- 1Department of Dermatology, Institute of Dermatology, Peking University Shenzhen Hospital, Shenzhen Peking University-The Hong Kong University of Science and Technology Medical Center, Shenzhen, China
- 2College of Life Science and Oceanography, Shenzhen University, Shenzhen, China
- 3Department of Epidemiology and Biostatistics, Institute of Basic Medical Sciences Chinese Academy of Medical Sciences, School of Basic Medicine Peking Union Medical College, Beijing, China
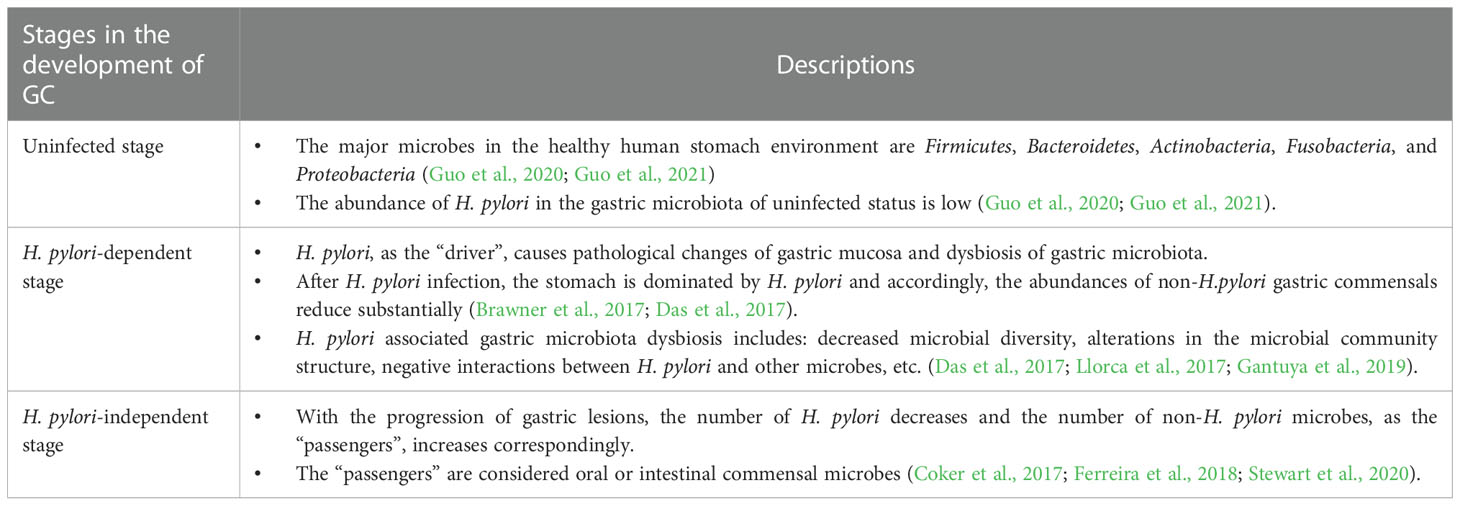
Gastric cancer (GC) is one of the leading causes of cancer-related deaths worldwide. The gastric microbiota plays a critical role in the development of GC. First, Helicobacter pylori (H. pylori) infection is considered a major risk factor for GC. However, recent studies based on microbiota sequencing technology have found that non-H. pylori microbes also exert effects on gastric carcinogenesis. Following the infection of H. pylori, gastric microbiota dysbiosis could be observed; the stomach is dominated by H. pylori and the abundances of non-H. pylori microbes reduce substantially. Additionally, decreased microbial diversity, alterations in the microbial community structure, negative interactions between H. pylori and other microbes, etc. occur, as well. With the progression of gastric lesions, the number of H. pylori decreases and the number of non-H. pylori microbes increases correspondingly. Notably, H. pylori and non-H. pylori microbes show different roles in different stages of gastric carcinogenesis. In the present mini-review, we provide an overview of the recent findings regarding the role of the gastric microbiota, including the H. pylori and non-H. pylori microbes, in the development of GC.
Introduction
Gastric cancer (GC) is one of the leading causes of cancer-related deaths worldwide, ranking fifth in incidence and third in mortality of cancers (Bray et al., 2018). According to World Health Organization International Agency for Research on Cancer (WHO-IARC), the annual burden of GC will increase to approximately 1.8 million new cases and 1.3 million deaths by 2040. Compared with those in 2020, the numbers of new cases and deaths will increase by approximately 63% and 66%, respectively (Morgan et al., 2022). Helicobacter pylori (H. pylori) infection is a critical risk factor for GC (Amieva and Peek, 2016) and H. pylori was classified by the WHO-IARC as a type I carcinogen (WHO-IARC, 1994). In recent years, sequencing-based studies focusing on microbiota have shown that patients with GC have gastric microbiota dysbiosis, including reduced microbial diversity, altered microbial community structure, altered compositions, and abnormal bacterial interactions (Gantuya et al., 2020; Kadeerhan et al., 2021). Furthermore, non-H. pylori microbes might also promote gastric lesions and even GC (Coker et al., 2017; Yu et al., 2017; Ferreira et al., 2018; Kadeerhan et al., 2021). The interactions between H. pylori and other microbes may be also involved in gastric carcinogenesis.
In the present mini-review, we aim to discuss the recent findings regarding the role of gastric microbiota, including H. pylori and non-H. pylori microbes, in the development of GC.
H. pylori infection, eradication, and GC
H. pylori is a gram-negative, flagellated, microaerophilic bacterium belonging to the Campylobacterota phylum, which was first identified in 1982 (Warren and Marshall, 1983). H. pylori colonizes in the stomach and becomes the predominant microbe in stomach after infection (Schulz et al., 2018). In terms of the global epidemiology of H. pylori infection, according to a global meta-analysis (Hooi et al., 2017), there were about 4.4 billion H. pylori-positive cases worldwide in 2015. The prevalence rate of H. pylori infection varied by region, with the highest prevalence rate in Africa (70.1%, 95% CI: 62.6-77.7%) and the lowest prevalence rate in Oceania (24.4%, 95% CI: 18.5-30.4%). Furthermore, for the temporal trend of H. pylori infection, the prevalence in different regions is stable or decreasing, especially in the developed world and in children (Burucoa and Axon, 2017; Hooi et al., 2017).
H. pylori infection is considered a major risk factor for gastric carcinogenesis. Overall, a large-scale pooled analysis of case-control studies nested within prospective cohorts showed that H. pylori infection was associated with nearly six-fold increased risk of non-cardia cancer (Helicobacter and Cancer Collaborative Group, 2001). The mechanism that H. pylori induces GC has been explored (Ishaq and Nunn, 2015; Talebi Bezmin Abadi, 2016). First, H. pylori primarily triggers the transition from normal mucosa to non-atrophic gastritis and then initiates precancerous lesions (Díaz et al., 2018). The responses after infection are mainly mediated through the action of bacterial virulence factors, including cytotoxin-associated gene A (CagA), vacuolating cytotoxin A (VacA), and other outer membrane proteins (Díaz et al., 2018; Alipour, 2021). CagA has multiple effects on epithelial cells, including stimulating cell proliferation, reducing epithelial cell apoptosis, etc. (Saadat et al., 2007; Nagy et al., 2009; Buti et al., 2011). Additionally, inflammatory cells can be recruited and oxygen species-induced damage can be induced after CagA and the type IV secretion system (T4SS) activate the inflammatory signaling (Viala et al., 2004; Chaturvedi et al., 2011). VacA can also cause alterations of cells, such as vacuolization and promoting immune regulation (Willhite et al., 2003; Yang et al., 2022). Further, the urease production by H. pylori and the glandular atrophy induced by H. pylori infection lead to reduced acid production and shifts in gastric pH value. As a result, the bacterial colonization environment in the stomach changes and gastric microbiota dysbiosis may occur (Schulz et al., 2015; Noto and Peek, 2017). The above-mentioned effects promote GC development.
For H. pylori-positive cases, eradication therapy could be given (Fallone et al., 2016; Malfertheiner et al., 2017; Liu et al., 2018). The effect of H. pylori eradication therapy on the GC risk has been evaluated. You et al. reported that, based on a randomized trial with a follow-up of 7.3 years, H. pylori treatment resulted in statistically significant decreases in the combined prevalence of severe chronic atrophic gastritis, intestinal metaplasia, dysplasia, or GC (OR = 0.77, 95% CI: 0.62-0.95) (You et al., 2006). With a follow-up of 22 years for this randomized trial, this team found that the protective effect of H pylori treatment on GC incidence (OR= 0.48, 95% CI: 0.32-0.71) and GC death (HR= 0.62, 95% CI: 0.39-0.99) persisted 22 years post-intervention (Li et al., 2019). Additionally, a recent well-designed meta-analysis enrolling randomized controlled trials (RCTs) with 10 or more years of follow-up found that the GC incidence decreased significantly with H. pylori eradication therapy (RR=0.54, 95% CI: 0.41-0.72); on the other hand, eradication of H. pylori showed significant reductions in GC mortality (RR=0.66, 95% CI: 0.46-0.95) (Ford et al., 2022).
H. pylori associated gastric microbiota dysbiosis
The gastrointestinal microbiota refers to microorganisms lived in the gastrointestinal tracts, which is critical to many aspects of human health (Clemente et al., 2012; Valdes et al., 2018). For human immune, the microbiota is key to the induction, training, and function of the host immune system (Belkaid and Hand, 2014; Ling et al., 2022). Regarding the gastric microbiota, due to the high acidity of the stomach, the human stomach was once assumed to be a sterile organ (Espinoza et al., 2018). However, H. pylori is able to colonize the human gastric mucosa and survive in the highly acidic environment of the stomach (Schulz et al., 2015). With the advent of novel techniques for analyzing the microbial community, the unique features of the gastric microbiota have been identified that the major microbes in the healthy human stomach environment are Firmicutes, Bacteroidetes, Actinobacteria, Fusobacteria, and Proteobacteria (Guo et al., 2020; Guo et al., 2021).
For H. pylori-infected individuals, the stomach is dominated by H. pylori and accordingly, the abundances of non-H. pylori microbes reduce substantially (Brawner et al., 2017; Das et al., 2017). In addition to the changes of microbial composition, other phenomena of gastric microbiota dysbiosis have also been found. For the microbial alpha diversity, Gantuya et al. reported that individuals infected with H. pylori showed significant decreased microbial diversity compared with H. pylori-negative individuals (Gantuya et al., 2019). Another study found that there was a negative association between the gastric microbiome diversity and Helicobacter abundance (Das et al., 2017). In addition to microbial alpha diversity, infection with H. pylori results in alterations of the microbial community structure (beta diversity). According to a population-based study, the H. pylori positive group and negative group were clearly separated according to beta diversity (Llorca et al., 2017). Furthermore, studies focusing on the microbial ecological interactions found shifts of the interactions between H. pylori and other microbes in the stomach environment. In detail, according to an Indian study using16S rRNA gene sequencing, the network analyses showed that Helicobacter had negative interactions with other microbes of the gastric microbiome (Das et al., 2017); another Chinese study reported similar findings (Guo et al., 2020). Regarding the numbers of interactions, Coker et al. found that H. pylori infection reduces the number of gastric microbiome interactions (Coker et al., 2017). However, all the above-mentioned findings were based on statistical analyses of sequencing data. Thus, we need more clinical data supporting current presented concept (Rivas-Ortiz et al., 2017).
For H. pylori-positive individuals, the H. pylori eradication could reverse gastric microbiota dysbiosis and exert beneficial effects on the gastric microbiota (Guo et al., 2022). Firstly, for the reduced gastric microbial diversity among H. pylori-positive cases, the diversity could increase significantly after successful eradication of H. pylori (Guo et al., 2020; Mao et al., 2021). Also, significant differences were observed for the microbial community structure (the beta diversity) following eradication (Guo et al., 2020; Sung et al., 2020b; Mao et al., 2021; Watanabe et al., 2021; Yuan et al., 2021). For the gastric microbiota composition, after removing H. pylori in the stomach environment, the gastric commonly dominant commensals are enriched (Guo et al., 2020; Shin et al., 2020). Different changes of specific microbes were reported, which may be resulted from different population, sequence methods, and sampling details. The common reported commensals included Firmicutes, Streptococcus, Prevotella., etc. (He et al., 2019; Guo et al., 2020; Mao et al., 2021; Watanabe et al., 2021; Yuan et al., 2021). In terms of interactions between gastric commensal bacteria, a reduction in these interactions was reported after eradication of H. pylori (Sung et al., 2020b; Yuan et al., 2021), which were also based on statistical analyses of sequencing data and required further validation. Moreover, due to the development of bioinformatics, microbiota function could be predicted and analyzed. According to the bioinformatic analysis of functional capacity, the bacteria reproduction-related pathways are down-regulated and pathways of gastric acid secretion, etc. are up-regulated (He et al., 2019; Guo et al., 2020), indicating beneficial effect of eradication on the recovery of gastric microbiota. In combination with the prevention effect of H. pylori eradication on GC, the alterations in gastric microbiota after eradication may contribute to the reduction in GC risk; further studies with long-term follow-up are needed (Guo et al., 2022).
The overall features of the gastric microbiota associated with GC
In recent years, the characterization of the gastric microbiota associated with GC has been identified, indicating that gastric microbiota dysbiosis occur in gastric carcinogenesis (Yang et al., 2021). In the year of 2009, the team of Prof. Engstrand compared the gastric microbiota of patients with GC and controls using the terminal restriction fragment length polymorphism (T-RFLP) and 16S rRNA gene cloning and sequencing. They found that diversity indices of GC microbiota were not significantly different from that in controls according to the T-RFLP. In terms of gastric microbiota composition of GC, the abundance of H. pylori was low and the GC microbiota was dominated by the following genera: Streptococcus, Lactobacillus, Veillonella and Prevotella (Dicksved et al., 2009). However, the sample size of this study was small (only ten patients and five controls); additionally, 16S rRNA sequencing technology and related procedures are not yet developed and extensively used, therefore this work is an initial investigation of this field.
In following decade, other findings have been reported. Firstly, the gastric microbial diversity alteration in GC has been the most focused topic. Several studies reported that compared with the gastritis status, gastric microbial diversity is significantly reduced; analyses showed that the microbial community structure (beta diversity) is significantly altered in GC patients (Coker et al., 2017; Ferreira et al., 2018). Similarly, according to studies based on comparison between GC tissues and non-cancerous tissues, GC tissues also have reduced diversity and shifted microbiota structure (Chen et al., 2019). However, the conclusions are inconsistent across studies. For instants, two studies showed that the alpha diversity of GC gastric microbiota was increased (Eun et al., 2014; Linz et al., 2017). The difference of results may be caused by different populations, sampling sites and stage of gastric disease.
In addition to microbial diversity analysis, with the development of bioinformatics, more in-depth analysis methods have been developed and used. The function prediction analyses have been applied to explore potential mechanisms of gastric carcinogenesis. The most studies did function prediction analyses using PICRUSt (Langille et al., 2013). Ferreira et al. identified the presence of a nitrosating microbial community in GC cases, indicating that nitrate-reducing bacteria may contribute to gastric carcinogenesis (Ferreira et al., 2018). Meanwhile, a switch towards purine metabolism, D-alanine metabolism, drug metabolism, etc. in GC were reported in another study (Coker et al., 2017). These findings suggested that the microorganisms in the stomach may contribute to the development of GC through specific functional effects. Similarly, these findings need further validation of mechanisms.
The non-H. pylori microbes associated with GC
In addition to H. pylori, more and more studies have been focusing on other non-H. pylori gastric microorganisms. Similar to the bacterial driver-passenger model in the development of colorectal cancer (Tjalsma et al., 2012), the hypothesis of GC has been proposed that: H. pylori, as the “driver”, causes pathological changes of gastric mucosa and dysbiosis of gastric microbiota; with the progression of gastric lesions, the number of H. pylori decreases and the number of other microorganisms in the stomach, i.e. non-H. pylori microbes as the “passengers”, increases correspondingly. These non-H. pylori microbes play an important role in the pathogenesis of GC.
The above hypothesis has been confirmed in animal research. An animal study using hypergastrinemic insulin-gastrin (INS-GAS) transgenic mice found that compared with the specific pathogen free (SPF) INS-GAS mice, the duration of gastric lesions development was longer for germ-free INS-GAS mice; compared with INS-GAS mice infected with H. pylori only, INS-GAS mice with complex gastric microbiota had more severe gastric lesions and an earlier onset of gastrointestinal intraepithelial neoplasia (Lofgren et al., 2011). Another INS-GAS mice-based study reported that INS-GAS mice coinfected with H. pylori and other intestinal bacteria had a higher rate of development of gastrointestinal intraepithelial neoplasia than those infected with H. pylori alone (Lertpiriyapong et al., 2014). These findings indicate the potential role of non-H. pylori microbes and the interactions between H. pylori and non-H. pylori microbes in gastric carcinogenesis.
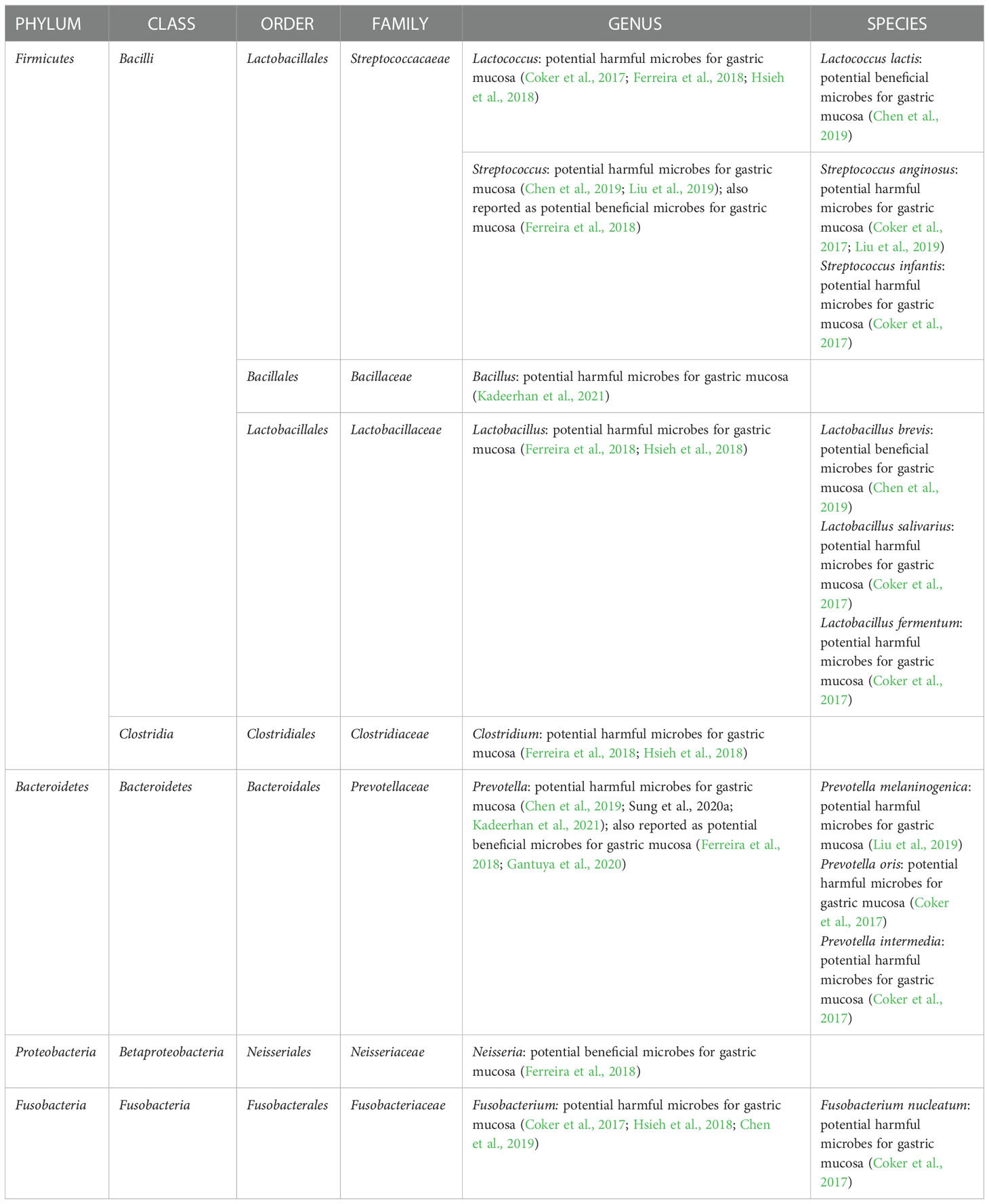
More researchers are paying attention to human studies as the hypothesis is supported in animal studies. In a population-based study using the 16S rRNA gene sequencing method, compared with individuals with gastritis, GC showed gastric microbiota dysbiosis and a lower abundance of Helicobacter and the over-representation of intestinal commensals was seen in GC gastric microbiota. In detail, 16 enriched taxa and 13 depleted taxa in GC according to the LEfSe analysis (Ferreira et al., 2018). Another study comparing gastric microbiota of GC patients and superficial gastritis reported that 21 bacterial taxa were enriched in GC and 10 bacterial taxa were depleted in GC. Specifically, enrichment of oral microbes was observed in the stomach of GC (Coker et al., 2017). In addition to above two cross-sectional studies, a cohort study with a 4-year follow-up reported that Helicobacter abundance was lower in the subjects with progression of gastric lesions compared with non-progression group. Specifically, the remarkable decline in Helicobacter was observed after the progression to stage of dysplasia/GC compared with non-progression controls (Kadeerhan et al., 2021). The key non-H. pylori microbes associated with GC are summarized in Table 1. However, inconsistent results were found, necessitating additional validations.
Furthermore, based on the current findings, a panel of differential gastric bacteria can be developed to distinguish GC and the progression of GC with outstanding performance. A recently published meta-analysis, which enrolled six independent studies, reported that eight bacterial taxa could serve as a panel of biomarkers to discriminate GC from superficial gastritis with an area under the curve (AUC) of 0.850 (Liu et al., 2022). Regarding the progression of GC, Kadeerhan et al. reported a combination of four genera (Bacillus, Capnocytophaga, Helicobater, Prevotella) with age and sex to distinguish subjects after lesion progression from non-progression controls (AUC = 0.927) (Kadeerhan et al., 2021). In addition to a panel of bacteria, a new single index called Microbial Dysbiosis Index (MDI) has been presented. MDI is calculated by log (total abundance of genera increased in GC/total abundance of genera decreased in GC); a higher value of MDI means a higher risk of GC. The application of MDI has been applied in the evaluation of GC: the GC gastric microbiota had a higher MDI and the findings were confirmed in the validation cohorts (Ferreira et al., 2018).
The different roles of H. pylori and non-H. pylori microbes in gastric carcinogenesis
The progression of gastric carcinogenesis is detailed in Figure 1. Like bacterial driver-passenger model of colorectal cancer, the development of GC showed similar change pattern of gastric microbiota. Thus, H. pylori and non-H. pylori microbes show different roles in different stages of gastric carcinogenesis. First of all, the load of H. pylori in the stomach increases after the initial infection, especially in the active gastritis stage (Stewart et al., 2020). Interestingly, the H. pylori load decreases with the progression of gastric lesions. A population-based study showed that a lower Helicobacter abundance was observed in subjects with the progression of gastric lesions (Kadeerhan et al., 2021); another study reported that the abundance of Helicobacter was substantially lower in GC patients than gastritis (Ferreira et al., 2018). This phenomenon could be explained that, following H. pylori infection, due to the persistence of inflammation and the loss of acid-secreting parietal cells, the gastric environment becomes more favorable for the colonization of other bacteria and progression of lesions are accelerated (Polk and Peek, 2010). In detail, with the development of gastric lesions, oral or intestinal commensal microbes are enriched (Coker et al., 2017; Ferreira et al., 2018; Stewart et al., 2020). However, by the late stage of gastric precancerous lesions, the stomach environment is no longer suitable for H. pylori and the abundance H. pylori of decreases. This phenomenon has been confirmed in human studies (Ferreira et al., 2018; Kadeerhan et al., 2021). The key roles of H. pylori in different stages of gastric carcinogenesis were shown in the Table 2. In addition to the overall description of the progression of gastric carcinogenesis, the roles of certain bacteria remain to be clarified and further mechanism investigation is needed for a deeper understanding of this issue.
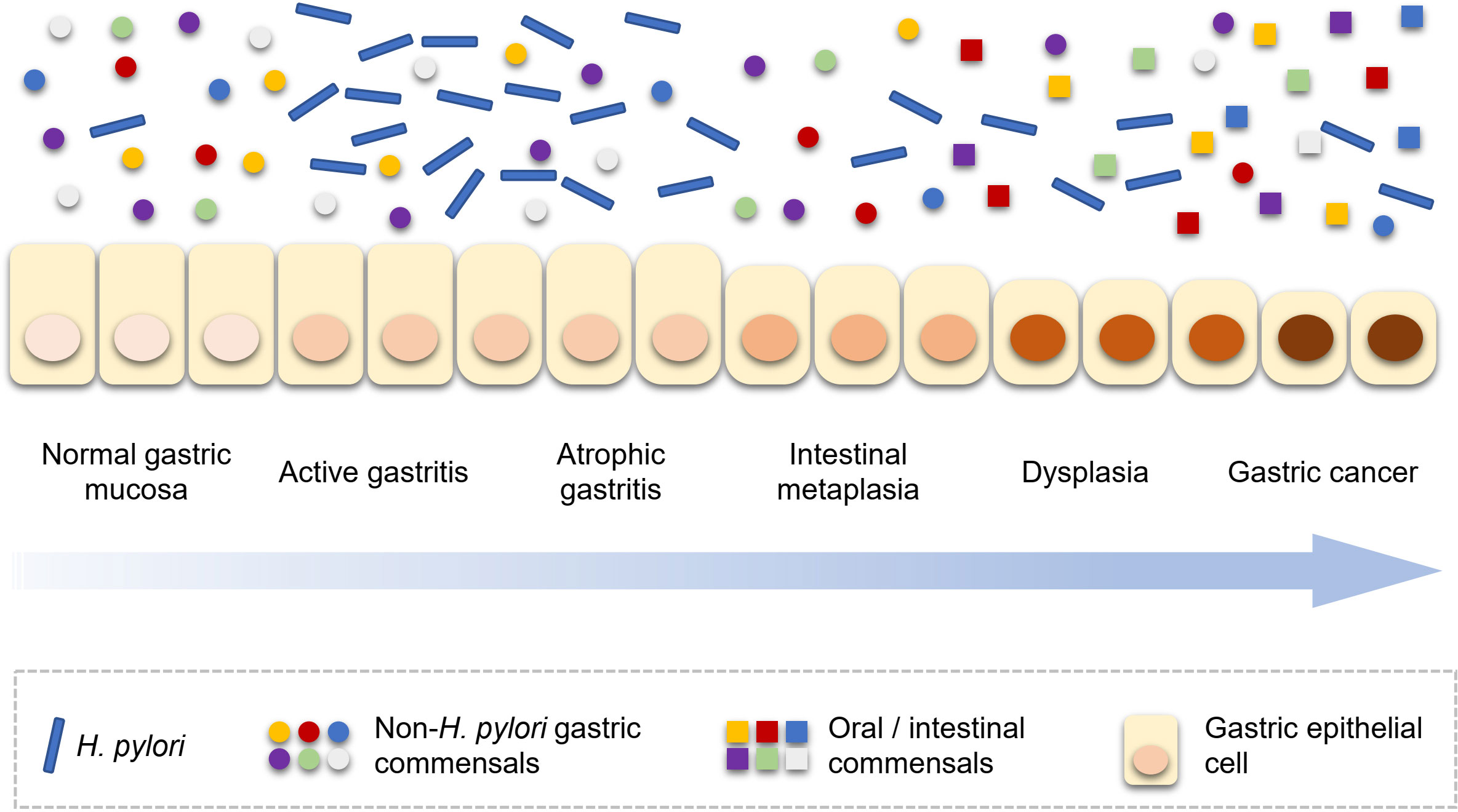
Figure 1 H. pylori and non-H. pylori microbes in the development of gastric carcinogenesis. H. pylori, Helicobacter pylori.
Future perspectives
Non-H. pylori microbes and their interactions may also play a critical role in the development of GC. However, inconsistent findings were reported for non-H. pylori microbes associated with GC. Accordingly, further mechanism investigation is needed to validate these potential GC-associated non-H. pylori microbes, such as animal studies. Additionally, most human studies are case-control studies, which compared gastric microbiota of gastric mucosa between GC patients and control population. Due to this study design, we cannot infer a causal relationship between gastric microbiota dysbiosis and development and progression of GC. In other words, it is unclear whether gastric microbiota dysbiosis causes GC or whether GC causes gastric microbiota dysbiosis. Therefore, cohort studies with long-term follow-up are needed to confirm the major findings.
Author contributions
YG drafted the manuscript, conceptualized the idea, and revised the manuscript. X-SC and M-GZ performed the literature search and revised the manuscript. YG and M-GZ contributed to drawing the figure. BY critically revised the manuscript and supervised the study. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82103727), the fellowship of China Postdoctoral Science Foundation (No. 2021M702221), Guangdong Basic and Applied Basic Research Foundation (No. 2022A1515010957 and 2021A1515011558), Shenzhen Sanming Project (No. SZSM201812059), Shenzhen Key Medical Discipline Construction Fund (No. SZXK040), Shenzhen Science and Technology Program (No. RCBS20210706092408008).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alipour, M. (2021). Molecular mechanism of helicobacter pylori-induced gastric cancer. J. Gastrointest Cancer 52 (1), 23–30. doi: 10.1007/s12029-020-00518-5
Amieva, M., Peek, R. M., Jr. (2016). Pathobiology of helicobacter pylori-induced gastric cancer. Gastroenterology 150 (1), 64–78. doi: 10.1053/j.gastro.2015.09.004
Belkaid, Y., Hand, T. W. (2014). Role of the microbiota in immunity and inflammation. Cell 157 (1), 121–141. doi: 10.1016/j.cell.2014.03.011
Brawner, K. M., Kumar, R., Serrano, C. A., Ptacek, T., Lefkowitz, E., Morrow, C. D., et al. (2017). Helicobacter pylori infection is associated with an altered gastric microbiota in children. Mucosal Immunol. 10 (5), 1169–1177. doi: 10.1038/mi.2016.131
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., Jemal, A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68 (6), 394–424. doi: 10.3322/caac.21492
Burucoa, C., Axon, A. (2017). Epidemiology of helicobacter pylori infection. Helicobacter 22 (Suppl 1). doi: 10.1111/hel.12403
Buti, L., Spooner, E., van der Veen, A. G., Rappuoli, R., Covacci, A., Ploegh, H. L. (2011). Helicobacter pylori cytotoxin-associated gene a (CagA) subverts the apoptosis-stimulating protein of p53 (ASPP2) tumor suppressor pathway of the host. Proc. Natl. Acad. Sci. U.S.A. 108 (22), 9238–9243. doi: 10.1073/pnas.1106200108
Chaturvedi, R., Asim, M., Romero-Gallo, J., Barry, D. P., Hoge, S., de Sablet, T., et al. (2011). Spermine oxidase mediates the gastric cancer risk associated with helicobacter pylori CagA. Gastroenterology 141 (5), 1696–1708.e1691-1692. doi: 10.1053/j.gastro.2011.07.045
Chen, X. H., Wang, A., Chu, A. N., Gong, Y. H., Yuan, Y. (2019). Mucosa-associated microbiota in gastric cancer tissues compared with non-cancer tissues. Front. Microbiol. 10. doi: 10.3389/fmicb.2019.01261
Clemente, J. C., Ursell, L. K., Parfrey, L. W., Knight, R. (2012). The impact of the gut microbiota on human health: An integrative view. Cell 148 (6), 1258–1270. doi: 10.1016/j.cell.2012.01.035
Coker, O. O., Dai, Z., Nie, Y., Zhao, G., Cao, L., Nakatsu, G., et al. (2017). Mucosal microbiome dysbiosis in gastric carcinogenesis. Gut 67 (6), 1024–1032. doi: 10.1136/gutjnl-2017-314281
Das, A., Pereira, V., Saxena, S., Ghosh, T. S., Anbumani, D., Bag, S., et al. (2017). Gastric microbiome of Indian patients with helicobacter pylori infection, and their interaction networks. Sci. Rep. 7 (1), 15438. doi: 10.1038/s41598-017-15510-6
Díaz, P., Valenzuela Valderrama, M., Bravo, J., Quest, A. F. G. (2018). Helicobacter pylori and gastric cancer: Adaptive cellular mechanisms involved in disease progression. Front. Microbiol. 9. doi: 10.3389/fmicb.2018.00005
Dicksved, J., Lindberg, M., Rosenquist, M., Enroth, H., Jansson, J. K., Engstrand, L. (2009). Molecular characterization of the stomach microbiota in patients with gastric cancer and in controls. J. Med. Microbiol. 58 (Pt 4), 509–516. doi: 10.1099/jmm.0.007302-0
Espinoza, J. L., Matsumoto, A., Tanaka, H., Matsumura, I. (2018). Gastric microbiota: An emerging player in helicobacter pylori-induced gastric malignancies. Cancer Lett. 414, 147–152. doi: 10.1016/j.canlet.2017.11.009
Eun, C. S., Kim, B. K., Han, D. S., Kim, S. Y., Kim, K. M., Choi, B. Y., et al. (2014). Differences in gastric mucosal microbiota profiling in patients with chronic gastritis, intestinal metaplasia, and gastric cancer using pyrosequencing methods. Helicobacter 19 (6), 407–416. doi: 10.1111/hel.12145
Fallone, C. A., Chiba, N., van Zanten, S. V., Fischbach, L., Gisbert, J. P., Hunt, R. H., et al. (2016). The Toronto consensus for the treatment of helicobacter pylori infection in adults. Gastroenterology 151 (1), 51–69 e14. doi: 10.1053/j.gastro.2016.04.006
Ferreira, R. M., Pereira-Marques, J., Pinto-Ribeiro, I., Costa, J. L., Carneiro, F., Machado, J. C., et al. (2018). Gastric microbial community profiling reveals a dysbiotic cancer-associated microbiota. Gut 67 (2), 226–236. doi: 10.1136/gutjnl-2017-314205
Ford, A. C., Yuan, Y., Moayyedi, P. (2022). Long-term impact of helicobacter pylori eradication therapy on gastric cancer incidence and mortality in healthy infected individuals: A meta-analysis beyond 10 years of follow-up. Gastroenterology 63 (3), 754–756.e751. doi: 10.1053/j.gastro.2022.05.027
Gantuya, B., El-Serag, H. B., Matsumoto, T., Ajami, N. J., Oyuntsetseg, K., Azzaya, D., et al. (2019). Gastric microbiota in helicobacter pylori-negative and -positive gastritis among high incidence of gastric cancer area. Cancers (Basel) 11 (4). doi: 10.3390/cancers11040504
Gantuya, B., El Serag, H. B., Matsumoto, T., Ajami, N. J., Uchida, T., Oyuntsetseg, K., et al. (2020). Gastric mucosal microbiota in a Mongolian population with gastric cancer and precursor conditions. Aliment Pharmacol. Ther. 51 (8), 770–780. doi: 10.1111/apt.15675
Guo, Y., Cao, X.-S., Guo, G.-Y., Zhou, M.-G., Yu, B. (2022). Effect of helicobacter pylori eradication on human gastric microbiota: A systematic review and meta-analysis. Front. Cell. Infection Microbiol. 12. doi: 10.3389/fcimb.2022.899248
Guo, Y., Li, X., Wang, Z., Yu, B. (2021). Gut microbiota dysbiosis in human hypertension: A systematic review of observational studies. Front. Cardiovasc. Med. 8 (414). doi: 10.3389/fcvm.2021.650227
Guo, Y., Zhang, Y., Gerhard, M., Gao, J. J., Mejias-Luque, R., Zhang, L., et al. (2020). Effect of helicobacter pylori on gastrointestinal microbiota: A population-based study in linqu, a high-risk area of gastric cancer. Gut 69 (9), 1598–1607. doi: 10.1136/gutjnl-2019-319696
Helicobacter and Cancer Collaborative Group (2001). Gastric cancer and helicobacter pylori: A combined analysis of 12 case control studies nested within prospective cohorts. Gut 49 (3), 347–353. doi: 10.1136/gut.49.3.347
He, C., Peng, C., Wang, H., Ouyang, Y., Zhu, Z., Shu, X., et al. (2019). The eradication of helicobacter pylori restores rather than disturbs the gastrointestinal microbiota in asymptomatic young adults. Helicobacter 24 (4), e12590. doi: 10.1111/hel.12590
Hooi, J. K. Y., Lai, W. Y., Ng, W. K., Suen, M. M. Y., Underwood, F. E., Tanyingoh, D., et al. (2017). Global prevalence of helicobacter pylori infection: Systematic review and meta-analysis. Gastroenterology 153 (2), 420–429. doi: 10.1053/j.gastro.2017.04.022
Hsieh, Y. Y., Tung, S. Y., Pan, H. Y., Yen, C. W., Xu, H. W., Lin, Y. J., et al. (2018). Increased abundance of clostridium and fusobacterium in gastric microbiota of patients with gastric cancer in Taiwan. Sci. Rep. 8 (1), 158. doi: 10.1038/s41598-017-18596-0
Ishaq, S., Nunn, L. (2015). Helicobacter pylori and gastric cancer: A state of the art review. Gastroenterol. Hepatol. Bed Bench 8 (Suppl 1), S6–s14.
Kadeerhan, G., Gerhard, M., Gao, J. J., Mejías-Luque, R., Zhang, L., Vieth, M., et al. (2021). Microbiota alteration at different stages in gastric lesion progression: A population-based study in linqu, China. Am. J. Cancer Res. 11 (2), 561–575.
Langille, M. G., Zaneveld, J., Caporaso, J. G., McDonald, D., Knights, D., Reyes, J. A., et al. (2013). Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 31 (9), 814–821. doi: 10.1038/nbt.2676
Lertpiriyapong, K., Whary, M. T., Muthupalani, S., Lofgren, J. L., Gamazon, E. R., Feng, Y., et al. (2014). Gastric colonisation with a restricted commensal microbiota replicates the promotion of neoplastic lesions by diverse intestinal microbiota in the helicobacter pylori INS-GAS mouse model of gastric carcinogenesis. Gut 63 (1), 54–63. doi: 10.1136/gutjnl-2013-305178
Ling, Z., Xiao, H., Chen, W. (2022). Gut microbiome: The cornerstone of life and health. Advanced Gut Microbiome Res. 2022, 9894812. doi: 10.1155/2022/9894812
Linz, B., Purbojati, R. W., Paulo, D. F., Gaultier, N. E., Subramanian, P., Hasan, N. A., et al. (2017). Dysbiosis of the microbiome in gastric carcinogenesis. Sci. Rep. 7 (1), 15957. doi: 10.1038/s41598-017-16353-x
Liu, C., Ng, S. K., Ding, Y., Lin, Y., Liu, W., Wong, S. H., et al. (2022). Meta-analysis of mucosal microbiota reveals universal microbial signatures and dysbiosis in gastric carcinogenesis. Oncogene 41 (28), 3599–3610. doi: 10.1038/s41388-022-02377-9
Liu, X., Shao, L., Liu, X., Ji, F., Mei, Y., Cheng, Y., et al. (2019). Alterations of gastric mucosal microbiota across different stomach microhabitats in a cohort of 276 patients with gastric cancer. EBioMedicine 40, 336–348. doi: 10.1016/j.ebiom.2018.12.034
Liu, W. Z., Xie, Y., Lu, H., Cheng, H., Zeng, Z. R., Zhou, L. Y., et al. (2018). Fifth Chinese national consensus report on the management of helicobacter pylori infection. Helicobacter 23 (2), e12475. doi: 10.1111/hel.12475
Li, W. Q., Zhang, J. Y., Ma, J. L., Li, Z. X., Zhang, L., Zhang, Y., et al. (2019). Effects of helicobacter pylori treatment and vitamin and garlic supplementation on gastric cancer incidence and mortality: follow-up of a randomized intervention trial. Bmj 366, l5016. doi: 10.1136/bmj.l5016
Llorca, L., Perez-Perez, G., Urruzuno, P., Martinez, M. J., Iizumi, T., Gao, Z., et al. (2017). Characterization of the gastric microbiota in a pediatric population according to helicobacter pylori status. Pediatr. Infect. Dis. J. 36 (2), 173–178. doi: 10.1097/inf.0000000000001383
Lofgren, J. L., Whary, M. T., Ge, Z., Muthupalani, S., Taylor, N. S., Mobley, M., et al. (2011). Lack of commensal flora in helicobacter pylori-infected INS-GAS mice reduces gastritis and delays intraepithelial neoplasia. Gastroenterology 140 (1), 210–220. doi: 10.1053/j.gastro.2010.09.048
Malfertheiner, P., Megraud, F., O'Morain, C. A., Gisbert, J. P. (2017). Management of helicobacter pylori infection-the maastricht V/Florence consensus report. Gut 66 (1), 6–30. doi: 10.1136/gutjnl-2016-312288
Mao, L. Q., Zhou, Y. L., Wang, S. S., Chen, L., Hu, Y., Yu, L. M., et al. (2021). Impact of helicobacter pylori eradication on the gastric microbiome. Gut Pathog. 13 (1), 60. doi: 10.1186/s13099-021-00460-2
Morgan, E., Arnold, M., Camargo, M. C., Gini, A., Kunzmann, A. T., Matsuda, T., et al. (2022). The current and future incidence and mortality of gastric cancer in 185 countries 2020-40: A population-based modelling study. EClinicalMedicine 47, 101404. doi: 10.1016/j.eclinm.2022.101404
Nagy, T. A., Frey, M. R., Yan, F., Israel, D. A., Polk, D. B., Peek, R. M., Jr. (2009). Helicobacter pylori regulates cellular migration and apoptosis by activation of phosphatidylinositol 3-kinase signaling. J. Infect. Dis. 199 (5), 641–651. doi: 10.1086/596660
Noto, J. M., Peek, R. M., Jr. (2017). The gastric microbiome, its interaction with helicobacter pylori, and its potential role in the progression to stomach cancer. PloS Pathog. 13 (10), e1006573. doi: 10.1371/journal.ppat.1006573
Polk, D. B., Peek, R. M., Jr. (2010). Helicobacter pylori: Gastric cancer and beyond. Nat. Rev. Cancer 10 (6), 403–414. doi: 10.1038/nrc2857
Rivas-Ortiz, C. I., Lopez-Vidal, Y., Arredondo-Hernandez, L. J. R., Castillo-Rojas, G. (2017). Genetic alterations in gastric cancer associated with helicobacter pylori infection. Front. Med. (Lausanne) 4. doi: 10.3389/fmed.2017.00047
Saadat, I., Higashi, H., Obuse, C., Umeda, M., Murata-Kamiya, N., Saito, Y., et al. (2007). Helicobacter pylori CagA targets PAR1/MARK kinase to disrupt epithelial cell polarity. Nature 447 (7142), 330–333. doi: 10.1038/nature05765
Schulz, C., Koch, N., Schutte, K., Pieper, D. H., Malfertheiner, P. (2015). H. pylori and its modulation of gastrointestinal microbiota. J. Dig Dis. 16 (3), 109–117. doi: 10.1111/1751-2980.12233
Schulz, C., Schutte, K., Koch, N., Vilchez-Vargas, R., Wos-Oxley, M. L., Oxley, A. P. A., et al. (2018). The active bacterial assemblages of the upper GI tract in individuals with and without helicobacter infection. Gut 67 (2), 216–225. doi: 10.1136/gutjnl-2016-312904
Shin, C. M., Kim, N., Park, J. H., Lee, D. H. (2020). Changes in gastric corpus microbiota with age and after helicobacter pylori eradication: A long-term follow-up study. Front. Microbiol. 11. doi: 10.3389/fmicb.2020.621879
Stewart, O. A., Wu, F., Chen, Y. (2020). The role of gastric microbiota in gastric cancer. Gut Microbes 11 (5), 1220–1230. doi: 10.1080/19490976.2020.1762520
Sung, J. J. Y., Coker, O. O., Chu, E., Szeto, C. H., Luk, S. T. Y., Lau, H. C. H., et al. (2020b). Gastric microbes associated with gastric inflammation, atrophy and intestinal metaplasia 1 year after helicobacter pylori eradication. Gut 69 (9), 1572–1580. doi: 10.1136/gutjnl-2019-319826
Talebi Bezmin Abadi, A. (2016). Helicobacter pylori and gastric cancer. Front. Med. (Lausanne) 3. doi: 10.3389/fmed.2016.00036
Tjalsma, H., Boleij, A., Marchesi, J. R., Dutilh, B. E. (2012). A bacterial driver-passenger model for colorectal cancer: Beyond the usual suspects. Nat. Rev. Microbiol. 10 (8), 575–582. doi: 10.1038/nrmicro2819
Valdes, A. M., Walter, J., Segal, E., Spector, T. D. (2018). Role of the gut microbiota in nutrition and health. BMJ 361, k2179. doi: 10.1136/bmj.k2179
Viala, J., Chaput, C., Boneca, I. G., Cardona, A., Girardin, S. E., Moran, A. P., et al. (2004). Nod1 responds to peptidoglycan delivered by the helicobacter pylori cag pathogenicity island. Nat. Immunol. 5 (11), 1166–1174. doi: 10.1038/ni1131
Warren, J. R., Marshall, B. (1983). Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet 1 (8336), 1273–1275.
Watanabe, T., Nadatani, Y., Suda, W., Higashimori, A., Otani, K., Fukunaga, S., et al. (2021). Long-term persistence of gastric dysbiosis after eradication of helicobacter pylori in patients who underwent endoscopic submucosal dissection for early gastric cancer. Gastric Cancer 24 (3), 710–720. doi: 10.1007/s10120-020-01141-w
WHO-IARC (1994). “Schistosomes, liver flukes and helicobacter pylori,” in IARC working group on the evaluation of carcinogenic risks to humans, vol. 61. (Lyon: World Health Organization International Agency for Research on Cancer), 1–241.
Willhite, D. C., Cover, T. L., Blanke, S. R. (2003). Cellular vacuolation and mitochondrial cytochrome c release are independent outcomes of helicobacter pylori vacuolating cytotoxin activity that are each dependent on membrane channel formation. J. Biol. Chem. 278 (48), 48204–48209. doi: 10.1074/jbc.M304131200
Yang, Y., Ji, R., Zhao, X., Cao, X., Wang, Q., Jiang, Q., et al. (2021). Alterations in gastric mucosal microbiota in gastric carcinogenesis: A systematic review and meta-analysis. Front. Med. (Lausanne) 8. doi: 10.3389/fmed.2021.754959
Yang, Y., Shu, X., Xie, C. (2022). An overview of autophagy in helicobacter pylori infection and related gastric cancer. Front. Cell Infect. Microbiol. 12. doi: 10.3389/fcimb.2022.847716
You, W. C., Brown, L. M., Zhang, L., Li, J. Y., Jin, M. L., Chang, Y. S., et al. (2006). Randomized double-blind factorial trial of three treatments to reduce the prevalence of precancerous gastric lesions. J. Natl. Cancer Inst 98 (14), 974–983. doi: 10.1093/jnci/djj264
Yuan, Z., Xiao, S., Li, S., Suo, B., Wang, Y., Meng, L., et al. (2021). The impact of helicobacter pylori infection, eradication therapy, and probiotics intervention on gastric microbiota in young adults. Helicobacter 26 (6), e12848. doi: 10.1111/hel.12848
Keywords: gastric cancer, gastric microbiota, Helicobacter pylori, carcinogenesis, dysbiosis
Citation: Guo Y, Cao X-S, Zhou M-G and Yu B (2023) Gastric microbiota in gastric cancer: Different roles of Helicobacter pylori and other microbes. Front. Cell. Infect. Microbiol. 12:1105811. doi: 10.3389/fcimb.2022.1105811
Received: 23 November 2022; Accepted: 21 December 2022;
Published: 10 January 2023.
Edited by:
Xin Zhou, Stanford University, United SatesReviewed by:
Kai Fu, Johnson & Johnson, United StatesAmin Talebi Bezmin Abadi, Tarbiat Modares University, Iran
Copyright © 2023 Guo, Cao, Zhou and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Guo, eWFuZ2d1b2FubnlAMTYzLmNvbQ==; Bo Yu, ZHJib3l1X2Rlcm1AMTI2LmNvbQ==
 Yang Guo
Yang Guo Xue-Shan Cao2
Xue-Shan Cao2 Meng-Ge Zhou
Meng-Ge Zhou Bo Yu
Bo Yu