
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Comput. Sci. , 04 January 2024
Sec. Human-Media Interaction
Volume 5 - 2023 | https://doi.org/10.3389/fcomp.2023.1237531
This article is part of the Research Topic Re-Imagining Mediated Human Building Interaction and Sensory Environments View all 6 articles
Technological advancements in physiological body sensor networks (i.e., biometric tracking wearables) and simulated environments (i.e., VR) have led to increased research in the field of neuroarchitecture, specifically investigating the effects of architectural forms, defined here as subtle variations in the shape or configuration of the interior built environment, on neurological responses. While this research field is still in its nascent stages, early findings suggest that certain architectural forms may impact physiological stress responses. Physiological stress has, in turn, been implicated in the development of certain diseases, including cardiovascular disease, cancer, chronic kidney disease, non-alcoholic fatty liver disease and autoimmune and neurodegenerative disorders. To aid future research, particularly into the relationship between media architecture and physiological stress, this paper conducts a systematic review following PRISMA-P guidelines on studies that evaluated physiological stress responses to architectural form using clinical biomarkers. The review identifies the specific clinical biomarkers used to evaluate physiological stress responses to architectural forms and the distinct categories of architectural forms that have, to date, been correlated with elevated stress responses: curvature, enclosure and proportion. Although these studies' findings imply that the identified architectural forms influence physiological stress, their generalisability is arguably constrained by several factors. These constraints include the paucity of research in this area, the lack of uniformity in the definition and measurement of these architectural forms, the varying contextual settings, the unisensory approach of research methodologies, and the duration of exposure under evaluation. The review concludes that clinical biomarkers may be used to measure the impact of architectural form on physiological stress; however, future research should strive for standardized approaches in defining and measuring architectural forms in order to increase the transferability and robustness of results.
In developed nations, individuals spend over 90% of their time indoors (Schweizer et al., 2007). In some areas, such as the UK, this increases to an average time spent indoors of 95.6% (Schweizer et al., 2007). Moreover, certain vulnerable segments of the European population, such as the elderly, infants, young children, and those with compromised immunity, could potentially allocate 100% of their time indoors (Vardoulakis et al., 2015, cited in Vardoulakis et al., 2015). As people increasingly spend a significant portion of their time in and around the built environment, it becomes crucial to understand how architectural design choices may impact human physiology.
Technological innovations in physiological body sensor networks and simulated environments (virtual reality or VR) have provided researchers in the field of neuroarchitecture with the tools to measure the impact of architectural design on physiological responses (Borgianni and Maccioni, 2020). Researchers have examined a variety of neurological responses to a wide range of architectural features,1 such as architectural styles (Choo et al., 2017; Coburn et al., 2017), spatial geometry (Vartanian et al., 2013, 2015; Shemesh et al., 2017, 2021), height and enclosure (Fich et al., 2014; Vartanian et al., 2015; Shemesh et al., 2017, 2021), architectural façade and landmark recognition (Rounds et al., 2020), interior space design (Fich et al., 2014; Banaei et al., 2017, 2019), lighting (Shin et al., 2015; Ergan et al., 2019; Lu et al., 2020), ambient conditions (Choi et al., 2019; Guan et al., 2020), materiality (Tsunetsugu et al., 2002) and chromaticity (Küller et al., 2009; Ergan et al., 2019).
Recently, researchers have begun to use clinical biomarkers to empirically examine physiological stress responses to the built environment (Guidi et al., 2021). Physiological stress responses refers to the intricate array of bodily adjustments initiated when an individual encounters physical or psychological stressors capable of disturbing the body's equilibrium, known as homeostasis (Chu et al., 2023). Clinical biomarkers (sometimes referred to as “psychophysiological measures”) are objective empirical measurements of bodily functions that predict clinically relevant processes (Aronson and Ferner, 2017). For the purposes of this paper, these objective empirical measurements are simply referred to here as “biomarkers.” The most common biomarkers used to measure physiological stress responses are blood pressure, heart rate and respiratory rate increases, skin conductance and body temperature, salivary cortisol, and brain activity (Persiani et al., 2021). These techniques have all been used in the field of neuroarchitecture. A number of neuroarchitecture studies suggest that visual exposure to variations in architectural form, defined here as subtle variations in the geometric shape or configuration of the interior built environment, may mediate physiological stress responses (Kim and Kim, 2022).
These findings are significant, given the causal relationship between physiological stress responses and systemic inflammation, the latter of which is associated with higher morbidity and mortality rates (Mariotti, 2015). Emerging research in the field of neuroimmunology suggests that physiological stress responses may result in long-term physiological damage (O'Callaghan, and Miller, 2019) and the development of a number of severe health conditions, including neuroinflammatory disorders (Dhabhar, 2008; Frank et al., 2019). Physiological stress responses engage in a bidirectional dialogue between the central nervous system (responsible for physiological stress responses) and the immune system (responsible for inflammatory processes in the body, including neuroinflammation; Munhoz et al., 2008; DiSabato et al., 2016). Physiological stressors have been found to induce alterations in immune function (Wohleb and Godbout, 2013) via the nervous system (Frank-Cannon et al., 2009; Grippo and Scotti, 2013), resulting in proinflammatory responses in the peripheral organ systems (i.e., liver, kidneys, lungs, and heart) and the brain (Lurie, 2018; Stenvinkel et al., 2021). Conversely, reductions in physiological stress have been found to mitigate these negative impacts by reversing inflammation via the same pathways (Rosenkranz et al., 2016).
To date, there has been a paucity of research into our physiological response to media architecture.2 Media architecture employs dynamic architectural elements, such as programmable lights and digital surfaces, creating structures that are capable of dynamically altering their form and appearance in response to various circumstances (Foth and Caldwell, 2018). The emergence of media architecture may open a window of opportunity for reducing physiological stress induced by specific architectural forms. For example, if architectural forms can indeed induce physiological stress, the interactive and dynamic nature of media architecture could be strategically utilized to counteract these effects. The integration of digital technologies allows the creation of environments that adjust according to their occupants' physiological responses. Consider a room design where wall curvature and proportions could dynamically adapt to its occupants' physiological stress levels, based on real-time feedback from biometric sensors. This adjustment could be as simple as the room appearing more spacious or the walls adopting a softer curvature. Given the growing trend toward biophilic design, which promotes our innate affinity for natural elements (Salingaros, 2019), digital responses could incorporate elements reminiscent of natural environments. The interplay of sunlight and shadow, the gentle movement of leaves, or the visual impression of natural materials could all be digitally replicated and adjusted to promote wellbeing (Yin et al., 2020).
However, it is essential to recognize that the integration of media architecture may have the potential to trigger our physiological stress responses. As digital technologies become more immersive, the risk of exacerbating physiological stress responses increases incrementally. Media architecture's dynamic nature might overstimulate occupants, causing disorientation or discomfort (Valentine, 2023a). For instance, the occurrence of an excessive multiplicity of sensory stimuli has the potential to precipitate sensory overload. Sensory overload, or sensory overstimulation, is a neurological phenomenon characterized by the excessive stimulation of one or more sensory modalities, resulting in the sensory system's inability to adequately process and respond to the incoming sensory information (Scheydt et al., 2017). This condition can be triggered by an intense, prolonged, or complex array of sensory stimuli, and is often associated with certain neurodivergent conditions, such as autism spectrum disorder (Balasco et al., 2020), post-traumatic stress disorder (Harricharan et al., 2017), attention deficit hyperactivity disorder and sensory processing disorder (Miller et al., 2012). In these instances, it is possible that the dynamic, and often unpredictable, architectural elements used in media architecture may increase stress responses rather than alleviating them. To the best of the author's knowledge, no research has directly examined physiological stress responses to media architecture.
To aid future research into the impact of architectural form on physiological stress, this paper conducts a review of studies that isolate and test physiological stress responses to specific architectural forms using clinical biomarkers. As this review is limited to considering the geometric shape or configuration of the interior built environment, the review does not consider architectural features such as lighting, materiality, thermal comfort, or color. Although these features invariably impact human physiology and are worthy of further investigation, they remain outside the scope of this review. The review aims to identify (i) the clinical biomarkers used to measure physiological stress responses to architectural form, (ii) categories of architectural forms that are correlated with elevated stress response, and (iii) limitations with existing research methods.
The review is conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Protocols (PRISMA-P) guidelines. PRISMA-P is a widely used methodological and analytical framework for systematic reviews that maintains consistency in systematic review methods across health disciplines. In brief, the review reveals that electroencephalography [Banaei et al., 2017; Kim et al., 2021 (Alpha to Beta Wave Ratio); Shemesh et al., 2021; Cruz-Garza et al., 2022], Functional Magnetic Resonance Imaging (Vartanian et al., 2015), Heart Rate Variability (Fich et al., 2014; Abd-Alhamid et al., 2020; Li et al., 2022), Galvanic Skin Response (Abd-Alhamid et al., 2020; Shemesh et al., 2021), Pupil Dilation (Shemesh et al., 2021), and Salivary Cortisol (Fich et al., 2014; Li et al., 2022) are the primary clinical biomarkers engaged. The review further identifies curvature (Banaei et al., 2017; Shemesh et al., 2021; Li et al., 2022), proportion (Kim et al., 2021; Shemesh et al., 2021), and enclosure (Fich et al., 2014; Vartanian et al., 2015; Abd-Alhamid et al., 2020; Kim et al., 2021; Cruz-Garza et al., 2022) as categories of architectural forms that have been linked to physiological stress. However, it is difficult to definitively categorize each architectural form measured or its impact on physiological stress given the lack of uniformity in the definition and measurement of these architectural forms, indicating variations in interpretive frameworks. Moreover, the transferability of these findings are further limited by the contextual and behavioral nature of architectural experiences.
The analysis process was divided into four stages. First, studies investigating stress responses to architectural forms were selected for inclusion. Second, the clinical biomarkers and methods used to examine physiological stress responses in these studies were gathered. Third, the architectural features examined in these studies were isolated. Fourth, robustness of the methodological approaches engaged to measure physiological stress responses to architectural forms were analyzed. To begin, the following section sets out the methodology employed and the limitations of this review.
In the present review, PRISMA-P was used to analyse controlled studies which isolated and tested physiological stress responses to individual architectural forms (i.e., subtle variations in the geometric shape or configuration of the interior built environment) to the exclusion of characteristics such as lighting, materiality and biophilic design. Variations in architectural form in both physical and virtual environments were considered.
PRISMA-P is a research protocol developed to enhance the transparency, quality and rigor of systematic reviews and meta-analyses (Shamseer et al., 2015). As Shamseer et al. (2015, p.1) state “protocols of systematic reviews and meta-analyses allow for planning and documentation of review methods, act as a guard against arbitrary decision making during review conduct, enable readers to assess for the presence of selective reporting against completed reviews, and, when made publicly available, reduce duplication of efforts and potentially prompt collaboration.” For the present review, PRISMA-P was particularly helpful in clearly defining the population of interest (i.e., healthy individuals), the intervention (i.e., the particular architectural forms and settings), comparator (i.e., no restriction was placed) and outcomes (i.e., impact on physiological stress response as measured using clinical biosensors such as EEG, HRV and GSR).
However, examining the impact of architectural form on physiological stress responses presents a multifaceted challenge, and its exploration is not without limitations. PRISMA-P is primarily designed for clinical and health-related studies, and its adaptation to architectural research might inadvertently overlook critical contextual factors intrinsic to the built environment. Architectural forms' influence on physiological stress responses is influenced by various variables, including cultural, social, and psychological dimensions that might not fit neatly into the PRISMA-P structure. Therefore, researchers should approach this investigation cautiously, acknowledging the need for a more tailored and interdisciplinary approach to fully capture the intricate interplay between architectural design and human neurophysiology. However, for the specific purposes of this review, PRISMA-P provided a rigorous framework to identify, categorize and analyse relevant studies.
A search strategy was developed using the PRISMA-P protocol under the headings of Population, Intervention, Comparison, and Outcome (PICO) (Moher et al., 2015).
a. Population—Included populations consisted of healthy individuals. No demographic restrictions were placed on the population.
b. Intervention—This review identified papers which evaluate stress responses to architectural form (i.e., geometric configuration). Studies which evaluated indoor environmental quality (i.e., auditory or environmental noise, thermal comfort, air quality or presence of volatile organic compounds and lighting), street design (i.e., traffic routing, sidewalk configuration), biophilia design or color were not considered. Studies which evaluated the impacts of urban greenery or exposure to indoor plants were also not considered. Additionally, studies which grouped intervention characteristics together were also not considered. For example, Ergan et al. (2019) measured the combined responses of lighting, signage, and light bounce, while Jin and Juan (2021) measured the impacts of Feng Shui, which incorporates a combined number of architectural criteria. Future research may wish to consider the impacts of these combined and confounding variables on stress responses; however, they remain outside the scope of this review. Both virtual and physical environments were considered.
c. Comparator—No restriction was placed on the control/comparator. This means that the selection of the control or comparator group was left unrestricted, allowing for a wider range of options and variations in the studies being reviewed. This approach could enhance the generalizability and variability of the findings in the systematic review.
d. Outcome—Studies which measured stress responses using clinical biomarkers (i.e., EEG, fMRI, HRV, GSR, pupil dilation, salivary cortisol) were included. Self-reported stress levels were excluded.
Study selection was undertaken with the removal of duplicates and the initial screening of the title and abstract against eligibility criteria using a checklist. This was achieved using the web-based Rayyan tool.
Three electronic bibliographic databases were searched: SCOPUS, Web of Science and Science Direct. PubMed was also searched, though it was not included in the formal database search due to algorithmic deviations in search criteria. Hand searches of reference lists of included studies were also made. Google Scholar was searched for additional gray literature. The review inclusion criteria were for studies in English conducted between January 2013 and January 2023. A table of relevant key conceptual search terms is displayed in Table 1.
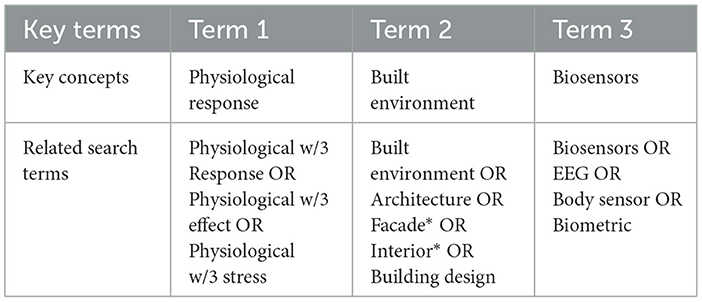
Table 1. Search criteria and linguistic variations for topic “measuring the physiological response to the built environment using biosensors”.
Results from databases and other sources, including gray literature, identified n = 1,334 potentially relevant studies after removal of duplicates. These results can be broken down as follows: PUBMED (n = 330), Science Direct (n = 273), SCOPUS (n = 551), Web of Science (n = 180) and records identified through other sources i.e., Google Scholar, cited articles etc. (n = 152). These records were screened by title and abstract (n = 269). Following full-text screening, n = 9 sources were included for data extraction. From these sources, the relevant clinical biomarkers and architectural forms were identified. The search process is summarized in the PRISMA-P flow diagram below (Figure 1).
To recall, the aim of this systematic review was three-fold: (i) to determine the specific clinical biomarkers utilized for evaluating physiological stress responses to architectural forms (ii) to identify distinct categories of architectural forms that are correlated with elevated stress responses, employing clinical biomarkers, and (iii) to analyse the robustness of the methodological approaches engaged to measure physiological stress responses to architectural forms. This section presents an overview of the review findings.
The review revealed the clinical biomarkers and biosensors most commonly used to measure stress responses to particular architectural forms (see Table 2 below). The most common biomarkers used to measure stress responses are pupil dilation, heart rate and heart rate variability (HRV), skin conductance and body temperature, salivary cortisol, Galvanic Skin Response (GSR), brain activity, and functional Magnetic Resonance Imaging (fMRI) (Persiani et al., 2021).
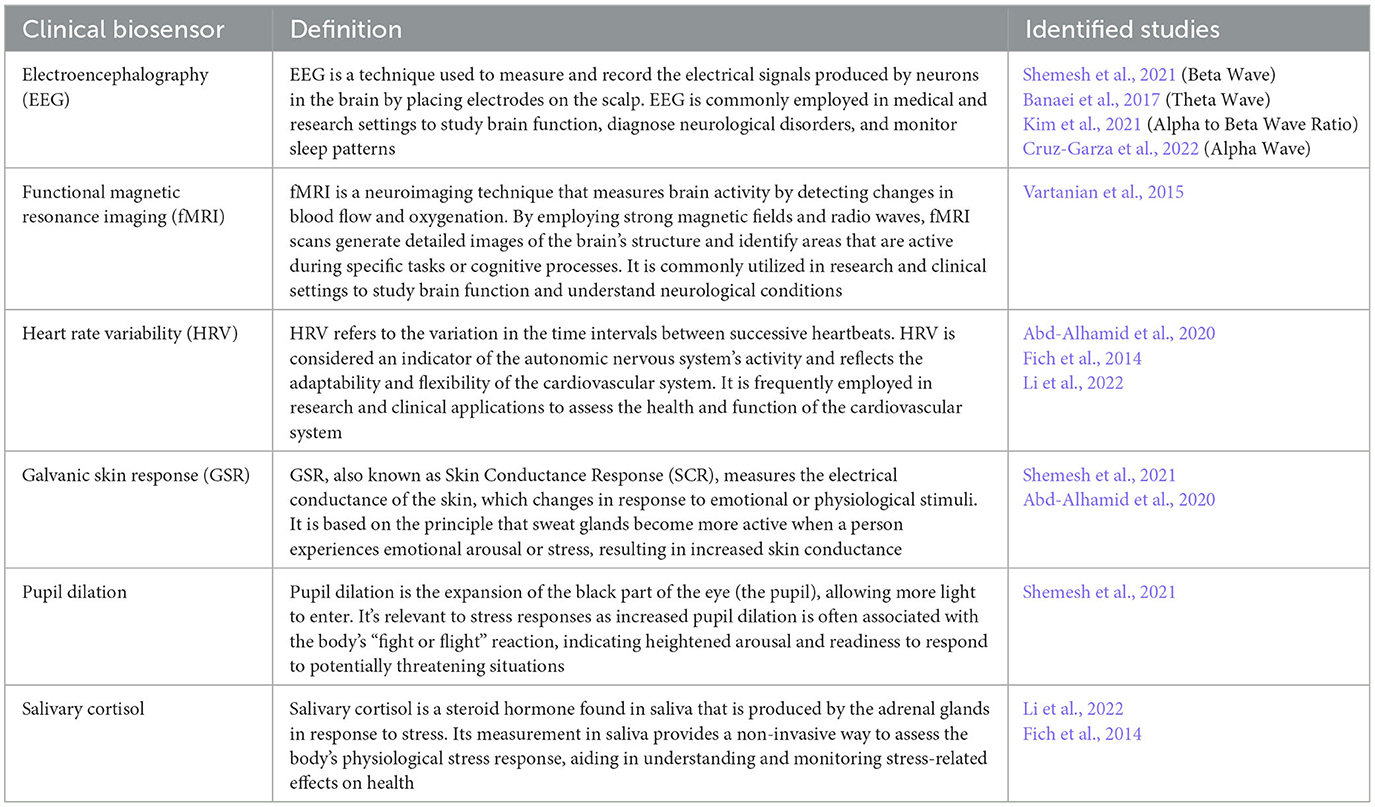
Table 2. Identified clinical biomarkers utilized for evaluating physiological stress responses to architectural forms.
Increased stress responses may be indicated by greater pupil dilation, which shows heightened cognitive and emotional arousal, a reduced HRV, increased levels of the stress hormone - salivary cortisol, and changes in GSR (with stressors resulting in increases in skin conductance levels). When it comes to measuring brain activity, EEG is used to measure activity in the brain waves—alpha, beta, delta, gamma, and theta. Changes in EEG activity indicating stress include increased beta wave activity and decreased alpha wave activity. A high ratio of alpha to beta waves (RAB) suggests a more relaxed state, while a low ratio indicates a more alert and active state. Therefore, an increase in beta waves relative to alpha waves is often interpreted as an indicator of heightened arousal or stress. Theta waves are typically observed during periods of intensified mental and emotional activity, while gamma waves are associated with heightened arousal states, including anxiety and fear. Finally, fMRI scans measure neurological activity by detecting changes associated with blood flow (Glover, 2011). Neural activity and energy metabolism (i.e., cerebral blood flow) are coupled, meaning that when a region of the brain is engaged, blood flow to that area increases (Logothetis et al., 2001). In short, with fMRI, it becomes possible to identify which area of the brain is being activated during a particular activity. The changes that indicate increased stress are increased activation in regions such as the amygdala, insula, and anterior cingulate cortex, which are associated with the processing of emotional and stress-related stimuli.
The review identified the most common architectural stimuli used to assess physiological stress responses to architectural forms (Table 3). Furthermore, it identified the most frequently evaluated categories of architectural forms that exhibit a notable correlation with augmented stress reactions, as confirmed by the assessment of clinical biomarkers (Table 4). The identified architectural forms have been separated into three categories using engineering terminology: curvature, proportion, and enclosure. For the purposes of this review, engineering terminology was adopted as the precise definition of the parameters used to characterize particular architectural forms (and variations in same) varied across individual studies (discussed further below). While engineering terminology may not be homologous to the identified architectural variables it is suitably analogous and provides a useful means to categorize these variables in a coherent fashion.
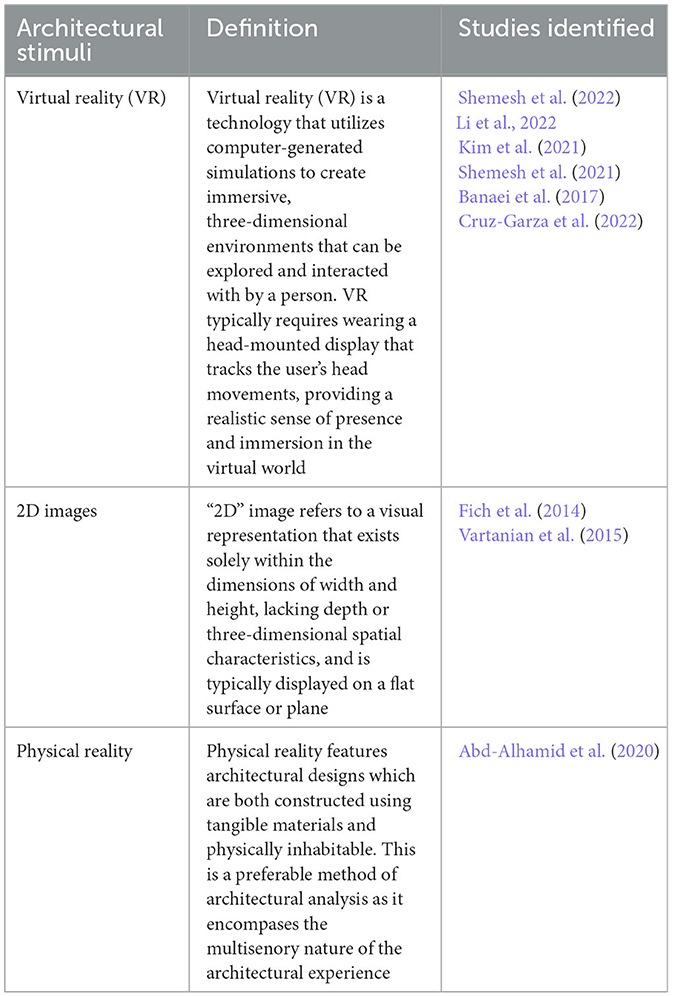
Table 3. Identified architectural stimuli utilized for evaluating physiological stress responses to architectural forms.
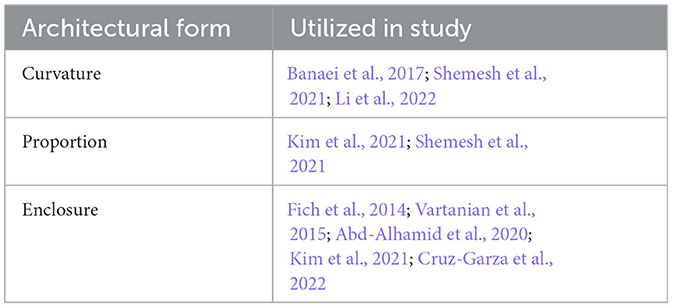
Table 4. Identified categories of architectural forms that have demonstrated a correlation with elevated stress responses.
However, given the inherently contextual nature of architecture, the application of engineering terminology requires careful consideration. For instance, curvature, as per engineering standards, signifies “the rate of directional change of a curve relative to its distance along the curve” (Whitman College, 2023). Nonetheless, the term curvature can encompass diverse architectural designs, such as the juncture of walls and ceilings in a vaulted structure, or the design of the header in an arched window. Despite these shortcomings, engineering terminology (curvature, proportion, and enclosure) was purposefully employed to establish a coherent framework for presenting the outcomes.
To recall, the linear or curvilinear quality of walls within architectural structures can be defined as “the rate of change of direction of a curve with respect to distance along the curve” (Whitman College, 2023). Among the studies reviewed, three studies (Banaei et al., 2017; Shemesh et al., 2021; Li et al., 2022) measured stress responses to variations in curvature using clinical biomarkers.
Shemesh et al. (2021) examined the physiological and emotional responses of 112 participants to 27 different virtual scenes that varied in scale, curvature, proportion, and protrusion using multiple clinical biomarkers. The study measured EEG (Beta Waves), pupil dilation, and GSR. The architectural environment consisted of a white room without ornamentation or openings. The study defined the degree of curvature in the VR environment by combining the criteria of zero curvature and asymmetry. The degree of wall and ceiling curvature of the interior room was controlled using a script in Grasshopper (software tool). Specifically, the interior walls and ceiling were adjusted from “orthogonal to round” and “sharp to curvy,” measured on the basis of a scale between 0 and 4. The findings of the study demonstrated a positive correlation between minimal wall curvature and increased physiological stress. The participant's exhibited augmented pupil diameter and an increase in peak measurements of maximal GSR amplitude when exposed to low curvature. However, no statistically significant results were reported concerning Beta Wave activity. Collectively, these outcomes imply that minimal wall curvature might be linked to an elevated physiological stress response.
An earlier study by Banaei et al. (2017) measured physiological responses to variations in architectural form, including degrees of wall curvature. Twenty-five different form clusters were extracted from 343 interior images of living rooms from different architectural epochs with varying architectural styles. To assess the impact of the stimuli, 17 participants' brain activity was measured using EEG as they walked through a VR environment. Unlike Shemesh et al. (2021), which used a curvature scale between 0 and 4, curvature in this study was defined using a binary: linear or curved. Linear was defined as a 3D solid with a width <20 cm, that was attached to another surface by its large face. The definition of curvature was not explicitly stated, however, the degree of curvature appeared to relate to either a curved connection between the ceiling and walls, curved archways, or a curved wall. While the study did not directly measure stress responses the findings suggest that greater curvature resulted in higher pleasure and emotional arousal rates, these were reflected in higher theta wave activity of the anterior cingulate cortex (ACC). Based on the findings of this study, it can be inferred that the anterior cingulate cortex (ACC) plays a role in the cognitive processing of architectural experiences. However, the specific association between theta wave activity and physiological stress remains inconclusive as theta wave activity is associated with a range of emotional arousal, including fear and anxiety (Lapomarda et al., 2022).
Most recently, Li et al. (2022) investigated the effects of transitional spaces on stress recovery using a VR environment. Forty participants were randomly assigned to one of three conditions: a curvilinear transitional space, a linear transitional space, or a control condition with no transitional space. Li et al. (2022) differed from both Banaei et al. (2017) and Shemesh et al. (2021) in that they assessed transitional spaces (partly indoor and partly outdoor). Additionally, Li et al. (2022) defined “curve” as having a round shape according to the Oxford Learner's dictionaries. Two transitional spaces were assessed: a cafe and a plaza. The area of the cafe space included a range of elements, like overhead weather shelters, benches, tables, planters, flooring, and lights. Within this cafe space, two distinct environments were established: a linear environment (B linear) and a curved environment (B curved). Similarly, the transitional area of the plaza featured overhead shelters, flowerbeds, and landscape treatment. In the plaza space, analogous to the cafe space, two environments were formed: a linear environment (A linear) and a curved environment (A curved). The study distinguished between linear and curved environments based on the predominant shape of their components. Stress responses were assessed using the Trier Social Stress Test (TSST), which included measures of salivary cortisol, heart rate variability, and subjective stress ratings. The study found that both curvilinear and linear transitional spaces had a positive effect on stress recovery compared to the control condition. However, no significant difference in physiological activity was recorded between the curvilinear and linear transitional space types. As a result, further scholarly investigation is merited.
Proportion is defined as “the relationship between one thing and another in size, amount, or degree” (Merriam-Webster, 2023), and is indicated in architectural design by variations in ceiling height, aspect ratio, and scale. The following two studies examined the impact of proportion on physiological stress using clinical biomarkers (Kim et al., 2021; Shemesh et al., 2022).
In Kim et al. (2021), an arousal response was measured using EEG and virtual reality, exploring changes in ceiling height, window area ratio, and aspect ratio in 33 female participants who experienced a private postpartum care room via virtual reality, during which an Alpha/Beta Wave (RAB) ratio was recorded and a self-report questionnaire was administered. A reduced ratio of alpha/beta (RAB) typically indicates higher stress levels or reduced relaxation states, whereas an increased RAB generally signifies lower stress levels and reduced arousal responses. Aspect ratio “means the ratio between the length and the width of a plane” (Kim et al., 2021, p. 7). Two scenarios with varying aspect ratios were created. The aspect ratio of Type A was 1:1.6 (3 m × 4.81 m), where the space was long seen from the door, while the aspect ratio of Type B was 1.6:1 (4.81 m × 3 m), where the space was wide. The precise ceiling height was defined as the height from the floor finish to the ceiling finish. Three ceiling height variations were defined: 2.3 m, 2.7 m, and 3 m. Window ratio was determined using the following formula: window area/(exterior envelope wall area + window area) × 100%. Five types of window ratios were defined: 20%, 40%, 60%, 80%, and 100% (discussed further in 4.2.3 below). The study found that in a room with an aspect ratio of 1:1.6, the window ratio of 60% induced the least arousal response when the ceiling height was 2.3 m. However, when the ceiling heights were 2.7 and 3.0 m, the window ratio of 80% induced the least arousal response. In a room with an aspect ratio of 1.6:1, when the ceiling heights were 2.3 and 2.7 m the window ratios of 100 and 80% induced the least arousal response, respectively. The window ratios induced the least arousal response when the ceiling heights were 2.3 and 2.7 m, whereas the window ratios of 40%, 60%, and 100% induced comparable arousal responses when the ceiling height was 3.0 m.
As noted above, Shemesh et al. (2022) conducted a study on the emotional impact of architectural geometries using biometric measurements: pupil dilation, galvanic skin response and EEG. Their findings regarding proportion found narrow spaces (2m × 4 m × 4 m) were associated with larger mean pupil diameter and maximal pupil diameter, indicating increased physiological stress. In addition, the study found heightened Beta Wave activity in narrow virtual environments. These results suggest that narrow spaces elicit greater physiological stress responses than wide spaces (6 m × 4 m × 4 m).
In the present context, the term “enclosure” pertains to the diverse configurations and dimensions of windows within a building. It specifically encompasses the size and arrangement of windows within the building envelope or facade. However, there is an intersection between proportion and enclosure. For example, specific room proportions, such as narrower dimensions, can amplify the sense of enclosure. Additionally, certain studies (Vartanian et al., 2015; Kim et al., 2021; Shemesh et al., 2021) manipulated both proportion and enclosure concurrently, highlighting their interconnected nature. The following five studies examined the impact of enclosure on physiological stress using clinical biomarkers (Fich et al., 2014; Vartanian et al., 2015; Abd-Alhamid et al., 2020; Kim et al., 2021; Cruz-Garza et al., 2022).
Fich et al. (2014) utilized a virtual Trier Social Stress Test to examine salivary cortisol, a stress hormone, and HRV responses in 49 healthy male participants with no architectural training, aged 19 to 31. Subjects were placed in two virtual environment test rooms and made to undergo three psychosocial stress-inducing activities (an incomplete instruction by the committee about one of the tasks, preparing and presenting a speech, and taking a basic maths exam), each of which lasted for 5 min. Room one had no openings, while room two had three large openings with a view of a simple horizon line. The specific size of the openings were not reported. The participants in the enclosed space responded with pronounced increases in salivary cortisol (73% increase), heart rate (15.3% increase) and decreased recovery speed, as compared to the participants in open rooms. The results indicate increased acute stress responses in enclosed architectural forms.
Subsequently, Vartanian et al. (2015) examined the effects of ceiling height and perceived enclosure areas on neurological activity using fMRI scans. The study examined the neural activity of 18 participants as they were exposed to 200 two-dimensional (2D) photographs of architectural forms. The study reported that “half of the spaces had high ceilings and the other half had low ceilings. Similarly, half of the spaces were enclosed and the other half open. This resulted in the following four conditions: open high ceiling, open low ceiling, enclosed high ceiling, and enclosed low ceiling” (Vartanian et al., 2015, p. 14). However, similarly to Fich et al. (2014), the explicit definitions or dimensions for “high ceiling,” “low ceiling,” “enclosed,” and “open” were not provided. The results indicated that the enclosed rooms elicited increased acute activation in the anterior midcingulate cortex (aMCC), which receives direct input from the amygdala, a part of the brain that governs fear responses. Although the aMCC receives inputs from many areas of the brain and is engaged in various cognitive and emotional integration processes, the author states that “Vogt's (2005) analysis of 23 neuroimaging studies showing peak activations within the cingulate gyrus demonstrated that the specific region activated in the present study is associated with processing fear” (Vartanian et al., 2015, p. 16). Put simply, it appears that enclosed rooms may trigger a fear based stress response. While Vartanian et al. (2015) did explore aspects of proportion, their empirical observations (i.e., activated structures involved in visuospatial exploration and attention in the dorsal stream) remain unassociated with physiological stress responses, consequently precluding their inclusion within the present review.
More recently, Abd-Alhamid et al. (2020) utilized skin conductance, heart rate, and HRV to assess the restorative effect of different levels of enclosure by providing a window view observation period after a stressful task on 32 participants. Unlike the studies outlined above, Abd-Alhamid manipulated the degree of enclosure by changing the viewing distance from the window as opposed to the size of the opening (Vartanian et al., 2015) or the number of openings (Fich et al., 2014). Instead, the window view was observed from three different viewing locations: close (0.8 m), middle (2.18 m), and far (3.55 m). The window measured 1.4 m × 1.4 m on a wall measuring 3.3 m × 2.85 m. A windowless environment was used as a baseline. Abd-Alhamid et al. (2020) detected significant changes in skin conductance, with higher values observed when participants viewed objects up close compared to when they viewed objects at middle and far distances. Moreover, decreased indicators of heart rate and HRV were observed in the middle and far viewing locations when contrasted with the close viewing location. These physiological changes suggest that the size of window views can have a significant impact on the stress-recovery process and overall wellbeing of building occupants, with smaller windows resulting in increased physiological stress responses.
Building on the previous research, Cruz-Garza et al. (2022) conducted a study to evaluate the effects of window positioning, quantity, and size, on human stress and anxiety levels using EEG in classrooms. Biometric measurements of stress responses were taken from participants during a VR experience and then integrated into a normative dataset composed of the participants' backgrounds and other demographic information. Similar to Fich et al. (2014) and Cruz-Garza et al. (2022) changed the number of windows, as opposed to the size of a single window. Subjects fitted with VR were exposed to four room types, each for a period of 2 min: (i) windowless; (ii) one window; (iii) two windows, and (iv) windowless but with an increased room width. Three different window arrangements were assessed: (a) no window view (“neutral” condition), (b) a side view tangential to the instruction, and (c) direct forward views located behind the instruction area. In both of the window conditions (b and c), the same image of natural exterior scenery was used, consisting of a view of tree-tops and clouds with no motion distractions, buildings, or other human-made elements. Two options for the classroom dimensions were evaluated: (a) narrow classroom of 15 ft by 50 ft, designated as the “neutral” condition, and (b) wide classroom of 22 ft by 50 ft. The ceiling's height was not manipulated in the study; it was held constant at 12 ft in each of the classroom variants. The exact size of the window was not stated. EEG data showed increased oscillatory activity in all recorded channels when exposed to rooms with windows. Although this study did not directly consider stress responses, alpha band power (8–12 Hz) increased when exposed to a room with a greater width, indicating an increased state of relaxation. In sum, the results suggest a potential positive relationship between the windowless and more enclosed spaces and physiological stress levels.
Lastly, to recall, Kim et al. (2021) conducted an EEG study involving 33 female participants who were exposed to VR in private postpartum care rooms. Alongside proportion, the study also investigated the relationship between the degree of enclosure, characterized by the aspect ratio, ceiling height, and window ratio, and RAB activity. Window ratio was determined using the following formula: window area/(exterior envelope wall area + window area) × 100%. Five types of window ratios were defined: 20%, 40%, 60%, 80%, and 100% (discussed in 4.2.3 below). The findings revealed that rooms with higher degrees of enclosure, indicated by lower ceiling heights, smaller window ratios, and length-to-width aspect ratios greater than one, exhibited reduced RAB wave activity. The decrease in the RAB signifies higher stress levels or reduced relaxation states. However, it is essential to acknowledge the complexity in interpreting the study's results due to the interconnected nature of window size, ceiling height, and aspect ratio. Further research is warranted to explore the specific mechanisms underlying these relationships and their implications for creating more comfortable and supportive postpartum care environments.
The results from the systematic review of the impact of architectural forms on physiological stress responses highlight an important and nuanced relationship between the built environment and human physiology. The findings from this review suggest that certain architectural forms, specifically curvature, enclosure and proportion, may impact physiological stress responses. However, the field of neuroarchitecture is in its infancy, and the techniques for measuring the stress implications of architectural form are still developing. It is evident from the review that there is still considerable scope for further research into human's physiological responses to the built environment. The following discussion highlights a number of critical concerns.
First, there is a limited amount of research examining the impact of architectural form on physiological stress responses. It is noteworthy to acknowledge that the findings pertaining to curvature were not robust, with only one (Shemesh et al., 2021) out of the three scrutinized studies demonstrating a discernible distinction between the impact of curved and linear configurations on physiological stress responses. Furthermore, the studies examining the impact of proportion on physiological stress responses were conducted with a limited sample size, encompassing merely two studies (Kim et al., 2021; Shemesh et al., 2021). While the reviewed studies evidence the potential to measure physiological stress responses to architectural forms using clinical biomarkers, further evidence is needed to substantiate a link between particular architectural forms and physiological stress outcomes.
Second, the precise characterization of curvature, proportion, and enclosure appear to lack uniformity in the reviewed studies. For example, when measuring the effect of curvature on physiological stress responses, Shemesh et al. (2021) defined curvature on a scale from 0 to 4, while Banaei et al. (2017) and Li et al. (2022) defined curvature as a binary (i.e., curved or linear). Moreover, the architectural forms examined in these studies differed greatly, with Shemesh et al. (2021) examining only wall and ceiling curvature while Banaei et al. (2017) and Li et al. (2022) included curvature of archways, and other more decorative features. Similarly, in the studies examining the impact of proportion on physiological stress responses, Kim et al. (2021) altered enclosure by changing space aspect ratio, ceiling height, and window ratio to study their effects on arousal response. In contrast, Shemesh et al. (2022) investigated the emotional impact of narrow spaces (2 m × 4 m × 4 m) compared to wide spaces (6 m × 4 m × 4 m), focusing on proportion as a key architectural element. This inconsistency in definitions or metrics indicates potential variation in interpretive frameworks utilized in these studies, leading to a number of issues. For instance, when measurement terms and criteria are not standardized, it becomes challenging to replicate studies and obtain consistent results. This lack of standardization makes it harder to establish cause-and-effect relationships. Likewise, without standardized metrics, it becomes difficult to compare findings across studies. This hinders the ability to identify trends, patterns, or divergent outcomes, which are crucial for advancing scientific understanding. Going forwards, it would be helpful for future research to actively work toward establishing standardized metrics denoted with consistent terminology.
Third, the reviewed studies examine a variety of settings, including a classroom (Fich et al., 2014; Cruz-Garza et al., 2022) and a postpartum care room (Kim et al., 2021). Some of the reviewed studies examined the impact of the stimuli in isolation and did not consider the context of the environment. For instance, Shemesh et al. (2021) examined the physiological effects of scale, curvature and proportion using multiple clinical biomarkers in a plain white room without any ornamentation, openings (i.e., windows or doors) or contextual indicators (i.e., a bedroom or a work environment). Due to these variations in the settings examined, the findings from one setting may not be transferable to another. For instance, the specific conditions in which an individual experiences certain spaces, such as a postpartum care room, may differ substantially from one another. The context specific nature of the settings thus further limits the ability to generalize the findings of these studies.
Fourth, of reviewed studies, six used virtual reality (Banaei et al., 2017; Kim et al., 2021; Shemesh et al., 2021, 2022; Cruz-Garza et al., 2022; Li et al., 2022) and two of the reviewed studies used 2D visual stimuli (Fich et al., 2014; Vartanian et al., 2015), while only one used physical architecture (Abd-Alhamid et al., 2020). Researchers often use VR and 2D images as proxy stimuli for physical architecture in architectural health research. This allowed the authors to manipulate architectural variables easily. However, it should be noted that VR and 2D images may fail to capture the multisensory nature of physical architecture, and therefore, the transferability of these findings may be limited (Valentine, 2023a). Additionally, VR has been known to induce physiological stress and subsequent distress and discomfort, often referred to as “cybersickness” or “simulator sickness” (Howard and Van Zandt, 2021). The reviewed studies did not control for these variables.
Fifth, the reviewed studies focus only on acute, short term physiological responses and did not consider the long term impacts of exposure to stress inducing architectural forms. However, a recent article has hypothesized that chronic exposure to stress inducing architectural forms may result in allostatic overloading (Valentine, 2023b). Allostatic overloading refers to the chronic or repeated exposure to stress-inducing events which may overwhelm the body's regulatory system, resulting in damage to organs and tissues (Mariotti, 2015). As Valentine (2023b) highlights, future research examining the impact of architectural form on physiological stress responses will need to integrate additional clinical biomarkers, as well as clinimetrics, in order to fully capture the effects of architectural form on human physiology.
The results from the systematic review highlight the complex relationship between the built environment and human health, specifically regarding the impact of architectural forms on physiological stress responses. The findings suggest that certain architectural forms, including curvature, enclosure, and proportion, may have the potential to amplify or reduce physiological stress. Additionally, this systematic review underscores the utility of clinical biomarkers in assessing physiological stress reactions to architectural configurations. However, to date, there is a paucity of research on the relationship between physiological stress and architectural form, an absence of uniformity in the metrics engaged to measure and define architectural forms, and a need to account for the contextually distinctive nature of architectural encounters. Moving forward, further research is needed: to standardize the metrics used to define particular architectural forms, to assess the impact of long-term exposure to architectural forms on physiological responses using longitudinal studies, and to produce a more robust body of scholarship on the stress-inducing and reducing impact of architectural forms.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The author confirms being the sole contributor of this work and has approved it for publication.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. ^The term “architectural features” here refers to specific characteristics of the built environment, i.e., thermal comfort, lighting and material composition.
2. ^However, see Zielinska-Dabkowska (2014), this study investigated concerns regarding media architecture negatively affecting urban nighttime environments.
Abd-Alhamid, F., Kent, M., Calautit, J., and Wu, Y. (2020). Evaluating the impact of viewing location on view perception using a virtual environment. Build. Environ. 180, 106932. doi: 10.1016/j.buildenv.2020.106932
Aronson, J. K., and Ferner, R. E. (2017). Biomarkers—a general review. Curr. Protoc. Pharmacol. 76, 9.23.1–9.23.17. doi: 10.1002/cpph.19
Balasco, L., Provenzano, G., and Bozzi, Y. (2020). Sensory abnormalities in autism spectrum disorders: a focus on the tactile domain, from genetic mouse models to the clinic. Front. Psychiatry 10, 1016. doi: 10.3389/fpsyt.2019.01016
Banaei, M., Ahmadi, A., Gramann, K., and Hatami, J. (2019). Emotional evaluation of architectural interior forms based on personality differences using virtual reality. Front. Archit. Res. 9, 138–147. doi: 10.1016/j.foar.2019.07.005
Banaei, M., Hatami, J., Yazdanfar, A., and Gramann, K. (2017). Walking through architectural spaces: the impact of interior forms on human brain dynamics. Front. Hum. Neurosci. 11, 477. doi: 10.3389/fnhum.2017.00477
Borgianni, Y., and Maccioni, L. (2020). Review of the use of neurophysiological and biometric measures in experimental design research. Artif. Intell. Eng. Des. Anal. Manuf. 34, 248–285. doi: 10.1017/S0890060420000062
Choi, Y., Kim, M., and Chun, C. (2019). Effect of temperature on attention ability based on electroencephalogram measurements. Build. Environ. 147, 299–304. doi: 10.1016/j.buildenv.2018.10.020
Choo, H., Nasar, J. L., Nikrahei, B., and Walther, D. B. (2017). Neural codes of seeing architectural styles. Sci. Rep. 7, 40201. doi: 10.1038/srep40201
Chu, B., Marwaha, K., Sanvictores, T., and Ayers, D. (2023). Physiology, Stress Reaction. StatPearls Publishing. Available online at: http://www.ncbi.nlm.nih.gov/books/NBK541120/ (accessed May 21, 2023).
Coburn, A., Vartanian, O., and Chatterjee, A. (2017). Buildings, beauty, and the brain: a neuroscience of architectural experience. J. Cogn. Neurosci. 29, 1521–1531. doi: 10.1162/jocn_a_01146
Cruz-Garza, J. G., Darfler, M., Rounds, J. D., Gao, E., and Kalantari, S. (2022). EEG-based investigation of the impact of room size and window placement on cognitive performance. J. Build. Eng. 53. doi: 10.1016/j.jobe.2022.104540
Dhabhar, F. S. (2008). Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection versus immunopathology. Allergy Asthma Clin. Immunol. 4, 2–11. doi: 10.1186/1710-1492-4-1-2
DiSabato, D. J., Quan, N., and Godbout, J. P. (2016). Neuroinflammation: the devil is in the details. J. Neurochem. 139 (Suppl. 2), 136–153. doi: 10.1111/jnc.13607
Ergan, S., Radwan, A., Zou, Z., Tseng, H. A., and Han, X. (2019). Quantifying human experience in architectural spaces with integrated virtual reality and body sensor networks. J. Comput. Civ. Eng. 33. doi: 10.1061/(ASCE)CP.1943-5487.0000812
Fich, L. B., Jönsson, P., Kirkegaard, P. H., Wallergård, M., Garde, A. H., Hansen, Å., et al. (2014). Can architectural design alter the physiological reaction to psychosocial stress? A virtual TSST experiment. Physiol. Behav. 135, 91–97. doi: 10.1016/j.physbeh.2014.05.034
Foth, M., and Caldwell, G. A. (2018). “More-than-human media architecture,” in Proceedings of the 4th Media Architecture Biennale Conference (New York, NY: ACM), 66–75. doi: 10.1145/3284389.3284495
Frank, M. G., Fonken, L. K., Watkins, L. R., and Maier, S. F. (2019). Microglia: neuroimmune-sensors of stress. Semin. Cell Dev. Biol. 94, 176–185. doi: 10.1016/j.semcdb.2019.01.001
Frank-Cannon, T. C., Alto, L. T., McAlpine, F. E., and Tansey, M. G. (2009). Does neuroinflammation fan the flame in neurodegenerative diseases? Mol. Neurodegen. 4:47. doi: 10.1186/1750-1326-4-47
Glover, G. H. (2011). Overview of functional magnetic resonance imaging. Neurosurg. Clin. N. Am. 22, 133–139. doi: 10.1016/j.nec.2010.11.001
Grippo, A. J., and Scotti, M.-A. L. (2013). Stress and neuroinflammation. Inflamm. Psychiatry 28, 20–32. doi: 10.1159/000343965
Guan, H., Hu, S., Lu, M., He, M., Zhang, X., Liu, G., et al. (2020). Analysis of human electroencephalogram features in different indoor environments. Build. Environ. 186, 107328. doi: 10.1016/j.buildenv.2020.107328
Guidi, J., Lucente, M., Sonino, N., and Fava, G. A. (2021). allostatic load and its impact on health: a systematic review. Psychother. Psychosom. 90, 11–27. doi: 10.1159/000510696
Harricharan, S., Nicholson, A. A., Densmore, M., Théberge, J., McKinnon, M. C., Neufeld, R. W. J., et al. (2017). Sensory overload and imbalance: resting-state vestibular connectivity in PTSD and its dissociative subtype. Neuropsychologia 106, 169–178. doi: 10.1016/j.neuropsychologia.2017.09.010
Howard, M. C., and Van Zandt, E. C. (2021). A meta-analysis of the virtual reality problem: unequal effects of virtual reality sickness across individual differences. Virtual Real. 25, 1221–1246. Scopus. doi: 10.1007/s10055-021-00524-3
Jin, Z. K., and Juan, Y. K. (2021). Is Fengshui a science or superstition? A new approach combining the physiological and psychological measurement of indoor environments. Build. Environ. 201, 107992. doi: 10.1016/j.buildenv.2021.107992
Kim, J., and Kim, N. (2022). Quantifying emotions in architectural environments using biometrics. Appl. Sci. 12, 9998. doi: 10.3390/app12199998
Kim, S., Park, H., and Choo, S. (2021). Effects of changes to architectural elements on human relaxation-arousal responses: based on VR and EEG. Int. J. Environ. Res. Public Health 18, 4305. doi: 10.3390/ijerph18084305
Küller, R., Mikellides, B., and Janssens, J. (2009). Color, arousal, and performance—a comparison of three experiments. Color Res. Appl., 34, 141–152. doi: 10.1002/col.20476
Lapomarda, G., Valer, S., Job, R., and Grecucci, A. (2022). Built to last: theta and delta changes in resting-state EEG activity after regulating emotions. Brain Behav. 12, e2597. doi: 10.1002/brb3.2597
Li, Z., Huang, X., and White, M. (2022). Effects of the visual character of transitional spaces on human stress recovery in a virtual reality environment. Int. J. Environ. Res. Public Health. 19, 13143. doi: 10.3390/ijerph192013143
Logothetis, N. K., Pauls, J., Augath, M., Trinath, T., and Oeltermann, A. (2001). Neurophysiological investigation of the basis of the fMRI signal. Nature 412, 150–157. doi: 10.1038/35084005
Lu, M., Hu, S., Mao, Z., Liang, P., Xin, S., Guan, H., et al. (2020). Research on work efficiency and light comfort based on EEG evaluation method. Build. Environ. 183, 107122. doi: 10.1016/j.buildenv.2020.107122
Lurie, D. I. (2018). An integrative approach to neuroinflammation in psychiatric disorders and neuropathic pain. J. Exp. Neurosci. 12, 1–11. doi: 10.1177/1179069518793639
Mariotti, A. (2015). The effects of chronic stress on health: new insights into the molecular mechanisms of brain-body communication. Future Sci. OA 1:FSO23. doi: 10.4155/fso.15.21
Merriam-Webster (2023). Definition of PROPORTION. Available online at: https://www.merriam-webster.com/dictionary/proportion (accessed May 27, 2023).
Miller, L. J., Nielsen, D. M., and Schoen, S. A. (2012). Attention deficit hyperactivity disorder and sensory modulation disorder: a comparison of behavior and physiology. Res. Dev. Disabil. 33, 804–818. doi: 10.1016/j.ridd.2011.12.005
Moher, D., Shamseer, L., Clarke, M., Ghersi, D., Liberati, A., Petticrew, M., et al. (2015). Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 4, 1. doi: 10.1186/2046-4053-4-1
Munhoz, C. D., Garc?a-Bueno, B., Madrigal, J. L. M., Lepsch, L. B., Scavone, C., and Leza, J. C. (2008). Stress-induced neuroinflammation: mechanisms and new pharmacological targets. Brazil. J. Med. Biol. Res. 41, 1037–1046. doi: 10.1590/S0100-879X2008001200001
O'Callaghan, J. P., and Miller, D. B. (2019). Neuroinflammation disorders exacerbated by environmental stressors. Metabolism 100:153951. doi: 10.1016/j.metabol.2019.153951
Persiani, S. G. L., Kobas, B., Koth, S. C., and Auer, T. (2021). Biometric data as real-time measure of physiological reactions to environmental stimuli in the built environment. Energies 14, 1. doi: 10.3390/en14010232
Rosenkranz, M. A., Lutz, A., Perlman, D. M., Bachhuber, D. R. W., Schuyler, B. S., MacCoon, D. G., and Davidson, R. J. (2016). Reduced stress and inflammatory responsiveness in experienced meditators compared to a matched healthy control group. Psychoneuroendocrinology 68, 117–125. doi: 10.1016/j.psyneuen.2016.02.013
Rounds, J. D., Cruz-Garza, J. G., and Kalantari, S. (2020). Using posterior EEG theta band to assess the effects of architectural designs on landmark recognition in an urban setting. Front. Hum. Neurosci. 14, 537. doi: 10.3389/fnhum.2020.584385
Salingaros, N. A. (2019). The biophilic healing index predicts effects of the built environment on our wellbeing. J. Biourban. 8, 13–34.
Scheydt, S., Müller Staub, M., Frauenfelder, F., Nielsen, G. H., Behrens, J., Needham, I., et al. (2017). Sensory overload: a concept analysis. Int. J. Ment. Health Nurs. 26, 110–120. doi: 10.1111/inm.12303
Schweizer, C., Edwards, R. D., Bayer-Oglesby, L., Gauderman, W. J., Ilacqua, V., Jantunen, M. J., et al. (2007). Indoor time–microenvironment–activity patterns in seven regions of Europe. J. Expo. Sci. Environ. Epidemiol. 17, 170–181. doi: 10.1038/sj.jes.7500490
Shamseer, L., Moher, D., Clarke, M., Ghersi, D., Liberati, A., Petticrew, M., et al. (2015). Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 349, g7647. doi: 10.1136/bmj.g7647
Shemesh, A., Leisman, G., Bar, M., and Grobman, Y. J. (2021). A neurocognitive study of the emotional impact of geometrical criteria of architectural space. Archit. Sci. Rev. 64, 1–14. doi: 10.1080/00038628.2021.1940827
Shemesh, A., Leisman, G., Bar, M., and Grobman, Y. J. (2022). The emotional influence of different geometries in virtual spaces: a neurocognitive examination. J. Environ. Psychol. 81, 101802. doi: 10.1016/j.jenvp.2022.101802
Shemesh, A., Talmon, R., Karp, O., Amir, I., Bar, M., Grobman, Y. J., et al. (2017). Affective response to architecture–investigating human reaction to spaces with different geometry. Archit. Sci. Rev. 60, 116–125. doi: 10.1080/00038628.2016.1266597
Shin, Y.-B., Woo, S.-H., Kim, D.-H., Kim, J., Kim, J.-J., Park, J. Y., et al. (2015). The effect on emotions and brain activity by the direct/indirect lighting in the residential environment. Neurosci. Lett. 584, 28–32. doi: 10.1016/j.neulet.2014.09.046
Stenvinkel, P., Chertow, G. M., Devarajan, P., Levin, A., Andreoli, S. P., Bangalore, S., et al. (2021). Chronic inflammation in chronic kidney disease progression: role of Nrf2. Kidney Int. Rep. 6, 1775–1787. doi: 10.1016/j.ekir.2021.04.023
Tsunetsugu, Y., Miyazaki, Y., and Sato, H. (2002). The visual effects of wooden interiors in actual-size living rooms on the autonomic nervous activities. J. Physiol. Anthropol. Appl. Human Sci. 21, 297–300. doi: 10.2114/jpa.21.297
Valentine, C. (2023a). Architectural allostatic overloading: exploring a connection between architectural form and allostatic overloading. Int. J. Environ. Res. Public Health 20, 9. doi: 10.3390/ijerph20095637
Valentine, C. (2023b). Health implications of virtual architecture: an interdisciplinary exploration of the transferability of findings from neuroarchitecture. Int. J. Environ. Res. Public Health 20, 2735. doi: 10.3390/ijerph20032735
Vardoulakis, S., Dimitroulopoulou, C., Thornes, J., Lai, K.-M., Taylor, J., Myers, I., et al. (2015). Impact of climate change on the domestic indoor environment and associated health risks in the UK. Environ. Int. 85, 299–313. doi: 10.1016/j.envint.2015.09.010
Vartanian, O., Navarrete, G., Chatterjee, A., Fich, L. B., Gonzalez-Mora, J. L., Leder, H., et al. (2015). Architectural design and the brain: effects of ceiling height and perceived enclosure on beauty judgments and approach-avoidance decisions. J. Environ. Psychol. 41, 10–18. doi: 10.1016/j.jenvp.2014.11.006
Vartanian, O., Navarrete, G., Chatterjee, A., Fich, L. B., Leder, H., Modroño, C., et al. (2013). Impact of contour on aesthetic judgments and approach-avoidance decisions in architecture. PubMed 110, 10446–10453. doi: 10.1073/pnas.1301227110
Vogt, B. A. (2005). Pain and emotion interactions in subregions of the cingulate gyrus. Nat. Rev. Neurosci. 6, 7. doi: 10.1038/nrn1704
Whitman College (2023). 13.3 Arc Length and Curvature. Whitman College Introduction to Calculus. Available online at: https://www.whitman.edu/mathematics/calculus_online/chapter00.html and https://www.whitman.edu/mathematics/calculus_online/section13.03.html (accessed May 22, 2023).
Wohleb, E. S., and Godbout, J. P. (2013). Basic aspects of the immunology of neuroinflammation. Modern Trends Pharmacopsychiatry 28, 1–19. doi: 10.1159/000343964
Yin, J., Yuan, J., Arfaei, N., Catalano, P. J., Allen, J. G., and Spengler, J. D. (2020). Effects of biophilic indoor environment on stress and anxiety recovery: a between-subjects experiment in virtual reality. Environ. Int. 136:105427. doi: 10.1016/j.envint.2019.105427
Zielinska-Dabkowska, K. M. (2014). “Critical perspectives on media architecture: Is it still possible to design projects without negatively affecting urban nighttime environments and will the future remain dynamic, bright and multi-colored?” in Proceedings of the 2nd Media Architecture Biennale Conference: World Cities (New York, NY: ACM), 101–108. doi: 10.1145/2682884.2682895
Keywords: architecture, architectural health, physiological stress, clinical biomarkers, architectural form, neuroarchitecture
Citation: Valentine C (2024) The impact of architectural form on physiological stress: a systematic review. Front. Comput. Sci. 5:1237531. doi: 10.3389/fcomp.2023.1237531
Received: 09 June 2023; Accepted: 07 December 2023;
Published: 04 January 2024.
Edited by:
Ava Fatah gen Schieck, University College London, United KingdomReviewed by:
Isabella Pasqualini, Swiss Federal Institute of Technology Lausanne, SwitzerlandCopyright © 2024 Valentine. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cleo Valentine, Y3J2MjlAY2FtLmFjLnVr
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.