Ushijima M, Matsuura M, et al. Pulmonary Carcinoids and Low-Grade Gastrointestinal Neuroendocrine Tumors Show Common MicroRNA Expression Profiles, Different From Adenocarcinomas and Small Cell Carcinomas. Neuroendocrinology (2018) 106(1):47–57. doi: 10.1159/000461582
90. Ranade AR, Cherba D, Sridhar S, Richardson P, Webb C, Paripati A, et al. MicroRNA 92a-2*: A Biomarker Predictive for Chemoresistance and Prognostic for Survival in Patients With Small Cell Lung Cancer. J Thorac Oncol (2010) 5(8):1273–8. doi: 10.1097/JTO.0b013e3181dea6be
91. Malczewska A, Kidd M, Matar S, Kos-Kudla B, Modlin IM. A Comprehensive Assessment of the Role of miRNAs as Biomarkers in Gastroenteropancreatic Neuroendocrine Tumors. Neuroendocrinology (2018) 107(1):73–90. doi: 10.1159/000487326
92. Li A, Yu J, Kim H, Wolfgang CL, Canto MI, Hruban RH, et al. MicroRNA Array Analysis Finds Elevated Serum miR-1290 Accurately Distinguishes Patients With Low-Stage Pancreatic Cancer From Healthy and Disease Controls. Clin Cancer Res (2013) 19(13):3600–10. doi: 10.1158/1078-0432.Ccr-12-3092
93. Thorns C, Schurmann C, Gebauer N, Wallaschofski H, Kümpers C, Bernard V, et al. Global MicroRNA Profiling of Pancreatic Neuroendocrine Neoplasias. Anticancer Res (2014) 34(5):2249–54.
94. Roldo C, Missiaglia E, Hagan JP, Falconi M, Capelli P, Bersani S, et al. MicroRNA Expression Abnormalities in Pancreatic Endocrine and Acinar Tumors are Associated With Distinctive Pathologic Features and Clinical Behavior. J Clin Oncol (2006) 24(29):4677–84. doi: 10.1200/jco.2005.05.5194
95. Lee Y, Kim H, Kim H, Lee J, Paik K, Kang J, et al. High Expression of microRNA-196a Indicates Poor Prognosis in Resected Pancreatic Neuroendocrine Tumor. Medicine (2015) 94:e2224. doi: 10.1097/MD.0000000000002224
96. Miller HC, Frampton AE, Malczewska A, Ottaviani S, Stronach EA, Flora R, et al. MicroRNAs Associated With Small Bowel Neuroendocrine Tumours and Their Metastases. Endocrine-Relat Cancer (2016) 23(9):711–26. doi: 10.1530/erc-16-0044
97. Li SC, Essaghir A, Martijn C, Lloyd RV, Demoulin JB, Oberg K, et al. Global microRNA Profiling of Well-Differentiated Small Intestinal Neuroendocrine Tumors. Modern Pathol (2013) 26(5):685–96. doi: 10.1038/modpathol.2012.216
98. Bowden M, Zhou CW, Zhang S, Brais L, Rossi A, Naudin L, et al. Profiling of Metastatic Small Intestine Neuroendocrine Tumors Reveals Characteristic miRNAs Detectable in Plasma. Oncotarget (2017) 8(33):54331–44. doi: 10.18632/oncotarget.16908
99. Lloyd KA, Moore AR, Parsons BN, O’Hara A, Boyce M, Dockray GJ, et al. Gastrin-Induced miR-222 Promotes Gastric Tumor Development by Suppressing P27kip1. Oncotarget (2016) 7(29):45462–78. doi: 10.18632/oncotarget.9990
100. Wang M, Xia X, Chu W, Xia L, Meng T, Liu L, et al. Roles of miR-186 and PTTG1 in Colorectal Neuroendocrine Tumors. Int J Clin Exp Med (2015) 8(12):22149–57.
101. Mitsuhashi K, Yamamoto I, Kurihara H, Kanno S, Ito M, Igarashi H, et al. Analysis of the Molecular Features of Rectal Carcinoid Tumors to Identify New Biomarkers That Predict Biological Malignancy. Oncotarget (2015) 6(26):22114–25. doi: 10.18632/oncotarget.4294
102. Rapa I, Votta A, Felice B, Righi L, Giorcelli J, Scarpa A, et al. Identification of MicroRNAs Differentially Expressed in Lung Carcinoid Subtypes and Progression. Neuroendocrinology (2015) 101(3):246–55. doi: 10.1159/000381454
103. Dizdar L, Werner TA, Drusenheimer JC, Möhlendick B, Raba K, Boeck I, et al. BRAF(V600E) Mutation: A Promising Target in Colorectal Neuroendocrine Carcinoma. Int J Cancer (2019) 144(6):1379–90. doi: 10.1002/ijc.31828
104. Mertens F, Johansson B, Fioretos T, Mitelman F. The Emerging Complexity of Gene Fusions in Cancer. Nat Rev Cancer (2015) 15(6):371–81. doi: 10.1038/nrc3947
105. Stransky N, Cerami E, Schalm S, Kim JL, Lengauer C. The Landscape of Kinase Fusions in Cancer. Nat Commun (2014) 5(1):4846. doi: 10.1038/ncomms5846
106. Pagani F, Randon G, Guarini V, Raimondi A, Prisciandaro M, Lobefaro R, et al. The Landscape of Actionable Gene Fusions in Colorectal Cancer. Int J Mol Sci (2019) 20(21):5319. doi: 10.3390/ijms20215319
107. Karlsson A, Brunnström H, Lindquist KE, Jirström K, Jönsson M, Rosengren F, et al. Mutational and Gene Fusion Analyses of Primary Large Cell and Large Cell Neuroendocrine Lung Cancer. Oncotarget (2015) 6(26):22028–37. doi: 10.18632/oncotarget.4314
108. Zhou Z, Zhu L, Niu X, Shen S, Zhao Y, Zhang J, et al. Comparison of Genomic Landscapes of Large Cell Neuroendocrine Carcinoma, Small Cell Lung Carcinoma, and Large Cell Carcinoma. Thorac Cancer (2019) 10(4):839–47. doi: 10.1111/1759-7714.13011
109. Shamir ER, Devine WP, Pekmezci M, Umetsu SE, Krings G, Federman S, et al. Identification of High-Risk Human Papillomavirus and Rb/E2F Pathway Genomic Alterations in Mutually Exclusive Subsets of Colorectal Neuroendocrine Carcinoma. Modern Pathol (2019) 32(2):290–305. doi: 10.1038/s41379-018-0131-6
110. Koh J, Nam SK, Kwak Y, Kim G, Kim KK, Lee BC, et al. Comprehensive Genetic Features of Gastric Mixed Adenoneuroendocrine Carcinomas and Pure Neuroendocrine Carcinomas. J Pathol (2021) 253(1):94–105. doi: 10.1002/path.5556
111. Cook JR, Dehner LP, Collins MH, Ma Z, Morris SW, Coffin CM, et al. Anaplastic Lymphoma Kinase (ALK) Expression in the Inflammatory Myofibroblastic Tumor: A Comparative Immunohistochemical Study. Am J Surg Pathol (2001) 25(11):1364–71. doi: 10.1097/00000478-200111000-00003
112. Huang EJ, Reichardt LF. Trk Receptors: Roles in Neuronal Signal Transduction. Annu Rev Biochem (2003) 72(1):609–42. doi: 10.1146/annurev.biochem.72.121801.161629
113. Tognon C, Knezevich SR, Huntsman D, Roskelley CD, Melnyk N, Mathers JA, et al. Expression of the ETV6-NTRK3 Gene Fusion as a Primary Event in Human Secretory Breast Carcinoma. Cancer Cell (2002) 2(5):367–76. doi: 10.1016/s1535-6108(02)00180-0
114. Drilon A, Li G, Dogan S, Gounder M, Shen R, Arcila M, et al. What Hides Behind the MASC: Clinical Response and Acquired Resistance to Entrectinib After ETV6-NTRK3 Identification in a Mammary Analogue Secretory Carcinoma (MASC). Ann Oncol (2016) 27(5):920–6. doi: 10.1093/annonc/mdw042
115. Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, et al. Studies of the HER-2/Neu Proto-Oncogene in Human Breast and Ovarian Cancer. Sci (New York NY) (1989) 244(4905):707–12. doi: 10.1126/science.2470152.Citedin:Pubmed
116. Gravalos C, Jimeno A. HER2 in Gastric Cancer: A New Prognostic Factor and a Novel Therapeutic Target. Ann Oncol (2008) 19(9):1523–9. doi: 10.1093/annonc/mdn169
117. Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in Combination With Chemotherapy Versus Chemotherapy Alone for Treatment of HER2-Positive Advanced Gastric or Gastro-Oesophageal Junction Cancer (ToGA): A Phase 3, Open-Label, Randomised Controlled Trial. Lancet (Lond Engl) (2010) 376(9742):687–97. doi: 10.1016/s0140-6736(10)61121-x
118. Swain SM, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M, et al. Pertuzumab, Trastuzumab, and Docetaxel in HER2-Positive Metastatic Breast Cancer. N Engl J Med (2015) 372(8):724–34. doi: 10.1056/NEJMoa1413513
119. Inno A, Bogina G, Turazza M, Bortesi L, Duranti S, Massocco A, et al. Neuroendocrine Carcinoma of the Breast: Current Evidence and Future Perspectives. Oncologist (2016) 21(1):28–32. doi: 10.1634/theoncologist.2015-0309
120. Irelli A, Sirufo MM, Morelli L, D’Ugo C, Ginaldi L, De Martinis M. Neuroendocrine Cancer of the Breast: A Rare Entity. J Clin Med (2020) 9(5):1452. doi: 10.3390/jcm9051452
121. Ishida M, Sekine S, Taniguchi H, Fukagawa T, Katai H, Kushima R. Consistent Absence of HER2 Expression, Regardless of HER2 Amplification Status, in Neuroendocrine Carcinomas of the Stomach. Histopathology (2014) 64(7):1027–31. doi: 10.1111/his.12348.Citedin:Pubmed
122. Yamaguchi M, Hirose K, Hirai N. HER2 Expression in Gastrointestinal Carcinoid Tumors: High in Intestinal But Not in Gastric Tumors. Surg Today (2007) 37(3):270–1. doi: 10.1007/s00595-006-3387-2
123. Yamashita S, Abe H, Kunita A, Yamashita H, Seto Y, Ushiku T. Programmed Cell Death Protein 1/Programmed Death Ligand 1 But Not HER2 Is a Potential Therapeutic Target in Gastric Neuroendocrine Carcinoma. Histopathology (2021) 78(3):381–91. doi: 10.1111/his.14230
124. Gilbert JA, Adhikari LJ, Lloyd RV, Rubin J, Haluska P, Carboni JM, et al. Molecular Markers for Novel Therapies in Neuroendocrine (Carcinoid) Tumors. Endocrine-Relat Cancer (2010) 17(3):623–36. doi: 10.1677/erc-09-0318
125. Iyoda A, Travis WD, Sarkaria IS, Jiang S-X, Amano H, Sato Y, et al. Expression Profiling and Identification of Potential Molecular Targets for Therapy in Pulmonary Large-Cell Neuroendocrine Carcinoma. Exp Ther Med (2011) 2(6):1041–5. doi: 10.3892/etm.2011.343
126. Kaufman HL, Russell J, Hamid O, Bhatia S, Terheyden P, D’Angelo SP, et al. Avelumab in Patients With Chemotherapy-Refractory Metastatic Merkel Cell Carcinoma: A Multicentre, Single-Group, Open-Label, Phase 2 Trial. Lancet Oncol (2016) 17(10):1374–85. doi: 10.1016/s1470-2045(16)30364-3
127. D’Angelo SP, Russell J, Lebbé C, Chmielowski B, Gambichler T, Grob JJ, et al. Efficacy and Safety of First-Line Avelumab Treatment in Patients With Stage IV Metastatic Merkel Cell Carcinoma: A Preplanned Interim Analysis of a Clinical Trial. JAMA Oncol (2018) 4(9):e180077. doi: 10.1001/jamaoncol.2018.0077
128. Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-Line Atezolizumab Plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med (2018) 379(23):2220–9. doi: 10.1056/NEJMoa1809064
129. Yao JC, Strosberg J, Fazio N, Pavel ME, Bergsland E, Ruszniewski P, et al. Spartalizumab in Metastatic, Well/Poorly-Differentiated Neuroendocrine Neoplasms. Endocrine-Relat Cancer (2021) 28(3):161–72. doi: 10.1530/erc-20-0382
130. Patel SP, Othus M, Chae YK, Giles FJ, Hansel DE, Singh PP, et al. A Phase II Basket Trial of Dual Anti-CTLA-4 and Anti-PD-1 Blockade in Rare Tumors (DART SWOG 1609) in Patients With Nonpancreatic Neuroendocrine Tumors. Clin Cancer Res (2020) 26(10):2290–6. doi: 10.1158/1078-0432.ccr-19-3356
131. Goel A, Boland CR. Epigenetics of Colorectal Cancer. Gastroenterology (2012) 143(6):1442–60. doi: 10.1053/j.gastro.2012.09.032
132. Smyrk TC, Watson P, Kaul K, Lynch HT. Tumor-Infiltrating Lymphocytes are a Marker for Microsatellite Instability in Colorectal Carcinoma. Cancer (2001) 91(12):2417–22. doi: 10.1002/1097-0142(20010615)91:12<2417::AID-CNCR1276>3.0.CO;2-U
133. Gatalica Z, Snyder CL, Yeatts K, Xiao N, Holterman D, Lynch HT. Programmed Death 1 (PD-1) Lymphocytes and Ligand (PD-L1) in Colorectal Cancer and Their Relationship to Microsatellite Instability Status. J Clin Oncol (2014) 32(15_suppl):3625–5. doi: 10.1200/jco.2014.32.15_suppl.3625
134. Sehgal R, Sheahan K, O’Connell PR, Hanly AM, Martin ST, Winter DC. Lynch Syndrome: An Updated Review. Genes (2014) 5(3):497–507. doi: 10.3390/genes5030497
135. Berindan-Neagoe I, Monroig Pdel C, Pasculli B, Calin GA. MicroRNAome Genome: A Treasure for Cancer Diagnosis and Therapy. CA: Cancer J Clin (2014) 64(5):311–36. doi: 10.3322/caac.21244
Keywords: neuroendocrine tumors, neuroendocrine carcinoma (NEC), next-generation sequencing (NGS), PD-L1, high microsatellite instability (MSI-H)
Citation: Prisciandaro M, Antista M, Raimondi A, Corti F, Morano F, Centonze G, Sabella G, Mangogna A, Randon G, Pagani F, Prinzi N, Niger M, Corallo S, Castiglioni di Caronno E, Massafra M, Bartolomeo MD, Braud Fd, Milione M and Pusceddu S (2022) Biomarker Landscape in Neuroendocrine Tumors With High-Grade Features: Current Knowledge and Future Perspective. Front. Oncol. 12:780716. doi: 10.3389/fonc.2022.780716
Received: 21 September 2021; Accepted: 10 January 2022;
Published: 04 February 2022.
Edited by:
Alex Giakoustidis, Aristotle University of Thessaloniki, GreeceReviewed by:
Francesco Enrico D’Amico, University of Padua, ItalyMoran Amit, University of Texas MD Anderson Cancer Center, United States
Copyright © 2022 Prisciandaro, Antista, Raimondi, Corti, Morano, Centonze, Sabella, Mangogna, Randon, Pagani, Prinzi, Niger, Corallo, Castiglioni di Caronno, Massafra, Bartolomeo, Braud, Milione and Pusceddu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michele Prisciandaro, bWljaGVsZS5wcmlzY2lhbmRhcm9AaXN0aXR1dG90dW1vcmkubWkuaXQ=
†These authors share senior authorship
 Michele Prisciandaro
Michele Prisciandaro Maria Antista1
Maria Antista1 Alessandra Raimondi
Alessandra Raimondi Giovanni Centonze
Giovanni Centonze Alessandro Mangogna
Alessandro Mangogna Giovanni Randon
Giovanni Randon Monica Niger
Monica Niger Filippo de Braud
Filippo de Braud Massimo Milione
Massimo Milione Sara Pusceddu
Sara Pusceddu