
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Comput. Sci. , 01 July 2022
Sec. Human-Media Interaction
Volume 4 - 2022 | https://doi.org/10.3389/fcomp.2022.869123
This article is part of the Research Topic Recognizing the State of Emotion, Cognition and Action from Physiological and Behavioural Signals View all 11 articles
A commentary has been posted on this article:
Commentary: A review on the role of affective stimuli in event-related frontal alpha asymmetry
Frontal alpha asymmetry refers to the difference between the right and left alpha activity over the frontal brain region. Increased activity in the left hemisphere has been linked to approach motivation and increased activity in the right hemisphere has been linked to avoidance or withdrawal. However, research on alpha asymmetry is diverse and has shown mixed results, which may partly be explained by the potency of the used stimuli to emotionally and motivationally engage participants. This review gives an overview of the types of affective stimuli utilized with the aim to identify which stimuli elicit a strong approach-avoidance effect in an affective context. We hope this contributes to better understanding of what is reflected by alpha asymmetry, and in what circumstances it may be an informative marker of emotional state. We systematically searched the literature for studies exploring event-related frontal alpha asymmetry in affective contexts. The search resulted in 61 papers, which were categorized in five stimulus categories that were expected to differ in their potency to engage participants: images & sounds, videos, real cues, games and other tasks. Studies were viewed with respect to the potency of the stimuli to evoke significant approach-avoidance effects on their own and in interaction with participant characteristics or condition. As expected, passively perceived stimuli that are multimodal or realistic, seem more potent to elicit alpha asymmetry than unimodal stimuli. Games, and other stimuli with a strong task-based component were expected to be relatively engaging but approach-avoidance effects did not seem to be much clearer than the studies using perception of videos and real cues. While multiple factors besides stimulus characteristics determine alpha asymmetry, and we did not identify a type of affective stimulus that induces alpha asymmetry highly consistently, our results indicate that strongly engaging, salient and/or personally relevant stimuli are important to induce an approach-avoidance effect.
When examining the emotional experience of individuals with a certain product, task or situation, they are commonly asked about it. For instance, in food research, usage of explicit, verbal questionnaires is by far the most common way to assess consumers' emotional experience (Lagast et al., 2017; Kaneko et al., 2018). However, explicit, verbal measures have their shortcomings. Firstly, social desirability and self-presentational concerns can influence self-reported measures (Gawronski and de Houwer, 2014). Dell et al. (2012) found that respondents were about 2.5 times more likely to favor a technology believed to be developed by the interviewer than an exactly identical alternative. Furthermore, questionnaires usually reflect summative emotions post-interaction (Lottridge et al., 2012). Explicit measures are not well-suited for continuous monitoring to understand how emotional experience changes over time, such as during the interaction with a product. Continuous self-reporting is demanding and adds another task, and affects the emotional experience itself. To overcome such limitations, researchers have been arguing for the use of implicit measures (Gawronski and de Houwer, 2014), such as those inferred from spontaneous behavior or physiological signals. These allow for more objective measures that are not affected by response biases and continuous observation of the individual's emotional or affective state (Reuderink et al., 2013).
The circumplex model of affect characterizes emotions by valence and arousal (Russell, 1980). Valence refers to pleasantness, i.e., the degree of positive or negative affect, whereas arousal refers to the energetic component of the emotion (alertness). Research has consistently linked skin conductance to arousal (Christopoulos et al., 2019; Bartolomé-Tomás et al., 2020). Also, other types of physiological responses have been found to generally map better on arousal rather than valence (Mauss and Robinson, 2009). Valence has been found to be more difficult to assess using physiological measures. In this regard, asymmetric frontal cortical activation is of particular interest for implicitly measuring emotional processes (Coan and Allen, 2004; Harmon-Jones et al., 2010; Diaz and Bell, 2012). Early research has reported high incidence of negative affect in individuals with unilateral left hemispheric brain damage (Alford, 1933; Goldstein, 2004). These patients showed increased negative responses, fear and pessimism about the future. On the other hand, patients with unilateral right hemisphere damage displayed euphoric reactions (Denny-brown et al., 1952), such as inappropriate presentation of positive affect and laughing (Scherer and Ekman, 1984). In the late 70s, patterns of emotion processing have been associated with differences in the EEG alpha band (8–12 Hz) between the left and right frontal cortex, and was termed frontal alpha asymmetry (Tucker et al., 1981; Ahern and Schwartz, 1985; Davidson et al., 1985). Note that alpha power is inversely related to brain activity, such that low alpha activity is taken as an indication of high regional brain activation (Cook et al., 1998; Allen et al., 2004a).
Initial research focused on an affective explanation of frontal alpha asymmetry responses to stimuli. Larger relative left hemispheric activation was argued to be associated with positively valenced stimuli and increased right hemispheric activation with negatively valenced stimuli (Briesemeister et al., 2013). Next to this valence model, the approach-avoidance, or approach-withdrawal, model was explored. In this model, activity in the right frontal cortex has been related to avoidance motivation, a tendency to withdraw from a certain stimulus, and activity in the left frontal cortex with approach motivation toward a stimulus (Davidson et al., 1990; Davidson and Irwin, 1999; Coan and Allen, 2003; Davidson, 2004; Alves et al., 2008; Harmon-Jones et al., 2010; Diaz and Bell, 2012). Since approach motivation is often associated with positive valence and avoidance with negative valence, the expected cortical activity patterns of these two theories overlap in many cases (Reuderink et al., 2013). Studies that specifically disentangled valence and approach-avoidance motivation were in line with the approach-avoidance model (Carver and Harmon-Jones, 2009; Berkman and Lieberman, 2010). The defining difference was found in the hemispheric activation pattern in response to anger (Reuderink et al., 2013). Anger as a negatively valenced emotion was found to be lateralized in the left hemisphere just like happiness instead of the right hemisphere as would be expected based on valence motivation (Davidson, 1984). Further support for the approach-avoidance model was found in transcranial magnetic stimulation experiments (Rutherford and Lindell, 2011).
Frontal alpha asymmetry as a tool to monitor motivational processes related to emotion would be desirable in a variety of application fields, such as marketing (including evaluating public service announcements, e.g., Inguscio et al., 2021), product design (e.g., cosmetics—Gabriel et al., 2021), human-computer interfaces, gaming and the diagnosis of affective disorders (Briesemeister et al., 2013). Another upcoming application and research area where frontal alpha asymmetry is highly relevant, is neuroesthetics (Babiloni et al., 2015; Cartocci et al., 2018, 2021; Daly et al., 2019). However, it is important to realize that frontal alpha asymmetry is not specific for motivational processes, but is also moderated by e.g., unilateral hand contractions (Harmon-Jones et al., 2010) and seating position (Baldwin and Penaranda, 2012). Variations in such factors between studies may underlie diverse results in recent literature, together with differences in data recording (e.g., noise, number of participants, recording length), processing and analysis methods (Smith et al., 2017). Additionally, researchers have used a wide variety of stimuli that were hypothesized to induce frontal alpha asymmetry and found mixed results. This review focuses on the factor of affective stimuli potentially affecting the approach-avoidance effect as measured by alpha asymmetry in the context of emotion. We expect that stimuli may crucially affect frontal alpha asymmetry through their potential to emotionally and motivationally engage the recorded individuals. Since frontal alpha asymmetry describes an approach- avoidance effect, affective stimuli that are strongly motivating in either of the directions are expected to produce clear results. Although it is extremely difficult to quantify this a priori (Brouwer et al., 2015b), we think some general expectations can be formulated for stimulus categories that are prevalent in alpha asymmetry emotional research.
We expect affective stimuli to induce strong approach-avoidance effects when they are engaging and realistic. In that sense, real stimuli that are part of an engaging task would be most effective. We expect that stimuli that are only perceived are less potent than active tasks. Within the “perception” category, we expect images and sounds (i.e., sensory unimodal stimuli that represent a certain object or situation) to be less potent than videos (bi-modal), followed by real cues (multimodal and realistic; the actual object or situation itself). Within the “action” category, we expect that games may be particularly engaging tasks and therefore elicit strong approach-avoidance effects. Finally, clearer effects of affective stimuli on alpha asymmetry are expected if the stimuli are particularly relevant for the participants under study (e.g., food is likely to produce stronger approach motivation for individuals who have not eaten for a long time compared to individuals who have).
To date there is no review focused on the stimuli that can evoke an approach-avoidance effect measured by frontal alpha asymmetry. Hence, as of yet it is unclear which types of affective stimuli elicit a strong approach or avoidance effect. Exploring this will help to understand better what is reflected by frontal alpha asymmetry, under which circumstances frontal alpha asymmetry can be expected to be an informative marker of emotion and what causes the diversity in literature in order to unify conflicting results.
Literature was searched on Scopus using the keywords “alpha AND asymmetry” AND (“approach” OR “avoidance” OR “withdrawal”) AND (“affect” OR “emotion”) and yielded 144 documents. Additionally, given our special interest in this measure from the perspective of studying food related emotion (Kaneko et al., 2018; Modica et al., 2018; Songsamoe et al., 2019), a search on Scopus using the terms (“alpha” AND “asymmetry”) AND “food” was conducted as well, resulting in 28 more papers. Out of the resulting 172 documents only those that had measured frontal alpha asymmetry related to an event or a stimulus (i.e., not resting alpha asymmetry only) were included. Furthermore, studies using a machine learning approach without separately reporting on the exact alpha asymmetry results were excluded. This resulted in the inclusion of 61 papers. Figure 1 visualizes the search and selection procedure.
The 61 selected studies were divided into five stimulus categories that were expected to systematically differ in their effectiveness to engage the subjects: 1. Images & sounds, 2. Videos, 3. Real cues, 4. Games, 5. Other tasks (Imagery; Modifying facial expression; Speech, reading and writing). While most studies involve some task, studies in the category “Games” and “Other tasks” specifically designed tasks to elicit a certain emotional state: performing the task serves as the main stimulus, and in case of games, the resulting or expected outcome in addition to performing the task.
Papers are summarized and evaluated per stimulus type. A summarized description of all 61 studies can be found in Table 1. Studies were rated based on whether the stimulus alone induced an alpha asymmetry approach-avoidance effect (one before last column in Table 1) and if applicable, whether alpha asymmetry approach-avoidance effects were found for, or in interaction with certain conditions or participant subgroups (last column in Table 1). Effects are indicated by “++” for a significant effect, “+” in case of a trend and “0” for no effect. Supplementary Table 1 contains information on the context or goal of the 61 studies and more details about the stimuli and results. Furthermore, since cortical hemispheric specialization of emotion may differ between left- and right-handed individuals (Harmon-Jones et al., 2008; Walsh et al., 2017), handedness is indicated in the “Participants” column of Supplementary Table 1 for all studies that report it. Most studies use right-handed participants and those that reported to have included left-handed persons stated that the results did not change by doing so.
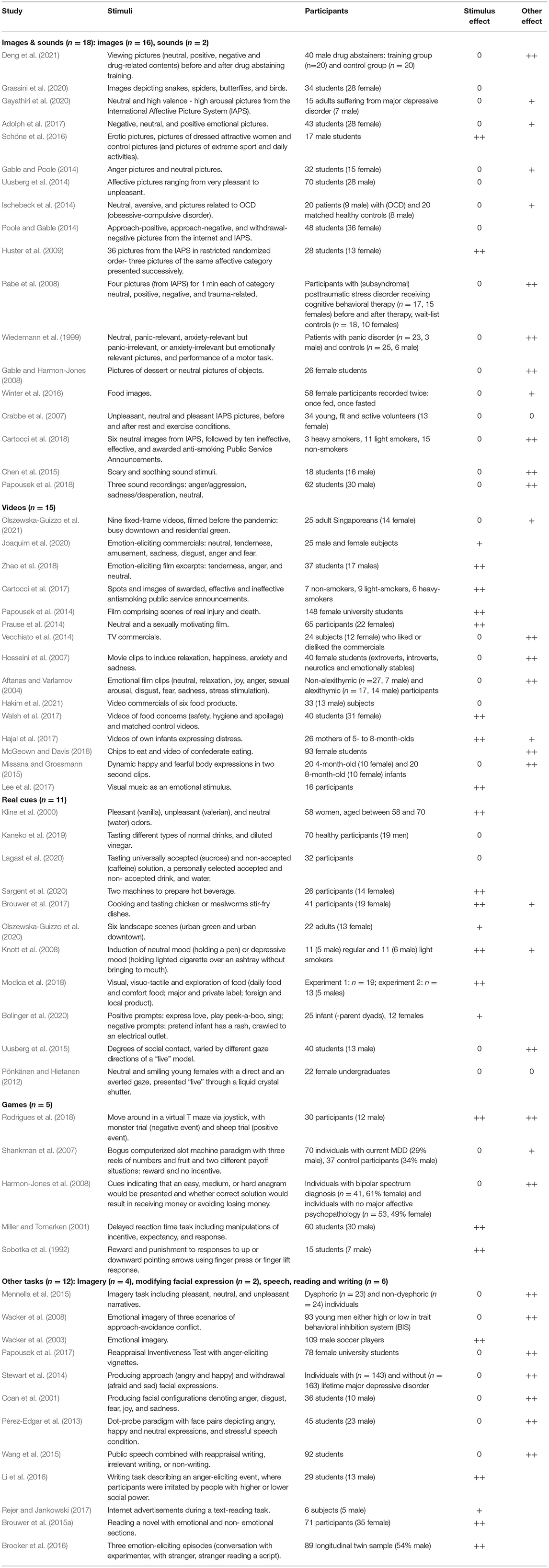
Table 1. Overview of studies arranged by stimulus types with (the hypothesized) approach-avoidance effect indicated by ++ (significant), + (trend) and 0 (none) of the stimulus alone, and/or other effects involving the stimulus. Note that many studies were set up for studying the ‘other' effect (e.g. interaction with person characteristics or interaction between stimuli and other condition).
Figure 2 presents the percentage of studies showing a significant effect of stimuli alone and in interaction with other conditions or participant subgroups, separately for each of the five stimulus categories. In the next sections, studies are discussed per stimulus category.
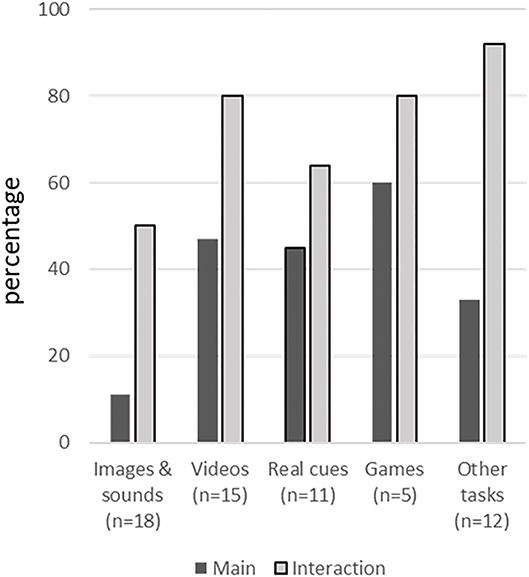
Figure 2. Percentage of studies showing a significant effect of stimuli alone and in interaction with other conditions or participant subgroups, separately for each stimulus category.
Most studies using sensory unimodal stimuli used visual (n = 16) rather than auditory (n = 2) stimuli. Studies utilizing unimodal stimuli appeared to show a significant effect only when the stimulus was particularly relevant for the participant group. This pattern can be seen in several clinical and substance-related studies.
Rabe et al. (2008) described that patients with posttraumatic stress disorder from motor vehicle accidents had increased right-sided activation during exposure to trauma-related pictures compared to neutral pictures. Cognitive behavioral therapy led to a significant reduction of right anterior activation for the group receiving therapy (n = 17) compared to wait-list controls (n = 18) in response to the trauma stimulus. Likewise, Wiedemann et al. (1999) conducted a study where patients with panic disorder (n = 23) compared to healthy controls (n = 25) were confronted with neutral (mushroom), panic-relevant (emergency situation), anxiety-relevant but panic-irrelevant (spider), or anxiety-irrelevant but emotionally relevant pictures (erotic image). They found a significant decrease of right compared to left frontal alpha power in response to the emergency picture category for the group with panic disorder but not for the healthy control group. Gayathiri et al. (2020) reported elevated right hemispheric activity, indicating avoidance, when individuals with major depressive disorder (n = 15) viewed images of high valence and arousal relative to neutral ones. Contrary to these studies, Ischebeck et al. (2014) did not find differences between twenty patients with obsessive compulsive disorder and twenty matched controls during viewing neutral, aversive and OCD-related images.
In Deng et al. (2021), drug abstainers' (n = 40) responses to drug-related images were compared to positive, negative, and neutral pictures in the context of evaluating the effect of a training on emotion regulation. While there was no main effect of picture type, improved alpha asymmetry scores for negative and drug-related pictures were found for the training group pre-and post-training. Cartocci et al. (2018) found higher frontal alpha asymmetry for heavy smokers compared to light smokers and non-smokers when viewing effective public service announcement (PSA) pictures. In their earlier study frontal alpha asymmetry for different PSA images did not differ (Cartocci et al., 2017), which might be attributed to several differences between the studies, such as a lower number of participants in the earlier study (n = 22 vs. n = 39).
The previously described pattern of responses only in groups for whom the stimuli are relevant is likely also important for non-clinical samples. (Schöne et al. 2016) asked seventeen male students to view erotic pictures of high salience as well as depictions of dressed attractive women and found significant results of picture category. Winter et al. (2016) used food images to assess the effect of hedonic hunger and restrained eating on frontal alpha asymmetry with 58 female participants. They found that higher restraint scores were associated with increased right frontal asymmetry and higher hedonic hunger was associated with increased left frontal asymmetry. Additionally, they found that overweight compared to normal weight individuals displayed greater left asymmetry. However, for the condition of fasted and fed state no differences emerged. Gable and Harmon-Jones (2008) did report for 26 female students that while dessert pictures alone did not evoke significant asymmetric activation, more time since eaten and dessert liking related to increased left frontal asymmetry for dessert pictures.
Our search resulted in two studies using auditory stimuli only. Papousek et al. (2018) (n = 62 students) explored inter-individual differences in frontal alpha asymmetry to other people's affect using sound recordings of three categories: anger (shouting), sadness (crying) and neutral (trivial everyday sounds) as a reference condition. Results show that individuals with higher compared to lower level of antagonism (assessed by a Personality Inventory) had less relative right frontal activation (approach) in response to the anger stimulus, whereas subjects with higher levels of detachment displayed greater relative right hemisphere activation (withdrawal) to the crying stimulus. Similarly, in Chen et al. (2015) a sample of 18 students listened to scary and soothing sounds. Subjects who showed a greater withdrawal response to scary sounds displayed a decreased pleasant state, and participants with higher approach motivation showed an increased pleasant state.
As expected, studies using video stimuli showed more often strong approach-avoidance effects than studies using unimodal stimuli (images & sounds), both as effect of the stimuli alone and in interaction with participant characteristics and conditions.
Unlike the anti-smoking image stimuli as described above in section Images and Sounds (Cartocci et al., 2017), anti-smoking video announcements induced effects in alpha asymmetry. Video announcements that had independently been classified as “awarded” induced an increased approach-avoidance effect compared to independently classified “ineffective” and “effective” ones. Another study on this topic (Cartocci et al., 2019) found that smokers showed stronger alpha-asymmetry avoidance than non-smokers in response to anti-smoking videos, highlighting again the importance of the interaction between stimuli and participant characteristics in approach-avoidance effects.
Not all studies using advertising videos have shown positive results. Joaquim et al. (2020) have reported only a trend in correlation between the asymmetry index for low alpha frequency band and negative emotions elicited by commercials viewed by 25 subjects. In a study by Hakim et al. (2021) 33 subjects watched skits from a comedy series followed by commercials of food products and later completed a choice task consisting of six products altogether. Results show that frontal alpha asymmetry as recorded during commercial viewing did not significantly differ for neither closely nor distantly ranked products. Vecchiato et al. (2014) showed six TV commercials (duration around 30 s) to 15 volunteers. Participants were divided into “LIKE” and “DISLIKE” group according to their pleasantness response rating. As expected, the “LIKE” group displayed increased left hemisphere activity compared to the “DISLIKE” group.
Walsh et al. (2017) recruited 40 students and showed them breakfast meal videos of 40 s in duration. The clips contained emotion-eliciting events with hygiene, safety, and spoilage concerns and almost identical controls without such concerns. For the spoilage videos they found greater right hemisphere activation indicating avoidance response when compared to its matched control. For the hygiene and safety videos they did not find significant differences. In another food related study, McGeown and Davis (2018) recorded the brain activity of 93 female students while watching a confederate consuming potato chips, followed by conducting a visual-probe task with non-food and food items of high craving ratings. Overweight participants (based on BMI) compared to leaner counterparts showed increased left frontal alpha asymmetry during the confederate video and greater attentional bias toward food pictures.
Most video studies used videos to induce basic types of emotion. Zhao et al. (2018) presented three film clips (duration of around 80 s each) to 37 students to elicit tenderness, anger and neutrality. They found greater left frontal activation during the tenderness film clip. The anger eliciting film clip led to expected greater right frontal activation. Papousek et al. (2014) displayed a film with a duration of ~10 min consisting of scenes of severely injured, mourning and dying people, to 148 female students. The expected effect of a right-sided shift of dorsolateral prefrontal asymmetry was found. In Prause et al. (2014) 65 students viewed a neutral film (10 min duration) followed by a sexual film (3 min duration). Increased alpha power was found in the left hemisphere (i.e., approach) during sexual compared to neutral films. Furthermore, self-reported mental sexual arousal and alpha asymmetry were positively correlated. In a study by Hajal et al. (2017) 26 mothers of 5- to 8-month-olds watched a 15-min video composed of 10 s clips of their own infants expressing distress. They found an association between greater right frontal asymmetry shift (from baseline to infant distress video) and higher self-reported sadness.
A large proportion of studies specifically examined the interaction between stimulus and groups of participants with certain characteristics. Hosseini et al. (2007) showed four video clips (duration of 3 min each) to induce relaxation, happiness, anxiety and sadness. Their sample consisted of 40 female students equally divided into four groups: extroverted, introverted, neurotic and emotionally stable. They found that right frontal asymmetry was associated with negative affect for the introvert and emotionally stable groups. Aftanas and Varlamov (2004) showed 10 film neutral and emotional clips each of 1.5–4.5 min duration to individuals with alexithymia (n = 17), a personality trait characterized by difficulties in emotional self-regulation, and non-alexithymic (n = 27) participants. In all cases subjects with alexithymia showed greater reactivity of the right hemisphere to the emotional clips relative to neutral, suggestive of increased avoidance motivation. Olszewska-Guizzo et al. (2021) found no significant effect for frontal alpha asymmetry for video type (nature exposure and busy public spaces), but a significant decrease of frontal alpha asymmetry as recorded following a national lockdown with a Stay-at-Home order compared to before the pandemic (n = 22). Missana and Grossmann (2015) studied a sample of 20 4-month-old and 20 8-month-old infants, and found that only the older infant group showed increased left-sided frontal alpha asymmetry in response to point-lighted display of happy body expressions and higher right-sided activation in response to fearful body expressions.
We expected that in general, real cues should produce stronger approach-avoidance effects than videos. However, the proportion of studies finding significant effects is similar.
Kaneko et al. (2019) and Lagast et al. (2020), who explored the effect of different types of drinks on frontal alpha asymmetry in, respectively, 70 and 32 participants, observed no significant effects. However, odors as researched by Kline et al. (2000) recording EEG in 58 women have led to increased relative left frontal activation for the pleasant stimulus (vanilla) when compared to unpleasant (valerian) and neutral (water).
Two neuromarketing studies utilizing real cues reported significant results for frontal alpha asymmetry. Modica et al. (2018) compared different categories of food items: daily and comfort food, major and private brands, and foreign and local products in two experiments (n = 19 and n = 13). They found increased tendency for approaching comfort compared to daily food, and foreign compared to local products during visual exploration and visual and tactile exploration phases. In addition, the private label compared to major brand also showed higher approach in the visual and tactile exploration phases. Similarly, Sargent et al. (2020) compared two machines to prepare hot beverages, one from a market leader and the other from a follower machine in an office setting (n = 26). It was shown that the market leader machine's user interface was preferred, indicated by self-reports and supported by significant valence measured by frontal alpha asymmetry and arousal extracted from electrodermal activity measures. Another study using a real food-related stimulus, was conducted by Brouwer et al. (2017), where 41 participants cooked and tasted two stir fry dishes. For one the main ingredient was chicken (hypothesized to induce approach) and for the other mealworms (hypothesized to evoke avoidance). The expected effect of food condition was found in frontal alpha asymmetry throughout the entire cooking and tasting session, significantly during the frying interval.
In a substance study, Knott et al. (2008), exposed 11 regular and 11 light smokers to a neutral and a cigarette-cue (holding a pen and holding a lighted cigarette above an ashtray respectively), while EEG was recorded. Results show that particularly regular female smokers exhibited withdrawal-related negative affect to holding the cigarette compared to holding the pen.
Three studies in our selection used real social interaction cues. In a study with 25 infant-parent dyades, Bolinger et al. (2020) used positive (e.g., parent played peek-a-boo with the infant) and negative prompts (e.g., parent pretended that the infant has rash on his/her face) and found significantly increased right-sided frontal alpha asymmetry (reflecting avoidance or withdrawal) for the negative prompts. No effects were observed for positive and neutral stimuli. Uusberg et al. (2015) and Pönkänen and Hietanen (2012) explored how eye-contact is related to frontal alpha asymmetry. In Uusberg et al. (2015) (n = 40) the degree of social contact was varied by gaze direction and as expected, neuroticism was related to stronger right-sided activation in response to direct gaze. In (Pönkänen and Hietanen 2012) (n = 22) the expected left-sided asymmetry in response to direct gaze was not observed.
Finally, Olszewska-Guizzo et al. (2020) passively exposed 22 adults to pre-selected real landscape scenes, consisting of six park scenes and three busy urban spaces. They found a non-significant trend in the expected direction with higher approach motivation for park compared to urban spaces.
Games were expected to be the most potent inducers of approach-avoidance effects. Indeed, this category seems to result the in the largest proportion of significant results for main stimulus effects, but we should note the modest number of studies in this category (n = 5).
Rodrigues et al. (2018) asked 30 participants to move freely around in a virtual T-maze using a joystick. The maze contained monsters and sheep (emotionally negative and positive trials, respectively). The results aligned with the approach-avoidance model, with more left frontal alpha activation during the positive event condition and increased right frontal alpha activation in the negative condition. In Miller and Tomarken (2001), 60 participants underwent a delayed reaction time task with manipulations of the incentive, expectancy, and response. They found that variations in monetary incentives led to the expected changes in alpha asymmetry, i.e., more relative left frontal activation during reward conditions, and shifts to right frontal activation during punishment conditions. Similarly, Sobotka et al. (1992) manipulated reward and punishment in a sample of 15 students. Reward trials were associated with higher activation in the left frontal hemisphere and during punishment trials higher right-sided activation was found.
Two studies in the games category recorded from clinical samples. Shankman et al. (2007) used a slot machine game with reward and no incentive outcomes. Participants included 70 individuals with major depression and 37 controls. No differences in hemispheric asymmetry for the two outcome conditions were observed, and no overall difference between the depressed and non-depressed group. However, they found a trend between age of depression onset and hypothesized approach during reward trials. Participants with early depression onset seemed to exhibit less left frontal activity (less approach) during reward conditions compared to participants with late-onset depression and the control group. Harmon-Jones et al. (2008) explored frontal cortical responses of 41 individuals with bipolar disorders and 53 controls. For this they used anagrams of different difficulty levels (easy, medium and hard) and valence (win money or avoid losing money). They found that as expected, individuals with bipolar disorder showed greater left frontal activation in preparation for the hard-win task compared to controls. Furthermore, while non-bipolar subjects showed a decrease in left frontal activation from medium to hard win trials, those on the bipolar disorder spectrum did not.
Tasks in this category entailed imagery (n = 4), modifying facial expression (n = 2) and speech, reading and writing tasks (n = 6). Overall, “other tasks” stimuli seemed quite potent in eliciting effects in interaction with participant group or condition, but relatively few main effects were reported.
Four studies used a variety of emotional imagery tasks, and all reported significant results. Mennella et al. (2015) measured EEG of a clinical sample of 23 dysphoric and 24 non-dysphoric individuals during pleasant, neutral and unpleasant narratives. They found reduced left relative to right activity irrespective of emotional condition in the dysphoric group compared to the control group, but no main effect of the different emotional tasks. Wacker et al. (2008) found significant approach-avoidance effects using emotional imagery scripts of three approach-avoidance conflict scenarios and a sample of 93 men with either high or low behavioral inhibition system (BIS) sensitivity. Their results showed that only the group high in trait BIS sensitivity had a significant change toward right-sided activation for the imagery compared to the pre-stimulus phase. In addition, Wacker et al. (2003) induced vivid imagery with relevant soccer scripts in a sample of 109 active, male soccer players. They found significant changes in the alpha band toward left frontal activation for the group with anger-inducing scripts and toward right frontal activation for the control and fear-withdrawal stimuli. Papousek et al. (2017) used a type of imagery task, where female university students (n = 78) looked at anger-eliciting vignettes supplemented by matching photographs and were instructed to imagine the depicted situation happening to them. Subsequently, they wrote down possible ways to appraise the situation to diminish anger. In a comparison task, they were asked to generate novel ideas to use a conventional, emotionally neutral object. Participants with greater capacity to generate reappraisal showed greater left-sided activity in the pre-frontal cortex. No difference was found between the two types of emotional task.
Two studies aimed to induce different emotions using facial expression tasks. Both reported significant effects. In Coan et al. (2001) students' (n = 36) facial configurations of anger, disgust, fear, joy and sadness matched the expected frontal activation patterns, i.e., less left frontal activity in withdrawal states compared to approach and control states. In Stewart et al. (2014) a participant group with major depressive disorder (n = 143) showed less left frontal activity during approach and withdrawal conditions than a control group (n = 163).
Six studies used speech, reading and writing tasks. (Pérez-Edgar et al. 2013) presented face pairs depicting angry, happy and neutral expressions in a dot-probe paradigm, followed by speech preparation to 45 students. Relative EEG asymmetry was calculated between the speech preparation and baseline. Increased right frontal alpha activation was associated with avoidance of happy, and attentional bias toward angry faces in the dot-probe task. Brooker et al. (2016) conducted a longitudinal twin study (n = 89) with three emotion eliciting episodes: conversation with the experimenter, with a stranger and listening to a stranger reading a script. They found that children showed increased asymmetry scores, consistent with approach, during conversing with a stranger and experimenter compared to the stranger script episodes.
In Wang et al. (2015) 92 students were informed that they had to give a speech to elicit anxiety, and they were asked to imagine the speech scenario or think of previous embarrassing experiences. This was followed by a possible writing task depending on the group: reappraisal writing, irrelevant writing and no writing. Afterwards they were asked to re-imagine embarrassing speech scenarios. Compared to the irrelevant writing group, the reappraisal writing group had lower frontal alpha asymmetry scores during the writing manipulation period and higher “approach” frontal alpha asymmetry scores following re-exposure to stress. Li et al. (2016) also used a writing task. Participants (n = 29 students) were instructed to think of a situation when they were irritated by people with higher or lower social power. As expected, they found a significant association between high social power and increased left frontal alpha asymmetry compared to the low social power condition.
In Rejer and Jankowski (2017) six subjects performed a reading task, which was interrupted by internet advertisements. This caused changes in frontal alpha asymmetry though the direction of change differed between subjects. In Brouwer et al. (2015a) 71 participants performed a reading task of a novel where emotional and non-emotional sections were pre-defined. Higher frontal alpha asymmetry was found for high compared to low emotional sections.
The aim of this review was to investigate what types of affective stimuli are effective in inducing an approach-avoidance response in frontal alpha asymmetry, in the hope that this will contribute to better understanding and application of alpha asymmetry. We reviewed findings in the affective alpha asymmetry literature following five types of commonly used stimuli that were expected to differ in their effectiveness to engage the subjects: (1) Images & sounds, (2) Videos, (3) Real cues, (4) Games and (5) Other tasks. The first three of these categories represent studies where participants' task mostly consisted of passively perceiving the stimuli, going from unimodal and less realistic, to multimodal and more realistic, where we expected this to be associated with an increasing level of affective engagement and therewith, potency to induce approach-avoidance effects. Tasks were expected to be more motivationally engaging overall, in particular games.
As expected, unimodal images and sounds appeared to be the least potent to induce clear effects—significant effects were almost only reported when the stimulus was particularly relevant for the participant group. Also as expected, studies using video stimuli showed strong approach-avoidance effects more often than studies using images and sounds, both as effect of the stimuli alone and in interaction with participant characteristics and conditions. The proportion of studies finding significant effects using real cues did not seem larger, but was approximately similar, to studies using videos. As expected, the proportion of significant results for main stimulus effects was largest for games, but we should note the modest number of studies in this category, and we conclude they are in the same order as videos and real cues. “Other tasks” stimuli seemed quite potent in eliciting effects in interaction with participant group or condition, but relatively few main effects were reported. Many studies that did not report an effect of stimulus alone reported stimulus effects in association with participant characteristics or other conditions. This makes sense in that the motivational aspect of stimuli is never completely determined by a stimulus itself, but affective approach-avoidance responses arise as an interplay between stimuli and an individual who has certain characteristics and finds him/herself in a certain situation. This aligns with ideas of Coan and colleagues and the capability model, stating that motivational tendency in an individual should be studied within a clear motivational context (Coan et al., 2006). Below, we discuss our results in more detail.
In general, viewing static images may be expected to be not very emotionally and motivationally engaging. The findings of this review revealed that picture presentation could induce approach-avoidance effects if the images were particularly emotionally relevant for the sample group, for instance anxiety-relevant pictures shown to patients with panic disorder (Wiedemann et al., 1999). Thus, for a general sample group, affective images alone might be insufficient to create motivational engagement while stimulus-relevant personal characteristics can potentiate frontal alpha asymmetry (Harmon-Jones et al., 2006; Gable and Harmon-Jones, 2008; Uusberg et al., 2014; Rejer and Jankowski, 2017). Consistent with this, significant correlations have been found between frontal alpha asymmetry and differences in emotive tendencies (e.g., dessert liking) or personality traits (Wacker et al., 2008; for examples see Gable and Harmon-Jones, 2008; Uusberg et al., 2015; Winter et al., 2016). As one of the exceptions, (Schöne et al. 2016) showed that presentation of erotic pictures to male students lead to clear alpha asymmetry results, even in a brief (3 s) picture presentation task. They argue that in this case, pictures are the actual desired object themselves, and therefore create a relatively strong approach motivation in contrast to pictures that are a depiction of something that is desirable, such as food. Huster et al. (2009) aimed to improve motivational engagement for pictures by successively displaying three pictures of the same affective category, and found a main effect. Showing pictures of the same category successively also allowed for computation of frontal alpha asymmetry over a longer time period, which may have increased the robustness of the measure (Huster et al., 2009). Note that this points to another overall difference between studies that use images and other stimuli besides expected engagement—the generally short interval per stimulus that used to determine alpha asymmetry may be another factor explaining weak alpha asymmetry results for images.
From the engagement perspective, and consistent with the reasoning by (Schöne et al. 2016) as mentioned above, we expected real cues to be particularly effective as they are not just a depiction of something creating a tendency to approach or avoid, but can be the genuine objects to approach or avoid. Indeed, experiments using food, odors and cigarettes found significant effects for frontal alpha asymmetry. However, those employing landscapes and tasting drinks did not. In these studies, noise caused by movement could have prevented clear results. Because body movement causes noise in EEG signals, stimuli employing movement can be expected to be less effective in producing an alpha asymmetry approach-avoidance effect. In Olszewska-Guizzo et al. (2020) participants went from one scene to the other, leading to long time intervals between recordings and hence noisy comparisons between conditions. Furthermore, in Kaneko et al. (2019) participants took sips from cups themselves, which led to noise through movement. On the other hand, Lagast et al. (2020) minimized such movements by using plastic tubes but were still not able to find a significant approach-avoidance effect. Also, results of studies in other stimulus categories did not suggest that in general, modest amounts of movement prohibit finding alpha asymmetry effects.
Out of scope for the current review that focussed on the role of affective stimuli, but also relevant for the approach-avoidance alpha asymmetry effect are data recording, processing, and analysis (for an extensive review, see Smith et al., 2017). A few essential points of consideration are the EEG recording length (Towers and Allen, 2009), selection of the electrode reference (Hagemann and Naumann, 2001; Hagemann, 2004; Stewart et al., 2010) and the reliability of the EEG measurement (Hagemann et al., 2002; Allen et al., 2004a,b). With novel wearable EEG monitoring devices and processing techniques, recordings in less controlled environments are becoming more reliable (e.g., see Aricò et al., 2018; Pion-Tonachini et al., 2019), but controlled experiments and lab-grade equipment will have some advantage on signal quality. Furthermore, aspects of the design besides choice of stimulus such as the number and duration of trials and baselines, analysis (e.g., exact definition of the alpha band and methods for artifact removal) are not standardized and can lead to big differences.
This brings us to the limitations of this literature review. One is that experiments are very diverse and thus difficult to compare. We focused on the overall effect of affective stimulus category. For almost every stimulus category, studies were identified that reported no effect of stimuli on alpha asymmetry at all; but glancing through these studies did not bring to light one obvious factor underlying these null results.
Second, even though keywords were clear, it was noted that not all relevant papers were captured through the search. We do not claim that we here provide an exhaustive overview, and our results should be taken as indicative. Still, we believe that the inclusion of 61 papers results in a representative review of the literature.
Thirdly, we should note that while our choice of stimulus categories was not arbitrary, other choices and definitions of stimulus categories would have been possible as well and could have influenced the conclusions. Also, our categories were not exactly exclusive and sometimes overlapping, e.g., the cooking and tasting experiment by Brouwer et al. (2017) could be arguably belonging to tasks rather than real cues. In such cases, the stimulus' affective content led to the final categorization decision. We hope that our summarizing Supplementary Table facilitates potential follow-up research, viewing the results from possible other perspectives.
Furthermore, most of the papers reviewed here reported significant alpha asymmetry approach-avoidance results, or trends in that direction. Papers that reported null findings possibly did not include the keywords used in our search. An example is Walden et al. (2015), where frontal theta activity was studied as a function of approach-avoidance affective autobiographical memory recall. They mention in a footnote that no effect on alpha-asymmetry was observed. In addition, many of such findings were probably withheld from publication in the first place, commonly known as publication bias. Not reporting null-findings is a general problem that could lead to another research group investigating the same line of thought, leading to null findings again, ultimately wasting resources, distorting literature and damaging the integrity of knowledge (Joober et al., 2012). Furthermore, negative outcomes are valuable for science since they force critical reflection, validation of current thinking and direct new approaches (Matosin et al., 2014). Therefore, researchers should be more encouraged and journals more open to publish manuscripts reporting negative results. Taking into account the likely underreporting of null findings, and the finding that roughly 50% of studies reporting a solid effect of stimulus only for four of the five categories, where this percentage was even considerably lower for the images & sounds category, we can conclude that alpha asymmetry approach-avoidance is not an easy to find phenomenon, especially not when tested in general populations without further manipulation of context to increase stimulus relevance.
Despite of the aforementioned limitations, the exploration of frontal alpha asymmetry as an indicator of affective approach-avoidance can benefit marketing, human-computer interfaces and the diagnosis of affective disorders. Frontal alpha asymmetry may provide a more objective and continuous measure of mental state than traditional methods that are influenced by social factors and may affect the mental state itself. This review confirmed that overall, strongly engaging, salient and/or personally relevant stimuli are important to induce an approach-avoidance effect and that the selection of stimuli accounts for part of the diversity in alpha asymmetry research. More work is required to gain a better understanding of other factors influencing frontal alpha asymmetry as a marker of emotion.
PS, IS, DK, and A-MB: conceptualization. PS: literature search, summarizing literature, and writing first draft. All authors contributed to revising the article and approved the submitted version.
This research was funded by the Kikkoman Europe R&D Laboratory B.V. The authors declare that this study received funding from Kikkoman Europe R&D Laboratory B.V. Other than that one of the co-authors (Daisuke Kaneko) was employed by Kikkoman Europe R&D Laboratory B.V., the funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
DK was employed by Kikkoman Europe R&D Laboratory B.V.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We gratefully acknowledge comments of Dimitra Dodou on a previous version of this work.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcomp.2022.869123/full#supplementary-material
Adolph, D., von Glischinski, M., Wannemüller, A., and Margraf, J. (2017). The influence of frontal alpha-asymmetry on the processing of approach- and withdrawal-related stimuli—A multichannel psychophysiology study. Psychophysiology 54, 1295–1310. doi: 10.1111/psyp.12878
Aftanas, L., and Varlamov, A. (2004). Associations of alexithymia with anterior and posterior activation asymmetries during evoked emotions: EEG evidence of right hemisphere “electrocortical effort.” Int. J. Neurosci. 114, 1443–1462. doi: 10.1080/00207450490509230
Ahern, G. L., and Schwartz, G. E. (1985). Differential lateralization for positive and negative emotion in the human brain: EEG spectral analysis. Neuropsychologia 23, 745–755. doi: 10.1016/0028-3932(85)90081-8
Alford, L. B. (1933). Localization of consciousness and emotion. Am. J. Psychiatry 89, 789–799. doi: 10.1176/ajp.89.4.789
Allen, J. J. B., Coan, J. A., and Nazarian, M. (2004a). Issues and assumptions on the road from raw signals to metrics of frontal EEG asymmetry in emotion. Biol. Psychol. 67, 183–218. doi: 10.1016/j.biopsycho.2004.03.007
Allen, J. J. B., Urry, H. L., Hitt, S. K., and Coan, J. A. (2004b). The stability of resting frontal electroencephalographic asymmetry in depression. Psychophysiology 41, 269–280. doi: 10.1111/j.1469-8986.2003.00149.x
Alves, N. T., Fukusima, S. S., and Aznar-Casanova, J. A. (2008). Models of brain asymmetry in emotional processing. Psychol. Neurosci. 1, 63–66. doi: 10.3922/j.psns.2008.1.010
Aricò, P., Borghini, G., Di Flumeri, G., Sciaraffa, N., and Babiloni, F. (2018). Passive BCI beyond the lab: current trends and future directions. Physiol. Meas. 39, 08TR02. doi: 10.1088/1361-6579/aad57e
Babiloni, F., Rossi, D., Cherubino, P., Trettel, A., Picconi, D., Maglione, A. G., et al. (2015). “The first impression is what matters: a neuroaesthetic study of the cerebral perception and appreciation of paintings by Titian,” in Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS. (Titian: Institute of Electrical and Electronics Engineers Inc.), 7990–7993.
Baldwin, C. L., and Penaranda, B. N. (2012). Adaptive training using an artificial neural network and EEG metrics for within- and cross-task workload classification. Neuroimage 59, 48–56. doi: 10.1016/j.neuroimage.2011.07.047
Bartolomé-Tomás, A., Sánchez-Reolid, R., Fernández-Sotos, A., Latorre, J. M., and Fernández-Caballero, A. (2020). Arousal detection in elderly people from electrodermal activity using musical stimuli. Sensors 20, 4788. doi: 10.3390/s20174788
Berkman, E. T., and Lieberman, M. D. (2010). Approaching the bad and avoiding the good: Lateral prefrontal cortical asymmetry distinguishes between action and valence. J. Cogn. Neurosci. 22, 1970–1979. doi: 10.1162/jocn.2009.21317
Bolinger, E., Ngo, H. V., Kock, V., Wassen, D. T., Matuz, T., et al. (2020). Affective cortical asymmetry at the early developmental emergence of emotional expression. eNeuro 7, 1–10. doi: 10.1523/ENEURO.0042-20.2020
Briesemeister, B. B., Tamm, S., Heine, A., and Jacobs, A. M. (2013). Approach the good, withdraw from the bad—A review on frontal alpha asymmetry measures in applied psychological research. Psychology 4, 261–267. doi: 10.4236/psych.2013.43A039
Brooker, R. J., Davidson, R. J., and Goldsmith, H. H. (2016). Maternal negative affect during infancy is linked to disrupted patterns of diurnal cortisol and alpha asymmetry across contexts during childhood. J. Exp. Child Psychol. 142, 274–290. doi: 10.1016/j.jecp.2015.08.011
Brouwer, A. M., Hogervorst, M., Reuderink, B., van der Werf, Y., and van Erp, J. (2015a). Physiological signals distinguish between reading emotional and non-emotional sections in a novel. Brain Comp. Interf. 2, 76–89. doi: 10.1080/2326263X.2015.1100037
Brouwer, A. M., Hogervorst, M. A., Grootjen, M., van Erp, J. B. F., and Zandstra, E. H. (2017). Neurophysiological responses during cooking food associated with different emotions. Food Qual. Prefer. 62, 307–316. doi: 10.1016/j.foodqual.2017.03.005
Brouwer, A. M., Zander, T. O., van Erp, J. B. F., Korteling, J. E., and Bronkhorst, A. W. (2015b). Using neurophysiological signals that reflect cognitive or affective state: six recommendations to avoid common pitfalls. Front. Neurosci. 9, 136. doi: 10.3389/fnins.2015.00136
Cartocci, G., Caratù, M., Modica, E., Maglione, A. G., Rossi, D., Cherubino, P., et al. (2017). Electroencephalographic, heart rate, and galvanic skin response assessment for an advertising perception study: application to antismoking public service announcements. J. Vis. Exp. 126, 55872. doi: 10.3791/55872
Cartocci, G., Modica, E., Rossi, D., Cherubino, P., Maglione, A. G., Colosimo, A., et al. (2018). neurophysiological measures of the perception of antismoking public service announcements among young population. Front. Hum. Neurosci. 12, 231. doi: 10.3389/fnhum.2018.00231
Cartocci, G., Modica, E., Rossi, D., Inguscio, B., Arico, P., Levy, A. C. M., et al. (2019). Antismoking campaigns? Perception and gender differences: a comparison among EEG indices. Comput. Intell. Neurosci. 2019, 7348795. doi: 10.1155/2019/7348795
Cartocci, G., Rossi, D., Modica, E., Maglione, A. G., Martinez Levy, A. C., Cherubino, P., et al. (2021). Neurodante: poetry mentally engages more experts but moves more non-experts, and for both the cerebral approach tendency goes hand in hand with the cerebral effort. Brain Sci. 11, 1–25. doi: 10.3390/brainsci11030281
Carver, C. S., and Harmon-Jones, E. (2009). Anger is an approach-related affect: evidence and implications. Psychol. Bull. 135, 183–204. doi: 10.1037/a0013965
Chen, X., Takahashi, I., Okita, Y., Hirata, H., and Sugiura, T. (2015). Psychological response to sound stimuli evaluated by EEG: joint consideration of AAE model and comfort vector model. J. Psychophysiol. 29, 112–118. doi: 10.1027/0269-8803/a000142
Christopoulos, G. I., Uy, M. A., and Yap, W. J. (2019). The body and the brain: measuring skin conductance responses to understand the emotional experience. Organ. Res. Methods 22, 394–420. doi: 10.1177/1094428116681073
Coan, J. A., and Allen, J. J. B. (2003). Frontal EEG asymmetry and the behavioral activation and inhibition systems. Psychophysiology 40, 106–114. doi: 10.1111/1469-8986.00011
Coan, J. A., and Allen, J. J. B. (2004). Frontal EEG asymmetry as a moderator and mediator of emotion. Biol. Psychol. 67, 7–50. doi: 10.1016/j.biopsycho.2004.03.002
Coan, J. A., Allen, J. J. B., and Harmon-Jones, E. (2001). Voluntary facial expression and hemispheric asymmetry over the frontal cortex. Psychophysiology 38, 912–925. doi: 10.1111/1469-8986.3860912
Coan, J. A., Allen, J. J. B., and McKnight, P. E. (2006). A capability model of individual differences in frontal EEG asymmetry. Biol. Psychol. 72, 198–207. doi: 10.1016/j.biopsycho.2005.10.003
Cook, I. A., O'Hara, R., Uijtdehaage, S. H. J., Mandelkern, M., and Leuchter, A. F. (1998). Assessing the accuracy of topographic EEG mapping for determining local brain function. Electroencephalogr. Clin. Neurophysiol. 107, 408–414. doi: 10.1016/S0013-4694(98)00092-3
Crabbe, J. B., Smith, J. C., and Dishman, R. K. (2007). Emotional & electroencephalographic responses during affective picture viewing after exercise. Physiol. Behav. 90, 394–404. doi: 10.1016/j.physbeh.2006.10.001
Daly, I., Williams, D., Hwang, F., Kirke, A., Miranda, E. R., and Nasuto, S. J. (2019). Electroencephalography reflects the activity of sub-cortical brain regions during approach-withdrawal behaviour while listening to music. Sci. Rep. 9, 1–22. doi: 10.1038/s41598-019-45105-2
Davidson, R. J., (1984). “Affect, cognition, and hemispheric specialization,” in Emotion, Cognition, and Behavior, eds. C. R. Izard, J. Kagan, and R. B. Zajonc (New York, NY: Cambridge University Press), 320–365.
Davidson, R. J. (2004). What does the prefrontal cortex “do” in affect: perspectives on frontal EEG asymmetry research. Biol. Psychol. 67, 219–234. doi: 10.1016/j.biopsycho.2004.03.008
Davidson, R. J., Ekman, P., Saron, C. D., Senulis, J. A., and Friesen, W. V. (1990). Approach-withdrawal and cerebral asymmetry: emotional expression and brain physiology I. J. Pers. Soc. Psychol. 58, 330–341. doi: 10.1037/0022-3514.58.2.330
Davidson, R. J., and Irwin, W. (1999). The functional neuroanatomy of emotion and affective style. Trends Cogn. Sci. 3, 11–21. doi: 10.1016/S1364-6613(98)01265-0
Davidson, R. J., Schaffer, C. E., and Saron, C. (1985). Effects of lateralized presentations of faces on self-reports of emotion and EEG asymmetry in depressed and non-depressed subjects. Psychophysiology 22, 353–364. doi: 10.1111/j.1469-8986.1985.tb01615.x
Dell, N., Vaidyanathan, V., Medhi, I., Cutrell, E., and Thies, W., (2012). ““yours is better!” Participant response bias in HCI,” in Conference on Human Factors in Computing Systems - Proceedings, eds J. A. Konstan, E. H. Chi, and K. Hook (New York, NY, USA: ACM), 1321–1330.
Deng, Y., Hou, L., Chen, X., and Zhou, R. (2021). Working memory training improves emotion regulation in drug abstainers: evidence from frontal alpha asymmetry. Neurosci. Lett. 742, 135513. doi: 10.1016/j.neulet.2020.135513
Denny-brown, D., Meyer, J. S., and Horenstein, S. (1952). The significance of perceptual rivalry resulting from parietal lesion. Brain 75, 432–471. doi: 10.1093/brain/75.4.432
Diaz, A., and Bell, M. A. (2012). Frontal EEG asymmetry and fear reactivity in different contexts at 10 months. Dev. Psychobiol. 54, 536–545. doi: 10.1002/dev.20612
Gable, P., and Harmon-Jones, E. (2008). Relative left frontal activation to appetitive stimuli: considering the role of individual differences. Psychophysiology 45, 275–278. doi: 10.1111/j.1469-8986.2007.00627.x
Gable, P. A., and Poole, B. D. (2014). Influence of trait behavioral inhibition and behavioral approach motivation systems on the LPP and frontal asymmetry to anger pictures. Soc. Cogn. Affect. Neurosci. 9, 182–190. doi: 10.1093/scan/nss130
Gabriel, D., Merat, E., Jeudy, A., Cambos, S., Chabin, T., Giustiniani, J., et al. (2021). Emotional effects induced by the application of a cosmetic product: a real-time electrophysiological evaluation. Appl. Sci. 11, 4766. doi: 10.3390/app11114766
Gawronski, B., and de Houwer, J., (2014). “Implicit measures in social and personality psychology,” in Handbook of Research Methods in Social and Personality Psychology, eds H. Reis and C. Judd (Cambridge: Cambridge University Press), 283–310.
Gayathiri, R. R., Bhuvana Devi, M., Kavya, G. A., Veezhinathan, M., and Geethanjali, B., (2020). “EEG based visualization and analysis of emotional processing in major depressive disorder,” in 2020 6th International Conference on Advanced Computing and Communication Systems, ICACCS 2020. (Institute of Electrical and Electronics Engineers Inc.), 336–341.
Goldstein, K. (2004). The Organism: A Holistic Approach to Biology Derived From Pathological Data in Man. American Book Publishing
Grassini, S., Sikka, P., Revonsuo, A., and Koivisto, M. (2020). Subjective ratings of fear are associated with frontal late positive potential asymmetry, but not with early brain activity over the occipital and centro-parietal cortices. Psychophysiology. 57, e13665. doi: 10.1111/psyp.13665
Hagemann, D. (2004). Individual differences in anterior EEG asymmetry: methodological problems and solutions. Biol. Psychol. 67, 157–182. doi: 10.1016/j.biopsycho.2004.03.006
Hagemann, D., and Naumann, E. (2001). The effects of ocular artifacts on (lateralized) broadband power in the EEG. Clin. Neurophysiol. 112, 215–231. doi: 10.1016/S1388-2457(00)00541-1
Hagemann, D., Thayer, J. F., Naumann, E., and Bartussek, D. (2002). Does resting electroencephalograph asymmetry reflect a trait? An application of latent state-trait theory. J. Person. Soc. Psychol. 82, 619–641. doi: 10.1037/0022-3514.82.4.619
Hajal, N. J., Cole, P. M., and Teti, D. M. (2017). Maternal responses to infant distress: linkages between specific emotions and neurophysiological processes. Parenting 17, 200–224. doi: 10.1080/15295192.2017.1336001
Hakim, A., Klorfeld, S., Sela, T., Friedman, D., Shabat-Simon, M., and Levy, D. J. (2021). Machines learn neuromarketing: improving preference prediction from self-reports using multiple EEG measures and machine learning. Int. J. Res. Market. 38, 770–791. doi: 10.1016/j.ijresmar.2020.10.005
Harmon-Jones, E., Abramson, L. Y., Nusslock, R., Sigelman, J. D., Urosevic, S., Turonie, L. D., et al. (2008). Effect of bipolar disorder on left frontal cortical responses to goals differing in valence and task difficulty. Biol. Psychiatry 63, 693–698. doi: 10.1016/j.biopsych.2007.08.004
Harmon-Jones, E., Gable, P. A., and Peterson, C. K. (2010). The role of asymmetric frontal cortical activity in emotion-related phenomena: a review and update. Biol. Psychol. 84, 451–462. doi: 10.1016/j.biopsycho.2009.08.010
Harmon-Jones, E., Lueck, L., Fearn, M., and Harmon-Jones, C. (2006). The effect of personal relevance and approach-related action expectation on relative left frontal cortical activity. Psychol. Sci. 17, 434–440. doi: 10.1111/j.1467-9280.2006.01724.x
Hosseini, S. M., Fallah, P. A., Tabatabaei, S. K. R., Ladani, S. H. G., and Heise, C. (2007). Brain activity, personality traits and affect: electrocortical activity in reaction to affective film stimuli. J. Appl. Sci. 7, 3743–3749. doi: 10.3923/jas.2007.3743.3749
Huster, R. J., Stevens, S., Gerlach, A. L., and Rist, F. (2009). A spectralanalytic approach to emotional responses evoked through picture presentation. Int. J. Psychophysiol. 72, 212–216. doi: 10.1016/j.ijpsycho.2008.12.009
Inguscio, B. M. S., Cartocci, G., Modica, E., Rossi, D., Martinez-Levy, A. C., Cherubino, P., et al. (2021). Smoke signals: a study of the neurophysiological reaction of smokers and non-smokers to smoking cues inserted into antismoking public service announcements. Int. J. Psychophysiol. 167, 22–29. doi: 10.1016/j.ijpsycho.2021.06.010
Ischebeck, M., Endrass, T., Simon, D., and Kathmann, N. (2014). Altered frontal EEG asymmetry in obsessive-compulsive disorder. Psychophysiology 51, 596–601. doi: 10.1111/psyp.12214
Joaquim, M. S., Maçorano, R., Canais, F., Ramos, R., Fred, A. L., Torrado, M., et al. (2020). “Learning data representation and emotion assessment from physiological data,” in ICASSP, IEEE International Conference on Acoustics, Speech and Signal Processing - Proceedings. (Institute of Electrical and Electronics Engineers Inc.), 3452–3456.
Joober, R., Schmitz, N., Annable, L., and Boksa, P. (2012). Publication bias: What are the challenges and can they be overcome? J. Psychiatry Neurosci. 37, 149–152. doi: 10.1503/jpn.120065
Kaneko, D., Hogervorst, M., Toet, A., van Erp, J. B. F., Kallen, V., and Brouwer, A. M. (2019). Explicit and implicit responses to tasting drinks associated with different tasting experiences. Sensors (Basel). 19, 4397. doi: 10.3390/s19204397
Kaneko, D., Toet, A., Brouwer, A. M., Kallen, V., and van Erp, J. B. F. (2018). Methods for evaluating emotions evoked by food experiences: a literature review. Front. Psychol. 9, 911. doi: 10.3389/fpsyg.2018.00911
Kline, J. P., Blackhart, G. C., Woodward, K. M., Williams, S. R., and Schwartz, G. E. R. (2000). Anterior electroencephalographic asymmetry changes in elderly women in response to a pleasant and an unpleasant odor. Biol. Psychol. 52, 241–250. doi: 10.1016/S0301-0511(99)00046-0
Knott, V. J., Naccache, L., Cyr, E., Fisher, D. J., McIntosh, J. F., Millar, A. M., et al. (2008). Craving-induced EEG reactivity in smokers: effects of mood induction, nicotine dependence and gender. Neuropsychobiology 58, 187–199. doi: 10.1159/000201716
Lagast, S., de Steur, H., Gadeyne, S., Hödl, S., Staljanssens, W., Vonck, K., et al. (2020). Heart rate, electrodermal responses and frontal alpha asymmetry to accepted and non-accepted solutions and drinks. Food Qual. Prefer. 82, 103893. doi: 10.1016/j.foodqual.2020.103893
Lagast, S., Gellynck, X., Schouteten, J. J., de Herdt, V., and de Steur, H. (2017). Consumers' emotions elicited by food: a systematic review of explicit and implicit methods. Trends Food Sci. Technol. 69, 172–189. doi: 10.1016/j.tifs.2017.09.006
Lee, I. E., Latchoumane, C. -F. V., and Jeong, J. (2017). Arousal rules: An empirical investigation into the aesthetic experience of cross-modal perception with emotional visual music. Front. Psychol. 8, 440. doi: 10.3389/fpsyg.2017.00440
Li, D., Wang, C., Yin, Q., Mao, M., Zhu, C., and Huang, Y. (2016). Frontal cortical asymmetry may partially mediate the influence of social power on anger expression. Front. Psychol. 7, 73. doi: 10.3389/fpsyg.2016.00073
Lottridge, D., Chignell, M., and Yasumura, M. (2012). Identifying emotion through implicit and explicit measures: Cultural differences, cognitive load, and immersion. IEEE Transact. Affect. Comp. 3, 199–210. doi: 10.1109/T-AFFC.2011.36
Matosin, N., Frank, E., Engel, M., Lum, J. S., and Newell, K. A. (2014). Negativity towards negative results: a discussion of the disconnect between scientific worth and scientific culture. Dis. Models Mech. 7, 171–173. doi: 10.1242/dmm.015123
Mauss, I. B., and Robinson, M. D. (2009). Measures of emotion: a review. Cogn. Emot. 23, 209–237. doi: 10.1080/02699930802204677
McGeown, L., and Davis, R. (2018). Frontal EEG asymmetry moderates the association between attentional bias towards food and body mass index. Biol. Psychol. 136, 151–160. doi: 10.1016/j.biopsycho.2018.06.001
Mennella, R., Messerotti Benvenuti, S., Buodo, G., and Palomba, D. (2015). Emotional modulation of alpha asymmetry in dysphoria: results from an emotional imagery task. Int. J. Psychophysiol. 97, 113–119. doi: 10.1016/j.ijpsycho.2015.05.013
Miller, A., and Tomarken, A. J. (2001). Task-dependent changes in frontal brain asymmetry: effects of incentive cues, outcome expectancies, and motor responses. Psychophysiology 38, 500–511. doi: 10.1111/1469-8986.3830500
Missana, M., and Grossmann, T. (2015). Infants' emerging sensitivity to emotional body expressions: Insights from asymmetrical frontal brain activity. Dev. Psychol. 51, 151–160. doi: 10.1037/a0038469
Modica, E., Cartocci, G., Rossi, D., Levy, A. C. M., Cherubino, P., Magilone, A. G., et al. (2018). Neurophysiological responses to different product experiences. Comput. Intell. Neurosci. 2018, 1–10. doi: 10.1155/2018/9616301
Olszewska-Guizzo, A., Fogel, A., Escoffier, N., and Ho, R. (2021). Effects of COVID-19-related stay-at-home order on neuropsychophysiological response to urban spaces: beneficial role of exposure to nature? J. Environ. Psychol. 75, 101590. doi: 10.1016/j.jenvp.2021.101590
Olszewska-Guizzo, A., Sia, A., Fogel, A., and Ho, R. (2020). Can exposure to certain urban green spaces trigger frontal alpha asymmetry in the brain?—Preliminary findings from a passive task EEG study. Int. J. Environ. Res. Public Health 17, 394. doi: 10.3390/ijerph17020394
Papousek, I., Aydin, N., Rominger, C., Feyaerts, K., Schmid-Zalaudek, K., Lackner, H. K., et al. (2018). DSM-5 personality trait domains and withdrawal versus approach motivational tendencies in response to the perception of other people's desperation and angry aggression. Biol. Psychol. 132, 106–115. doi: 10.1016/j.biopsycho.2017.11.010
Papousek, I., Weiss, E. M., Perchtold, C. M., Weber, H., de Assunção, V. L., Schulter, G., et al. (2017). The capacity for generating cognitive reappraisals is reflected in asymmetric activation of frontal brain regions. Brain Imaging Behav. 11, 577–590. doi: 10.1007/s11682-016-9537-2
Papousek, I., Weiss, E. M., Schulter, G., Fink, A., Reiser, E. M., and Lackner, H. K. (2014). Prefrontal EEG alpha asymmetry changes while observing disaster happening to other people: cardiac correlates and prediction of emotional impact. Biol. Psychol. 103, 184–194. doi: 10.1016/j.biopsycho.2014.09.001
Pérez-Edgar, K., Kujawa, A., Nelson, S. K., Cole, C., and Zapp, D. J. (2013). The relation between electroencephalogram asymmetry and attention biases to threat at baseline and under stress. Brain Cogn. 82, 337–343. doi: 10.1016/j.bandc.2013.05.009
Pion-Tonachini, L., Kreutz-Delgado, K., and Makeig, S. (2019). ICLabel: an automated electroencephalographic independent component classifier, dataset, and website. Neuroimage 198, 181–197. doi: 10.1016/j.neuroimage.2019.05.026
Pönkänen, L. M., and Hietanen, J. K. (2012). Eye contact with neutral and smiling faces: effects on autonomic responses and frontal EEG asymmetry. Front. Hum. Neurosci. 6, 122. doi: 10.3389/fnhum.2012.00122
Poole, B. D., and Gable, P. A. (2014). Affective motivational direction drives asymmetric frontal hemisphere activation. Exp. Brain Res. 232, 2121–2130. doi: 10.1007/s00221-014-3902-4
Prause, N., Staley, C., and Roberts, V. (2014). Frontal alpha asymmetry and sexually motivated states. Psychophysiology 51, 226–235. doi: 10.1111/psyp.12173
Rabe, S., Zoellner, T., Beauducel, A., Maercker, A., and Karl, A. (2008). Changes in brain electrical activity after cognitive behavioral therapy for posttraumatic stress disorder in patients injured in motor vehicle accidents. Psychosom. Med. 70, 13–19. doi: 10.1097/PSY.0b013e31815aa325
Rejer, I., and Jankowski, J. (2017). Brain activity patterns induced by interrupting the cognitive processes with online advertising. Cogn. Process. 18, 419–430. doi: 10.1007/s10339-017-0815-8
Reuderink, B., Mühl, C., and Poel, M. (2013). Valence, arousal and dominance in the EEG during game play. Int. J. Auton. Adapt. Commun. Syst. 6, 45–62. doi: 10.1504/IJAACS.2013.050691
Rodrigues, J., Müller, M., Mühlberger, A., and Hewig, J. (2018). Mind the movement: frontal asymmetry stands for behavioral motivation, bilateral frontal activation for behavior. Psychophysiology 55. doi: 10.1111/psyp.12908
Russell, J. A. (1980). A circumplex model of affect. J. Pers. Soc. Psychol. 39, 1161–1178. doi: 10.1037/h0077714
Rutherford, H. J. V., and Lindell, A. K. (2011). Thriving and surviving: approach and avoidance motivation and lateralization. Emot. Rev. 3, 333–343. doi: 10.1177/1754073911402392
Sargent, A., Watson, J., Ye, H., Suri, R., and Ayaz, H. (2020). Neuroergonomic assessment of hot beverage preparation and consumption: an EEG and EDA study. Front. Hum. Neurosci. 14, 175. doi: 10.3389/fnhum.2020.00175
Schöne, B., Schomberg, J., Gruber, T., and Quirin, M. (2016). Event-related frontal alpha asymmetries: electrophysiological correlates of approach motivation. Exp. Brain Res. 234, 559–567. doi: 10.1007/s00221-015-4483-6
Shankman, S. A., Klein, D. N., Tenke, C. E., and Bruder, G. E. (2007). Reward sensitivity in depression: a biobehavioral study. J. Abnorm. Psychol. 116, 95–104. doi: 10.1037/0021-843X.116.1.95
Smith, E. E., Reznik, S. J., Stewart, J. L., and Allen, J. J. B. (2017). Assessing and conceptualizing frontal EEG asymmetry: an updated primer on recording, processing, analyzing, and interpreting frontal alpha asymmetry. Int. J. Psychophysiol. 111, 98–114. doi: 10.1016/j.ijpsycho.2016.11.005
Sobotka, S. S., Davidson, R. J., and Senulis, J. A. (1992). Anterior brain electrical asymmetries in response to reward and punishment. Electroencephalogr. Clin. Neurophysiol. 83, 236–247. doi: 10.1016/0013-4694(92)90117-Z
Songsamoe, S., Saengwong-ngam, R., Koomhin, P., and Matan, N. (2019). Understanding consumer physiological and emotional responses to food products using electroencephalography (EEG). Trends Food Sci. Technol. 93, 167–173. doi: 10.1016/j.tifs.2019.09.018
Stewart, J. L., Bismark, A. W., Towers, D. N., Coan, J. A., and Allen, J. J. B. (2010). Resting frontal EEG asymmetry as an endophenotype for depression risk: sex-specific patterns of frontal brain asymmetry. J. Abnorm. Psychol. 119, 502–512. doi: 10.1037/a0019196
Stewart, J. L., Coan, J. A., Towers, D. N., and Allen, J. J. B. (2014). Resting and task-elicited prefrontal EEG alpha asymmetry in depression: support for the capability model. Psychophysiology 51, 446–455. doi: 10.1111/psyp.12191
Towers, D. N., and Allen, J. J. B. (2009). A better estimate of the internal consistency reliability of frontal EEG asymmetry scores. Psychophysiology 46, 132–142. doi: 10.1111/j.1469-8986.2008.00759.x
Tucker, M., Stenslie, C. E., Roth, R. S., and Shearer, S. L. (1981). Right frontal lobe activation and right hemisphere performance: decrement during a depressed mood. Arch. Gen. Psychiatry 38, 169–174. doi: 10.1001/archpsyc.1981.01780270055007
Uusberg, A., Uibo, H., Tiimus, R., Sarapuu, H., Kreegipuu, K., and Allik, J. (2014). Approach-avoidance activation without anterior asymmetry. Front. Psychol. 5, 192. doi: 10.3389/fpsyg.2014.00192
Uusberg, H., Allik, J., and Hietanen, J. K. (2015). Eye contact reveals a relationship between neuroticism and anterior EEG asymmetry. Neuropsychologia 73, 161–168. doi: 10.1016/j.neuropsychologia.2015.05.008
Vecchiato, G., Cherubino, P., Maglione, A. G., Ezquierro, M. T. H., Marinozzi, F., Bini, F., et al. (2014). How to measure cerebral correlates of emotions in marketing relevant tasks. Cognit. Comput. 6, 856–871. doi: 10.1007/s12559-014-9304-x
Wacker, J., Chavanon, M. L., Leue, A., and Stemmler, G. (2008). Is running away right? The behavioral activation-behavioral inhibition model of anterior asymmetry. Emotion 8, 232–249. doi: 10.1037/1528-3542.8.2.232
Wacker, J., Heldmann, M., and Stemmler, G. (2003). Separating emotion and motivational direction in fear and anger: effects on frontal asymmetry. Emotion 3, 167–193. doi: 10.1037/1528-3542.3.2.167
Walden, K., Pornpattananangkul, N., Curlee, A., McAdams, D. P., and Nusslock, R. (2015). Posterior versus frontal theta activity indexes approach motivation during affective autobiographical memories. Cogn. Affect. Behav. Neurosci. 15, 132–144. doi: 10.3758/s13415-014-0322-7
Walsh, A. M., Duncan, S. E., Bell, M. A., O'Keefe, S. F., and Gallagher, D. L. (2017). Integrating implicit and explicit emotional assessment of food quality and safety concerns. Food Qual. Prefer. 56, 212–224. doi: 10.1016/j.foodqual.2016.11.002
Wang, F., Wang, C., Yin, Q., Wang, K., Li, D., Mao, M., et al. (2015). Reappraisal writing relieves social anxiety and may be accompanied by changes in frontal alpha asymmetry. Front. Psychol. 6, 1604. doi: 10.3389/fpsyg.2015.01604
Wiedemann, G., Pauli, P., Dengler, W., Lutzenberger, W., Birbaumer, N., and Buchkremer, G. (1999). Frontal brain asymmetry as a biological substrate of emotions in patients with panic disorders. Arch. Gen. Psychiatry 56, 78–84. doi: 10.1001/archpsyc.56.1.78
Winter, S. R., Feig, E. H., Kounios, J., Erickson, B., Berkowitz, S., and Lowe, M. R. (2016). The relation of hedonic hunger and restrained eating to lateralized frontal activation. Physiol. Behav. 163, 64–69. doi: 10.1016/j.physbeh.2016.04.050
Keywords: alpha asymmetry, EEG, approach-avoidance, emotion, motivation, computational psychophysiology, affective computing, mental state monitoring
Citation: Sabu P, Stuldreher IV, Kaneko D and Brouwer A-M (2022) A Review on the Role of Affective Stimuli in Event-Related Frontal Alpha Asymmetry. Front. Comput. Sci. 4:869123. doi: 10.3389/fcomp.2022.869123
Received: 03 February 2022; Accepted: 30 May 2022;
Published: 01 July 2022.
Edited by:
Siyuan Chen, University of New South Wales, AustraliaReviewed by:
Peter König, Osnabrück University, GermanyCopyright © 2022 Sabu, Stuldreher, Kaneko and Brouwer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anne-Marie Brouwer, YW5uZS1tYXJpZS5icm91d2VyQHRuby5ubA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.