- 1Department of Clinical Laboratory, The Third Clinical Medical College of the Three Gorges University, Gezhouba Central Hospital of Sinopharm, Yichang, China
- 2Department of Nephrology, The Third Clinical Medical College of the Three Gorges University, Gezhouba Central Hospital of Sinopharm, Yichang, China
- 3Department of Neurology, The Third Clinical Medical College of the Three Gorges University, Gezhouba Central Hospital of Sinopharm, Yichang, China
- 4Department of Medical Business, The Third Clinical Medical College of the Three Gorges University, Gezhouba Central Hospital of Sinopharm, Yichang, China
- 5Department of Pharmacy, The Third Clinical Medical College of the Three Gorges University, Gezhouba Central Hospital of Sinopharm, Yichang, China
- 6Department of Pain and Rehabilitation, The Third Clinical Medical College of the Three Gorges University, Gezhouba Central Hospital of Sinopharm, Yichang, China
- 7Department of Ultrasound, The Third Clinical Medical College of the Three Gorges University, Gezhouba Central Hospital of Sinopharm, Yichang, China
- 8Department of Endocrinology, The Third Clinical Medical College of the Three Gorges University, Gezhouba Central Hospital of Sinopharm, Yichang, China
Background: The N-terminal pro B type natriuretic peptide (NT-proBNP) is important for prognosis of heart failure in patients with chronic kidney disease (CKD). However, the NT-proBNP level is easily affected by renal insufficiency, which limits its clinical use.
Methods: This study included 396 patients with CKD. Plasma levels of NT-proBNP and cystatin C (CysC) were measured during hospitalization. The echocardiographic parameters were also detected. Patients were divided into the heart failure group and control group according to the European Society of Cardiology Guideline on Chronic Heart Failure 2021. Multiple modeling analysis of the values of NT-proBNP and CysC, including NT-proBNP/Cyscn and NT-proBNP/nCysC was performed. The receiver operating characteristic (ROC) curve, combined with the cardiac function, was used to determine the formula with the best diagnostic efficiency. Then, the sensitivity and specificity of new predictors for cardiac insufficiency in CKD patients were calculated. Pearson correlation analysis was used to analyze the relationship between new predictors and the NT-proBNP level. The clinical data of CKD patients from another local hospital were used to validate the new predictors and the cut-off values.
Results: An elevated NT-proBNP/CysC1.53 ratio was an independent risk factor for cardiac dysfunction in CKD and the best predictor derived from multiple modeling analysis. There was no correlation between the NT-proBNP/CysC1.53 ratio and the NT-proBNP level (r = 0.376, p = 6.909). The area under the ROC curve for the NT-proBNP/CysC1.53 ratio was 0.815 (95% confidence interval: 0.772–0.858), and for a cut-off point of 847.964, this ratio had a sensitivity of 78.24%, and a specificity of 69.44%. When applied to the data of CKD patients from another local hospital, the NT-proBNP to CysC1.53 ratio had a sensitivity of 70.27% and a specificity of 67.74%.
Conclusion: The NT-proBNP to CysC1.53 ratio was superior to NT-proBNP alone for predicting cardiac dysfunction in patients with CKD.
Introduction
Chronic kidney disease (CKD) is diagnosed when the estimated glomerular filtration rate (eGFR) is <60 mL/min/1.73 m2 for 3 consecutive months, or abnormal renal structure or function other than decreased eGFR for over 3 months (1). The main cause of death among patients with CKD is cardiovascular disease, including myocardial infarction and heart failure (HF) (2–4). When HF occurs in CKD patients, the retention of sodium and fluid leads to increases in vascular tension and cardiac preload (5). The increase in ventricular pressure induces the release of biomarkers, such as natriuretic peptide (6). Therefore, biomarkers of myocardial stretching are often used for the diagnosis and prognosis of HF (7). Brain natriuretic peptide (BNP) and N-terminal pro B type natriuretic peptide (NT-proBNP) are important indicators for the diagnosis, prediction, and treatment evaluation of HF, and NT-proBNP is superior to BNP in this prognostic assessment (8–11). Although they both are important indicators of HF (12), NT-proBNP is rarely used as a diagnostic biomarker for HF in patients with end-stage renal disease (ESRD) (13), because ESRD patients without HF have high levels of NT-proBNP due to decreased renal elimination, volume overload, hypertension, and increased left ventricular hypertrophy (14). NT-proBNP is mostly eliminated by glomerular filtration, which explains the strong influence of renal function on NT-proBNP levels (15).
When using NT-proBNP for the diagnosis of HF in patients with renal insufficiency, the diagnostic cut-off value must be adjusted according to the eGFR (16). The cut-off value of NT-proBNP for the diagnosis of acute HF (AHF) in patients with CKD stages 3–5 (eGFR <60 mL/min/1.73m2) is higher than that in patients with CKD stages 1–2 (eGFR >60 mL/min/1.73m2), ranging from 1,200–6,000 pg/mL, and both points are higher than the standard “Januzzi cut-off point.” In general, the specificity and sensitivity of the NT-proBNP concentration for the diagnosis of AHF in patients with CKD stages 3–5 are low (7). To date, there have been no large-scale prospective clinical trials determining the diagnostic cut-off value of NT-proBNP for HF in CKD patients (17).
It is important to use a reliable serological indicator to predict HF in CKD patients with different eGFRs. Low molecular-weight proteins have been used to estimate the value of eGFR (18), and the most promising biomarker is cystatin C (CysC) (19). CysC is a non-glycosylated, 13-kDa basic protein that belongs to the cystatin superfamily of cysteine protease inhibitors. It is produced by all nucleated cells, unaffected by muscle mass (unlike creatinine) (20), and considered a replacement for serum creatinine (sCr) for the estimation of eGFR (21). CycC is also used for early diagnosis of renal damage (22).
In the present study, we investigated the prognostic potential of the ratio of NT-proBNP to CysC compared with NT-proBNP alone for cardiac dysfunction in Chinese CKD patients.
Materials and Methods
Study Population
The clinical records of 938 adults who underwent serum CysC measurement and cardiac biomarker measurement simultaneously between September 2020 and August 2021 in Gezhouba Central Hospital of Sinopharm, China, were retrospectively reviewed. Individuals who were diagnosed with CKD, aged above 18 years, and had complete medical records were eligible for this study. This study was approved by the Hospital Ethics Committee, and informed consent was obtained from all patients. To minimize the confounding effects of circulating biomarkers, patients who met any of the following criteria were excluded: (1) diagnosed with acute myocardial infarction, acute HF, atrial fibrillation, or unstable angina within 1 month of enrollment; (2) diagnosed with malignant arrhythmia or hemodynamic changes; (3) diagnosed with primary liver disease complicated with HF; (4) diagnosed with an end-stage malignant tumor; (5) diagnosed with primary thyroid disease; (6) pregnant; or (7) diagnosed with blood system diseases. Finally, 396 patients were included in the study. Among them, there were 123 (31.06%) cases of diabetic nephropathy, 75 (18.94%) cases of chronic glomerulonephritis, 87 (21.97%) cases of hypertensive nephropathy, 41 (10.35%) cases of nephrotic syndrome, 39 (9.85%) cases of IgA nephropathy, 12 (3.03%) cases of polycystic kidney, 8 (2.02%) cases of after renal transplantation, and 11 (2.78%) cases of other types of kidney diseases. A total of 98 (24.74%) patients had been diagnosed with HF during previous hospitalization.
During hospitalization, all patients with CKD were given a low-salt (3–5 g/day), low-fat, high-quality, low-protein diet and medications to control blood pressure, blood glucose, and blood lipids. All patients were given 3–5 compound ketoic acid tablets orally (three times per day). Patients with proteinuria (n = 156) were given huangkui capsule (three times per day). Seventy-two patients received hormone and immunosuppressant therapy. Spironolactone (20 mg, once daily) was routinely given to CKD patients with previously diagnosed HF. Beta-blockers (12.5–25 mg, twice daily) and angiotensin-converting enzyme inhibitor (ACEI)/angiotensin receptor blocker (ARB) drugs were used in antihypertensive treatment for patients with eGFR >30%. Calcium channel blocker drugs were used in antihypertensive treatment for patients with eGFR <30%. All enrolled patients were treated with cardiac intensification and diuretic therapy for acute HF during the observation period. Serum potassium was regularly monitored in all patients treated with concomitant spironolactone and angiotensin converting enzyme inhibitors or angiotensin receptor blocker.
During the follow-up period, 57 patients showed clinical symptoms of HF, including 25 patients with newly diagnosed HF and 32 patients diagnosed with HF during previous hospitalization. Thirty-nine patients required hospitalization, while the other 18 were in stable condition after outpatient cardiotonic and diuretic treatment. During the follow-up period, all patients with HF clinical symptoms were continually followed after their condition became stable, and HF was treated according to aforementioned regimes. Serum potassium was routinely monitored in all patients treated with concomitant spironolactone and ACEI/ARB.
Biomarker Assessment
The levels of highly sensitive cardiac troponin T (hs-cTnT) and NT-proBNP were measured by the Cobas 601 automatic chemiluminescence analyzer (Roche, Inc.). The levels of CysC, serum urea and sCr were measured using the 7600 automatic biochemical analyzer (Hitachi, Inc.). The levels of hemoglobin (Hb) were measured using the XN-1000 automatic blood cell analyzer (Sysmex, Inc.).
Echocardiography
On the day that blood tests were performed, echocardiography also was performed using the GE Vivid E90 Ultrasound System (M5S probe, 1.7~3.3 MHz; GE Healthcare) with participants laying on their left side and staying calm during the test. The following parameters were measured: left ventricular end-diastolic dimension (LVDd), left ventricular end-systolic dimension (LVDs), left ventricular ejection fraction (LVEF), left atrial volume index (LAVI), left ventricular mass index (LMVI), global area strain (GAS), global longitudinal strain (GLS), left ventricular fractional shortening (FS), stroke volume (SV), and the echocardiographic ratio of early diastolic mitral inflow velocity to early diastolic mitral annulus velocity (E/e').
Diagnostic Criteria for HF
HF was diagnosed based on the symptoms and/or signs of HF, echocardiography report, and the Heart Failure Association (HFA)-PEFF (P, pre-test assessment; E, echocardiography and natriuretic peptide score; F1, functional testing; F2, final etiology) diagnostic algorithm (23). The algorithm consists of three domains: functional, morphological, and biomarker domains. For each domain, 2 points are scored when the main criteria are met, while 1 point is scored when minor criteria are met. Points from different domains are then summed. A total score of ≥5 is considered a diagnosis of HF preserved ejection fraction (HFpEF); while a score of ≤1 indicates an unlikely diagnosis of HFpEF (24). The diagnosis of HF with mildly reduced ejection fraction (HFmrEF) requires the presence of symptoms and/or signs of HF, the LVEF between 40 and 50%, elevated levels of natriuretic peptides (BNP ≥35 pg/mL or NT-proBNP ≥125 pg/mL), and other evidence of structural heart disease (25). The diagnosis of HF with reduced ejection fraction (HFrEF) requires the presence of symptoms and/or signs of HF and a reduced ejection fraction (LVEF ≤40%) (25).
Statistical Analysis
SPSS 22.0 software (SPSS, Inc.) was used for data analysis. The levels of NT-proBNP were log-transformed, and then statistical analysis was performed to eliminate the influence of extreme values. Normally distributed data are expressed as ± s. Data that were not normally distributed [i.e., NT-proBNP levels, CysC levels, sCr, eGFR, and the ratio of NT-proBNP/CysC1.53 (new predictors)] are expressed as median (P25~P75) values. An independent sample t-test was used to compare the results between two groups. Multiple modeling analysis of the values of NT-proBNP and CysC, including NT-proBNP/CysCn and NT-proBNP/nCysC, was performed. The ROC curve, combined with the cardiac function, was used to determine formula with the best diagnostic efficiency. Then, the sensitivity and specificity of new predictors for cardiac insufficiency in CKD patients were calculated. ROC curve analysis was used to analyze echocardiography indicators with statistical significance. The area under the ROC curve (AUC) was calculated to evaluate the ability of these factors to predict HF in patients with CKD. The optimal diagnostic cut-off point was determined by the “Youden index” (sensitivity + specificity−1). The significance level was α = 0.05, and p < 0.05 was considered statistically significant.
Results
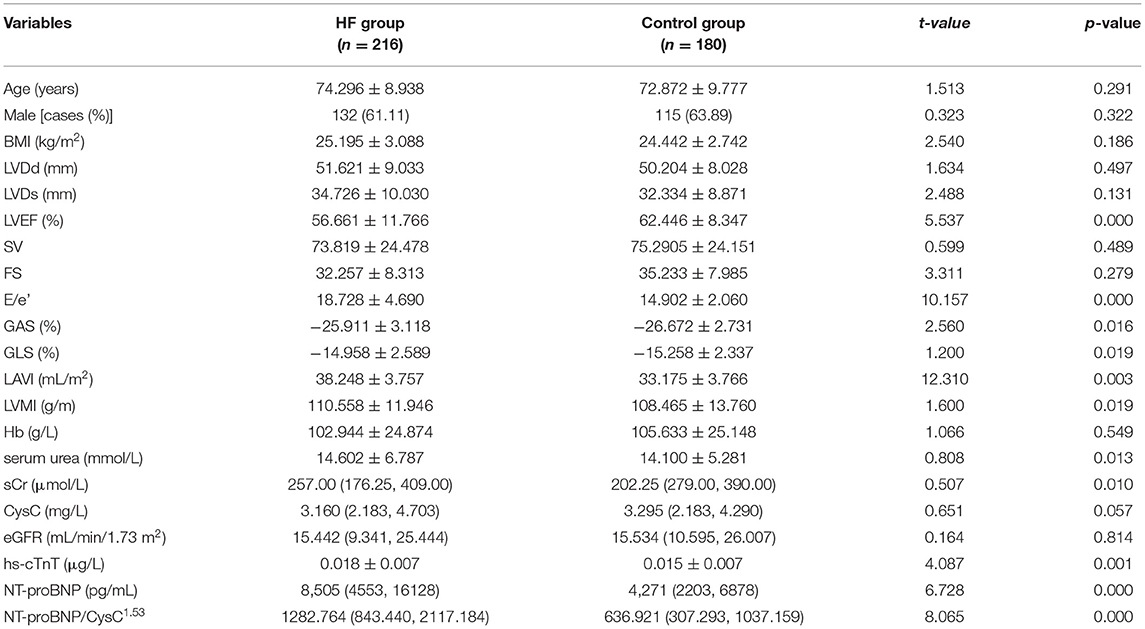
The baseline characteristics of 396 hospitalized patients with CKD are summarized in Table 1. Patients were divided into two groups according to their cardiac function assessed following the European Society of Cardiology (ESC) Guidelines for Chronic Heart Failure 2021: the HF group (n = 216) and the control group (n = 180).
The ROC curve showed that the cut-off value of the NT-proBNP/CysC1.53 ratio was 847.964.
Pearson correlation analysis showed that the level of NT-proBNP was not correlated with the NT-proBNP/CysC1.53 ratio (r = 0.376, p = 6.909; Figure 1).
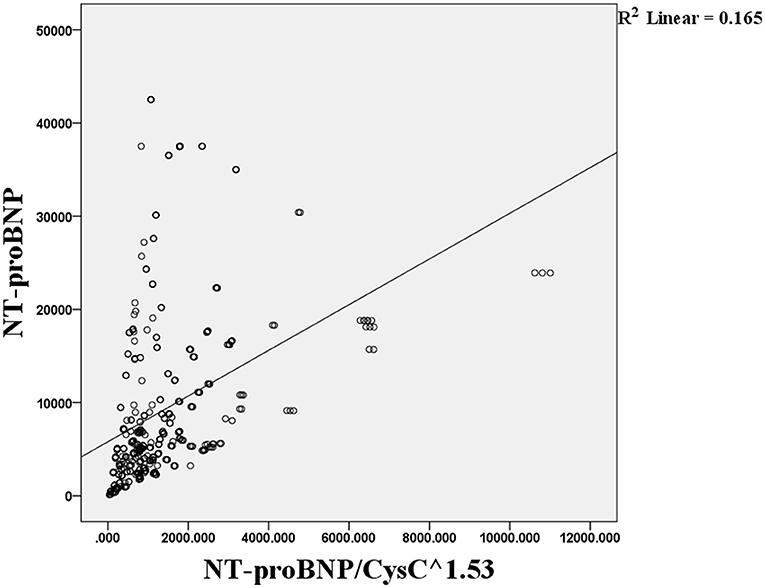
Figure 1. Pearson correlation analysis of the association between the NT-proBNP level and NT-proBNP/CysC1.53 ratio.
The E/e', GAS, GLS, LAVI, LVMI, serum urea, sCr, hs-cTnT, NT-proBNP, and NT-proBNP/CysC1.53 ratio in the HF group were significantly higher than those in the control group, while the LVEF was significantly lower than that in the control group (p < 0.05). No significant differences in age, CysC, LVDd, and LVDS were observed between the two groups (p > 0.05).
Then, we used ROC curve analysis to identify the predictors that were statistically different between the two groups. The ROC curves for eight potentially predictive factors (NT-proBNP/CysC1.53, NT-proBNP, GAS, GLS, LVMI, LAVI, E/e', and LVEF) were plotted. The following AUC values for the eight factors were calculated: AUC (NT-proBNP/CysC1.53) = 0.815, AUC (LAVI) = 0.798, AUC (E/e') = 0.747, AUC (NT-proBNP) = 0.726, AUC (LVEF) = 0.646, AUC (GAS) = 0.570, AUC (LVMI) = 0.561, and AUC (GLS) = 0.535. The AUC for the NT-proBNP/CysC1.53 ratio was greater than those for NT-proBNP and LVEF. The diagnostic cut-off value for the NT-proBNP/CysC1.53 ratio was 847.964, with a sensitivity of 78.24% and a specificity of 69.44%. The diagnostic cut-off value for NT-proBNP was 8,198 pg/mL, with a sensitivity of 52.31% and a specificity of 84.44% (Figure 2).
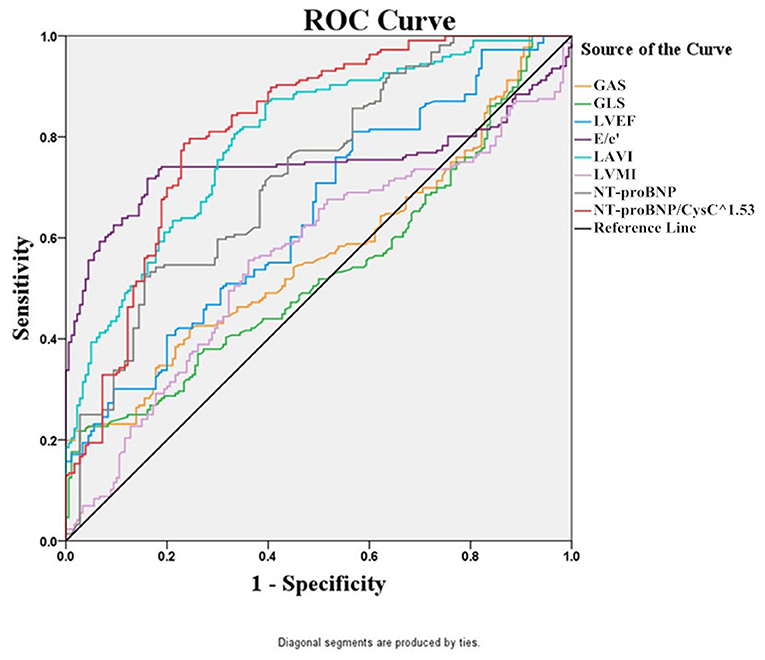
Figure 2. The ROC curves of NT-proBNP/CysC1.53, NT-proBNP, GAS, GLS, LVMI, LAVI, E/e', and LVEF for the diagnosis of HF in patients with CKD.
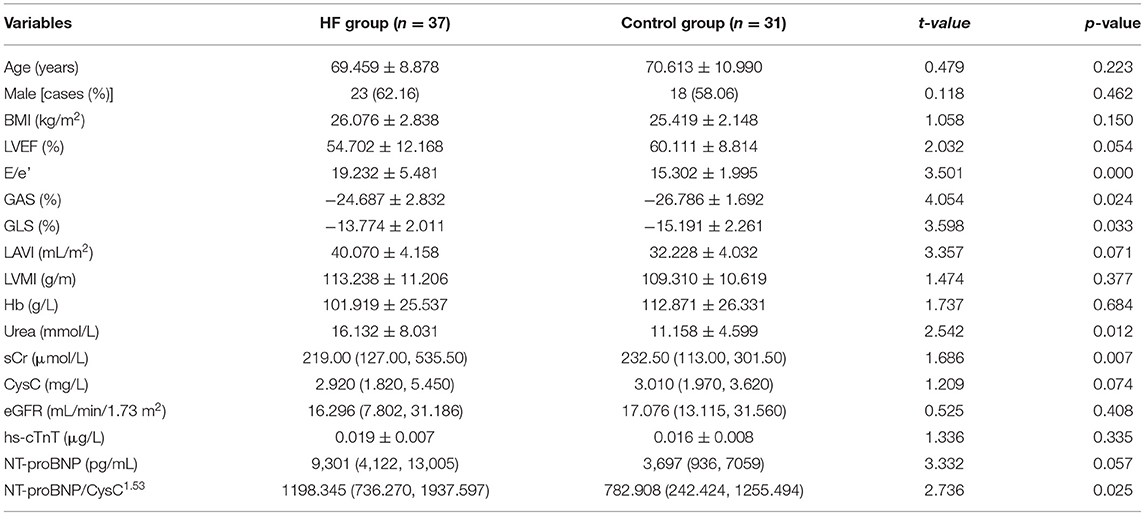
The clinical data of CKD patients from another local hospital were used to validate the new predictors and the truncation value. The baseline characteristics of 68 hospitalized patients with CKD are summarized in Table 2. The results showed a sensitivity of 70.27% and a specificity of 67.74%.
Discussion
Renal function and cardiac function are interdependent in patients with CKD. HF has been identified as an independent risk factor for all-cause mortality in hospitalized CKD patients. The morbidity and mortality rates are high in CKD complicated with cardiac insufficiency and have been increasing in recent years (26). Thus, early diagnosis of cardiac insufficiency may improve the prognosis of patients with CKD.
HF can be divided into HFrEF, HFmrEF, and HFpEF. It has been reported that HFpEF accounts for more than half of all hospital admissions for HF (27). Early diagnosis of HFpEF is more difficult than that of HFrEF (LVEF ≤ 40%) and HFmrEF. The widely used New York Heart Association Classification System classifies HF based on subjective feelings of patients; therefore, the results may not be accurate. Even when the objective HFA-PEFF diagnostic algorithm is used, a cardiovascular ultrasound system (e.g., GE Vivid E90 Ultrasound System designed by GE Healthcare) is always needed for the classification of HF. However, in China, most primary and intermediate hospitals do not have such equipment. NT-proBNP is a diagnostic marker for HF, and its serum levels are closely associated with renal insufficiency and age. Therefore, the diagnostic cut-off value of NT-proBNP for HF should be adjusted based on the level of eGFR. However, no large-scale prospective clinical trials have provided an accurate cut-off value of NT-porBNP for the diagnosis of HF in patients with CKD (17).
By comparing the baseline characteristics of the HF and control groups, we found that the NT-proBNP/CysC1.53 ratio differed significantly between the two groups. Patients with HF showed a significantly higher NT-proBNP/CysC1.53 ratio than the control group, suggesting that a high NT-proBNP/CysC1.53 may be related to the occurrence of cardiac dysfunction in CKD. Our results also demonstrated that the NT-proBNP/CysC1.53 ratio was a more reliable predictor of HF than NT-proBNP, GAS, GLS, LVMI, LAVI, E/e', and LVEF in patients with CKD. According to the Kidney Disease: Improving Global Outcomes (KDIGO) 2012 clinical practice guideline for the evaluation and management of CKD (1), CysC is the best indicator of eGFR and has been used for eGFR calculation. Therefore, in this study, we used both NT-proBNP and CysC to evaluate the cardiac function of patients with CKD. To our knowledge, this is the first study to explore the potential of NT-proBNP combined with CysC as a predictor of HF in CKD patients.
A previous study (28) proposed that LVEF is an indicator of cardiac insufficiency in patients with early diagnosed CDK. However, the use of LVEF as a diagnostic marker cannot rule out cases with HFpEF. In the present study, patients with HFpEF, HFmrEF, and HFrEF were included in the HF group for the analysis of cardiac function in CKD patients, and thus, this study may provide more comprehensive results.
Gao et al. (29) proposed that the level of NT-proBNP can indicate HF in different eGFR intervals. However, whether the same cut-off value of NT-proBNP can be used for the diagnosis of HF over a wide range of eGFR values remains unknown. Moreover, they only included patients with HFrEF (LVEF ≤40%). In our study, both NT-proBNP and CysC levels were used to predict cardiac insufficiency, which may better reflect individual differences.
We further validated the results using the clinical data of patients from another local hospital. The NT-proBNP/CysC1.53 ratio accurately determined the cardiac function of these patients.
It should be noted that the diagnostic criteria that we have derived in this study were simpler than those recently published in ESC Chronic Heart Failure Guidelines 2021 and require fewer tests. In addition, laboratory test results have less interference and are more accurate than the results of ultrasound scans, and therefore, may have a wider range of applications.
The present study has some limitations. There were only 9 (2.27%) cases with stage 1–2 CKD. Due to compliance issues, patients with symptoms or signs of HF and with a HFA score between 2 and 4 did not undergo a diastolic pressure test or invasive hemodynamic measurement. Because there were only 12 (3.03%) cases with symptoms or signs of HF and with a HFA score between 2 and 4, it might not significantly affect the overall results. We only recruited a small number of Chinese subjects from another local hospital to validate the results, which may not represent the population in other areas. Taking into account the differences in regions, populations, and examinations (e.g., altitude, ethnicity, equipment, reagent), it is recommended that the current findings be validated in a local population. In summary, our study showed that the NT-proBNP/CysC1.53 ratio was a predictor of cardiac insufficiency in patients with CKD and might be used for the early detection of HF in this population in clinical settings.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of Gezhouba Central Hospital of Sinopharm. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
SW, XW, and JL conceived and designed research. ML, CL, XY, HA, and TY collected data and conducted research. SW and YZ analyzed and interpreted data. YH and ML wrote the initial paper. SW, ML, YZ, and XL revised the paper. SW had primary responsibility for final content. All authors read and approved the final manuscript.
Funding
This study was supported by the 2017 Three Gorges University Youth Science Foundation Project (No. KJ2017A011).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
NT-proBNP, N-terminal pro B type natriuretic peptide; CKD, chronic kidney disease; CysC, cystatin C; ESC, European Society of Cardiology; ROC, receiver operating characteristic; eGFR, estimated glomerular filtration rate; HF, heart failure; BNP, Brain natriuretic peptide; ESRD, end-stage renal disease; AHF, acute heart failure; sCr, serum creatinine; hs-cTnT, highly sensitive cardiac troponin T; LVDd, left ventricular end-diastolic dimension; LVDs, left ventricular end-systolic dimension; LVEF, left ventricular ejection fraction; LMVI, left ventricular mass index; GAS, global area strain; GLS, global longitudinal strain; LAVI, left atrial volume index; FS, left ventricular fractional shortening; SV, stroke volume; E/e', the echocardiographic ratio of early diastolic mitral inflow velocity to early diastolic mitral annulus velocity; HFA, Heart Failure Association; PEFF, pre-test assessment; pre-test assessment; functional testing in case of uncertainty; final etiology; HFpEF, heart failure preserved ejection fraction; HFmrEF, heart failure with reduced ejection fraction; HFrEF, heart failure with reduced ejection fraction; AUC, area under the receiver operating characteristic curve; KDIGO, Kidney Disease: Improving Global Outcomes.
References
1. Levin A, Stevens P, Bilous RW, Coresh J, Francisco A, Jong PE, et al. Kidney disease: improving global outcomes (KDIGO) CKD work group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Inter Suppl. (2013) 3:1–150. doi: 10.1038/kisup.2012.73
2. Stacy SR, Suarez-Cuervo C, Berger Z, Wilson LM, Yeh HC, Bass EB, et al. Role of troponin in patients with chronic kidney disease and suspected acute coronary syndrome: a systematic review. Ann Intern Med. (2014) 161:502–12. doi: 10.7326/m14-0746
3. Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. (2010) 375:2073–81. doi: 10.1016/s0140-6736(10)60674-5
4. Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. (2001) 286:421–6. doi: 10.1001/jama.286.4.421
5. Srisawasdi P, Vanavanan S, Charoenpanichkit C, Kroll MH. The effect of renal dysfunction on BNP, NT-proBNP, and their ratio. Am J Clin Pathol. (2010) 133:14–23. doi: 10.1309/ajcp60htpgigfcnk
6. Bargnoux AS, Klouche K, Fareh J, Barazer I, Villard-Saussine S, Dupuy AM, et al. Prohormone brain natriuretic peptide (proBNP), BNP and N-terminal-proBNP circulating levels in chronic hemodialysis patients. Correlation with ventricular function, fluid removal and effect of hemodiafiltration. Clin Chem Lab Med. (2008) 46:1019–24. doi: 10.1515/cclm.2008.192
7. Schaub JA, Coca SG, Moledina DG, Gentry M, Testani JM, Parikh CR. Amino-Terminal pro-B-type natriuretic peptide for diagnosis and prognosis in patients with renal dysfunction: a systematic review and meta-analysis. JACC Heart Fail. (2015) 3:977–89. doi: 10.1016/j.jchf.2015.07.014
8. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. (2017) 70:776–803. doi: 10.1016/j.jacc.2017.04.025
9. Tsutsui H, Isobe M, Ito H, Ito H, Okumura K, Ono M, et al. JCS 2017/JHFS 2017 guideline on diagnosis and treatment of acute and chronic heart failure- digest version. Circ J. (2019) 83:2084–184. doi: 10.1253/circj.CJ-19-0342
10. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC guidelines for the diagnosis treatment of acute chronic heart failure: the task force for the diagnosis treatment of acute chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. (2016) 18:891–975. doi: 10.1002/ejhf.592
11. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. (2013) 62:e147–239. doi: 10.1016/j.jacc.2013.05.019
12. Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. (2002) 347:161–7. doi: 10.1056/NEJMoa020233
13. Maisel A, Mueller C, Adams K Jr, Anker SD, Aspromonte N, Cleland JG, et al. State of the art: using natriuretic peptide levels in clinical practice. Eur J Heart Fail. (2008) 10:824–39. doi: 10.1016/j.ejheart.2008.07.014
14. David S, Kümpers P, Seidler V, Biertz F, Haller H, Fliser D. Diagnostic value of N-terminal pro-B-type natriuretic peptide (NT-proBNP) for left ventricular dysfunction in patients with chronic kidney disease stage 5 on haemodialysis. Nephrol Dial Transplant. (2008) 23:1370–7. doi: 10.1093/ndt/gfm700
15. Alehagen U, Lindstedt G, Eriksson H, Dahlström U. Utility of the amino-terminal fragment of pro-brain natriuretic peptide in plasma for the evaluation of cardiac dysfunction in elderly patients in primary health care. Clin Chem. (2003) 49:1337–46. doi: 10.1373/49.8.1337
16. Mueller C, McDonald K, de Boer RA, Maisel A, Cleland JGF, Kozhuharov N, et al. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur J Heart Fail. (2019) 21:715–31. doi: 10.1002/ejhf.1494
17. Han X, Zhang S, Chen Z, Adhikari BK, Zhang Y, Zhang J, et al. Cardiac biomarkers of heart failure in chronic kidney disease. Clin Chim Acta. (2020) 510:298–310. doi: 10.1016/j.cca.2020.07.040
18. Little DJ, Mascio HM, Altenburg RJ, Moon DS, Deressa WT, Wong S, et al. Implementing GFR estimation guidelines using cystatin C: a quality improvement project. Am J Kidney Dis. (2016) 67:711–2. doi: 10.1053/j.ajkd.2015.10.014
19. Sjöström P, Tidman M, Jones I. Determination of the production rate and non-renal clearance of cystatin C and estimation of the glomerular filtration rate from the serum concentration of cystatin C in humans. Scand J Clin Lab Invest. (2005) 65:111–24. doi: 10.1080/00365510510013523
20. Herget-Rosenthal S, Marggraf G, Hüsing J, Göring F, Pietruck F, Janssen O, et al. Early detection of acute renal failure by serum cystatin C. Kidney Int. (2004) 66:1115–22. doi: 10.1111/j.1523-1755.2004.00861.x
21. Tong Y, Liu X, Guan M, Wang M, Zhang L, Dong D, et al. Evaluation of serological indicators and glomerular filtration rate equations in Chinese cancer patients. Med Sci Monit. (2017) 23:2949–60. doi: 10.12659/msm.902138
22. Hu C, Li D, Yin W, Zuo X. Evaluation of cystatin C-derived glomerular filtration rate equations in Chinese population. Scand J Clin Lab Invest. (2019) 79:629–34. doi: 10.1080/00365513.2019.1689575
23. Seferović PM, Polovina M, Bauersachs J, Arad M, Gal TB, Lund LH, et al. Heart failure in cardiomyopathies: a position paper from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. (2019) 21:553–76. doi: 10.1002/ejhf.1461
24. Pieske B, Tschöpe C, de Boer RA, Fraser AG, Anker SD, Donal E, et al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J. (2019) 40:3297–317. doi: 10.1093/eurheartj/ehz641
25. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis andtreatment of acute and chronic heart failure. Eur Heart J. (2021) 42:3599–726. doi: 10.1093/eurheartj/ehab368
26. Liu Y, He YT, Tan N, Chen JY, Liu YH, Yang DH, et al. Preprocedural N-terminal pro-brain natriuretic peptide (NT-proBNP) is similar to the Mehran contrast-induced nephropathy (CIN) score in predicting CIN following elective coronary angiography. J Am Heart Assoc. (2015) 4:e001410. doi: 10.1161/jaha.114.001410
27. Van Riet EE, Hoes AW, Wagenaar KP, Limburg A, Landman MA, Rutten FH. Epidemiology of heart failure: the prevalence of heart failure and ventricular dysfunction in older adults over time. A systematic review. Eur J Heart Fail. (2016) 18:242–52. doi: 10.1002/ejhf.483
28. Mishra RK, Yang W, Roy J, Anderson AH, Bansal N, Chen J, et al. Kansas city cardiomyopathy questionnaire score is associated with incident heart failure hospitalization in patients with chronic kidney disease without previously diagnosed heart failure: chronic renal insufficiency cohort study. Circ Heart Fail. (2015) 8:702–8. doi: 10.1161/circheartfailure.115.002097
Keywords: chronic kidney disease, heart failure, NT-proBNP, CysC, combined diagnostic index
Citation: Wang S, Li M, Wang X, Luo J, Zou Y, Hu Y, Liu X, Ao H, Yao X, Li C and Yang T (2021) The Ratio of NT-proBNP to CysC1.53 Predicts Heart Failure in Patients With Chronic Kidney Disease. Front. Cardiovasc. Med. 8:731864. doi: 10.3389/fcvm.2021.731864
Received: 28 June 2021; Accepted: 15 October 2021;
Published: 12 November 2021.
Edited by:
Vasundhara Kain, University of South Florida, United StatesReviewed by:
Ralph Knöll, AstraZeneca, SwedenChristoph Edlinger, University Hospital Salzburg, Austria
Copyright © 2021 Wang, Li, Wang, Luo, Zou, Hu, Liu, Ao, Yao, Li and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Hu, MTQ0NDI0MTEyOEBxcS5jb20=
 Sheng Wang1
Sheng Wang1 Yang Hu
Yang Hu