- 1China Animal Health and Epidemiology Center, Qingdao, China
- 2College of Veterinary Medicine, Qingdao Agricultural University, Qingdao, China
In recent years, the emergence and widespread dissemination of the coronavirus SARS-CoV-2 has posed a significant threat to global public health and social development. In order to safely and effectively prevent and control the spread of coronavirus diseases, a profound understanding of virus-host interactions is paramount. Cellular autophagy, a process that safeguards cells by maintaining cellular homeostasis under diverse stress conditions. Xenophagy, specifically, can selectively degrade intracellular pathogens, such as bacteria, fungi, viruses, and parasites, thus establishing a robust defense mechanism against such intruders. Coronaviruses have the ability to induce autophagy, and they manipulate this pathway to ensure their efficient replication. While progress has been made in elucidating the intricate relationship between coronaviruses and autophagy, a comprehensive summary of how autophagy either benefits or hinders viral replication remains elusive. In this review, we delve into the mechanisms that govern how different coronaviruses regulate autophagy. We also provide an in-depth analysis of virus-host interactions, particularly focusing on the latest data pertaining to SARS-CoV-2. Our aim is to lay a theoretical foundation for the development of novel coronavirus vaccines and the screening of potential drug targets.
1 Introduction
In 2019, Wuhan, Hubei, China, witnessed the emergence of a novel coronavirus, dubbed COVID-19, whose exceptionally contagious nature triggered a rapid dissemination of the disease across the globe, persisting as a pandemic in the epicenter of the outbreak (Huang et al., 2020; Zhu et al., 2020). Subsequently, the Coronavirus Study Group of the International Committee on Taxonomy of Viruses (ICTV) categorized this virus under the β-coronavirus genus, designating it as Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). As of December 2021, global statistics indicate a staggering 271 million confirmed cases and 5.32 million fatalities, while a viable vaccine against SARS-CoV-2 remains elusive. Furthermore, coronaviruses pose a significant threat to economic animals, inflicting immense economic losses on the global livestock industry.
1.1 Introduction to coronaviruses
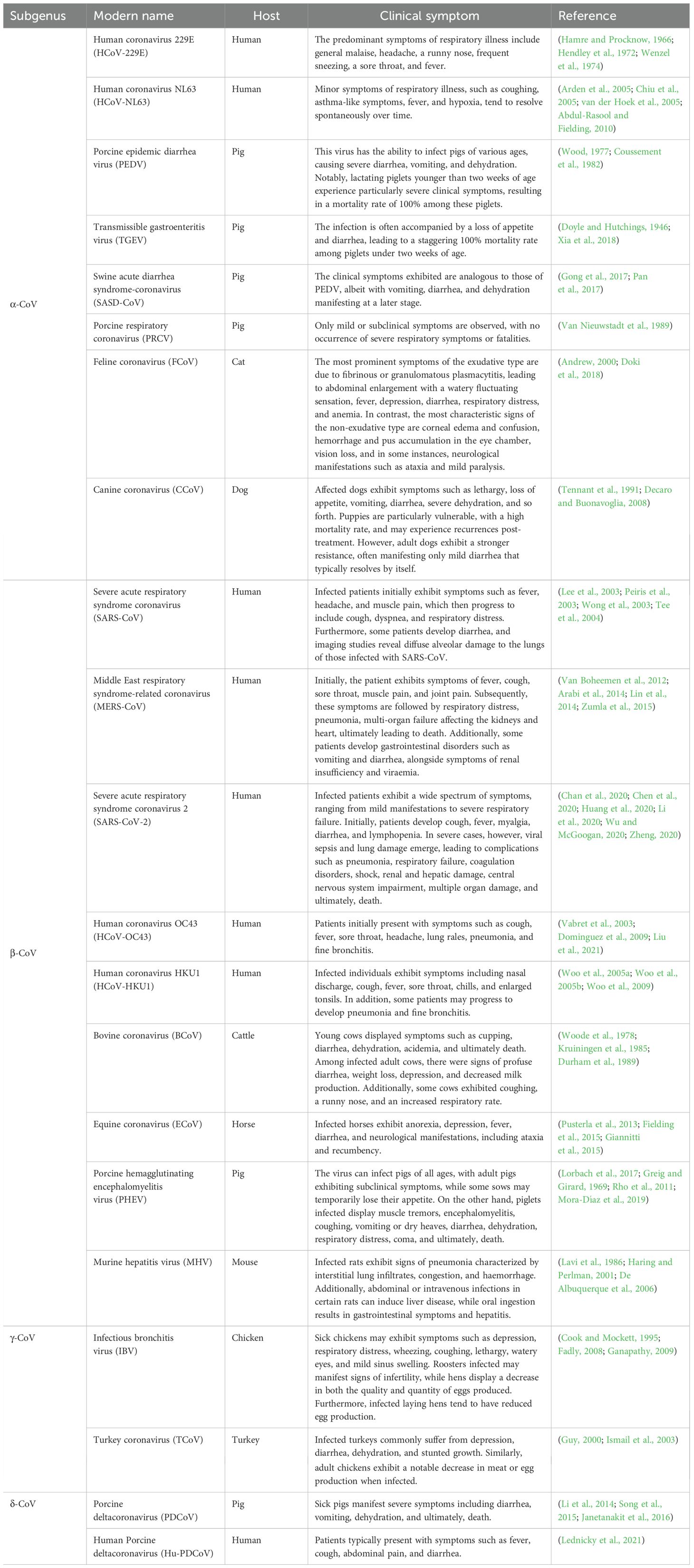
The coronavirus is classified under the Nidovirales order and falls within the Orthocoronavirus subfamily of the Coronavirus family (CoV). This expansive family is further segmented into four distinct viral genera: Alphacoronavirus (Alpha-CoV, α-CoV), Betacoronavirus (Beta-CoV, β-CoV), Gammacoronavirus (Gamma-CoV, γ-CoV), and Deltacoronavirus (Delta-CoV, δ-CoV) (Schoeman and Fielding, 2019). Coronaviruses possess a diverse range of hosts, encompassing mammals, birds, rodents, ruminants, and more. These infections in hosts can trigger a spectrum of illnesses, including respiratory disease, intestinal disease, hepatitis, and neurological disorders. Notably, α-CoV and β-CoV predominantly target mammals, while γ-CoV and δ-CoV predominantly affect avian species (Gorbalenya et al., 2004; Woo et al., 2010; Woo et al., 2012). Here, we offer a concise overview of the specific characteristics associated with the various coronaviruses (Table 1).
Coronavirus is a single-stranded, positive-sense RNA virus that is enveloped and possesses a genome size ranging approximately from 24,500 to 31,800 bp (Mihindukulasuriya et al., 2008). Its genome is characterized by a cap structure at the 5’ end and a polyadenylated tail at the 3’ end, sandwiched between which lies an intermediate structure composed of seven overlapping open reading frames (ORFs). These ORFs encode three primary non-structural proteins: replicase proteins (ORF1a and ORF1b), the auxiliary protein ORF3, and four structural proteins: spike protein (S), envelope protein (E), membrane protein (M), and nucleocapsid protein (N). The genes encoding ORF1a and ORF1b constitute approximately two-thirds of the entire genome and can be further dissected into 15-16 non-structural proteins (nsp), primarily involved in viral replication processes (Wang et al., 2020). The initial strategy employed by the coronavirus to circumvent the host’s immune defense system involves suppressing the production of IFNα/β, marking a crucial juncture for the virus to achieve successful replication. Nsp1 facilitates mRNA degradation and impedes host cell protein translation, thereby suppressing the innate immune response (Nakagawa and Makino, 2021). Among these nsps, Nsp3, the largest multi-domain protein, harbors a PLPro/Deubiquitinase structural domain that cleaves viral polyproteins and mitigates the innate immune response (Imbert et al., 2008; Neuman et al., 2014). The coronavirus establishes a “replication factory” known as a double-membrane vesicle (DMV), where Nsp3 and Nsp4 play pivotal roles in its formation. This DMV serves as a secure environment for viral replication, shielding viral RNA and encapsulating replication complexes to evade detection by host sensors (Knoops et al., 2008; Oudshoorn et al., 2017). Furthermore, Nsp15 facilitates viral genome replication and transcription by reducing the accumulation of negative-strand RNA and dsRNA, thereby aiding in the evasion of detection by host RNA sensors (Gao et al., 2021). Nsp16 possesses 2′-O-methyltransferase activity, enabling viral mRNA to mimic host mRNA and thus escape the interferon (IFN) response (Züst et al., 2011). Additionally, the coronavirus targets the double-stranded RNA sensor (RIG-I) and melanoma differentiation-associated protein 5 (MDA5). Notably, the N protein of SARS-CoV, SARS-CoV-2, and MERS-CoV interacts with TRIM25 E3 ubiquitin ligase, disrupting the K63-linked ubiquitination of RIG-I and preventing its activation (Chang et al., 2020; Chen et al., 2020; Xue et al., 2022). PACT, a dsRNA binding protein, activates RIG-I or MDA5, but the N proteins of PDCoV, MHV, and SARS-CoV directly bind to PACT, impeding the interaction between RIG-I/MDA5 and dsRNA or PACT, and thus inhibiting type I IFN activation (Chen et al., 2019). Moreover, the proteases PLpro and 3CLpro from MERS-CoV, SARS-CoV, and HCoV-NL63 inhibit multiple steps of type I IFN signal transduction by directly acting on signal transduction proteins or promoting their degradation through ubiquitin modification manipulations (such as interfering with IFN regulatory factor 3 and MAVS) (Barretto et al., 2005; Yang et al., 2014).
As the initial structural proteins in coronavirus replication, S proteins play a pivotal role in viral attachment and invasion. S proteins bind to specific receptors, including angiotensin-converting enzyme 2 (ACE2) (Li et al., 2003; Li et al., 2005; Zhou P. et al., 2020), aminopeptidase N (APN) (Tusell et al., 2007; Park et al., 2015), 9-O-acetylated sialic acid (Liu et al., 2015; Li et al., 2016), dipeptidyl peptidase 4(DPP4) (Li et al., 2017), and the carcinoembryonic antigen cell adhesion molecule (CEACAM1) (Dveksler et al., 1993), which facilitate the fusion between coronavirus membranes and host cell membranes, enabling viral entry into host cells. Furthermore, the S protein of coronaviruses is prone to amino acid mutations, leading to the emergence of novel strains with enhanced transmissibility and pathogenicity. There are 36 amino acid sites in the porcine epidemic diarrhea virus (PEDV) S protein that have a mutation frequency of more than 90%, and the high frequency of mutations makes it easier for the virus to escape from immune pressure, which may be the cause of recurrent outbreaks of piglet diarrhoea in pig farms (Yu et al., 2023). After the synthesis of viral subgenomic RNA, the membrane-associated structural proteins S, E, and M undergo translation and are inserted into the endoplasmic reticulum. From there, these proteins relocate to the endoplasmic reticulum-Golgi intermediate compartment. During this process, the N protein binds to the newly synthesized positive-sense RNA, forming a ribonucleoprotein complex. Following this, the M protein organizes the S, E, and N proteins into mature viral particles through protein-protein interactions, ultimately releasing them outside the cell via exocytosis (Fehr and Perlman, 2015; Hartenian et al., 2020). The E proteins, being the smallest structural proteins, primarily participate in virus assembly and budding (Masters, 2006; Nieto-Torres et al., 2011) (Figure 1).
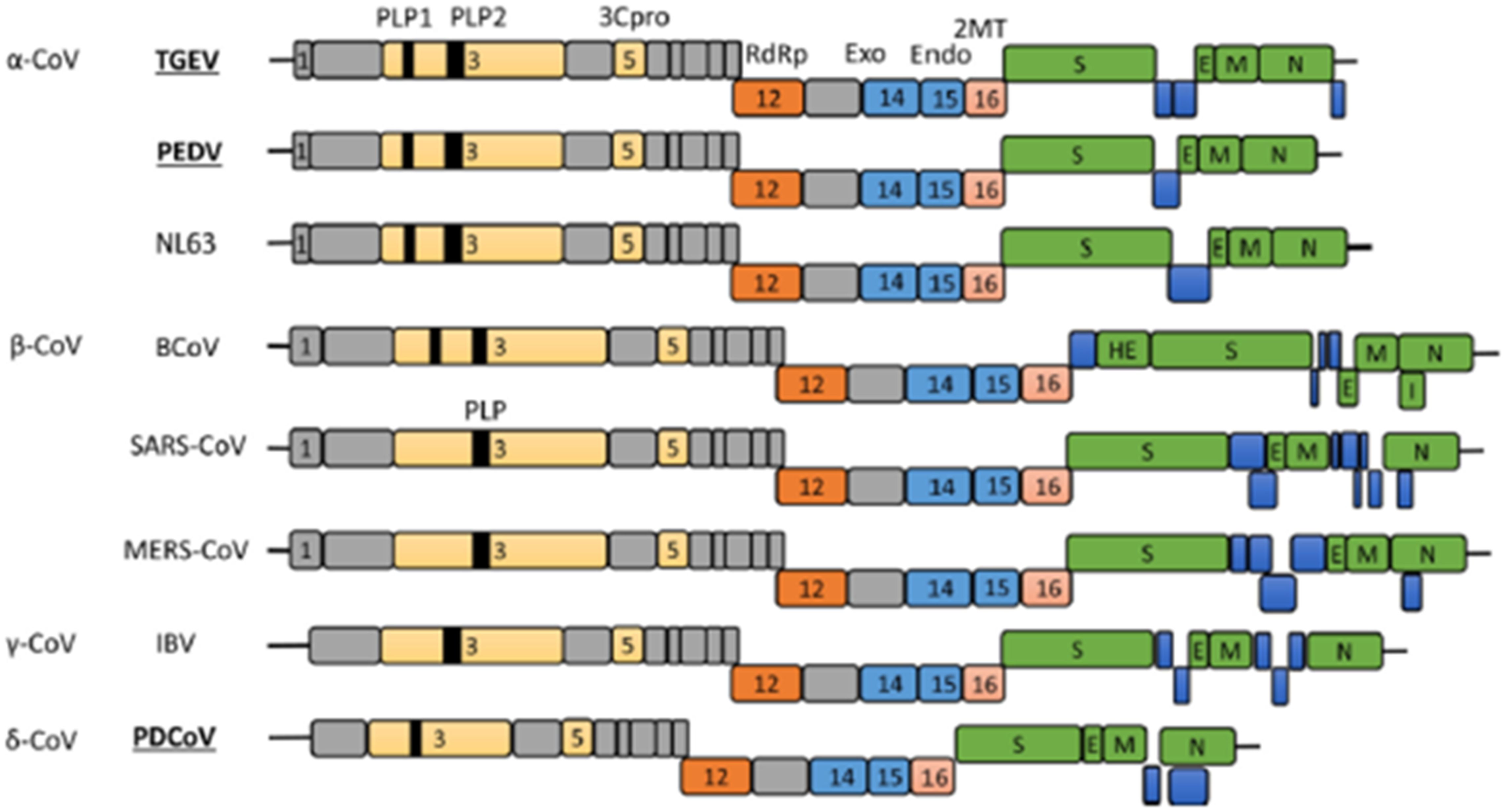
Figure 1. Coronavirus genome arrangement (Zhang and Yoo, 2016).
2 Autophagy
Autophagy holds a pivotal role in cellular growth and development. As an evolutionarily conserved lysosome-reliant metabolic pathway within eukaryotic cells, autophagy functions to transport senescent or impaired organelles and proteins to the lysosome for degradation. The resulting degraded small-molecule products are then utilized in sustaining cellular material recycling and maintaining intracellular homeostasis (Rubinsztein et al., 2011; Wei et al., 2018; Levine and Kroemer, 2019). Furthermore, autophagy serves as a crucial component of the body’s defense mechanism, playing a vital role in resisting the invasion of pathogenic microorganisms. Upon viral invasion, cells rely on autophagy to transport viral proteins, nucleic acids, and other components to the lysosomes for degradation (Levine, 2005; Choi et al., 2018; Yang and Klionsky, 2020). Generally, autophagy encompasses three primary types: macroautophagy, microautophagy, and molecular chaperone-mediated autophagy.
Typical autophagy comprises several sequential stages: initiation, nucleation, structural extension of the phagocytic vesicle membrane to encapsulate the cargo, closure of the phagocytic vesicle membrane to form autophagosomes, fusion of autophagosomes with lysosomes to yield autophagolysosomes, and ultimately, the in vivo degradation of autophagic contents within the autophagolysosomes (Levy et al., 2017; Yin et al., 2019). The upstream signaling pathways of autophagy, Akt-mTOR and AMPK-mTOR, govern the afferent activation of autophagy signals. These signals are relayed to the autophagy initiation complex, the ULK (ATG1) complex, which comprises ULK1, ATG13, FIP200, and ATG101. Upon activation, the ULK complex proceeds to activate the VPS34 complex (composed of VPS34, VPS15, and Beclin-1). The VPS34 complex facilitates phagocytic vesicle membrane elongation via binding to ATG14, and subsequently, closure of the membrane to form autophagic lysosomes through the interaction with UVRAG. This binding mediates the maturation of autophagic vesicles and further closure of the phagocytic vesicle membrane to form autophagic lysosomes (Glick et al., 2010; Mizushima et al., 2011). ATG5-ATG12-ATG16L1 and LC3-II, as crucial membrane components of phagocytic vesicles, are recruited to the vesicle membrane during the membrane extension phase. Following phagocytic vesicle closure, the ATG5-ATG12-ATG16L1 complex detaches from the membrane (Klionsky et al., 2021). LC3-I serves as the precursor of LC3. Upon cleavage by ATG4, the C-terminal glycine residue is exposed and subsequently binds with phosphatidylethanolamine (PE) to form LC3-II. LC3-II is also recognized as a marker molecule for autophagosomes (Kirisako et al., 1999; Kirisako et al., 2000). Ultimately, the contents within the autophagic vesicles, after fusing with lysosomes, undergo degradation, and the resulting macromolecular precursors are recycled for reuse. Alongside LC3-II, the specific substrate of autophagy, P62, also plays a role in mediating selective autophagic processes, including mitochondrial autophagy. Therefore, the expression levels of LC3-II and P62 can serve as indicators, reflecting the extent of autophagic vesicle formation and the efficacy of autophagic lysosomes in degrading autophagic substrates (Klionsky et al., 2021).
Increasing evidence suggests that a diverse array of pathogenic microorganisms, encompassing viruses, have evolved numerous tactics to modulate host cell autophagy (Jackson, 2015; Lennemann and Coyne, 2015). The Influenza A virus (IAV) orchestrates autophagy by intricately modulating the AKT-mTOR signaling cascade and influencing the expression of HSP90AA1 via its NP and M2 proteins. This regulatory mechanism subsequently fosters the replication of IAV (Wang et al., 2019). Similarly, the capsid protein VP2 of the Foot-and-Mouth Disease Virus (FMDV) engages in a functional interaction with the heat shock protein HSPB1, thereby activating the EIF2S1-ATF4-AKT-MTOR pathway. This activation triggers a sequential cascade reaction that induces autophagy and ultimately enhances the replication of FMDV (Sun et al., 2018). Similarly, the Japanese encephalitis virus (JEV) facilitates the infection of host cells by suppressing autophagy. The decrease in autophagy-related proteins, namely ATG5 and ATG7, results in the accumulation of JEV RNA, an elevation in viral titers, and an augmentation in cell mortality. Notably, viral replication complexes co-localize with markers of ERAD regulation, specifically EDEM1 and the LC3-I. Furthermore, the downregulation of non-lipidated LC3 diminishes viral titers (Sharma et al., 2014). Although previous studies have predominantly focused on the interplay between human coronaviruses and autophagy, there is a scarcity of reports exploring the interactions between various classes of coronaviruses and autophagy. In this paper, we comprehensively review the interactions between different coronavirus species and autophagy, conducting a meticulous systematic study of coronavirus infections’ impact on autophagic processes and their underlying mechanisms.
3 α-CoVs
Among the research on alpha-coronaviruses (α-CoVs), there has been a more profound examination of PEDV, TGEV, SADS-CoV, and HCoV-NL63. Therefore, we have consolidated the mechanisms in which the pertinent proteins of these viruses regulate autophagy through diverse pathways (Figure 2).
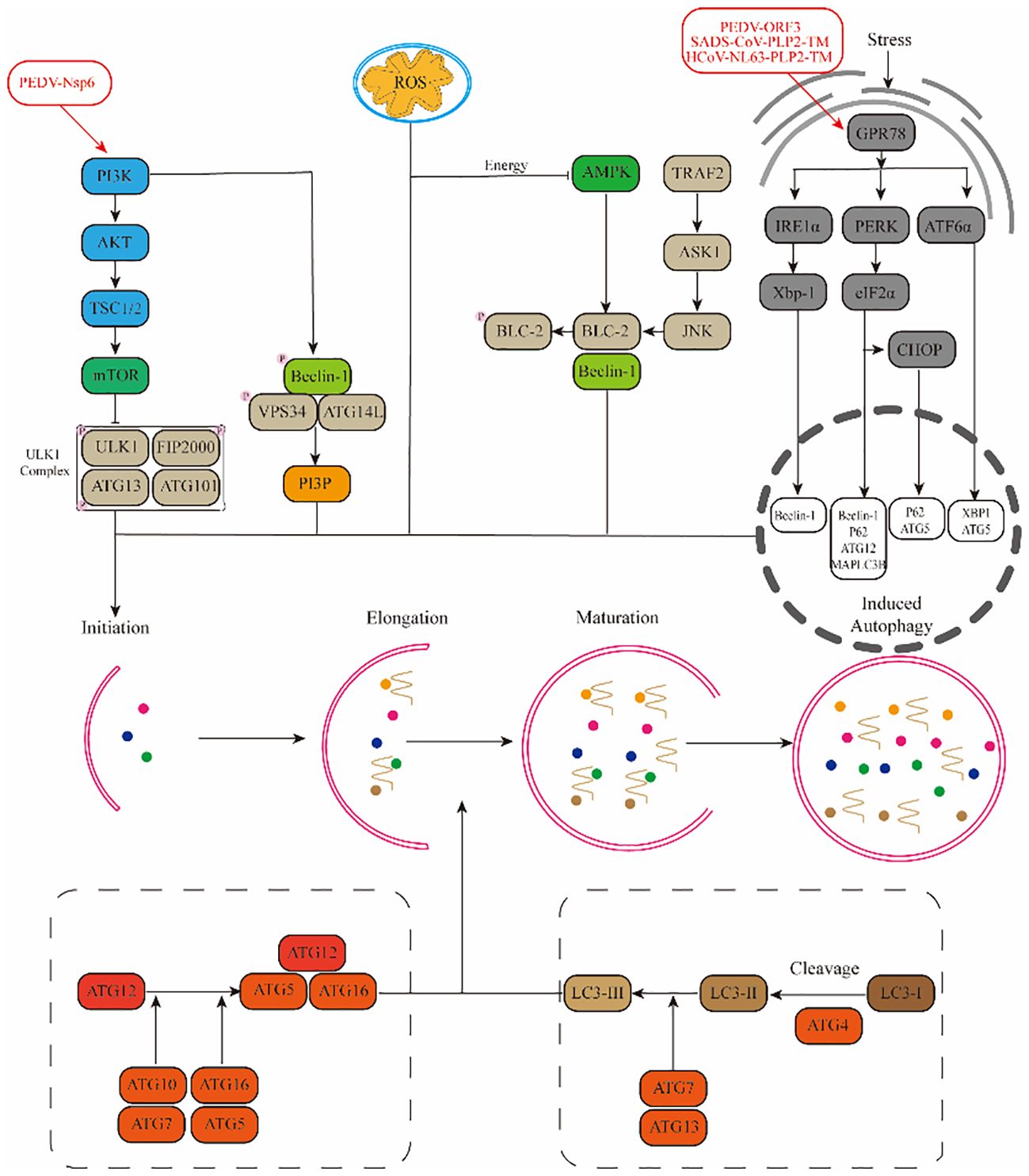
Figure 2. Mechanisms by which α-CoVs regulate autophagy through different signalling pathways.(1) Viruses promote autophagy through the PI3K-AKT-mTOR and AMPK-mTOR-ULK1 signalling pathways. (2) ROS regulate autophagy occurrence through downstream pathways. (3) Viruses promote autophagy through stress pathways: RE1α-XBP1 and PERK-eIF2α.
3.1 PEDV
Porcine Epidemic Diarrhea Virus (PEDV), a coronavirus notorious for inducing vomiting, diarrhea, dehydration, and even mortality in pigs, exhibits a staggering prevalence of up to 100% among newborn piglets (Wood, 1977). Research has shown that PEDV infection in Vero cells can activate the autophagy pathway, leading to a downregulation in the phosphorylation levels of mTOR and its downstream effectors 4EBP1 and p70S6K (Guo X. et al., 2016). Further investigations revealed a substantial increase in the number of autophagic vesicles in PEDV-infected Vero cells, indicating a positive correlation between autophagy and NF-κB activation (Guo et al., 2017). A similar trend was observed in PEDV-infected IPEC-J2 cells, where autophagy activation promoted PEDV replication. However, Kong et al. presented a contrasting view, demonstrating that upon PEDV infection in Vero and LLC-PK1 cells, upregulated BST2 recruited the autophagy-associated cargo protein MARCH8 to catalyze the ubiquitination of the PEDV N protein. This process subsequently inhibited PEDV replication through the selective autophagic degradation of the N protein (Kong et al., 2020). Among the viral proteins of PEDV, Nsp3, Nsp6, and ORF3 are the primary inducers of autophagy activation. Nsp3 primarily inhibits the formation of autophagic streams by hindering the fusion of autophagic vesicles with lysosomes, while Nsp6 functions as an inhibitor of the PI3K/Akt/mTOR pathway to activate autophagy (Chen et al., 2014; Lin et al., 2020). On the other hand, the ORF3 protein upregulates GPR78 protein expression, activating the PERK-eIF2α pathway, triggering endoplasmic reticulum stress, and thereby activating autophagy (Zou et al., 2019).
3.2 TGEV
Transmissible Gastroenteritis Virus (TGEV) is an acute and highly contagious porcine enteric virus that infects pigs of various breeds and ages, resulting in symptoms such as vomiting, diarrhea, dehydration, intestinal villous atrophy, and high mortality rates among piglets (Turlewicz-Podbielska and Pomorska-Mól, 2021). Research has indicated that TGEV infection triggers an autophagic response in PK15 and ST cells, leading to an increase in monolayer and bilayer vesicle structures, which contribute to the formation of autophagic vesicles (Guo L. et al., 2016). Further investigations revealed that TGEV-induced autophagy may stem from the induction of mitochondrial autophagy by the N protein, which mitigates oxidative stress and apoptosis triggered by TGEV infection (Zhu et al., 2016).
3.3 SADS-CoV
Swine Acute Diarrhea Syndrome Coronavirus (SADS-CoV) is a novel porcine coronavirus that poses a significant threat to piglets, often resulting in severe mortality (Gong et al., 2017). In a study conducted by Zeng et al., the global gene expression profile of Vero cells infected with SADS-CoV was analyzed using RNA-seq, revealing a notable downregulation in the expression levels of PI3K and Akt genes (Zeng et al., 2021). Furthermore, it was discovered that SADS-CoV promotes autophagy in Vero cells by inhibiting the activation of the Akt/mTOR pathway (Zeng et al., 2022). Additionally, the Coronavirus-associated papain-like protease PLP2 (PLP2-TM) within SADS-CoV triggers endoplasmic reticulum stress through its interaction with GRP78 and subsequently induces autophagy via the activation of the IRE1-JNK-Beclin1 pathway (Shi et al., 2023).
3.4 HCoV-NL63
The Human Coronavirus (HCoV-NL63) also triggers autophagy. Specifically, PLP2-TM, through its interaction with Beclin1, enhances the accumulation of autophagosomes and impedes the fusion between autophagosomes and lysosomes, thereby inducing incomplete autophagy (Chen et al., 2014). Additionally, the non-structural protein Nsp3 of HCoV-NL63 relies on papain activity to induce autophagy but exerts an inhibitory effect on the autophagic flow (Chen et al., 2014).
In summary, both PEDV and TGEV infections are capable of stimulating the production of autophagic vesicles within susceptible cells, and the resulting autophagy represents a comprehensive autophagic process, commonly referred to as complete autophagy. A notable distinction arises in the specific interaction between BST2 and the N protein of these viruses; BST2 selectively engages with the N protein of PEDV to facilitate its autophagic degradation, whereas no such interaction occurs with the N protein of TGEV. Similarly, both the PLP2-TM domain of SADS-CoV and HCoV-NL63 have the ability to induce autophagy, but they differ in their outcomes. SADS-CoV triggers a complete autophagy process, where autophagosome accumulation is intimately linked to the hydrolysis of autophagic cargo. Conversely, HCoV-NL63 induces an incomplete autophagy, a process that ultimately impedes the successful maturation of autophagosomes into autophagolysosomes.
Recent investigations have unveiled intriguing differences in the replication mechanisms among various coronaviruses, further revealing that the influence of autophagy on these mechanisms varies significantly across cell types. Specifically, TGEV is capable of eliciting mitochondrial autophagy in IPEC cells, a process mediated by DJ-1, a versatile redox-sensitive protein. This autophagic response alleviates oxidative stress and apoptosis, thereby fostering viral replication (Zhu et al., 2016). In contrast, when TGEV infects PK-15 cells, the induced autophagy serves to impede viral replication. Analogously, PEDV induces autophagy in IPEC cells via the PI3K/AKT/mTOR signaling cascade, which in turn amplifies viral replication. However, when IPEC cells are treated with rapamycin, a potent autophagy inducer, it increases autophagic flux to a level that ultimately leads to the suppression of viral infection, illustrating the complexity and cell-specific nature of these interactions (Ko et al., 2017).
4 β-CoVs
In contrast to α-CoVs, the study of autophagy induced by β-CoVs is comparatively intricate, encompassing primarily SARS-CoV-2, SARS-CoV, MERS-CoV, MHV, and PHEV. We hereby provide a concise overview of the intricate interactions between β-CoVs and autophagy (Figure 3).
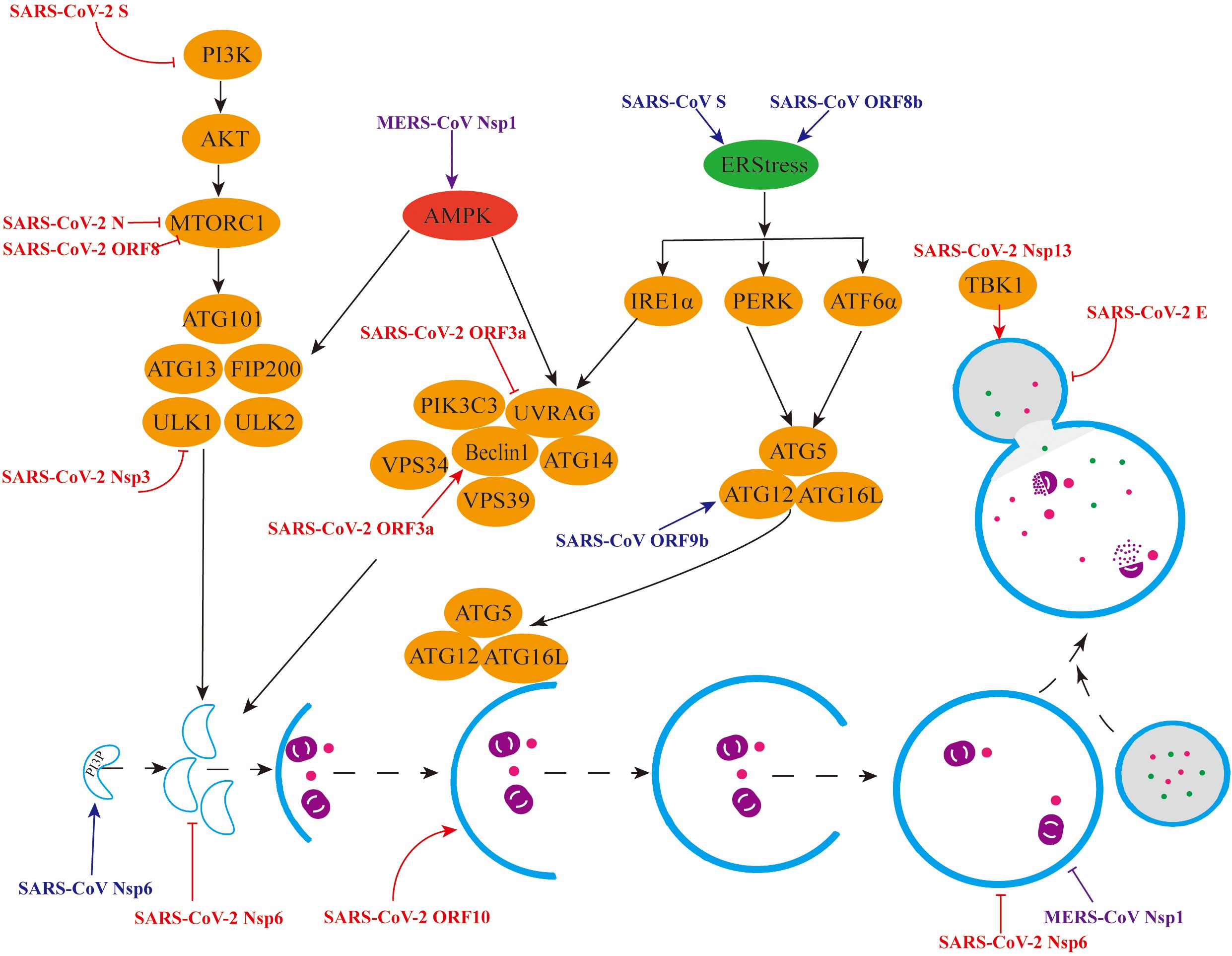
Figure 3. Mechanisms by which β-CoVs regulate autophagy through different signalling pathways. (1) Red colour indicates regulation of the autophagy pathway by individual SARS-CoV-2 viral proteins. (2) Blue colour indicates the promotion of autophagy by individual SARS-CoV viral proteins. (3) Purple colour indicates the different regulation of the autophagy pathway by the MERS-CoV Nsp1 protein.
4.1 SARS-CoV-2
Since the global outbreak of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in 2019, the epidemic has persisted, posing a significant threat to human and animal health. Due to its high priority, research on SARS-CoV-2, particularly in relation to autophagy, has been extensively conducted. Metabolomic analyses have revealed a significant upregulation of proteins associated with autophagy in SARS-CoV-2-infected cells, encompassing proteins involved in autophagy initiation (AMPK, TSC2, ULK1) and those critical for membrane nucleation and autophagy precursor formation (Beclin1, VPS34, ATG14) (Gassen et al., 2021). Furthermore, Krogan et al. demonstrated, through phosphoproteomic analysis using quantitative mass spectrometry, that SARS-CoV-2 infection promotes the activation of CK2 and p38/MAPK signaling pathways in Vero cells, leading to the production of various cytokines and the inhibition of the PI3K/Akt pathway, thus indicating that SARS-CoV-2 infection enhances autophagy (Bouhaddou et al., 2020).
Multiple proteins encoded by SARS-CoV-2 have been identified to modulate autophagy through diverse mechanisms. At the cellular level, the non-structural protein Nsp3 is capable of cleaving the autophagy initiator protein ULK1, thereby hindering the formation of the ULK1 complex and subsequently suppressing autophagy (Mohamud et al., 2021). Moreover, apart from inhibiting ULK1-mediated autophagy, the deubiquitination of Nsp3 may disrupt the cellular process of selective autophagy, ultimately fostering viral replication. Nsp6 functions to inhibit autophagy by interacting with VAMP7, ESYT2, ATP2A2, and TBK1, thereby suppressing the production of pre-autophagosomal structures (Kumar et al., 2021). Additionally, Nsp13 directly targets TBK1 and recruits it to P62 for autophagic degradation, thereby inhibiting type I IFN production (Sui et al., 2022). The ORF3a protein interacts with VPS39 and the HOPS complexes, inhibiting the fusion between autophagosomes and lysosomes, which subsequently impedes autophagic degradation. Notably, the accumulation of autophagy volume facilitates virus trafficking and excretion via lysosomal exocytosis (Chen et al., 2021). Conversely, during the autophagic degradation phase, ORF3a, ORF7a, and Nsp6 hinder autophagic flow by compromising lysosomal function, further suppressing autophagy (Miao et al., 2021).
The viral proteins of SARS-CoV-2 can also facilitate autophagy production. Specifically, the structural protein S binds to the receptor ACE2, stimulates ROS production, and inhibits the activation of the PI3K/Akt/mTOR pathway, thereby promoting autophagy (Li F. et al., 2021). Additionally, the structural protein E activates the ER pathway, leading to the phosphorylation of the translation initiation factor eIF-2α, which in turn promotes LC3 lipidation and induces autophagy (Waisner et al., 2023). The structural protein N interacts with LARP1 and inhibits the activation of the mTORC1 pathway, ultimately inducing autophagy (Gordon et al., 2020). ORF3a plays a dual role in autophagy, not only inhibiting autophagy production but also inducing it. This protein can interact with HMGB1 and induce RETREG1/FAM134B-mediated reticulophagy through the HMGB1-Beclin1 pathway (Zhang et al., 2022). Furthermore, the ORF8 protein induces the production of autophagic vesicles and degrades MHC-I via the Beclin1-mediated autophagy pathway (Zhang et al., 2021). Additionally, ORF8 interacts with the FKBP7 protein to inhibit the mTORC1 pathway, leading to the induction of autophagy (Gordon et al., 2020). Lastly, the overexpression of ORF10 promotes LC3 accumulation in mitochondria and induces mitochondrial autophagy by binding to the mitochondrial autophagy receptor NIX (Li et al., 2022).
Clinically, Ramachandran et al. observed that the viral proteins of SARS-CoV-2, including M, Nsp6, ORF3a, ORF9c, and ORF10, selectively target the endoplasmic reticulum and mitochondria, leading to autophagy in cardiomyocytes, suppression of mitochondrial function, disruption of intracellular Ca2+ homeostasis, decreased cell viability, and ultimately cellular death (Ramachandran et al., 2022). Li et al. uncovered through RNA sequencing analysis of peripheral blood mononuclear cells (PBMCs) from SARS-CoV-2-infected patients that the virus disrupts the expression of genes related to cellular stress responses (ER and HSF1) while also inducing autophagy-related genes. Notably, the virus-induced heat-shock response was intricately linked to autophagy (Li S. et al., 2021). Barbati et al. reported autophagy defects in PBMCs from SARS-CoV-2-infected patients, wherein the expression of LC3-II and p62 was significantly upregulated compared to normal cells. Moreover, the expression of these markers positively correlated with lymphocyte apoptosis and negatively correlated with lymphocyte counts, indicating that SARS-CoV-2-induced autophagy hijacking contributes to apoptosis (Barbati et al., 2022). Recently, LC3, SQSTM1, and BECN1 have emerged as potential new tools for differentiating patients with moderate to severe SARS-CoV-2 infection from asymptomatic individuals. Xia et al. found that patients with LC3 concentrations below 5.5 ng/mL should be hospitalized promptly for treatment (Fang et al., 2021). Conversely, BECN1 levels positively correlated with disease severity in SARS-CoV-2 patients, exhibiting significantly higher levels compared to the normal group, thus indicating its potential as a marker for assessing SARS-CoV-2 disease severity (Okuyan et al., 2021).
4.2 SARS-CoV
In 2003, China reported a devastating respiratory disease, known as Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV), which posed a significant threat to human health. By April 4th, 2003, a total of 2,353 cases had been documented, resulting in a mortality rate of approximately 4 per cent (Lee et al., 2003). SARS-CoV has been found to induce autophagy, and the viral proteins it encodes can modulate this process through diverse mechanisms. Wileman et al. discovered that the non-structural protein Nsp6 is capable of inducing LC3 to produce autophagosomes. Moreover, Nsp6 recruits the effector protein DFCP1 by upregulating the expression of PI3P, which subsequently facilitates the nucleation of detached membranes (Cottam et al., 2011). Remarkably, the diameter of Nsp6-induced autophagosomes is notably smaller compared to those induced by starvation (Cottam et al., 2014). Furthermore, research has demonstrated that SARS-CoV infection is associated with selective autophagy in mitochondria, and the ORF9b protein specifically induces autophagy through the classical autophagic pathway (Shi et al., 2014).
SARS-CoV infection disrupts the functionality of the endoplasmic reticulum, subsequently triggering an unfolded protein response (UPR). Notably, the S proteins encoded by SARS-CoV stimulate the activation of diverse UPR transcription factors, such as GRP78, GRP94, and C/EBP homologues (Chan et al., 2006). Additionally, the ORF3a protein plays a pivotal role in lysosomal membrane permeabilization, promoting the release of lysosomal tissue proteases, which leads to lysosomal impairment and dysfunction (Yue et al., 2018). Moreover, the lysine residue at position 77, which is dependent on the ORF8b protein, forms insoluble intracellular aggregates. These aggregated ORF8b proteins induce endoplasmic reticulum stress and lysosomal damage, ultimately enhancing autophagic flow through the nuclear translocation of TFEB (Shi et al., 2019).
4.3 MERS-CoV
In 2012, Saudi Arabia reported a highly pathogenic coronavirus known as the Middle East Respiratory Syndrome coronavirus (MERS-CoV), which is transmitted from dromedary camels to humans (De Wit et al., 2016). Kindrachuk et al. conducted a peptide kinome array and functional network analysis on human hepatocytes infected with MERS-CoV, revealing that MAPK/ERK1/2 and PI3K/Akt/mTOR signaling pathways were specifically regulated. This suggests that MERS-CoV infection may have implications on autophagy processes (Kindrachuk et al., 2015). Furthermore, Gassen et al. observed an accumulation of autophagosomes in Vero cells infected with MERS-CoV. Notably, the SKP2 protein promotes BECN1 ubiquitination, leading to proteasomal degradation of BECN1, thereby inhibiting the fusion of autophagosomes with lysosomes and ultimately impeding autophagy flow (Gassen et al., 2019). Additionally, the non-structural protein Nsp1 enhances ROS expression in cells, activating the MAPK pathway and inhibiting the mTOR pathway, thereby inducing autophagy (Feng et al., 2022). However, Nsp1 also inhibits lysosomal acidification, which further impedes autophagic flow (Feng et al., 2022).
4.4 MHV
Mouse hepatitis virus (MHV) pioneered as the first viral model to delve into the intricate correlation between coronaviruses and autophagy, specifically for investigating viral replication mechanisms and immune responses (de Haan et al., 2005). While Prentice et al. observed that MHV triggers autophagy and necessitates ATG5 to bolster viral replication (Prentice et al., 2004). Zhao et al.’s findings offer a contrasting perspective, revealing that MHV can replicate in BMM cells devoid of ATG5, thereby indicating that the autophagy process induced by MHV is not reliant on ATG5 (Zhao et al., 2007).
4.5 PHEV
Porcine hemagglutinating encephalomyelitis virus (PHEV), a virus that has the potential to impact the nervous and digestive systems of pigs, triggers symptoms such as vomiting, anorexia, profound lethargy, ataxia, and ultimately leads to the demise of infected pigs within 2-3 days of disease onset (Roe and Alexander, 1958). Li et al., utilizing neuroblastoma cells with ULK1 knockout, discovered that the formation of PHEV-induced autophagosomes does not necessitate the participation of ULK1, indicating that PHEV-induced autophagy represents a non-canonical process, independent of the AMPK-mTORC1-ULK1 signaling pathway (Li Z. et al., 2021). Ding et al. further revealed that PHEV infection in Neuro-2a cells induces autophagy, with the production being temporally dependent. However, PHEV infection impedes the fusion between autophagosomes and lysosomes, thereby inhibiting the progression of autophagic flux (Ding et al., 2017).
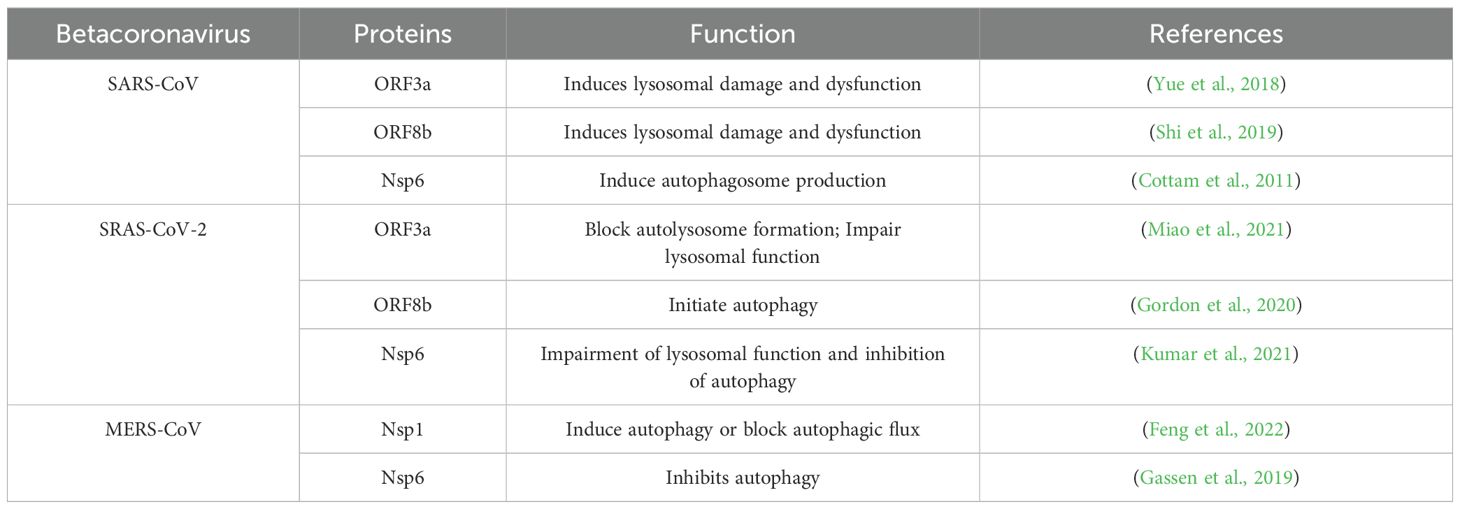
Since the outbreak of SARS in 2003, emerging coronaviruses have garnered extensive attention, with various coronavirus proteins exhibiting distinct mechanisms in activating or inhibiting autophagy processes, as illustrated in Table 2. Nevertheless, numerous coronaviruses remain understudied, resulting in a profound lack of comprehension regarding the intricacies of autophagy in the context of coronavirus infections and related pathologies. Consequently, the intricate relationship between autophagy and coronaviruses necessitates further exhaustive exploration.
5 γ-CoV
5.1 IBV
Infectious bronchitis virus (IBV), a coronavirus that targets avian species, poses a significant threat to the poultry industry, causing a reduction in the productivity of laying hens and broilers, resulting in considerable economic losses (Broadfoot et al., 1956). There is compelling evidence that IBV exhibits intricate interactions with autophagy. Cottam et al. uncovered that the non-structural protein Nsp6 triggers autophagosome formation and facilitates the fusion of autophagosomes with lysosomes via the activation of class III PI3K (Cottam et al., 2011). Concurrently, Majer et al. discovered that IBV stimulates the formation of autophagosomes, which, in turn, amplifies autophagic signaling in Vero cells (Maier et al., 2013).
6 δ-CoV
6.1 PDCoV
In 2012, a novel porcine deltacoronavirus, designated PDCoV, was initially documented in Hong Kong (He et al., 2020). Upon infection with PDCoV, the virus induced the formation of double membrane vesicles and rearrangements of vesicle membranes, with a notable increase in the number of autophagosome-like vesicles detected within the cytoplasm. These observations hint that PDCoV might induce autophagy (Qin et al., 2019). In a proteomics-based study, Zhou et al. examined PDCoV-infected porcine small intestinal epithelial cells (IPEC-J2) and discovered that autophagy-related signaling pathways, including PI3K-Akt and mTOR, were activated during the infection process. This suggests that PDCoV triggers autophagy in IPEC-J2 cells (Zhou X. et al., 2020). Additionally, PDCoV infection in LLC-PK1 cells activates the p38 signaling pathway, ultimately leading to complete autophagy, which facilitates viral replication (Duan et al., 2021).
7 Concluding remarks
To date, a total of eight human coronaviruses and 13 animal coronaviruses have been reported. Coronaviruses are capable of inducing cell death in infected cells via diverse pathways, including apoptosis, necrosis, and autophagy (Yue et al., 2018). Despite the existing literature on the interplay between viruses and autophagy, the intricate mechanism of how viruses harness autophagy to facilitate their replication remains to be comprehensively understood. Additionally, autophagy can significantly influence cellular biological processes, and autophagic cell death, as a mode of programmed cell death, not only directly contributes to cell demise but also interacts with necrosis, apoptosis, ferroptosis, and other modes of cell death to expedite cell mortality (Gao et al., 2016). Whether these biological processes induced by autophagy have an impact on the replication cycle of coronaviruses merits further investigation.
In this review, our primary focus is on the viral regulation of autophagy and the intricate mechanisms underlying autophagy-virus interactions. However, several challenges persist that hinder our ability to gain a deeper understanding of the nexus between autophagy and coronavirus infection, necessitating further exploration. Firstly, the current research landscape on coronaviruses and autophagy mechanisms lacks the requisite experimental conditions. Specifically, a significant portion of the experimental data, particularly those pertaining to highly pathogenic coronaviruses, have been derived primarily from cellular models, leaving room for debate on whether these findings align with the outcomes of in vivo infection studies. Secondly, the impact of autophagy on viral replication remains contentious, a complexity that may be attributed to variations in cell type, strain type, and the timing of infection. Lastly, whether coronaviruses can trigger other forms of autophagy, such as chaperone-mediated autophagy (CMA) and microautophagy, remains an open question that demands further scrutiny.
In the context of the interplay between SARS-CoV-2 and autophagy, a range of drugs that modulate autophagy have been developed to target SARS-CoV-2 infection (Min et al., 2020; Hui et al., 2021; Yuen et al., 2021). Notably, Chloroquine, which functions as an inhibitor of the autophagy pathway, has been proposed as a biological treatment option for SARS-CoV-2 infection. For animals, autophagy plays a regulatory role in growth, reproduction, and production performance, to a certain extent, contingent upon the timing and degree of autophagy induction (Tesseraud et al., 2021). Recent studies have revealed that specific autophagy-related components and vesicles play a crucial role in coronavirus infection and replication. Furthermore, considering the diverse expression levels of autophagy-related genes and the abundance of autophagic vesicles across different cell types, alternative and complementary pathways may potentially enable the resumption of infection and replication processes after autophagy inhibition (Sun et al., 2023). Notably, high levels of autophagy inhibition or activation can lead to various cellular dysfunctions, including excessive endoplasmic reticulum stress and acute inflammatory responses. These findings suggest that conventional autophagy inhibitors may not effectively hinder SARS-CoV-2 infection or alleviate acute inflammatory responses. Instead, combination therapy involving autophagy-related drugs and other medications may yield superior therapeutic outcomes. Specifically, targeting specific autophagy mechanisms or organelle components in combination with other drugs may represent a promising new direction for the development of autophagy-based treatments for COVID-19 in the future.
In summary, autophagy emerges as a potential target in the treatment of coronavirus infections, and a comprehensive understanding of its interactions with coronaviruses is crucial for future therapeutic advancements.
Author contributions
JY: Writing – original draft. SG: Writing – original draft. JL: Funding acquisition, Writing – review & editing. YZ: Software, Writing – original draft. JX: Investigation, Software, Writing – original draft. YW: Data curation, Writing – original draft. SL: Software, Writing – original draft. XY: Data curation, Writing – original draft. ZW: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Research and Development of Technology for Blocking, Prevention and Control of Major Exotic Animal Diseases (2022YFD1800500).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdul-Rasool, S., Fielding, B. C. (2010). Understanding human coronavirus HCoV-NL63. Open Virol. J. 4, 76. doi: 10.2174/1874357901004010076
Andrew, S. E. (2000). Feline infectious peritonitis. Vet. Clinics North America: Small Anim. Pract. 30, 987–1000. doi: 10.1016/s0195-5616(00)05002-6
Arabi, Y. M., Arifi, A. A., Balkhy, H. H., Najm, H., Aldawood, A. S., Ghabashi, A. (2014). Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Ann. Internal Med. 160, 389–397. doi: 10.7326/M13-2486
Arden, K. E., Arden, K. E., Nissen, M. D., Sloots, T. P., Mackay, I. M. (2005). New human coronavirus, HCoV-NL63, associated with severe lower respiratory tract disease in Australia. J. Med. Virol. 75, 455–462. doi: 10.1002/(ISSN)1096-9071
Barbati, C., Celia, A. I., Colasanti, T., Vomero, M. Speziali M., Putro, E., et al. (2022). Autophagy hijacking in PBMC from COVID-19 patients results in lymphopenia. Front. Immunol. 13, 903498. doi: 10.3389/fimmu.2022.903498
Barretto, N., Jukneliene, D., Ratia, K., Chen, Z., Mesecar, A. D., Baker, S. C., et al. (2005). The papain-like protease of severe acute respiratory syndrome coronavirus has deubiquitinating activity. J. Virol. 79, 15189–15198. doi: 10.1128/JVI.79.24.15189-15198.2005
Bouhaddou, M., Memon, D., Meyer, B., White, K. M., Rezelj, V. V., Marrero, M. C., et al. (2020). The global phosphorylation landscape of SARS-CoV-2 infection. Cell 182, 685–712. e19. doi: 10.1016/j.cell.2020.06.034
Broadfoot, D., Pomeroy, B., Smith, W., Jr (1956). Effects of infectious bronchitis in baby chicks. Poultry Sci. 35, 757–762. doi: 10.3382/ps.0350757
Chan, C.-P., Siu, K.-L., Chin, K.-T., Yuen, K.-Y., Zheng, B., Jin, D.-Y., et al. (2006). Modulation of the unfolded protein response by the severe acute respiratory syndrome coronavirus spike protein. J. Virol. 80, 9279–9287. doi: 10.1128/JVI.00659-06
Chan, J. F.-W., Yuan, S., Kok, K.-H., To, K. K.-W., Chu, H., Yang, J., et al. (2020). A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 395, 514–523. doi: 10.1016/S0140-6736(20)30154-9
Chang, C.-Y., Liu, H. M., Chang, M.-F., Chang, S. C. (2020). Middle East respiratory syndrome coronavirus nucleocapsid protein suppresses type I and type III interferon induction by targeting RIG-I signaling. J. Virol. 94. doi: 10.1128/jvi.00099-20
Chen, X., Wang, K., Xing, Y., Tu, J., Yang, X., Zhao, Q., et al. (2014). Coronavirus membrane-associated papain-like proteases induce autophagy through interacting with Beclin1 to negatively regulate antiviral innate immunity. Protein Cell 5, 912–927. doi: 10.1007/s13238-014-0104-6
Chen, J., Qi, T., Liu, L., Ling, Y., Qian, Z., et al. (2020). Clinical progression of patients with COVID-19 in Shanghai, China. J. Infect. 80, e1–e6. doi: 10.1016/j.jinf.2020.03.004
Chen, J., Fang, P., Wang, M., Peng, Q., Ren, J., Wang, D., et al. (2019). Porcine deltacoronavirus nucleocapsid protein antagonizes IFN-β production by impairing dsRNA and PACT binding to RIG-I. Virus Genes 55, 520–531. doi: 10.1007/s11262-019-01673-z
Chen, K., Xiao, F., Hu, D., Ge, W., Tian, M., Wang, W., et al. (2020). SARS-CoV-2 nucleocapsid protein interacts with RIG-I and represses RIG-mediated IFN-β production. Viruses 13, 47. doi: 10.3390/v13010047
Chen, D., Zheng, Q., Sun, L., Ji, M., Li, Y., Deng, H., et al. (2021). ORF3a of SARS-CoV-2 promotes lysosomal exocytosis-mediated viral egress. Dev. Cell 56, 3250–3263. e5. doi: 10.1016/j.devcel.2021.10.006
Chiu, S. S., Hung Chan, K., Wing Chu, K., Kwan, S, , W., Guan Poon, Y. Man L. L., et al. (2005). Human coronavirus NL63 infection and other coronavirus infections in children hospitalized with acute respiratory disease in Hong Kong, China. Clin. Infect. Dis. 40, 1721–1729. doi: 10.1086/430301
Choi, Y., Bowman, J. W., Jung, J. U. (2018). Autophagy during viral infection—a double-edged sword. Nat. Rev. Microbiol. 16, 341–354. doi: 10.1038/s41579-018-0003-6
Cook, J. K., Mockett, A. (1995). Epidemiology of infectious bronchitis virus. The Coronaviridae 1995, 317–335. doi: 10.1007/978-1-4899-1531-3_15
Cottam, E. M., Maier, H. J., Manifava, M., Vaux, L. C., Chandra-Schoenfelder, P., Gerner, W., et al. (2011). Coronavirus nsp6 proteins generate autophagosomes from the endoplasmic reticulum via an omegasome intermediate. Autophagy 7, 1335–1347. doi: 10.4161/auto.7.11.16642
Cottam, E. M., Whelband, M. C., Wileman, T. (2014). Coronavirus NSP6 restricts autophagosome expansion. Autophagy 10, 1426–1441. doi: 10.4161/auto.29309
Coussement, W., Ducatelle, R., Debouck, P., and Hoorens, J. (1982). Pathology of experimental CV777 coronavirus enteritis in piglets. I. Histological and histochemical study. Vet. Pathol. 19, 46–56. doi: 10.1177/030098588201900108
De Albuquerque, N., Baig, E., Ma, X., Zhang, J., He, W., Rowe, A., et al. (2006). MurineHepatitis virus strain 1 produces a clinically relevant model of severe acute respiratory syndrome in A/J mice. J. Virol. 80, 10382–10394. doi: 10.1128/JVI.00747-06
Decaro, N., Buonavoglia, C. (2008). An update on canine coronaviruses: viral evolution and pathobiology. Vet. Microbiol. 132, 221–234. doi: 10.1016/j.vetmic.2008.06.007
de Haan, C. A. M., Li, Z., te Lintelo, E., Bosch, B. J., Haijema, B. J., Rottier, P. J. M., et al. (2005). Murine coronavirus with an extended host range uses heparan sulfate as an entry receptor. J. Virol. 79, 14451–14456. doi: 10.1128/JVI.79.22.14451-14456.2005
De Wit, E., Van Doremalen, N., Falzarano, D., Munster, V. J. (2016). SARS and MERS: recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 14, 523–534. doi: 10.1038/nrmicro.2016.81
Ding, N., Zhao, K., Lan, Y., Li, Z., Lv, X., Su, J., et al. (2017). Induction of atypical autophagy by porcine hemagglutinating encephalomyelitis virus contributes to viral replication. Front. Cell. Infect. Microbiol. 7, 56. doi: 10.3389/fcimb.2017.00056
Doki, T., Yabe, M., Takano, T., Hohdatsu, T. (2018). Differential induction of type I interferon by type I and type II feline coronaviruses in vitro. Res. Vet. Sci. 120, 57–62. doi: 10.1016/j.rvsc.2018.09.002
Dominguez, S. R., Robinson, C. C., Holmes, K. V. (2009). Detection of four human coronaviruses in respiratory infections in children: a one-year study in Colorado. J. Med. Virol. 81, 1597–1604. doi: 10.1002/jmv.21541
Doyle, L., Hutchings, L. (1946). A transmissible gastroenteritis in pigs. J. Am. Vet. Med. Assoc. 108, 257–259.
Duan, C., Liu, Y., Hao, Z., Wang, J. (2021). Ergosterol peroxide suppresses porcine deltacoronavirus (PDCoV)-induced autophagy to inhibit virus replication via p38 signaling pathway. Vet. Microbiol. 257, 109068. doi: 10.1016/j.vetmic.2021.109068
Durham, P. J., Hassard, L. E., Armstrong, K. R., Naylor, J. M. (1989). Coronavirus-associated diarrhea (winter dysentery) in adult cattle. Can. Vet. J. 30, 825.
Dveksler, G. S., Pensiero, M. N., Dieffenbach, C. W., Cardellichio, C. B., Basile, A. A., Elia, P. E., et al. (1993). Mouse hepatitis virus strain A59 and blocking antireceptor monoclonal antibody bind to the N-terminal domain of cellular receptor. Proc. Natl. Acad. Sci. U.S.A. 90, 1716–1720. doi: 10.1073/pnas.90.5.1716
Fang, S., Zhang, L., Liu, Y., Xu, W., Wu, W., Huang, Z., et al. (2021). Lysosome activation in peripheral blood mononuclear cells and prognostic significance of circulating LC3B in COVID-19. Briefings Bioinf. 22, 1466–1475. doi: 10.1093/bib/bbab043
Fehr, A. R., Perlman, S. (2015). Coronaviruses: an overview of their replication and pathogenesis. Coronaviruses: Methods Protoc. 2015, 1–23. doi: 10.1007/978-1-4939-2438-7_1
Feng, Y., Pan, Z., Wang, Z., Lei, Z., Yang, S., Zhao, H., et al. (2022). MERS-CoV nsp1 regulates autophagic flux via mTOR signalling and dysfunctional lysosomes. Emerging Microbes Infect. 11, 2529–2543. doi: 10.1080/22221751.2022.2128434
Fielding, C., Higgins, J. K., Higgins, J. C., McIntosh, S., Scott, E., Giannitti, F., et al. (2015). Disease associated with equine coronavirus infection and high case fatality rate. J. Vet. Internal Med. 29, 307–310. doi: 10.1111/jvim.2015.29.issue-1
Ganapathy, K. (2009). Diagnosis of infectious bronchitis in chickens. In Pract. 31, 424–431. doi: 10.1136/inpract.31.9.424
Gao, M., Monian, P., Pan, Q., Zhang, W., Xiang, J., Jiang, X., et al. (2016). Ferroptosis is an autophagic cell death process. Cell Res. 26, 1021–1032. doi: 10.1038/cr.2016.95
Gao, B., Gong, X., Fang, S., Weng, W., Wang, H., Chu, H., et al. (2021). Inhibition of anti-viral stress granule formation by coronavirus endoribonuclease nsp15 ensures efficient virus replication. PloS Pathog. 17, e1008690. doi: 10.1371/journal.ppat.1008690
Gassen, N. C., Niemeyer, D., Muth, D., Corman, V. M., Martinelli, S., Gassen, A., et al. (2019). SKP2 attenuates autophagy through Beclin1-ubiquitination and its inhibition reduces MERS-Coronavirus infection. Nat. Commun. 10, 5770. doi: 10.1038/s41467-019-13659-4
Gassen, N. C., Papies, J., Bajaj, T., Emanuel, J., Dethloff, F., Chua, R. L., et al. (2021). SARS-CoV-2-mediated dysregulation of metabolism and autophagy uncovers host-targeting antivirals. Nat. Commun. 12, 3818. doi: 10.1038/s41467-021-24007-w
Giannitti, F., Diab, S., Mete, A., Stanton, J. B., Fielding, L, Crossley, B., et al. (2015). Necrotizing enteritis and hyperammonemic encephalopathy associated with equine coronavirus infection in equids. Vet. Pathol. 52, 1148–1156. doi: 10.1177/0300985814568683
Glick, D., Barth, S., Macleod, K. F. (2010). Autophagy: cellular and molecular mechanisms. J. Pathol. 221, 3–12. doi: 10.1002/path.v221:1
Gong, L., Li, J., Zhou, Q., Xu, Z., Chen, L., Zhang, Y., et al. (2017). A new bat-HKU2–like coronavirus in swine, China, 2017. Emerging Infect. Dis. 23, 1607. doi: 10.3201/eid2309.170915
Gorbalenya, A. E., Snijder, E. J., Spaan, W. J. (2004). Severe acute respiratory syndrome coronavirus phylogeny: toward consensus. J. Virol. 78, 7863–7866. doi: 10.1128/JVI.78.15.7863-7866.2004
Gordon, D. E., Jang, G. M., Bouhaddou, M., Xu, J., Obernier, K., White, K. M., et al. (2020). A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 583, 459–468. doi: 10.1038/s41586-020-2286-9
Greig, A., Girard, A. (1969). Serological comparison of hemagglutinating encephalomyelitis viruses isolated from different outbreaks. Can. J. Comp. Med. 33, 25.
Guo, X., Hu, H., Chen, F., Li, Z., Ye, S., Cheng, S., et al. (2016). iTRAQ-based comparative proteomic analysis of Vero cells infected with virulent and CV777 vaccine strain-like strains of porcine epidemic diarrhea virus. J. Proteomics 130, 65–75. doi: 10.1016/j.jprot.2015.09.002
Guo, L., Yu, H., Gu, W., Luo, X., Li, R., Zhang, J., et al. (2016). Autophagy negatively regulates transmissible gastroenteritis virus replication. Sci. Rep. 6, 23864. doi: 10.1038/srep23864
Guo, X., Zhang, M., Zhang, X., Tan, X., Guo, H., Zeng, W., et al. (2017). Porcine epidemic diarrhea virus induces autophagy to benefit its replication. Viruses 9, 53. doi: 10.3390/v9030053
Guy, J. S. (2000). Turkey coronavirus is more closely related to avian infectious bronchitis virus than to mammalian coronaviruses: a review. Avian Pathol. 29, 207–212. doi: 10.1080/03079450050045459
Hamre, D., Procknow, J. J. (1966). A new virus isolated from the human respiratory tract. Proc. Soc. Exp. Biol. Med. 121, 190–193. doi: 10.3181/00379727-121-30734
Haring, J., Perlman, S. (2001). Mouse hepatitis virus. Curr. Opin. Microbiol. 4, 462–466. doi: 10.1016/S1369-5274(00)00236-8
Hartenian, E., Nandakumar, D., Lari, A., Ly, M., Tucker, J. M., Glaunsinger, B. A., et al. (2020). The molecular virology of coronaviruses. J. Biol. Chem. 295, 12910–12934. doi: 10.1074/jbc.REV120.013930
He, W.-T., Ji, X., He, W., Dellicour, S., Wang, S., Li, G., et al. (2020). Genomic epidemiology, evolution, and transmission dynamics of porcine deltacoronavirus. Mol. Biol. Evol. 37, 2641–2654. doi: 10.1093/molbev/msaa117
Hendley, J. O., Fishburne, H. B., Gwaltney, J. M., Jr (1972). Coronavirus infections in working adults: eight-year study with 229 E and OC 43. Am. Rev. Respir. Dis. 105, 805–811.
Huang, C., Wang, Y., Li, X., Ren, L., Zhao, J., Hu, Y., et al. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395, 497–506. doi: 10.1016/S0140-6736(20)30183-5
Hui, X., Zhang, L., Cao, L., Huang, K., Zhao, Y., Zhang, Y., et al. (2021). SARS-CoV-2 promote autophagy to suppress type I interferon response. Signal Transduct. Targeted Ther. 6, 180. doi: 10.1038/s41392-021-00574-8
Imbert, I., Snijder, E. J., Dimitrova, M., Guillemot, J.-C., Lécine, P., Canard, B., et al. (2008). The SARS-Coronavirus PLnc domain of nsp3 as a replication/transcription scaffolding protein. Virus Res. 133, 136–148. doi: 10.1016/j.virusres.2007.11.017
Ismail, M., Tang, Y., Saif, Y. (2003). Pathogenicity of Turkey coronavirus in Turkeys and chickens. Avian Dis. 47, 515–522. doi: 10.1637/5917
Jackson, W. T. (2015). Viruses and the autophagy pathway. Virology 479, 450–456. doi: 10.1016/j.virol.2015.03.042
Janetanakit, T., Lumyai, M., Bunpapong, N., Boonyapisitsopa, S., Chaiyawong, S., Nonthabenjawan, N., et al. (2016). Porcine deltacoronavirus, Thailand, 2015. Emerging Infect. Dis. 22, 757. doi: 10.3201/eid2204.151852
Kindrachuk, J., Ork, B., Mazur, S., Holbrook, M. R., Frieman, M. B., Traynor, D., et al. (2015). Antiviral potential of ERK/MAPK and PI3K/AKT/mTOR signaling modulation for Middle East respiratory syndrome coronavirus infection as identified by temporal kinome analysis. Antimicrobial Agents Chemother. 59, 1088–1099. doi: 10.1128/AAC.03659-14
Kirisako, T., Baba, M., Ishihara, N., Miyazawa, K., Ohsumi, M., Yoshimori, T., et al. (1999). Formation process of autophagosome is traced with Apg8/Aut7p in yeast. J. Cell Biol. 147, 435–446. doi: 10.1083/jcb.147.2.435
Kirisako, T., Ichimura, Y., Okada, H., Kabeya, Y., Mizushima, N., Yoshimori, T., et al. (2000). The reversible modification regulates the membrane-binding state of Apg8/Aut7 essential for autophagy and the cytoplasm to vacuole targeting pathway. J. Cell Biol. 151, 263–276. doi: 10.1083/jcb.151.2.263
Klionsky, D. J., Abdel-Aziz, A. K., Abdelfatah, S., Abdellatif, M., Abdoli, A., Abel, S., et al. (2021). Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 17, 1–382. doi: 10.1080/15548627.2020.1797280
Knoops, K., Kikkert, M., van den Worm, S. H. E., Zevenhoven-Dobbe, J. C., Van Der Meer, Y., Koster, A. J., et al. (2008). SARS-coronavirus replication is supported by a reticulovesicular network of modified endoplasmic reticulum. PloS Biol. 6, e226. doi: 10.1371/journal.pbio.0060226
Ko, S., Gu, M. J., Kim, C. G., Kye, Y. C., Lim, Y., Lee, J. E., et al. (2017). Rapamycin-induced autophagy restricts porcine epidemic diarrhea virus infectivity in porcine intestinal epithelial cells. Antiviral Res. 146, 86–95. doi: 10.1016/j.antiviral.2017.08.010
Kong, N., Shan, T., Wang, H., Jiao, Y., Zuo, Y., Li, L., et al. (2020). BST2 suppresses porcine epidemic diarrhea virus replication by targeting and degrading virus nucleocapsid protein with selective autophagy. Autophagy 16, 1737–1752. doi: 10.1080/15548627.2019.1707487
Kruiningen, H. V., Hiestand, L., Hill, D. L., Tilton, R. C., Ryan, R. W. (1985). Winter dysentery in dairy cattle: recent findings.
Kumar, S., Javed, R., Mudd, M., Pallikkuth, S., Lidke, K. A., Jain, A., et al. (2021). Mammalian hybrid pre-autophagosomal structure HyPAS generates autophagosomes. Cell 184, 5950–5969. e22. doi: 10.1016/j.cell.2021.10.017
Lavi, E., Gilden, D. H., Highkin, M. K., Weiss, S. R. (1986). The organ tropism of mouse hepatitis virus A59 in mice is dependent on dose and route of inoculation. Lab. Anim. Sci. 36, 130–135.
Lednicky, J. A., Tagliamonte, M. S., White, S. K., Elbadry, M. A., Alam, M. M., Stephenson, C. J., et al. (2021). Independent infections of porcine deltacoronavirus among Haitian children. Nature 600, 133–137. doi: 10.1038/s41586-021-04111-z
Lee, N., Hui, D., Wu, A., Chan, P., Cameron, P., Joynt, G. M., et al. (2003). A major outbreak of severe acute respiratory syndrome in Hong Kong. New Engl. J. Med. 348, 1986–1994. doi: 10.1056/NEJMoa030685
Lennemann, N. J., Coyne, C. B. (2015). Catch me if you can: the link between autophagy and viruses. PloS Pathog. 11, e1004685. doi: 10.1371/journal.ppat.1004685
Levine, B. (2005). Eating oneself and uninvited guests: autophagy-related pathways in cellular defense. Cell 120, 159–162. doi: 10.1016/j.cell.2005.01.005
Levine, B., Kroemer, G. (2019). Biological functions of autophagy genes: a disease perspective. Cell 176, 11–42. doi: 10.1016/j.cell.2018.09.048
Levy, J. M. M., Towers, C. G., Thorburn, A. (2017). Targeting autophagy in cancer. Nat. Rev. Cancer 17, 528–542. doi: 10.1038/nrc.2017.53
Li, W., Moore, M. J., Vasilieva, N., Sui, J., Wong, S. K., Berne, M. A., et al. (2003). Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 426, 450–454. doi: 10.1038/nature02145
Li, F., Li, W., Farzan, M., Harrison, S. C. (2005). Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science 309, 1864–1868. doi: 10.1126/science.1116480
Li, G., Chen, Q., Harmon, K. M., Yoon, K.-J., Schwartz, K. J., Hoogland, M. J., et al. (2014). Full-length genome sequence of porcine deltacoronavirus strain USA/IA/2014/8734. Genome Announcements 2, e00278-14. doi: 10.1128/genomea.00278-14
Li, W., van Kuppeveld, F. J. M., He, Q., Rottier, P. J. M., Bosch, B.-J. (2016). Cellular entry of the porcine epidemic diarrhea virus. Virus Res. 226, 117–127. doi: 10.1016/j.virusres.2016.05.031
Li, C., Li, W., Lucio de Esesarte, E., Guo, H., van den Elzen, P., Aarts, E., et al. (2017). Cell attachment domains of the porcine epidemic diarrhea virus spike protein are key targets of neutralizing antibodies. J. Virol. 91, e00273-17. doi: 10.1128/JVI.00273-17
Li, J., Gong, X., Wang, Z., Chen, R., Li, T., Zeng, D., et al. (2020). Clinical features of familial clustering in patients infected with 2019 novel coronavirus in Wuhan, China. Virus Res. 286, 198043. doi: 10.1016/j.virusres.2020.198043
Li, F., Li, J., Wang, P.-H., Yang, N., Huang, J., Ou, J., et al. (2021). SARS-CoV-2 spike promotes inflammation and apoptosis through autophagy by ROS-suppressed PI3K/AKT/mTOR signaling. Biochim. Biophys. Acta (BBA)-Molecular Basis Dis. 1867, 166260. doi: 10.1016/j.bbadis.2021.166260
Li, S., Liu, W., Chen, Y., Wang, L., An, W., An, X., et al. (2021). Transcriptome analysis of cepharanthine against a SARS-CoV-2-related coronavirus. Briefings Bioinf. 22, 1378–1386. doi: 10.1093/bib/bbaa387
Li, Z., Gao, F., Lan, Y., Guan, J., Zhang, J., Lu, H., et al. (2021). Porcine Hemagglutinating encephalomyelitis virus triggers neural autophagy independently of ULK1. J. Virol. 95. doi: 10.1128/jvi.00851-21
Li, X., Hou, P., Ma, W., Wang, X., Wang, H., Yu, Z., et al. (2022). SARS-CoV-2 ORF10 suppresses the antiviral innate immune response by degrading MAVS through mitophagy. Cell. Mol. Immunol. 19, 67–78. doi: 10.1038/s41423-021-00807-4
Lin, M.-H., Chuang, S.-J., Chen, C.-C., Cheng, S.-C., Cheng, K.-W., Lin, C.-H., et al. (2014). Structural and functional characterization of MERS coronavirus papain-like protease. J. Biomed. Sci. 21, 1–8. doi: 10.1186/1423-0127-21-54
Lin, H., Li, B., Liu, M., Zhou, H., He, K., Fan, H., et al. (2020). Nonstructural protein 6 of porcine epidemic diarrhea virus induces autophagy to promote viral replication via the PI3K/Akt/mTOR axis. Vet. Microbiol. 244, 108684. doi: 10.1016/j.vetmic.2020.108684
Liu, C., Tang, J., Ma, Y., Liang, X.Yang Y., Peng, G., et al. (2015). Receptor usage and cell entry of porcine epidemic diarrhea coronavirus. J. Virol. 89, 6121–6125. doi: 10.1128/JVI.00430-15
Liu, D. X., Liang, J. Q., Fung, T. S. (2021). Human coronavirus-229E,-OC43,-NL63, and-HKU1 (Coronaviridae). Encyclopedia Virol. p, 428. doi: 10.1016/B978-0-12-809633-8.21501-X
Lorbach, J. N., Wang, L., Nolting, J. M., Benjamin, M. G., Killian, M. L., Zhang, Y., et al. (2017). Porcine hemagglutinating encephalomyelitis virus and respiratory disease in exhibition swine, Michigan, USA, 2015. Emerging Infect. Dis. 23, 1168. doi: 10.3201/eid2307.170019
Maier, H. J., Cottam, E. M., Stevenson-Leggett, P., Wilkinson, J. A., Harte, C. J., Wileman, T., et al. (2013). Visualizing the autophagy pathway in avian cells and its application to studying infectious bronchitis virus. Autophagy 9, 496–509. doi: 10.4161/auto.23465
Masters, P. S. (2006). The molecular biology of coronaviruses. Adv. Virus Res. 66, 193–292. doi: 10.1016/S0065-3527(06)66005-3
Miao, G., Zhao, H., Li, Y., Ji, M., Chen, Y., Shi, Y., et al. (2021). ORF3a of the COVID-19 virus SARS-CoV-2 blocks HOPS complex-mediated assembly of the SNARE complex required for autolysosome formation. Dev. Cell 56, 427–442. e5. doi: 10.1016/j.devcel.2020.12.010
Mihindukulasuriya, K. A., Wu, G., St. Leger, J., Nordhausen, R. W., Wang, D. (2008). Identification of a novel coronavirus from a beluga whale by using a panviral microarray. J. Virol. 82, 5084–5088. doi: 10.1128/JVI.02722-07
Min, J. S., Kim, D. E., Jin, Y.-H., Kwon, S. (2020). Kurarinone inhibits HCoV-OC43 infection by impairing the virus-induced autophagic flux in MRC-5 human lung cells. J. Clin. Med. 9, 2230. doi: 10.3390/jcm9072230
Mizushima, N., Yoshimori, T., Ohsumi, Y. (2011). The role of Atg proteins in autophagosome formation. Annu. Rev. Cell Dev. Biol. 27, 107–132. doi: 10.1146/annurev-cellbio-092910-154005
Mohamud, Y., Xue, Y. C., Liu, H., Ng, C. S., Bahreyni, A., Jan, E., et al. (2021). The papain-like protease of coronaviruses cleaves ULK1 to disrupt host autophagy. Biochem. Biophys. Res. Commun. 540, 75–82. doi: 10.1016/j.bbrc.2020.12.091
Mora-Díaz, J. C., Piñeyro, P. E., Houston, E., Zimmerman, J., Giménez-Lirola, L. G. (2019). Porcine hemagglutinating encephalomyelitis virus: a review. Front. Vet. Sci. 6, 53. doi: 10.3389/fvets.2019.00053
Nakagawa, K., Makino, S. (2021). Mechanisms of coronavirus nsp1-mediated control of host and viral gene expression. Cells 10, 300. doi: 10.3390/cells10020300
Neuman, B. W., Chamberlain, P., Bowden, F., Joseph, J. (2014). Atlas of coronavirus replicase structure. Virus Res. 194, 49–66. doi: 10.1016/j.virusres.2013.12.004
Nieto-Torres, J. L., DeDiego, M. L., Álvarez, E., Jiménez-Guardeño, J. M., Regla-Nava, J. A., Llorente, M., et al. (2011). Subcellular location and topology of severe acute respiratory syndrome coronavirus envelope protein. Virology 415, 69–82. doi: 10.1016/j.virol.2011.03.029
Okuyan, H. M., Dogan, S., Bal, T., Çabalak, M. (2021). Beclin-1, an autophagy-related protein, is associated with the disease severity of COVID-19. Life Sci. 278, 119596. doi: 10.1016/j.lfs.2021.119596
Oudshoorn, D., Rijs, K., Limpens, R. W. A. L., Groen, K., Koster, A. J., Snijder, E. J., et al. (2017). Expression and cleavage of middle east respiratory syndrome coronavirus nsp3-4 polyprotein induce the formation of double-membrane vesicles that mimic those associated with coronaviral RNA replication. MBio 8, e01658-17-17. doi: 10.1128/mbio.01658-17
Pan, Y., Tian, X., Qin, P., Wang, B., Zhao, P., Yang, Y.-L., et al. (2017). Discovery of a novel swine enteric alphacoronavirus (SeACoV) in southern China. Vet. Microbiol. 211, 15–21. doi: 10.1016/j.vetmic.2017.09.020
Park, J.-E., Park, E.-S., Yu, J.-E., Rho, J., Paudel, S., Hyun, B.-H., et al. (2015). Development of transgenic mouse model expressing porcine aminopeptidase N and its susceptibility to porcine epidemic diarrhea virus. Virus Res. 197, 108–115. doi: 10.1016/j.virusres.2014.12.024
Peiris, J. S. M., Chu, C.-M., Cheng, V. C.-C., Chan, K. S., Hung, I. F. N., Poon, L. L. M., et al. (2003). Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet 361, 1767–1772. doi: 10.1016/S0140-6736(03)13412-5
Prentice, E., Jerome, W. G., Yoshimori, T., Mizushima, N., Denison, M. R. (2004). Coronavirus replication complex formation utilizes components of cellular autophagy. J. Biol. Chem. 279, 10136–10141. doi: 10.1074/jbc.M306124200
Pusterla, N., Mapes, S., Wademan, C., White, A., Ball, R., Sapp, K., et al. (2013). Emerging outbreaks associated with equine coronavirus in adult horses. Vet. Microbiol. 162, 228–231. doi: 10.1016/j.vetmic.2012.10.014
Qin, P., Du, E.-Z., Luo, W.-T., Yang, Y.-L., Zhang, Y.-Q., Wang, B., et al. (2019). Characteristics of the life cycle of porcine deltacoronavirus (PDCoV) in vitro: replication kinetics, cellular ultrastructure and virion morphology, and evidence of inducing autophagy. Viruses 11, 455. doi: 10.3390/v11050455
Ramachandran, K., Maity, S., Muthukumar, A. R., Kandala, S., Tomar, D., Abd El-Aziz, T. M., et al. (2022). SARS-CoV-2 infection enhances mitochondrial PTP complex activity to perturb cardiac energetics. IScience 25, 103722. doi: 10.1016/j.isci.2021.103722
Rho, S., Moon, H. J., Park, S. J., Kim, H. K., Keum, H. O., Han, J. Y., et al. (2011). Detection and genetic analysis of porcine hemagglutinating encephalomyelitis virus in South Korea. Virus Genes 42, 90–96. doi: 10.1007/s11262-010-0551-y
Roe, C., Alexander, T. (1958). A disease of nursing pigs previously unreported in Ontario. Can. J. Comp. Med. Vet. Sci. 22, 305.
Rubinsztein, D. C., Mariño, G., Kroemer, G. (2011). Autophagy and aging. Cell 146, 682–695. doi: 10.1016/j.cell.2011.07.030
Schoeman, D., Fielding, B. C. (2019). Coronavirus envelope protein: current knowledge. Virol. J. 16, 1–22. doi: 10.1186/s12985-019-1182-0
Sharma, M., Bhattacharyya, S., Nain, M., Kaur, M., Sood, V., Gupta, V., et al. (2014). Japanese encephalitis virus replication is negatively regulated by autophagy and occurs on LC3-I-and EDEM1-containing membranes. Autophagy 10, 1637–1651. doi: 10.4161/auto.29455
Shi, C.-S., Qi, H.-Y., Boularan, C., Huang, N.-N., Abu-Asab, M., Shelhamer, J. H., et al. (2014). SARS-coronavirus open reading frame-9b suppresses innate immunity by targeting mitochondria and the MAVS/TRAF3/TRAF6 signalosome. J. Immunol. 193, 3080–3089. doi: 10.4049/jimmunol.1303196
Shi, C.-S., Nabar, N. R., Huang, N.-N., Kehrl, J. H. (2019). SARS-Coronavirus Open Reading Frame-8b triggers intracellular stress pathways and activates NLRP3 inflammasomes. Cell Death Discovery 5, 101. doi: 10.1038/s41420-019-0181-7
Shi, D., Zhou, L., Shi, H., Zhang, J., Zhang, J., Zhang, L., et al. (2023). Autophagy is induced by swine acute diarrhea syndrome coronavirus through the cellular IRE1-JNK-Beclin 1 signaling pathway after an interaction of viral membrane-associated papain-like protease and GRP78. PloS Pathog. 19, e1011201. doi: 10.1371/journal.ppat.1011201
Song, D., Zhou, X, Peng, Q., Chen, Y., Zhang, F., Huang, T., et al. (2015). Newly emerged porcine deltacoronavirus associated with diarrhoea in swine in China: Identification, prevalence and full-length genome sequence analysis. Transboundary Emerging Dis. 62, 575–580. doi: 10.1111/tbed.2015.62.issue-6
Sui, C., Xiao, T., Zhang, S., Zeng, H., Zheng, Y., Liu, B., et al. (2022). SARS-CoV-2 NSP13 inhibits type I IFN production by degradation of TBK1 via p62-dependent selective autophagy. J. Immunol. 208, 753–761. doi: 10.4049/jimmunol.2100684
Sun, P., Zhang, S., Qin, X., Chang, X., Cui, X., Li, H., et al. (2018). Foot-and-mouth disease virus capsid protein VP2 activates the cellular EIF2S1-ATF4 pathway and induces autophagy via HSPB1. Autophagy 14, 336–346. doi: 10.1080/15548627.2017.1405187
Sun, Q., Li, X., Kuang, E. (2023). Subversion of autophagy machinery and organelle-specific autophagy by SARS-CoV-2 and coronaviruses. Autophagy 19, 1055–1069. doi: 10.1080/15548627.2022.2116677
Tee, A. K., Oh, H. M. L., Hui, K. P., Lien, C. T. C., Narendran, K., Heng, B. H., et al. (2004). Atypical SARS in geriatric patient. Emerging Infect. Dis. 10, 261. doi: 10.3201/eid1002.030322
Tennant, B., Gaskell, R. M., Kelly, D. F., Carter, S. D., Gaskell, C. J. (1991). Canine coronavirus infection in the dog following oronasal inoculation. Res. Vet. Sci. 51, 11–18. doi: 10.1016/0034-5288(91)90023-H
Tesseraud, S., Avril, P., Bonnet, M., Bonnieu, A., Cassar-Malek, I., Chabi, B., et al. (2021). Autophagy in farm animals: current knowledge and future challenges. Autophagy 17, 1809–1827. doi: 10.1080/15548627.2020.1798064
Turlewicz-Podbielska, H., Pomorska-Mól, M. (2021). Porcine coronaviruses: overview of the state of the art. Virologica Sin. 36, 833–851. doi: 10.1007/s12250-021-00364-0
Tusell, S. M., Schittone, S. A., Holmes, K. V. (2007). Mutational analysis of aminopeptidase N, a receptor for several group 1 coronaviruses, identifies key determinants of viral host range. J. Virol. 81, 1261–1273. doi: 10.1128/JVI.01510-06
Vabret, A., Mourez, T., Gouarin, S., Petitjean, J., Freymuth, F. (2003). An outbreak of coronavirus OC43 respiratory infection in Normandy, France. Clin. Infect. Dis. 36, 985–989. doi: 10.1086/cid.2003.36.issue-8
Van Boheemen, S., De Graaf, M., Lauber, C., Bestebroer, T. M., Raj, V. S., Zaki, A. M., et al. (2012). Genomic characterization of a newly discovered coronavirus associated with acute respiratory distress syndrome in humans. MBio 3. doi: 10.1128/mbio.00473-12
van der Hoek, L., Sure, K., Ihorst, G., Stang, A., Pyrc, K., Jebbink, M. F., et al. (2005). Croup is associated with the novel coronavirus NL63. PloS Med. 2, e240. doi: 10.1371/journal.pmed.0020240
Van Nieuwstadt, A., Zetstra, T., Boonstra, J. (1989). Infection with porcine respiratory coronavirus does not fully protect pigs against intestinal transmissible gastroenteritis virus. Vet. Rec. 125, 58–60. doi: 10.1136/vr.125.3.58
Waisner, H., Grieshaber, B., Saud, R., Henke, W., Stephens, E. B., Kalamvoki, M., et al. (2023). SARS-CoV-2 harnesses host translational shutoff and autophagy to optimize virus yields: The role of the envelope (E) protein. Microbiol. Spectr. 11, e03707-22. doi: 10.1128/spectrum.03707-22
Wang, R., Zhu, Y., Zhao, J., Ren, C., Li, P., Chen, H., et al. (2019). Autophagy promotes replication of influenza A virus in vitro. J. Virol. 93, 10.1128/jvi.01984-18. doi: 10.1128/jvi.01984-18
Wang, Y., Grunewald, M., Perlman, S. (2020). Coronaviruses: an updated overview of their replication and pathogenesis. Coronaviruses: Methods Protoc. 2020, 1–29. doi: 10.1007/978-1-0716-0900-2_1
Wei, Y., Liu, M., Li, X., Liu, J., Li, H. (2018). Origin of the autophagosome membrane in mammals. BioMed. Res. Int. 2018, 1-9. doi: 10.1155/2018/1012789
Wenzel, R. P., Hendley, J. O., Davies, J. A., Gwaltney Jr, J. M. (1974). Coronavirus infections in military recruits: three-year study with coronavirus strains OC43 and 229E. Am. Rev. Respir. Dis. 109, 621–624.
Wong, K. T., Antonio, G. E., Hui, D. S. C., Lee, N., Yuen, E. H. Y., Wu, A., et al. (2003). Thin-section CT of severe acute respiratory syndrome: evaluation of 73 patients exposed to or with the disease. Radiology 228, 395–400. doi: 10.1148/radiol.2283030541
Woo, P. C. Y., Lau, S. K. P., Chu, C.-m., Chan, K.-h., Tsoi, H.-w., Huang, Y., et al. (2005a). Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J. Virol. 79, 884–895. doi: 10.1128/JVI.79.2.884-895.2005
Woo, P. C., Lau, S. K., Tsoi, H. W., Huang, Y., Poon, R. W., Chu, C. M., et al. (2005b). Clinical and molecular epidemiological features of coronavirus HKU1–associated community-acquired pneumonia. J. Infect. Dis. 192, 1898–1907. doi: 10.1086/jid.2005.192.issue-11
Woo, P. C. Y., Lau, S. K. P., Yip, C. C. Y., Huang, Y., Yuen, K.-Y. (2009). More and more coronaviruses: human coronavirus HKU1. Viruses 1, 57–71. doi: 10.3390/v1010057
Woo, P. C. Y., Huang, Y., Lau., S. K. P., Yuen, K.-Y. (2010). Coronavirus genomics and bioinformatics analysis. Viruses 2, 1804–1820. doi: 10.3390/v2081803
Woo, P. C. Y., Lau, S. K. P., Lam, C. S.F., Lau, C. C. Y., Tsang, A. K. L., Lau, J. H. N., et al. (2012). Discovery of seven novel Mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. J. Virol. 86, 3995–4008. doi: 10.1128/JVI.06540-11
Wood, E. (1977). An apparently new syndrome of porcine epidemic diarrhoea. Vet. Rec. 1977, 243-244. doi: 10.1136/vr.100.12.243
Woode, G., Bridger, J., Meyling, A. (1978). Significance of bovine coronavirus infection. Veterinary Record. 102, 15-16. doi: 10.1136/vr.102.1.15
Wu, Z., McGoogan, J. M. (2020). Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. jama 323, 1239–1242. doi: 10.1001/jama.2020.2648
Xia, L., Yang, Y., Wang, J., Jing, Y., Yang, Q. (2018). Impact of TGEV infection on the pig small intestine. Virol. J. 15, 1–7. doi: 10.1186/s12985-018-1012-9
Xue, W., Ding, C., Qian, K., Liao, Y. (2022). The interplay between coronavirus and type I IFN response. Front. Microbiol. 12, 805472. doi: 10.3389/fmicb.2021.805472
Yang, X., Chen, X., Bian, G., Tu, J., Xing, Y., Wang, Y., et al. (2014). Proteolytic processing, deubiquitinase and interferon antagonist activities of Middle East respiratory syndrome coronavirus papain-like protease. J. Gen. Virol. 95, 614–626. doi: 10.1099/vir.0.059014-0
Yang, Y., Klionsky, D. J. (2020). Autophagy and disease: unanswered questions. Cell Death Differentiation 27, 858–871. doi: 10.1038/s41418-019-0480-9
Yin, H.-C., Shao, S.-l., Jiang, X.-j., Xie, P.-y., Sun, W.-s., Yu, T.-f., et al. (2019). Interactions between autophagy and DNA viruses. Viruses 11, 776. doi: 10.3390/v11090776
Yu, J., Chen, P., Liu, R., Lao, M., Zhu, J., Zhou, S., et al. (2023). Newly characterized porcine epidemic diarrhea virus GII subtype strain. Transboundary Emerging Dis. 2023, 14. doi: 10.1155/2023/5544724
Yue, Y., Nabar, N. R., Shi, C.-S., Kamenyeva, O., Xiao, X., Hwang, I.-Y., et al. (2018). SARS-Coronavirus Open Reading Frame-3a drives multimodal necrotic cell death. Cell Death Dis. 9, 904. doi: 10.1038/s41419-018-0917-y
Yuen, C.-K., Wong, W.‐M., Mak, L.‐F., Wang, X., Chu, H., Yuen, K.‐Y., et al. (2021). Suppression of SARS-CoV-2 infection in ex-vivo human lung tissues by targeting class III phosphoinositide 3-kinase. J. Med. Virol. 93, 2076–2083. doi: 10.1002/jmv.26583
Zeng, S., Peng, O., Sun, R., Xu, Q., Hu, F., Zhao, Y., et al. (2021). Transcriptional landscape of vero E6 cells during early swine acute diarrhea syndrome coronavirus infection. Viruses 13, 674. doi: 10.3390/v13040674
Zeng, S., Zhao, Y, Peng, O, Xia, Y., Xu, Q., Li, H., et al. (2022). Swine acute diarrhea syndrome coronavirus induces autophagy to promote its replication via the Akt/mTOR pathway. Iscience 25. doi: 10.1016/j.isci.2022.105394
Zhang, Y., Chen, Y., Li, Y., Huang, F., Luo, B., Yuan, Y., et al. (2021). The ORF8 protein of SARS-CoV-2 mediates immune evasion through down-regulating MHC-I. Proc. Natl. Acad. Sci. 118, e2024202118. doi: 10.1073/pnas.2024202118
Zhang, X., Yang, Z., Pan, T., Long, X., Sun, Q., Wang, P.-H., et al. (2022). SARS-CoV-2 ORF3a induces RETREG1/FAM134B-dependent reticulophagy and triggers sequential ER stress and inflammatory responses during SARS-CoV-2 infection. Autophagy 18, 2576–2592. doi: 10.1080/15548627.2022.2039992
Zhang, Q., Yoo, D. (2016). Immune evasion of porcine enteric coronaviruses and viral modulation of antiviral innate signaling. Virus Research. 226, 128-141. doi: 10.1016/j.virusres.2016.05.015
Zhao, Z., Thackray, L. B., Miller, B. C., Lynn, T. M., Becker, M. M., Ward, E., et al. (2007). Coronavirus replication does not require the autophagy gene ATG5. Autophagy 3, 581–585. doi: 10.4161/auto.4782
Zheng, J. (2020). SARS-CoV-2: an emerging coronavirus that causes a global threat. Int. J. Biol. Sci. 16, 1678. doi: 10.7150/ijbs.45053
Zhou, P., Yang, X.-L., Wang, X.-G., Hu, B., Zhang, L., Zhang, W., et al. (2020). A pneumonia outbreak associated with a new coronavirus of probable bat origin 579, 7798, 270–273. doi: 10.1038/s41586-020-2012-7
Zhou, X., Zhou, L., Ge, X., Guo, X., Han, J., Zhang, Y., et al. (2020). Quantitative proteomic analysis of porcine intestinal epithelial cells infected with porcine deltacoronavirus using iTRAQ-coupled LC-MS/MS. J. Proteome Res. 19, 4470–4485. doi: 10.1021/acs.jproteome.0c00592
Zhu, L., Mou, C., Yang, X., Lin, J., Yang, Q. (2016). Mitophagy in TGEV infection counteracts oxidative stress and apoptosis. Oncotarget 7, 27122. doi: 10.18632/oncotarget.v7i19
Zhu, N., Zhang, D., Wang, W., Li, X., Yang, B., Song, J., et al. (2020). A novel coronavirus from patients with pneumonia in China, 2019. New Engl. J. Med. 382, 727–733. doi: 10.1056/NEJMoa2001017
Zou, D., Xu, J., Duan, X., Xu, X., Li, P., Cheng, L., et al. (2019). Porcine epidemic diarrhea virus ORF3 protein causes endoplasmic reticulum stress to facilitate autophagy. Vet. Microbiol. 235, 209–219. doi: 10.1016/j.vetmic.2019.07.005
Zumla, A., Hui, D. S., Perlman, S. (2015). Middle East respiratory syndrome. Lancet 386, 995–1007. doi: 10.1016/S0140-6736(15)60454-8
Keywords: coronavirus, SARS-CoV-2, autophagy, virus replication, immune response
Citation: Yu J, Ge S, Li J, Zhang Y, Xu J, Wang Y, Liu S, Yu X and Wang Z (2024) Interaction between coronaviruses and the autophagic response. Front. Cell. Infect. Microbiol. 14:1457617. doi: 10.3389/fcimb.2024.1457617
Received: 01 July 2024; Accepted: 18 October 2024;
Published: 22 November 2024.
Edited by:
Xiaoyong Chen, Zhejiang Normal University, ChinaReviewed by:
Wuxiang Guan, Chinese Academy of Sciences (CAS), ChinaXinyu Zhu, Duke University, United States
Copyright © 2024 Yu, Ge, Li, Zhang, Xu, Wang, Liu, Yu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiliang Wang, V2FuZ3poaWxpYW5nQGNhaGVjLmNu
 Jiarong Yu
Jiarong Yu Shengqiang Ge1
Shengqiang Ge1 Yongqiang Zhang
Yongqiang Zhang Jiao Xu
Jiao Xu