- 1Division of Orthopaedics and Traumatology, Department of Orthopaedics, Nanfang Hospital, Southern Medical University, Guangzhou, China
- 2Guangdong Provincial Key Laboratory of Bone and Cartilage Regenerative Medicine, Nanfang Hospital, Southern Medical University, Guangzhou, China
- 3Department of Hospital Management, Southern Medical University, Guangzhou, China
- 4Department of Emergency Trauma Center, Nanfang Hospital, Southern Medical University, Guangzhou, China
Background: Previous studies have indicated that nitric oxide synthase 2 (NOS2) genetic variations are involved in delayed fracture healing and fracture non-union. Whether these genetic variants associate with the development of osteomyelitis (OM) remains unclear. Here, we analyzed the potential relationships between NOS2 genetic variations and the risk of developing post-traumatic OM (PTOM) in a Chinese Han population.
Methods: Altogether 704 participants, including 336 PTOM patients and 368 healthy controls, were genotyped of rs2297514 and rs2248814 of the NOS2 gene using the SNaPshot genotyping method.
Results: Outcomes showed that the frequency of allele C of rs2297514 in the patient group was significantly lower than that in the control group (48.7% vs. 54.5%, P = 0.029, OR = 0.792, 95% CI 0.642 – 0.976). In addition, significant associations were found between rs2297514 and susceptibility to PTOM by the recessive model (P = 0.007, OR = 0.633, 95% CI 0.453 – 0.884), and the homozygous model (P = 0.039, OR = 0.648, 95% CI 0.429 – 0.979). Moreover, patients with the CC genotype of rs2297514 had lower inflammatory biomarkers levels than the TT genotype, especially for the C-reactive protein (CRP) level (median: 4.1 mg/L vs. 8.9 mg/L, P = 0.027). However, no significant relationship was noted between rs2248814 and the risk of developing PTOM.
Conclusion: In this Chinese cohort, rs2297514 is correlated with a decreased risk of PTOM development, with genotype CC as a protective factor.
1 Introduction
Osteomyelitis (OM), a hard-to-treat, deep-bone infection, remains a significant healthcare problem worldwide (Muthukrishnan et al., 2019). According to the infection route, OM can occur following perioperative and contiguous conditions, hematogenous spread, and vascular insufficiency-related disorders (e.g., diabetic foot) (Lew and Waldvogel, 1997). Post-traumatic OM (PTOM) remains one primary cause of OM, the incidence of which ranges from 0% to 55%, depending on multiple systematic and local factors of the individuals (Hogan et al., 2013). Despite great advances in surgical techniques, PTOM still poses challenges to orthopaedic surgeons, which primarily attributed to its characteristic of high heterogeneity (Jiang et al., 2020). Early and accurate diagnosis is sometimes difficult, and treatment is always tricky, with high risks of limb deformity and infection recurrence (Panteli and Giannoudis, 2016). Thus, how to reduce the incidence and increase the cure rate is of great clinical significance, which is built on comprehensive understanding of its pathogenesis.
PTOM pathogenesis is complex and associated with both extrinsic and intrinsic factors (Beck-Broichsitter et al., 2015). Most of the previous studies focused on the environmental factors, ignoring the potential role of host factors in developing PTOM. Recently, increasing evidence has suggested that as a representative of host factors, single nucleotide variations (SNVs) were also linked to PTOM development. Such SNVs included but were not limited to rs689466 in cyclooxygenase-2 (COX-2) gene (Wang et al., 2017), rs16944, rs2234663, rs1143627, rs4251961, rs1800796, and rs2234663 in interleukin (IL) genes (Alves De Souza et al., 2017; Jiang et al., 2020). These findings demonstrated that SNVs might play essential roles in PTOM development.
The nitric oxide synthase 2 (NOS2) enzyme, encoded by the NOS2 gene, is responsible for synthesizing nitric oxide (NO) in the human body. As a reactive free radical, NO mediates multiple biological processes, such as neurotransmission, antitumoral and antimicrobial activities (Huang et al., 2018). In addition, a previous study (Zhu et al., 2001) reported that NOS2 might also participate in the bone fracture healing process, implying that NOS2 plays a role in bone metabolism. Moreover, two recent studies reported NOS2 SNVs associated with the susceptibility to delayed fracture-healing (Sathyendra et al., 2014) and even fracture non-union (Huang et al., 2018), which confirmed the important role of NOS2 in the fracture healing process.
It is known that PTOM is a bone metabolism-related disorder, characterized by inflammatory bone destruction with or without new bone formation. We speculated that NOS2 genetic SNVs might also participate in the occurrence of PTOM. Therefore, in the present study, we investigated the potential relationships between NOS2 genetic SNVs, rs2297514 and rs2248814, and susceptibility to PTOM in a Chinese Han population.
2 Materials and methods
2.1 Study design, setting, definition, inclusion, and exclusion criteria
The present study was designed as a case-control analysis, with comparison conducted between PTOM patients and healthy controls. Included patients were those who had sought medical attention for PTOM in our hospital between January 2016 and December 2019. Participants in the control group were healthy adults. PTOM is defined as a chronic and persistent inflammatory bone disease by infecting microorganisms, characterized by progressive bone destruction and sequestrum formation following trauma and/or orthopaedic surgery, with infection duration exceeding ten weeks (Metsemakers et al., 2018). PTOM was diagnosed concerning any of the confirmatory criteria outlined by the International Fracture-Related Infection (FRI) Consensus Group (Govaert et al., 2020), including wound breakdown to the bone or the implant, sinus or fistula connecting the bone or the implant, positive pathogen culture outcomes, and positive histopathology test outcomes. Patients with OM following diabetic foot or hematogenous spread, and those who refused to participate were excluded. All the included participants or their legal guardians had signed the informed consent form. This study, conducted following the tenets of the 1964 Helsinki declaration, was approved by the medical ethical committee of Nanfang Hospital, Southern Medical University (NFEC-2019-087).
2.2 DNA extraction and SNV genotyping
Peripheral blood samples (5ml each) were collected in the ethylene diamine tetraacetic acid (EDTA) and stored at –80° C. Then, the genomic DNA of each sample was extracted from leukocytes according to the instructions of the Flexi Gene-DNA Kit (Qiagen, Valencia, CA). Two tag SNVs in the NOS2 gene (rs2297514 and rs2248814) were genotyped using the Multiplex SNaPshot system (Applied Biosystems, Foster City, USA). The forward (F), reverse (R), and extension primers used for polymerase chain reactions (PCR) and extension reactions were as follows: For rs2297514: F: 5’-GCACAGATCAATGAAACCTGC-3’, R: 5’-CGTCTACTCTTGGTTAACCAC-3’, extension primer: 5’-CTGAGAGAGGAAGTGGAGCAGATGCT-3’. For rs2248814: F: 5’-GTCTCCGCTTCTCGTCCT-3’, R: 5’-GGGTGTGAAGGGTCCTCTAC-3’, extension primer: 5’-AGCGGGGTCCTGGCTTGGCTC-3’. The detailed procedure of the SNaPshot genotyping method was described previously (Jiang et al., 2016).
2.3 Outcome parameters
Primary outcome measures were comparisons between PTOM patients and healthy controls regarding genotype distribution, mutant allele frequency, and four genetic models (dominant, recessive, homozygous, and heterozygous models) of the two NOS2 SNVs (rs2297514 and rs2248814). Secondary outcomes were the preoperative serological levels of white blood cell (WBC) count, percentage of polymorphonuclear leukocytes (PMN%), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), procalcitonin (PCT), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and serum amyloid A (SAA), among different genotypes of the two NOS2 SNVs. In addition, clinical characteristics of the PTOM cohort were summarized.
2.4 Statistical analysis
Statistical analysis was conducted using the Statistical Product and Service Solutions software (version 17.0, SPSS Inc., Chicago, IL, USA). Data distribution was first evaluated for normality by the Kolmogorov-Smirnov test. Continuous variables were presented as mean ± standard deviation (SD) or median with interquartile range (IQR) based on data distribution. For normally distributed data, Student’s t-test or one-way analysis of variance (ANOVA) was used to compare differences between two groups or among three groups. Otherwise, the Mann-Whitney U or Kruskal-Wallis H tests were applied. Post-hoc multiple comparisons were conducted using LSD/Dunnett’s T3 following one-way ANOVA or the Mann-Whitney U test following the Kruskal-Wallis H test.
The genotype distributions of the healthy controls were tested to confirm the Hardy-Weinberg Equilibrium (HWE) using the chi-square test. The chi-square test or Fisher’s exact test was used to compare genotype distribution, mutant allele frequency, and the four genetic models, with corresponding odds ratios (ORs) and 95% confidence intervals (CIs) between the patients and healthy controls. All reported values were 2-sided with a P value of less than 0.05, which was considered statistically significant.
3 Results
3.1 Demographics and clinical characteristics
Altogether 468 patients diagnosed with chronic OM (COM) and 368 healthy controls were screened for inclusion, with no statistical differences in sex ratio (0.31 vs. 0.37, P = 0.25) or median age [48 (IQR 33, 59) years vs. 46 (IQR 37, 52) years, P = 0.08] between the patients and controls. Of the 468 COM patients, 132 were categorized as non-PTOM (70 having diabetic-foot related OM and 62 having hematogenous spread-related OM), with the remaining 336 patients included for analysis. Among the 336 PTOM patients, traffic accidents accounted for 40% of all injury types, with the tibia (59%) as the most frequent infection site. The positive rate of intraoperative sample culture was 57%, with Staphylococcus aureus (46%) being the most frequently detected one.
3.2 HWE test outcomes
The NOS2 genetic variation rs2297514 genotype distribution of the healthy controls failed in the HWE (P = 0.04), while the distribution of rs2248814 of the healthy controls was in the HWE (P = 0.248).
3.3 Potential links between NOS2 gene SNVs and the susceptibility to PTOM
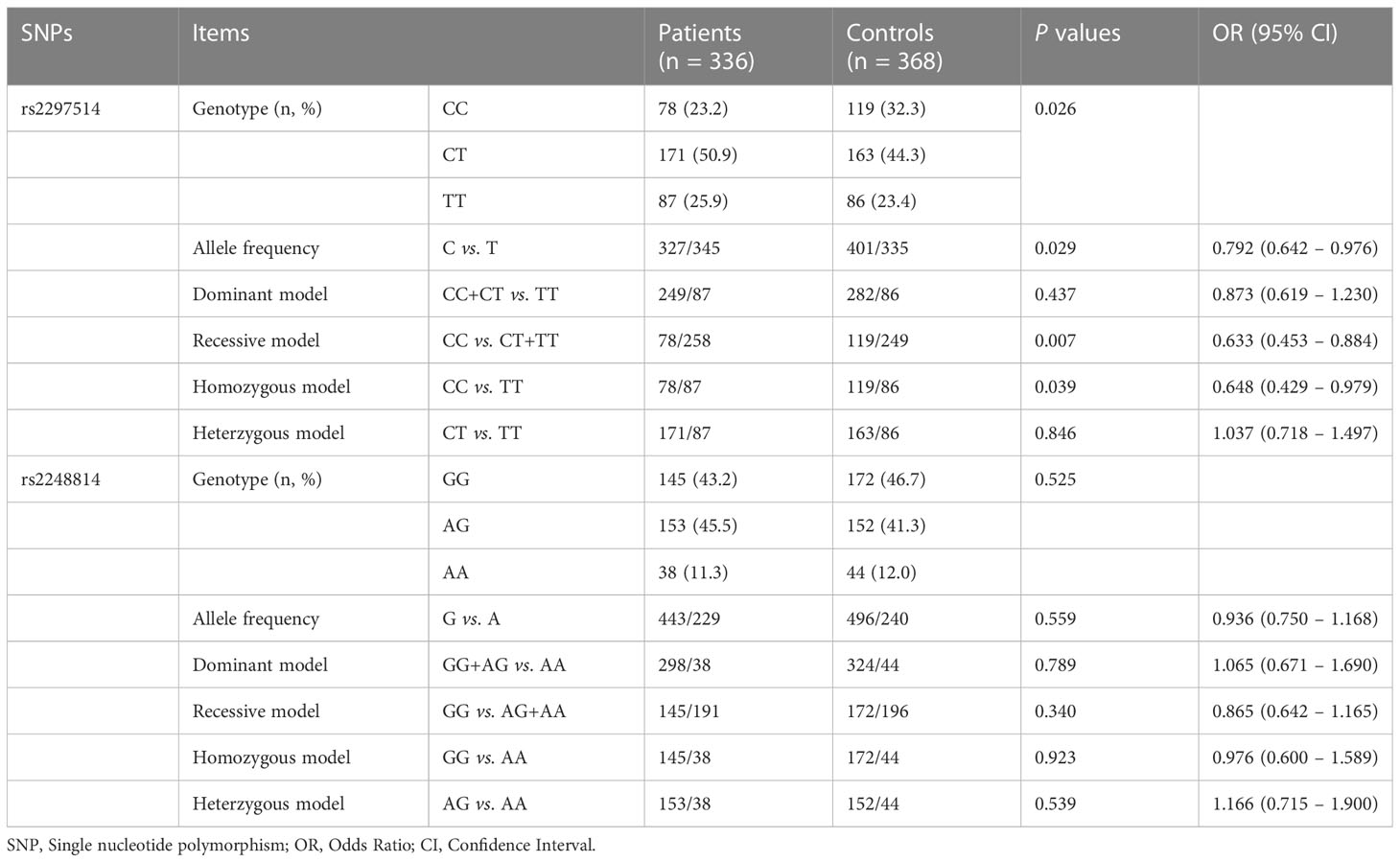
Regarding rs2297514, outcomes revealed a significant difference in genotype distribution between the patients and healthy controls (P = 0.026). Further comparison outcome showed that the allele C frequency in the patient group was significantly lower than that in the control group (48.7% vs. 54.5%, P = 0.029, OR = 0.792, 95% CI 0.642-0.976), demonstrating such a mutant allele may be protective. Additionally, significant links were found between rs2297514 and susceptibility to PTOM by recessive (P = 0.007, OR = 0.633, 95% CI 0.453 – 0.884) and homozygous (P = 0.039, OR = 0.648, 95% CI 0.429 – 0.979) models (Table 1). These suggest that the CC genotype of rs2297514 may be a protective factor against PTOM.
As for rs2248814, no significant relationships were found between this SNP site and the risk of developing PTOM in this Chinese cohort, neither by outcomes of genotype distribution and allele frequency nor by results of the four genetic models (Table 1).
3.4 Preoperative serological levels of inflammatory biomarkers among different genotypes of the two NOS2 SNV Sites among the PTOM patients
Significant differences were identified regarding the medial levels of ESR (P = 0.042) and CRP (P < 0.001) among the three genotypes of rs2297514. Outcomes of post hoc multiple comparisons by Mann-Whitney U test demonstrated that the medial CRP level of patients with the CC genotype was relatively lower than that of the TT genotype (4.1 mg/L vs. 8.9 mg/L, P = 0.027). In addition, patients with the CT genotype had significantly lower levels of ESR (P = 0.012) and CRP (P < 0.001) and a relatively lower IL-6 (P = 0.038) level than those of the TT genotype (Table 2). Concerning rs2248814, the only positive result was that patients with AG genotype had a relatively higher level of TNF-α than that of the GG genotype (P = 0.037) (Table 2).
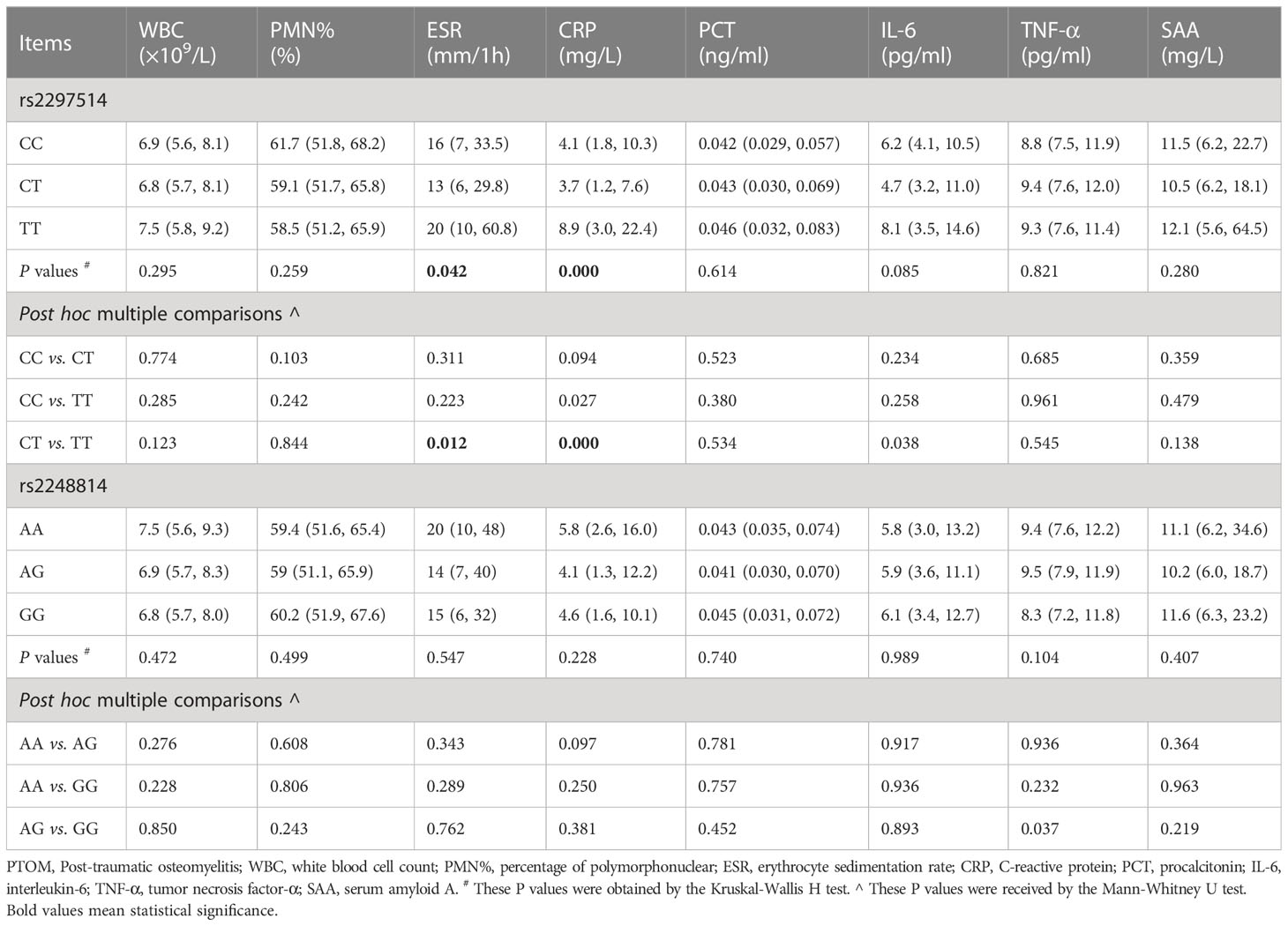
Table 2 Preoperative serological levels of inflammatory biomarkers among different genotypes of rs2297514 and rs2248814 in the PTOM patients.
4 Discussion
As mentioned previously, successful management of PTOM still represents significant challenges, as early and accurate diagnosis is sometimes difficult. Treatment is often tricky, with infection recurrence risk as high as 20 to 30% (Panteli and Giannoudis, 2016). In addition, such a disorder also brings socioeconomic problems. According to a recent survey of a group of Belgian patients (Iliaens et al., 2021), the direct hospital-related medical care costs of FRI are eight times that of non-FRI of long bone fractures. While in the USA, treatment of bone infection can be up to $150,000 per patient and up to 1.62 billion a year by 2020 (Muthukrishnan et al., 2019). Thus, such heavy economic burdens aggravate the negative influences of PTOM on patients, both physically and psychologically (Walter et al., 2022). Therefore, how to decrease the morbidity and increase the cure rate is clinical significance, built on a comprehensive understanding of PTOM pathogenesis.
Whether PTOM occurs depends on the complex interactions between extrinsic and intrinsic factors, while previous studies primarily focused on extrinsic factors. As a typical representative of intrinsic factors, growing evidence has shown that SNVs also involve in the development of PTOM. Here, we analyzed potential relationships between NOS2 gene SNVs and susceptibility to PTOM in a Chinese Han population. Outcomes of 704 subjects demonstrated that rs2297514 might be correlated with a reduced risk of PTOM development, with genotype CC as a protective factor. In contrast, the present failed to find a positive link between rs2248814 and the risk of PTOM development in this Chinese cohort. Our findings can be discussed with the following aspects.
First, we found that rs2297514 was associated with a decreased susceptibility to PTOM in this population, with mutant allele C and genotype CC as protective factors. Aside from the current study, two previous studies (Sathyendra et al., 2014; Huang et al., 2018) reported that this SNV was related to the fracture healing process. In 2014, Sathyendra et al. (2014) included 62 participants from the USA and screened 144 SNVs in potentially osteogenic genes. They observed that rs2297514 was linked to an elevated risk of developing atrophic delayed fracture-healing (P = 0.015, OR = 3.98), with CT genotype and allele T as risk factors. Later in 2018, Huang et al. (2018) also analyzed this SNV in the development of fracture non-union among a Chinese Han population. Similarly, they also noted that rs2297514 was correlated to an increased susceptibility to fracture non-union, with the T allele as a risk factor. Our present study shared similarities with the two investigations; the mutant allele C as a protective factor in the present study means that the wild-type allele T may be a risk factor for PTOM. However, we found it is the genotype TT, instead of CT, that was identified as a risk factor. Several possible factors might account for the differences, such as different orthopaedic disorders, different ethnicities, and even different numbers of participants.
Second, we failed to find any significant correlations between rs2248814 and the risk of PTOM development in this Chinese cohort, which was in accordance with the study by Huang et al. (2018). However, Sathyendra et al. (2014) reported that such an SNV also increased the risk of delayed fracture healing in an American population. In addition to PTOM, rs2248814 was also reported to be related to several different disorders. Hancock et al. (2008) found that this SNV was a genetic risk factor for Parkinson’s disease. While Velez et al. (2009) observed that rs2248814, interacting with rs1327474, contributed to pulmonary tuberculosis susceptibility in African-Americans. Lim et al. (2013) indicated NOS2 genetic SNVs (rs2248814 and rs2072324) associated with a sustained virological response to peginterferon plus ribavirin therapy for chronic hepatitis C in Taiwanese Chinese. However, in a recent study (Brookes et al., 2020) focusing on potential relationships between NOS2 genetic SNVs and susceptibility to the Achilles tendon injuries, the rs2248814 variant might not be linked to the risk of developing Achilles tendinopathy or Achilles tendon rupture. Nonetheless, considering these findings were derived from a single study, more investigations should be conducted to certify these outcomes.
Third, we found PTOM patients with the CC genotype of rs2297514 had relatively lower inflammatory biomarkers (apart from PMN%) levels than those with TT genotype, implying that such an SNV participating in the development of PTOM may partly via its influences on peripheral levels of inflammatory indicators. Interestingly, patients with the CT genotype had significantly lower levels of ESR and CRP and a relatively lower IL-6 than those with the TT genotype, though the heterozygous model found no significant association. Thus, whether the CT genotype is a risk or a protective factor requires further investigations with a larger sample size.
Although we compared serological levels of eight different inflammatory biomarkers among different genotypes of rs2297514 and rs2248814, it is not enough to uncover the underlying mechanisms. It is known that NO, the synthesis of which is regulated by NOS2, is a reactive free radical that plays as an important mediator in neurotransmission, antimicrobial and antitumoral activities (Huang et al., 2018). In addition, previous studies (Zhu et al., 2001; Sathyendra et al., 2014; Huang et al., 2018) had indicated the important role of NOS2 in bone metabolism. Based on these, we speculate that one potential mechanism of NOS2 SNVs involving in the pathogenesis of PTOM is NOS2 SNVs may influence expression levels of the NOS2 protein, the latter of which not only affect antimicrobial abilities of the human body, but also affect the bone metabolism process. Of course, future in-depth research is necessary to uncover the detailed mechanisms.
Our study also has several limitations. First, the sample size of the current research remains limited. More eligible patients and controls should be recruited for analysis to obtain more accurate conclusions. Second, the genotype distribution of rs2297514 among the healthy controls was not in HWE; thus, a cautious attitude should be taken, and future studies should testify to such outcomes. Third, we only focused on PTOM; whether such SNVs involve in the occurrence of other OM types, including hematogenous-related OM, and diabetic foot OM, requires further studies. Meanwhile, only two SNVs of the NOS2 gene were analyzed; whether another SNVs in this gene play a role in the development of PTOM also needs investigation.
5 Conclusions
In summary, we found that NOS2 genetic SNV rs2297514 is associated with a decreased susceptibility to PTOM in this Chinese Han population, with the genotype of CC as a protective factor. Also, such an SNV may play a role partly via its influences on peripheral blood levels of inflammatory biomarkers. Furthermore, the current study failed to find enough evidence to support the hypothesis that rs2248814 is related to PTOM occurrence, which needs to be certified by future studies.
Data availability statement
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by Southern Medical University Nanfang Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
C-SS and PZ contributed equally to this study. NJ and Y-JH designed the study. C-SS, Q-RL, Y-YH, and C-QP conducted the experiment. C-SS and NJ performed the statistical analysis. C-SS, PZ, and Q-RL participated in the sample collections. C-SS and PZ drafted the manuscript. NJ and Y-JH contributed to the manuscript revision. All authors contributed to the article approved the submitted version.
Funding
This research was funded by Guangdong Provincial Science and Technology Project, grant number: 2020A0505100039, Guangzhou Science and Technology Project, grant number 202002020001, and Xinjiang Uygur Autonomous Region Science and Technology Support Project, grant number: 2022E02040.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alves De Souza, C., Queiroz Alves De Souza, A., Queiroz Alves De Souza, M. D. S., Dias Leite, J. A., Silva De Morais, M., Barem Rabenhorst, S. H. (2017). A link between osteomyelitis and IL1RN and IL1B polymorphisms-a study in patients from northeast Brazil. Acta orthop 88 (5), 556–561. doi: 10.1080/17453674.2017.1348439
Beck-Broichsitter, B. E., Smeets, R., Heiland, M. (2015). Current concepts in pathogenesis of acute and chronic osteomyelitis. Curr. Opin. Infect. Dis. 28 (3), 240–245. doi: 10.1097/QCO.0000000000000155
Brookes, C., Ribbans, W. J., El Khoury, L. Y., Raleigh, S. M. (2020). Variability within the human iNOS gene and Achilles tendon injuries: evidence for a heterozygous advantage effect. J. Sci. Med. Sport 23 (4), 342–346. doi: 10.1016/j.jsams.2019.11.001
Govaert, G. A. M., Kuehl, R., Atkins, B. L., Trampuz, A., Morgenstern, M., Obremskey, W. T., et al. (2020). Fracture-related infection consensus G: diagnosing fracture-related infection: current concepts and recommendations. J. orthop Trauma 34 (1), 8–17. doi: 10.1097/BOT.0000000000001614
Hancock, D. B., Martin, E. R., Vance, J. M., Scott, W. K. (2008). Nitric oxide synthase genes and their interactions with environmental factors in parkinson’s disease. Neurogenetics 9 (4), 249–262. doi: 10.1007/s10048-008-0137-1
Hogan, A., Heppert, V. G., Suda, A. J. (2013). Osteomyelitis. Arch. Orthop Trauma Surg. 133 (9), 1183–1196. doi: 10.1007/s00402-013-1785-7
Huang, W., Zhang, K., Zhu, Y., Wang, Z., Li, Z., Zhang, J. (2018). Genetic polymorphisms of NOS2 and predisposition to fracture non-union: a case control study based on han Chinese population. PloS One 13 (3), e0193673. doi: 10.1371/journal.pone.0193673
Iliaens, J., Onsea, J., Hoekstra, H., Nijs, S., Peetermans, W. E., Metsemakers, W. J. (2021). Fracture-related infection in long bone fractures: a comprehensive analysis of the economic impact and influence on quality of life. Injury 52 (11), 3344–3349. doi: 10.1016/j.injury.2021.08.023
Jiang, N., Li, S. Y., Ma, Y. F., Hu, Y. J., Lin, Q. R., Yu, B. (2020). Associations between interleukin gene polymorphisms and risks of developing extremity posttraumatic osteomyelitis in Chinese han population. Mediators Inflammation 2020, 3278081. doi: 10.1155/2020/3278081
Jiang, N., Zhao, X. Q., Qin, C. H., Hu, Y. J., Wang, L., Xie, G. P., et al. (2016). Association of vitamin d receptor gene TaqI, BsmI, FokI and ApaI polymorphisms and susceptibility to extremity chronic osteomyelitis in Chinese population. Injury 47 (8), 1655–1660. doi: 10.1016/j.injury.2016.06.005
Lew, D. P., Waldvogel, F. A. (1997). Osteomyelitis. N Engl. J. Med. 336 (14), 999–1007. doi: 10.1056/NEJM199704033361406
Lim, Y. P., Peng, C. Y., Liao, W. L., Hung, D. Z., Tien, N., Chen, C. Y., et al. (2013). Genetic variation in NOS2A is associated with a sustained virological response to peginterferon plus ribavirin therapy for chronic hepatitis c in Taiwanese Chinese. J. Med. Virol. 85 (7), 1206–1214. doi: 10.1002/jmv.23598
Metsemakers, W. J., Kuehl, R., Moriarty, T. F., Richards, R. G., Verhofstad, M. H. J., Borens, O., et al. (2018). Infection after fracture fixation: current surgical and microbiological concepts. Injury 49 (3), 511–522. doi: 10.1016/j.injury.2016.09.019
Muthukrishnan, G., Masters, E. A., Daiss, J. L., Schwarz, E. M. (2019). Mechanisms of immune evasion and bone tissue colonization that make staphylococcus aureus the primary pathogen in osteomyelitis. Curr. Osteoporos Rep. 17 (6), 395–404. doi: 10.1007/s11914-019-00548-4
Panteli, M., Giannoudis, P. V. (2016). Chronic osteomyelitis: what the surgeon needs to know. EFORT Open Rev. 1 (5), 128–135. doi: 10.1302/2058-5241.1.000017
Sathyendra, V., Donahue, H. J., Vrana, K. E., Berg, A., Fryzel, D., Gandhi, J., et al. (2014). Single nucleotide polymorphisms in osteogenic genes in atrophic delayed fracture-healing: a preliminary investigation. J. Bone Joint Surg. Am. volume 96 (15), 1242–1248. doi: 10.2106/JBJS.M.00453
Velez, D. R., Hulme, W. F., Myers, J. L., Weinberg, J. B., Levesque, M. C., Stryjewski, M. E., et al. (2009). Gilbert JR et al: NOS2A, TLR4, and IFNGR1 interactions influence pulmonary tuberculosis susceptibility in African-americans. Hum. Genet. 126 (5), 643–653. doi: 10.1007/s00439-009-0713-y
Walter, N., Rupp, M., Baertl, S., Hinterberger, T., Alt, V. (2022). Prevalence of psychological comorbidities in bone infection. J. Psychosom Res. 157, 110806. doi: 10.1016/j.jpsychores.2022.110806
Wang, L., Jiang, N., Lin, Q. R., Qin, C. H., Hu, Y. J., Yu, B. (2017). Cyclooxygenase-2 (COX-2) polymorphism rs689466 may contribute to the increased susceptibility to post-traumatic osteomyelitis in Chinese population. Infect. Dis. 49 (11-12), 817–823. doi: 10.1080/23744235.2017.1347816
Keywords: post-traumatic osteomyelitis, fracture-related infection, Nos2, single nucleotide polymorphisms, rs2297514, case-control study
Citation: Song C-s, Zhang P, Lin Q-r, Hu Y-y, Pan C-q, Jiang N and Hu Y-j (2023) Nitric oxide synthase 2 genetic variation rs2297514 associates with a decreased susceptibility to extremity post-traumatic osteomyelitis in a Chinese Han population. Front. Cell. Infect. Microbiol. 13:1177830. doi: 10.3389/fcimb.2023.1177830
Received: 02 March 2023; Accepted: 12 June 2023;
Published: 03 July 2023.
Edited by:
Hongyi Shao, Beijing Jishuitan Hospital, ChinaReviewed by:
Anna Benini, University of Verona, ItalyMinwei Zhao, Peking University Third Hospital, China
Copyright © 2023 Song, Zhang, Lin, Hu, Pan, Jiang and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nan Jiang, aG54eWpuQHNtdS5lZHUuY24=; Yan-jun Hu, aHV5YW5qdW40NzUwQHNtdS5lZHUuY24=
†These authors have contributed equally to this work
 Chen-sheng Song1,2†
Chen-sheng Song1,2† Ping Zhang
Ping Zhang Nan Jiang
Nan Jiang Yan-jun Hu
Yan-jun Hu