- 1Institute of Antibiotics, Huashan Hospital, Fudan University, Shanghai, China
- 2Key Laboratory of Clinical Pharmacology of Antibiotics, Ministry of Health, Shanghai, China
- 3Department of Laboratory Medicine, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, China
Providencia rettgeri is a nosocomial pathogen associated with urinary tract infections related to hospital-acquired Infections. In recent years, P. rettgeri clinical strains producing New Delhi Metallo-β-lactamase (NDM) and other β-lactamase which reduce the efficiency of antimicrobial therapy have been reported. However, there are few reports of P. rettgeri co-producing two metallo-β-lactamases in one isolate. Here, we first reported a P. rettgeri strain (P138) co-harboring blaNDM-1, blaVIM-1, and blaOXA-10. The specie were identified using MALDI-TOF MS. The results of antimicrobial susceptibility testing by broth microdilution method indicated that P. rettgeri P138 was resistant to meropenem (MIC = 64μg/ml), imipenem (MIC = 64μg/ml), and aztreonam (MIC = 32μg/ml). Conjugation experiments revealed that the blaNDM-1-carrying plasmid was transferrable. The carbapenemase genes were detected using PCR and confirmed by PCR-based sequencing. The complete genomic sequence of the P. rettgeri was identified using Illumina (Illumina, San Diego, CA, USA) short-read sequencing (150bp paired-end reads), and many common resistance genes had been identified, including blaNDM-1, blaVIM-1, blaOXA-10, aac(6’)-Il, aadA5, ant(2’’)-Ia, aadA1, aac(6’)-Ib3, aadA1, aph(3’)-Ia, aac(6’)-Ib-cr, qnrD1, qnrA1, and catA2. The blaNDM-1 gene was characterized by the following structure: IS110–TnpA–IntI1–aadB–IS91–GroEL–GroES–DsbD–PAI–ble–blaNDM-1–IS91–QnrS1–IS110. Blast comparison revealed that the blaNDM-1 gene structure shared >99% similarity with plasmid p5_SCLZS62 (99% nucleotide identity and query coverage). In summary, we isolated a P. rettgeri strain coproducing blaNDM-1, blaVIM-1, and blaOXA-10. To the best of our acknowledge, this was first reported in the world. The occurrence of the strain needs to be closely monitored.
Introduction
Providencia rettgeri is an opportunistic human pathogen, unlike other Enterobacterales, it is a little-known pathogen, which is mainly associated with hospital-acquired infections including catheter-related urinary tract infections, bacteremia, meningitis, diarrhea, and eye infections (Yoh et al., 2005; Tada et al., 2014). Treatment of these infections is challenging, as they are intrinsically resistant to multiple antibiotics including first-generation cephalosporins, amoxicillin-clavulanic acid, nitrofurantoin, tigecycline, and polymyxins. Imipenem, amikacin, and cefepime are effective against more than 90% of the isolates (Lee et al., 2007; Sharma et al., 2017). However, in recent years P. rettgeri has become increasingly carbapenemase producers carrying the carbapenem-resistant genes like blaNDM, blaVIM, and so on (Piza-Buitrago et al., 2020). The emergence of multidrug-resistant of P. rettgeri strains poses a serious threat to public health.
The widespread of metallo-β-lactamases (MBLs) remain a severe challenge in health care settings because the hydrolysis of β-lactams by MBL enzymes cannot be prevented by clinically available β-lactamase inhibitors, including avibactam, relebactam, and vaborbactam (Wu et al., 2019). New Delhi Metallo-β-lactamases were the most predominant MBL among Enterobacterales clinical isolates which were initially identified in Klebsiella pneumoniae in 2009 in a Swedish patient (Pillai et al., 2011). Currently, although blaNDM-1 was commonly related to K. pneumoniae (Han et al., 2020), E. coli, Enterobacter cloacae, and Citrobacter freundii strains in China (Yong et al., 2009; Zhang et al., 2021), reports on blaNDM-1 producing P. rettgeri are rare. The spread of plasmid-bearing MBL possess a great challenge for clinical treatment because these multidrug-resistant isolates will result in limitations on treatment options (Oteo et al., 2014; Piza-Buitrago et al., 2020). Here, we report the co-existence of the carbapenemase genes blaNDM-1, blaVIM-1, and blaOXA-10 in a P. rettgeri clinical isolate in China.
Materials and Methods
Species Identification, Antimicrobial Susceptibility Testing, and Confirmation of Carbapenemase Production
Species identification was performed using MALDI-TOF MS (bioMérieux, France). The minimal inhibitory concentration (MIC) was determined by the broth microdilution method according to the guidelines of the Clinical Laboratory Standards Institute (CLSI) (Clinical and Laboratory Standards Institute, 2021). The strains E. coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853 were used as quality controls for antimicrobial susceptibility testing. Quality control and interpretation of the results were based on 2021 CLSI breakpoints (CLSI, 2021) for all the antimicrobial agents with the exception of cefepime-tazobactam, tigecycline, and polymyxin B. Cefepime- tazobactam MICs were interpreted using CLSI breakpoints for cefepime for comparison purposes only. Tigecycline and polymyxin B MICs were interpreted using the European Committee for Antimicrobial Susceptibility Testing (EUCAST) criteria (EUCAST, 2021). Carbapenemase production was phenotypically detected using imipenem- 3-aminobenzeneboronic acid/EDTA double disk synergy test. The existence of the carbapenemase genes (KPC, NDM, OXA, IMP, and VIM) was confirmed by NG-Test Carba-5 and PCR-based sequencing, as previously described (Poirel et al., 2011b; Weiß et al., 2017).
Conjugation Assay and Plasmid Sequencing
Conjugation experiments were performed to explore the transferability of the plasmid using azide-resistant E. coli J53 as a recipient strain. The conjugants were selected on Mueller-Hinton (MH) agar supplemented with azide (100 mg/L) and ampicillin (50 mg/L). The conjugation frequency was calculated according to the number of conjugants per initial donor bacteria. The presence of the blaNDM-1, blaVIM-1, blaOXA-10 in conjugants was confirmed by PCR and PCR-based sequencing. The Qiagen Midi kit (Qiagen, Hilden, Germany) was used to extract the plasmid of the conjugant and the plasmid was sequenced using Illumina (Illumina, San Diego, CA, USA) short-read sequencing (150bp paired-end reads). SPAdes 3.12.0 was used to de novo assemble the sequencing reads, and the open reading frame prediction and annotation were done with RAST version 2.0 (https://rast.nmpdr.org) and BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi). The plasmid replicon was determined using the PCR-based replicon typing method (Carattoli et al., 2005). Plasmid comparisons were performed using BRIG (http://brig.sourceforge.net) (Alikhan et al., 2011) and Easyfig tools (http://mjsull.github.io/Easyfig) (Sullivan et al., 2011). Plasmids carrying blaNDM-1 were circularized using PCR and Sanger sequencing to fill in gaps between contigs. The conjugation elements were detected using oriTfinder, a web-based tool for the identification of origin of transfers in DNA sequences of bacterial mobile genetic elements (https://tool-mml.sjtu.edu.cn/oriTfinder/oriTfinder.html) (Li et al., 2018).
Whole Genome Sequencing and Bioinformatics Analysis
The isolates’ genomic DNA was obtained by using one commercial kit, according to the manufacturer’s recommendation: Qiagen for P138. And the genomic DNA was sequenced using Illumina (Illumina, San Diego, CA, USA) short-read sequencing (150bp paired-end reads). Reads were trimmed with sickle (GitHub), subsequently, they were de novo assembled using SPAdes 3.12.0. Antimicrobial resistance genes analysis was performed using BacWGSTdb (http://bacdb.cn/BacWGSTdb/analysis_single.php) and the annotation process was done using RAST version 2.0 (https://rast.nmpdr.org).
Results
Overview of the P. rettgeri Clinical Isolate
The P. rettgeri strain P138 was isolated from a 51-year-old female patient that was admitted to a public hospital for the treatment of cervical cancer in 2019 in Sichuan Province in the southwest of China. A hysterectomy was performed for this patient. At the same time, due to the dense adhesion between the patient’s bilateral ureters and the paravaginal tissue, stents were put in the bilateral ureters. On the day before the operation, cefathiamidine (2g Q8h) was used for seven days for prophylaxis. On the ninth day after the operation, the patient developed a fever, an E. coli and the P. rettgeri strain P138 were isolated from urine culture, therapeutic regimen switched to levofloxacin (0.5g QD) and ceftizoxime (2g Q12h) for 2 days. Two days later, the patient’s body temperature returned to normal and the infection was controlled. Finally, the patient recovered and was discharged successfully.
The antimicrobial susceptibility profiles of P. rettgeri P138 are presented in Table 1. The isolate was resistant to all tested antimicrobial agents including amikacin (MIC >128μg/ml), cefoperazone-sulbactam (MIC >128μg/ml), aztreonam (MIC = 32μg/ml), piperacillin-tazobactam (MIC ≥256μg/ml), meropenem (MIC =64μg/ml), imipenem (MIC =64μg/ml), ceftazidime-avibactam (MIC ≥64μg/ml), tigecycline (MIC = 2μg/ml), and polymyxin B (MIC > 16μg/ml).
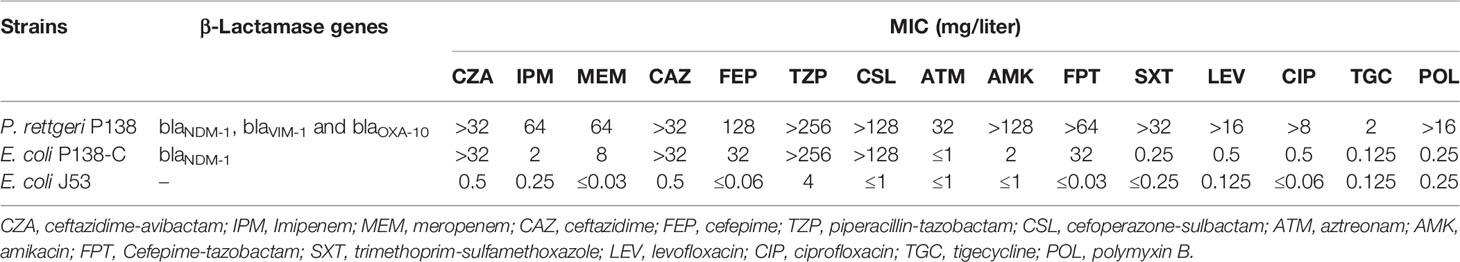
Table 1 Susceptibility of P. rettgeri clinical isolate, conjugant, and recipient to antimicrobial agents.
Carbapenemase Genes and Conjugation Experiments
PCR-based sequencing demonstrated the presence of blaNDM-1, blaVIM-1, and blaOXA-10 in P. rettgeri strain P138. According to the results of Conjugation Experiments, conjugants were positive for blaNDM-1 but negative for blaVIM-1 and blaOXA-10, making the conjugants resistant to meropenem (MIC = 8μg/ml) and ceftazidime-avibactam (MIC = >32μg/ml), intermediate to imipenem (MIC = 2μg/ml). The meropenem, imipenem, and ceftazidime-avibactam MICs of conjugants increased at least 256, 8, 128-fold respectively, compared with the recipient E. coli J53 (Table 1). The conjugation frequency is 2.47 × 10–5 (The conjugation frequency was calculated according to the number of conjugants per initial donor bacteria). Lots of modules associated with conjugation were detected in pP138-NDM, like the oriT gene (origin of transfer gene), relaxase, type IV coupling protein (TraD), and type IV secretion system (T4SS).
WGS Analysis and Characterization of Plasmid Sequence Carrying blaNDM-1 Gene
According to the whole-genome sequencing analysis, many common resistance genes had been identified, including the carbapenemase genes blaNDM-1, blaVIM-1 and blaOXA-10, the aminoglycoside resistance genes aac(6’)-Il, aadA5, ant(2’’)-Ia, aadA1, aac(6’)-Ib3, aadA1, aph(3’)-Ia and aac(6’)-Ib-cr, the fluoroquinolone resistance genes qnrD1 and qnrA1 and the phenicol resistance gene catA2. The sequencing of the conjugant’s plasmid localized blaNDM-1 on a plasmid of 120,528 bp, belonging to the IncC type. Four resistance genes were identified in the plasmid pP138-NDM, blaNDM-1, qnrA1, sul1, and ant(2’’)-Ia, conferring resistance to carbapenems, quinolones, sulphonamides, and aminoglycosides, respectively. BLAST comparison disclosed that the blaNDM−1 gene environment of the plasmid pP138-NDM shared >99% similarity with plasmid p5_SCLZS62 (99% nucleotide identity and query coverage), isolated from a Raoultella planticola strain from Sichuan, China (GenBank accession number CP082173). In both plasmids, blaNDM-1 and qnrA1 were located in an identical multidrug resistance region (MRR). The MRR was flanked by genes of IS110 family transposase on both sides, and also contained IS91. TnAs3, which belongs to the Tn3 family was also found in pP138-NDM. The full genetic environment surrounding blaNDM-1 is: IS110–TnpA–IntI1–aadB–IS91–GroEL–GroES–DsbD–PAI–ble–blaNDM-1–IS91–QnrS1–IS110.
In several plasmids with similar sequences (Figure 1), pSAL-19-0623_NDM (99% nucleotide identity and query coverage), an IncA/C2-type blaNDM-1 carrying plasmid with 276,695 bp in a carbapenem-resistant Salmonella strain from Singapore (GenBank accession numbers NZ_CP020913) (Octavia et al., 2020). They all showed resistance to meropenem and ceftazidime/avibactam, the only difference is that the strain P138 in our study was resistant to aztreonam, and this is most likely mediated by blaOXA-10.
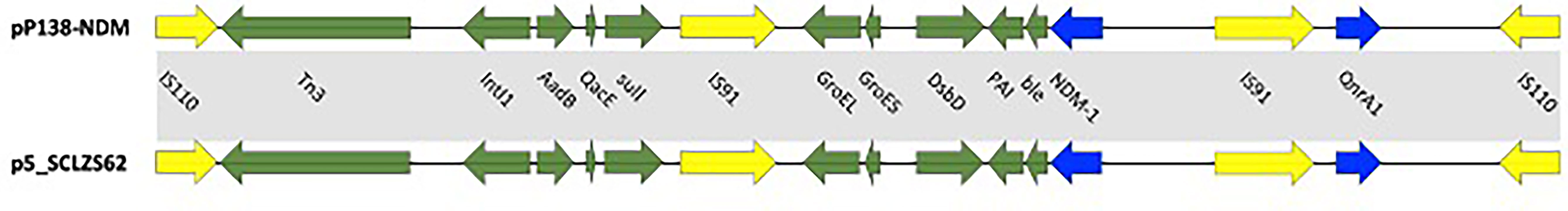
Figure 1 Circular comparison between plasmid pP138-NDM (MZ670000) and other similar plasmids. Plasmid pP138-NDM (the outer circle) was used by the BRIG software as a reference plasmid to perform the sequence alignment with BLASTN. The different colors indicate different plasmids and are listed in the color key.
Discussion
The first isolate of NDM-1 producing P. rettgeri was reported in Israel in 2013 (Gefen-Halevi et al., 2013). Since then, NDM-1-producing P. rettgeri has been reported in various parts of the world (Pillai et al., 2011; Barrios et al., 2013; Carvalho-Assef et al., 2013; Mataseje et al., 2014; Pasteran et al., 2014; An et al., 2016). Reports in Nepal (Tada et al., 2014) as well as reports in Colombia (Marquez-Ortiz et al., 2017; Piza-Buitrago et al., 2020), and Korea (Shin et al., 2018), commonly associate P. rettgeri with high resistance rates to carbapenems. This resistance characteristic in P. rettgeri is commonly associated with the production of blaNDM-1. Recently, Piza-Buitrago et al. reported two NDM-1, VIM-2, and OXA-10 coproducing P. rettgeri strains GMR-RA257 and GMR-RA1153, similar to the drug resistance spectrum in our study, with resistance to the carbapenems imipenem and meropenem, and this was highly probable caused by the production of NDM-1 and VIM-1 (Piza-Buitrago et al., 2020). However, as to OXA-10, it seemed to have a limited effect on the hydrolysis of carbapenems, according to a study in Nigeria in 2011, a P. rettgeri isolate co-producing blaOXA-10, blaVEB-1, and blaCMY-4 genes with no presence of the MBL genes was susceptible to carbapenems (Aibinu et al., 2011).
The moving elements can aggregate and combine with resistance genes, resulting in multiple resistance transfer of plasmids (Partridge, 2011). Different from previous studies often associated blaNDM-1 with Tn125, especially ISAba125 (Nordmann et al., 2011; Poirel et al., 2011a; Nordmann et al., 2012), in our study, was TnAs3, which is relatively rare reported. As to insertion sequences, IS26 is widely distributed and it is often combined with Tn125 family transposons (Poirel et al., 2011a; Zheng et al., 2021), in P. rettgeri isolate P138, blaNDM-1 was associated at its 3’-end and 5’ -end with IS110 that is also relative rare reported. This further reflects the diversity of genetic elements, which leads to the wide spread of resistance genes among bacteria. Gene encoding small multidrug resistance (SMR) efflux transporter was also found in the MRR, such transmembrane proteins were frequently found in Gram-negative and Gram-positive bacteria where they were deduced to be associated with the efflux system (Kazama et al., 1998) (Figure 2).
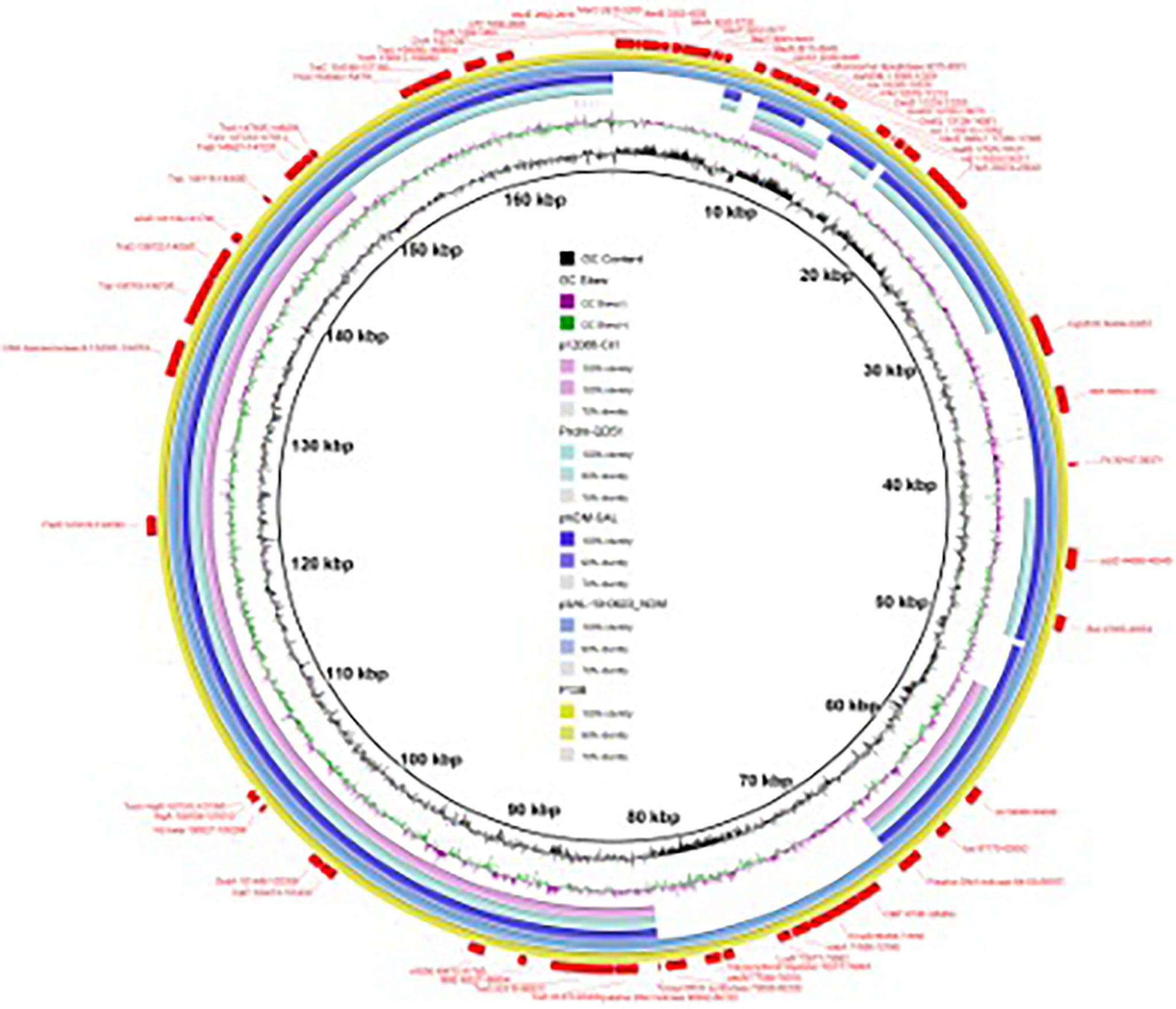
Figure 2 Multidrug resistance region (MRR) of blaNDM-1 in the plasmid pP138-NDM (MZ670000) and p5_SCLZS62 (CP082173). Resistance genes are indicated by blue symbols. Transposon-related genes and insertion sequences are indicated by yellow symbols. Other genes are indicated by violet symbols. Light gray shading indicated homologous regions (>99% DNA identity).
In our study, we reported a carbapenem resistant P. rettgeri isolate P138, co-harboring blaNDM-1, blaVIM-1, and blaOXA-10, combined with the previous results (Pillai et al., 2011; Barrios et al., 2013; Carvalho-Assef et al., 2013; Mataseje et al., 2014; Pasteran et al., 2014; Tada et al., 2014; An et al., 2016; Marquez-Ortiz et al., 2017; Piza-Buitrago et al., 2020), the presence of MBL genes as blaNDM-1, blaVIM-1, and blaVIM-2 contribute significantly to carbapenem resistance in P. rettgeri, while blaOXA-10 plays a relatively weak role. With the increasing number of such multi-drug resistant bacteria, especially these showed resistance to carbapenems like imipenem, meropenem, and new combination of antimicrobials like ceftazidime-avibactam, the clinical treatment options are limited, so the initial effective anti-infection treatment is important to reduce the mortality of infection caused by CRE. In the future, the laboratory should strengthen the monitoring of carbapenemase, and perform combined antimicrobial susceptibility tests to seek an effective therapeutic regime for the infection caused by CRE strain.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, MZ670000.
Ethics Statement
The study protocol was approved by the Institutional Review Board of Huashan Hospital, Fudan University (Number: 2018-408).
Author Contributions
FH and HY designed the study. SS and XH collected clinical samples and performed the experiments. SS, LD, YY, RH, QS, DY, YG, and XZ analyzed data. SS wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (81871690, and 81861138051), and Shanghai Public Health System Construction Three-Year Action Plan (2020-2022), Discipline leader Grant (GWV-10.2-XD02). The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aibinu, I. E., Pfeifer, Y., Ogunsola, F., Odugbemi, T., Koenig, W., Ghebremedhin, B. (2011). Emergence of β-Lactamases OXA-10, VEB-1 and CMY in Providencia Spp. From Nigeria. J. Antimicrob. Chemother. 66 (8), 1931–1932. doi: 10.1093/jac/dkr197
Alikhan, N. F., Petty, N. K., Ben Zakour, N. L., Beatson, S. A. (2011). BLAST Ring Image Generator (BRIG): Simple Prokaryote Genome Comparisons. BMC Genomics 12, 402. doi: 10.1186/1471-2164-12-402
An, J., Guo, L., Zhou, L., Ma, Y., Luo, Y., Tao, C., et al. (2016). NDM-Producing Enterobacteriaceae in a Chinese Hospita-2015: Identification of NDM-Producing Citrobacterwerkmanii and Acquisition of blaNDM-1-Carrying Plasmid In Vivo in a Clinical Escherichia Coli Isolate. J. Med. Microbiol. 65 (11), 1253–1259. doi: 10.1099/jmm.0.000357
Barrios, H., Garza-Ramos, U., Reyna-Flores, F., Sanchez-Perez, A., Rojas-Moreno, T., Garza-Gonzalez, E., et al. (2013). Isolation of Carbapenem-Resistant NDM-1-Positive Providencia Rettgeri in Mexico. J. Antimicrob. Chemother. 68 (8), 1934–1936. doi: 10.1093/jac/dkt124
Carattoli, A., Bertini, A., Villa, L., Falbo, V., Hopkins, K. L., Threlfall, E. J. (2005). Identification of Plasmids by PCR-Based Replicon Typing. J. Microbiol. Methods 63 (3), 219–228. doi: 10.1016/j.mimet.2005.03.018
Carvalho-Assef, A. P., Pereira, P. S., Albano, R. M., Berião, G. C., Chagas, T. P., Timm, L. N., et al. (2013). Isolation of NDM-Producing Providencia Rettgeri in Brazil. J. Antimicrob. Chemother. 68 (12), 2956–2957. doi: 10.1093/jac/dkt298
Clinical and Laboratory Standards Institute. (2021). Performance Standards for Antimicrobial Susceptibility Testing, CLSI Supplement M100. 31th (PA, Wayne: Clinical and Laboratory Standards Institute).
European Committee on Antimicrobial Susceptibility Testing. (2021). Breakpoint Tables for Interpretation of MICs and Zone Diameters Version 11.0, Valid From 2021-01-01. Available at: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_11.0_Breakpoint_Tables.pdf.
FDA. (2019). Tigecycline-Injection Products. Available at: https://www.fda.gov/drugs/development-resources/tigecycline-injection-products.
Gefen-Halevi, S., Hindiyeh, M. Y., Ben-David, D., Smollan, G., Gal-Mor, O., Azar, R., et al. (2013). Isolation of Genetically Unrelated blaNDM-1-Positive Providencia Rettgeri Strains in Israel. J. Clin. Microbiol. 51 (5), 1642–1643. doi: 10.1128/jcm.00381-13
Han, R., Shi, Q., Wu, S., Yin, D., Peng, M., Dong, D., et al. (2020). Dissemination of Carbapenemases (KPC, NDM, OXA-48, IMP, and VIM) Among Carbapenem-Resistant Enterobacteriaceae Isolated From Adult and Children Patients in China. Front. Cell Infect. Microbiol. 10, 314. doi: 10.3389/fcimb.2020.00314
Kazama, H., Hamashima, H., Sasatsu, M., Arai, T. (1998). Distribution of the Antiseptic-Resistance Genes qacE and qacE Delta 1 in Gram-Negative Bacteria. FEMS Microbiol. Lett. 159 (2), 173–178. doi: 10.1111/j.1574-6968.1998.tb12857.x
Lee, H. W., Kang, H. Y., Shin, K. S., Kim, J. (2007). Multidrug-Resistant Providencia Isolates Carrying blaPER-1, blaVIM-2, and armA. J. Microbiol. 45 (3), 272–274.
Li, X., Xie, Y., Liu, M., Tai, C., Sun, J., Deng, Z., et al. (2018). Oritfinder: A Web-Based Tool for the Identification of Origin of Transfers in DNA Sequences of Bacterial Mobile Genetic Elements. Nucleic Acids Res. 46 (W1), W229–w234. doi: 10.1093/nar/gky352
Marquez-Ortiz, R. A., Haggerty, L., Sim, E. M., Duarte, C., Castro-Cardozo, B. E., Beltran, M., et al. (2017). First Complete Providencia Rettgeri Genome Sequence, the NDM-1-Producing Clinical Strain Rb151. Genome Announc. 5 (3), e01472–e01416. doi: 10.1128/genomeA.01472-16
Mataseje, L. F., Boyd, D. A., Lefebvre, B., Bryce, E., Embree, J., Gravel, D., et al. (2014). Complete Sequences of a Novel blaNDM-1-Harbouring Plasmid From Providencia Rettgeri and an FII-Type Plasmid From Klebsiella Pneumoniae Identified in Canada. J. Antimicrob. Chemother. 69 (3), 637–642. doi: 10.1093/jac/dkt445
Nordmann, P., Dortet, L., Poirel, L. (2012). Carbapenem Resistance in Enterobacteriaceae: Here Is the Storm! Trends Mol. Med. 18 (5), 263–272. doi: 10.1016/j.molmed.2012.03.003
Nordmann, P., Poirel, L., Walsh, T. R., Livermore, D. M. (2011). The Emerging NDM Carbapenemases. Trends Microbiol. 19 (12), 588–595. doi: 10.1016/j.tim.2011.09.005
Octavia, S., Chew, K. L., Chew, K. L., Lin, R. T. P., Teo, J. W. P. (2020). Multidrug-Resistant Salmonella Enterica Serovar London Carrying blaNDM-1 Encoding Plasmid From Singapore. Clin. Microbiol. Infect. 26 (7), 963–966. doi: 10.1016/j.cmi.2020.01.033
Oteo, J., Miró, E., Pérez-Vázquez, M., Navarro, F. (2014). Evolution of Carbapenemase-Producing Enterobacteriaceae at the Global and National Level: What Should be Expected in the Future? Enferm Infecc Microbiol. Clin. 32 Suppl 4, 17–23. doi: 10.1016/s0213-005x(14)70170-3
Partridge, S. R. (2011). Analysis of Antibiotic Resistance Regions in Gram-Negative Bacteria. FEMS Microbiol. Rev. 35 (5), 820–855. doi: 10.1111/j.1574-6976.2011.00277.x
Pasteran, F., Meo, A., Gomez, S., Derdoy, L., Albronoz, E., Faccone, D., et al. (2014). Emergence of Genetically Related NDM-1-Producing Providencia Rettgeri Strains in Argentina. J. Glob Antimicrob. Resist. 2 (4), 344–345. doi: 10.1016/j.jgar.2014.07.003
Pillai, D. R., McGeer, A., Low, D. E. (2011). New Delhi Metallo-β-Lactamase-1 in Enterobacteriaceae: Emerging Resistance. Cmaj 183 (1), 59–64. doi: 10.1503/cmaj.101487
Piza-Buitrago, A., Rincón, V., Donato, J., Saavedra, S. Y., Duarte, C., Morero, J., et al. (2020). Genome-Based Characterization of Two Colombian Clinical Providencia Rettgeri Isolates Co-Harboring NDM-1, VIM-2, and Other β-Lactamases. BMC Microbiol. 20 (1), 345. doi: 10.1186/s12866-020-02030-z
Poirel, L., Dortet, L., Bernabeu, S., Nordmann, P. (2011a). Genetic Features of blaNDM-1-Positive Enterobacteriaceae. Antimicrob. Agents Chemother. 55 (11), 5403–5407. doi: 10.1128/aac.00585-11
Poirel, L., Walsh, T. R., Cuvillier, V., Nordmann, P. (2011b). Multiplex PCR for Detection of Acquired Carbapenemase Genes. Diagn. Microbiol. Infect. Dis. 70 (1), 119–123. doi: 10.1016/j.diagmicrobio.2010.12.002
Sharma, D., Sharma, P., Soni, P. (2017). First Case Report of Providencia Rettgeri Neonatal Sepsis. BMC Res. Notes 10 (1), 536. doi: 10.1186/s13104-017-2866-4
Shin, S., Jeong, S. H., Lee, H., Hong, J. S., Park, M. J., Song, W. (2018). Emergence of Multidrug-Resistant Providencia Rettgeri Isolates Co-Producing NDM-1 Carbapenemase and PER-1 Extended-Spectrum β-Lactamase Causing a First Outbreak in Korea. Ann. Clin. Microbiol. Antimicrob. 17 (1), 20. doi: 10.1186/s12941-018-0272-y
Sullivan, M. J., Petty, N. K., Ben Zakour, S. A. (2011). Easyfig: A Genome Comparison Visualizer. Bioinformatics 27, 1009–1010. doi: 10.1093/bioinformatics/btr039
Tada, T., Miyoshi-Akiyama, T., Dahal, R. K., Sah, M. K., Ohara, H., Shimada, K., et al. (2014). NDM-1 Metallo-β-Lactamase and ArmA 16s rRNA Providencia Rettgeri Clinical Isolates in Nepal. BMC Infect. Dis. 14, 56. doi: 10.1186/1471-2334-14-56
Weiß, D., Engelmann, I., Braun, S. D., Monecke, S., Ehricht, R. (2017). A Multiplex Real-Time PCR for the Direct, Fast, Economic and Simultaneous Detection of the Carbapenemase Genes blaKPC, blaNDM, blaVIM and blaOXA-48. J. Microbiol. Methods 142, 20–26. doi: 10.1016/j.mimet.2017.08.017
Wu, W., Feng, Y., Tang, G., Qiao, F., McNally, A., Zong, Z. (2019). NDM Metallo-β-Lactamases and Their Bacterial Producers in Health Care Settings. Clin. Microbiol. Rev. 32 (2), e00115–e00118. doi: 10.1128/cmr.00115-18
Yoh, M., Matsuyama, J., Ohnishi, M., Takagi, K., Miyagi, H., Mori, K., et al. (2005). Importance of Providencia Species as a Major Cause of Travellers’ Diarrhoea. J. Med. Microbiol. 54 (Pt 11), 1077–1082. doi: 10.1099/jmm.0.45846-0
Yong, D., Toleman, M. A., Giske, C. G., Cho, H. S., Sundman, K., Lee, K., et al. (2009). Characterization of a New Metallo-Beta-Lactamase Gene, blaNDM-1, and a Novel Erythromycin Esterase Gene Carried on a Unique Genetic Structure in Klebsiella Pneumoniae Sequence Type 14 From India. Antimicrob. Agents Chemother. 53 (12), 5046–5054. doi: 10.1128/aac.00774-09
Zhang, T., Lin, Y., Li, P., Li, Z., Liu, X., Li, J., et al. (2021). Characterization of Plasmid Co-Harboring NDM-1 and SHV-12 From a Multidrug-Resistant Citrobacter Freundii Strain ZT01-0079 in China. Infect. Drug Resist. 14, 947–952. doi: 10.2147/idr.S301736
Zheng, X. R., Sun, Y. H., Zhu, J. H., Wu, S. L., Ping, C., Fang, L. X., et al. (2021). Two Novel blaNDM-1-Harbouring Transposons on Ppry2001-Like Plasmids Coexisting With a Novel Cfr-Encoding Plasmid in Food Animal Source Enterobacteriaceae. J. Glob Antimicrob. Resist. 26, 222–226. doi: 10.1016/j.jgar.2021.06.006
Keywords: Providencia rettgeri, blaNDM-1, blaVIM-1, blaOXA-10, Mobile gene elements
Citation: Shen S, Huang X, Shi Q, Guo Y, Yang Y, Yin D, Zhou X, Ding L, Han R, Yu H and Hu F (2022) Occurrence of NDM-1, VIM-1, and OXA-10 Co-Producing Providencia rettgeri Clinical Isolate in China. Front. Cell. Infect. Microbiol. 11:789646. doi: 10.3389/fcimb.2021.789646
Received: 05 October 2021; Accepted: 29 November 2021;
Published: 03 January 2022.
Edited by:
Guo-bao Tian, Sun Yat-sen University, ChinaReviewed by:
Abid Ali, University of Pittsburgh Medical Center, United StatesJian Sun, South China Agricultural University, China
Fangyou Yu, Tongji University, China
Copyright © 2022 Shen, Huang, Shi, Guo, Yang, Yin, Zhou, Ding, Han, Yu and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hua Yu, eXVodWEyMDAyQDE2My5jb20=; Fupin Hu, aHVmdXBpbkBmdWRhbi5lZHUuY24=
†These authors have contributed equally to this work, Author order was determined both alphabetically and in order of increasing seniority
 Siquan Shen
Siquan Shen Xiangning Huang
Xiangning Huang Qingyu Shi
Qingyu Shi Yan Guo1,2
Yan Guo1,2 Fupin Hu
Fupin Hu