- 1 Department of Microbiology, Biocenter, University of Würzburg, Würzburg, Germany
- 2 Department of Medical Microbiology, University Medical Center Groningen, Groningen, Netherlands
Staphylococcus aureus uses a plethora of virulence factors to accommodate a diversity of niches in its human host. Aside from the classical manifestations of S. aureus-induced diseases, the pathogen also invades and survives within mammalian host cells.The survival strategies of the pathogen are as diverse as strains or host cell types used. S. aureus is able to replicate in the phagosome or freely in the cytoplasm of its host cells. It escapes the phagosome of professional and non-professional phagocytes, subverts autophagy, induces cell death mechanisms such as apoptosis and pyronecrosis, and even can induce anti-apoptotic programs in phagocytes. The focus of this review is to present a guide to recent research outlining the variety of intracellular fates of S. aureus.
Introduction
Most manifestations of Staphylococcus aureus disease involve extracellular bacteria (furuncles, carbuncles, impetigo, abscesses, septicemia, necrotizing pneumonia) or biofilm formation (catheter-induced infective endocarditis, atherosclerosis). Aside from this S. aureus infections have a second face: there is accumulating evidence that S. aureus is able to survive within its hosts cells and thus might be termed a facultative intracellular pathogen. Intracellularity of S. aureus has been implied as immune-evasive strategy thereby escaping detection by professional phagocytes.
Internalization of S. aureus by Host Cells
Invasion of non-professional phagocytes by S. aureus is mediated by a zipper-type mechanism. To date many bacterial adhesins have been identified with Fibronectin (Fn)-binding proteins A and B (FnBPA, FnBPB) constituting the major staphylococcal adhesins for non-professional phagocytes such as epithelial, endothelial cells, fibroblasts, osteoblasts, and keratinocytes (Dziewanowska et al., 1999; Jevon et al., 1999; Lammers et al., 1999; Peacock et al., 1999; Sinha et al., 1999; Fowler et al., 2000; Ahmed et al., 2001; Kintarak et al., 2004; Sinha and Fraunholz, 2010; Edwards et al., 2011; Figure 1, Map Item 1). Fibronectin-bridging between FnBPs and α5β1 integrins on the host cell surface is sufficient to induce zipper-type uptake of staphylococci (Sinha et al., 2000). However, FnBPs also have been shown to directly bind to human heat shock protein 60 (Hsp60) exposed on the cellular surface (Dziewanowska et al., 2000). FnBP-independent invasion was observed in S. aureus Newman, which produces C-terminally truncated FnBPs that are not covalently anchored to the cell wall of S. aureus. It has been shown that strain Newman gets internalized by epithelial cells and fibroblasts mediated by extracellular adherence protein (Eap; Harraghy et al., 2003) with its cellular receptor still not identified to date. Recently, the staphylococcal autolysin (Atl) was identified to function as adhesin/invasin with heat shock cognate protein Hsc70 being the direct cellular receptor (Hirschhausen et al., 2010). Further, wall teichoic acids (WTA) seem to be important for establishment of nasal colonization and there is evidence that a scavenger receptor is involved in WTA binding (Weidenmaier et al., 2004, 2005, 2008). Clumping factor B (ClfB) has been shown to bind to cytokeratins in the extracellular matrix (ECM) of host cells (O’Brien et al., 2002; Wertheim et al., 2008; Haim et al., 2010), and staphylococcal protein A can directly interact with tumor necrosis factor α receptor 1 (TNFR1; Claro et al., 2011). To what extent the internalization of the pathogen is mediated by WTA, ClfB, protein A, and a body of other molecules interacting with the ECM of host cells is not known thus far.
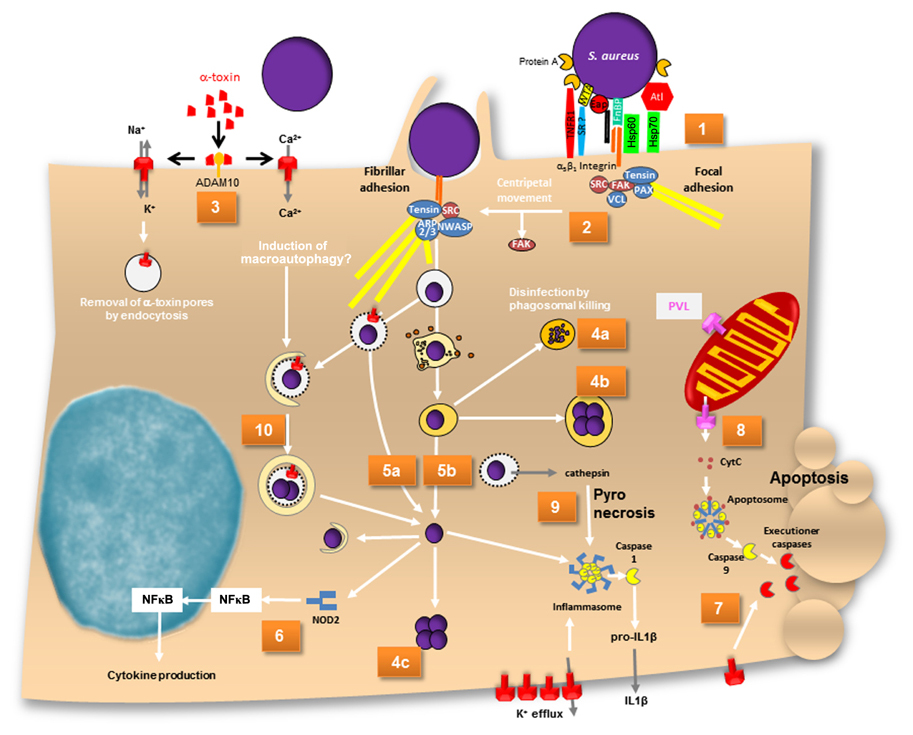
FIGURE 1. A map of intracellular fates of S. aureus. (1) α5β1 integrins are sequestered by FnBP-dependent fibronectin cross-linking at focal adhesions. (2) Centripetal movement and loss of FAK lead to development of fibrillar adhesions, at which phagocytic cups are formed and bacteria are eventually endocytosed. (3) Assembly of α-toxin pores on the plasma membrane of host cells leads is dependent on ADAM10. α-Toxin pores are permeable for cations. Ca2+ has been reported to induce macroautophagy. (4a) Bacteria are disinfected by phagolysosomes or (4b) survive and grow within endosomes or (4c) in the cytoplasm after phagosomal escape. (5a) Phagosomal escape can be mediated by α-toxin in cystic fibrosis cells and (5b) also by a combination of phenol-soluble modulins and phospholipases. (6) Cytoplasmic S. aureus peptidoglycan is recognized by NOD2, which activates NFκB and results in cytokine production. (7) The mode of cell death induced by S. aureus is not completely understood. While caspase-independent cell death exists, α-toxin is capable of inducing extrinsic apoptosis. Upon alpha-toxin induced potassium efflux caspase 2 has recently been shown to lead to mitochondrial outer membrane permeabilization. (8) PVL has been reported to permeabilize mitochondrial outer membrane thereby releasing cytochrome c and thus inducing the apoptosome in a Bax-independent pathway of intrinsic apoptosis. Caspase 9 subsequently activates executioner caspases. (9) Cathepsin release from permeabilized phagosomes activates the inflammasome. Activated caspase 1 leads to IL1β maturation and inflammatory pyronecrotic cell death. (10) Toxin-permeabilized endocytic vesicles are targeted by autophagy. During autophagy an isolation membrane engulfs leaky endosomes or cytoplasmically located bacteria. Within these autophagosomes bacterial replicate and eventually escape the organelle ultimately leading to host cell death. ADAM, a metalloprotease and disintegrin; ARP2/3, actin-related protein 2 and 3; Atl, autolysin; CytC, cytochrome c; Eap, extracellular adherence protein; FAK, focal adhesion kinase; FnBP, fibronectin-binding protein; HSP, heat shock protein; IL, interleukin; NF?B, nuclear factor κB; NWASP, neural Wiskott–Aldrich syndrome protein; PAX, paxillin; SR, scavenger receptor; VCL, vinculin; WTA, wall teichoic acid.
Since FnBPs contribute to the adherence of S. aureus to intact endothelium in vivo (Laschke et al., 2005; Kerdudou et al., 2006; Edwards et al., 2010), we can assume that staphylococcal invasion of epithelia or the endothelium is relevant in natural infections. The interaction of FnBP with ECM Fn is mediated by tandem β zipper structures via the binding of multiple fibronectin molecules by the repetitively arranged modules within a single FnBP (Schwarz-Linek et al., 2003; Rudino-Pinera et al., 2004; Bingham et al., 2008). As a result FnBP/Fn sequester α5β1 integrins on the host cell surface. The resulting receptor clustering relays signals that result in cytoskeletal rearrangements (Agerer et al., 2005; Schröder et al., 2006b). The rearrangements initiated at focal adhesions, which are remodeled to fibrillar adhesions by loss of focal adhesion kinase (FAK), paxillin, and vinculin. The rearrangement is accompanied by a centripetal movement of S. aureus on the host cell surface that were observed by videomicroscopy (Schröder et al., 2006a). The repeated generation of actin comet tails beneath adherent staphylococci or FnbA-coated beads and the formation of actin cups without internalization of staphylococci is interpreted by the authors as a delay of phagocytosis (Schröder et al., 2006a; Figure 1, Map Item 2). Invasion signaling further involves src kinase (Agerer et al., 2003). Extracellular signal-regulated kinases (ERK) and c-Jun N-terminal kinase (JNK) but not mitogen-activated protein kinase (MAPK) p38 are required in osteoblasts (Ellington et al., 2001), whereas in HEp-2 cells p38 MAPK was found to be upregulated alongside ERK (Li et al., 2009). Further, phosphorylation of transcription factor c-Jun, but not of Elk-1 or ATF-2 has been demonstrated during invasion of osteoblasts (Ellington et al., 2001). The phosphoinositide-3-kinase (PI3K)-Akt pathway is active during S. aureus internalization by bovine endothelial cells (Oviedo-Boyso et al., 2011).
Eventually, S. aureus gets endocytosed by professional as well as non-professional cells where the pathogen faces a variety of intracellular fates.
Staphylococcus aureus Intracellular Persistence and Growth
The fates of the pathogen and the infected host cell depend on staphylococcal isolate and genotype (Krut et al., 2003) as well as differential susceptibility of host cells to virulence factors, host cell gene expression, etc. For example, S. aureus produces different hemolysins. The majority of bovine mastitis strains were phenotypically positive for the sphingomyelinase β-toxin, whereas only a minority of human strains isolated from cases of septicemia or nasal carriage was positive for β-toxin (Aarestrup et al., 1999). There seems to exist a selective pressure for S. aureus strains colonizing humans to acquire β-toxin converting phage (Goerke et al., 2009). This is most likely due to staphylococcal complement inhibitor SCIn and the chemotaxis inhibitor protein CHIPS (and additional factors) that are usually found to be encoded by the respective phage genomes. Purified β-toxin, selectively kills monocytes (Bhakdi et al., 1996) and destroys platelets, but barely affects other cell types (Wadstrom and Mollby, 1972). Similarly, most human cell types are fairly insensitive to the pore-former α-toxin, whereas human leukocytes and cells from other mammalian species are highly susceptible (Bhakdi and Tranum-Jensen, 1991). The metalloprotease ADAM10 has been recently identified as receptor of α-toxin monomers (Wilke and Bubeck Wardenburg, 2010; Inoshima et al., 2011; Figure 1, Map Item 3). The differential specificities of α-toxin possibly reflect ADAM10 expression differences in the respective cells or might result from differential capability of host cells to remove α-toxin pores by endocytosis (Husmann et al., 2009).
Staphylococcus aureus survival within host cells was highly dependent on multiplicity of infection (MOI; e.g., Mohammed et al., 2007; Schwartz et al., 2009; Pang et al., 2010) and also the growth phase of the bacteria used for infection (Schwartz et al., 2009). Green-fluorescent protein (GFP)-expressing S. aureus displayed bleaching of the fluorescent protein, which indicated degradation of the bacteria in polymorphonuclear neutrophils (PMN; Figure 1, Map Item 4a). The loss of fluorescence was not strain-specific and was seen in each of several different strains of S. aureus, including nosocomial and community-associated methicillin-resistant strains. When rapidly growing S. aureus was used for infection, the bacteria were found to be more susceptible to GFP bleaching (Schwartz et al., 2009). This indicated that these bacteria were cleared more efficiently. Bacterial disinfection was mainly dependent on hypochlorous acid (HOCl; Schwartz et al., 2009). Phagosomal acidification and digestion of S. aureus within professional phagocytes is required for MyD88-dependent toll-like receptor (TLR) responses to infection (Abdelzaher et al., 2010).
Not all bacterial cells are disinfected by the phagolysosomes. S. aureus has been reported to persist inside phagocytes or endothelial cells for prolonged periods (Hamill et al., 1986; Lowy et al., 1988; Vann and Proctor, 1988; Buisman et al., 1991; Hiemstra et al., 1992; Schröder et al., 2006a; Garzoni et al., 2007; Kubica et al., 2008; Tuchscherr et al., 2011; reviewed in Sendi and Proctor, 2009). Persistence is most often attributed to small colony variants (SCVs) of S. aureus. SCVs often present a metabolically quiescent, non-hemolytic, non-pigmented phenotype characterized by reversible auxotrophies in heme biosynthetic pathways or in oxidative phosphorylation (Proctor et al., 1994) as well as a defined transcriptome (Garzoni et al., 2007) and proteome (Kriegeskorte et al., 2011). Also, SCVs are generally found to be mutants in the accessory gene regulator locus (agr), thus failing to produce a variety of quorum sensing-controlled virulence factors. SCVs grow slowly and hence are more resistant to a variety of antibiotics (reviewed in Sendi and Proctor, 2009). Further, SCVs display a thick cell wall (Bulger and Bulger, 1967) and an up-regulation of alternative sigma-factor σB (Moisan et al., 2006), which enables S. aureus to cope with a variety of environmental stressors (Horsburgh et al., 2002). There is increasing evidence that S. aureus can persist in vivo in human infections presumably owing to its extreme durability and resistance against a variety of environmental conditions and thus can serve as a potential source for recurrent infection (Proctor et al., 1995; von Eiff et al., 2001; Kipp et al., 2003; Schröder et al., 2006a; reviewed in Garzoni and Kelley, 2009; Sendi and Proctor, 2009). SCVs of S. aureus even have been shown to survive and grow within host cell phagosomes (Schröder et al., 2006a; Figure 1, Map Item 4b). Also, the complementation of rsbU in laboratory strains restored activity of the alternative sigma-factor σB and led to intracellular growth of S. aureus within phagolysosomes of THP-1 phagocytes (Olivier et al., 2009). Contrasting these reports, staphylococcal growth has been described after pathogen translo-cation to the host cell cytoplasm (Figure 1, Map Item 4c): S. aureus strain Newman is able to escape the phagosome and persists within human monocyte-derived macrophages (hMDM) which resulted in host cell lysis on day 5 after infection (Kubica et al., 2008). The authors postulate that this survival within phagocytes might constitute a route for dissemination of staphylococcal infection. This is further corroborated by the identification of cytopro-tective effects on macrophages after phagocytosis of S. aureus. Thus, the up-regulation of anti-apoptotic factors upon staphy-lococcal infection is responsible for extended phagocyte lifetime (Koziel et al., 2009). Both studies suggest that S. aureus might penetrate deeper into the tissue and even disseminate to different sites within “Trojan horse” phagocytes (Koziel et al., 2009). Survival within PMN is reported to depend on the accessory regulator Sar1, which was crucial to S. aureus survival inside spacious vacuoles, whereas sar- strains were localizing to so-called “tight vacuoles” (Gresham et al., 2000). Such different vacuoles can also be observed innon-professional phagocytes (Sinha and Fraunholz, 2010). The large vacuoles also are reminiscent of spacious Liste-ria-containing phagosomes, which were found to be non-acidified and non-degradative niches in macrophages (Birmingham et al., 2008). However, a more thorough characterization of the different vacuolar locations in intracellular S. aureus infections is lacking.
Staphylococcus aureus Phagosomal Escape
Phagocytosed bacterial pathogens evade lysosomal killing, e.g., by disintegration of the organelle membrane in order to translocate into the host cell cytoplasm. Listeria monocytogenes co-opts the pore-forming toxin (PFT) listeriolysin O (LLO) and phos-pholipases (reviewed in Dramsi and Cossart, 2002; Schnupf and Portnoy, 2007), and Group A streptococci use the PFT strep-tolysin O (Hakansson et al., 2005). Phagosomal escape of S. aureus initially has been described by Bayles et al. (1998) and the agr-dependency of this process has been demonstrated (Qazi et al., 2001; Shompole et al., 2003; Jarry and Cheung, 2006; Kubica et al., 2008). The membrane destructive function of staphylococcal a-toxin thus suggested an involvement of the pore-former in phagosomal membrane disruption. The requirement for phago-somal escape and intracellular bacterial survival has been reported in CFT-1, a cystic fibrosis (CF) lung cell line (Jarry and Cheung, 2006) as well as in macrophages (Kubica et al., 2008; Figure 1, Map Item 5a). In CFT-1, S. aureus translocates into the cytoplasm in an α-toxin-dependent manner and the bacteria replicate within the cytoplasm (Kahl et al., 2000; Jarry et al., 2008). However, in the LCSFN cell line complemented with the wild-type CF transmem-brane conductance regulator, CFTR, α-toxin has no effect (Jarry and Cheung, 2006). Further, it has been demonstrated that neither α-toxin overexpressing strains (Lám et al., 2010) nor laboratory strains inducibly expressing α-toxin (Giese et al., 2009, 2011) are capable of releasing staphylococci into the host cell cytoplasm. By expression of the amphiphilic 26 amino-acid (AA) peptide δ-toxin in the non-cytotoxic laboratory strain S. aureus RN4220 S. aureus was capable of escape in rates similar to that of heterolo-gously expressed LLO (Giese et al., 2011). δ-Toxin is encoded by the agr-effector RNAIII and is translated about 1 h after transcription of RNAIII (Balaban and Novick, 1995). It is capable of lysing bacterial protoplasts, lysosomes, lipid spherules, mitochondria, and erythrocytes in vitro in a temperature-independent manner. Its activity and mode of action is comparable to that of non-ionic detergents (Kreger and Bernheimer, 1971; Kreger et al., 1971; Rahal Jr., 1972; Kapral, 1976; reviewed in Verdon et al., 2009). δ-Toxin is encoded by RNAIII, the agr effector, and thus might constitute an immediate response to space limitation by phagosomal engulfment (Figure 1, Map Item 5b). However, membrane disruption by δ-toxin depended on the presence of the staphylococcal sphingomyelinase, β-toxin (Giese et al., 2011), which cleaves sph-ingomyelins (SM) to phosphorylcholine and ceramide moieties. δ-Toxin hardly binds to negatively charged phospholipids, binds strongly to liquid-disordered domains and poorly to cholesterol and sphingomyelin liquid-ordered raft domains (Pokorny et al., 2006). In one model, β-toxin thus may cleave SM to ceramides, which tend to accumulate in membrane microdomains. The hydrophobic nature of ceramide-rich domains thus might constitute regions of δ-toxin assembly, which eventually lead to target membrane permeabilization (Figure 2). S. aureus strain USA300 LAC, however, is escape proficient yet does not encode a functional β-toxin due to lysogeny of a β-converting phage (Diep et al., 2006). We thus have to hypothesize alternative factors that can act in phagosomal escape, such as a variety of lipases encoded by the staphylococcal genome or phenol-soluble modulins (PSMs; see below). Alternatively, prophages might be lost, e.g., during exposure to phagosomal reactive oxygen species, and then might contribute to phagosomal escape. A similar activation mechanism is used by Streptococcus pneumoniae, which produces hydrogen peroxide and thereby lyses S. aureus by a “remote control” prophage activation (Selva et al., 2009).
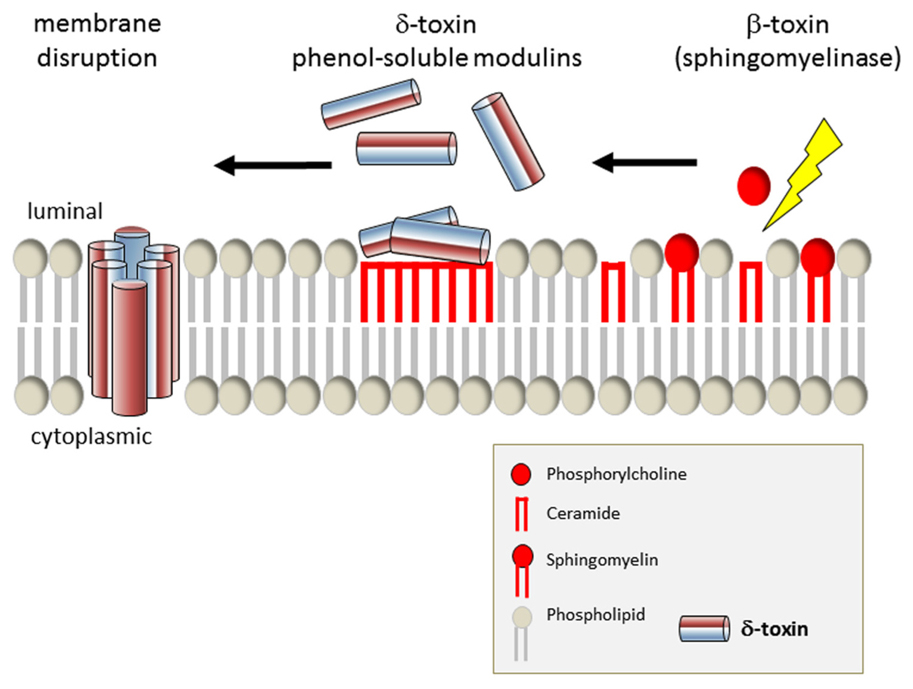
FIGURE 2. Model of phagosomal escape by S. aureus by synergistic action of δ-toxin and β-toxin. After cleavage of sphingomyelin to ceramide and phosphocholine, δ-toxin is capable of interacting more efficiently with the outer leaflet of the eukaryotic plasma membrane. δ-Toxin accumulates in the resulting hydrophobic ceramide membrane domains and eventually permeabilizes the target membrane.
For complete hemolysis of erythrocytes in sheep blood agar plates either a shift to 4°C (hot-cold hemolysis), osmotic stress, or synergistic toxins are necessary. δ-Toxin belong to the class of PSMs, which initially had been shown to be present within a hot-phenol extraction of S. epidermidis extracts (Otto et al., 2004) and only later had been identified in S. aureus by sequence homology (Wang et al., 2007). The major groups of PSMs are divided into two operons referred to as PSMα and PSMβ. The PSMα operon is comprised of four open reading frames (ORFs) with approximately 20 AA. PSMβ encodes two ORFs, which are about 40 AA in length (Wang et al., 2007). The expression of PSMβ has been demonstrated to result in phagosomal escape in a gain-of-function study (Giese et al., 2011). Just like δ-toxin, PSMα and PSMβ are agr-dependently expressed. It recently was shown that the staphylococcal agr system is confinement induced and thus comprises not only a quorum sensing system but also a diffusion sensing system active at the single cell level (Carnes et al., 2009). δ-Toxin and PSMs are hence produced upon phagosomal confinement and thus also low numbers of endocytosed staphy-lococci should be able to mount a toxin response in order to avoid lysosomal killing. Whereas gain of function assays demonstrated PSMβ activity in phagosomal escape (Giese et al., 2011), in vivo studies suggests a prominent role of PSMα, with the third ORF of the operon, PSMα3, being the most virulent principle (Wang et al., 2007). Despite these incongruities a common theme emerges: amphiphilic PSMs are involved in phagosomal escape. One possibility to explain the observed differences is the postu-lation of alternative pathways of phagosomal escape mechanisms for S. aureus.
Molecular patterns of pathogens that reside in the cytoplasm of their hosts are detected by nucleotide-binding and oligomerization domain proteins NOD1 and NOD2, which detect γ-D-glutamyl-diaminopimelic acid and muramyl dipeptide, pep-tidoglycan components of Gram-negative and Gram-positive bacteria. Peptidoglycan binding leads to a conformation change in NOD proteins, which initiates the recruitment of ubiquitin lig-ases and kinases and ultimately results in nuclear translocation of NFκB and activator protein 1 and expression of inflammatory genes (reviewed in Strober et al., 2006). NOD2 signaling upon S. aureus infection has been found to induce cytokine production (Kapetanovic et al., 2007) and thus might contribute to induction of inflammation, e.g., in the lung (Gomez and Prince, 2008; Figure 1; Map Item 6). NOD2-deficient mice exhibit a delayed inflammatory response and impaired bacterial clearance after infection with S. aureus (Hruz et al., 2009). α-Toxin facilitates NOD2-dependent recognition of S. aureus muramyl dipeptide (Hruz et al., 2009), possibly by interfering with phagosomal integrity.
The observation that S. aureus can translocate into the cytoplasm of host cells and grow without an immediately ensuing cell death (e.g., Kubica et al., 2008) illustrates that phagosomal escape is not identical with cytotoxicity Thus, the link between phagosomal escape and host cell death still needs to be elucidated.
Staphylococcus aureus-Induced Host Cell Death
The classical separation of host cell death into programed cell death (PCD; apoptosis) and accidental cell death or necrosis has long been superseded after the identifying a large variety of cell death mechanisms (for a reviews, see Taylor et al., 2008; Ting et al., 2008a; Bergsbaken et al., 2009). Many of which are defined by key factors that allow the assessment of death pathway activation following bacterial infection (e.g., reviewed in Rudel et al., 2010), although the synchronous activity of virulence factors from the staphylococcal arsenal renders unequivocal correlations between toxin and associated mode of cell death a daunting task. When virulent S. aureus strains are added to host cells in tissue culture, host cell death occurs via mechanisms that have been mainly identified as apoptotic (Bayles et al., 1998; Menzies and Kourteva, 1998, 2000; Wesson et al., 1998, 2000; Kahl et al., 2000; Nuzzo et al., 2000; Tucker et al., 2000; Haslinger et al., 2003; Genestier et al., 2005; Figure 1, Map Item 7). α-Toxin is both required and sufficient for induction of leukocyte cell death, either apoptotic or necrotic (Bantel et al., 2001; Essmann et al., 2003; Haslinger et al., 2003). Leukocytes are sensitive to staphylococcal α-toxin. Already low toxin doses induce apoptosis accompanied by a breakdown of the mitochondrial transmembrane potential (Bantel et al., 2001; Haslinger et al., 2003). A recent study suggests that caspase-2 acts as an initiator caspase during cell death of non-professional phagocytes. Caspase 2 was induced by potassium efflux due to pore-forming toxins such as staphylococcal alpha-toxin and aerolysin (Imre et al., 2012). By contrast, high doses induce necrotic cell death (Walev et al., 1993; Bantel et al., 2001; Essmann et al., 2003; Haslinger et al., 2003). Human endothelial cells are virtually insensitive to the action of S. aureus α-toxin, however, comparatively low numbers of S. aureus cells with a combined invasive and strongly hemolytic phenotype readily induce apoptotic cell death in HUVEC (Haslinger-Löffler et al., 2005). This suggests that cell death mechanisms are activated from within their intracellular location. The effect is highly specific, since fixed, non-hemolytic, rifampin-treated or weakly invasive staphylococci are not cytotoxic toward endothelial cells (Haslinger-Löffler et al., 2005). Multiple other studies indicate that S. aureus might kill its other types of host cells from within (Bayles et al., 1998; Menzies and Kourteva, 1998; Wesson et al., 1998; Nuzzo et al., 2000; Tucker et al., 2000; Krut et al., 2003; Haslinger-Löffler et al., 2005; Chatterjee et al., 2008; Jarry et al., 2008; Kubica et al., 2008; Lám et al., 2010). The virulence factors required for S. aureus-induced apoptosis in endothelial cells depend on agr and the alternative stress–response sigma-factor σB (Wesson et al., 1998; Qazi et al., 2001; Shompole et al., 2003; Jarry and Cheung, 2006; Kubica et al., 2008), but mainly seem to be independent of SarA (Haslinger-Löffler et al., 2005; Jarry et al., 2008) - although there are contradicting results on the involvement of SarA (Wesson et al., 1998).
Phage-encoded Panton-Valentine leukocidin (PVL) predominantly destroys leukocytes, although there also is some species specificity for human and rabbit PMN (Loffler et al., 2010). In PMN, PVL induced a rapid caspase-9/3-dependent cell death in vitro (Genestier et al., 2005). The authors further identified to a mitochondrial localization of the PVL toxin. Isolated mitochondria were permeabilized for pro-apoptotic factors such as cytochrome c (CytC) by PVL which suggested that PVL is able to create pores in the mitochondrial outer membrane and thus triggers a Bax-independent mitochondrial pathway of host cell apoptosis (Genestier et al., 2005; Figure 1, Map Item 8). During the intrinsic pathway of apoptosis release of CytC from injured mitochondria leads to activation of apoptosis-activating factor-1 (APAF-1). Oligomerizing CytC/APAF-1 recruits and subsequently activates pro-caspase 9. Caspase 9 then proteolytically activates effector caspases, which finally cleave their respective substrates resulting in membrane blebbing, and DNA fragmentation (reviewed in Rudel et al., 2010). Similarly, α-toxin has been described to activate caspases via the intrinsic death pathway (Bantel et al., 2001; Haslinger et al., 2003) independently of death receptor signaling (CD95/Fas/APO-1). Bcl-2 overexpressing Jurkat cells were protected from α-toxin mediated cell death (Bantel et al., 2001; Essmann et al., 2003) although recent result suggest that the function of Bcl-2 in autophagy might be responsible for the observed phenomena (Schnaith et al., 2007; Mestre et al., 2010).
Interestingly, S. aureus also seems to be able to exert anti-apoptotic host cell responses. The pathogen was shown to suppress staurosporine-induced apoptosis in hMDM although early apoptotic features such as phosphatidylserine display at the outer plasma membrane leaflet, reduced mitochondrial membrane potential, CytC release, and caspase-3 activation are still observed (Koziel et al., 2009). S. aureus infection strongly upregulated the expression of mitochondrial membrane potential stabilizing Bcl-2 and Mcl-1 gene products. Since also heat-killed S. aureus was able to suppress apoptosis in the host cells staphylococcal products such as lipoteichoic acid or peptidoglycan might activate the macrophages through intracellular pattern recognition sensors such as nucleotide oligomerization domain receptors (NOD; Kapetanovic et al., 2007; Koziel et al., 2009).
Aside from apoptosis S. aureus is also able to induce pyronecrosis (Figure 1; Map Item 9). There, caspase-1 is activated as part of an inflammasome, which further consists of NOD-like receptor protein 3 (NLRP3) and the adaptor protein, apoptosis-associated speck-like protein containing a caspase-associated recruitment domain (ASC). S. aureus can function as a stimulus for NLRP3 (Munoz-Planillo et al., 2009), however, the molecular identity of the stimulating signal is not known thus far (Mariathasan et al., 2006; Ting et al., 2008b; Wright and Nair, 2010). Lysosomal permeabilization is one NLRP3-activating principle, wherein release of the lysosomal protease cathepsin B into the cytoplasm contributes to NLRP3 activation (reviewed in Willingham and Ting, 2008; Bergsbaken et al., 2009). Thus, it seems likely, that lysosomal rupture or permeabilization by S. aureus toxins releases cathepsin which then activates the inflammasome. Indeed staphylococcal α, β, and γ-hemolysins have been shown to be important activation of the NLRP3 inflammasome (Craven et al., 2009; Munoz-Planillo et al., 2009; Kebaier et al., 2012). The pore-forming α-and γ-toxins of S. aureus permeabilize membranes and thus might be involved in cathepsin release and subsequent inflammasome activation. α-Toxin further is known to permeabilize the plasma membrane for potassium ions. In turn, potassium efflux activates the inflammasome (Petrilli et al., 2007). β-Toxin has been shown to be involved in phagosomal escape (Giese et al., 2011) and thus also might act in cathepsin release.
Staphylococcus aureus and the Subversion of Autophagy
Autophagy sequesters cytoplasmic contents via an isolation membrane. Engulfment of cargo by the so-called phagophore forms double membrane-bound autophagic vesicles that eventually fuse with lysosomes to yield autolysosomes. Autophagy serves for degradation of organelles or self-digestion during nutrient limiting conditions such as starvation and is generally thought to constitute a cellular survival mechanism. During bacterial infections autophagy disposes of leaky vesicles or intracellular bacteria, however, bacterial pathogens have found multiple ways to subvert autophagy (reviewed in Dorn et al., 2002; Kirkegaard et al., 2004; Levine, 2005; Campoy and Colombo, 2009; Orvedahl and Levine, 2009; Ogawa et al., 2011).
Staphylococcus aureus was shown to interact with autophagosomes in a rather unique way (Schnaith et al., 2007) when compared to autophagy-subvertive strategies of other bacterial pathogens (Campoy and Colombo, 2009; Ogawa et al., 2011). S. aureus inhibits fusion of phagosomes with lysosomes. It permeabilizes HeLa phagosomes by a mechanism dependent on Staphylococcus-secreted toxins. The leaky phagosomes are targeted by autophagy and within autophagosomes S. aureus replicates. Eventually the bacteria escape from their intracellular confinement into the host cell cytoplasm in an agr-dependent manner (Schnaith et al., 2007; Figure 1, Map Item 9). Finally, host cell death is induced, which is independent of a caspase activation cascade but was blocked by overexpression of anti-autophagic Bcl-2. Induction of autophagy by rapamycin resulted in an increased number of recovered colony-forming units, whereas inhibition with wortmann in reduced the colonies recovered from the intracellular environment. S. aureus-induced autophagy resulted in a vacuolization of the host cell cytoplasm (“Swiss cheese phenotype”). agr-deficient S. aureus fail to induce autophagy, which results in maturation of bacteria-containing phagosomes followed by lysosomal degradation of the pathogens. α-Toxin is able to permeabilize membranes for Ca2+, an inducer of autophagy (Brady et al., 2007) and autophagy targets phagosomes perforated by α-toxin in Chinese hamster ovary cells (Mestre et al., 2010). Whereas latter observation is in line with the results obtained by Schnaith et al. (2007) it contrasts findings that α-toxin is not sufficient to permeabilize HeLa phagosomes (Jarry and Cheung, 2006; Giese et al., 2009; Lám et al., 2010).
Conclusion
With about 200–300 virulence factors, S. aureus is able to exert a multitude of effects upon its eukaryotic host cells. Although many details have emerged through ground-breaking and recent research, only a minority of pathogenicity factors of S. aureus has been functionally annotated to date. Particularly the assessment of intracellular staphylococcal virulence is often hampered by the difficulty to discriminate between toxin effects that result from extracellular or intracellular bacteria, although inducible toxin-expressing might provide valuable tools for molecular dissection of host–pathogen interactions.
With our advancing knowledge of cell death mechanisms ground-breaking experiments will have to be re-evaluated in order to understand the mechanisms of S. aureus-induced host cell killing. When comparing experimental research originating from different labs the influence of a body of factors should be taken into account that could lead to different infection outcomes:
Staphylococcus aureus strain used for infection, its growth phase at the time of infection (and hence the bacterial growth medium), as well as MOI are important, whereas on the host side the cell type and hence the protein profile will drastically influence infection outcome by altering host cell susceptibility to bacterial toxins, response to pathogen-associated molecular patterns, expression of receptors, caspases, and other host factors.
In order to identify activities of single toxins or virulence factors gain-of-function studies can be useful, e.g., using the a pathogenic S. carnosus as toxin delivery vehicle. However, such strategies will not easily identify pathways during which an orchestrated interplay of multiple virulence factors is required. However, novel high-throughput sequencing technologies of transposon insertions (Gawronski et al., 2009; van Opijnen et al., 2009) open up new possibilities for analysis of genome-wide mutant libraries of clinically relevant strains. Using such novel tools we will be able to address a lot of open questions with regard to intracellular staphylococcal infections: do different adhesins result in employment of different uptake mechanisms into non-professional phagocytes as these would subsequently result in different infection outcomes? What is the nature of tight and spacious vacuoles (Gresham et al., 2000) that are occupied by, for example, sarA- and sarA+ S. aureus, respectively? Which alternative pathways for phagosomal escape do exist? Which modes of cell death are activated by a single strain in different host cell types or different strains in a single host cell line? There is still a lot to learn about the versatile facultative intracellular pathogen, Staphylococcus aureus.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Aarestrup, F. M., Larsen, H. D., Eriksen, N. H., Elsberg, C. S., and Jensen, N. E. (1999). Frequency of alpha- and beta-haemolysin in Staphylococcus aureus of bovine and human origin. A comparison between pheno- and genotype and variation in phenotypic expression. APMIS 107, 425–430.
Abdelzaher, A. M., Wright, M. E., Ortega, C., Solo-Gabriele, H. M., Miller, G., Elmir, S., Newman, X., Shih, P., Bonilla, J. A., Bonilla, T. D., Palmer, C. J., Scott, T., Lukasik, J., Harwood, V. J., Mcquaig, S., Sinigalliano, C., Gidley, M., Plano, L. R., Zhu, X., Wang, J. D., and Fleming, L. E. (2010). Presence of pathogens and indicator microbes at a non-point source subtropical recreational marine beach. Appl. Environ. Microbiol. 76, 724–732.
Agerer, F., Lux, S., Michel, A., Rohde, M., Ohlsen, K., and Hauck, C. R. (2005). Cellular invasion by Staphylococcus aureus reveals a functional link between focal adhesion kinase and cortactin in integrin-mediated internalisation. J. Cell Sci. 118, 2189–2200.
Agerer, F., Michel, A., Ohlsen, K., and Hauck, C. R. (2003). Integrin-mediated invasion of Staphylococcus aureus into human cells requires Src family protein-tyrosine kinases. J. Biol. Chem. 278, 42524–42531.
Ahmed, S., Meghji, S., Williams, R. J., Henderson, B., Brock, J. H., and Nair, S. P. (2001). Staphylococcus aureus fibronectin binding proteins are essential for internalization by osteoblasts but do not account for differences in intracellular levels of bacteria. Infect Immun. 69, 2872–2877.
Balaban, N., and Novick, R. P. (1995). Translation of RNAIII, the Staphylococcus aureus agr regulatory RNA molecule, can be activated by a 3′-end deletion. FEMS Microbiol Lett. 133, 155–161.
Bantel, H., Sinha, B., Domschke, W., Peters, G., Schulze-Osthoff, K., and Janicke, R. U. (2001). alpha-Toxin is a mediator of Staphylococcus aureus-induced cell death and activates caspases via the intrinsic death pathway independently of death receptor signaling. J. Cell Biol. 155, 637–648.
Bayles, K. W., Wesson, C. A., Liou, L. E., Fox, L. K., Bohach, G. A., and Trumble, W. R. (1998). Intracellular Staphylococcus aureus escapes the endosome and induces apoptosis in epithelial cells. Infect. Immun. 66, 336–342.
Bergsbaken, T., Fink, S. L., and Cookson, B. T. (2009). Pyroptosis: host cell death and inflammation. Nat. Rev. Microbiol. 7, 99–109.
Bhakdi, S., Bayley, H., Valeva, A., Walev, I., Walker, B., Kehoe, M., and Palmer, M. (1996). Staphylococcal alpha-toxin, streptolysin-O, and Escherichia coli hemolysin: prototypes of pore-forming bacterial cytolysins. Arch. Microbiol. 165, 73–79.
Bhakdi, S., and Tranum-Jensen, J. (1991). Alpha-toxin of Staphylococcus aureus. Microbiol. Rev. 55, 733–751.
Bingham, R. J., Rudino-Pinera, E., Meenan, N. A., Schwarz-Linek, U., Turkenburg, J. P., Hook, M., Garman, E. F., and Potts, J. R. (2008). Crystal structures of fibronectin-binding sites from Staphylococcus aureus FnBPA in complex with fibronectin domains. Proc. Natl. Acad. Sci. U.S.A. 105, 12254–12258.
Birmingham, C. L., Canadien, V., Kaniuk, N. A., Steinberg, B. E., Higgins, D. E., and Brumell, J. H. (2008). Listeriolysin O allows Listeria monocytogenes replication in macrophage vacuoles. Nature 451, 350–354.
Brady, N. R., Hamacher-Brady, A., Yuan, H., and Gottlieb, R. A. (2007). The autophagic response to nutrient deprivation in the hl-1 cardiac myocyte is modulated by Bcl-2 and sarco/endoplasmic reticulum calcium stores. FEBS J. 274, 3184–3197.
Buisman, H. P., Buys, L. F., Langermans, J. A., Van Den Broek, P. J., and Van Furth, R. (1991). Effect of probenecid on phagocytosis and intracellular killing of Staphylococcus aureus and Escherichia coli by human monocytes and granulocytes. Immunology 74, 338–341.
Bulger, R. J., and Bulger, R. E. (1967). Ultra structure of small colony variants of a methicillin-resistant Staphylococcus aureus. J. Bacteriol. 94, 1244–1246.
Campoy, E., and Colombo, M. I. (2009). Autophagy in intracellular bacterial infection. Biochim. Biophys. Acta 1793, 1465–1477.
Carnes, E. C., Lopez, D. M., Donegan, N. P., Cheung, A., Gresham, H., Timmins, G. S., and Brinker, C. J. (2009). Confinement-induced quorum sensing of individual Staphylococcus aureus bacteria. Nat. Chem. Biol. 6, 41–45.
Chatterjee, I., Kriegeskorte, A., Fischer, A., Deiwick, S., Theimann, N., Proctor, R. A., Peters, G., Herrmann, M., and Kahl, B. C. (2008). In vivo mutations of thymidylate synthase (encoded by thyA) are responsible for thymidine dependency in clinical small-colony variants of Staphylococcus aureus. J. Bacteriol. 190, 834–842.
Claro, T., Widaa, A., O’Seaghdha, M., Miajlovic, H., Foster, T. J., O’Brien, F. J., and Kerrigan, S. W. (2011). Staphylococcus aureus protein A binds to osteoblasts and triggers signals that weaken bone in osteomyelitis. PLoS ONE 6, e18748. doi: 10.1371/journal. pone.0018748
Craven, R. R., Gao, X., Allen, I. C., Gris, D., Bubeck Wardenburg, J., Mcelvania-Tekippe, E., Ting, J. P., and Duncan, J. A. (2009). Staphylococcus aureus α-hemolysin activates the NLRP3-inflammasome in human and mouse monocytic cells. PLoS ONE 4, e7446. doi: 10.1371/journal. pone.0007446
Diep, B. A., Gill, S. R., Chang, R. F., Phan, T. H., Chen, J. H., Davidson, M. G., Lin, F., Lin, J., Carleton, H. A., Mongodin, E. F., Sensabaugh, G. F., and Perdreau-Remington, F. (2006). Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet 367, 731–739.
Dorn, B. R., Dunn, W. A. Jr., and Progulske-Fox, A. (2002). Bacterial interactions with the autophagic pathway. Cell. Microbiol. 4, 1–10.
Dramsi, S., and Cossart, P. (2002). Listeriolysin O: a genuine cytolysin optimized for an intracellular parasite. J. Cell Biol. 156, 943–946.
Dziewanowska, K., Carson, A. R., Patti, J. M., Deobald, C. F., Bayles, K. W., and Bohach, G. A. (2000). Staphylococcal fibronectin binding protein interacts with heat shock protein 60 and integrins: role in internalization by epithelial cells. Infect. Immun. 68, 6321–6328.
Dziewanowska, K., Patti, J. M., Deobald, C. F., Bayles, K. W., Trumble, W. R., and Bohach, G. A. (1999). Fibronectin binding protein and host cell tyrosine kinase are required for internalization of Staphylococcus aureus by epithelial cells. Infect. Immun. 67, 4673–4678.
Edwards, A. M., Potter, U., Meenan, N. A., Potts, J. R., and Massey, R. C. (2011). Staphylococcus aureus keratinocyte invasionisdependent upon multiple high-affinity fibronectin-binding repeats within FnBPA. PLoS ONE 6, e18899. doi: 10.1371/journal. pone.0018899
Edwards, A. M., Potts, J. R., Josefsson, E., and Massey, R. C. (2010). Staphylococcus aureus host cell invasion and virulence in sepsis is facilitated by the multiple repeats within FnBPA. PLoS Pathog. 6, e1000964. doi: 10.1371/journal.ppat.1000964
Ellington, J. K., Elhofy, A., Bost, K. L., and Hudson, M. C. (2001). Involvement of mitogen-activated protein kinase pathways in Staphylococcus aureus invasion of normal osteoblasts. Infect. Immun. 69, 5235–5242.
Essmann, F., Bantel, H., Totzke, G., Engels, I. H., Sinha, B., Schulze-Osthoff, K., and Janicke, R. U. (2003). Staphylococcus aureus alpha-toxin-induced cell death: predominant necrosis despite apoptotic caspase activation. Cell Death Differ. 10, 1260–1272.
Fowler, T., Wann, E. R., Joh, D., Johansson, S., Foster, T. J., and Hook, M. (2000). Cellular invasion by Staphylococcus aureus involves a fibronectin bridge between the bacterial fibronectin-binding MSCRAMMs and host cell beta1 integrins. Eur. J. Cell Biol. 79, 672–679.
Garzoni, C., Francois, P., Huyghe, A., Couzinet, S., Tapparel, C., Charbonnier, Y., Renzoni, A., Lucchini, S., Lew, D. P., Vaudaux, P., Kelley, W. L., and Schrenzel, J. (2007). A global view of Staphylococcus aureus whole genome expression upon internalization in human epithelial cells. BMC Genomics 8, 171. doi: 10.1186/ 1471-2164-8-171
Garzoni, C., and Kelley, W. L. (2009). Staphylococcus aureus: new evidence for intracellular persistence. Trends Microbiol. 17, 59–65.
Gawronski, J. D., Wong, S. M., Giannoukos, G., Ward, D. V., and Akerley, B. J. (2009). Tracking insertion mutants within libraries by deep sequencing and a genome-wide screen for Haemophilus genes required in the lung. Proc. Natl. Acad. Sci. U.S.A. 106, 16422–16427.
Genestier, A. L., Michallet, M. C., Prévost, G., Bellot, G., Chalabreysse, L., Peyrol, S., Thivolet, F., Etienne, J., Lina, G., Vallette, F. M., Vandenesch, F., and Genestier, L. (2005). Staphylococcus aureus Panton-Valentine leukocidin directly targets mitochondria and induces Bax-independent apoptosis of human neutrophils. J. Clin. Invest. 115, 3117–3127.
Giese, B., Dittmann, S., Paprotka, K., Levin, K., Weltrowski, A., Biehler, D., Lam, T. T., Sinha, B., and Fraunholz, M. J. (2009). Staphylococcal alpha-toxin is not sufficient to mediate escape from phagolysosomes in upper-airway epithelial cells. Infect. Immun. 77, 3611–3625.
Giese, B., Glowinski, F., Paprotka, K., Dittmann, S., Steiner, T., Sinha, B., and Fraunholz, M. J. (2011). Expression of delta-toxin by Staphylococcus aureus mediates escape from phago-endosomes of human epithelial and endothelial cells in the presence of beta-toxin. Cell. Microbiol. 13, 316–329.
Goerke, C., Pantucek, R., Holtfreter, S., Schulte, B., Zink, M., Grumann, D., Broker, B. M., Doskar, J., and Wolz, C. (2009). Diversity of prophages in dominant Staphylococcus aureus clonal lineages. J. Bacteriol. 191, 3462–3468.
Gomez, M. I., and Prince, A. (2008). Airway epithelial cell signaling in response to bacterial pathogens. Pediatr. Pulmonol. 43, 11–19.
Gresham, H. D., Lowrance, J. H., Caver, T. E., Wilson, B. S., Cheung, A. L., and Lindberg, F. P. (2000). Survival of Staphylococcus aureus inside neutrophils contributes to infection 1. J. Immunol. 164, 3713–3722.
Haim, M., Trost, A., Maier, C. J., Achatz, G., Feichtner, S., Hintner, H., Bauer, J. W., and Onder, K. (2010). Cytokeratin 8 interacts with clumping factor B: a new possible virulence factor target. Microbiology 156, 3710–3721.
Hakansson, A., Bentley, C. C., Shakhnovic, E. A., and Wessels, M. R. (2005). Cytolysin-dependent evasion of lysosomal killing. Proc. Natl. Acad. Sci. U.S.A. 102, 5192–5197.
Hamill, R. J., Vann, J. M., and Proctor, R. A. (1986). Phagocytosis of Staphylococcus aureus by cultured bovine aortic endothelial cells: model for postadherence events in endovascular infections. Infect. Immun. 54, 833–836.
Harraghy, N., Hussain, M., Haggar, A., Chavakis, T., Sinha, B., Herrmann, M., and Flock, J. I. (2003). The adhesive and immunomodulating properties of the multifunctional Staphylococcus aureus protein Eap. Microbiology 149, 2701–2707.
Haslinger-Löffler, B., Kahl, B. C., Grundmeier, M., Strangfeld, K., Wagner, B., Fischer, U., Cheung, A. L., Peters, G., Schulze-Osthoff, K., and Sinha, B. (2005). Multiple virulence factors are required for Staphylococcus aureus-induced apoptosis in endothelial cells. Cell. Microbiol. 7, 1087–1097.
Haslinger, B., Strangfeld, K., Peters, G., Schulze-Osthoff, K., and Sinha, B. (2003). Staphylococcus aureus alpha-toxin induces apoptosis in peripheral blood mononuclear cells: role of endogenous tumour necrosis factor-alpha and the mitochondrial death pathway. Cell. Microbiol. 5, 729–741.
Hiemstra, P. S., Annema, A., Schippers, E. F., and Van Furth, R. (1992). Pertussis toxin partially inhibits phagocytosis of immunoglobulin G-opsonized Staphylococcus aureus by human granulocytes but does not affect intracellular killing. Infect. Immun. 60, 202–205.
Hirschhausen, N., Schlesier, T., Schmidt, M. A., Gotz, F., Peters, G., and Heilmann, C. (2010). A novel staphylococcal internalization mechanism involves the major autolysin Atl and heat shock cognate protein Hsc70 as host cell receptor. Cell. Microbiol. 12, 1746–1764.
Horsburgh, M. J., Aish, J. L., White, I. J., Shaw, L., Lithgow, J. K., and Foster, S. J. (2002). sigmaB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J. Bacteriol. 184, 5457–5467.
Hruz, P., Zinkernagel, A. S., Jenikova, G., Botwin, G. J., Hugot, J. P., Karin, M., Nizet, V., and Eckmann, L. (2009). NOD2 contributes to cutaneous defense against Staphylococcus aureus through alpha-toxin-dependent innate immune activation. Proc. Natl. Acad. Sci. U.S.A. 106, 12873–12878.
Husmann, M., Beckmann, E., Boller, K., Kloft, N., Tenzer, S., Bobkiewicz, W., Neukirch, C., Bayley, H., and Bhakdi, S. (2009). Elimination of a bacterial pore-forming toxin by sequential endocytosis and exocytosis. FEBS Lett. 583, 337–344.
Inoshima, I., Inoshima, N., Wilke, G. A., Powers, M. E., Frank, K. M., Wang, Y., and Bubeck Wardenburg, J. (2011). A Staphylococcus aureus pore-forming toxin subverts the activity of ADAM10 to cause lethal infection in mice. Nat. Med. 17, 1310–1314.
Imre, G., Heering, J., Takeda, A. N., Husmann, M., Thiede, B., zu Heringdorf, D. M., Green, D. R., der Goot, F. G., Sinha, B., Dötsch, V., and Rajalingam, K. (2012). Caspase-2 is an initiator caspase responsible for pore-forming toxin-mediated apoptosis. EMBO J. (in press).
Jarry, T. M., and Cheung, A. L. (2006). Staphylococcus aureus escapes more efficiently from the phagosome of a cystic fibrosis bronchial epithelial cell line than from its normal counterpart. Infect. Immun. 74, 2568–2577.
Jarry, T. M., Memmi, G., and Cheung, A. L. (2008). The expression of alpha-haemolysin is required for Staphylococcus aureus phagosomal escape after internalization in CFT-1 cells. Cell. Microbiol. 10, 1801–1814.
Jevon, M., Guo, C., Ma, B., Mordan, N., Nair, S. P., Harris, M., Henderson, B., Bentley, G., and Meghji, S. (1999). Mechanisms of internalization of Staphylococcus aureus by cultured human osteoblasts. Infect. Immun. 67, 2677–2681.
Kahl, B. C., Goulian, M., Van Wamel, W., Herrmann, M., Simon, S. M., Kaplan, G., Peters, G., and Cheung, A. L. (2000). Staphylococcus aureus RN6390 replicates and induces apoptosis in a pulmonary epithelial cell line. Infect. Immun. 68, 5385–5392.
Kapetanovic, R., Nahori, M. A., Balloy, V., Fitting, C., Philpott, D. J., Cavaillon, J. M., and Adib-Conquy, M. (2007). Contribution of phagocytosis and intracellular sensing for cytokine production by Staphylococcus aureus-activated macrophages. Infect. Immun. 75, 830–837.
Kapral, F. A. (1976). Effect of fatty acids on Staphylococcus aureus delta-toxin hemolytic activity. Infect. Immun. 13, 114–119.
Kebaier, C., Chamberland, R. R., Allen, I. C., Gao, X., Broglie, P. M., Hall, J. D., Jania, C., Doerschuk, C. M., Tilley, S. L., and Duncan, J. A. (2012). Staphylococcus aureus alpha-hemolysin mediates virulence in a murine model of severe pneumonia through activation of the NLRP3 inflammasome. J. Infect. Dis. 205, 807–817.
Kerdudou, S., Laschke, M. W., Sinha, B., Preissner, K. T., Menger, M. D., and Herrmann, M. (2006). Fibronectin binding proteins contribute to the adherence of Staphylococcus aureus to intact endothelium in vivo. Thromb. Haemost. 96, 183–189.
Kintarak, S., Whawell, S. A., Speight, P. M., Packer, S., and Nair, S. P. (2004). Internalization of Staphylococcus aureus by human keratinocytes. Infect. Immun. 72, 5668– 5675.
Kipp, F., Ziebuhr, W., Becker, K., Krimmer, V., Hobeta, N., Peters, G., and von Eiff, C. (2003). Detection of Staphylococcus aureus by 16S rRNA directed in situ hybridisation in a patient with a brain abscess caused by small colony variants. J. Neurol. Neurosurg. Psychiatry 74, 1000–1002.
Kirkegaard, K., Taylor, M. P., and Jackson,W. T. (2004). Cellular autophagy: surrender, avoidance and subversion by microorganisms. Nat. Rev. Microbiol. 2, 301–314.
Koziel, J., Maciag-Gudowska, A., Mikolajczyk, T., Bzowska, M., Sturdevant, D. E., Whitney, A. R., Shaw, L. N., Deleo, F. R., and Potempa, J. (2009). Phagocytosis of Staphylococcus aureus by macrophages exerts cytoprotective effects manifested by the upregulation of antiapoptotic factors. PLoS ONE 4, e5210. doi: 10.1371/journal. pone.0005210
Kreger, A. S., and Bernheimer, A. W. (1971). Disruption of bacterial protoplasts and spheroplasts by staphylococcal delta hemolysin. Infect. Immun. 3, 603–605.
Kreger, A. S., Kim, K. S., Zaboretzky, F., and Bernheimer, A. W. (1971). Purification and properties of staphylococcal delta hemolysin. Infect. Immun. 3, 449–465.
Kriegeskorte, A., Konig, S., Sander, G., Pirkl, A., Mahabir, E., Proctor, R. A., von Eiff, C., Peters, G., and Becker, K. (2011). Small colony variants of Staphylococcus aureus reveal distinct protein profiles. Proteomics 11, 2476–2490.
Krut, O., Utermohlen, O., Schlossherr, X., and Kronke, M. (2003). Strain-specific association of cytotoxic activity and virulence of clinical Staphylococcus aureus isolates. Infect. Immun. 71, 2716–2723.
Kubica, M., Guzik, K., Koziel, J., Zarebski, M., Richter, W., Gajkowska, B., Golda, A., Maciag-Gudowska, A., Brix, K., Shaw, L., Foster, T., and Potempa, J. (2008). A potential new pathway for Staphylococcus aureus dissemination: the silent survival of S. aureus phagocytosed by human monocyte-derived macrophages. PLoS ONE 3, e1409. doi: 10.1371/journal.pone.0001409
Lám, T. T., Giese, B., Chikkaballi, D., Kuhn, A., Wolber, W., Pané-Farré, J., Schäfer, D., Engelmann, S., Fraunholz, M., and Sinha, B. (2010). Phagolysosomal integrity is generally maintained after Staphylococcus aureus invasion of nonprofessional phagocytes but is modulated by strain 6850. Infect. Immun. 78, 3392–3403.
Lammers, A., Nuijten, P. J. M., and Smith, H. E. (1999). The fibronectin binding proteins of Staphylococcus aureus are required for adhesion to and invasion of bovine mammary gland cells. FEMS Microbiol. Lett. 180, 103–109.
Laschke, M. W., Kerdudou, S., Herrmann, M., and Menger, M. D. (2005). Intravital fluorescence microscopy: a novel tool for the study of the interaction of Staphylococcus aureus with the microvascular endothelium in vivo. J. Infect. Dis. 191, 435–443.
Levine, B. (2005). Eating oneself and uninvited guests: autophagy-related pathways in cellular defense. Cell 120, 159–162.
Li, M., Rigby, K., Lai, Y., Nair, V., Peschel, A., Schittek, B., and Otto, M. (2009). Staphylococcus aureus mutant screen reveals interaction of the human antimicrobial peptide dermcidin with membrane phospholipids. Antimicrob. Agents Chemother. 53, 4200–4210.
Loffler, B., Hussain, M., Grundmeier, M., Bruck, M., Holzinger, D., Varga, G., Roth, J., Kahl, B. C., Proctor, R. A., and Peters, G. (2010). Staphylococcus aureus Panton-Valentine leukocidin is a very potent cytotoxic factor for human neutrophils. PLoS Pathog. 6, e1000715. doi: 10.1371/journal. ppat.1000715
Lowy, F. D., Fant, J., Higgins, L. L., Ogawa, S. K., and Hatcher, V. B. (1988). Staphylococcus aureus – human endothelial cell interactions. J. Ultrastruct. Mol. Struct. Res. 98, 137–146.
Mariathasan, S., Weiss, D. S., Newton, K., Mcbride, J., O’Rourke, K., Roose-Girma, M., Lee, W. P., Weinrauch, Y., Monack, D. M., and Dixit, V. M. (2006). Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440, 228–232.
Menzies, B. E., and Kourteva, I. (1998). Internalization of Staphylococcus aureus by endothelial cells induces apoptosis. Infect. Immun. 66, 5994–5998.
Menzies, B. E., and Kourteva, I. (2000). Staphylococcus aureus alpha-toxin induces apoptosis in endothelial cells. FEMS Immunol. Med. Microbiol. 29, 39–45.
Mestre, M. B., Fader, C. M., Sola, C., and Colombo, M. I. (2010). Alpha-hemolysin is required for the activation of the autophagic pathway in Staphylococcus aureus-infected cells. Autophagy 6, 110–125.
Mohammed, K. A., Nasreen, N., and Antony, V. B. (2007). Bacterial induction of early response genes and activation of proapoptotic factors in pleural mesothelial cells. Lung 185, 355–365.
Moisan, H., Brouillette, E., Jacob, C. L., Langlois-Begin, P., Michaud, S., and Malouin, F. (2006). Transcription of virulence factors in Staphylococcus aureus small-colony variants isolated from cystic fibrosis patients is influenced by SigB. J. Bacteriol. 188, 64–76.
Munoz-Planillo, R., Franchi, L., Miller, L. S., and Nunez, G. (2009). A critical role for hemolysins and bacterial lipoproteins in Staphylococcus aureus-induced activation of the Nlrp3 inflammasome. J. Immunol. 183, 3942–3948.
Nuzzo, I., Sanges, M. R., Folgore, A., and Carratelli, C. R. (2000). Apoptosis of human keratinocytes after bacterial invasion. FEMS Immunol. Med. Microbiol. 27, 235–240.
O’Brien, L. M., Walsh, E. J., Massey, R. C., Peacock, S. J., and Foster, T. J. (2002). Staphylococcus aureus clumping factor B (ClfB) promotes adherence to human type I cytokeratin 10: implications for nasal colonization. Cell. Microbiol. 4, 759–770.
Ogawa, M., Mimuro, H., Yoshikawa, Y., Ashida, H., and Sasakawa, C. (2011). Manipulation of autophagy by bacteria for their own benefit. Microbiol. Immunol. 55, 459–471.
Olivier, A. C., Lemaire, S., Van Bambeke, F., Tulkens, P. M., and Oldfield, E. (2009). Role of rsbU and staphyloxanthin in phagocytosis and intracellular growth of Staphylococcus aureus in human macrophages and endothelial cells. J. Infect. Dis. 200, 1367–1370.
Orvedahl,A., and Levine, B.(2009). Eating the enemy within: autophagy in infectious diseases. Cell Death Differ. 16, 57–69.
Otto, M., O’Mahoney, D. S., Guina, T., and Klebanoff, S. J. (2004). Activity of Staphylococcus epidermidis phenol-soluble modulin peptides expressed in Staphylococcus carnosus. J. Infect. Dis. 190, 748–755.
Oviedo-Boyso, J., Cortes-Vieyra, R., Huante-Mendoza, A., Yu, H. B., Valdez-Alarcon, J. J., Bravo-Patino, A., Cajero-Juarez, M., Finlay, B. B., and Baizabal-Aguirre, V. M. (2011). The phosphoinositide-3-kinase-Akt signaling pathway is important for Staphylococcus aureus internalization by endothelial cells. Infect. Immun. 79, 4569–4577.
Pang, Y. Y., Schwartz, J., Thoendel, M., Ackermann, L. W., Horswill, A. R., and Nauseef, W. M. (2010). agr-Dependent interactions of Staphylococcus aureus USA300 with human polymorphonuclear neutrophils. J. Innate Immun. 2, 546–559.
Peacock, S. J., Foster, T. J., Cameron, B. J., and Berendt, A. R. (1999). Bacterial fibronectin-binding proteins and endothelial cell surface fibronectin mediate adherence of Staphylococcus aureus to resting human endothelial cells. Microbiology 145(Pt. 12), 3477–3486.
Petrilli, V., Papin, S., Dostert, C., Mayor, A., Martinon, F., and Tschopp, J. (2007). Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 14, 1583– 1589.
Pokorny, A., Yandek, L. E., Elegbede, A. I., Hinderliter, A., and Almeida, P. F. (2006). Temperature and composition dependence of the interaction of delta-lysin with ternary mixtures of sphingomyelin/cholesterol/POPC. Biophys. J. 91, 2184–2197.
Proctor, R. A., Balwit, J. M., and Vesga, O. (1994). Variant subpopulations of Staphylococcus aureus as cause of persistent and recurrent infections. Infect. Agents Dis. 3, 302–312.
Proctor, R. A., Van Langevelde, P., Kristjansson, M., Maslow, J. N., and Arbeit, R. D. (1995). Persistent and relapsing infections associated with small-colony variants of Staphylococcus aureus. Clin. Infect. Dis. 20, 95–102.
Qazi, S. N. A., Counil, E., Morrissey, J., Rees, C. E. D., Cockayne, A., Winzer, K., Chan, W. C., Williams, P., and Hill, P. J. (2001). agr expression precedes escape of internalized Staphylococcus aureus from the host endosome. Infect. Immun. 69, 7074–7082.
Rahal, J. J. Jr. (1972). Comparative effects of purified staphylococcal alpha and delta toxins on mitochondrial metabolism. J. Infect. Dis. 126, 96–103.
Rudel, T., Kepp, O., and Kozjak-Pavlovic, V. (2010). Interactions between bacterial pathogens and mitochondrial cell death pathways. Nat. Rev. Microbiol. 8, 693–705.
Rudino-Pinera, E., Schwarz-Linek, U., Potts, J. R., and Garman, E. F. (2004). Twinned or not twinned, that is the question: crystallization and preliminary crystallographic analysis of the 2F1(3)F1 module pair of human fibronectin. Acta Crystallogr. D Biol. Crystallogr. 60, 1341–1345.
Schnaith, A., Kashkar, H., Leggio, S. A., Addicks, K., Krönke, M., and Krut, O. (2007). Staphylococcus aureus subvert autophagy for induction of caspase-independent host cell death. J. Biol. Chem. 282, 2695–2706.
Schnupf, P., and Portnoy, D. A. (2007). Listeriolysin O: a phagosome-specific lysin. Microbes Infect. 9, 1176–1187.
Schröder, A., Kland, R., Peschel, A., von Eiff, C., and Aepfelbacher, M. (2006a). Live cell imaging of phagosome maturation in Staphylococcus aureus infected human endothelial cells: small colony variants are able to survive in lysosomes. Med. Microbiol. Immunol. 195, 185–194.
Schröder, A., Schröder, B., Roppenser, B., Linder, S., Sinha, B., Fassler, R., and Aepfelbacher, M. (2006b). Staphylococcus aureus fibronectin binding protein-A induces motile attachment sites and complex actin remodeling in living endothelial cells. Mol. Biol. Cell 17, 5198–5210.
Schwartz, J., Leidal, K. G., Femling, J. K., Weiss, J. P., and Nauseef, W. M. (2009). Neutrophil bleaching of GFP-expressing staphylococci: probing the intraphagosomal fate of individual bacteria. J. Immunol. 183, 2632–2641.
Schwarz-Linek, U., Werner, J. M., Pickford, A. R., Gurusiddappa, S., Kim, J. H., Pilka, E. S., Briggs, J. A., Gough, T. S., Hook, M., Campbell, I. D., and Potts, J. R. (2003). Pathogenic bacteria attach to human fibronectin through a tandem beta-zipper. Nature 423, 177–181.
Selva, L., Viana, D., Regev-Yochay, G., Trzcinski, K., Corpa, J. M., Lasa, I., Novick, R. P., and Penades, J. R. (2009). Killing niche competitors by remote-control bacteriophage induction. Proc. Natl. Acad. Sci. U.S.A. 106, 1234–1238.
Sendi, P., and Proctor, R. A. (2009). Staphylococcus aureus as an intracellular pathogen: the role of small colony variants. Trends Microbiol. 17, 54–58.
Shompole, S., Henon, K. T., Liou, L. E., Dziewanowska, K., Bohach, G. A., and Bayles, K. W. (2003). Biphasic intracellular expression of Staphylococcus aureus virulence factors and evidence for Agr-mediated diffusion sensing. Mol. Microbiol. 49, 919–927.
Sinha, B., Francois, P., Que, Y. A., Hussain, M., Heilmann, C., Moreillon, P., Lew, D., Krause, K. H., Peters, G., and Herrmann, M. (2000). Heterologously expressed Staphylococcus aureus fibronectin-binding proteins are sufficient for invasion of host cells. Infect. Immun. 68, 6871–6878.
Sinha, B., François, P. P., Nüße, O., Foti, M., Hartford, O. M., Vaudaux, P., Foster, T. J., Lew, D. P., Herrmann, M., and Krause, K.-H. (1999). Fibronectin-binding protein acts as Staphylococcus aureus invasin via fibronectin bridging to integrin a5β1. Cell. Microbiol. 1, 101–117.
Sinha, B., and Fraunholz, M. (2010). Staphylococcus aureus host cell invasion and post-invasion events. Int. J. Med. Microbiol. 300, 170–175.
Strober, W., Murray, P. J., Kitani, A., and Watanabe, T. (2006). Signalling pathways and molecular interactions of NOD1 and NOD2. Nat. Rev. Immunol. 6, 9–20.
Taylor, R. C., Cullen, S. P., and Martin, S. J. (2008). Apoptosis: controlled demolition at the cellular level. Nat. Rev. Mol. Cell Biol. 9, 231–241.
Ting, J. P., Lovering, R. C., Alnemri, E. S., Bertin, J., Boss, J. M., Davis, B. K., Flavell, R. A., Girardin, S. E., Godzik, A., Harton, J. A., Hoffman, H. M., Hugot, J. P., Inohara, N., Mackenzie, A., Maltais, L. J., Nunez, G., Ogura, Y., Otten, L. A., Philpott, D., Reed, J. C., Reith, W., Schreiber, S., Steimle, V., and Ward, P. A. (2008a). The NLR gene family: a standard nomenclature. Immunity 28, 285–287.
Ting, J. P., Willingham, S. B., and Bergstralh, D. T. (2008b). NLRs at the intersection of cell death and immunity. Nat. Rev. Immunol. 8, 372–379.
Tuchscherr, L., Medina, E., Hussain, M., Volker, W., Heitmann, V., Niemann, S., Holzinger, D., Roth, J., Proctor, R. A., Becker, K., Peters, G., and Loffler, B. (2011). Staphylococcus aureus phenotype switching: an effective bacterial strategy to escape host immune response and establish a chronic infection. EMBO Mol. Med. 3, 129–141.
Tucker, K. A., Reilly, S. S., Leslie, C. S., and Hudson, M. C. (2000). Intracellular Staphylococcus aureus induces apoptosis in mouse osteoblasts. FEMS Microbiol. Lett. 186, 151–156.
Vann, J. M., and Proctor, R. A. (1988). Cytotoxic effects of ingested Staphylococcus aureus on bovine endothelial cells: role of S. aureus α-hemolysin. Microb. Pathog. 4, 443–453.
van Opijnen, T., Bodi, K. L., and Camilli, A. (2009). Tn-seq: high-throughput parallel sequencing for fitness and genetic interaction studies in microorganisms. Nat. Methods 6, 767–772.
Verdon, J., Girardin, N., Lacombe, C., Berjeaud, J. M., and Hechard, Y. (2009). delta-hemolysin, an update on a membrane-interacting peptide. Peptides 30, 817–823.
von Eiff, C., Peters, G., and Proctor, R. A. (2001). “Small colony variants of Staphylococcus aureus: mechanisms for production, biology of infection, and clinical significance,” in Staphylococcus aureus Infection and Disease, eds A. L. Honeyman, H. Friedman, and M. Bendinelli (New York, NY: Kluwer Academic/Plenum), 17–33.
Wadstrom, T., and Mollby, R. (1972). Some biological properties of purified staphylococcal haemolysins. Tox -icon 10, 511–519.
Walev, I., Martin, E., Jonas, D., Mohamadzadeh, M., Muller-Klieser, W., Kunz, L., and Bhakdi, S. (1993). Staphylococcal alpha-toxin kills human keratinocytes by permeabilizing the plasma membrane for monovalent ions. Infect. Immun. 61, 4972–4979.
Wang, R., Braughton, K. R., Kretschmer, D., Bach, T. H., Queck, S. Y., Li, M., Kennedy, A. D., Dorward, D. W., Klebanoff, S. J., Peschel, A., Deleo, F. R., and Otto, M. (2007). Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat. Med. 13, 1510–1514.
Weidenmaier, C., Kokai-Kun, J. F., Kristian, S. A., Chanturiya, T., Kalbacher, H., Gross, M., Nicholson, G., Neumeister, B., Mond, J. J., and Peschel, A. (2004). Role of teichoic acids in Staphylococcus aureus nasal colonization, a major risk factor in nosocomial infections. Nat. Med. 10, 243–245.
Weidenmaier, C., Kokai-Kun, J. F., Kulauzovic, E., Kohler, T., Thumm, G., Stoll, H., Gotz, F., and Peschel, A. (2008). Differential roles of sortase-anchored surface proteins and wall teichoic acid in Staphylococcus aureus nasal colonization. Int. J. Med. Microbiol. 298, 505–513.
Weidenmaier, C., Peschel, A., Xiong, Y. Q., Kristian, S. A., Dietz, K., Yeaman, M. R., and Bayer, A. S. (2005). Lack of wall teichoic acids in Staphylococcus aureus leads to reduced interactions with endothelial cells and to attenuated virulence in a rabbit model of endocarditis. J. Infect. Dis. 191, 1771–1777.
Wertheim, H. F., Walsh, E., Choudhurry, R., Melles, D. C., Boelens, H. A., Miajlovic, H., Verbrugh, H. A., Foster, T., and Van Belkum, A. (2008). Key role for clumping factor B in Staphylococcus aureus nasal colonization of humans. PLoS Med. 5, e17. doi: 10.1371/journal.pmed. 0050017
Wesson, C. A., Deringer, J., Liou, L. E., Bayles, K. W., Bohach, G. A., and Trumble, W. R. (2000). Apoptosis induced by Staphylococcus aureus in epithelial cells utilizes a mechanism involving caspases 8 and 3. Infect. Immun. 68, 2998–3001.
Wesson, C. A., Liou, L. E., Todd, K. M., Bohach, G. A., Trumble, W. R., and Bayles, K. W. (1998). Staphylococcus aureus Agr and Sar global regulators influence internalization and induction of apoptosis. Infect. Immun. 66, 5238–5243.
Wilke, G. A., and Bubeck Wardenburg, J. (2010). Role of a disintegrin and metalloprotease 10 in Staphylococcus aureus alpha-hemolysin-mediated cellular injury. Proc. Natl. Acad. Sci. U.S.A. 107, 13473–13478.
Willingham, S. B., and Ting, J. P. (2008). NLRs and the dangers of pollution and aging. Nat. Immunol. 9, 831–833.
Keywords: Staphylococcus aureus, phagocytosis, phagosomal escape, autophagy, host cell death, bacterial persistence
Citation: Fraunholz M and Sinha B (2012) Intracellular Staphylococcus aureus: live-in and let die. Front. Cell. Inf. Microbio. 2:43. doi: 10.3389/fcimb.2012.00043
Received: 20 January 2012; Paper pending published: 04 February 2012;
Accepted: 15 March 2012; Published online: 24 April 2012.
Edited by:
Martin John McGavin, University of Western Ontario, CanadaReviewed by:
Ross Fitzgerald, Roslin Institute, UKSung Ouk Kim, University of Western Ontario, Canada
Copyright: © 2012 Fraunholz and Sinha. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Martin Fraunholz, Department of Microbiology, Biocenter, University of Würzburg, Am Hubland, D-97074 Würzburg, Germany. e-mail:bWFydGluLmZyYXVuaG9sekB1bmktd3VlcnpidXJnLmRl

