
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell. Infect. Microbiol. , 28 February 2024
Sec. Antibiotic Resistance and New Antimicrobial drugs
Volume 14 - 2024 | https://doi.org/10.3389/fcimb.2024.1360852
This article is part of the Research Topic Developments in non-antibiotic antimicrobials in the era of drug-resistant bacteria View all 10 articles
Helicobacter pylori (H. pylori) eradication is pivotal for alleviating gastric mucosal inflammation and preventing the progression of gastric diseases. While antibiotic-based therapies have achieved significant success in H. pylori eradication, challenges such as antibiotic resistance, drug toxicity, side effects, nonadherence, inapplicability, and disruption of gastrointestinal microflora have emerged. Updated therapies are urgently needed to suppress H. pylori. Nature has provided multitudinous therapeutic agents since ancient times. Natural products can be a potential therapy endowed with H. pylori eradication efficacy. We summarize the basic information, possible mechanisms, and the latest research progress of some representative natural products in H. pylori eradication, highlighting their safety, accessibility, efficiency, and ability to overcome limitations associated with antibiotic application. This review highlights the potential therapeutic advantages of incorporating ethnomedicine into anti-H. pylori regimens. The findings of this review may provide insights into the development of novel natural products and expand the therapeutic options available for H. pylori eradication.
Helicobacter pylori (H. pylori) infection is a major cause of gastric mucosa injury. Approximately 1% of infected individuals will progress to gastric cancer (de Vries et al., 2008). Therefore, the WHO classified H. pylori as a class I carcinogen in 1994 (Cancer, 1994). According to statistics, approximately half of the world’s population is affected by H. pylori infection, which accounts for 15% of the global cancer burden (Shah et al., 2021). Therefore, this pathogen exerts a profound influence on society and the economy, and it is crucial to find an effective method for H. pylori eradication to avoid potential accompanying diseases. Some risk factors have been reported to be associated with H. pylori infection, such as smoking, unboiled water and uncooked food intaking, poor socioeconomic conditions, and early childhood exposure to the bacterium (Leja et al., 2019; Yuan et al., 2022). Antibiotic combination therapy is needed to eradicate H. pylori, for example, the standard PPI-clarithromycin-containing triple therapy and bismuth quadruple therapies (Malfertheiner et al., 2017). However, in the context of a global increase in antibiotic consumption, antibiotic resistance of H. pylori has reached a crisis point, which poses a severe threat to the current regimens (Fallone et al., 2016). Consequently, clarithromycin-resistant strains of H. pylori were recognized by the WHO in 2017 as one of 12 priority pathogens in urgent need of novel antibiotics or alternatives (Tacconelli et al., 2018). As a result, new therapeutic therapies are imperative.
Complementary and alternative treatments with features of lower toxicity and low cost are attractive, and natural compounds have long been viewed as vital candidates for anti-H. pylori regimens. Researchers in gastroenterology or bacteriology domains have shown remarkable interest in the antimicrobial activities of natural products, especially for the possible lessened likelihood of developing genetic resistance. With a view to identifying more medicinal natural products and their synthetic variations, it is ideal to take a deep look into ethnomedicine, for example, traditional Chinese medicine (TCM). Numerous in vitro and in vivo studies have been conducted to investigate the therapeutic effect of ethnomedicine and their corresponding natural products against H. pylori. For incidence, the in vitro efficacy of artemisone and artemisinin derivatives against H. pylori was demonstrated in the work by Sisto et al (Sisto et al., 2016), while it also indicated a synergistic effect combined with standard drugs. In addition, Krzyzek et al. identified myricetin as a natural substance that hampers the morphological transition of H. pylori from spiral to coccoid forms and therefore increases susceptibility to antibiotics (Krzyżek et al., 2021). Confronting the antibiotic resistance affecting H. pylori eradication, such studies elucidate applicable natural products as alternative therapies. Great progress has been made in this field, but few natural product-based regimens have been recommended by clinical guidelines, and the mechanisms behind ethnomedicine are still not clearly explained. Consequently, the keywords “H. pylori”, “natural products” and “ethnomedicine” were comprehensively searched in PubMed, Wed of Science databases, China National Knowledge Infrastructure (CNKI), and Scopus databases. The publications were screened and those related to the theme of this review were included. Based on these relevant studies, we summarized the general situation and possible therapeutic mechanisms of natural products potentially with anti-H. pylori properties to serve as a reference for the development of novel drugs and expand therapeutic options against the bacterium (Figure 1).
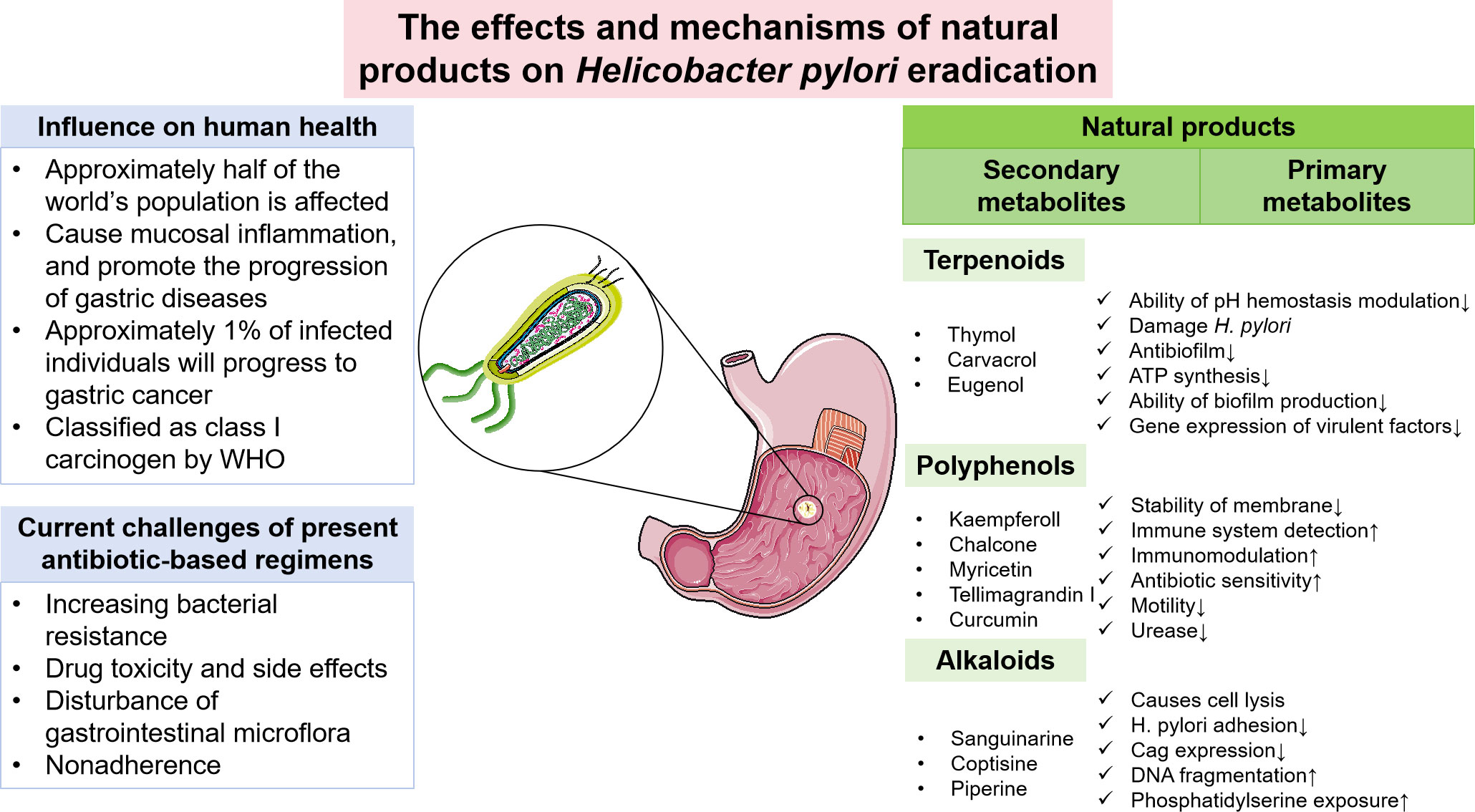
Figure 1 Current challenges of H. pylori eradication and the potential role of natural products in eradicating H. pylori.
H. pylori is a gram-negative, spiral, and microaerophilic bacterium that was discovered by Australian scientists Warren and Marshall in 1982 (Marshall and Warren, 1984). While the majority of bacteria are incapable of colonizing in such an acidic environment, H. pylori has evolved unique features that allow it to thrive in the exclusive ecological niche of the human stomach (Ansari and Yamaoka, 2019). These features include its mobility, helical shape, and ability to produce large amounts of urease, which neutralizes gastric hydrochloric acid by hydrolyzing urea into bicarbonate and ammonia (Solnick and Schauer, 2001). Furthermore, H. pylori possesses a chemotaxis system that enables it to detect pH gradients as well as sheathed polar flagella, which aid in reaching less acidic gastric mucosal surfaces (Roszczenko-Jasińska et al., 2020). Once there, adhesins such as blood-antigen binding protein A (BabA) facilitate the attachment of the bacteria to gastric epithelial cells (Sousa et al., 2022). In addition to factors involved in establishing infections, many clinical isolates of H. pylori produce virulence factors such as VacA, CagA, and cagPAI within the host cell cytoplasm. These factors play significant roles in immune evasion and disease induction (Sukri et al., 2020). Infected gastric tissues also exhibit elevated levels of reactive oxygen species (ROS), leading to gastric inflammation with the production of various mediators (Kang and Kim, 2017).
H. pylori eradication is reported to help alleviate mucosal inflammation, restore normal mechanisms governing acid secretion, and stop the progression of gastric diseases (Sugano et al., 2015). Accordingly, the present consensus among all major gastroenterological societies is that H. pylori infection should be eradicated in individuals who tested positive unless there are compelling reasons (Sugano et al., 2015; Matsumoto et al., 2019; Shah et al., 2021). Since the microorganism was discovered, a wide variety of therapeutic strategies have been proposed to tackle H. pylori, such as clarithromycin triple therapy, bismuth or nonbismuth-based quadruple therapy, and rifabutin-based triple therapy (Cardos et al., 2021).
The option of eradication regimes should be determined according to the geographical area, in the context of regional differences in antibiotic resistance patterns, as well as the different common host genotypes of drug-metabolizing enzymes of the local population. In regions with low clarithromycin resistance (<15%), the Maastricht consensus report still endorses standard triple therapy as the primary treatment option (Malfertheiner et al., 2017), which consists of a proton pump inhibitor (PPI), amoxicillin, and clarithromycin or metronidazole. However, the regime is inapplicable in high clarithromycin resistance regions without preceding antimicrobial susceptibility testing. Metronidazole is an ideal substitute for clarithromycin in regions with low to intermediate metronidazole resistance rates (Malfertheiner et al., 2017). When high resistance to both antibiotics is observed, bismuth quadruple or nonbismuth quadruple, concomitant (PPI, amoxicillin, clarithromycin, and nitroimidazole) therapies are favorable options instead (Sugano et al., 2015; Malfertheiner et al., 2017).
Despite the significant progress made by antibiotic-based therapies, the present regimens are faced with a series of challenges, for example, high resistance to antibiotics, nonadherence, drug toxicity and side effects, and disturbance of gastrointestinal microflora (Matsumoto et al., 2019).
Increasing bacterial resistance to commonly used antimicrobial agents is one of the most common reasons for eradication failure, which has reached alarming levels worldwide and severely threatens the efficacy of treatment. Based on a systematic review and meta-analysis involving 65 countries, the incidence of primary and secondary resistance to clarithromycin, metronidazole, and levofloxacin is greater than 15% in most WHO regions (Savoldi et al., 2018). This threshold is commonly used to select alternative treatment regimens (Savoldi et al., 2018). Likewise, a recent prospective study covering 18 European countries also showed alarming H. pylori resistance to commonly used antibiotics and revealed a positive correlation between macrolide and quinolone consumption and corresponding H. pylori resistance (Megraud et al., 2021). Accordingly, the last few decades have witnessed the unacceptable fact that H. pylori eradication rates of standard triple therapies experienced a sharp decrease from 80-90% in the 1990s to below 70%, and other regimens also encounter similar hurdles (O'Morain et al., 2018). To maximize the eradication rate and ensure the prudent use of antibiotics, individual antibiotic susceptibility testing seems to be a feasible solution, which is especially recommended in cases needing salvage therapies (O'Morain et al., 2018). Moreover, it is beneficial to collect regional antibiotic consumption data and clinical outcomes to provide local resistance reports and update guidelines for infection (Matsumoto et al., 2019).
In addition to the emergence of antibiotic-resistant strains, the possible adverse effects and nonadherence of some patients also impose restrictions on the utilization of conventional antibiotic-based eradication therapies. Antibiotics, especially at high dosages, may induce many side effects. For example, antibiotics are known to affect the balance of gut microbiota through both direct and indirect mechanisms. While eradicating targeted pathogens, antibiotics also discriminately kill or inhibit subsets of commensal microbes or disrupt the homeostasis of the symbiosis and codependency relationships among different subsets of gut microbiota (Zhang et al., 2019). In addition, some individuals may experience adverse reactions, including nausea, allergic reactions to antibiotics, and severe complications (liver and/or kidney dysfunction) (Takeuchi et al., 2014). The level of patient compliance is another crucial contributing factor for treating the infection, which can be influenced by barriers such as physical intolerance of medication, lack of understanding of the prescription, and high pill burden (Shah et al., 2021). The threshold of adherence for successful eradication varies depending on individual factors, but it was demonstrated that patients need to hold to at least >60% to >90% of the prescribed course to guarantee the eradication rate (Shah et al., 2021). Furthermore, host genetic polymorphisms of drug-metabolizing enzymes also account for the eradication failure of H. pylori infection. The metabolism-enhancing phenotypes of CYP2C19 are responsible for the rapid metabolism of some PPIs and thus influence the reduction in intragastric acidity. The optimal intragastric pH for H. pylori replication is between 6 and 8; thus, alkalizing the gastric environment can increase susceptibility to antibiotics in populations with metabolism-enhancing phenotypes of CYP2C19 (Safavi et al., 2015; Shah et al., 2021).
Due to the current persistence and rise of antibiotic resistance and other restrictions on current therapies, traditional antibiotic-based therapies have been severely weakened, and the development of novel and more effective antimicrobial compounds and novel strategies is drawing increasing attention worldwide (Matsumoto et al., 2019). However, owing to the complex biology of the pathogen, the high cost of research and the lack of financial support, the discovery and development of new therapeutics is not without challenges. To reduce the cost of developing new candidates for H. pylori eradication, it is beneficial to attach importance to the exploitation of natural product libraries and conduct thorough ethnobotanical and ethnomedical analyses, which may offer valuable inspiration for new treatments.
In light of the challenges faced by currently used H. pylori antibiotic-based eradication therapies, research on alternative treatment approaches is gaining popularity. With vast resources, nature has provided multitudinous therapeutic agents since ancient times and is seen as a promising source in the search for new compounds endowed with anti-H. pylori potential.
Ethnomedicine is the study of naturally obtained drugs based on traditional knowledge and practices of various ethnic groups (Lai et al., 2022), usually supported by ancient medical classics or passed down orally over generations. Generally, acquired from empirical observations and beliefs of indigenous peoples, the theoretical systems of ethnomedicine differ from scientific medicine, and some are still currently inexplicable by science. Nevertheless, ethnomedicine has been indispensable in health maintenance in households for centuries and is still widely used for primary health needs in extensive regions (Buenz et al., 2018).
Natural products are a series of bioactive chemical compounds produced by living organisms from nature, usually serving as the primary and secondary metabolites in biochemical pathways (Katz and Baltz, 2016). Typical examples of natural products include alkaloids, flavonoids, saponins, terpenes, etc. Although not universally recognized, natural products are widely acknowledged as the active components of numerous ethnomedicines, and the pertinent cutting-edge research findings have input vigor into the domain of drug discovery and development.
Several natural products and ethnomedicine have demonstrated antimicrobial activity through diverse scientific research as well as multiple generations of medical practice. Even before the discovery of H. pylori, a wide range of plants and substances have been employed to address gastric symptoms that are currently considered to be associated with the infection of this pathogen (Takagi and Harada, 1969; Escobedo-Hinojosa et al., 2012). Today, with a more in-depth knowledge of H. pylori after decades of exploration, ethnomedicine is still being used as a guide for the search for effective natural products and treatment approaches to cope with the infection (Vale and Oleastro, 2014), and some patent medicines have already been recommended as supplements in clinical guidelines. For example, traditional Chinese medicine and Kampo are recommended as treatment options by countries with prevalent use of traditional medicine (Helicobacter pylori Study Group, 2022; Miwa et al., 2022). The bioactivity of some natural products in inhibition of H. pylori has been summarized in Table 1.
Natural products are usually divided into two categories: primary metabolites and secondary metabolites. Including carbohydrates, lipids, proteins, and nucleic acids, primary metabolites are indispensable and directly involved in the life cycle of organisms (Chaturvedi and Gupta, 2021). Few of them, however, demonstrate pharmacologic actions against microorganisms. Several polysaccharides have exhibited antiadhesive properties against H. pylori, likely by inhibiting the crucial docking process of adhesins of H. pylori and gastric epithelium (Gottesmann et al., 2020).
In contrast, secondary metabolites are compounds that are not needed for life, usually not only possessing a wide range of biological functions but also having special mechanisms of action (Li et al., 2021). The scientific community has invested significant expectations in the utilization of secondary metabolites, and a large number of studies have been performed to reveal the pharmacological value of these compounds. We summarized the basic information and possible mechanisms of some representative secondary metabolites for H. pylori eradication (Table 2 and Figure 2). The section below describes the latest research progress of secondary metabolites for H. pylori eradication.
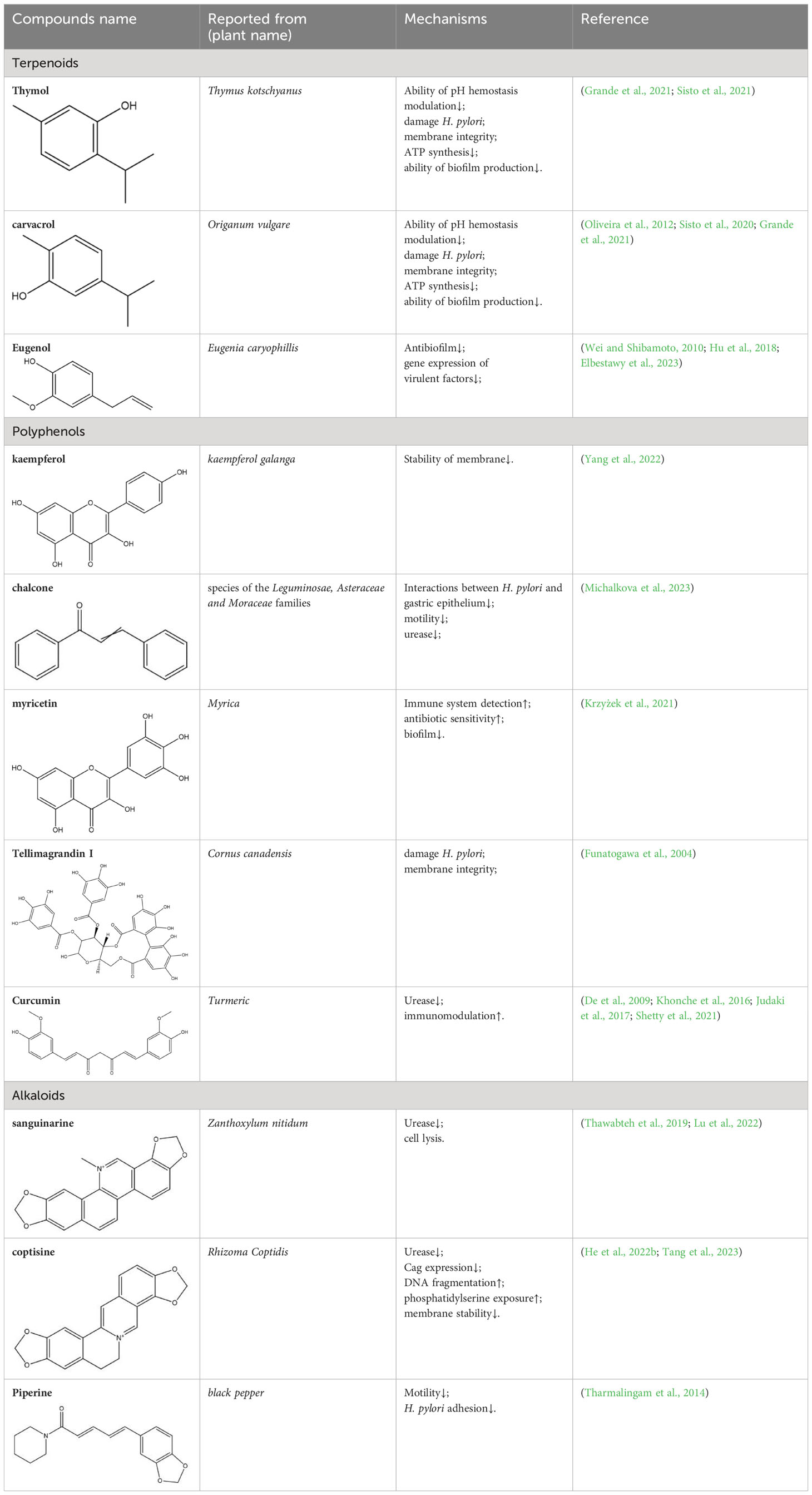
Table 2 Summarization of the basic information and possible mechanisms of some representative secondary metabolite for H. pylori eradication.
Terpenes are a large class of plant secondary metabolites formed with five-carbon isoprene (C5H8), and classifications of terpenoids are based on the number of isoprene units in their structure (Khan et al., 2018). As widely diffused chemicals in plants, many types of terpenoids have been shown to have anti-H. pylori properties, and here, we will update the reports of the antimicrobial effects of some terpenoids.
Monoterpenoids are a series of natural products that possess two isoprenes in their molecules, which are the prevailing components of the essential oils of pine, lemon, thyme, tea tree, etc., and many of them have demonstrated gastroprotective activity in vivo as well as antimicrobial properties in vitro (Rozza et al., 2011). Typical examples with anti-H. pylori properties include limonene and β-pinene, which are predominant components in Citrus lemon (Rutaceae) essential oil and have been demonstrated to exert antibacterial effects in vitro. Limonene was shown to elevate mucus secretion by ensuring adequate PGE2 levels, thus limiting the colonization of H. pylori and isolating gastric mucosa from ulcerative factors (Moraes et al., 2009). Additionally, monoterpenes demonstrate anti-inflammatory capacity by regulating oxidative stress and the inflammatory response of gastric mucus. Consequently, the expression of MPO, NF-κB, and proinflammatory cytokines such as TNF-a, IL-6, and IL-1β is decreased, while that of IL-10 is increased (de Souza et al., 2019). β-Pinene can also promote mucus production, relieve oxidative stress and inflammation and inhibit Nf-κb expression to exert its gastroprotective properties (de Souza et al., 2019). Similarly, α-pinene manifests gastroprotective properties with modulation of oxidative stress and PGE2 and histamine levels in vivo (Al-Sayed et al., 2021). β-Myrcene also possesses the capacity to block the growth of H. pylori, and the corresponding MIC of the monoterpene is 500 μg/mL (Wei and Shibamoto, 2010). For gastroprotective effects, β-myrcene prevents gastric damage by the marked upregulation of antioxidant enzyme activity, with decreased activity of superoxide dismutase (SOD) and increased levels of glutathione peroxidase (GPx), glutathione reductase (GR), and total glutathione (Bonamin et al., 2014). Moreover, terpenoid phenols, thymol and carvacrol, are reported antimicrobial agents with MIC ranges of 64-128 µg/mL and 16-64 µg/mL (Grande et al., 2021). A specific concentration of carvacrol or thymol is observed to cause an increase in membrane fluidity, leakage of essential ions, and limited ATP synthesis in pathogens, which is partly due to the hydrophobic character and may also be attributed to the presence of a hydroxyl group and a system of delocalized electrons in their structures, causing a decrease in the membrane pH gradient and thus interfering with ATP synthesis (Ultee et al., 2002). Additionally, the inhibition of carbonic anhydrase (CA), a series of enzymes that catalyze the equilibrium between CO2 hydration, may also play a crucial role in the antibacterial mechanism of the two terpenoids. H. pylori CAs are pivotal in the modulation of pH hemostasis, the integrity of the bacterial membrane, and the ability to produce biofilms, which are promising druggable targets in eliminating pathogenic microorganisms (Grande et al., 2021). Last, Eugenol is thought to be one of the most active natural products against H. pylori, with MICs ranging from 23.0 to 51.0 µg/mL (Elbestawy et al., 2023). Previous studies indicate that eugenol possesses antibiofilm activities against H. pylori and can downregulate the expression of virulence factors, while eugenol can also relieve H. pylori-related gastritis by its anti-inflammatory activities (Hu et al., 2018).
Sesquiterpenoids are another group of predominant secondary metabolites in various plant essential oils. For instance, over 70% of the components present in cedarwood essential oil are sesquiterpenoids, including α-, β-cedrene, thujopsene, cedrol, and cuparene, and the compound group displayed excellent efficacy in inhibiting urease activity and H. pylori growth (Korona-Glowniak et al., 2020), which may indicate that the anti-H. pylori activity of sesquiterpenoids. Additionally, with a similar structure to the major components in cedarwood essential oil, patchouli alcohol, a tricyclic sesquiterpenoid, exhibited antimicrobial properties toward Streptococcus mutans via the inhibition of DNA polymerase in a previous study (Takao et al., 2012), which may also offer a clue for the anti-H. pylori activity of sesquiterpenoids.
Similarly, triterpenoids have also gained attention for their various biological functions, including anti-inflammatory, antitumor, antiviral and antimicrobial activities (Dzubak et al., 2006). Triterpene glycosides (saponin) are good examples of anti-H. pylori activity of triterpenoids. For instance, glycyrrhizic acid is a representative triterpenoid saponin enriched in Glycyrrhiza glabra, whose major metabolite, glycyrrhetinic acid, was reported to exhibit rapid anti-H. pylori property in vitro (Wittschier et al., 2009). Glycyrrhetinic acid is reportedly in possession of cytotoxic effects and can impair H. pylori growth. Nevertheless, some studies have indicated that long-term intake of saponin may lead to an increased risk of gastric lesions (Périco et al., 2015). Therefore, the use of saponin-rich plants as an antiulcer ethnomedicine should be under careful consideration.
Tetraterpenoids are terpenoids that consist of a C40 structure, and carotenoids are a well-known subclass of tetraterpenoids that possess potent antioxidant properties. Previous research has explored the potential of some carotenoids in the treatment of H. pylori-related gastric diseases, including β-carotene (Bae et al., 2021), astaxanthin (Davinelli et al., 2019; Kim and Kim, 2021; Lee et al., 2022), and lycopene (Jang et al., 2012; Kim et al., 2018a). Carotenoids can be divided into two groups by the presence of oxygen, namely, xanthophylls and carotenes, and here, we will present β-carotene and astaxanthin as representatives. Abundant in orange-colored fruits and vegetables, β-carotene is a well-known carotenoid with marked antioxidant capacity, which may be structurally ascribed to the numerous conjugated double bonds (Kang and Kim, 2017). β-carotene intake can suppress NADPH oxidase and stimulate antioxidant enzyme activity, thus contributing to the suppression of H. pylori-induced ROS generation as well as the level of iNOS and COX-2 expression. As a result, β-carotene exerts its anti-inflammatory effects by suppressing ROS-mediated inflammatory signaling (including MAPKs and NF-κB), which functions in the prevention of inflammatory damage (Jang et al., 2009; Park et al., 2019; Bae et al., 2021). However, previous studies have mainly focused on the ability of β-carotene to modulate gastric carcinogenesis, and existing evidence has not shown explicit antimicrobial activity of β-carotene against H. pylori, which needs further exploration. In addition, astaxanthin is a xanthophyll carotenoid commonly found in crustaceans such as shrimp, crabs, and lobster (Davinelli et al., 2019), which is reported to have 10-fold stronger antioxidant activity than β-carotene. In vivo, a study using BALB/cA mice showed that an astaxanthin-rich algal meal could inhibit the colonization of H. pylori and suppress inflammation in gastric tissues of H. pylori-infected mice (Bennedsen et al., 2000; Naguib, 2000; Wang et al., 2000). Similarly, astaxanthin demonstrates its ability to prevent oxidative stress-mediated inflammation through ROS reduction (Kim et al., 2018b). Some research also illustrated that astaxanthin could induce a shift in the Th1/Th2 response pattern, thus enhancing the clearance of H. pylori (Davinelli et al., 2019). Additionally, a significant ability to inhibit H+, K+-ATPase was observed in astaxanthin esters, indicating a possible mechanism of H. pylori inhibition and a potent future of astaxanthin modification (Kamath et al., 2008).
Polyphenols are another ubiquitous spectrum of natural products abundant in green tea, propolis, cranberry, etc (Gao et al., 2021; Ngan et al., 2021; Widelski et al., 2022), and can be grouped into classes such as flavonoids and tannoids. Structurally, polyphenols possess several hydroxyl groups on aromatic rings, which contribute to their antioxidant properties to reduce ROS production (Bonacorsi et al., 2012). Several investigations into the mechanisms of anti-H. pylori capacities have shown that polyphenols may lead to a decrease in urease activity, inhibition of cytotoxic activity as well as its binding with the gastric mucosa, and the rupture of outer membrane (Burger et al., 2002; Lin et al., 2005; Yahiro et al., 2005; Nohynek et al., 2006). According to a previous study, polyphenols as an adjuvant in the treatment of H. pylori infection can significantly improve the eradication rate (Wang et al., 2023; Wu et al., 2023), while no evidence has been found to suggest an increased risk of side effects. Consequently, polyphenols are suggested as an alternative treatment approach for H. pylori infection.
Among the polyphenol family, flavonoids are one of the most important and vast groups of compounds with a basic skeleton comprising two benzene rings linked through a heterocyclic ring, involving over 9000 species of molecules (Gonzalez et al., 2021). Many flavonoids have been shown to possess promising antimicrobial capacity against H. pylori, including kaempferol, chalcone, myricetin, taxifolin, and other compounds with proven validity (Krzyżek et al., 2021; Stenger Moura et al., 2021; Yang et al., 2022; Michalkova et al., 2023). Flavonoids contribute to several antibacterial mechanisms, such as the inhibition of crucial enzymes for colonization, survival, and reproduction (Asha et al., 2013; Steinmann et al., 2013; Egas et al., 2018), and interference with the fluidity and stability of the cytoplasmic membrane (Tsuchiya and Iinuma, 2000; Yang et al., 2022). In addition, flavonoids can engage in the modulation of several intracellular pathways, such as MAPK and NF-κB, thus attenuating the level of pro-inflammatory cytokines induced by H. pylori infection and improving gastric inflammation status (Wang and Huang, 2013). In addition to their antimicrobial and anti-inflammatory effects, flavonoids are capable of acting synergistically with antibiotics commonly utilized in the treatment of H. pylori infection (Krzyżek et al., 2021).
Tannoids (or tannins) are naturally occurring plant polyphenolic substances that bind to proteins, amino acids, alkaloids, and precipitate them and are highly expected antimicrobial biomolecules (Kurhekar, 2016), and previous studies have demonstrated the in vitro anti-H. pylori activity of the compounds (Wang, 2014; Cardoso et al., 2018). According to Funatogawa et al.’s work, monomeric hydrolyzable tannoids inclusive of tellimagrandin I and II exhibit strong antibacterial activity by damaging the membrane of H. pylori (Funatogawa et al., 2004). In addition, tannoids can also serve as inflammatory mediators by reducing nitric oxide levels and exerting anti-inflammatory activity on gastric mucosa (Cardoso et al., 2018).
Curcumin, the principal ingredient isolated from turmeric, has been used in many Asian regions as an herbal remedy for various diseases (Kwiecien et al., 2019). In vivo, an experiment in a mouse model has proven a notably reduced number of H. pylori colonizing mucosa, which may be attributed to decreased activity of lipid peroxide, MPO and urease and increased level of immunomodulation (De et al., 2009). Recent evidence offered by randomized clinical trials also revealed that triple therapy with curcumin can improve symptoms of dyspepsia, attenuate oxidative stress, and prevent mucosa against inflammation, and some showed that adjunctive therapy with curcumin can improve the eradication rate of H. pylori (Khonche et al., 2016; Judaki et al., 2017). Additionally, the good interaction between curcumin and targeted virulence factors such as Ureα/β subunits is implicated in the prevention of H. pylori survival and colonization (Shetty et al., 2021). However, the oral bioactivity of curcumin as an alternative remedy for H. pylori elimination is weakened by its limited aqueous solubility and retention time, for which an effective delivery system is needed to ensure enough therapeutic window at the site of infection, and chitosan (CS) polymer may be a practical solution (Ejaz et al., 2022).
Alkaloids are a wide range of naturally occurring molecules that contain at least one nitrogen atom and several hydrogen-carbon groups in their structures, forming heterocyclic rings. To date, over 10,000 kinds of alkaloids have been identified among 300 plant families (Mahapatra et al., 2019). A variety of compounds in alkaloid families have shown multiple therapeutic effects, for example, their antitumor, antimicrobial, anti-inflammatory and antidiabetic properties (Wu et al., 2018). Numerous previous studies have noted diverse representatives of pharmaceutically used alkaloids, among which some are recognized as promising antimicrobial agents, such as berberine, coptisine, palmatine, and quinolone alkaloids (Hamasaki et al., 2000; Wen et al., 2022). Some studies have focused on the mechanisms of anti-H. pylori properties, which will be discussed in the following section.
The inhibition of urease activity is a common mechanism of alkaloids against H. pylori. According to the significant reduction in anti-urease activity after adding sulfhydryl-containing reagents or competitive Ni2+-binding restrainers, sanguinarine (a natural alkaloid enriched in Zanthoxylum nitidum) is supposed to suppress urease by targeting Ni2+ and thiol (Lu et al., 2022). Similarly, coptisine could also interact with both active center Ni2+ and sulfhydryl groups in amino acid residues to inhibit urease activity (He et al., 2022b). In addition to urease inhibition, coptisine also demonstrates various antibacterial mechanisms, including decreasing Cag expression and inducing DNA fragmentation (Tang et al., 2023). Moreover, many alkaloids, such as coptisine and squalamine, can cause disruption of the cell membrane of H. pylori, which is especially beneficial for synergistic therapy with commonly used antibiotics (such as amoxicillin), providing extra portals for some compounds to enter the bacteria (Krzyzek et al., 2021; Tang et al., 2023). Piperine, an alkaloid present in black pepper, has shown a diminution of H. pylori motility as well as adhesion to gastric cells, contributing to the suppression of H. pylori growth (Tharmalingam et al., 2014).
Like other natural products, alkaloids can also exert diverse anti-inflammatory effects, including the reduction of certain proinflammatory cytokines (IL-2, IL-6, IL-17, CXCL1) (Tang et al., 2023), the regulation of macrophage activity (Yang et al., 2021), and the modulation of inflammatory signaling pathways (Alam et al., 2019; Haftcheshmeh et al., 2022). In addition to anti-inflammatory properties, some studies have investigated the gastroprotective effects of alkaloids and reported improvements in the ulcer area of rats, with increased prostaglandin E2 (PGE2) as well as decreased platelet-activating factor (PAF) (Wang et al., 2017). All of these features may relieve the symptoms and health hazards induced by H. pylori.
Given that only a limited fraction of plant species have been scientifically studied, there still lies great potential in discovering new promising drugs, which are likely to be included in ethnomedicine and are perceived to be an ideal substitute or supplement for current recommended treatment.
Ethnomedicine distinguishes itself from traditional therapies by its accessibility, relatively affordable price and widespread availability. In addition, many consumers perceive herbal medicine as a more natural and thus safer option than synthetic drugs to address diseases, particularly in regions where traditional medicine has been widely practiced for a long time. Although herbal medicine cannot necessarily avoid adverse effects, evidence has proven the safety advantages of natural products over the traditional therapies of combining multiple antibiotics (Deng et al., 2022; Liu et al., 2022). Some studies have investigated the efficacy and safety of the combination of standard therapy and some ethnomedicine medications, which was demonstrated to have a higher eradication rate and fewer side effects than using an antibiotic-based regimen (Bao et al., 2022). In addition to the eradication efficiency, combination medication possesses the superiority of relieving the symptoms of H. pylori-associated gastritis, with the concept of overall adjustment and multitargeting curative effects (Li et al., 2021). Moreover, another appealing reason for adding ethnomedicine to treatments is that pharmaceutical synergism may lessen the likelihood of antibiotic resistance, which can also be attributed to the multiple target effects of ethnomedicine (Ngo et al., 2013; Li et al., 2021). Ultimately, the use of ethnomedicine has noteworthy value in the H. pylori eradication of specific populations. For example, it is relatively inappropriate to employ conventional antibiotic-based regimens in vulnerable elderly individuals who would be unable to endure the possible adverse effects of high-dose antibiotics and acid suppression. Thus, ethnomedicine, with milder side effects, is regarded as an ideal therapeutic solution.
Apart from the aforementioned advantages from the patients’ perspective, the screening process for new pharmaceuticals also gains from the higher success rate of natural product libraries and the information provided by ethnomedicine. Natural products already possess biological functions since they are mainly primary and secondary metabolites, which leads to a higher possibility of discovering biologically appropriate compounds in natural product libraries than exploring traditional combinatorial chemistry (Sukuru et al., 2009; Buenz et al., 2018). The screening of polyketide metabolites is a good illustration of the efficiency with a hit rate of 0.3% (more than 20 commercial drugs in just over 7000 known structures), while the hit rate for HTS of synthetic compound libraries is less than 0.001% (Weissman and Leadlay, 2005). Additionally, with its empirical knowledge and historical trials, the ethnomedicine system may also facilitate the identification of bioactive compounds by indicating possible screening directions (Buenz et al., 2018).
With the advances of new technologies such as virtual screening, bioinformatics, and artificial intelligence, bioprospecting is currently equipped with innovative powerful tools and undergoing rapid evolution. For instance, in the work of He et al., virtual screening and molecular modeling were utilized to identify ligands that interact with CagA protein to discover potential natural compound candidates (He et al., 2022a). Bioinformatics approaches, including genomics, proteomics, and metabolomics, are also widely implemented in natural product biosynthesis and production, and some researchers bet on artificial intelligence projects for drug innovation inspiration (Ngo et al., 2013; Merwin et al., 2020; Zhang et al., 2020).
While ethnomedicine may provide valuable insights into potential natural remedies or complementary approaches, there are several challenges associated with the development of ethnomedicine as a treatment for H. pylori. It has been generally accepted that the premise of TCM treatment is syndrome differentiation, which is the cornerstone of treatment. However, due to the complexity of TCM syndrome types, it is difficult to develop a widely accepted TCM syndrome type differentiation system at present (Li et al., 2021; Elbehiry et al., 2023). Thus, it is necessary to summarize the experience from current clinical practice and integrate experts’ opinions to form consensus about syndrome differentiation for H. pylori-associated diseases. In addition, basic animal experimental studies were also limited by the complexity of TCM syndrome types. Currently, most animal experimental studies on disease-syndrome combinations only have a single syndrome type, which is inconsistent with the complex syndrome type of TCM that is more common in clinical practice. Future studies should explore and develop new research models containing complex disease-syndrome combinations to be in line with the current majority clinical practice. Furthermore, the combination of natural products and Western medicine is becoming increasingly common in modern clinical research. This approach can achieve the goal of increasing efficiency and reducing toxicity, highlighting the advantages of combination therapy. Phytochemicals from ethnomedicine formulas and their compositional herb medicines exhibit great potential for the development of novel anti-H. pylori drugs. However, the current investigation of the combination of natural products and Western medicine for treating H. pylori is still insufficient. Researchers should try to combine some herbal monomers that have clear chemical structures or active ingredients in formulas to develop novel drugs for treating H. pylori infection based on the compatibility principle of ethnomedicine prescriptions (Li et al., 2021). Last, the effectiveness and safety of natural products can vary significantly (Périco et al., 2015), and it is important to consider potential interactions with other medications and any possible side effects. There is currently little clinical trial data regarding their safety and efficacy. Therefore, more multicenter, double-blind, randomized, and controlled clinical trials should be conducted to validate the effectiveness and safety of these natural product prescriptions in the treatment of H. pylori. Well-designed clinical trials and experimental studies would be beneficial for facilitating a better understanding of the mechanism of natural products and promoting the modernization of natural products in the treatment of H. pylori-associated diseases (Yang et al., 2023).
Confronting the challenge presented by H. pylori antibiotic resistance, ethnomedicine is a particularly noteworthy choice of complementary therapy. The scientists and practitioners should continue working collaboratively to bring this ancient wisdom up-to-date and make full use of it in H. pylori eradication. In this review, we comprehensively summarized the anti-H. pylori function and mechanisms of natural products, and analyzed the therapeutic advantages of incorporating ethnomedicine into anti-H. pylori regimens, including safety, accessibility, efficiency and restriction of antibiotic resistance. Natural products can also provide clues for novel antimicrobial drug discovery. This review can provide insights into the development of natural products and expand the therapeutic options available for H. pylori eradication. However, there is still some limitation. The language of the included studies was limited to English, so some potential eligible studies published in other languages might be neglected.
RD: Data curation, Formal analysis, Investigation, Methodology, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. XC: Conceptualization, Data curation, Formal analysis, Investigation, Validation, Visualization, Writing – original draft, Software. SZ: Investigation, Writing – review & editing, Methodology, Validation. QZ: Investigation, Project administration, Supervision, Writing – review & editing, Resources. YS: Conceptualization, Funding acquisition, Resources, Supervision, Writing – review & editing, Methodology, Project administration.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Beijing-Tianjin-Hebei Basic Research Cooperation project, Beijing Natural Science Foundation of China (J230002), the National Natural Science Foundation of China (81700496), Key Laboratory of Epidemiology of Major Diseases (Peking University), Ministry of Education, and the special fund of the Beijing Clinical Key Specialty Construction Program, P. R. China (2021).
We are grateful to the Research Center of Clinical Epidemiology (Peking University Third Hospital, China) for supporting our study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Alam, M. B., Ju, M.-K., Kwon, Y.-G., Lee, S. H. (2019). Protopine attenuates inflammation stimulated by carrageenan and LPS via the MAPK/NF-κB pathway. Food Chem. Toxicol. 131, 110583. doi: 10.1016/j.fct.2019.110583
Al-Sayed, E., Gad, H. A., El-Kersh, D. M. (2021). Characterization of Four Piper Essential Oils (GC/MS and ATR-IR) Coupled to Chemometrics and Their anti-Helicobacter pylori Activity. ACS Omega 6, 25652–25663. doi: 10.1021/acsomega.1c03777
Ansari, S., Yamaoka, Y. (2019). Helicobacter pylori Virulence Factors Exploiting Gastric Colonization and its Pathogenicity. Toxins 11, 677. doi: 10.3390/toxins11110677
Asha, M. K., Debraj, D., Prashanth, D., Edwin, J. R., Srikanth, H. S., Muruganantham, N., et al. (2013). In vitro anti-Helicobacter pylori activity of a flavonoid rich extract of Glycyrrhiza glabra and its probable mechanisms of action. J. Ethnopharmacol. 145, 581–586. doi: 10.1016/j.jep.2012.11.033
Bae, S., Lim, J. W., Kim, H. (2021). β-carotene inhibits expression of matrix metalloproteinase-10 and invasion in Helicobacter pylori-infected gastric epithelial cells. Molecules 26, 1567. doi: 10.3390/molecules26061567
Bao, Z., Wu, G., Du, J., Ye, Y., Zheng, Y., Wang, Y., et al. (2022). The comparative efficacy and safety of 9 traditional Chinese medicines combined with standard quadruple therapy for Helicobacter pylori-associated gastritis: a systematic review and network meta-analysis. Ann. Trans. Med. 10, 1349–1349. doi: 10.21037/atm-22-5421
Bennedsen, M., Wang, X., Willén, R., Wadström, T., Andersen, L. P. (2000). Treatment of H. pylori infected mice with antioxidant astaxanthin reduces gastric inflammation, bacterial load and modulates cytokine release by splenocytes. Immunol. Lett. 70, 185–189. doi: 10.1016/S0165-2478(99)00145-5
Bergonzelli, G. E., Donnicola, D., Porta, N., Corthésy-Theulaz, I. E. (2003). Essential oils as components of a diet-based approach to management of Helicobacter infection. Antimicrob. Agents Chemother. 47, 3240–3246. doi: 10.1128/aac.47.10.3240-3246.2003
Bodet, C., Burucoa, C., Rouillon, S., Bellin, N., Taddeo, V. A., Fiorito, S., et al. (2014). Antibacterial activities of oxyprenylated chalcones and napthtoquinone against helicobacter pylori. Nat. Prod. Commun. 9, 1934578X1400900920. doi: 10.1177/1934578x1400900920
Bonacorsi, C., Raddi, M. S., Da Fonseca, L. M., Sannomiya, M., Vilegas, W. (2012). Effect of Byrsonima crassa and phenolic constituents on Helicobacter pylori-induced neutrophils oxidative burst. Int. J. Mol. Sci. 13, 133–141. doi: 10.3390/ijms13010133
Bonamin, F., Moraes, T. M., Dos Santos, R. C., Kushima, H., Faria, F. M., Silva, M. A., et al. (2014). The effect of a minor constituent of essential oil from Citrus aurantium: the role of β-myrcene in preventing peptic ulcer disease. Chem. Biol. Interact. 212, 11–19. doi: 10.1016/j.cbi.2014.01.009
Buenz, E. J., Verpoorte, R., Bauer, B. A. (2018). The ethnopharmacologic contribution to bioprospecting natural products. Annu. Rev. Pharmacol. Toxicol. 58, 509–530. doi: 10.1146/annurev-pharmtox-010617-052703
Burger, O., Weiss, E., Sharon, N., Tabak, M., Neeman, I., Ofek, I. (2002). Inhibition of Helicobacter pylori adhesion to human gastric mucus by a high-molecular-weight constituent of cranberry juice. Crit. Rev. Food Sci. Nutr. 42, 279–284. doi: 10.1080/10408390209351916
Cancer, I. (1994). Schistosomes, liver flukes and Helicobacter pylori. IARC working group on the evaluation of carcinogenic risks to humans. IARC Monogr. Eval. Carcinog Risks Hum. 61, 218–220.
Cardos, I. A., Zaha, D. C., Sindhu, R. K., Cavalu, S. (2021). Revisiting therapeutic strategies for H. pylori treatment in the context of antibiotic resistance: focus on alternative and complementary therapies. Molecules 26, 6078. doi: 10.3390/molecules26196078
Cardoso, O., Donato, M. M., Luxo, C., Almeida, N., Liberal, J., Figueirinha, A., et al. (2018). Anti-Helicobacter pylori potential of Agrimonia eupatoria L. and Fragaria vesca. J. Funct. Foods 44, 299–303. doi: 10.1016/j.jff.2018.03.027
Chaturvedi, S., Gupta, P. (2021). “Plant secondary metabolites for preferential targeting among various stressors of metabolic syndrome,” in Studies in Natural Products Chemistry. Ed. Atta Ur, R. (Amsterdam, Netherlands: Elsevier), 221–261.
Darmani, H., Smadi, E. A. M., Bataineh, S. M. B. (2020). Blue light emitting diodes enhance the antivirulence effects of curcumin against helicobacter pylori. J. Med. Microbiol. 69, 617–624. doi: 10.1099/jmm.0.001168
Davinelli, Melvang, Andersen, Scapagnini, Nielsen (2019). Astaxanthin from shrimp cephalothorax stimulates the immune response by enhancing IFN-γ, IL-10, and IL-2 secretion in splenocytes of Helicobacter pylori-infected mice. Mar. Drugs 17, 382. doi: 10.3390/md17070382
De, R., Kundu, P., Swarnakar, S., Ramamurthy, T., Chowdhury, A., Nair, G. B., et al. (2009). Antimicrobial Activity of Curcumin against Helicobacter pylori Isolates from India and during Infections in Mice. Antimicrobial Agents Chemotherapy 53, 1592–1597. doi: 10.1128/AAC.01242-08
Deng, G., Wu, Y., Song, Z., Li, S., Du, M., Deng, J., et al. (2022). Tea polyphenol liposomes overcome gastric mucus to treat Helicobacter pylori infection and enhance the intestinal microenvironment. ACS Appl. Materials Interfaces 14, 13001–13012. doi: 10.1021/acsami.1c23342
de Souza, M. C., Vieira, A. J., Beserra, F. P., Pellizzon, C. H., Nóbrega, R. H., Rozza, A. L. (2019). Gastroprotective effect of limonene in rats: Influence on oxidative stress, inflammation and gene expression. Phytomedicine 53, 37–42. doi: 10.1016/j.phymed.2018.09.027
de Vries, A. C., Van Grieken, N. C. T., Looman, C. W. N., Casparie, M. K., De Vries, E., Meijer, G. A., et al. (2008). Gastric cancer risk in patients with premalignant gastric lesions: a nationwide cohort study in the Netherlands. Gastroenterology 134, 945–952. doi: 10.1053/j.gastro.2008.01.071
Dzubak, P., Hajduch, M., Vydra, D., Hustova, A., Kvasnica, M., Biedermann, D., et al. (2006). Pharmacological activities of natural triterpenoids and their therapeutic implications. Natural Product Rep. 23, 394. doi: 10.1039/b515312n
Egas, V., Salazar-Cervantes, G., Romero, I., Méndez-Cuesta, C. A., Rodríguez-Chávez, J. L., Delgado, G. (2018). Anti-Helicobacter pylori metabolites from Heterotheca inuloides (Mexican arnica). Fitoterapia 127, 314–321. doi: 10.1016/j.fitote.2018.03.001
Ejaz, S., Ejaz, S., Shahid, R., Noor, T., Shabbir, S., Imran, M. (2022). Chitosan-curcumin complexation to develop functionalized nanosystems with enhanced antimicrobial activity against hetero-resistant gastric pathogen. Int. J. Biol. Macromolecules 204, 540–554. doi: 10.1016/j.ijbiomac.2022.02.039
Elbehiry, A., Marzouk, E., Aldubaib, M., Abalkhail, A., Anagreyyah, S., Anajirih, N., et al. (2023). Helicobacter pylori infection: current status and future prospects on diagnostic, therapeutic and control challenges. Antibiotics (Basel) 12 (2), 191. doi: 10.3390/antibiotics12020191
Elbestawy, M. K. M., El-Sherbiny, G. M., Moghannem, S. A. (2023). Antibacterial, Antibiofilm and Anti-Inflammatory Activities of Eugenol Clove Essential Oil against Resistant Helicobacter pylori. Molecules 28, 2448. doi: 10.3390/molecules28062448
Escandón, R. A., Del Campo, M., López-Solis, R., Obreque-Slier, E., Toledo, H. (2016). Antibacterial effect of kaempferol and (–)-epicatechin on helicobacter pylori. Eur. Food Res. Technol. 242, 1495–1502. doi: 10.1007/s00217-016-2650-z
Escobedo-Hinojosa, W. I., Del Carpio, J. D., Palacios-Espinosa, J. F., Romero, I. (2012). Contribution to the ethnopharmacological and anti-Helicobacter pylori knowledge of Cyrtocarpa procera Kunth (Anacardiaceae). J. Ethnopharmacology 143, 363–371. doi: 10.1016/j.jep.2012.07.001
Fallone, C. A., Chiba, N., Van Zanten, S. V., Fischbach, L., Gisbert, J. P., Hunt, R. H., et al. (2016). The Toronto consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology 151 (1), 51–69.e14. doi: 10.1053/j.gastro.2016.04.006
Funatogawa, K., Hayashi, S., Shimomura, H., Yoshida, T., Hatano, T., Ito, H., et al. (2004). Antibacterial activity of hydrolyzable tannins derived from medicinal plants against Helicobacter pylori. Microbiol. Immunol. 48, 251–261. doi: 10.1111/j.1348-0421.2004.tb03521.x
Gao, T., Hou, M., Zhang, B., Pan, X., Liu, C., Sun, C., et al. (2021). Effects of cranberry beverages on oxidative stress and gut microbiota in subjects with Helicobacter pylori infection: a randomized, double-blind, placebo-controlled trial. Food Funct. 12, 6878–6888. doi: 10.1039/D1FO00467K
Gonzalez, A., Casado, J., Lanas, A. (2021). Fighting the antibiotic crisis: flavonoids as promising antibacterial drugs against Helicobacter pylori infection. Front. Cell. Infection Microbiol. 11. doi: 10.3389/fcimb.2021.709749
Gottesmann, M., Goycoolea, F. M., Steinbacher, T., Menogni, T., Hensel, A. (2020). Smart drug delivery against Helicobacter pylori: pectin-coated, mucoadhesive liposomes with antiadhesive activity and antibiotic cargo. Appl. Microbiol. Biotechnol. 104, 5943–5957. doi: 10.1007/s00253-020-10647-3
Grande, R., Carradori, S., Puca, V., Vitale, I., Angeli, A., Nocentini, A., et al. (2021). Selective inhibition of Helicobacter pylori carbonic anhydrases by Carvacrol and Thymol could impair biofilm production and the release of outer membrane vesicles. Int. J. Mol. Sci. 22, 11583. doi: 10.3390/ijms222111583
Haftcheshmeh, S. M., Abedi, M., Mashayekhi, K., Mousavi, M. J., Navashenaq, J. G., Mohammadi, A., et al. (2022). Berberine as a natural modulator of inflammatory signaling pathways in the immune system: Focus on NF-κB , JAK/STAT, and MAPK. Phytotherapy Res. 36, 1216–1230. doi: 10.1002/ptr.7407
Hamasaki, N., Ishii, E., Tominaga, K., Tezuka, Y., Nagaoka, T., Kadota, S., et al. (2000). Highly Selective Antibacterial Activity of Novel Alkyl Quinolone Alkaloids from a Chinese Herbal Medicine, Gosyuyu (Wu-Chu-Yu), against Helicobacter pylori In Vitro. Microbiol. Immunol. 44, 9–15. doi: 10.1111/j.1348-0421.2000.tb01240.x
He, S., Almalki, A. A., Rafeeq, M. M., Sain, Z. M., Alqosaibi, A. I., Alnamshan, M. M., et al. (2022a). Targeting cytotoxin-associated antigen A, a virulent factor of helicobacter pylori-associated gastric cancer: structure-based in silico screening of natural compounds. Molecules 27, 732. doi: 10.3390/molecules27030732
He, Y., Zhang, X., Li, M., Zheng, N., Zhao, S., Wang, J. (2022b). Coptisine: A natural plant inhibitor of ruminal bacterial urease screened by molecular docking. Sci. Total Environ. 808, 151946. doi: 10.1016/j.scitotenv.2021.151946
Helicobacter Pylori Study Group, C.S.O.G., Chinese Medical Association (2022). 2022 Chinese national clinical practice guideline on Helicobacter pylori eradication treatment. Chin. J. Digestion 135 (24), 2899–2910. doi: 10.1097/CM9.0000000000002546
Hu, Q., Zhou, M., Wei, S. (2018). Progress on the antimicrobial activity research of clove oil and eugenol in the food antisepsis field. J. Food Sci. 83, 1476–1483. doi: 10.1111/1750-3841.14180
Jang, S. H., Lim, J. W., Kim, H. (2009). Beta-carotene inhibits Helicobacter pylori-induced expression of inducible nitric oxide synthase and cyclooxygenase-2 in human gastric epithelial AGS cells. J. Physiol. Pharmacol. 60 (Suppl 7), 131–137.
Jang, S. H., Lim, J. W., Morio, T., Kim, H. (2012). Lycopene inhibits Helicobacter pylori-induced ATM/ATR-dependent DNA damage response in gastric epithelial AGS cells. Free Radic. Biol. Med. 52, 607–615. doi: 10.1016/j.freeradbiomed.2011.11.010
Judaki, A., Rahmani, A., Feizi, J., Asadollahi, K., Hafezi Ahmadi, M. R. (2017). Curcumin in combination with triple therapy regimes ameliorates oxidative stress and histopathologic changes in chronic gastritis-associated Helicobacter pylori infection. Arquivos Gastroenterologia 54, 177–182. doi: 10.1590/s0004-2803.201700000-18
Kamath, B. S., Srikanta, B. M., Dharmesh, S. M., Sarada, R., Ravishankar, G. A. (2008). Ulcer preventive and antioxidative properties of astaxanthin from Haematococcus pluvialis. Eur. J. Pharmacol. 590, 387–395. doi: 10.1016/j.ejphar.2008.06.042
Kang, H., Kim, H. (2017). Astaxanthin and β-carotene in Helicobacter pylori-induced gastric inflammation: A mini-review on action mechanisms. J. Cancer Prev. 22, 57–61. doi: 10.15430/JCP.2017.22.2.57
Katz, L., Baltz, R. H. (2016). Natural product discovery: past, present, and future. J. Ind. Microbiol. Biotechnol. 43, 155–176. doi: 10.1007/s10295-015-1723-5
Khan, M. S. A., Khundmiri, S. U. K., Khundmiri, S. R., Al-Sanea, M. M., Mok, P. L. (2018). Fruit-derived polysaccharides and terpenoids: recent update on the gastroprotective effects and mechanisms. Front. Pharmacol. 9. doi: 10.3389/fphar.2018.00569
Khonche, A., Biglarian, O., Panahi, Y., Valizadegan, G., Soflaei, S., Ghamarchehreh, M., et al. (2016). Adjunctive therapy with curcumin for peptic ulcer: a randomized controlled trial. Drug Res. 66, 444–448. doi: 10.1055/s-0042-109394
Kim, S. H., Kim, H. (2021). Inhibitory effect of astaxanthin on gene expression changes in Helicobacter pylori-infected human gastric epithelial cells. Nutrients 13, 4281. doi: 10.3390/nu13124281
Kim, J. H., Lee, J., Choi, I. J., Kim, Y.-I., Kwon, O., Kim, H., et al. (2018a). Dietary carotenoids intake and the risk of gastric cancer: A case—Control study in Korea. Nutrients 10, 1031. doi: 10.3390/nu10081031
Kim, S., Lim, J., Kim, H. (2018b). Astaxanthin inhibits mitochondrial dysfunction and interleukin-8 expression in Helicobacter pylori-infected gastric epithelial cells. Nutrients 10, 1320. doi: 10.3390/nu10091320
Korona-Glowniak, I., Glowniak-Lipa, A., Ludwiczuk, A., Baj, T., Malm, A. (2020). The in vitro activity of essential oils against Helicobacter pylori growth and urease activity. Molecules 25, 586. doi: 10.3390/molecules25030586
Krausse, R., Bielenberg, J., Blaschek, W., Ullmann, U. (2004). In vitro anti-helicobacter pylori activity of extractum liquiritiae, glycyrrhizin and its metabolites. J. Antimicrob. Chemother. 54, 243–246. doi: 10.1093/jac/dkh287
Krzyżek, P., Migdał, P., Paluch, E., Karwańska, M., Wieliczko, A., Gościniak, G. (2021). Myricetin as an Antivirulence Compound Interfering with a Morphological Transformation into Coccoid Forms and Potentiating Activity of Antibiotics against Helicobacter pylori. Int. J. Mol. Sci. 22 (5), 2695. doi: 10.3390/ijms22052695
Krzyzek, P., Junka, A., Slupski, W., Dolowacka-Jozwiak, A., Plachno, B. J., Sobiecka, A., et al. (2021). Antibiofilm and Antimicrobial-Enhancing Activity of Chelidonium majus and Corydalis cheilanthifolia Extracts against Multidrug-Resistant Helicobacter pylori. Pathogens 10 (8), 1033. doi: 10.3390/pathogens10081033
Kwiecien, S., Magierowski, M., Majka, J., Ptak-Belowska, A., Wojcik, D., Sliwowski, Z., et al. (2019). Curcumin: A potent protectant against esophageal and gastric disorders. Int. J. Mol. Sci. 20, 1477. doi: 10.3390/ijms20061477
Lai, J., Tang, Y., Yang, F., Chen, J., Huang, F. H., Yang, J., et al. (2022). Targeting autophagy in ethnomedicine against human diseases. J. Ethnopharmacol 282, 114516. doi: 10.1016/j.jep.2021.114516
Lee, J., Lim, J. W., Kim, H. (2022). Astaxanthin inhibits matrix metalloproteinase expression by suppressing PI3K/AKT/mTOR activation in Helicobacter pylori-infected gastric epithelial cells. Nutrients 14, 3427. doi: 10.3390/nu14163427
Leja, M., Grinberga-Derica, I., Bilgilier, C., Steininger, C. (2019). Review: Epidemiology of Helicobacter pylori infection. Helicobacter 24 Suppl 1, e12635. doi: 10.1111/hel.12635
Li, Y., Li, X., Tan, Z. (2021). An overview of traditional Chinese medicine therapy for Helicobacter pylori-related gastritis. Helicobacter 26, e12799. doi: 10.1111/hel.12799
Lin, Y. T., Kwon, Y. I., Labbe, R. G., Shetty, K. (2005). Inhibition of Helicobacter pylori and associated urease by oregano and cranberry phytochemical synergies. Appl. Environ. Microbiol. 71, 8558–8564. doi: 10.1128/AEM.71.12.8558-8564.2005
Liu, Q., Tang, J., Chen, S., Hu, S., Shen, C., Xiang, J., et al. (2022). Berberine for gastric cancer prevention and treatment: Multi-step actions on the Correa's cascade underlie its therapeutic effects. Pharmacol. Res. 184, 106440. doi: 10.1016/j.phrs.2022.106440
Lu, Q., Zhang, Z., Xu, Y., Chen, Y., Li, C. (2022). Sanguinarine, a major alkaloid from Zanthoxylum nitidum (Roxb.) DC., inhibits urease of Helicobacter pylori and jack bean: Susceptibility and mechanism. J. Ethnopharmacol 295, 115388. doi: 10.1016/j.jep.2022.115388
Mahady, G. B., Pendland, S. L., Stoia, A., Chadwick, L. R. (2003). In vitro susceptibility of helicobacter pylori to isoquinoline alkaloids from sanguinaria canadensis and hydrastis canadensis. Phytother. Res. 17, 217–221. doi: 10.1002/ptr.1108
Mahapatra, A. D., Bhowmik, P., Banerjee, A., Das, A., Ojha, D., Chattopadhyay, D. (2019). “Ethnomedicinal wisdom: an approach for antiviral drug development,” in New look to phytomedicine (Amsterdam, Netherlands: Elsevier), 35–61.
Malfertheiner, P., Megraud, F., O'morain, C. A., Gisbert, J. P., Kuipers, E. J., Axon, A. T., et al. (2017). Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 66, 6–30. doi: 10.1136/gutjnl-2016-312288
Marshall, B. J., Warren, J. R. (1984). Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1, 1311–1315. doi: 10.1016/S0140-6736(84)91816-6
Matsumoto, H., Shiotani, A., Graham, D. Y. (2019). Current and future treatment of Helicobacter pylori infections. Adv. Exp. Med. Biol. 1149, 211–225. doi: 10.1007/5584_2019_367
Megraud, F., Bruyndonckx, R., Coenen, S., Wittkop, L., Huang, T.-D., Hoebeke, M., et al. (2021). Helicobacter pylori resistance to antibiotics in Europe in 2018 and its relationship to antibiotic consumption in the community. Gut 70, 1815–1822. doi: 10.1136/gutjnl-2021-324032
Merwin, N. J., Mousa, W. K., Dejong, C. A., Skinnider, M. A., Cannon, M. J., Li, H., et al. (2020). DeepRiPP integrates multiomics data to automate discovery of novel ribosomally synthesized natural products. Proc. Natl. Acad. Sci. U.S.A. 117, 371–380. doi: 10.1073/pnas.1901493116
Michalkova, R., Kello, M., Cizmarikova, M., Bardelcikova, A., Mirossay, L., Mojzis, J. (2023). Chalcones and gastrointestinal cancers: experimental evidence. Int. J. Mol. Sci. 24, 5964. doi: 10.3390/ijms24065964
Miwa, H., Nagahara, A., Asakawa, A., Arai, M., Oshima, T., Kasugai, K., et al. (2022). Evidence-based clinical practice guidelines for functional dyspepsia 2021. J. Gastroenterol. 57, 47–61. doi: 10.1007/s00535-021-01843-7
Moraes, T. M., Kushima, H., Moleiro, F. C., Santos, R. C., Rocha, L. R. M., Marques, M. O., et al. (2009). Effects of limonene and essential oil from Citrus aurantium on gastric mucosa: role of prostaglandins and gastric mucus secretion. Chemico-biological Interact. 180, 499–505. doi: 10.1016/j.cbi.2009.04.006
Naguib, Y. M. (2000). Antioxidant activities of astaxanthin and related carotenoids. J. Agric. Food Chem. 48, 1150–1154. doi: 10.1021/jf991106k
Ngan, L. T. M., Tan, M. T., Hoang, N. V. M., Thanh, D. T., Linh, N. T. T., Hoa, T. T. H., et al. (2021). Antibacterial activity of Hibiscus rosa-sinensis L. red flower against antibiotic-resistant strains of Helicobacter pylori and identification of the flower constituents. Braz. J. Med. Biol. Res. 54 (7), e10889. doi: 10.1590/1414-431x2020e10889
Ngo, L. T., Okogun, J. I., Folk, W. R. (2013). 21st Century natural product research and drug development and traditional medicines. Natural Product Rep. 30, 584. doi: 10.1039/c3np20120a
Nohynek, L. J., Alakomi, H.-L., Kähkönen, M. P., Heinonen, M., Helander, I. M., Oksman-Caldentey, K.-M., et al. (2006). Berry phenolics: antimicrobial properties and mechanisms of action against severe human pathogens. Nutr. Cancer 54, 18–32. doi: 10.1207/s15327914nc5401_4
O'Morain, N. R., Dore, M. P., O'connor, A. J. P., Gisbert, J. P., O'morain, C. A. (2018). Treatment of Helicobacter pylori infection in 2018. Helicobacter 23 (Suppl 1), e12519. doi: 10.1111/hel.12519
Oliveira, I. S., Da Silva, F. V., Viana, A. F. S. C., Dos Santos, M. R. V., Quintans-Júnior, L. J., Martins, M. D. C. C., et al. (2012). Gastroprotective activity of carvacrol on experimentally induced gastric lesions in rodents. Naunyn-Schmiedeberg's Arch. Pharmacol. 385, 899–908. doi: 10.1007/s00210-012-0771-x
Park, Y., Lee, H., Lim, J. W., Kim, H. (2019). Inhibitory effect of β-carotene on Helicobacter pylori-induced TRAF expression and hyper-proliferation in gastric epithelial cells. Antioxidants 8, 637. doi: 10.3390/antiox8120637
Périco, L. L., Heredia-Vieira, S. C., Beserra, F. P., De Cássia Dos Santos, R., Weiss, M. B., Resende, F. A., et al. (2015). Does the gastroprotective action of a medicinal plant ensure healing effects? An integrative study of the biological effects of Serjania marginata Casar. (Sapindaceae) in rats. J. Ethnopharmacology 172, 312–324. doi: 10.1016/j.jep.2015.06.025
Roszczenko-Jasińska, P., Wojtyś, M. I., Jagusztyn-Krynicka, E. K. (2020). Helicobacter pylori treatment in the post-antibiotics era-searching for new drug targets. Appl. Microbiol. Biotechnol. 104, 9891–9905. doi: 10.1007/s00253-020-10945-w
Rozza, A. L., Moraes Tde, M., Kushima, H., Tanimoto, A., Marques, M. O., Bauab, T. M., et al. (2011). Gastroprotective mechanisms of Citrus lemon (Rutaceae) essential oil and its majority compounds limonene and β-pinene: involvement of heat-shock protein-70, vasoactive intestinal peptide, glutathione, sulfhydryl compounds, nitric oxide and prostaglandin E2. Chem. Biol. Interact. 189, 82–89. doi: 10.1016/j.cbi.2010.09.031
Safavi, M., Shams-Ardakani, M., Foroumadi, A. (2015). Medicinal plants in the treatment of Helicobacter pylori infections. Pharm. Biol. 53, 939–960. doi: 10.3109/13880209.2014.952837
Savoldi, A., Carrara, E., Graham, D. Y., Conti, M., Tacconelli, E. (2018). Prevalence of antibiotic resistance in Helicobacter pylori: A systematic review and meta-analysis in world health organization regions. Gastroenterology 155, 1372–1382.e1317. doi: 10.1053/j.gastro.2018.07.007
Shah, S. C., Iyer, P. G., Moss, S. F. (2021). AGA clinical practice update on the management of refractory Helicobacter pylori infection: expert review. Gastroenterology 160, 1831–1841. doi: 10.1053/j.gastro.2020.11.059
Shetty, N. P., Prabhakaran, M., Srivastava, A. K. (2021). Pleiotropic nature of curcumin in targeting multiple apoptotic-mediated factors and related strategies to treat gastric cancer: A review. Phytotherapy Res. 35, 5397–5416. doi: 10.1002/ptr.7158
Sisto, F., Carradori, S., Guglielmi, P., Spano, M., Secci, D., Granese, A., et al. (2021). Synthesis and Evaluation of Thymol-Based Synthetic Derivatives as Dual-Action Inhibitors against Different Strains of H. pylori and AGS Cell Line. Molecules 26 1829. doi: 10.3390/molecules26071829
Sisto, F., Carradori, S., Guglielmi, P., Traversi, C. B., Spano, M., Sobolev, A. P., et al. (2020). Synthesis and biological evaluation of carvacrol-based derivatives as dual inhibitors of H. pylori strains and AGS cell proliferation. Pharmaceuticals 13, 405. doi: 10.3390/ph13110405
Sisto, F., Scaltrito, M. M., Masia, C., Bonomi, A., Coccè, V., Marano, G., et al. (2016). In vitro activity of artemisone and artemisinin derivatives against extracellular and intracellular Helicobacter pylori. Int. J. Antimicrob. Agents 48, 101–105. doi: 10.1016/j.ijantimicag.2016.03.018
Solnick, J. V., Schauer, D. B. (2001). Emergence of diverse Helicobacter species in the pathogenesis of gastric and enterohepatic diseases. Clin. Microbiol. Rev. 14, 59–97. doi: 10.1128/CMR.14.1.59-97.2001
Sousa, C., Ferreira, R., Azevedo, N. F., Oleastro, M., Azeredo, J., Figueiredo, C., et al. (2022). Helicobacter pylori infection: from standard to alternative treatment strategies. Crit. Rev. Microbiol. 48, 376–396. doi: 10.1080/1040841X.2021.1975643
Steinmann, J., Buer, J., Pietschmann, T., Steinmann, E. (2013). Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea. Br. J. Pharmacol. 168, 1059–1073. doi: 10.1111/bph.12009
Stenger Moura, F. C., Cechinel-Filho, V., Greco, F. A., Venzon, L., Meurer, M. C., França, T., et al. (2021). Taxifolin and gastro-adhesive microparticles containing taxifolin promotes gastric healing in vivo, inhibits Helicobacter pylori in vitro and proton pump reversibly in silico. Chem. Biol. Interact. 339, 109445. doi: 10.1016/j.cbi.2021.109445
Sugano, K., Tack, J., Kuipers, E. J., Graham, D. Y., El-Omar, E. M., Miura, S., et al. (2015). Kyoto global consensus report on Helicobacter pylori gastritis. Gut 64, 1353–1367. doi: 10.1136/gutjnl-2015-309252
Sukri, A., Hanafiah, A., Mohamad Zin, N., Kosai, N. R. (2020). Epidemiology and role of Helicobacter pylori virulence factors in gastric cancer carcinogenesis. APMIS 128, 150–161. doi: 10.1111/apm.13034
Sukuru, S. C., Jenkins, J. L., Beckwith, R. E., Scheiber, J., Bender, A., Mikhailov, D., et al. (2009). Plate-based diversity selection based on empirical HTS data to enhance the number of hits and their chemical diversity. J. Biomol Screen 14, 690–699. doi: 10.1177/1087057109335678
Tacconelli, E., Carrara, E., Savoldi, A., Harbarth, S., Mendelson, M., Monnet, D. L., et al. (2018). Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 18, 318–327. doi: 10.1016/S1473-3099(17)30753-3
Takagi, K., Harada, M. (1969). [Pharmacological studies on herb paeony root. II. Anti-inflammatory effect, inhibitory effect on gastric juice secretion, preventive effect on stress ulcer, antidiuretic effect of paeoniflorin and combined effects with licorice component Fm 100]. Yakugaku Zasshi 89, 887–892. doi: 10.1248/yakushi1947.89.7_887
Takao, Y., Kuriyama, I., Yamada, T., Mizoguchi, H., Yoshida, H., Mizushina, Y. (2012). Antifungal properties of Japanese cedar essential oil from waste wood chips made from used sake barrels. Mol. Med. Rep. 5, 1163–1168. doi: 10.3892/mmr.2012.821
Takeuchi, H., Trang, V. T., Morimoto, N., Nishida, Y., Matsumura, Y., Sugiura, T. (2014). Natural products and food components with anti-Helicobacter pylori activities. World J. Gastroenterol. 20, 8971–8978. doi: 10.3748/wjg.v20.i27.8971
Tang, Q., Ma, Z., Tang, X., Liu, Y., Wu, H., Peng, Y., et al. (2023). Coptisine inhibits Helicobacter pylori and reduces the expression of CagA to alleviate host inflammation in vitro and in vivo. J. Ethnopharmacol. 314, 116618. doi: 10.1016/j.jep.2023.116618
Tharmalingam, N., Kim, S.-H., Park, M., Woo, H. J., Kim, H. W., Yang, J. Y., et al. (2014). Inhibitory effect of piperine on Helicobacter pylori growth and adhesion to gastric adenocarcinoma cells. Infect. Agents Cancer 9, 43. doi: 10.1186/1750-9378-9-43
Thawabteh, A., Juma, S., Bader, M., Karaman, D., Scrano, L., Bufo, S., et al. (2019). The biological activity of natural alkaloids against herbivores, cancerous cells and pathogens. Toxins 11, 656. doi: 10.3390/toxins11110656
Tsuchiya, H., Iinuma, M. (2000). Reduction of membrane fluidity by antibacterial sophoraflavanone G isolated from Sophora exigua. Phytomedicine 7, 161–165. doi: 10.1016/S0944-7113(00)80089-6
Ultee, A., Bennik, M. H. J., Moezelaar, R. (2002). The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 68, 1561–1568. doi: 10.1128/AEM.68.4.1561-1568.2002
Vale, F. F., Oleastro, M. (2014). Overview of the phytomedicine approaches against Helicobacter pylori. World J. Gastroenterol. 20, 5594–5609. doi: 10.3748/wjg.v20.i19.5594
Villa-Ruano, N., Becerra-Martínez, E., Cruz-Durán, R., Zarate-Reyes, J. A., Landeta-Cortés, G., Romero-Arenas, O. (2018). Volatile profiling, insecticidal, antibacterial and antiproliferative properties of the essential oils of bursera glabrifolia leaves. Chem. Biodiversity 15, e1800354. doi: 10.1002/cbdv.201800354
Wang, Y. C. (2014). Medicinal plant activity on Helicobacter pylori related diseases. World J. Gastroenterol. 20, 10368–10382. doi: 10.3748/wjg.v20.i30.10368
Wang, Y.-C., Huang, K.-M. (2013). In vitro anti-inflammatory effect of apigenin in the Helicobacter pylori-infected gastric adenocarcinoma cells. Food Chem. Toxicol. 53, 376–383. doi: 10.1016/j.fct.2012.12.018
Wang, L., Wang, X., Zhang, S.-L., Zhu, X.-M., Liu, Y.-Q., Song, Z.-J., et al. (2017). Gastroprotective effect of palmatine against acetic acid-induced gastric ulcers in rats. J. Natural Medicines 71, 257–264. doi: 10.1007/s11418-016-1057-2
Wang, X., Willen, R., Wadstrom, T. (2000). Astaxanthin-rich algal meal and vitamin C inhibit Helicobacter pylori infection in BALB/cA mice. Antimicrob. Agents Chemothe 44, 2452–2457. doi: 10.1128/AAC.44.9.2452-2457.2000
Wang, Q., Yao, C., Li, Y., Luo, L., Xie, F., Xiong, Q., et al. (2023). Effect of polyphenol compounds on Helicobacter pylori eradication: a systematic review with meta-analysis. BMJ Open 13, e062932. doi: 10.1136/bmjopen-2022-062932
Wei, A., Shibamoto, T. (2010). Antioxidant/lipoxygenase inhibitory activities and chemical compositions of selected essential oils. J. Agric. Food Chem. 58, 7218–7225. doi: 10.1021/jf101077s
Weissman, K. J., Leadlay, P. F. (2005). Combinatorial biosynthesis of reduced polyketides. Nat. Rev. Microbiol. 3, 925–936. doi: 10.1038/nrmicro1287
Wen, J., Wu, S., Ma, X., Zhao, Y. (2022). Zuojin Pill attenuates Helicobacter pylori-induced chronic atrophic gastritis in rats and improves gastric epithelial cells function in GES-1 cells. J. Ethnopharmacol 285, 114855. doi: 10.1016/j.jep.2021.114855
Widelski, J., Okińczyc, P., Paluch, E., Mroczek, T., Szperlik, J., Żuk, M., et al. (2022). The Antimicrobial Properties of Poplar and Aspen–Poplar Propolises and Their Active Components against Selected Microorganisms, including Helicobacter pylori. Pathogens 11, 191. doi: 10.3390/pathogens11020191
Wittschier, N., Faller, G., Hensel, A. (2009). Aqueous extracts and polysaccharides from liquorice roots (Glycyrrhiza glabra L.) inhibit adhesion of Helicobacter pylori to human gastric mucosa. J. Ethnopharmacology 125, 218–223. doi: 10.1016/j.jep.2009.07.009
Wu, X., Li, X., Dang, Z., Jia, Y. (2018). Berberine demonstrates anti-inflammatory properties in Helicobacter pylori-infected mice with chronic gastritis by attenuating the Th17 response triggered by the B cell-activating factor. J. Cell Biochem. 119, 5373–5381. doi: 10.1002/jcb.26681
Wu, H., Sun, Q., Dong, H., Qiao, J., Lin, Y., Yu, C., et al. (2023). Gastroprotective action of the extract of Corydalis yanhusuo in Helicobacter pylori infection and its bioactive component, dehydrocorydaline. J. Ethnopharmacol 307, 116173. doi: 10.1016/j.jep.2023.116173
Yahiro, K., Shirasaka, D., Tagashira, M., Wada, A., Morinaga, N., Kuroda, F., et al. (2005). Inhibitory effects of polyphenols on gastric injury by Helicobacter pylori vacA toxin. Helicobacter 10, 231–239. doi: 10.1111/j.1523-5378.2005.00315.x
Yang, R. J., Li, J. J., Wang, J. R., Wang, Y. F., Ma, F. W., Zhai, R., et al. (2022). Kaempferol inhibits the growth of Helicobacter pylori in a manner distinct from antibiotics. J. Food Biochem. 46 (9), e14210. doi: 10.1111/jfbc.14210
Yang, L., Liu, X., Zhu, J., Zhang, X., Li, Y., Chen, J., et al. (2023). Progress in traditional Chinese medicine against chronic gastritis: From chronic non-atrophic gastritis to gastric precancerous lesions. Heliyon 9, e16764. doi: 10.1016/j.heliyon.2023.e16764
Yang, T., Wang, R., Liu, H., Wang, L., Li, J., Wu, S., et al. (2021). Berberine regulates macrophage polarization through IL-4-STAT6 signaling pathway in Helicobacter pylori-induced chronic atrophic gastritis. Life Sci. 266, 118903. doi: 10.1016/j.lfs.2020.118903
Yuan, C., Adeloye, D., Luk, T. T., Huang, L., He, Y., Xu, Y., et al. (2022). The global prevalence of and factors associated with Helicobacter pylori infection in children: a systematic review and meta-analysis. Lancet Child Adolesc. Health 6, 185–194. doi: 10.1016/S2352-4642(21)00400-4
Zhang, S., Chen, D.-C., Chen, L.-M. (2019). Facing a new challenge: the adverse effects of antibiotics on gut microbiota and host immunity. Chin. Med. J. 132, 1135–1138. doi: 10.1097/CM9.0000000000000245
Keywords: Helicobacter pylori, eradication, antibiotic resistance, ethnomedicine, natural products, complementary therapy
Citation: Deng R, Chen X, Zhao S, Zhang Q and Shi Y (2024) The effects and mechanisms of natural products on Helicobacter pylori eradication. Front. Cell. Infect. Microbiol. 14:1360852. doi: 10.3389/fcimb.2024.1360852
Received: 24 December 2023; Accepted: 13 February 2024;
Published: 28 February 2024.
Edited by:
Ritesh Sevalkar, University of Alabama at Birmingham, United StatesReviewed by:
Tooba Mahboob, UCSI University, MalaysiaCopyright © 2024 Deng, Chen, Zhao, Zhang and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanyan Shi, c2hpeWFueWFuQGJqbXUuZWR1LmNu
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.