
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Cell. Infect. Microbiol., 01 May 2023
Sec. Clinical Microbiology
Volume 13 - 2023 | https://doi.org/10.3389/fcimb.2023.1159891
Background: Extra-urogenital infections due to Mycoplasma hominis (M. hominis) are rare, particularly co-infection with Pseudomonas aeruginosa (P. aeruginosa). Herein, we report on a patient who was co-infected and successfully treated despite delayed treatment.
Case presentation: We reported the case of a 43-year-old man with M. hominis and P. aeruginosa co-infection after a traffic accident. The patient developed a fever and severe infection despite postoperative antimicrobial therapies. The blood culture of wound tissues was positive for P. aeruginosa. Meanwhile, culturing of blood and wound samples showed pinpoint-sized colonies on blood agar plates and fried-egg-type colonies on mycoplasma medium, which were identified as M. hominis by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) and 16S rRNA sequencing. Based on antibiotic susceptibility and symptoms, ceftazidime–avibactam and moxifloxacin were administered for P. aeruginosa infection. Meanwhile, after the failure of a series of anti-infective agents, M. hominis and P. aeruginosa co-infection was successfully treated with a minocycline-based regimen and polymyxin B.
Conclusion: The co-infection with M. hominis and P. aeruginosa was successfully treated with anti-infective agents despite delayed treatment, providing information for the management of double infection.
Mycoplasma hominis (M. hominis) commonly colonizes the genitourinary tract in a nonvirulent manner (Whitson et al., 2014). Nevertheless, it is an opportunistic pathogen that can cause a variety of genitourinary or extragenital infections, as well as neonatal infections (Poku, 2022; Zeng et al., 2022). However, this pathogen is commonly underestimated and overlooked in clinical settings due to undetermined pathogenic processes (Ahmed et al., 2021).
Clinically, abscesses, infections of the central nervous system, and bone and joint infections are common extragenital infections, especially in postoperative and immunocompromised patients (Meyer and Clough, 1993; Stabler et al., 2021). Although several extragenital infections due to M. hominis have been described, these infections are very rare (Hos et al., 2015), especially bacteremia. For instance, to date, only five cases of M. hominis were isolated from the blood over a 10-year period, as reported by the Public Health England reference laboratory (Chalker et al., 2021). Even in China, with the largest population, M. hominis was only detected in eight (0.7%) of 1,148 patients with bloodstream infection (Zeng et al., 2022). Due to the limited knowledge on M. hominis bacteremia, its clinical features, diagnosis, drug resistance, and treatment recommendations have not yet been fully established.
At present, the diagnosis of invasive M. hominis infections remains a clinical challenge owing to the lack of clear symptoms, especially for M. hominis bacteremia. As reported by Posse et al. (2018), the conventional method may fail to detect M. hominis bacteremia. Meanwhile, empirical antimicrobials generally cannot provide adequate antimicrobial coverage (Wildenbeest et al., 2016); thus, the typical broad-spectrum antibiotic regimens are ineffective for M. hominis infections (Whitson et al., 2014), let alone co-infection with another pathogen. Theory predicts increased virulence when multiple strains simultaneously infect the same host, which has major consequences for disease dynamics (Susi et al., 2015), but data on M. hominis co-infection are currently scarce. Here, we report on a patient who was co-infected with M. hominis and Pseudomonas aeruginosa (P. aeruginosa) and successfully treated with a minocycline-based regimen and polymyxin B despite delayed treatment.
A 43-year-old man was admitted to the intensive care unit in our hospital due to multiple open traumas caused by a traffic accident. The patient had a history of hypertension. At admission, the patient presented a constant body temperature of 36.5°C. Physical examination showed no obvious abnormality in the heart, lungs, or abdomen. He suffered multiple lacerations and fractures of the right acetabulum and inferior ramus of the pubis, accompanied by pelvic extraperitoneal hematoma. On the first day of hospitalization, he underwent surgery for multiple injuries. Based on empirical therapy, cefuroxime (1.5 g, every 8 h) was administrated for infection prevention, and anti-infective therapy with cefoperazone–sulbactam (3 g, every 8 h) and levofloxacin (500 mg, daily) was conducted for common bacteria and traumatic wet lung. On day 2, the patient developed a fever (38.4°C), with an increase of procalcitonin (PCT; 31.47 ng/ml) and lactic acid (8.58 mmol/L) after the operation. The antibiotic treatment was changed to meropenem (1.0 g, every 8 h) plus teicoplanin (0.4 g daily). On day 14, P. aeruginosa was positive in the bacterial culture of wound tissues (Figure 1A) and was identified as a multiple-resistant strain with antibiotic susceptibility testing (AST; Kirby–Bauer method), thus amikacin (0.4 g/day) was added to the anti-infective regimen. Despite antimicrobial therapies, a P. aeruginosa-positive blood culture was still identified as bloodstream infection on day 16; the dose of amikacin was therefore adjusted. On day 21, the patient underwent thigh amputation, surgical debridement, and drainage due to recurrent vascular rupture and aggravated cyanosis. Five days later, due to the less effective anti-infective therapy, P. aeruginosa-positive blood culture along with fever still existed; therefore, the patient received moxifloxacin to replace the teicoplanin and amikacin. Despite this management, fevers continued, and infection was still under suspicion. Ceftazidime–avibactam (2.5 g/day) was then administrated to replace the meropenem. The culture was P. aeruginosa negative for the 26th-day blood sample but still positive for wound tissues.
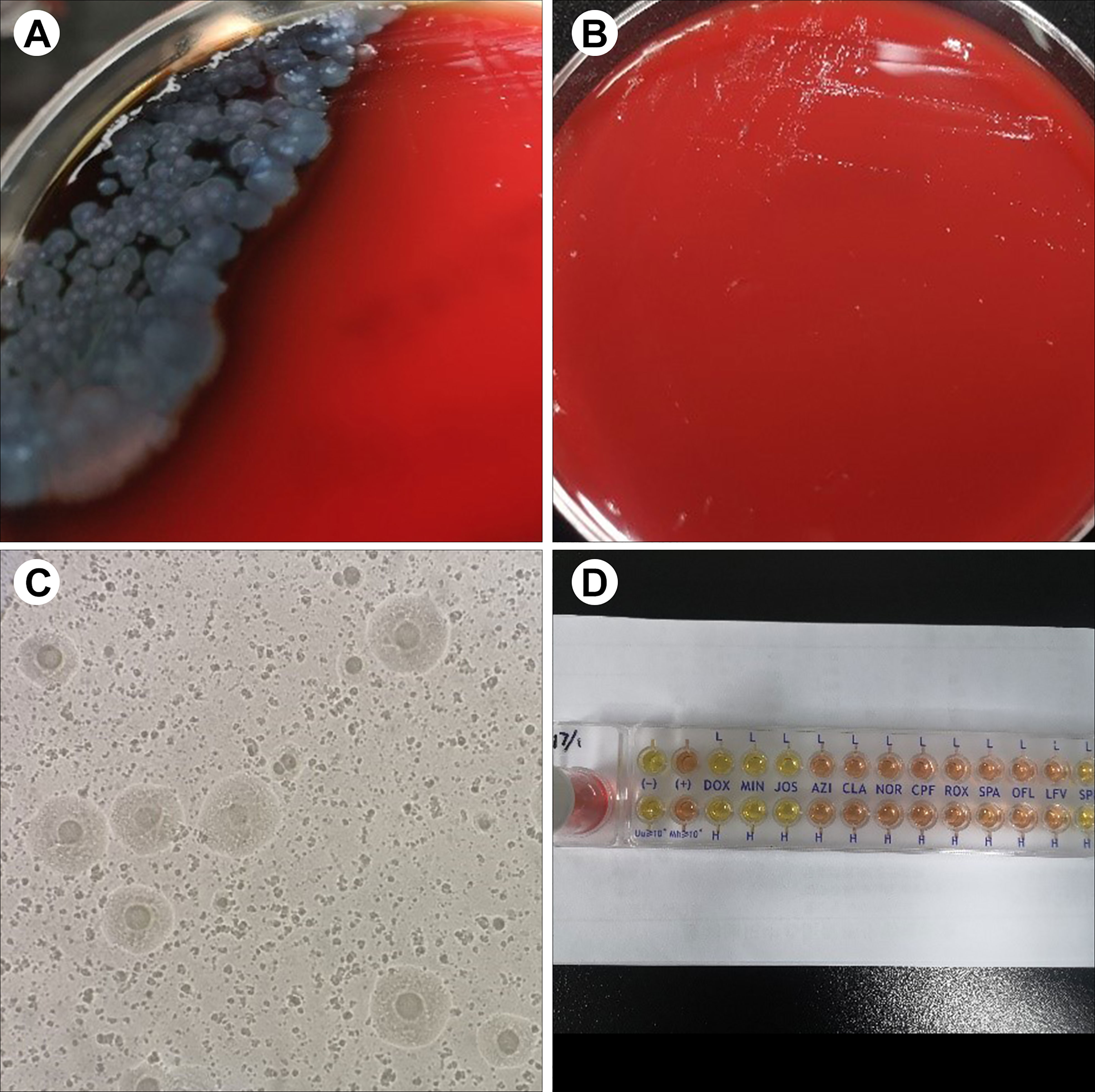
Figure 1 Representative results of sample culture and antibiotic susceptibility testing in patients. (A) On day 14, P. aeruginosa was positive in the blood culture and bacterial culture of wound tissues. (B) On day 28, tiny, nonhemolytic, and transparent colonies were observed on the Columbia blood agar plate. (C) Fried-egg-type colonies were observed on mycoplasma medium after 5 days of incubation. (D) The results of antibiotic susceptibility testing showed that M. hominis was susceptible to doxycycline, minocycline, and josamycin but resistant to azithromycin, clarithromycin, norfloxacin, ciprofloxacin, roxithromycin, sparfloxacin, spectinomycin, and levofloxacin.
Due to the uncontrolled infection, other pathogens were suspected. On day 26, tiny, nonhemolytic, and transparent colonies grew on the Columbia blood agar plate of four blood sample cultures (Figure 1B), possibly representing M. hominis. Gram staining of the blood smear showed no bacteria. A subculture of blood and wound tissue samples on mycoplasma medium presents as fried-egg-type colonies after 5 days of incubation (Figure 1C). Colonies were then identified to be M. hominis by the matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) and further confirmed by 16S rRNA sequencing (primers: 27F, AGAGTTTGATCMTGGCTCAG; 1492R, GGTTACCTTGTTACGACTT) and phylogenetic tree analysis (Figure 2; GenBank Accession No. OQ642125 for a strain isolated from a wound tissue sample and OQ642126 for a strain isolated from a blood sample). On day 31, therapy with polymyxin B in a dose of 5 × 105 U/day was initiated instead of ceftazidime–avibactam due to its shortage. In addition to the initial isolates of M hominis, two subsequent cultures obtained in the following week were also positive. The AST with a commercial kit (broth dilution method, Zhongaisheng, Hebei, China) showed that M. hominis was susceptible to doxycycline, minocycline, and josamycin but resistant to azithromycin, clarithromycin, norfloxacin, ciprofloxacin, roxithromycin, sparfloxacin, spectinomycin, and levofloxacin (Figure 1D). Based on the results of AST, minocycline (100 mg, twice/day), meropenem plus teicoplanin were started instead of moxifloxacin on the 33rd day of hospitalization. On the 37th hospital day, the hematology data and PCT level returned to normal, and infection was controlled. After starting minocycline-based therapy for 6 days, repeated cultures from the blood were M. hominis negative. Details regarding the diagnosis and treatment are shown in Figure 3.
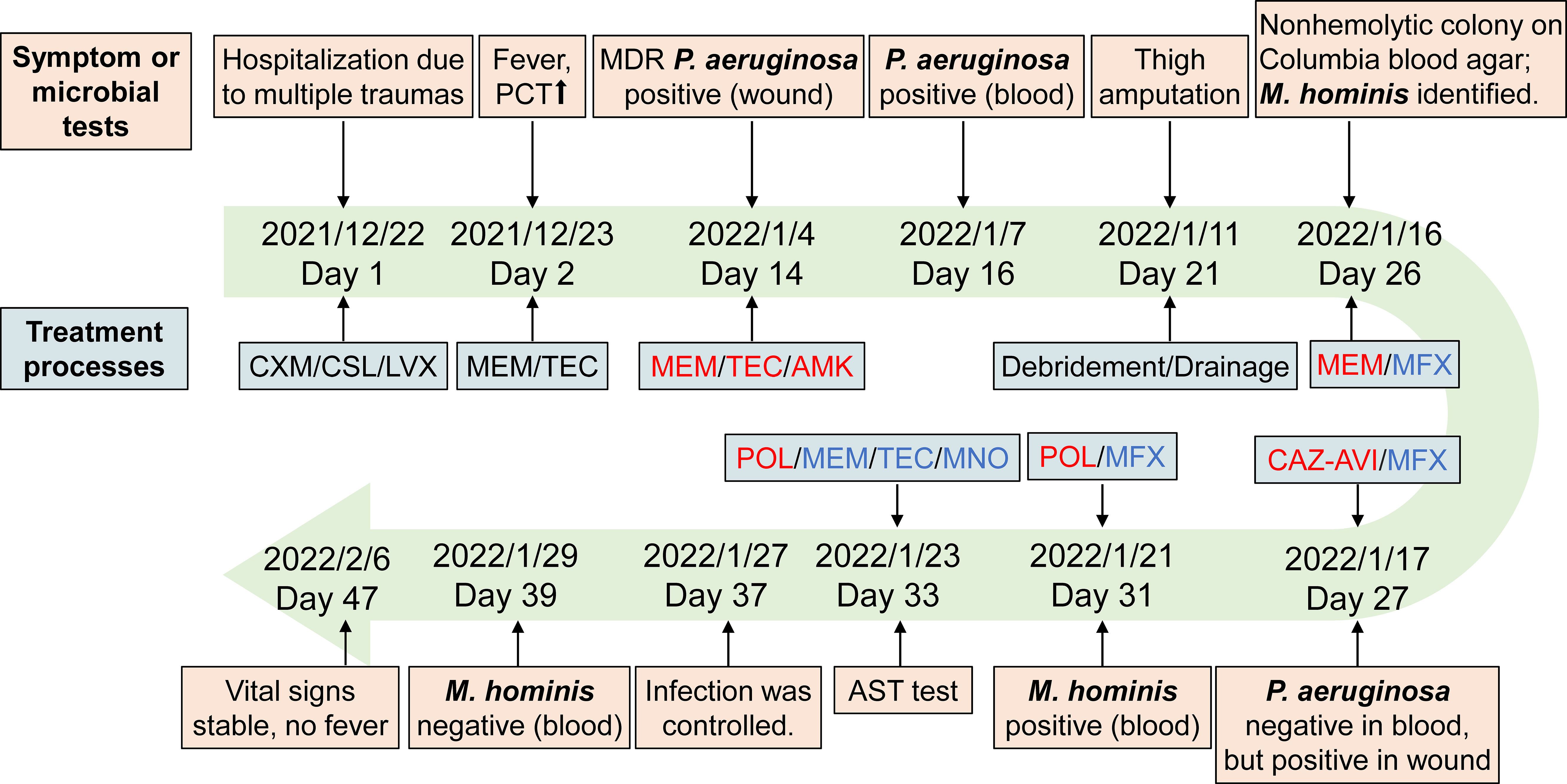
Figure 3 Timelines of patient’s diagnosis and treatment. Red, the anti-infective drugs for P. aeruginosa. Blue, the anti-infective drugs for M. hominis. Abbreviations: CXM, cefuroxime; CSL, cefoperazone-sulbactam; LVX, levofloxacin; MEM, meropenem; TEC, teicoplanin; AMK, amikacin; MFX, moxifloxacin; CAZ-AVI, ceftazidime–avibactam; POL, polymyxin B; MNO, minocycline.
Accumulating evidence has demonstrated that co-infection by multiple pathogen strains is a double trouble for global public health, which increases the risk of disease severity as well as the difficulty in clinical diagnosis and treatment (Kehr and Engelmann, 2015; Zhu et al., 2020). In this case report, we report on a patient who experienced double trouble, that is, M. hominis and P. aeruginosa co-infection. This co-infection was successfully treated with anti-infective agents despite delayed treatment.
Bloodstream infection due to M. hominis is rarely reported (Wang et al., 2022), let alone co-infection with another pathogen. In this report, the patient was co-infected with M. hominis and P. aeruginosa, which manifested as fever and an increase of PCT and lactic acid. These clinical manifestations are in accordance with the reported typical symptoms of M. hominis bloodstream infection (Zeng et al., 2022). P. aeruginosa blood infection was successfully treated with short-term antibiotics therapy. However, similar to previous cases (Henao-Martínez et al., 2012; Pailhoriès et al., 2014), the patient showed poor response to the wide antimicrobial treatments and developed persistent fever due to M. hominis infection. Additionally, delayed wound closure, another clinical manifestation of M. hominis infection, was also observed in our case, which was similar to the report by Adams et al. (2020). Thus, the identification of M. hominis should be considered in multiple-trauma patients who developed unexplained postoperative fever and protracted wound healing, particularly those who have a poor response to wide-spectrum antibiotics.
To date, the identification of M. hominis is challenging under conventional techniques (e.g., direct examination and culture of specimens), leading to the underestimation of this pathogen. Due to the absence of a cell wall, M. hominis, as an atypical bacteria, cannot be detected by Gram staining of clinical specimens. Conversely, in our case, P. aeruginosa infection was identified easily by Gram staining due to definite colony morphology, manifesting as yellow–green colonies. Accordingly, the P. aeruginosa infection was first treated appropriately. However, due to the uncontrolled infection, other pathogens were suspected. By the prolonged incubation (~5 days) on blood agar, tiny, nonhemolytic, and transparent colonies were observed, which is consistent with the previously reported colony morphology (Xiang and Lu, 2019; Wang et al., 2022). At this point, we need to remind readers that prolonged incubation is necessary to allow M. hominis colonies to develop, and in addition, translucent M. hominis colonies may be overlooked and should be examined carefully under reflected light. As recommended by Stabler et al. (2021), M. hominis should be suspected when Gram staining failed to detect microorganisms from pinpoint-sized, transparent colonies, warranting subculture onto mycoplasma medium. Previously, 16S rRNA sequencing and MALDI-TOF MS have been proven to be very useful for the rapid identification of M. hominis (Pereyre et al., 2013; Pailhoriès et al., 2014). Thus, with the suspicion of M. hominis, the colonies isolated from our case were sent to be identified by 16S rRNA sequencing and MALDI-TOF MS in order to prove the presence of microorganisms. As previously reported, a score of ≥ 1.70 rather than the classical threshold of ≥ 2.00 for Bruker MALDI-TOF MS was enough for accurate species-level identification of M. hominis (Pereyre et al., 2013); in our case, a score of 1.902 was achievable after 5 days of incubation on blood agar, supporting this result. Overall, M. hominis was definitely identified, which also supported the feasibility of MALDI-TOF MS or molecular methods in its rapid identification. Thus, for the rapid identification of this pathogen, we recommend that MALDI-TOF MS be necessary once colonies are isolated.
Empirical therapy for M. hominis infection includes the typical broad-spectrum antibiotic regimens (e.g., beta-lactam antimicrobials, vancomycin); however, these agents are generally ineffective since they act on cell wall metabolism (Yamaguchi et al., 2009; Bergin et al., 2017). So far, there has been no consensus on the treatment of M. hominis infection. In previously published cases, therapy/treatment options included drainage, debridement, and specific antibiotic therapy (Zhou et al., 2016). In our case, the patient underwent drainage and debridement because of ineffective anti-infective therapy. Thus, surgery may be considered a promising treatment option and should be performed promptly, as Yamaguchi et al. (2009) recommended. Several anti-infective agents, such as doxycycline (Henao-Martínez et al., 2012; Pailhoriès et al., 2014) and imipenem (Cuchý et al., 2000), have been used successfully in some cases of M. hominis infection; nevertheless, there is a lack of consensus about their efficacy. There are also conflicting views pointing out that specific antibiotic therapy for M. hominis was unnecessary (Cuchý et al., 2000). Even so, the present study supported that specific antibiotic therapy is preferable for the M. hominis infection since our patient was finally cured using antimicrobials. Despite the patient having been treated with various wide-spectrum antibiotics, he developed a persistent fever but excitingly recovered after adding minocycline, which was consistent with previous experience (Zhou et al., 2016). Actually, existing evidence has already indicated that tetracycline was considered to be the drug of choice for M. hominis infections (Myhre and Mårdh, 1983). On the basis of our report and existing evidence, tetracycline might be an effective option for M. hominis infection if the strain is not resistant.
Nevertheless, currently, the use of antimicrobials has led to a rapid increase in the emergence of resistant M. hominis strains (Cummings and McCormack, 1990; Pereyre et al., 2002; Meygret et al., 2018). For example, M. hominis are innately resistant to all agents acting on bacterial cell wall replication (e.g., β-lactams, sulfonamides, trimethoprim, rifampin) or folic acid synthesis (e.g., sulfonamides) (McCormack, 1993; Ahmed et al., 2021). The M. hominis isolates in our report were susceptible to doxycycline, minocycline, and coxacycline but are intrinsically resistant to a variety of antibiotic classes, such as azithromycin, roxithromycin, and levofloxacin. This antibiotic susceptibility profile is generally similar to the case reported by Reissier et al. (2016) and in line with the commonly reported antimicrobial susceptibility patterns of M. hominis (Kenny and Cartwright, 2001; Redelinghuys et al., 2014). Thereby, considering that the antibiotic susceptibility profile of each isolate varied irregularly (Cuchý et al., 2000; Pailhoriès et al., 2014), the appropriate AST testing for antimicrobial agents suitable for M. hominis infections, including macrolides, lincosamides, streptogramins, tetracyclines, and fluoroquinolones, should be applied before antibiotic therapy. Nevertheless, empirical therapy is necessary and required before antimicrobial test results.
In conclusion, extra-urogenital infections due to M. hominis are rare, especially co-infected with another pathogen. Despite the increased risk of disease severity and difficulty in treatment, the M. hominis and P. aeruginosa co-infection are treatable with appropriate antimicrobial agents. Moreover, this case highlighted the pathogenic potential of M. hominis in the bloodstream. M. hominis bloodstream infection is recommended to be considered in multiple-trauma patients with unexplained fever, particularly in the absence of response to wide-spectrum antibiotics.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Ethics committee of Shengli Oilfield Central Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Conception and design: S-MH and J-LW. Provision of study materials or patients: Y-RT and S-FP. Data analysis and interpretation: X-ZW and Y-YZ. Administrative support: S-FP. Manuscript writing and reviewing: all authors. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Adams, M., Bouzigard, R., Al-Obaidi, M., Zangeneh, T. T. (2020). Perinephric abscess in a renal transplant recipient due to mycoplasma hominis: case report and review of the literature. Transplant. Infect. Dis. 22 (5), e13308. doi: 10.1111/tid.13308
Ahmed, J., Rawre, J., Dhawan, N., Khanna, N., Dhawan, B. (2021). Mycoplasma hominis: an under recognized pathogen. Indian J. Med. Microbiol. 39 (1), 88–97. doi: 10.1016/j.ijmmb.2020.10.020
Bergin, S. M., Mendis, S. M., Young, B., Binti Izharuddin, E. (2017). Postoperative mycoplasma hominis brain abscess: keep it in mind! BMJ Case Rep. 2017, bcr2016218022. doi: 10.1136/bcr-2016-218022
Chalker, V. J., Sharratt, M. G., Rees, C. L., Bell, O. H., Portal, E., Sands, K., et al. (2021). Tetracycline resistance mediated by tet(M) has variable integrative conjugative element composition in mycoplasma hominis strains isolated in the united kingdom from 2005 to 2015. Antimicrob. Agents Chemother. 65 (4), e02513-20. doi: 10.1128/aac.02513-20
Cuchý, E., Cherta, I., Garau, J. (2000). Mycoplasma hominis catheter-related infection in a patient with multiple trauma. Clin. Microbiol. Infection 6 (2), 115–116. doi: 10.1046/j.1469-0691.2000.00022.x
Cummings, M. C., McCormack, W. M. (1990). Increase in resistance of mycoplasma hominis to tetracyclines. Antimicrob. Agents Chemother. 34 (12), 2297–2299. doi: 10.1128/aac.34.12.2297
Henao-Martínez, A. F., Young, H., Nardi-Korver, J. J. L., Burman, W. (2012). Mycoplasma hominis brain abscess presenting after a head trauma: a case report. J. Med. Case Rep. 6 (1), 253. doi: 10.1186/1752-1947-6-253
Hos, N. J., Bauer, C., Liebig, T., Plum, G., Seifert, H., Hampl, J. (2015). Autoinfection as a cause of postpartum subdural empyema due to mycoplasma hominis. Infection 43 (2), 241–244. doi: 10.1007/s15010-014-0713-2
Kehr, J., Engelmann, L. J. M. (2015). Double trouble? Towards an epistemology co-infection. Med. Anthropol. Theory 2 (1), 1–31. doi: 10.17157/mat.2.1.212
Kenny, G. E., Cartwright, F. D. (2001). Susceptibilities of mycoplasma hominis, m. pneumoniae, and ureaplasma urealyticum to GAR-936, dalfopristin, dirithromycin, evernimicin, gatifloxacin, linezolid, moxifloxacin, quinupristin-dalfopristin, and telithromycin compared to their susceptibilities to reference macrolides, tetracyclines, and quinolones. Antimicrob. Agents Chemother. 45 (9), 2604–2608. doi: 10.1128/aac.45.9.2604-2608.2001
McCormack, W. M. (1993). Susceptibility of mycoplasmas to antimicrobial agents: clinical implications. Clin. Infect. Dis. 17 Suppl 1, S200–S201. doi: 10.1093/clinids/17.supplement_1.s200
Meyer, R. D., Clough, W. (1993). Extragenital mycoplasma hominis infections in adults: emphasis on immunosuppression. Clin. Infect. Dis. 17 (Supplement_1), S243–S249. doi: 10.1093/clinids/17.Supplement_1.S243
Meygret, A., Le Roy, C., Renaudin, H., Bébéar, C., Pereyre, S. (2018). Tetracycline and fluoroquinolone resistance in clinical ureaplasma spp. and mycoplasma hominis isolates in France between 2010 and 2015. J. Antimicrobial Chemotherapy 73 (10), 2696–2703. doi: 10.1093/jac/dky238
Myhre, E. B., Mårdh, P. A. (1983). Treatment of extragenital infections caused by mycoplasma hominis. Sex Transm Dis. 10 (4 Suppl), 382–385.
Pailhoriès, H., Rabier, V., Eveillard, M., Mahaza, C., Joly-Guillou, M. L., Chennebault, J. M., et al. (2014). A case report of mycoplasma hominis brain abscess identified by MALDI-TOF mass spectrometry. Int. J. Infect. Dis. 29, 166–168. doi: 10.1016/j.ijid.2014.08.004
Pereyre, S., Gonzalez, P., Barbeyrac, B.d., Darnige, A., Renaudin, H., Charron, A., et al. (2002). Mutations in 23S rRNA account for intrinsic resistance to macrolides in mycoplasma hominis and mycoplasma fermentans and for acquired resistance to macrolides in m. hominis. Antimicrobial Agents Chemotherapy 46 (10), 3142–3150. doi: 10.1128/AAC.46.10.3142-3150.2002
Pereyre, S., Tardy, F., Renaudin, H., Cauvin, E., Del Prá Netto Machado, L., Tricot, A., et al. (2013). Identification and subtyping of clinically relevant human and ruminant mycoplasmas by use of matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 51 (10), 3314–3323. doi: 10.1128/jcm.01573-13
Poku, V. O. (2022). Maternal mortality: the role of mycoplasma hominis and its impact on neonatal health. Health Sci. Rev. 4, 100036. doi: 10.1016/j.hsr.2022.100036
Posse, T., Prieto, M., Cipolla, L., Kaufman, S. (2018). [Mycoplasma hominis bacteremia. an underestimated etiological agent]. Rev. Argent. Microbiologia 50 (1), 45–47. doi: 10.1016/j.ram.2017.02.009
Redelinghuys, M. J., Ehlers, M. M., Dreyer, A. W., Lombaard, H. A., Kock, M. M. (2014). Antimicrobial susceptibility patterns of ureaplasma species and mycoplasma hominis in pregnant women. BMC Infect. Dis. 14, 171. doi: 10.1186/1471-2334-14-171
Reissier, S., Masson, R., Guérin, F., Viquesnel, G., Petitjean-Lecherbonnier, J., Pereyre, S., et al. (2016). Fatal nosocomial meningitis caused by mycoplasma hominis in an adult patient: case report and review of the literature. Int. J. Infect. Dis. 48, 81–83. doi: 10.1016/j.ijid.2016.05.015
Stabler, S., Faure, E., Duployez, C., Wallet, F., Dessein, R., Guern, R. L. (2021). The brief case: mycoplasma hominis extragenital abscess. J. Clin. Microbiol. 59 (4), e02343–e02320. doi: 10.1128/JCM.02343-20
Susi, H., Barrès, B., Vale, P. F., Laine, A.-L. (2015). Co-Infection alters population dynamics of infectious disease. Nat. Commun. 6 (1), 5975. doi: 10.1038/ncomms6975
Wang, Q., Tang, X., van der Veen, S. (2022). Mycoplasma hominis bloodstream infection and persistent pneumonia in a neurosurgery patient: a case report. BMC Infect. Dis. 22 (1), 169. doi: 10.1186/s12879-022-07137-4
Whitson, W. J., Ball, P. A., Lollis, S. S., Balkman, J. D., Bauer, D. F. (2014). Postoperative mycoplasma hominis infections after neurosurgical intervention. J. Neurosurg. Pediatr. 14 (2), 212–218. doi: 10.3171/2014.4.peds13547
Wildenbeest, J. G., Said, I., Jaeger, B., van Hest, R. M., van de Beek, D., Pajkrt, D. (2016). Neonate with mycoplasma hominis meningoencephalitis given moxifloxacin. Lancet Infect. Dis. 16 (11), e261–e266. doi: 10.1016/S1473-3099(16)30162-1
Xiang, L., Lu, B. (2019). Infection due to mycoplasma hominis after left hip replacement: case report and literature review. BMC Infect. Dis. 19 (1), 50. doi: 10.1186/s12879-019-3686-z
Yamaguchi, M., Kikuchi, A., Ohkusu, K., Akashi, M., Sasahara, J., Takakuwa, K., et al. (2009). Abscess formation due to mycoplasma hominis infection after cesarean section. J. Obstetrics Gynaecology Res. 35 (3), 593–596. doi: 10.1111/j.1447-0756.2008.00993.x
Zeng, T., Wu, Y., Yang, Z., Luo, M., Xu, C., Liu, Z., et al. (2022). Clinical and microbiological characterization of bloodstream infections caused by mycoplasma hominis: an overlooked pathogen. Infect. Dis. Ther. 11 (3), 1003–1017. doi: 10.1007/s40121-022-00616-w
Zhou, M., Wang, P., Chen, S., Du, B., Du, J., Wang, F., et al. (2016). Meningitis in a Chinese adult patient caused by mycoplasma hominis: a rare infection and literature review. BMC Infect. Dis. 16 (1), 557. doi: 10.1186/s12879-016-1885-4
Keywords: Mycoplasma hominis, Pseudomonas aeruginosa, case report, infectious diseases, infection
Citation: Huang S-M, Tang Y-R, Wang J-L, Wang X-Z, Zhang Y-Y and Pan S-F (2023) Case Report: Double trouble: a rare case of successfully treated Mycoplasma hominis and Pseudomonas aeruginosa co-infection. Front. Cell. Infect. Microbiol. 13:1159891. doi: 10.3389/fcimb.2023.1159891
Received: 06 February 2023; Accepted: 13 April 2023;
Published: 01 May 2023.
Edited by:
Rodolfo García-Contreras, National Autonomous University of Mexico, MexicoReviewed by:
Mehdi Fatahi-Bafghi, Shahid Sadoughi University of Medical Sciences and Health Services, IranCopyright © 2023 Huang, Tang, Wang, Wang, Zhang and Pan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ji-Liang Wang, enh5eXdqbEAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.