- 1State Key Laboratory of Mariculture Biobreeding and Sustainable Goods, Yellow Sea Fisheries Research Institute (YSFRI), Chinese Academy of Fishery Sciences (CAFS), Qingdao, China
- 2Laoshan National Laboratory, Qingdao, China
- 3Key Laboratory of Maricultural Organism Disease Control, Ministry of Agriculture and Rural Affairs, Qingdao Key Laboratory of Mariculture Epidemiology and Biosecurity, Qingdao, Shandong, China
- 4School of Marine Science and Fisheries, Jiangsu Ocean University, Lianyungang, China
- 5Network of Aquaculture Centres in Asia-Pacific, Bangkok, Thailand
Recent reports have shown that wild crabs may be important hosts involved in the transmission and spread of the parasitic Hematodinium in cultured marine crustaceans. Therefore, monitoring the prevalence of Hematodinium infections in wild crabs is necessary to develop effective strategies for the prevention and control of Hematodinium disease. Here we report a wild crab species, Macrophthalmus (Macrophthalmus) abbreviatus Manning & Holthuis, 1981, as a new natural host for Hematodinium sp. infection. It is one of the common wild crab species dwelling in the ponds or waterways connected to the polyculture ponds located on the coast of Rizhao or Weifang, Shandong Peninsula, China. According to the results of PCR detection and phylogenetic analysis targeting the internal transcribed spacer 1 (ITS 1) region, these Hematodinium sp. isolates were identified as H. perezi and fell into the genotype II category within H. perezi. A high monthly prevalence of H. perezi infection was observed during the 2021–2022 field survey, ranging from 33.3% to 90.6% in M. abbreviatus originating from Weifang (n=304 wild crabs) and from 53.6% to 92.9% in those from Rizhao (n=42 wild crabs). Artificial inoculation infection experiments demonstrated that M. abbreviatus could be infected by H. perezi, and massive Hematodinium cells and typical histopathological changes were observed in the hepatopancreas and gill tissues of the infected crabs. To our knowledge, this is the first report of M. abbreviatus as a new natural host for H. perezi infection. Results in the present study extend the known host spectrum for this emerging parasite pathogen, and also provide valuable information for epidemic surveillance of the Hematodinium disease as well.
1 Introduction
The parasitic dinoflagellate Hematodinium spp., first reported in 1931 (Chatton and Poisson, 1931), infect more than 40 crustacean species worldwide (Li et al., 2021a). This parasite has been recognized as an important pathogen causing massive mortality and extensive economic losses to some economic crustaceans (Field et al., 1992; Taylor and Khan, 1995; Messick and Shields, 2000; Shields et al., 2007; Xu et al., 2007; Li et al., 2008; Ryazanova et al., 2010; Xu et al., 2010; Albalat et al., 2012; Small, 2012; Li et al., 2013; Smith et al., 2015; Stentiford et al., 2015; Wang et al., 2017; Huang et al., 2021; Ryazanova et al., 2021). Lack of knowledge in the pathogenic and transmission mechanisms of Hematodinium in crustaceans has hindered the development of effective management strategies for the Hematodinium disease (caused by the pathogenic Hematodinium spp.).
To date, six wild crab species, i.e. Helice tientsinensis, Uca arcuate, Hemigrapsus penicillatus, Helice wuana, Macrophthalmus japonicas, and Hemigrapsus takanoi, inhabiting the waterways connected to the polyculture ponds of marine crustaceans, have been identified as hosts for Hematodinium infections (Huang et al., 2019; Li et al., 2021b; Gong et al., 2023). The prevalence of H. perezi in H. tientsinensis (up to 30.1%) is much higher than in the other five wild crab species (up to 20.0%), and is positively correlated with the prevalence of H. perezi in cultured Portunus trituberculatus (Li et al., 2021b; Gong et al., 2023). H. perezi can be transmitted directly from H. tientsinensis to P. trituberculatus, as evidenced by the fact that healthy P. trituberculatus are infected by H. perezi, when being cohabitated with H. perezi infected-H. tientsinensis (Huang et al., 2021). The potential role of H. tientsinensis is highlighted in the transmission and maintenance of Hematodinium in the integrative culture systems (Huang et al., 2019; Huang et al., 2021).
In the years of 2021 and 2022, we carried out epidemiological surveys for the Hematodinium disease in wild and cultured populations of marine crustaceans, inhabiting along the coast (mainly in Rizhao and Weifang) of the Shandong Peninsula. This area is a major mariculture region for P. trituberculatus, contributing to over 80% of the gross outcome in northern China (data from China Fishery Statistical Yearbook in 2021). Among the wild crab species sampled in the surveys, M. abbreviatus was finally identified as a novel host for H. perezi, based on evidences from morphological observation, molecular detection, experimental challenge tests, and histopathological examination. The prevalence of H. perezi in M. abbreviatus was also presented. The results in the present study provide important information on the H. perezi epidemics, and will contribute to the development of an effective prevention and management strategy for this parasite in marine crustacean pond systems.
2 Materials and methods
2.1 Crab samples and the experimental conditions
During June to October in 2021 and June to August in 2022, a total of 346 M. abbreviatus crabs were collected from the waterways connecting to polyculture ponds, located on the coast of Rizhao or Weifang, Shandong Province, China. These crabs were transported to the laboratory in plastic tanks with constant aeration.
In the laboratory, crabs were housed individually in perforated plastic boxes (108×108 × 42 mm, 24 holes with diameter of 3 mm) and cultured with aeration in the tanks, containing 40 L of seawater (24 ± 1°C, salinity of 30 ppt). They were fed with clams, and the residuals of clam tissue were removed timely after each feeding. 80% of culturing seawater in each tank was exchanged every day. Crabs were acclimated for a week before artificial inoculation tests.
2.2 Hemolymph smear assay
Hemolymph smear assays were performed as described by Stentiford and Shields (2005). Briefly, 2–3 drops of hemolymph, drawn from the juncture (prior sterilization with 70% ethanol) between the basis and ischium of the 5th walking leg, were mixed with an aliquots of neutral red (0.04%, w/v). The hemolymph smear was then screened under a light microscope (Olympus, Japan) for diagnosing and observing the status of Hematodinium.
2.3 Molecular detections for Hematodinium
Genomic DNA (gDNA) of hemolymph (~30 μl) sampled from natural Hematodinium-infected crabs and abdominal muscle (~30 mg) sampled from artificial Hematodinium-infected crabs were extracted by using a TIANamp Blood DNA Kit (Tiangen, China) or a TIANamp Marine Animals DNA Kit (Tiangen, China) according to the manufacturer’s instructions, respectively.
Hematodinium were detected using nested PCR with two primer sets as previously described by Gong et al. (2023). The nested PCR targets an interval region between 18S ribosomal RNA gene and the first internal transcribed spacer (ITS 1). The outer primer set includes primers He.p-18sF1 (CAG CTC GTG CTG ATT ACG TCC C) and He.p-ITS1R1 (AAA GCC CTA ACC CCG CTA AAG G), and the inner primer set includes primers He.p-18sF2 (TCG TAA CAA GGT TTC CGT AGG T) and He.p-ITS1R2 (ATG GAG GAG TTC AGT GGT AGG C). The PCR reaction mix (25 μL) composed of 12.5 μl Premix PrimeSTAR HS (TaKaRa, Dalian, China), 1μl exacted gDNA (~10 ng), 1 μl forward and reverse primers (10 μM, each), and 9.5 μl sterile water, respectively. PCR amplifications were conducted at 95°C for 5 min, 35 cycles (94°C for 30 s, 60°C (for outer primers)/55°C (for inner primers) for 30 s, and 72°C for 60 s (for outer primers)/20 s (for inner primers)), and 72°C for 5 min. The amplicons were analyzed using 1.2% agarose gel electrophoresis, and positive products were further sequenced with the corresponding amplification primers in Sangon Biotech (Shanghai, China) Co., Ltd.
2.4 Phylogenetic analysis
Eight Hematodinium sp. ITS1 sequences (308-341 bp in length) were retrieved from naturally Hematodinium-infected M. abbreviatus in the present study, and deposited in GenBank with Accession numbers of OQ564394 (retrieved from one crab originating from Rizhao), and OQ564393, OQ564395-OQ564400 (retrieved from seven crabs originating from Weifang). These ITS 1 sequences were multiply aligned with other Hematodinium sp. sequences originated from different crustacean hosts (Li et al., 2021a; Li et al., 2021b; Gong et al., 2023), using the software Clustal X. A phylogenetic tree was constructed by using the neighbor-joining method with 1000 bootstrap replicates embedded in MEGA 7.0 (Kumar et al., 2016).
2.5 Challenging tests
To evaluate the susceptibility of M. abbreviatus to Hematodiniun sp., artificial inoculation infection experiments were performed according to the protocol in a previous report (Li et al., 2021b). Before inoculation infections, M. abbreviatus were examined for Hematodiniun using the hemolymph smear. Negatively examined M. abbreviatus were used for the challenge tests. Briefly, M. abbreviatus (n=15) was injected with 10 µl of hemolymph, extracted from a donor Helice tientsinensis heavily infected by H. perezi, and containing 1.46 x 104 parasites/µl, assessed by using a hemocytometer under a microscope. The gDNA extracted from a hemolymph sample was used for detection of Hematodiniun via the nested PCR assays. The PCR amplicons were confirmed after sequencing.
2.6 Histopathological analysis
Tissues (gills, heart, hepatopancreas, and muscle) dissected from Hematodiniun detection-negative or positive crabs were immediately immersed in Davidson’s AFA for 24 h. After fixation, these fixed tissues were transferred to serial alcohol and dimethylbenzene, and embedded in paraffin. The sections were stained with hematoxylin and eosin (H&E), using routine histological method as described by (Wheeler et al., 2007), and subsequently photographed under a light microscope (Olympus, Japan).
3 Results
3.1 Morphology of Hematodinium sp. in the hemolymph from naturally infected M. abbreviatus
Hematodinium sp. exhibited various shapes were observed in the hemolymph from the naturally Hematodinium-infected crabs, which indicates different developmental stages in the lifecycle of Hematodinium. They were ameboid trophonts (e.g., uninucleate, binuclear, trinuclear and multinucleate) (Figures 1A, B) and presumptive clump colonies (Figure 1C).
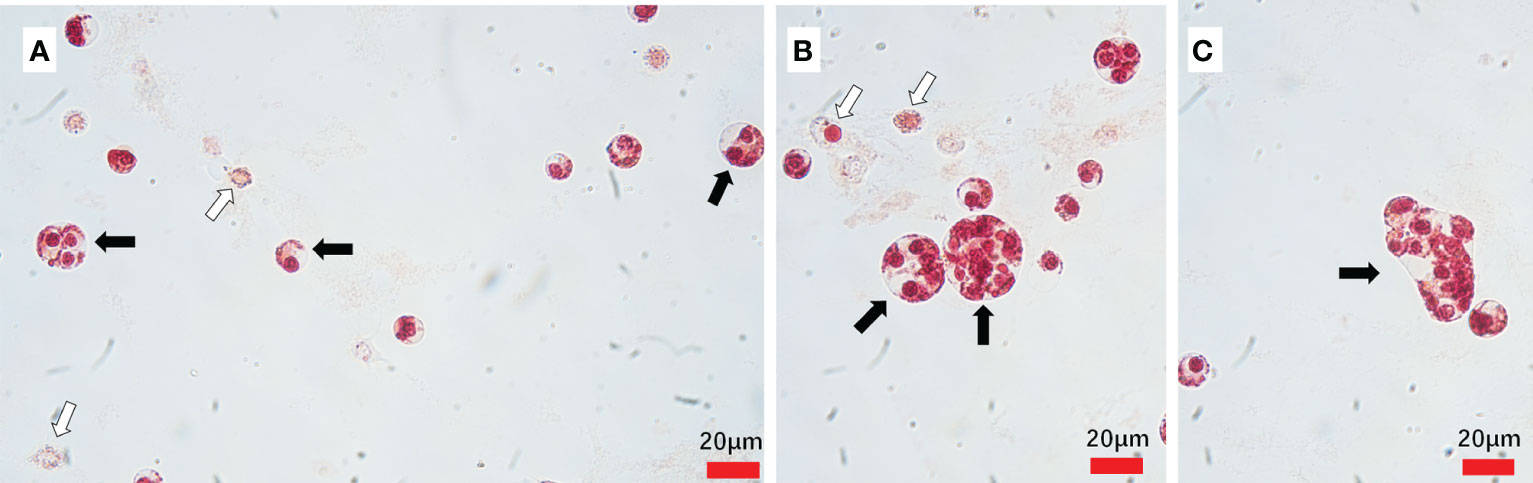
Figure 1 Hematodinium staining and examination in the hemolymph from naturally infected M. abbreviatus. Uninucleate, binuclear and trinuclear ameboid trophonts (A) Multinucleate ameboid trophonts (B) Putative clump colony (C). Black arrows indicate parasites and white arrow indicate host haemocytes.
3.2 Prevalence of Hematodinium infections in M. abbreviatus
Considerable prevalence of H. perezi infections existed in M. abbreviatus originated from Weifang (the averaged prevalence rate of 62.2%; n=304) or Rizhao (66.7%; n=42). On the month scale, the prevalence in specific varied from 33.3% to 90.6% in Weifang samples, and from 53.6% to 92.9% in Rizhao samples (Figure 2). The overall prevalence in these samples originated from the two regions during investigations in years 2021 and 2022 was 62.7% (n=346).
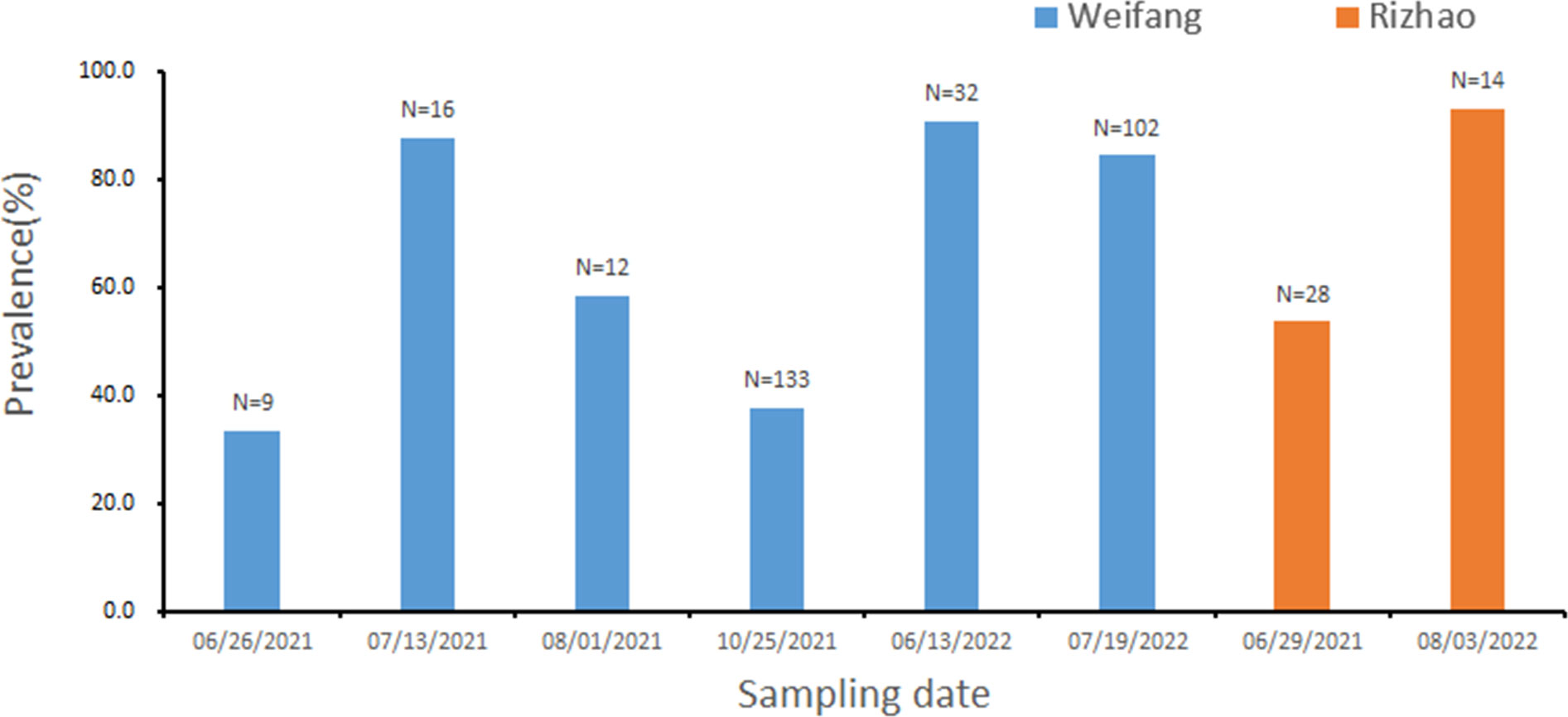
Figure 2 Prevalence of Hematodinium infections in M. abbreviatus, collected from Rizhao and Weifang during the sampling period. The total sample number are shown over each bar.
3.3 Phylogenetic and taxonomic analyses
Eight Hematodinium ITS 1 sequences (265 or 266 bp in length) were retrieved from M. abbreviatus collected from Weifang and Rizhao and they shared 99.6–100% sequence similarity with each other. In addition, multiple sequence alignments showed that they shared high similarity (97.8–100%) with those of H. perezi genotypes I and II, which infect crustacean species such as P. trituberculatus, S. paramamosain, P. monodon, and H. tientsinensis in China (Small et al., 2012; Wang et al., 2017; Huang et al., 2019; Li et al., 2021b), while they shared relatively lower similarity (95.9%-96.7%) with that of H. perezi genotype III isolates, infecting Callinectes sapidus in USA (Jensen et al., 2010), and other Hematodinium isolates (≤63.5%).
The phylogenetic analysis based on ITS1 sequences indicated that the eight Hematodinium isolates in the present study were all grouped into H. perezi genotype II (Figure 3), which together with that other isolates reported in China, e.g., P. trituberculatus, P. monodon, S. paramamosain, H. tientsinensisn, and H. takanoi (Small et al., 2012; Wang et al., 2017; Huang et al., 2019; Li et al., 2021b; Gong et al., 2023). Taken together, the Hematodinium isolates in M. abbreviatus were taxonomically identified as H. perezi and belonged to the genotype II.
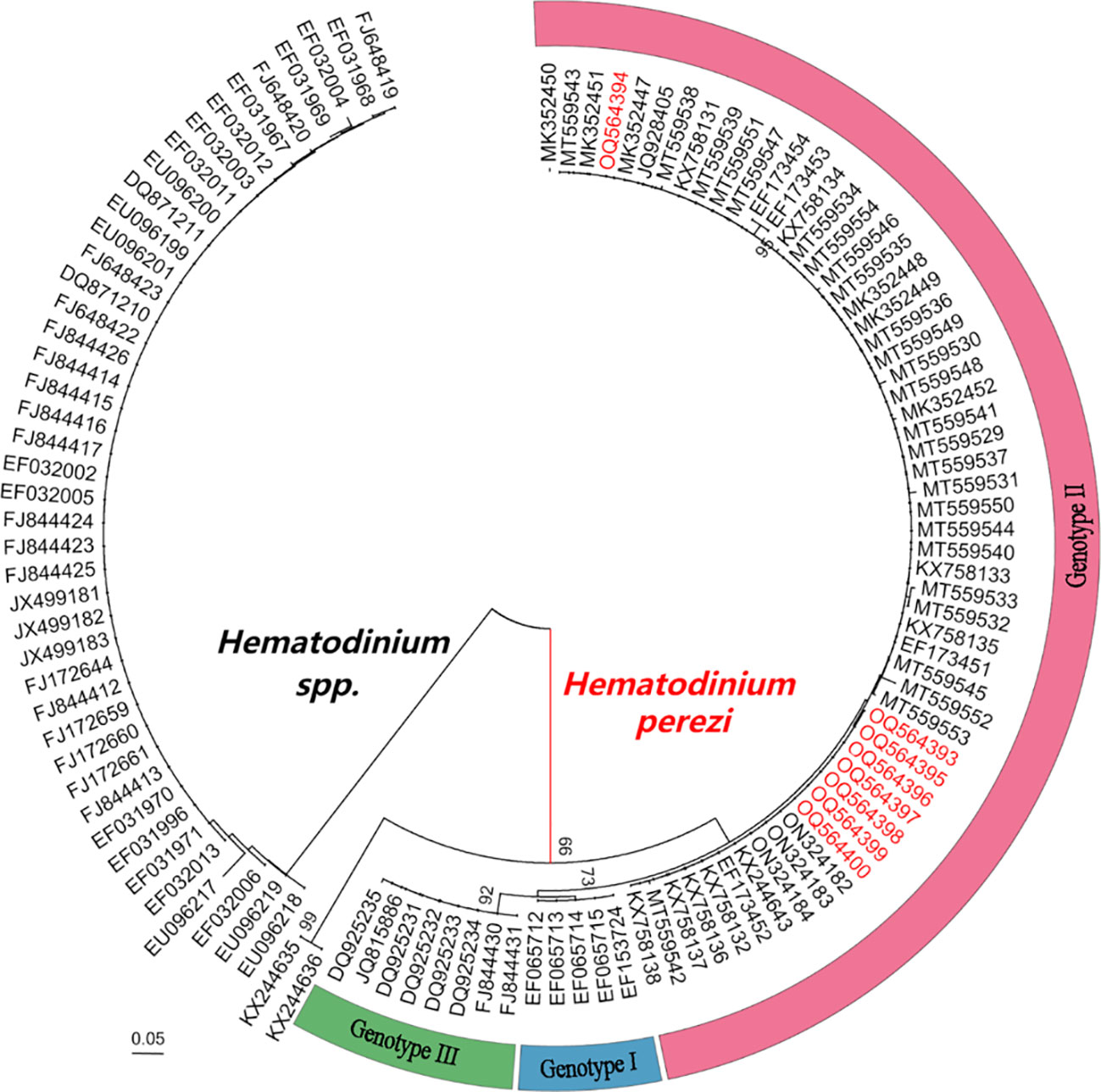
Figure 3 Neighbour-joining phylogenetic tree based on partial sequences of ITS 1 gene from Hematodinium spp. Hematodinium sequences were from previous reports (Li et al., 2021a; Li et al., 2021b; Gong et al., 2023). Bootstrap values above 60% are shown (1000 replications) at branch points. Bar = 0.05.
3.4 H. perezi confirmed in the hemolymph from artificially challenged M. abbreviatus
Massive Hematodinium ameboid cells were observed in the hemolymph of artificially inoculated H. perezi-infected crabs, whereas haemocytes were rare in this sample (Figures 4A, B). The hemolymph samples were positive for Hematodinium and identified as H. perezi based on the results of PCR and phylogenetic analysis.
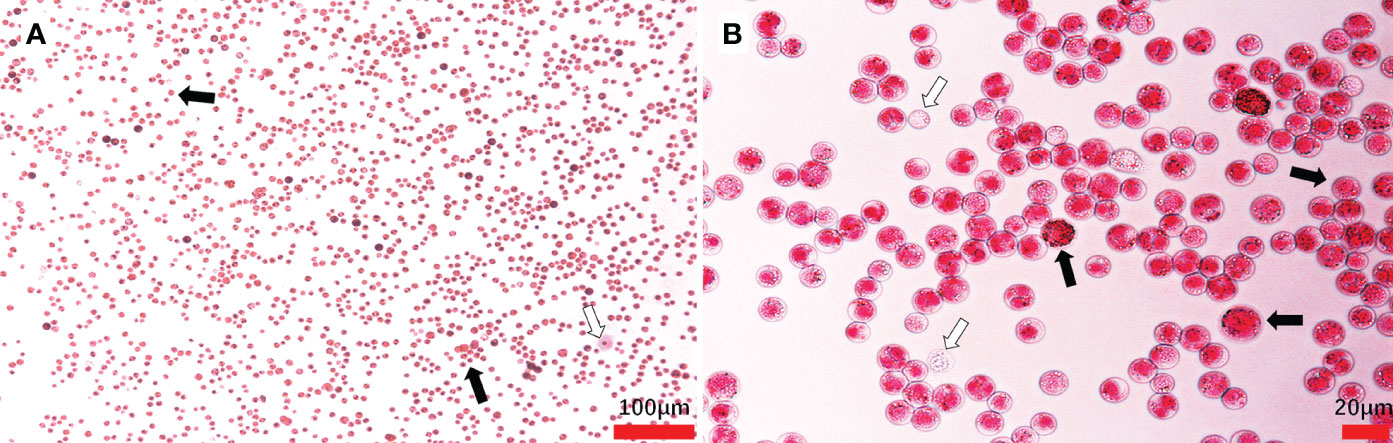
Figure 4 Hematodinium staining and examination in the hemolymph from artificially challenged M. abbreviatus. Black arrows indicate parasites and white arrows indicate haemocytes (A, B).
3.5 Histopathology caused by Hematodinium infections
The histopathology caused by natural and artificial H. perezi infections in M. abbreviatus was examined. Compared to the histology of Hematodinium-negative crabs (Figures 5A–D), obvious histopathological changes were observed in the tissues of hepatopancreas and gills of M. abbreviatus suffering from natural Hematodinium infection (Figures 6A–D). The prominent V-shaped chromosomes characteristic of syndinean dinoagellates were observed in hepatopancreas (Figure 6B) and gills (Figure 6D), whereas these parasites were rarely observed in heart and muscle, probably due to light infections. Granulomas were also observed in the diseased gills (Figure 6C).
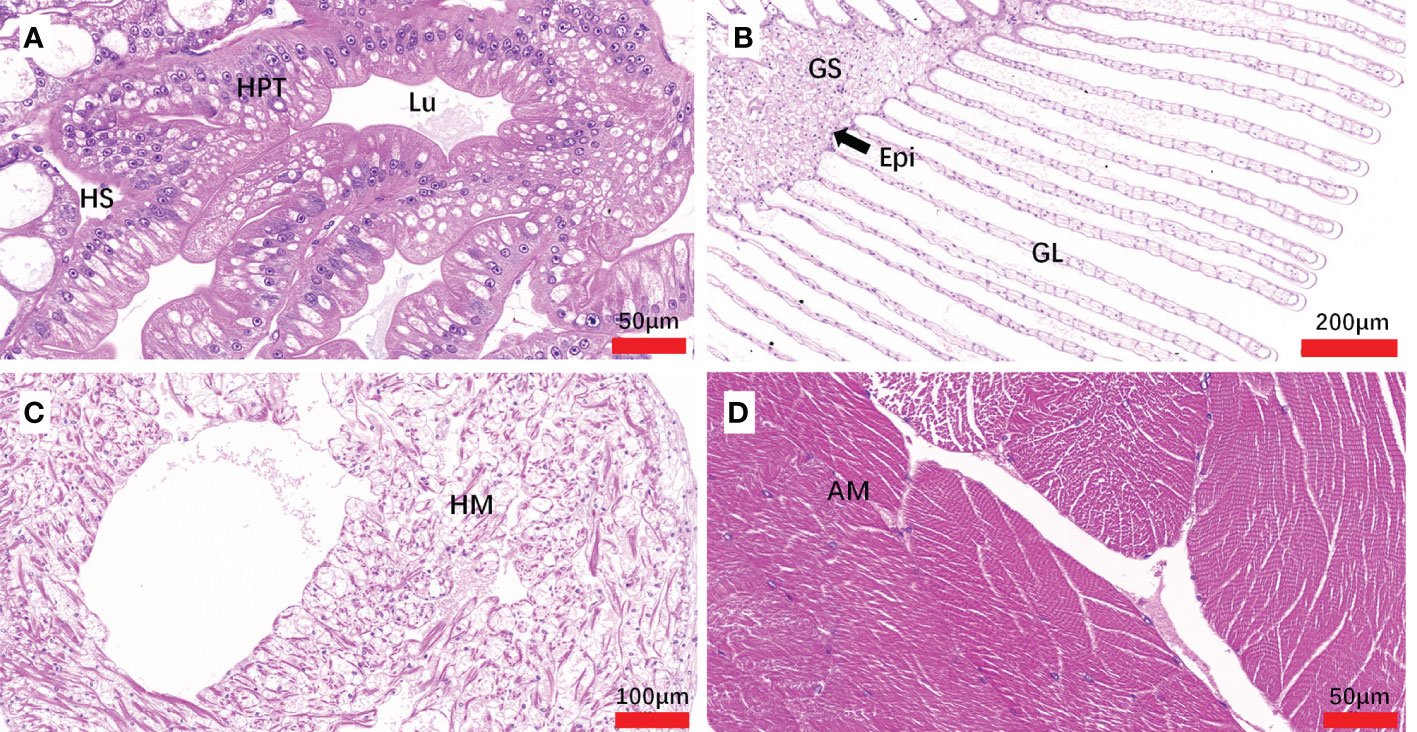
Figure 5 Sections of tissues from Hematodiniun infection-negative M. abbreviatus. Hepatopancrea (A), gill (B), heart (C) and abdominal muscle (D) tissus. H&E staining. HPT, Hepatopancreatic tubule; Lu, lumen of hepatopancreatic tubules; HS, hemal space; Epi, epithelium; GS, Gill stem; GL Gill lamellae; HM, heart muscle; AM, abdominal muscle.
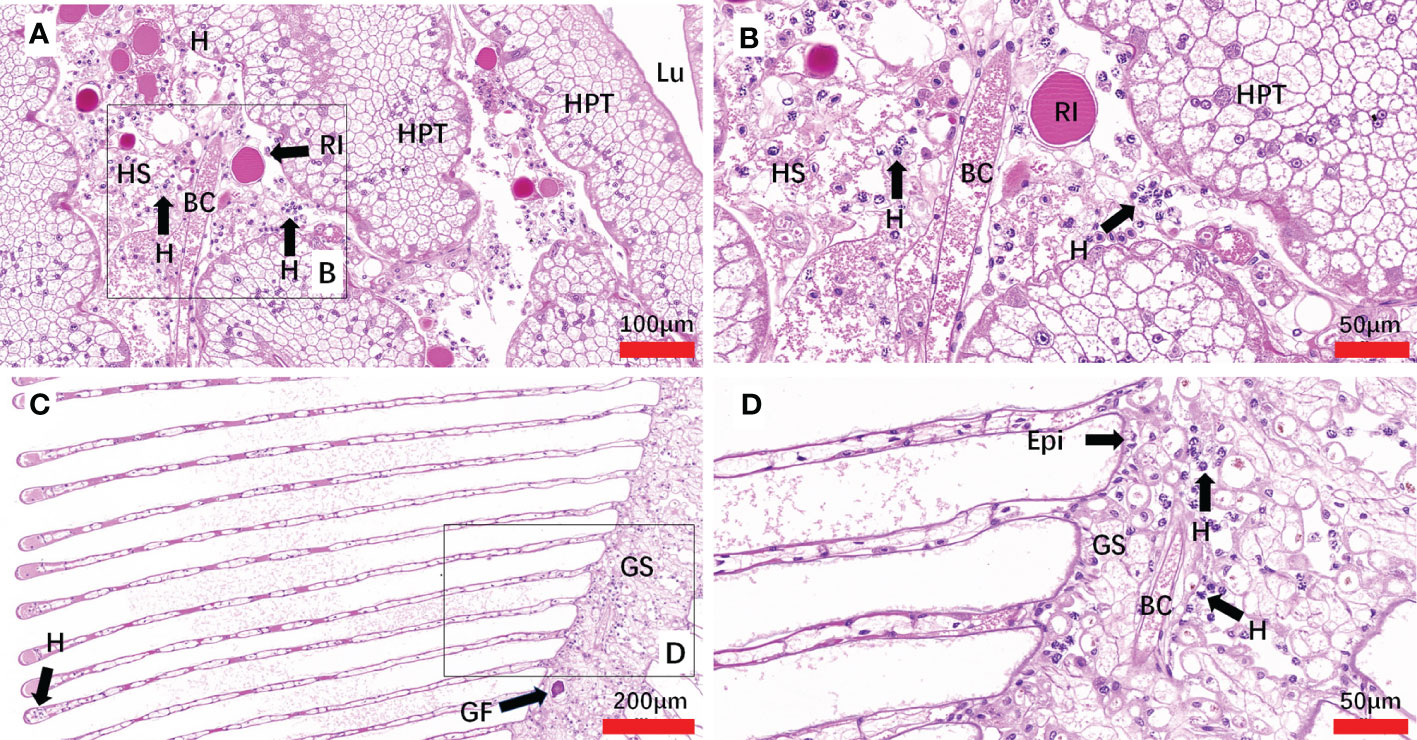
Figure 6 Sections of tissues from naturally Hematodinium-infected M. abbreviatus. Hepatopancrea (A, B) and gill (C, D). Note uninucleate ameboid trophonts with V-shaped chromatin in the nucleus were observed in Hepatopancrea (B) and gill (D). A granulomas was present in the gill tissues (C). H&E staining. HPT, Hepatopancreatic tubule; Lu, lumen of hepatopancreatic tubules; HS, hemal space; BC, blood channel; H, Hematodinium cells; P, fixed phagocytes; RI, reserve inclusion cells; GF, granuloma formation; Epi, epithelium; GS, Gill stem; GL Gill lamellae.
In inoculated crabs at 25-day post-infection, the hepatopancreas was severely altered due to the invasion of Hematodinium parasites, the spongy connective tissue around the hepatopancreatic tubules appeared to be diminished, and haemocytes were barely observed. The enlarged hemal space among the hepatopancreatic tubules was filled with massive numbers of parasites, while the hepatopancreatic tubules appeared intact and no parasites were observed within the lumen proper of the hepatopancreatic tubules. In addition, fixed phagocytes which were enlarged and activated were observed, probably in response of tissue degradation or parasite infection (Figures 7A, B). In the gills, a number of Hematodinium parasites were observed in the gill stem and filaments. The epithelium was deformed and appeared to be necrotic, and reduction or loss of spongy connective tissue was also observed. In some of the gill lamellae, necrosis of the connective tissue, and loss or necrosis of the trabecular cells were observed due to parasite infection (Figures 7C–F). The released spores were also observed between adjacent lamellae (Figure 7D). In abdominal muscle tissue, the intact structure of the muscle fibers was distorted and lost its normal dense appearance due to interstitial infiltrates of parasites (Figure 7G). In the heart tissue, the heart muscle lost its normal dense appearance and the myocardial fibers showed a reduction or loss of spongy connective tissue, separated by parasite cell invasion (Figure 7H).
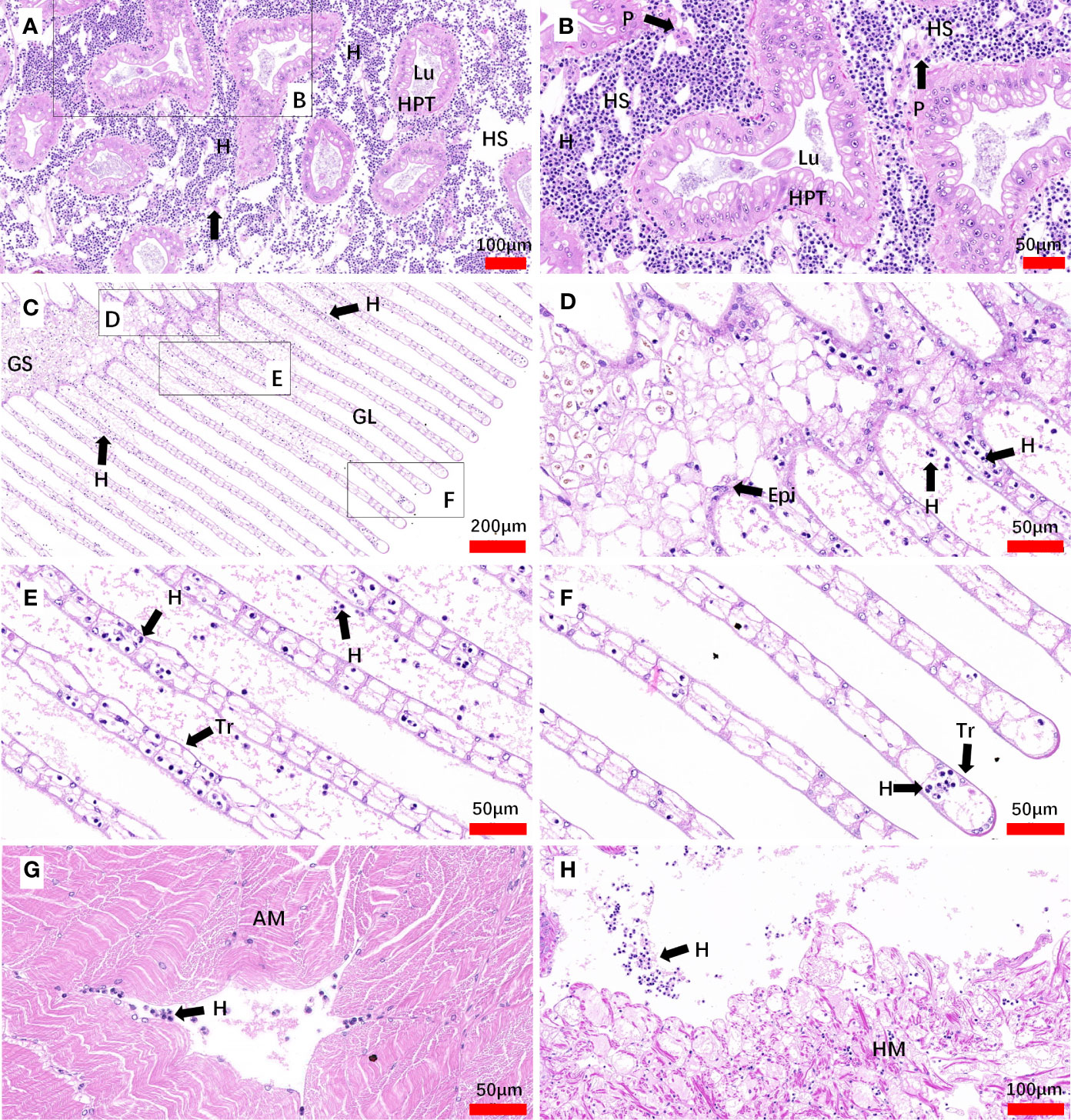
Figure 7 Sections of tissues from artificial inoculation Hematodinium-infected M. abbreviatus. Hepatopancrea (A, B), gill (C-F), abdominal muscle (G) and heart (H) tissus of infected crab. H&E staining. HPT, Hepatopancreatic tubule; Lu, lumen of hepatopancreatic tubules; HS, hemal space; H, Hematodinium cells; P, fixed phagocytes; Epi, epithelium; GS, Gill stem; GL Gill lamellae; Tr, trabecular cell; AM, abdominal muscle; HM, heart muscle.
4 Discussion
Since 2013, frequent outbreaks of the Hematodinium disease have led to serious economic losses to the cultured P. trituberculatus in coastal areas of the Shandong Peninsula. Considering the fact that hosts play an important role in occurrence and spread of parasitic diseases, it is important to conduct epidemic surveillance for hosts of Hematodinium sp. In the present study, we demonstrated that M. abbreviatus is a new natural host of H. perezi. This finding together with previous publications show that H. perezi genotype II can infect various marine crustaceans (e.g. H. takanoi, P. trituberculatus, H. tientsinensis, S. paramamosain and P. monodon) under a wide host range in China (Small et al., 2012; Wang et al., 2017; Huang et al., 2019; Li et al., 2021b; Gong et al., 2023). The occurrence and transmission mechanisms of Hematodinium parasites in the integrative culture systems are not yet clearly understood. Dinospores may play an important role in the transmission of this disease. A recent study on the transmission pattern indicated that H. perezi can be transmitted from H. tientsinensis to P. trituberculatus via waterborne transmission, while P. trituberculatus could not be infected by H. perezi via feeding tissues from H. perezi-infected H. tientsinensis (Li et al., 2021b).
It is noted that a similar trend in the prevalence of H. perezi infection was observed in both M. abbreviatus and P. trituberculatus collected simultaneously during the survey in Weifang in the present study. According to the results of PCR detection, the monthly prevalence of H. perezi infection in M. abbreviatus was generally higher than that in the cultured P. trituberculatus (unpublished data) in 2021. When compared with the data from a previous survey from June to October in 2018 in Qingdao, China (Li et al., 2021b), which is adjacent to the sampling site in the present study, the monthly prevalence of H. perezi in M. abbreviatus was also much higher than that in H. tientsinensis (9.4%-30.1%) and the cultured P. trituberculatus (3.0%-55.7%). In addition, M. abbreviatus is a migratory species in the integrative culture ponds and a dominant species of the most common wild crab species, dwelling in the polyculture ponds or in the waterways connected to the polyculture pond system along the coast of the Shandong Peninsula. Therefore, it is reasonable to assume that M. abbreviatus as well as other co-inhabiting crabs are likely important alternates or reservoir hosts involved in transmission and maintenance of Hematodinium. It requires further investigations to determine whether M. abbreviatus is more susceptible to Hematodinium infection compared to P. trituberculatus and/or other reported wild crabs, and the exact role of M. abbreviatus in the epizootiology of Hematodinium disease.
5 Conclusions
In summary, to our knowledge, this is the first report of naturally infected M. abbreviatus with H. perezi. M. abbreviatus with a high prevalence of H. perezi infections indicates that more attention should be paid to their potential risk in Hematodinium epizootics. The present study extends the known hosts for H. perezi, and also provides important information for monitoring and designing effective management strategies for Hematodinium disease in marine crustacean aquaculture in China.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
The animal study was approved by Since the Ethical Principles and Guidelines for the Use of Animals of the National Research Council of China apply to vertebrates only, there is no official standard for invertebrates. We adapted its principles to shrimp. All of the animal experiments were performed according to local government regulations and the protocols were approved by the Animal Ethics Committee of the Yellow Sea Fisheries Institute, Chinese Academy of Fishery Sciences. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
ZL: Investigation, Project administration, Writing – original draft, Formal analysis. GX: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization. HW: Formal analysis, Methodology, Supervision, Writing – original draft, Writing – review & editing. XL: Methodology, Writing – review & editing, Formal analysis. XW: Methodology, Software, Supervision, Writing – review & editing. AL: Methodology, Writing – review & editing. LZ: Methodology, Writing – review & editing. CS: Supervision, Writing – review & editing. QZ: Formal analysis, Writing – review & editing. JH: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Joint Research Fund of the National Natural Science Foundation of China (NNSFC)–Shandong Province (U1906214), the Central Public-Interest Scientific Institution Basal Research Fund, CAFS (NO. 2023TD42), and the Project of Species Conservation from the Ministry of Agriculture and Rural Affairs-Marine Fisheries Resources Collection and Preservation.
Acknowledgments
We thank Dr Meng Li (Institute of Oceanology, Chinese Academy of Sciences) for providing us the donor Helice tientsinensis with heavy infection by Hematodinium perezi.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Albalat, A., Gornik, S. G., Beevers, N., Atkinson, R. J. A., Miskin, D., Neil, D. M. (2012). Hematodinium sp. infection in Norway lobster Nephrops norvegicus and its effects on meat quality. Dis. Aquat. Organ. 100, 105–112. doi: 10.3354/dao02500
Chatton, E. P. L., Poisson, R. (1931). Sur l'existence dans la sang des crabes, de péridiniens parasites: Hematodinium perezi n.g., n. sp. (Syndinidae). C.R. Seances Soc. Biol. Paris 1931 105, 553–557.
Field, R., Chapman, C., Taylor, A., Neil, D., Vickerman, K. (1992). Infection of the Norway lobster Nephrops norvegicus by a Hematodinium-like species of dinoflagellate on the west coast of Scotland. Dis. Aquat. Organ 13, 1–15. doi: 10.3354/dao013001
Gong, M., Xie, G., Wang, H., Li, X., Li, A., Wan, X., et al. (2023). Hematodinium perezi naturally infects Asian brush-clawed crab (Hemigrapsus takanoi). J.Fish.Dis. 46, 67–74. doi: 10.1111/jfd.13718
Huang, Q., Li, M., Wang, F., Li, C. (2019). The parasitic dinoflagellate Hematodinium perezi infecting mudflat crabs, Helice tientsinensis, in polyculture system in China. J. Invertebr. Pathol. 166, 107229. doi: 10.1016/j.jip.2019.107229
Huang, Q., Li, M., Wang, F., Song, S., Li, C. (2021). Transmission pattern of the parasitic dinoflagellate Hematodinium perezi in polyculture ponds of coastal China. Aquaculture 538, 736549. doi: 10.1016/j.aquaculture.2021.736549
Jensen, P. C., Califf, K., Lowe, V., Hauser, L., Morado, J. F. (2010). Molecular detection of Hematodinium sp. in Northeast Pacific Chionoecetes spp. and evidence of two species in the Northern Hemisphere. Dis. Aquat. Organ. 89, 155–166. doi: 10.3354/dao02193
Kumar, S., Stecher, G., Tamura, K. (2016). MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874. doi: 10.1093/molbev/msw054
Li, M., Huang, Q., Lv, X., Song, S., Li, C. (2021b). The parasitic dinoflagellate Hematodinium infects multiple crustaceans in the polyculture systems of Shandong Province, China. J. Invertebr. Pathol. 178, 107523. doi: 10.1016/j.jip.2020.107523
Li, C., Li, M., Huang, Q. (2021a). The parasitic dinoflagellate Hematodinium infects marine crustaceans. Mar. Life. Sci. Technol. 3, 313–325. doi: 10.1007/s42995-020-00061-z
Li, C., Song, S., Liu, Y., Chen, T. (2013). Hematodinium infections in cultured Chinese swimming crab, Portunus trituberculatus, in northern China. Aquaculture 396, 59–65. doi: 10.1016/j.aquaculture.2013.02.022
Li, Y. Y., Xia, X. A., Wu, Q. Y., Liu, W. H., Lin, Y. S. (2008). Infection with Hematodinium sp. in mud crabs Scylla serrata cultured in low salinity water in southern China. Dis. Aquat. Organ. 82, 145–150. doi: 10.3354/dao01988
Messick, G. A., Shields, J. D. (2000). Epizootiology of the parasitic dinoflagellate Hematodinium sp. in the American blue crab Callinectes sapidus. Dis. Aquat. Organ. 43, 139–152. doi: 10.3354/dao043139
Ryazanova, T., Eliseikina, M., Kukhlevsky, A. (2021). First detection of Hematodinium sp. In spiny king crab Paralithodes brevipes, and new geographic areas for the parasite in tanner crab Chionoecetes bairdi, and red king crab Paralithodes camtschaticus. J. Invertebr. Pathol. 184, 107651. doi: 10.1016/j.jip.2021.107651
Ryazanova, T., Eliseikina, M., Kukhlevsky, A., Kharlamenko, V. (2010). Hematodinium sp. infection of red Paralithodes camtschaticus and blue Paralithodes platypus king crabs from the Sea of Okhotsk, Russia. J. Invertebr. Pathol. 105, 329–334. doi: 10.1016/j.jip.2010.07.009
Shields, J. D., Taylor, D. M., O’Keefe, P. G., Colbourne, E., Hynick, E. (2007). Epidemiological determinants in outbreaks of bitter crab disease (Hematodinium sp.) in snow crabs Chionoecetes opilio from Conception Bay, Newfoundland, Canada. Dis. Aquat. Organ. 77, 61–72. doi: 10.3354/dao01825
Small, H. J. (2012). Advances in our understanding of the global diversity and distribution of Hematodinium spp.–Significant pathogens of commercially exploited crustaceans. J. Invertebr. Pathol. 110, 234–246. doi: 10.1016/j.jip.2012.03.012
Small, H. J., Shields, J. D., Reece, K. S., Bateman, K., Stentiford, G. D. (2012). Morphological and molecular characterization of Hematodinium perezi (Dinophyceae: Syndiniales), a dinoflagellate parasite of the harbour crab, Liocarcinus depurator. J. Eukaryot. Microbiol. 59, 54–66. doi: 10.1111/j.1550-7408.2011.00592.x
Smith, A. L., Hirschle, L., Vogan, C. L., Rowley, A. F. (2015). Parasitization of juvenile edible crabs (Cancer pagurus) by the dinoflagellate, Hematodinium sp.: pathobiology, seasonality and its potential effects on commercial fisheries. Parasitology. 142, 428–438. doi: 10.1017/S0031182014001255
Stentiford, G., Neil, D., Albalat, A., Milligan, R., Bailey, N. (2015). The effect of parasitic infection by Hematodinium sp. on escape swimming and subsequent recovery in the Norway lobster, Nephrops norvegicus (L.). J. Crustacean. Biol. 35, 1–10. doi: 10.1163/1937240X-00002296
Stentiford, G. D., Shields, J. D. (2005). A review of the parasitic dinoflagellates Hematodinium species and Hematodinium-like infections in marine crustaceans. Dis. Aquat. Organ. 66, 47–70. doi: 10.3354/dao066047
Taylor, D., Khan, R. (1995). Observations on the occurrence of Hematodinium sp.(Dinoflagellata: Syndinidae), the causative agent of bitter crab disease in Newfoundland snow crab (Chionoecetes opilio). J. Invertebr. Pathol. 65, 283–288. doi: 10.1006/jipa.1995.1043
Wang, J., Li, M., Xiao, J., Xu, W., Li, C. (2017). Hematodinium spp. infections in wild and cultured populations of marine crustaceans along the coast of China. Dis. Aquat. Organ. 124, 181–191. doi: 10.3354/dao03119
Wheeler, K., Shields, J. D., Taylor, D. M. (2007). Pathology of Hematodinium infections in snow crabs (Chionoecetes opilio) from Newfoundland, Canada. J. Invertebr. Pathol. 95, 93–100. doi: 10.1016/j.jip.2007.01.002
Xu, W., Shi, H., Xu, H., Small, H. (2007). Preliminary study on the Hematodinium infection in cultured Portunus trituberculatus. Acta Hydrobiologica Sin. 31, 637–640. doi: 10.3321/j.issn:1000-3207.2007.05.004
Keywords: parasitic dinoflagellates, Hematodinium sp., Hematodinium perezi, Macrophthalmus abbreviatus, host
Citation: Liu Z, Xie G, Wang H, Li X, Wan X, Li A, Zhou L, Shi C, Zhang Q and Huang J (2024) Macrophthalmus (Macrophthalmus) abbreviatus Manning & Holthuis, 1981, a new natural host for Hematodinium perezi infection. Front. Cell. Infect. Microbiol. 13:1328872. doi: 10.3389/fcimb.2023.1328872
Received: 31 October 2023; Accepted: 14 December 2023;
Published: 04 January 2024.
Edited by:
Ceferino M. López Sández, University of Santiago de Compostela, SpainReviewed by:
Ji Hyung Kim, Gachon University, Republic of KoreaLusheng Xin, Jining Medical University, China
Copyright © 2024 Liu, Xie, Wang, Li, Wan, Li, Zhou, Shi, Zhang and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guosi Xie, eGllZ3NAeXNmcmkuYWMuY24=; Jie Huang, aHVhbmdqaWVAeXNmcmkuYWMuY24=
†These authors have contributed equally to this work
 Zhengmin Liu
Zhengmin Liu Guosi Xie
Guosi Xie Hailiang Wang
Hailiang Wang Xinshu Li4
Xinshu Li4 Xiaoyuan Wan
Xiaoyuan Wan Liqing Zhou
Liqing Zhou Qingli Zhang
Qingli Zhang Jie Huang
Jie Huang