
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 11 October 2021
Sec. Atherosclerosis and Vascular Medicine
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.757030
This article is part of the Research Topic Lipids and Inflammation in Health and Disease View all 26 articles
 Adam Hartley1
Adam Hartley1 Matthew Shun-Shin2
Matthew Shun-Shin2 Mikhail Caga-Anan1
Mikhail Caga-Anan1 Christopher Rajkumar2
Christopher Rajkumar2 Alexandra N. Nowbar2
Alexandra N. Nowbar2 Michael Foley2
Michael Foley2 Darrel P. Francis2
Darrel P. Francis2 Dorian O. Haskard1
Dorian O. Haskard1 Ramzi Y. Khamis1*†
Ramzi Y. Khamis1*† Rasha K. Al-Lamee2†
Rasha K. Al-Lamee2†Aim: Malondialdehyde-modified low-density lipoprotein (MDA-LDL) forms a significant component of oxidised LDL. The effects of exercise on levels of MDA-LDL and anti-MDA-LDL antibodies are not well-understood. Furthermore, it is not known whether these can be modified in patients with coronary artery disease by percutaneous coronary intervention (PCI).
Methods: The Objective Randomised Blinded Investigation with optimal medical Therapy of Angioplasty in stable angina (ORBITA) trial was the first blinded, multi-centre randomised trial of PCI vs. placebo procedure for angina relief. Serum samples were available at four time-points: pre-randomisation pre- (P1) and post- (P2) exercise and post-randomisation (6-weeks following the PCI or placebo procedure), pre- (P3) and post- (P4) exercise. ELISAs were performed using laboratory-developed assays for MDA-LDL (adjusted for Apolipoprotein B) and anti-MDA-LDL antibodies.
Results: One hundred ninety-six of the 200 patients (age 66.1 [SD 8.99] years, 28% female) with severe single vessel coronary artery disease suitable for PCI enrolled in the ORBITA trial had blood available for analysis. With exercise at pre-randomisation (P2–P1) there was no significant change in adjusted MDA-LDL (−0.001, 95% CI −0.004 to 0.001; p = 0.287); however, IgG and IgM anti-MDA-LDL significantly declined (−0.022, 95% CI −0.029 to −0.014, p < 0.0001; −0.016, 95% CI −0.024 to −0.008, p = 0.0002, respectively). PCI did not have a significant impact on either the pre-exercise values (P3 controlling for P1) of MDA-LDL (p = 0.102), IgG (p = 0.444) or IgM anti-MDA-LDL (p = 0.909). Nor did PCI impact the exercise induced changes in these markers (P4 controlling for P1, P2, and P3) for MDA-LDL (p = 0.605), IgG (p = 0.725) or IgM anti-MDA-LDL (p = 0.171). Pre-randomisation ischaemia on stress echo did not impact these interactions.
Conclusions: Exercise results in an acute reduction in anti-oxLDL antibodies in patients with severe single vessel coronary disease, possibly indicating an induction in homoeostatic clearance via the innate immune system. However, PCI did not ameliorate this effect.
Oxidised low-density lipoprotein (oxLDL) is found both within atherosclerotic plaques and the plasma of patients with cardiovascular disease (CVD). It has been shown, in observational studies, to be a significant predictor of cardiovascular outcome, reflecting a central role in atherogenesis (1–3).
Oxidation specific epitopes (OSEs) on oxLDL can act as “danger associated molecular patterns,” whereby they undergo recognition by members of the innate immune system such as C-reactive protein, complement proteins and both “natural” and “adaptive” antibodies (3). This provides an important homeostatic mechanism for oxLDL clearance (4). Anti-oxLDL antibodies of the IgM subclass have been generally found to confer protective benefit from coronary artery disease (CAD) and CVD, and indeed lower levels of IgM to one well-characterised epitope [malondialdehyde-modification of LDL (MDA-LDL)] have been linked with features of atherosclerotic plaques vulnerable to rupture (5). Conversely, the association of IgG anti-oxLDL antibodies with CVD is less clear (6).
Exercise, although of course widely accepted to carry significant health advantages, can transiently increase circulating oxLDL (7), and high levels of physical activity may counterintuitively contribute to atherogenesis in the presence of adequate substrate (8).
It has been demonstrated previously that percutaneous coronary intervention (PCI) can lead acutely to transiently higher levels of oxLDL in the plasma, attributed to either direct mechanical release/disruption from stented atherosclerotic plaque or increased generation (9). Whether or not this is clinically significant is unknown.
In this study we sought to assess the effects of exercise on plasma MDA-LDL in relation to anti-MDA-LDL antibodies. The availability of blood samples collected from CAD patients enrolled in the Objective Randomised Blinded Investigation with optimal medical Therapy of Angioplasty in stable angina (ORBITA) trial provided the unique opportunity of determining whether any changes with exercise we observed are influenced by coronary artery intervention (10).
The ORBITA trial was a blinded, multi-centre randomised trial of PCI (105 patients) vs. a placebo procedure (95 patients) for stable CAD. The full ORBITA study protocol has been described previously (10). In brief, patients were enrolled if they had severe (≥70%) single vessel stenoses suitable for PCI. Key exclusion criteria were angiographic stenosis ≥50% in a non-target vessel, acute coronary syndrome, previous coronary artery bypass graft surgery, left main stem coronary disease, severe valvular disease or left ventricular systolic impairment. After enrolment, patients received 6 weeks of intense anti-anginal medication optimisation and were then randomised 1:1 to undergo PCI or a placebo procedure. The primary endpoint was difference in exercise time increment between baseline and 6-week follow-up between the two groups. Dobutamine stress echocardiography and cardiopulmonary exercise testing were performed at pre-randomisation after medication optimisation and 6-weeks post-randomisation, as previously described (11). From this a stress echo score was calculated (11); in brief, each abnormal segment was assigned a single point and segments totalled.
The London Central Research Ethics Committee (13/LO/1340) approved the study and the investigation conformed to the principles outlined in the Declaration of Helsinki. Written consent was obtained from all patients prior to enrolment. The study is registered with ClinicalTrials.gov, number NCT02062593.
Blood samples were obtained on the day of pre-randomisation assessment (after the six-week medical optimisation run-in), prior to exercise (timepoint P1) and 3 h after exercise (P2). At post-randomisation assessment (6 weeks following the PCI or placebo procedure) further blood tests were obtained: before (P3) and 3 h after (P4) exercise (Figure 1). On both occasions exercise consisted of a cardiopulmonary exercise test, performed with the QUARK CPET breath-by-breath metabolic measurement system (COSMED, Rome, Italy). A physician and a physiologist, both blinded to treatment assignment, performed all tests. The test was continued until the development of limiting symptoms (angina, dyspnoea, or fatigue), heart rhythm or blood pressure abnormalities, or marked ST-segment deviation (≥0.20 mV associated with typical angina or in the first stage of exercise).
Blood plasma specimens were stored at −80°C and thawed to room temperature prior to use. Enzyme-linked immunosorbent assays (ELISA) were performed to assess for MDA-LDL, IgG anti-MDA-LDL, IgM anti-MDA-LDL, and Apolipoprotein B (ApoB), as have been conducted previously (5, 12). ApoB levels were assessed as a marker for dilutional change. All researchers were blinded to treatment allocation. Intra-plate and inter-plate coefficients of variance were <5 and <15%, respectively.
A sandwich ELISA protocol was adopted using LO1, an in-house generated IgG monoclonal mouse autoantibody, to detect MDA-LDL (13). ELISA plates were coated with 10 μg/mL of LO1 as the capture antibody prior to washing and blocking with 3% bovine serum albumin (BSA) in phosphate-buffered saline (PBS). Goat biotinylated polyclonal anti-ApoB antibody (1:2000) (Abcam, Cambridge, MA, USA) and horseradish peroxidase (HRP)-conjugated streptavidin (R&D Systems, Minneapolis, MN, USA) at 1:200 dilution were used for detection. Subsequently, for this assay and all other assays, 3,3′,5,5′-tetramethylbenzidine (TMB) (Sigma Aldrich, Poole, UK) was added and the reaction stopped with 0.5M H2SO4. Plates were read at 450 nm optical density using a Synergy HT microplate reader (BioTek, VT, USA).
ApoB was measured by ELISA in sandwich format. Plates were coated with polyclonal goat anti-human anti-ApoB (Abcam, Cambridge, MA, 1:2000). Detection was performed with goat biotinylated anti-ApoB antibody, prior to HRP-conjugated streptavidin and TMB as above.
Acid hydrolysis of malondialdehyde bis (dimethyl acetal) (Sigma-Aldrich, Poole, UK) was performed to produce 0.5 M MDA solution, which was subsequently incubated with native LDL at 37°C for 3 h, as described previously (14). This generated MDA-LDL, which was then eluted through a Zeba Spin desalting column (ThermoFisher Scientific, Waltham, MA, USA), and PBS/0.01% EDTA added to prevent additional oxidation.
ELISA plates were coated with 10 μg/mL MDA-LDL, prior to washing and blocking as above. The primary detection antibodies were either unlabelled mouse anti-human IgG (Southern Biotech, Birmingham, AL, USA, 1:2000), or biotinylated mouse anti-human IgM (Southern Biotech, Birmingham, AL, USA, 1:2000). The secondary detection antibody was HRP-conjugated polyclonal rabbit anti-mouse antibody (Dako, Cambridgeshire, UK, 1:2000) for IgG anti-MDA-LDL and HRP-conjugated streptavidin for IgM anti-MDA-LDL.
Concentrations of MDA-LDL and ApoB were derived by interpolation from log transformation of OD values onto a sigmoidal, four-parametric logistic curve using GraphPad Prism 8 (La Jolla, CA, USA). To correct for possible changes in plasma protein concentration due to exercise, each MDA-LDL concentration was adjusted by expressing the values as a ratio to ApoB concentration. MDA-LDL adjusted for ApoB is used throughout this study. Raw OD values were utilised for anti-MDA-LDL antibodies.
Continuous variables are presented as mean ± standard deviation (SD) or median with interquartile range (IQR), depending on the normality of the distribution. Categorical variables are presented as numbers with percentages.
Statistical analysis was performed using R 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria) using the modelling package “rms” (15, 16). The change in biomarkers with exercise from timepoint P1 to P2 and P3 to P4 were assessed using paired Student's t-test. The remainder of the analyses were performed using ordinary least squares regression modelling. The effect of PCI vs. placebo on the pre-exercise (P3) biomarkers was assessed by including the treatment arm, and the P1 time point in the model. The effect of PCI on the post-exercise biomarker was assessed by including treatment arm and the P1, P2, and P3 timepoint in the model. The effect of stress echo score on the pre-randomisation post-exercise (P2) biomarker was assessed by including the P1 timepoint, and the stress echo score with a restricted cubic spline with 3 knots. The effect of stress echo score on the placebo-controlled impact of PCI on the post-exercise (P4) biomarker levels was again assessed using modelling, including the treatment arm and pre-randomisation stress echo score (with a restricted cubic spline) and their interaction, and timepoints P1, P2, and P3.
The ORBITA trial randomised 200 patients to either PCI or placebo procedure between January 6, 2014, and August 11, 2017. From these patients, 196 had blood samples available for analysis. Table 1 displays baseline patient characteristics. The mean age in the PCI group was 66.0 (9.50) (mean [standard deviation, SD]) years and 66.2 (8.45) years in the placebo group. There were no substantial differences in baseline demographics between the PCI and placebo groups. At pre-randomisation assessment, 98% (192/196) of patients were taking aspirin and 95% (186/196) were taking a statin. 78% (153/196) of the study population were taking β-blockers whilst 91% (178/196) were taking calcium channel antagonists. The median number of anti-anginal medications was three prior to randomisation.
Table 2 displays the average values for adjusted MDA-LDL and anti-MDA-LDL antibodies at each study timepoint. There was no significant change with exercise (P1–P2) in ApoB (−9.189 OD, 95% CI −19.913 to 1.542; p = 0.09) or in MDA-LDL adjusted for ApoB levels (−0.001, 95% CI −0.004 to 0.001; p = 0.287; Figure 2A). On the other hand, there was a significant reduction in anti-MDA-LDL antibodies with exercise: IgG anti-MDA-LDL declined by −0.022 OD (95% CI −0.029 to −0.014; p < 0.0001; Figure 2B; whilst IgM anti-MDA-LDL reduced by −0.016 OD (95% CI −0.024 to −0.008; p = 0.0002; Figure 2C).
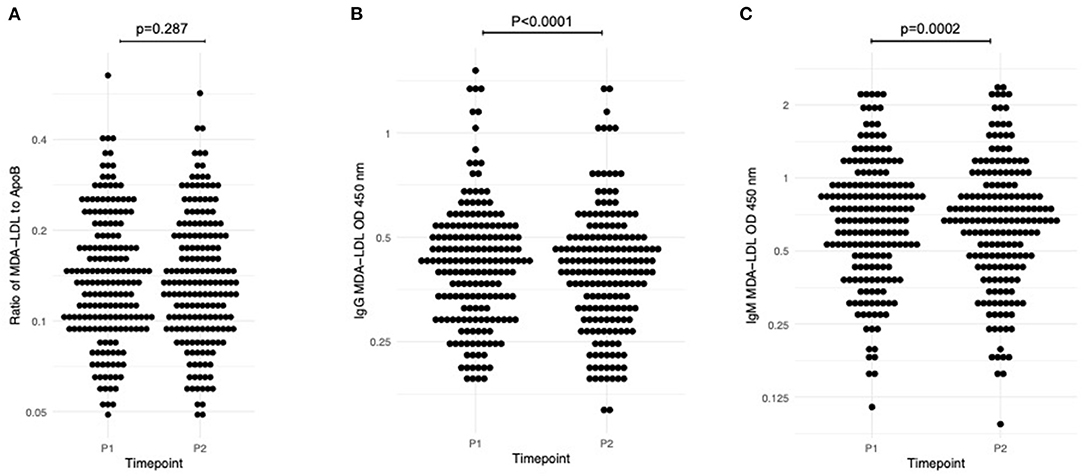
Figure 2. Dynamic change in measured biomarkers between P1 (baseline pre-randomisation assessment, pre-exercise) and P2 (baseline pre-randomisation assessment, post-exercise). (A) Adjusted MDA-LDL; (B) IgG anti-MDA-LDL; (C) IgM anti-MDA-LDL. One sample t-test used to assess significance.
PCI, when compared to placebo, did not have a significant impact on pre-exercise (P3 controlling for P1) adjusted MDA-LDL, IgG or IgM anti-MDA-LDL (p = 0.102, p = 0.444, p = 0.909 respectively). Similarly, PCI did not have a significant impact on post-exercise (P4 controlling for P3, P2, and P1) biomarker measurements (adjusted MDA-LDL p = 0.605, IgG anti-MDA-LDL p = 0.725, IgM anti-MDA-LDL p = 0.171). This is despite the exercise induced changes in the measured antibodies remaining in the whole study population when retested, with significant reductions in both IgG and IgM anti-MDA-LDL (−0.006, 95% CI −0.013 to −0.0004, p = 0.036; −0.015, 95% CI −0.024 to −0.006, p = 0.002, respectively. Again, there was no significant change in adjusted MDA-LDL (0.0007, 95% CI −0.003 to 0.004; p = 0.707.
We also examined for a relationship between oxidative biomarker change with exercise at baseline (P2 controlling for P1) and exercise intensity. There were no significant associations between MDA-LDL (p = 0.355), IgG (p = 0.386) or IgM (p = 0.657) anti-MDA-LDL antibodies with exercise duration. Similarly, there were no relationships between biomarker changes and maximum oxygen consumption (VO2 max) (MDA-LDL [p = 0.121], IgG anti-MDA-LDL [p = 0.701], IgM anti-MDA-LDL [p = 0.07]).
Of the patients included in the study, 180 had dobutamine stress echocardiography performed at baseline. The pre-randomisation stress echo score (with higher scores reflecting more abnormal myocardial segments) did not significantly affect the post-exercise (P2 controlling for P1) MDA-LDL (p = 0.349), IgG anti-MDA-LDL (p = 0.852) or IgM anti-MDA-LDL (p = 0.255). Furthermore, the pre-randomisation stress echo score did not affect the placebo-controlled impact of PCI on the post-exercise (P4) levels of adjusted MDA-LDL (p = 0.484), IgG anti-MDA-LDL (p = 0.750) or IgM anti-MDA-LDL (p = 0.759). Therefore, even patients with high levels of baseline ischaemia saw no significant effect of PCI on exercise-induced biomarker change.
In this study we find that high intensity exercise resulted in significant plasma oxidative biomarker changes in patients with severe CAD on intensively up-titrated anti-anginal medical therapy, with IgG and IgM anti-MDA-LDL antibodies significantly decreasing following exercise. PCI neither significantly influenced the biomarkers at baseline prior to exercise, nor their changes after exercise.
One explanation for the immune system modulation in this population is that the vigorous exercise acts as a severe cellular stressor, resulting in oxidative stress with increased free radical generation and MDA-LDL formation. Consequently, there is consumption of the circulating anti-oxLDL antibodies as they beneficially clear the increased antigenic load, forming complexes and trafficking them to the reticuloendothelial system for removal in a homeostatic clearance mechanism. These observations are consistent with other studies from our laboratory, which demonstrate acute reduction in anti-MDA-LDL antibodies following major vascular surgery and coronary artery bypass grafting (17).
The degree of myocardial ischaemia as assessed by stress echocardiography did not influence biomarker changes in this study. We also report no significant change in MDA-LDL, which possibly may be due to rapid immune clearance. Nonetheless, an acute increase in circulating oxLDL with exercise has been demonstrated previously in diseased populations, such as hypertensive hypercholesterolaemic patients (18), chronic heart failure (19), diabetes mellitus (20) and in aged populations (7, 21). A possible explanation for this discrepancy is the timing when samples are obtained; blood samples were taken 3 h following exercise in this study, rather than immediately post exercise as in many of these studies. As such, the acute rise prior to homeostatic clearance may be missed at the time of blood sampling. Another possible explanation is that the highly optimised medical therapy renders the patients relatively non-ischaemic, and as such the biomarkers behave as though they were not sampled from patients with severe CAD. Indeed, there is no consensus in the literature on exercise-induced oxLDL changes in healthy participants (22–27), and in some circumstances no changes in oxLDL levels, even following very high intensity exercise, have been found (28).
The chronic effect of regular exercise on baseline oxLDL levels has been examined, with studies reporting improved oxidised lipid profiles with sufficiently robust exercise (29–33). Moreover, exercise training has been demonstrated to counteract raised baseline oxLDL levels that are found in overweight or obese individuals (34), to combat the acute exercise-induced increased LDL oxidation that occurs with advancing age (21), and in patients with CVD undergoing cardiac rehabilitation, those who completed the 6-month exercise program had significantly lower baseline MDA-LDL levels than their counterparts (35). Additionally, MDA-LDL levels have been shown to vary inversely proportionally to daily pedometer step counts (36). Moreover, in animal models of exercise, it has been demonstrated that exercise can reduce oxidative stress (37) and even selectively increase the B-1 cell population and natural IgM levels (38).
One hypothesis to explain the apparent paradox between acute and chronic exercise and circulating oxLDL level is that recurrent plasma oxLDL exposure leads to greater homeostatic immune system induction. With a greater circulating anti-oxLDL antibody reservoir, there may have a better adapted clearance mechanism to cope with the oxidative stress of exercise once the antioxidant capacity is exceeded and go some way to explain why baseline oxLDL levels decline with greater fitness. Perhaps greater immune system induction could also underpin some of the cardiovascular benefit associated with exercise. Indeed, exercise-related cardiovascular events occur more commonly in inactive people with multiple cardiovascular risk factors than in well-trained athletes, who may have more honed innate immunity (39). Immunosenescence, ageing of the adaptive and innate immune systems, may also explain the greater LDL oxidation that occurs with exercise in advancing age (7, 40).
In our study we only measured samples at 3 h following single episodes of exercise and identified immune consumption. If samples were tested at multiple earlier timepoints after frequent exercise we may expect to see a rebound rise in anti-oxLDL antibodies, as has been reported in the literature (41, 42). However, in a recent study by Bachi and colleagues, trained athletes after running a marathon exhibited no change in IgM or IgG anti-MDA-LDL antibodies immediately or 72-h after the race, despite increases in plasma oxLDL (27). Again, perhaps the timepoints tested missed any small dynamic changes with the utilised assay, or the elite fitness of the participants means that their baseline anti-oxLDL antibody levels are already fully induced.
There is some plausibility to the theory of immune induction following an oxLDL stimulus: using PCI as an example (43), studies have shown long-lasting immune system induction to rapid oxLDL increases, with raised IgG and IgM anti-oxLDL antibodies present out to 6 months following the index procedure (9, 44). Whilst these studies demonstrated immunomodulation following PCI, we did not find such a trend in this study, with unchanged baseline or post-exercise oxidative biomarkers. It may be expected that PCI, through restoring unobstructed flow and therefore coronary perfusion, would attenuate the immune system induction seen with exercise. As above, this unexpected result may be due to sample timing (taken at 6-weeks following the procedure, where the peak level may have been missed), or an interaction with medical therapy, with a very high proportion of patients on a statin and well-titrated anti-anginal therapy. What is demonstrated however, given the lack of association between severity of baseline ischaemia and dynamic biomarker change with exercise, is that anti-MDA-LDL antibodies in themselves were not biomarkers of ischaemia per se, in this study.
The main limitations of this study lie in the fixed timing of blood samples for biomarker analysis. The availability of plasma samples immediately following exercise and the PCI/placebo procedure would shed further light onto the interaction of the stimuli and the immune system. Furthermore, the limited number of blood sampling timepoints makes it difficult to exactly elucidate when plasma levels peak and when they are cleared. Another caveat of this study is that MDA adduction of LDL is but one of a range of possible post-translational oxidative modifications on LDL; however, MDA-LDL does comprise a significant component of the oxLDL population (45).
Using blood samples obtained from the ORBITA trial, this study demonstrates that exercise results in an acute reduction in anti-oxLDL antibodies, possibly indicating an induction in homoeostatic clearance of oxLDL via the innate immune system. However, PCI did not ameliorate this effect.
The raw data supporting the conclusions of this article will be made available by the authors upon appropriate request following review by the corresponding author.
The studies involving human participants were reviewed and approved by London Central Research Ethics Committee (13/LO/1340). The patients/participants provided their written informed consent to participate in this study.
AH, MC-A, and RA-L: investigation. DH, DF, RA-L, and RK: supervision. AH, MC-A, and MS-S: data analysis. AH: writing—original draft. AH, MS-S, CR, AN, MF, DH, RK, and RA-L: writing—review and editing. All authors contributed to the article and approved the submitted version.
AH was funded by a Wellcome Trust Clinical Research Fellowship (220572/Z/20/Z). RK was funded by a BHF Clinical Research Fellowship (FS/17/16/32560) and had received a Wellcome Trust Clinical Research Fellowship (095034/Z/10/Z) (as part of the Wellcome Trust/GSK Fellowship programme). DH received professorial chair funding from the British Heart Foundation (BHF). AN was supported by the NIHR Academy.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ApoB, apolipoprotein B-100; BSA, bovine serum albumin; ELISA, enzyme-linked immunosorbent assay; LDL, low-density lipoprotein; LDS, lithium dodecyl sulphate; MDA, malondialdehyde; MDA-LDL, malondialdehyde-conjugated LDL; OD, optical density; OxLDL, oxidised low-density lipoprotein; RT, room temperature.
1. Tsimikas S, Kiechl S, Willeit J, Mayr M, Miller ER, Kronenberg F, et al. Oxidized phospholipids predict the presence and progression of carotid and femoral atherosclerosis and symptomatic cardiovascular disease: five-year prospective results from the Bruneck study. J Am Coll Cardiol. (2006) 47:2219–28. doi: 10.1016/j.jacc.2006.03.001
2. van Dijk RA, Kolodgie F, Ravandi A, Leibundgut G, Hu PP, Prasad A, et al. Differential expression of oxidation-specific epitopes and apolipoprotein(a) in progressing and ruptured human coronary and carotid atherosclerotic lesions. J Lipid Res. (2012) 53:2773–90. doi: 10.1194/jlr.P030890
3. Steinberg D, Witztum JL. Oxidized low-density lipoprotein and atherosclerosis. Arterioscler Thromb Vasc Biol. (2010) 30:2311–6. doi: 10.1161/ATVBAHA.108.179697
4. Upadhye A, Srikakulapu P, Gonen A, Hendrikx S, Perry HM, Nguyen AT, et al. Diversification and CXCR4-dependent establishment of the bone marrow B-1a cell pool governs atheroprotective igm production linked to human coronary atherosclerosis. Circ Res. (2019) 125:e55–70. doi: 10.1161/CIRCRESAHA.119.315786
5. van den Berg VJ, Haskard DO, Fedorowski A, Hartley A, Kardys I, Caga-Anan M, et al. IgM anti-malondialdehyde low density lipoprotein antibody levels indicate coronary heart disease and necrotic core characteristics in the Nordic Diltiazem (NORDIL) study and the Integrated Imaging and Biomarker Study 3 (IBIS-3). EBioMedicine. (2018) 36:63–72. doi: 10.1016/j.ebiom.2018.08.023
6. van den Berg VJ, Vroegindewey MM, Kardys I, Boersma E, Haskard D, Hartley A, et al. Anti-oxidized LDL antibodies and coronary artery disease: a systematic review. Antioxidants (Basel). (2019) 8:484. doi: 10.3390/antiox8100484
7. Medlow P, McEneny J, Murphy MH, Trinick T, Duly E, Davison GW. Lipoprotein subfraction oxidation in acute exercise and ageing. Free Radic Res. (2016) 50:345–53. doi: 10.3109/10715762.2015.1109084
8. Fernandez AB, Chaudhry W, Thompson PD. Coronary atherosclerosis in masters athletes: mechanisms and implications for cardiovascular disease risk. Curr Treat Options Cardiovasc Med. (2019) 21:87. doi: 10.1007/s11936-019-0798-0
9. Tsimikas S, Lau HK, Han KR, Shortal B, Miller ER, Segev A, et al. Percutaneous coronary intervention results in acute increases in oxidized phospholipids and lipoprotein(a): short-term and long-term immunologic responses to oxidized low-density lipoprotein. Circulation. (2004) 109:3164–70. doi: 10.1161/01.CIR.0000130844.01174.55
10. Al-Lamee R, Thompson D, Dehbi HM, Sen S, Tang K, Davies J, et al. Percutaneous coronary intervention in stable angina (ORBITA): a double-blind, randomised controlled trial. Lancet (London, England). (2018) 391:31–40. doi: 10.1016/S0140-6736(17)32714-9
11. Al-Lamee RK, Shun-Shin MJ, Howard JP, Nowbar AN, Rajkumar C, Thompson D, et al. Dobutamine stress echocardiography ischemia as a predictor of the placebo-controlled efficacy of percutaneous coronary intervention in stable coronary artery disease: the stress echocardiography-stratified analysis of ORBITA. Circulation. (2019) 140:1971–80. doi: 10.1161/CIRCULATIONAHA.119.042918
12. Khamis RY, Hughes AD, Caga-Anan M, Chang CL, Boyle JJ, Kojima C, et al. High serum immunoglobulin G and M levels predict freedom from adverse cardiovascular events in hypertension: a nested case-control substudy of the anglo-scandinavian cardiac outcomes trial. EBioMedicine. (2016) 9:372–80. doi: 10.1016/j.ebiom.2016.06.012
13. Chang SH, Johns M, Boyle JJ, McConnell E, Kirkham PA, Bicknell C, et al. Model IgG monoclonal autoantibody-anti-idiotype pair for dissecting the humoral immune response to oxidized low density lipoprotein. Hybridoma (Larchmt). (2012) 31:87–98. doi: 10.1089/hyb.2011.0058
14. Palinski W, Yla-Herttuala S, Rosenfeld ME, Butler SW, Socher SA, Parthasarathy S, et al. Antisera and monoclonal antibodies specific for epitopes generated during oxidative modification of low density lipoprotein. Arteriosclerosis (Dallas, Tex). (1990) 10:325–35. doi: 10.1161/01.ATV.10.3.325
15. Team RC. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing (2020).
16. FEH Jr. Regression Modeling Strategies. R package Version 6.1-0 (2020). Available online at: https://cran.r-project.org/web/packages/rms/index.html
17. Pandey SS, Hartley A, Caga-Anan M, Ammari T, Khan AH, Nguyen BA, et al. A Novel immunoassay for malondialdehyde-conjugated low-density lipoprotein measures dynamic changes in the blood of patients undergoing coronary artery bypass graft surgery. Antioxidants. (2021) 10:1298.
18. Caparevic Z, Kostic N, Celic V, Cosic Z, Marina D, Ilic S, et al. Effects of acute exercise on atherogenic lipids in untreated mild hypertensive patients. Vojnosanit Pregl. (2009) 66:313–8. doi: 10.2298/VSP0904313C
19. Jorde UP, Colombo PC, Ahuja K, Hudaihed A, Onat D, Diaz T, et al. Exercise-induced increases in oxidized low-density lipoprotein are associated with adverse outcomes in chronic heart failure. Journal of cardiac failure. (2007) 13:759–64. doi: 10.1016/j.cardfail.2007.06.724
20. Kostic N, Caparevic Z, Marina D, Ilic S, Radojkovic J, Cosic Z, et al. Clinical evaluation of oxidative stress in patients with diabetes mellitus type II—impact of acute exercise. Vojnosanit Pregl. (2009) 66:459–64. doi: 10.2298/VSP0906459K
21. Medlow P, McEneny J, Murphy MH, Trinick T, Duly E, Davison GW. Exercise training protects the LDL I subfraction from oxidation susceptibility in an aged human population. Atherosclerosis. (2015) 239:516–22. doi: 10.1016/j.atherosclerosis.2015.02.012
22. Mitsui T, Nakamura T, Ito T, Umemoto Y, Sakamoto K, Kinoshita T, et al. Exercise significantly increases plasma adrenaline and oxidized low-density lipoprotein in normal healthy subjects but not in persons with spinal cord injury. Arch Phys Med Rehabil. (2012) 93:725–7. doi: 10.1016/j.apmr.2011.08.046
23. Vuorimaa T, Ahotupa M, Irjala K, Vasankari T. Acute prolonged exercise reduces moderately oxidized LDL in healthy men. Int J Sports Med. (2005) 26:420–5. doi: 10.1055/s-2004-821142
24. Valimaki IA, Vuorimaa T, Ahotupa M, Vasankari T. Effect of continuous and intermittent exercises on oxidised HDL and LDL lipids in runners. Int J Sports Med. (2016) 37:1103–9. doi: 10.1055/s-0042-114703
25. Wetzstein CJ, Shern-Brewer RA, Santanam N, Green NR, White-Welkley JE, Parthasarathy S. Does acute exercise affect the susceptibility of low density lipoprotein to oxidation? Free Rad Biol Med. (1998) 24:679–82. doi: 10.1016/S0891-5849(97)00320-1
26. Wiecek M, Maciejczyk M, Szymura J, Szygula Z. Sex differences in oxidative stress after eccentric and concentric exercise. Redox Rep. (2017) 22:478–85. doi: 10.1080/13510002.2017.1304195
27. Bachi AL, Sierra AP, Rios FJ, Goncalves DA, Ghorayeb N, Abud RL, et al. Athletes with higher VO2max present reduced oxLDL after a marathon race. BMJ Open Sport Exerc Med. (2015) 1:bmjsem-2015-000014. doi: 10.1136/bmjsem-2015-000014
28. Benedetti S, Catalani S, Peda F, Luchetti F, Citarella R, Battistelli S. Impact of the 24-h ultramarathon race on homocysteine, oxidized low-density lipoprotein, and paraoxonase 1 levels in professional runners. PLos ONE. (2018) 13:e0192392. doi: 10.1371/journal.pone.0192392
29. Cornelissen VA, Arnout J, Holvoet P, Fagard RH. Influence of exercise at lower and higher intensity on blood pressure and cardiovascular risk factors at older age. J Hypertens. (2009) 27:753–62. doi: 10.1097/HJH.0b013e328322cf60
30. Koh Y, Park J, Carter R. Oxidized low-density lipoprotein and cell adhesion molecules following exercise training. Int J Sports Med. (2018) 39:83–8. doi: 10.1055/s-0043-118848
31. Kwasniewska M, Kostka T, Jegier A, Dziankowska-Zaborszczyk E, Leszczynska J, Rebowska E, et al. Regular physical activity and cardiovascular biomarkers in prevention of atherosclerosis in men: a 25-year prospective cohort study. BMC Cardiovasc Disord. (2016) 16:65. doi: 10.1186/s12872-016-0239-x
32. Tiainen S, Kiviniemi A, Hautala A, Huikuri H, Ukkola O, Tokola K, et al. Effects of a two-year home-based exercise training program on oxidized LDL and HDL lipids in coronary artery disease patients with and without type-2 diabetes. Antioxidants (Basel). (2018) 7:144. doi: 10.3390/antiox7100144
33. Vasankari TJ, Kujala UM, Vasankari TM, Ahotupa M. Reduced oxidized LDL levels after a 10-month exercise program. Med Sci Sports Exerc. (1998) 30:1496–501. doi: 10.1097/00005768-199810000-00005
34. Kosola J, Ahotupa M, Kyrolainen H, Santtila M, Vasankari T. Good aerobic or muscular fitness protects overweight men from elevated oxidized LDL. Med Sci Sports Exerc. (2012) 44:563–8. doi: 10.1249/MSS.0b013e31823822cc
35. Takashima A, Ise T, Yagi S, Iwase T, Kimura S, Ueda Y, et al. Cardiac rehabilitation reduces serum levels of oxidized low-density lipoprotein. Circ J Off J Jpn Circ Soc. (2014) 78:2682–7. doi: 10.1253/circj.CJ-14-0532
36. Kotani K, Taniguchi N. Pedometer step counts and oxidized low-density lipoprotein levels among asymptomatic subjects. Ann Clin Lab Sci. (2012) 42:435–8. Available online at: http://www.annclinlabsci.org/content/42/4/435.full
37. Navarro A, Gomez C, Lopez-Cepero JM, Boveris A. Beneficial effects of moderate exercise on mice aging: survival, behavior, oxidative stress, and mitochondrial electron transfer. Am J Physiol Regul Integr Comp Physiol. (2004) 286:R505–11. doi: 10.1152/ajpregu.00208.2003
38. Elphick GF, Wieseler-Frank J, Greenwood BN, Campisi J, Fleshner M. B-1 cell (CD5+/CD11b+) numbers and nIgM levels are elevated in physically active vs. sedentary rats. J Appl Physiol. (2003) 95:199–206. doi: 10.1152/japplphysiol.01054.2002
39. Giri S, Thompson PD, Kiernan FJ, Clive J, Fram DB, Mitchel JF, et al. Clinical and angiographic characteristics of exertion-related acute myocardial infarction. JAMA. (1999) 282:1731–6. doi: 10.1001/jama.282.18.1731
40. Bachi AL, Suguri VM, Ramos LR, Mariano M, Vaisberg M, Lopes JD. Increased production of autoantibodies and specific antibodies in response to influenza virus vaccination in physically active older individuals. Results Immunol. (2013) 3:10–6. doi: 10.1016/j.rinim.2013.01.001
41. Sasaki S, Matsuura T, Takahashi R, Iwasa T, Watanabe H, Shirai K, et al. Effects of regular exercise on neutrophil functions, oxidative stress parameters and antibody responses against 4-hydroxy-2-nonenal adducts in middle aged humans. Exerc Immunol Rev. (2013) 19:60–71. Available online at: http://eir-isei.de/2013/eir-2013-060-article.pdf
42. Garcia-Unzueta MT, Gutierrez-Sanchez JB, de Mier I, Amado JA, Berrazueta JR. Autoantibodies against oxidized LDL and serum total antioxidant status in active cyclists and ex-cyclists. Horm Metab Res. (2003) 35:541–5. doi: 10.1055/s-2003-42656
43. Fujii H, Shimizu M, Ino H, Yamaguchi M, Yasuda T, Fujino N, et al. Acute increases in plasma oxidized low-density lipoprotein immediately after percutaneous transluminal coronary angioplasty. Am J Cardiol. (2001) 87:102–3, a8. doi: 10.1016/S0002-9149(00)01281-9
44. Leibundgut G, Lee JH, Strauss BH, Segev A, Tsimikas S. Acute and long-term effect of percutaneous coronary intervention on serially-measured oxidative, inflammatory, and coagulation biomarkers in patients with stable angina. J Thromb Thrombolysis. (2016) 41:569–80. doi: 10.1007/s11239-016-1351-6
Keywords: atherosclerosis, percutaneous coronary intervention (PCI), exercise, LDL-cholesterol, oxidised LDL (oxLDL), malondialdehyde-modified LDL (MDA-LDL)
Citation: Hartley A, Shun-Shin M, Caga-Anan M, Rajkumar C, Nowbar AN, Foley M, Francis DP, Haskard DO, Khamis RY and Al-Lamee RK (2021) The Placebo-Controlled Effect of Percutaneous Coronary Intervention on Exercise Induced Changes in Anti-Malondialdehyde-LDL Antibody Levels in Stable Coronary Artery Disease: A Substudy of the ORBITA Trial. Front. Cardiovasc. Med. 8:757030. doi: 10.3389/fcvm.2021.757030
Received: 11 August 2021; Accepted: 16 September 2021;
Published: 11 October 2021.
Edited by:
Alexander Nikolaevich Orekhov, Institute for Aterosclerosis Research, RussiaReviewed by:
Tsuyoshi Ito, Nagoya City University, JapanCopyright © 2021 Hartley, Shun-Shin, Caga-Anan, Rajkumar, Nowbar, Foley, Francis, Haskard, Khamis and Al-Lamee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ramzi Y. Khamis, ci5raGFtaXNAaW1wZXJpYWwuYWMudWs=
†These authors share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.