- Graduate School of Frontier Sciences, The University of Tokyo, Chiba, Japan
Workplace stress is a pertinent problem in today's world. Preventing and overcoming stress is critical for a healthy lifestyle because it is linked to various health problems and can lead to poor work performance. Controlling your breathing is one of the most effective ways to promote relaxation. However, regulating one's breathing necessitates some training and is not something that everyone can do easily. As a result, we concentrated on the relaxing effect of breathing and developed a cushion-shaped device that displays the desired respiratory motion. We used the effect of inducing one's respiratory movements by watching others' respiratory movements. When the user hugged the device, it changed the user's respiratory rhythm and depth. We conducted a user study with this device, which revealed that presenting respiratory motion can induce the user's respiratory rhythm and depth without any pre-training. Furthermore, subjective evaluation and ECG data suggested that using this device during task breaks can improve the relaxation effect and thus task performance after the break.
1. Introduction
Stress in the workplace has become a significant problem in modern society. It may lead to depression (Yamaura et al., 2013); therefore, we need stress-reduction techniques that can be integrated into daily life. Accordingly, researchers have been working on several approaches to develop these techniques, one of which focuses on respiration.
For centuries, respiration techniques have been used to regulate physiological and psychological states; for example, systemized breathing maneuvers are incorporated into Oriental practices such as Zen meditation and Tai Chi. Various respiratory methods increase the time taken for expiration, such as abdominal breathing. The abdomen is inflated and deflated during the inspiration and exhalation of air, respectively. When deep breathing is combined with these exercises, the parasympathetic nervous system is activated, resulting in a relaxed state (Jerath et al., 2006).
Therefore, researchers have concluded that it is possible to relax one's body and mind by intentionally adjusting the rhythm of one's breathing (Joseph et al., 2005). However, these breathing techniques require practice and are not easy to master. As a result, many studies have looked into how external stimuli can change people's breathing patterns.
Respiration is greatly affected by physiological and ambient conditions. Rhythms of voluntary motions (Wilke et al., 1975), ambient vibrations and oscillations (such as those experienced by a baby in a cradle) (Omlin et al., 2016), and music (Sato et al., 2016) affect the pace of respiration. For example, Omlin et al. (2016) demonstrated that this pace could be increased by placing a working floor in a bed and swinging the user's body sideways in a fixed rhythm.
Furthermore, it has been demonstrated that the entrainment effect in interpersonal communication alters the rate of breathing. Discovered by Condon and Sander (1974), the entrainment effect is a communication phenomenon in which the speaker's body synchronizes with their speech ahead of time and the listener's movements mirror those of the speaker. Watanabe and Okubo (1997) reported that it is not only the body movements during the conversation but also the heartbeats and breathing rhythms of the speaker and listener that can be synchronized.
Given these phenomena, some researchers have developed a method to influence respiratory rhythm with vibration, sound, and lighting effects (Kini et al., 2003; Sato et al., 2015; Yu et al., 2018). These methods can instruct the user on the pace of breathing by presenting rhythmic sensory stimuli. However, this coaching requires the user to practice to adjust respiratory movements to the stimuli beforehand. They confirmed that this device could be used to train children to breathe.
Based on these findings, we aimed to develop a breath-control system that can control rhythm and depth of users' breathing without prior coaching. Thus, we propose a method that overrides the physical sensation of breathing. Physiological responses (such as pulse rate) can be altered by providing feedback that overrides physiological sensations (Costa et al., 2016; Ueoka and AlMutawa, 2018). We hypothesized that if we could create the illusion that the device's movements were an extension of the user's breathing, we could induce the user's respiratory movements through the stimuli presented by the device without prior training or awareness, as in previous studies that induced actual heartbeats by presenting vibrations that mimic heartbeats. Moreover, it is known that emotions such as joy and sadness can be aroused when simulated stimuli are presented that make us feel as if our physical reactions are changing (Valins, 1966; Fukushima and Kajimoto, 2012). For example, Yoshida et al. (2013) confirmed that the user's positive emotions were reinforced by presenting a mirror-like display with an image as if the user himself were laughing. Therefore, we thought that if we could make users recognize the breathing motion presented by the device as their bodily reaction, presenting a deep and slow breathing motion when they are relaxed would evoke a relaxed feeling, and it would improve the effect of rest.
To test this, we constructed a cushion-shaped device that expands and contracts in an imitation of respiratory motions (Figure 1). By embracing this cushion near the abdomen, it can feel like an extension of the body—this illusion overrides the natural rhythm and depth of respiration. Additionally, since this system is not dependent on the feedback of visual and auditory stimuli, it has the advantage of being easy-to-use in a daily environment. In this article, we build a prototype that uses the proposed method, and investigate its ability to control the rhythm and depth of breathing and thereby improve the effect of rest on cognitive task performance through a user study.
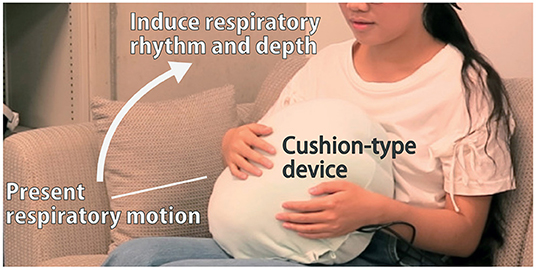
Figure 1. The cushion-type device presents breathing motion. By holding the device on the user's stomach, their breathing rhythm and depth can be modified.
This article is a revised version of the abstract we presented at Siggraph Asia 2018, Emerging Technologies (Ban et al., 2018), with additional details on the development of the device and experiments to verify the effectiveness of the device.
2. Related Work
2.1. Stress and Stress Relief Methods
Workplace stress has become a significant problem in modern society. Chronic stress leads to sustained physiological responses that are detrimental to health and an overproduction of the steroid hormone cortisol (Chandola et al., 2006). Chronically elevated cortisol levels, in turn, increase the risk of many health problems, such as anxiety, depression (Burke et al., 2005), abnormalities in the immune system (Padgett and Glaser, 2003), heart disease (Esler and Kaye, 2000), high blood pressure (Esler and Kaye, 2000), and diabetes mellitus (Lloyd et al., 2005).
As a result, much research has been done on reducing workplace stress and improving the effectiveness of relaxation during task breaks. These studies looked into various approaches, which can be divided into two categories: those that change the user's ambiance and those that work directly on the user's body.
The first category includes studies of visual stimulants such as the type of lighting (Liberman, 1990; Cocilovo, 1999) and nature-based imagery (Saito and Tada, 2007), haptic stimulants such as vibration (Yoshida, 1988; Kimura et al., 2017), olfactory stimulants including different aromas (Watanabe and Okubo, 1997), and auditory stimulants such as classical background music (Hanser, 1985), all of which are confirmed to have an impact on break-time relaxation and can hence be incorporated in private spaces. Effective implementation of these approaches in public spheres involving many people sharing the same space, however, would necessitate designing an ambiance that accounts for and finds common ground between everyone's individual preferences—which is inherently difficult. Furthermore, it is necessary to drastically reconstruct the environment in some cases, leading to increased costs.
On the other hand, the second category of approaches works directly on the mind and body of the user through biofeedback, which is a process enabling a user to be aware of and consciously control the state of their bodily functions (Brown, 1977; Frank et al., 2010). The biofeedback approach allows the user to monitor the negative effects of stress on their physiology, manage stress responses, and practice relaxation techniques to alleviate stress. In addition, research on some devices shows that they do not merely present the physiological state of the user, but utilize it to coach and guide the body function in the direction of increased relaxation (George et al., 2006; Yu et al., 2018). Unlike the first category, this category of methods has the advantage of being unaffected by individual preferences and can be directly applied to everybody.
Respiration, a physiological reaction controlled by the second category of techniques, has been observed in various studies. A key reason for this is that respiration is a semi-voluntary, controlled motion, unlike other bodily functions (such as the pulse rate). Several feedback techniques have been advanced to develop respiration in light of several stimuli that affect respiration.
2.2. Stimuli That Affect Respiration
Many studies have looked into the factors that influence respiration, which can be divided into two categories: internal and external factors.
Internal factors refer to those within one's body, such as the tempo and rhythm of respiration affecting each other. This is called locomotor-respiratory coupling. An example of this is the coordination between rhythm and tempo while running (Bramble and Carrier, 1983; Morin and Viala, 2002). This phenomenon occurs readily during high-intensity forms of exercise and leads to more efficient respiration. As a result, dyspnea and energy costs during exercise can be reduced (Hoffmann et al., 2012). In particular, in respiratory rehabilitation, it is common to use a respiratory teaching method that matches respiratory and lower limb movements with continuous training such as walking in order to control dyspnea (Daley et al., 2013). Wilke et al. (1975)reported that this synchronization is achieved by using smaller muscles that are not involved in respiration. They confirmed that the frequency and timing of the subjects' respiration were synchronized with their fingers tapping.
Concerning external factors, the pace of respiration is influenced by music (Sato et al., 2012) and other vibratory stimuli (Sato et al., 2015). Moreover, the entrainment effect in communication, as described in the previous section, also impacts the pace of breathing. The heartbeat and respiration of the speaker are closely attuned to those of the listener when they are directly facing each other in a conversation, but they occur as more independent patterns when the interlocutors are separated by a partition (Watanabe and Okubo, 1997). It has been reported that this effect occurs not only in verbal communication, but also in cooperative tasks such as playing musical instruments and sports (Yamamoto and Miyake, 2004).
2.3. Systems and Devices That Control Respiratory Rhythm
Systems and devices have been developed to control respiration with visual, auditory, and tactile feedback using the internal and external factors, as described above.
Studies on radiation-therapy have introduced respiration-coaching devices (George et al., 2006; Neicu et al., 2006; Park et al., 2009; Yu et al., 2018) that employ visual and audio cues; audio cues lead to a period of more stable respiration than visual cues (Kini et al., 2003). However, it is difficult to achieve stable control of respiration with music due to differences in the abilities of experienced and inexperienced musicians to induce respiratory control and variations in the effects experienced by different people even while listening to the same music (Sato et al., 2012).
Several applications have been developed for such breathing coaching systems, which can be performed while viewing the screen of a smartphone or smartwatch (Mani et al., 2015). Many of these applications encourage users to relax by breathing according to the respiratory rhythm presented by the screen. In addition, systems that provide breathing training in games have also been developed (Stafford et al., 2016). The advantage of these systems is that they can be used easily using only a smartphone. However, they require the user to consciously control their breathing in response to the information presented, which is a disadvantage for people who are not comfortable with such active efforts.
Respiration control by the feedback of vibratory stimuli has also been studied. Sato et al. (2015) showed that the respiratory timing of a user could be appropriately guided by using a tactile phantom sensation moving continuously on their backs. They attached two vibrators to the user's back—one facing up and the other facing down—and demonstrated that their breathing pace could be controlled by training them beforehand to breathe according to the perceived vibrations generated. This method can also be used to coach the pace of breathing while driving (Paredes et al., 2018). Besides, Choi et al. (2021) proposed a method of guiding the respiratory rate of a person moving in a car by presenting pneumatic pressure instead of vibration.
Omlin et al. (2016) tried to control the respiratory rhythm and reduce its pace with other forms of vibratory stimuli, such as the cradling motion of a bed. However, they were unable to do so.
Using tactile (rather than vibratory) stimuli to establish respiratory control, rhythm modification arising from holding a stuffed animal mimicking the motions of respiration has been studied (Uratani and Ohsuga, 2015). Researchers inserted an airbag inside the animal and enabled it to mimic respiratory motions by using an air compressor; respiratory coaching was demonstrated to be possible by holding this stuffed animal up to an infant. A similar technique has been incorporated in “Somnox,” a start-up company featuring a pillow-type product to help reduce breathing rates in a way that assists the user with falling asleep (Somnox, 2019).
Using steam, light, and sound, other tactile stimulation techniques have also been developed to make users more aware of their breathing. Zhu et al. (2017) reported that it is possible to make users conscious of their respiration by controlling the presentation timing of steam, light, and sound, putting them in a state of increased mindfulness.
Frey et al. (2018) constructed “Breeze,” a pendant that uses biofeedback technology to transmit respiratory information between users using light, vibration, and sound, strengthening the entrainment effect of respiration. Users of Breeze mimic each other's breathing patterns not only through the entrainment effect applicable to those in proximity to one another, but also when spread across remote locations. However, many users could not identify the vibration and instead interpreted it as noise.
The impact of the system's breath-coaching on task and other activity performance has also been investigated. Balters et al. (2018)found that coaching users to breathe faster while driving subjectively improved levels of arousal and concentration. Ghandeharioun and Picard (2017) also reported that breath-coaching using auditory and vibratory stimuli subjectively improved calmness and focus.
As described above, although there have been studies that attempted to induce respiratory rhythms using various types of feedback, few have actually achieved control of the respiration's rhythm and depth simultaneously. We address this issue by developing a device that presents breathing motions. “Somnox” is similar to our research in giving respiratory motion but differs from our research in that our approach can control both the rhythm and depth of the presented respiratory motion. Furthermore, it is unknown how the break-time respiratory guidance provided by these coaching systems affects task performance after a break. By examining these points, this study aims to fill in the gaps in the existing body of research.
3. Controlling Rhythm and Depth by Imitating Respiratory Motions
We developed a device to naturally control breathing patterns by overriding its physiological sensation with the device-induced sensation of the target patterns of breathing. This section first details the approach used in the selection of appropriate stimuli, and then describes design and hardware configuration, and the operation of the device.
3.1. Approach
This study presents a method to improve the effect of relaxation during breaks taken between tasks in the workplace. Since breaks are commonly taken across various jobs, enhancing their quality could help reduce the workplace-stress-levels of many people. As described in Section 2.3, we decided to present the breathing motion as a tactile stimulus to induce the rhythm and depth of breathing simultaneously. This approach is consistent with the “Use clear and consistent guiding metaphors.” in the guideline for respiratory guidance methods in information work proposed by Tabor et al. (2021).
3.2. Design
Previous research used two speaker units as actuators to guide respiratory movements (Sato et al., 2015). These actuators need to be securely fastened to the user's back, making them cumbersome for break-time use. To improve upon this aspect, we aimed to design a device that did not need to be attached to or detached from the user's person. Thus, the functional requirements of our device were set as follows: (1) ability to present respiratory motion, (2) no hassles of attachment and detachment, and (3) stand-alone operation.
In the first stage of the design, we decided on the mode of interaction that users would have with our device. For the movement of the device to feel like an extension of the user's own respiratory motions, it should be placed at a part of their body that moves during respiration.
Hence, the abdomen was chosen as the ideal location for the installation of the device. As shown in Figure 1, the device was shaped in the form of a cushion for comfort and ease of integration into daily life.
The next step was to figure out which actuation mode would best mimic the desired respiratory motions. There were two options for actuation: airbag or mechanism-based. The airbag method makes for smooth and soft stretching motions, demonstrated by a stuffed-toy-based device developed with this technique (Uratani and Ohsuga, 2015). However, this method requires the presence of an external pump to generate forces strong enough to sustain such motions; this necessity is at odds with the stand-alone requirement of the device.
As a result, we used a linear-motion mechanism with a rack-and-pinion actuator that can withstand the force applied by the user when hugging the device, allowing it to meet the stand-alone requirement. Furthermore, when compared to an air compressor, this method allows for finer control of the expansion and contraction motion timing and speed. The embedding of such a rigid mechanism into the cushion presented three difficulties: (1) maintaining the softness of the cushion, (2) suppressing the noise of the motor, and (3) arranging the power source and the rest of the mechanical components into the limited space within the cushion.
3.3. Hardware Configuration
The hardware configuration of the mechanism inside the cushion is shown in Figure 2, and the overall design of the device is detailed in this subsection. A base unit and a top unit made up the mechanism. Variations in the distance between these units caused the cushion to expand and contract, simulating the target respiratory patterns. A rack-and-pinion actuator and a geared DC motor (Tamiya, Inc., 3633K300) were installed to withstand the force exerted by the user's hugs. Linear motion was guided by two linear slides, while the distance between the units was measured with a linear potentiometer (Alps Electric Co., Ltd., RS6011DP6002). A microcontroller (DFRobot, Bluno) was used to control the motor and communicate with devices outside the cushion through an attached Bluetooth 4.0—Bluetooth Low Energy (BLE)—module. The motor was powered by a battery (Li-Po, 11.1 V, 850 mAh) for long-term and stand-alone operation. Two limit switches were installed at the lower and upper bounds of the stretching motion for safety reasons, with the operation stopping when the switches were pressed. An acrylic board was used for the frame considering its manufacturability and weight.
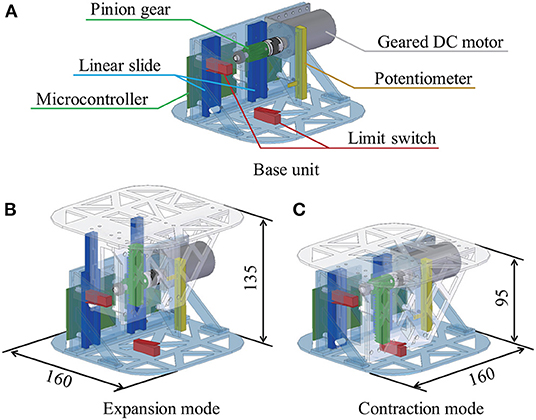
Figure 2. Hardware configuration and respiratory motion. The device consists of a base unit (A) and a top panel unit, and the top panel unit moves up and down as shown in (B,C) by meshing the pinion gear connected to the motor set in the base unit with the rack set in the top panel unit.
Figure 3 represents the device constructed as detailed above. The mechanical apparatus weighed 768 g, while the complete device (including the cotton filling and the cushion cover) weighed approximately 1,000 g. In terms of width, depth, and height, respectively, the dimensions of the equipment were 160 × 160 × 95 mm, while those of the entire cushion were 350 × 350 × 170 mm at maximum shrinkage. The maximum height of the mechanism was 135 mm. This means the device presented users with a respiratory depth of 40 mm. The fabric of the cushion cover was made of 93% polyester and 7% polyurethane. The inside of the cushion had a two-layer structure, as shown in Figure 3C, with the device stored in the center cavity and surrounded by polyester cotton. The cotton cushioning layer was made by sewing two cotton bags together at the top and bottom. This cushioning layer improved softness and muted the motor sound.
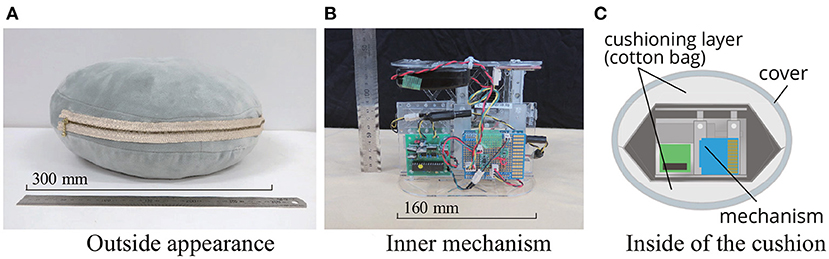
Figure 3. Appearance of the cushion-type device. The mechanism (B) is housed in the cushion (A). The inside of the cushion had a two-layer structure, and the mechanism was placed in a cavity surrounded by cushioning layer made of cotton (C). Due to the cushioning layer, the user did not feel the stiffness of the mechanism and the driving noise of the motor was reduced.
Device feasibility was tested by operating it continuously for 3 h, during which time it displayed stable operation.
3.4. Operation
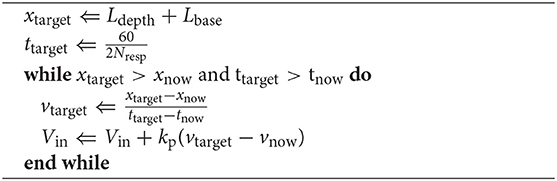
This subsection describes the control system of the mechanism. The user controls the rhythm (the number of breaths per minute : Nresp) and depth (Ldepth) of the respiratory motion of the device by connecting it to a smartphone via BLE. It is necessary to drive the pinion gear at a constant speed. However, because the force exerted by the user while hugging the cushion is time-variant, this was accomplished by adjusting the motor's input voltage in response to the device's hugging force. This voltage adjustment was performed according to Algorithm 1.
In Algorithm 1, x denotes the distance between the base and top plates measured using a potentiometer embedded in the device, while v denotes the velocity of the top plate. Lbase denotes the distance between the plates at the most contracted state of the device, which is 95 mm. xtarget and ttarget are defined by the user's input commands. The input voltage Vin was controlled by proportional control to maintain the pace of the cushion's motion. When xnow or xtarget reaches the target value, the mechanism is reversed to perform a contraction. Algorithm 1 describes only the expansion motion. However, it also similarly applies to the contraction phase of the mechanism.
To reduce the required maximum operation speed, the time-series waveform of x , on the periodic expansion and contraction of the device, was modified to be triangular instead of sinusoidal. Assuming the specifications of the motor to be fixed, a sinusoidal wave requires a higher speed for propagation than a triangular one in the center of oscillation. Therefore, a triangular waveform is optimal for fast respiratory motion. The deformable cotton filling in the cushion, as well as the human abdomen act as dampers; allowing the device to move naturally even when operating on a triangular wave. With the above control, the top panel of the device moves at a constant speed even when the force with which the user holds the device changes.
4. User Study
4.1. Overview
We conducted a user study of the device to investigate its ability to modify the rhythm and depth of respiration as desired and to ascertain if it could be used to improve the relaxation effect of workplace breaks. The following three concerns were addressed in this study:
• Q1: Is it possible for the device to change the user's respiratory rate by presenting movements mimicking respiration at a rate that is faster/slower than that of the user?
• Q2: Can the breathing depth of the user be modified by varying that of the cushion device?
• Q3: Can the user improve the effectiveness of breaks taken between tasks by holding the device during these breaks? The effectiveness of the break was assessed in this experiment based on three factors: performance change, biometric indicators (RR interval) during the break, and subjective mental workload of tasks.
4.2. Participants
Sixteen participants (twelve men and four women), aged 21 to 27 years, were enrolled in this study. They were screened for tiredness and depression to eliminate the effects of pre-existing physical or emotional stresses on task performance. The Ethics Committee of the University of Tokyo approved the experiments presented in this article (No. 18-177), and written informed consent was obtained from all the participants in this study.
The experiment took place in Kashiwa Campus, University of Tokyo. We prepared an experiment room with a desk and a chair, and set empty shelves against the wall. To minimize the influence of the outside environment on the users, we pulled down the window shade. The experiment was conducted from April 10th, 2019 to May 10th, 2019.
4.3. Experimental Procedure
To prevent the subjects from feeling conscious of their breathing, they were not informed that the purpose of the device was to regulate their respiration — they were only told about measuring the changes in their performance of tasks before and after breaks. The participants were then fitted with an electrocardiographic pad and a breathing measurement band (PLUX, biosignal Explorer) to measure their baseline values of breathing pace, depth, and degree of relaxation. Although measuring the respiratory flow rate in absolute terms is not possible with the breathing measurement band, changes in rhythm and depth can be determined from the deformation of the band wrapped around the chest (Figure 4).
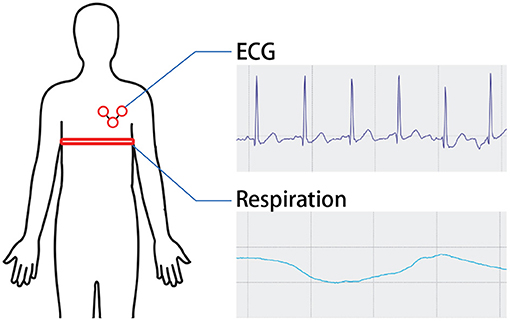
Figure 4. Physiological signal acquisition. The participants were then fitted with an electrocardiographic pad and a breathing measurement band to measure their baseline values of breathing pace, depth, and degree of relaxation.
The participants were asked to begin the experiment after it was confirmed that the ECG and respiratory parameters could be reliably measured using the pad and band. To investigate the ability of our method to modify the respiratory rhythm naturally, no pre-training was conducted to synchronize the proposed information with the respiratory rhythm, unlike in previous studies (Sato et al., 2015; Uratani and Ohsuga, 2015).
The participants were seated in a chair in front of a desk during the experiment, with only a monitor and a keyboard, as well as the cushion-shaped device, available for task execution (Figure 5). They were made to wear noise-canceling headphones and listen to white noise to suppress any background sounds during the experiment.
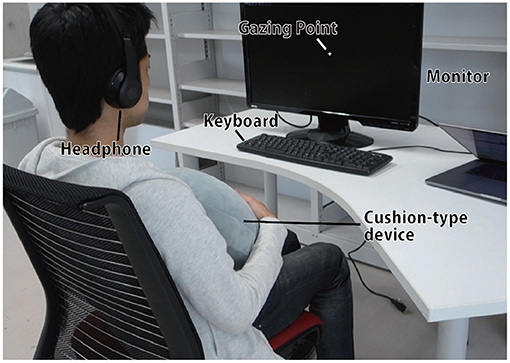
Figure 5. Experimental scene. Other than the desk, monitor, keyboard, and device, all other objects not related to the experiment were hidden from the user's eyes, and white noise was played from the head phone to suppress background sounds.
Experimental trials were performed in the order depicted in Figure 6. Participants were asked to rest for three mins, after which they were instructed to perform a cognitive task (task A) for 5 mins. Following this, they were told to hold the cushion for another 5 mins following this. In this duration, which was called the “breaking phase,” they were told to watch a gazing point displayed on the monitor. Data from the last 2 mins of these 5 mins were deemed the most stable data points, and were thus used in the analysis. Cognitive task A might affect the physiological state at the beginning of the breaking phase. Subsequently, another cognitive task (task B) was performed for 5 mins. The difference in the performance of cognitive tasks A and B and the stress index calculated from the ECG during the breaking phase were used to determine the effect of our device on break-time.
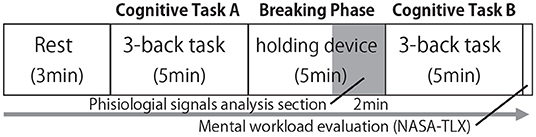
Figure 6. Flow of experimental trial. This process was considered as one trial, and each of the seven conditions was trailed once.
After completing the above steps, participants were asked to respond to their subjective mental workload throughout the task using the National Aeronautics and Space Administration Task Load Index (NASA-TLX) (Hart, 2006). This method was originally developed to measure the mental workload of pilots and consists of six aspects—mental demand (MD), physical demand (PD), temporal demand (TD), own performance (OP), effort (EF), and frustration level (FL)—; these aspects are closely related to the tasks executed by the subjects in this study.
Items were evaluated using the Visual Analog Scale (VAS) (Crichton, 2001); each item was scored by marking a point on a 12-cm line segment (whose endpoints denoted the lowest and highest possible values for that metric) and converting that point to a percentage value. The mental workload value was calculated using the adaptive weighted workload (AWWL) from these scores (Sato et al., 1998). The six metrics are ranked from 1 to 6, in the descending order of their scores; this ranking of each metric is used as its corresponding weighting factor for AWWL. The sum of the weights is then divided by 21, the sum of the coefficients, to obtain the average value (Figure 7). Therefore, the resulting mental workload will be a value between 0 and 100, with larger values indicating a larger mental workload. More effective rest during the breaking phase was expected to lead to lower mental workload values.
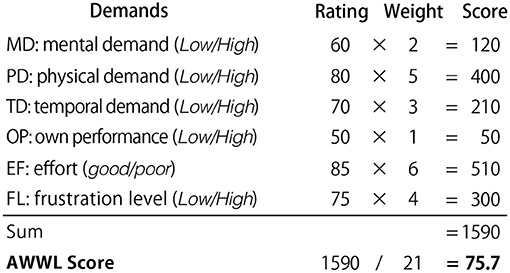
Figure 7. Calculation example of AWWL. Participants scored each of the six NASA-TLX indices on a scale of 0 to 100 using the VAS. These scores were weighted in descending order and divided by 21 to obtain the average. The resulting mental workload would be a value between 0 and 100, with larger values indicating a larger mental workload.
In this experiment, we used the N-back task as a cognitive task. This is one of the cognitive tasks and it is often used to assess cognitive abilities by measuring working memory capacity (Gazzaniga and Ivry, 2013). Working memory is an important cognitive system that allows the brain to temporarily focus on a given task and store and manipulate relevant information. Working memory is essential for complex cognitive tasks such as language comprehension, learning, and reasoning (Baddeley, 1992). If it can be clearly shown that the proposed device has a positive effect on working memory, it can be applied to various situations in the real workplace, so we used this task in our study.
This study used a 3-back task in which participants were presented with a sequence of numeric stimuli and asked to input number N steps before each trial, as shown in Figure 8. The stimuli themselves were natural numbers ranging from one to nine, and they were placed at two-second intervals. Participants typed their responses to them on a keyboard.
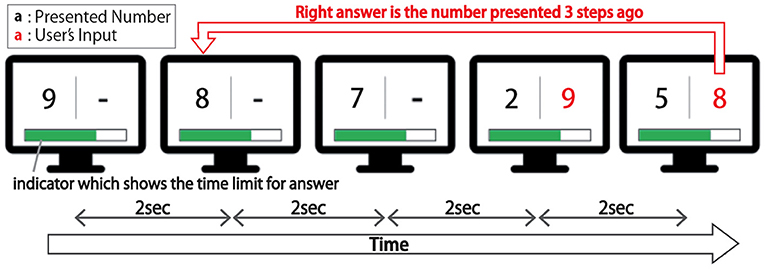
Figure 8. Schema of the 3-back task. In this task, participants were presented with a sequence of numeric stimuli and asked to input the number presented three steps before using a keyboard.
For the induction conditions in the breaking phase, three respiratory rhythms (8, 15, and 24 times per minute) were introduced, with two depths (14 and 32 mm) presented for each rhythm. The rhythms chosen correspond to commonly accepted values for relaxed, normal, and tense physiological states (Frey et al., 2018). The inhalation/exhalation (i/e) ratio was fixed to be 1.0 in this study.
The trial was also conducted under a condition in which holding the cushion device did not cause respiratory motion as a control condition. Therefore, participants did trials under a total of seven conditions: 3 (rhythms) ×2 (depths per rhythm) + 1 (“without respiratory motion.”) They experienced each condition once. To prevent the ordering effects from skewing the experimental results, the order of these seven conditions was counterbalanced using Latin hypercube sampling (Loh, 1996). In combinatorics and in experimental design, a Latin square is an n × n array filled with n different symbols, each occurring exactly once in each row and exactly once in each column. Using this method, where a row is a participant, and a column is the order of presentation, we can counterbalance the timing of the appearance of conditions. In other words, in an experiment with a within-participant design, when n conditions are presented to k*n participants, there will be no bias in the timing of the appearance of the condition among the participants. Next, by randomizing in the column direction, we can reduce the bias in the order of presentation, such as “condition B comes after condition A.” However, there were 16 participants in this experiment for seven conditions, so we randomized the order of condition presentation for the remaining two participants (16−2*7 = 2).
To recover from the experiment, we allowed a minimum of 30 min rest between conditions. We prohibited exercise and caffeine intake during the breaks but imposed no other restrictions. The length of each trial in each condition was about 20 to 25 min. Because of the long total experimental time, the seven trials were conducted in two conditions in the morning, followed by an interval of one and a half hours after lunch and then five conditions in the afternoon.
After completing the trials for all conditions, we collected the participants' comments by interviewing them. The questions were as follows: “Did you feel that your breathing fit the breathing motion presented by the device?” “Did you feel that the breathing motion presented by the device was your own?” “How did you feel when presented with a slow (or fast) breathing rhythm?” and “How did you feel when presented with large (or small) breathing motions?”
4.3.1. Physiological Signal Analysis
We looked at the respiratory rhythm (in terms of the number of breaths taken per minute), depth, and the RRI (RR interval) calculated from the ECG signals during the breaking phase to see how resting while holding this device affected the results. To process physiological signals, we employed biosppy (Carreiras et al., 2015), a widely used Python library for physiological processing and feature extraction. Low-pass filters were applied to the unprocessed signals as part of the preprocessing process. The biosppy library's built-in functions removed noise from raw ECG and respiration data. For ECG analysis, an FIR-type band-pass filter with a passband of 3 Hz and a stopband of 45 Hz was used, while for respiratory analysis, a Butterworth low-pass filter with a cutoff frequency of 5 Hz was used.
The rhythm was calculated by performing a fast Fourier transform (FFT) on the waveform of the respiratory motion recorded during the final two mins of the breaking phase, calculating peak frequency from the resulting spectrum, and then inverting this peak frequency value. On the other hand, depth was evaluated by calculating the distance between the valley and the peak observed during expiration and inspiration in the waveform. The average respiratory depth during the last two mins of the breaking phase was taken as the respiratory depth for each participant.
The RRI index represents the R-wave interval of the ECG. It reflects the nervous system's sympathetic and parasympathetic branches, which work to activate and rest the body, respectively. The sympathetic nervous system takes over stressful or demanding situations, causing a quickening of the heartbeat and a shortening of the RRI. On the other hand, in situations involving elements such as rest and relief, where the parasympathetic nervous system prevails, and the heartbeat slows down, while the RRI lengthens (Taelman et al., 2009). R-peaks were detected from filtered ECG signals. The RRI value was calculated in each condition by averaging the intervals between adjacent R-peaks in the last two mins of the breaking phase.
4.4. Result
4.4.1. Physiological Signal Evaluation
In this section, the respiratory rhythm presented by the device per minute is referred to as R (respiration count per minute), and the depth of respiratory motion presented by the device is referred to as D [mm]. First, we analyzed the results corresponding to Q1 and Q2. Figure 9 shows the box-plot of participants' respiratory rhythm under each condition, and Figure 9 shows that of participants' respiratory depth. This plot shows the quartiles, the median, and the mean.
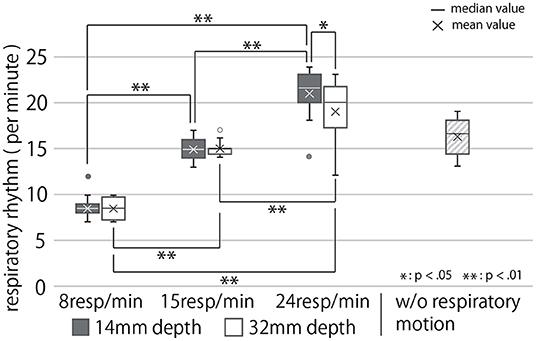
Figure 9. Participants' respiratory rhythm under each condition. Data more than 1.5 times the quartile range away from the quartile point are represented as outliers.
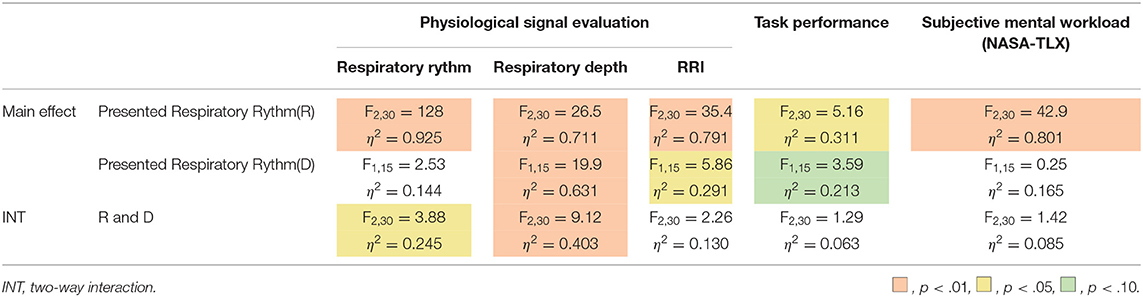
The breathing rhythm of the participants changed in response to the rhythm of the respiratory motion presented by the cushion device, as shown in this diagram. Results obtained in this section, on being subject to the Shapiro-Wilk test at a level of 5%, were found to be normally distributed. They were then analyzed using the two-way, repeated ANOVA and Tukey's multiple comparison tests to have a significance of 5%. The ANOVA test revealed the presented respiratory rhythm to have a significant main effect, presented depth to have no significant main effect, and both of them to have a significant interaction effect on the modified respiratory rhythm of the user (First row of Table 1). As Figure 9 shows, the post-hoc test exposed significant differences between all combinations of the presented respiratory rhythms for each presented depth of respiratory motion (p < 0.01). In addition, the respiratory rhythm was significantly slower in the D = 32 condition than in the D = 14 condition when R = 24 (p < 0.05).
By contrast, Figure 10 shows the box-plot of the modified respiratory depth of users under each condition.
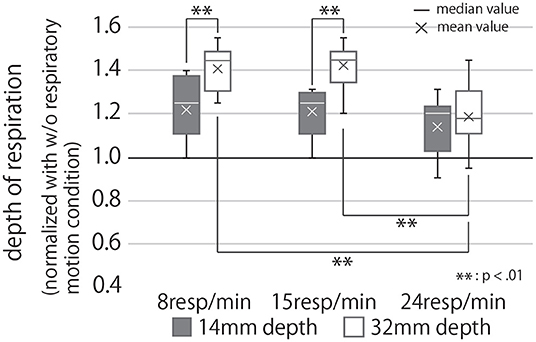
Figure 10. Participants' respiratory depth under each condition, normalized with the condition of no respiratory motion. If the value of the graph is greater than 1, it means that the depth of the participants' breathing was greater than when they were holding the device that did not present the respiratory motion.
Analysis of these results with a two-way repeated ANOVA test revealed the presented rhythm and depth to have significant main effects individually and, when combined, a significant interaction effect on the modified respiratory depth of the user (Second row of Table 1). The post-hoc test uncovered significant differences between the presented respiratory rhythm (R = 15 vs. R = 24, and R = 8 vs. R = 24) under the condition of D = 32 (p < 0.01). In other words, under D = 32, R = 24 had a significantly smaller breathing depth than the other two respiratory rhythm conditions (Figure 10). In addition, participants breathed significantly more deeply in the D = 32 condition than in the D = 14 condition under R = 8 and 15 conditions (p < 0.01).
As results for Q3, we analyzed RRI during breaks. Figure 11 shows the box-plot of RRI in each condition, normalized with the “without respiratory motion condition” for each participant.
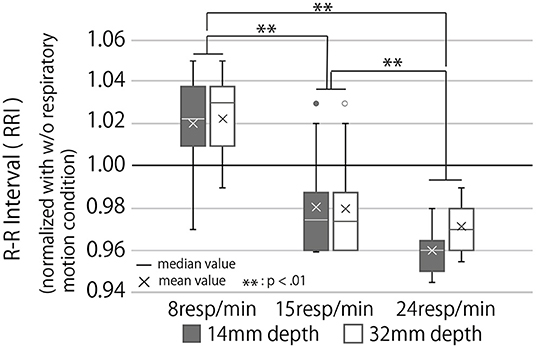
Figure 11. RR interval during the breaking phase under each condition normalized with the condition of no respiratory motion. The RRI is the interval between the R waves that appear with each heartbeat. A high average value of RRI indicates parasympathetic dominance and a low stress level.
The higher the RRI value, the more relaxed the participants and their breaks more effectively. Analysis of the RRI values with a two-way repeated ANOVA test revealed a significant main effect of the presented rhythm and a marginally significant main effect of the presented depth. Still, there was no significant interaction effect of depth and rhythm (Third row of Table 1). As Figure 11 shows, the post-hoc test revealed significant differences between all combinations of the presented respiratory rhythms (p < 0.01).
4.4.2. Evaluation of Task-Performance Levels
Figure 12 shows the change in cognitive task performance under each condition. The large value indicates an improvement in the performance of the task post-break; it can thus be determined that the effect of the break on relaxation and task performance is high. The figure also shows that breaks were more effective when R = 8 than when R = 24 and that the post-break performance was reduced when R = 24, regardless of the presented depth of the respiratory motion. Analyzing these results with a two-way repeated ANOVA test revealed the presented respiratory rhythm to have a significant main effect and a small effect size on task performance (Fourth row of Table 1). As Figure 12 shows, the post-hoc test revealed significant differences between the R = 8 and R = 24 conditions (p < 0.01).
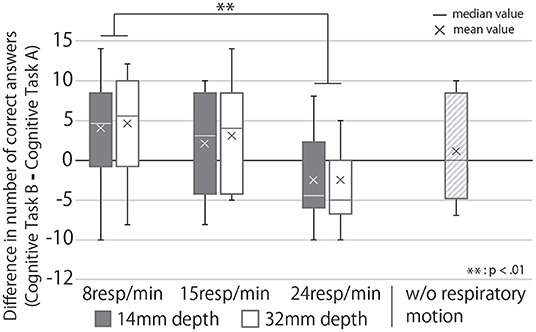
Figure 12. Change in task performance from before to after the break under each condition. If the score is greater than zero, the task performance after the break with the device was better than that before the break.
4.4.3. Subjective Mental Workload Evaluation
Figure 13 shows the subjective mental workload score of the participants, calculated by AWWL under each condition and normalized with the “without respiratory motion condition.” The larger this value, the greater the mental workload felt by the participants throughout the trial. It indicates that as breathing rhythms slowed, the subjective mental workload score also lowered. Analyzing these results with a two-way repeated ANOVA test revealed the presented respiratory rhythm to have a significant main effect and a small effect size on the score (Fifth row of Table 1). As Figure 13 shows, the post-hoc test revealed significant differences between all combinations of the presented respiratory rhythms (p < 0.01).
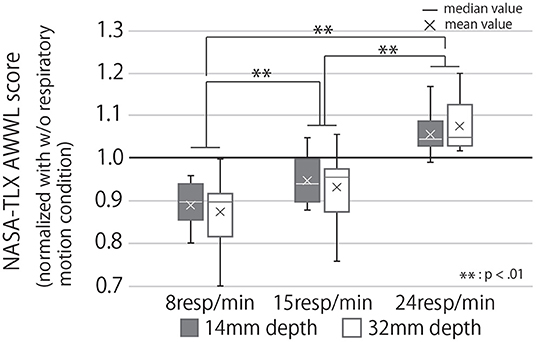
Figure 13. Subjective mental workload score calculated by AWWL under each condition, normalized with “w/o respiratory motion condition.” If the value of the graph is greater than 1, it means that the subjective mental workload for the task was greater than when the subject had a device that did not present the respiratory motion.
4.4.4. Participants' Comments
Based on the comments collected after the experiment, the following summarizes the participants' experiences: Thirteen of the sixteen participants naturally adjusted their breathing to the device's movement. At the same time, ten people said they felt the cushion's respiratory motion as if it were their own. During the presentation of a relaxed respiratory rhythm (R = 8), 10 participants reported feeling more comfortable with the presentation of larger respiratory motions than smaller ones; the opposite, while three participants reported they were more comfortable with the small respiratory motion. When presented with a relaxed breathing pace, all of the participants reported feeling calmer. However, when the device presented a fast breathing pace, nine out of sixteen participants reported feeling restless or anxious.
4.5. Discussion
The results demonstrate the ability of our device to induce a user's respiratory rhythm and depth without pre-training. As shown in Figure 9, the respiratory rhythm of the user changed according to the respiratory rhythm presented by the device, which is consistent with the findings of previous studies that the respiratory rhythm of the user is induced by the rhythm presented by other tactile stimuli such as vibration (Frey et al., 2018; Tabor et al., 2021). This result clarified Q1 shown in section 4.1. Since few studies have demonstrated ways to exert any significant natural control over the depth of breathing, this study contributes a new approach of inducing the breathing depth by using biofeedback. In particular, as Figure 10 shows, presenting deep respiratory motions through our device can induce similar deep-breathing in the user. This result clarified Q2.
Since deep-breathing leads to the resolution of stress and anxiety (Paul et al., 2007), this device has the possibility of having a higher stress-relieving effect than systems that merely modify the users respiratory rhythm. In fact, as shown in Figure 11 and Table 1, the RRI of the participants was observed to be significantly higher when presenting deep-breathing motions than when presenting shallow-breathing motions. In addition to the actual change in respiratory motion, 13 out of 16 participants responded that they naturally regulated their breathing according to the device's motion, suggesting that the device strongly induced the rhythm and depth of their breathing. Besides, the participants' comments indicate we could make the users recognize the respiratory motion presented by the device as their physical responses, as we intended.
The RRI results (Figure 11), change in performance before and after the break (Figure 12), and subjective mental workload (Figure 13), which were used as indicators of the effect of the break, confirmed Q3; it suggest that the device enhanced psychological and physiological relaxation during the break and improved task performance after the break.
The stimulating effect of the break is maximized on the presentation of slow respiratory rhythms, according to the results of all three indicators, while the RRI and subjective mental workload indicate that this effect can be achieved by increasing the depth of the respiratory motion presented. Furthermore, our device is suitable for daily use because it can improve relaxation even during 5-minute breaks. In the breaking phase, when the device presented a slow breathing rhythm, task performance was better after the break than before the break. This may be because the device was not used in the break before the cognitive task A, so the effect of the break before the task was lower than that of the break before the cognitive task B (breaking phase). Task performance after the break was slightly lower than before the break in the condition where the device did not display respiratory movements. This suggests that the improvement in task performance after the break could be due to the effect of the respiratory motion presentation rather than habituation from performing the task repeatedly. It has been reported that respiratory motion guidance by a device improves autonomic indices such as RRI (Sato et al., 2015). However, this experiment is the first to show that the respiratory motion induced by a device during a break can affect task performance and subjective mental workload for the task after the break, which is a contribution of our research.
On the other hand, upon presenting fast respiratory motions (R = 24), the user's breathing rhythm increased but did not synchronize with the presented rhythm. This is attributed to a lack of effective respiratory induction stemming not only from the presented rhythm being too fast, but also from the large gap between the presented and user rhythms. From the graph of R = 32 and D = 24 in Figure 9, it can be seen that the respiratory induction effect was especially low when fast and deep breathing movements were present; the high rates of device deformation could account for this under fast and deep respiratory conditions, which prevents the user from perceiving it as an extension of their body movements.
It should be noted here that on presenting a fast respiratory rhythm, users get restless and their post-break performance is lower than the case where no breathing motion is presented—nine out of 16 participants reported feeling impatient and anxious when they were presented with faster respiration rates. Therefore, it is also possible to induce a state of agitation in users with this device; this would be useful in cases where an agitated state of mind is required. It could also be used as an experimental device to study the relationship between breathing, task performance, and stress.
In summary, the above results and participant comments suggest that respiratory induction is very powerful when using our technique and device. Inducing the rhythm and depth of breathing with this device can improve the effectiveness of breaks between tasks, improve task performance after a break, and reduce subjective mental workload levels. By changing the breathing pace with this device, the user's physiological state can be changed to a calm, well-rested or excited state.
5. Limitation and Future works
In this article, we realized the system that can modify the respiratory rhythm and depth by presenting the respiratory motion with the cushion-type device. However, some points to improve the system and some points have not been verified yet.
First, as described in the Discussion, which factor of our device contributed to the respiratory depth and rhythm induction has not been clarified. Participants answered that they felt the motion of the device as if it was their own motion, but it has not been verified to what extent “a tactile override of a respiratory motion” is effective for respiratory induction compared with other previous methods, especially in the induction of respiratory rhythm. It is possible to find a technique that can powerfully induce respiration by examining this. Moreover, it remains unclear whether the expansion and contraction movement of the device felt by the user's hands acted on respiration or whether the pressure on the chest acted on respiration. We told participants to place their hands only lightly on the device's surface when holding it, so the device's motion presented almost no pressure on the chest. However, we should clarify which effect is more dominant on respiratory control in the future. For example, if we create a situation where there is a gap between the chest and the device so that the device's motion does not affect the chest, we can evaluate only the effect of the device's motion felt by the hand on breathing. Moreover, suppose the device is pressed against the user's chest by an external mechanism, and the user does not touch the device with their hands. In that case, we can evaluate only the effect of the pressure on the chest caused by the device's motion on breathing.
There are also limitations to the design of the experiment. In this experiment, we tried to reduce the order effect by taking enough breaks between trials and carefully explaining and practicing the task in advance. However, the presented conditions did not wholly eliminate the order effect. It will be necessary to plan experiments with a narrower number of conditions in the future because many participants will be required to cover all presentation orders.
Besides, the limitation of respiratory rhythm and depth, which this device can control, has not been clarified. Therefore, it is necessary to clarify this device's limit range of respiration induction through more precise verification in the future. The breathing guides in our study exclusively provided guidance and did not provide any type of feedback on performance. Previous work has shown that leading guidance is better than real-time feedback for influencing breathing behavior (Matthews et al., 2015). However, the design of the presented stimuli is different between the previous study and the present method. We use tactile stimuli instead of light to simulate the movement of the stomach during breathing. As a result, there's a chance that different real-time feedback effects will emerge. Specifically, it seems to be possible to realize the natural respiration induction by sensing the actual respiratory rhythm of the user in real time, synchronizing the pace of the respiratory motion of the cushion, and gradually inducing the respiratory rhythm to the target respiratory rhythm.
It is also important to note that the respiratory rhythm that a user feels comfortable with the presented respiratory motion may differ from person to person. Participants' comments indicated that not all of them preferred the slow respiratory rhythm presentation. We may solve this problem by continuously measuring physiological data during the use of the device, as Amores et al. (2018), and gradually adjusting the respiratory movements to make the user feel more relaxed.
Moreover, to avoid a huge number of experimental conditions, the inhalation/exhalation (i/e) ratio was fixed to be 1.0 in this experiment. However, previous work reported increased relaxation, stress reduction, mindfulness and positive energy when breathing with the low compared to the high i/e ratio (Van Diest et al., 2014). To improve the relaxation effect during use of our device and the improvement of task performance after use of it, the effects of varying the i/e ratio should be investigated in the future.
There are also future issues regarding the presentation of faster respiratory rhythm. In a similar attempt, Balters et al. (2018) reported that coaching one to breathe faster while driving improves subjective arousal and concentration; this contrasts with our experimental findings of decreased performance levels after the presentation of faster breathing. In addition to variations in the type of task and the cognitive load involved in both studies, the difference may also be because while they provided breath-coaching during the task, we induced breathing during the break before the task.
In this study, we tested the device's effectiveness under controlled conditions in a laboratory environment. However, to demonstrate the device's usefulness, it is necessary to test it in more chaotic environments such as actual offices and test it on people who are under extreme stress. In addition, we can expand the research from the viewpoint of improving the device. For example, changing the texture and color of the cushion may produce a higher resting effect, and adding other tactile information such as warmth may relax the user more.
6. Conclusion
By conceptualizing and designing a cushion-shaped device that mimics respiratory motion, we proposed a technique to optimize users' respiratory rhythm and depth to enhance the relaxation effect of workplace break-times and their post-break performance on tasks. A user study was conducted on this device, in which participants were asked to hug it. Their respiratory parameters, mental workload, and cognitive abilities based on task performance were measured. The experiment yielded the following findings:
• Presenting respiratory motion using the cushion-shaped device can modify the user's respiratory rhythm and depth.
• Using this device during breaks between tasks can enhance the relaxation effect of breaks in both subjective and physiological indices, and improve task performance after breaks.
These results strongly demonstrate that our device enables the user to control her/his own breathing rhythm and depth without the need for special training, so that it has the potential to release stress and improve productivity in daily life.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by The Ethics Committee of the University of Tokyo, the University of Tokyo. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
YB: designed the study and developed the device with assistance from HK, collected the data and analyzed the results, and wrote the manuscript with contributions from HK, RF, and SW. All authors reviewed and approved the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Amores, J., Hernandez, J., Dementyev, A., Wang, X., and Maes, P. (2018). “Bioessence: a wearable olfactory display that monitors cardio-respiratory information to support mental wellbeing,” in 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) (Honolulu, HI: IEEE), 5131–5134.
Balters, S., Murnane, E. L., Landay, J. A., and Paredes, P. E. (2018). Breath booster! exploring in-car, fast-paced breathing interventions to enhance driver arousal state. In Proceedings of the 12th EAI International Conference on Pervasive Computing Technologies for Healthcare (New York, NY), 128–137.
Ban, Y., Karasawa, H., Fukui, R., and Warisawa, S. (2018). “Relaxushion: controlling the rhythm of breathing for relaxation by overwriting somatic sensation,” in SIGGRAPH Asia 2018 Emerging Technologies (New York, NY), 1–2.
Burke, H. M., Davis, M. C., Otte, C., and Mohr, D. C. (2005). Depression and cortisol responses to psychological stress: a meta-analysis. Psychoneuroendocrinology 30, 846–856. doi: 10.1016/j.psyneuen.2005.02.010
Carreiras, C., Alves, A. P., Lourenço, A., Canento, F., Silva, H., Fred, A., et al. (2015–). BioSPPy: Biosignal Processing in Python. (accessed June 11, 2019).
Chandola, T., Brunner, E., and Marmot, M. (2006). Chronic stress at work and the metabolic syndrome: prospective study. Bmj 332, 521–525. doi: 10.1136/bmj.38693.435301.80
Choi, K. Y., Lee, J., ElHaouij, N., Picard, R., and Ishii, H. (2021). “aspire: Clippable, mobile pneumatic-haptic device for breathing rate regulation via personalizable tactile feedback,” in Extended Abstracts of the 2021 CHI Conference on Human Factors in Computing Systems (Yokohama), 1–8.
Cocilovo, A. (1999). Colored light therapy: overview of its history, theory, recent developments, and clinical applications combined with acupuncture. Am. J. Acupuncture 27, 71–84.
Condon, W. S., and Sander, L. W. (1974). Neonate movement is synchronized with adult speech: interactional participation and language acquisition. Science 183, 99–101.
Costa, J., Adams, A. T., Jung, M. F., Guimbretière, F., and Choudhury, T. (2016). “Emotioncheck: leveraging bodily signals and false feedback to regulate our emotions,” in Proceedings of the 2016 ACM International Joint Conference on Pervasive and Ubiquitous Computing (Heidelberg), 758–769.
Daley, M. A., Bramble, D. M., and Carrier, D. R. (2013). Impact loading and locomotor-respiratory coordination significantly influence breathing dynamics in running humans. PLoS ONE 8, e70752. doi: 10.1371/journal.pone.0070752
Esler, M., and Kaye, D. (2000). Sympathetic nervous system activation in essential hypertension, cardiac failure and psychosomatic heart disease. J. Cardiovasc. Pharmacol. 35, S1–S7. doi: 10.1097/00005344-200000004-00001
Frank, D. L., Khorshid, L., Kiffer, J. F., Moravec, C. S., and McKee, M. G. (2010). Biofeedback in medicine: who, when, why and how? Ment. Health Fam. Med. 7, 85.
Frey, J., Grabli, M., Slyper, R., and Cauchard, J. R. (2018). Breeze: Sharing biofeedback through wearable technologies. In Proceedings of the 2018 CHI Conference on Human Factors in Computing Systems (Montreal, QC: ACM), 645.
Fukushima, S., and Kajimoto, H. (2012). “Chilly chair: facilitating an emotional feeling with artificial piloerection,” in ACM SIGGRAPH 2012 Emerging Technologies (New York, NY), 1.
Gazzaniga, M., and Ivry, R. B. (2013). Cognitive Neuroscience: The Biology of the Mind: Fourth International Student Edition. New York, NY: WW Norton.
George, R., Chung, T. D., Vedam, S. S., Ramakrishnan, V., Mohan, R., Weiss, E., et al. (2006). Audio-visual biofeedback for respiratory-gated radiotherapy: impact of audio instruction and audio-visual biofeedback on respiratory-gated radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 65, 924–933. doi: 10.1016/j.ijrobp.2006.02.035
Ghandeharioun, A., and Picard, R. (2017). Brightbeat: effortlessly influencing breathing for cultivating calmness and focus. In Proceedings of the 2017 CHI Conference Extended Abstracts on Human Factors in Computing Systems (Denver, CO), 1624–1631.
Hart, S. G. (2006). “Nasa-task load index (nasa-tlx); 20 years later,” in Proceedings of the Human Factors and Ergonomics Society Annual Meeting, vol. 50 (Los Angeles, CA: Sage publications Sage CA), 904–908.
Hoffmann, C. P., Torregrosa, G., Bardy, B. G., and Balasubramaniam, R. (2012). Sound stabilizes locomotor-respiratory coupling and reduces energy cost. PLoS ONE 7, e45206. doi: 10.1371/journal.pone.0045206
Jerath, R., Edry, J. W., Barnes, V. A., and Jerath, V. (2006). Physiology of long pranayamic breathing: neural respiratory elements may provide a mechanism that explains how slow deep breathing shifts the autonomic nervous system. Med. Hypotheses 67, 566–571. doi: 10.1016/j.mehy.2006.02.042
Joseph, C. N., Porta, C., Casucci, G., Casiraghi, N., Maffeis, M., Rossi, M., et al. (2005). Slow breathing improves arterial baroreflex sensitivity and decreases blood pressure in essential hypertension. Hypertension 46, 714–718. doi: 10.1161/01.HYP.0000179581.68566.7d
Kimura, H., Kuramoto, A., Inui, Y., and Inou, N. (2017). Mechanical bed for investigating sleep-inducing vibration. J. Healthcare Eng. 2017, 2364659. doi: 10.1155/2017/2364659
Kini, V. R., Vedam, S. S., Keall, P. J., Patil, S., Chen, C., and Mohan, R. (2003). Patient training in respiratory-gated radiotherapy. Med. Dosimetry 28, 7–11. doi: 10.1016/S0958-3947(02)00136-X
Liberman, J. (1990). Light: Medicine of the Future: How We Can Use It To Heal Ourselves Now. New Mexico: Inner Traditions/Bear & Co.
Lloyd, C., Smith, J., and Weinger, K. (2005). Stress and diabetes: a review of the links. Diabetes Spectrum 18, 121–127.
Mani, M., Kavanagh, D. J., Hides, L., and Stoyanov, S. R. (2015). Review and evaluation of mindfulness-based iphone apps. JMIR mHealth uHealth 3, e4328. doi: 10.2196/mhealth.4328
Matthews, M., Snyder, J., Reynolds, L., Chien, J. T., Shih, A., Lee, J. W., and Gay, G. (2015). “Real-time representation versus response elicitation in biosensor data,” in Proceedings of the 33rd Annual ACM Conference on Human Factors in Computing Systems (Seoul), 605–608.
Morin, D., and Viala, D. (2002). Coordinations of locomotor and respiratory rhythms in vitro are critically dependent on hindlimb sensory inputs. J. Neurosci. 22, 4756–4765. doi: 10.1523/JNEUROSCI.22-11-04756.2002
Neicu, T., Berbeco, R., Wolfgang, J., and Jiang, S. B. (2006). Synchronized moving aperture radiation therapy (smart): improvement of breathing pattern reproducibility using respiratory coaching. Phys. Med. Biol. 51, 617. doi: 10.1088/0031-9155/51/3/010
Omlin, X., Crivelli, F., Heinicke, L., Zaunseder, S., Achermann, P., and Riener, R. (2016). Effect of rocking movements on respiration. PLoS ONE 11, e0150581. doi: 10.1371/journal.pone.0150581
Padgett, D. A., and Glaser, R. (2003). How stress influences the immune response. Trends Immunol. 24, 444–448. doi: 10.1016/S1471-4906(03)00173-X
Paredes, P. E., Zhou, Y., Hamdan, N. A.-H., Balters, S., Murnane, E., Ju, W., and Landay, J. A. (2018). Just breathe: in-car interventions for guided slow breathing. Proc. ACM Interact. Mob. Wearable Ubiquitous Technol. 2, 1–23. doi: 10.1145/3191760
Park, S.-H., Jang, D.-G., Son, D.-H., Zhu, W., and Hahn, M.-S. (2009). A biofeedback-based breathing induction system. In 2009 3rd International Conference on Bioinformatics and Biomedical Engineering (Beijing: IEEE), 1–4.
Paul, G., Elam, B., and Verhulst, S. J. (2007). A longitudinal study of students' perceptions of using deep breathing meditation to reduce testing stresses. Teach. Learn. Med. 19, 287–292. doi: 10.1080/10401330701366754
Saito, Y., and Tada, H. (2007). Effects of color images on stress reduction: Using images as mood stimulants. Japan J. Nurs. Sci. 4, 13–20. doi: 10.1111/j.1742-7924.2007.00068.x
Sato, N., Kamada, T., Miyake, S., Akatsu, J., Kumashiro, M., and Kume, Y. (1998). Power spectral analysis of heart rate variability in type a females during a psychomotor task. J. Psychosomatic Res. 45, 159–169.
Sato, T. G., Ohsuga, M., Boutani, H., and Moriya, T. (2015). Tactile phantom sensation for coaching respiration timing. IEEE Trans. Haptics 8, 119–125. doi: 10.1109/TOH.2015.2396896
Sato, T. G., Ohsuga, M., and Moriya, T. (2012). Increase in the timing coincidence of a respiration event induced by listening repeatedly to the same music track. Acoust. Sci. Technol. 33, 255–261. doi: 10.1250/ast.33.255
Sato, T. G., Watanabe, J., and Moriya, T. (2016). Presenting changes in acoustic features synchronously to respiration alters the affective evaluation of sound. Int. J. Psychophysiol. 110, 179–186. doi: 10.1016/j.ijpsycho.2016.08.003
Somnox, (2019). The Somnox Sleep Robot Scientific background. Available online at: https://somnox.com/wp-content/uploads/2019/06/20191203_Somnox_Whitepaper.pdf
Stafford, M., Lin, F., and Xu, W. (2016). “Flappy breath: A smartphone-based breath exergame,” in 2016 IEEE First International Conference on Connected Health: Applications, Systems and Engineering Technologies (CHASE) (Washington, DC), 332–333.
Tabor, A., Bateman, S., Scheme, E., Sadprasid, B., and Schraefel, M. (2021). “Understanding the design and effectiveness of peripheral breathing guide use during information work,” in Proceedings of the 2021 CHI Conference on Human Factors in Computing Systems (Yokohama), 1–13.
Taelman, J., Vandeput, S., Spaepen, A., and Van Huffel, S. (2009). “Influence of mental stress on heart rate and heart rate variability,” in 4th European Conference of the International Federation for Medical and Biological Engineering (Berlin: Springer), 1366–1369.
Ueoka, R., and AlMutawa, A. (2018). “Emotion hacking VR: amplifying scary VR experience by accelerating actual heart rate,” in International Conference on Human Interface and the Management of Information (Las Vegas, NV: Springer), 436–445.
Uratani, H., and Ohsuga, M. (2015). Development of respiration leading stuffed toy using airbags for children. Jpn J. Ergon. 51, 428–434. doi: 10.5100/JJE.51.428
Van Diest, I., Verstappen, K., Aubert, A. E., Widjaja, D., Vansteenwegen, D., and Vlemincx, E. (2014). Inhalation/exhalation ratio modulates the effect of slow breathing on heart rate variability and relaxation. Appl. Psychophysiol. Biofeedback 39, 171–180. doi: 10.1007/s10484-014-9253-x
Watanabe, T., and Okubo, M. (1997). “Evaluation of the entrainment between a speaker's burst-pause of speech and respiration and a listener's respiration in face-to-face communication,” in Proceedings 6th IEEE International Workshop on Robot and Human Communication. RO-MAN'97 SENDAI (Sendai: IEEE), 392–397.
Wilke, J. T., Lansing, R. W., and Rogers, C. A. (1975). Entrainment of respiration to repetitive finger tapping. Physiol. Psychol. 3, 345–349.
Yamamoto, T., and Miyake, Y. (2004). Analysis of musical communication between players in a musical cooperative performance. Trans. Soc. Instrum. Control Eng. 40, 563–572. doi: 10.9746/sicetr1965.40.563
Yamaura, K., Bi, Y., Ishiwatari, M., Oishi, N., Fukata, H., and Ueno, K. (2013). Sex differences in stress reactivity of hippocampal bdnf in mice are associated with the female preponderance of decreased locomotor activity in response to restraint stress. Zoolog. Sci. 30, 1019–1025. doi: 10.2108/zsj.30.1019
Yoshida, S., Tanikawa, T., Sakurai, S., Hirose, M., and Narumi, T. (2013). “Manipulation of an emotional experience by real-time deformed facial feedback,” in Proceedings of the 4th Augmented Human International Conference (New York, NY), 35–42.
Yoshida, Y. (1988). “Studies on the effects of low frequency vibration to the human body-physiological and psychological effects of low frequency horizontal vibration,” in Congress of the International Ergonomics Association Horizontal Vibration-Proc. of the 10th IEA (Sydney, NSW), 369–371.
Yu, B., Hu, J., Funk, M., and Feijs, L. (2018). Delight: biofeedback through ambient light for stress intervention and relaxation assistance. Pers. Ubiquitous Comput. 22, 787–805. doi: 10.1007/s00779-018-1141-6
Keywords: biofeedback, human computer interaction, relax, respiration control, tactile feedback
Citation: Ban Y, Karasawa H, Fukui R and Warisawa S (2022) Development of a Cushion-Shaped Device to Induce Respiratory Rhythm and Depth for Enhanced Relaxation and Improved Cognition. Front. Comput. Sci. 4:770701. doi: 10.3389/fcomp.2022.770701
Received: 04 September 2021; Accepted: 21 February 2022;
Published: 24 March 2022.
Edited by:
Antonio Frisoli, Sant'Anna School of Advanced Studies, ItalyReviewed by:
Giada Brianza, University of Sussex, United KingdomEleuda Nunez, University of Tsukuba, Japan
Copyright © 2022 Ban, Karasawa, Fukui and Warisawa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuki Ban, YmFuQGVkdS5rLnUtdG9reW8uYWMuanA=
 Yuki Ban
Yuki Ban Hiroyuki Karasawa
Hiroyuki Karasawa Rui Fukui
Rui Fukui Shin'ichi Warisawa
Shin'ichi Warisawa