- 1Department of Medical Engineering, Daping Hospital, Army Medical University, Chongqing, China
- 2State Key Laboratory of Ultrasound in Medicine and Engineering, College of Biomedical Engineering, Chongqing Medical University, Chongqing, China
- 3Chongqing Key Laboratory of Biomedical Engineering, College of Biomedical Engineering, Chongqing Medical University, Chongqing, China
Fluorescent nanomaterials (NMs) are widely used in imaging techniques in biomedical research. Especially in bioimaging systems, with the rapid development of imaging nanotechnology, precious metal clusters such as Au, Ag, and Cu NMs have emerged with different functional agents for biomedical applications. Compared with traditional fluorescent molecules, precious metal clusters have the advantages of high optical stability, easy regulation of shape and size, and multifunctionalization. In addition, NMs possess strong photoluminescent properties with good photostability, high release rate, and sub-nanometer size. They could be treated as fundamental agents in bioimaging usability. This review summarizes the recent advances in bioimaging utilization, it conveys that metal clusters refer to Au, Ag, and Cu fluorescent clusters and could provide a generalized overview of their full applications. It includes optical property measurement, precious metal clusters in bioimaging systems, and a rare earth element-doped heterogeneous structure illustrated in biomedical imaging with specific examples, that provide new and innovative ideas for fluorescent NMs in the field of bioimaging usability.
Introduction
Bioimaging technology is an important research tool to view biological functions in real-time and to elucidate various physiological functions of organisms (Karlas et al., 2021; Mazumder et al., 2022; Ju et al., 2023), this approach has minimal interference with life processes and allows the direct acquisition of microstructural images by using optical or electron microscopy (Yang et al., 2022; Sobhanan et al., 2023). The analysis of the resulting images is used to understand different physiological processes in biological cells. In addition, bioimaging is widely used to acquire data on the three-dimensional structure of an observed object without physical interaction (Klymchenko et al., 2021). Biological imaging covers a wide range of modalities, including X-ray, ultrasound, computed tomography (CT), positron emission computed tomography (PET), magnetic resonance imaging (MRI), etc (Wen et al., 2020). Among them, fluorescence imaging technology plays an important role in whole imaging medical works. Its advantages are divided into such categories as high sensitivity, easy observation, and simple instrumentation, which mainly utilize the change of fluorescence characteristics to obtain optical images (Collot, 2021). Also, it is necessary to introduce exogenous fluorescent materials as a contrast agent to calibrate specific cells, tissues, and organs (An et al., 2020; Zhang et al., 2020; Wang et al., 2021). Due to the lack of endogenous fluorescent materials in many biological structures and processes, it is difficult to utilize the intrinsic fluorescence of biological samples for imaging characterizations. Therefore, the potential of fluorescent agents is recognized as a new type of typical functional material, that could be utilized in this area. For example, in the field of bioimaging, they have the advantages of excellent fluorescence performance, tunability, multifunctionality, and high photostability (Cai et al., 2021; Guo et al., 2021). Fluorescene imaging technology can provide comprehensive detection methods from cells, isolated tissues, and living biological samples in terms of structural and dynamic information (Drozdov et al., 2022; Song et al., 2022) in the interdisciplinary fields of materials, optics, and biomedicines. Among them, noble metal nanoclusters, as a new type of fluorescent agent recently developed, have a broad development prospect in the field of bioimaging due to their unique optical properties, biocompatibility, high contrast, tunability, and targeting. In this review, we mainly summarize and outline the properties of precious metal clusters as fluorescent NMs in the context of recent trends and their application in bioimaging usability. Here, there are three different parts fully illustrated: 1) pure-phased metal clusters as fluorescent agents in optical measurements; 2) copper (Cu), silver (Ag), and gold (Au)- based clusters were utilized in bioimaging usability; 3) rare earth element-doped fluorescent materials in imaging applications. Based on the applied research on the functional materials of noble metal clusters, they will gradually come to be widely used in imaging analysis and therapies. Hence, based on the application research of precious metal clusters’ functional materials, it will graduallycome to have extensive application in imaging analysis and treatments.
Precious metal clusters as fluorescent agents in optical measurement
Fluorescent NMs are of interest for their multifunctional applications in solar cells, biomarkers, imaging, etc. Among them, metallic NMs exhibit strong fluorescence emission and offer great potential for the development of biomarkers and imaging. In particular, precious metal nanoparticles (NPs), which are represented by gold (Au), silver (Ag), and copper (Cu), with unique surface plasmon resonance (SPR) in the range of light from the ultraviolet (UV) to the near-infrared (NIR) (Chakraborty and Pradeep, 2017), and distinctive optical properties such as molecular absorption and strong luminescence, have attracted much attention (Babu Busi et al., 2022). Metal nanoclusters have improved luminescence efficiency and enhanced biocompatibility compared to metal nanoparticles and can escape the renal barrier. Among them, the fluorescent metal clusters exhibit tunable fluorescence from the visible to the near-infrared region. This tunable fluorescence occurs either through molecular-like electronic leaps within the conduction band or due to charge transfer leaps from the ligand to the metal nanoparticles. It was considered a class of fluorescent clusters with novel properties such as ultra-small morphologies, water-solubility, biocompatibility, and so on. By virtue of their usefulness, these precious metal clusters can complement or even replace traditional fluorescent probes in sensor fabrication. This can provide great opportunities for the advancement of imaging technology (Wang et al., 2018), represented by Au nanocluster (NCs), Ag NCs, and Cu NCs. Au NCs have recently been used for bioimaging and other biomedical applications due to their favorable intrinsic optical properties, highly stable chemical properties, and good biocompatibility (Chen et al., 2015). For example, Liu et al. prepared atom-precise Au NCs with 25 gold atoms and 18 peptide ligands, such that the cluster can be used as a NIR-II fluorophore (Liu et al., 2019). Especially in brain imaging, this study found that NIR-II imaging based on Au NCs is able to monitor many small blood vessels and can be used as NIR-II dyes for imaging. The preparation of cluster structures can be realized by using organic ligands in order to optimize their performance with better biological properties. In a recent research report, Obstarczyk et al. prepared ultrasmall Au NCs using 12-crown-4 ligand capped (Obstarczyk et al., 2023). Such nanoclusters were amphiphilic and could be successfully transferred between aqueous and organic solvents while maintaining their physicochemical integrity. They can be used as probes for light (because they emit near-infrared fluorescence) and electron microscopy (because of the high electron density of Au) in multimodal biological imaging (Figure 1A). Wang et al. used in situ self-assembly to biosynthesize fluorescent Au nanocluster-DNA (Au-DNA) complexes for precise bioimaging and safe, targeted cancer therapy (Wang et al., 2019).
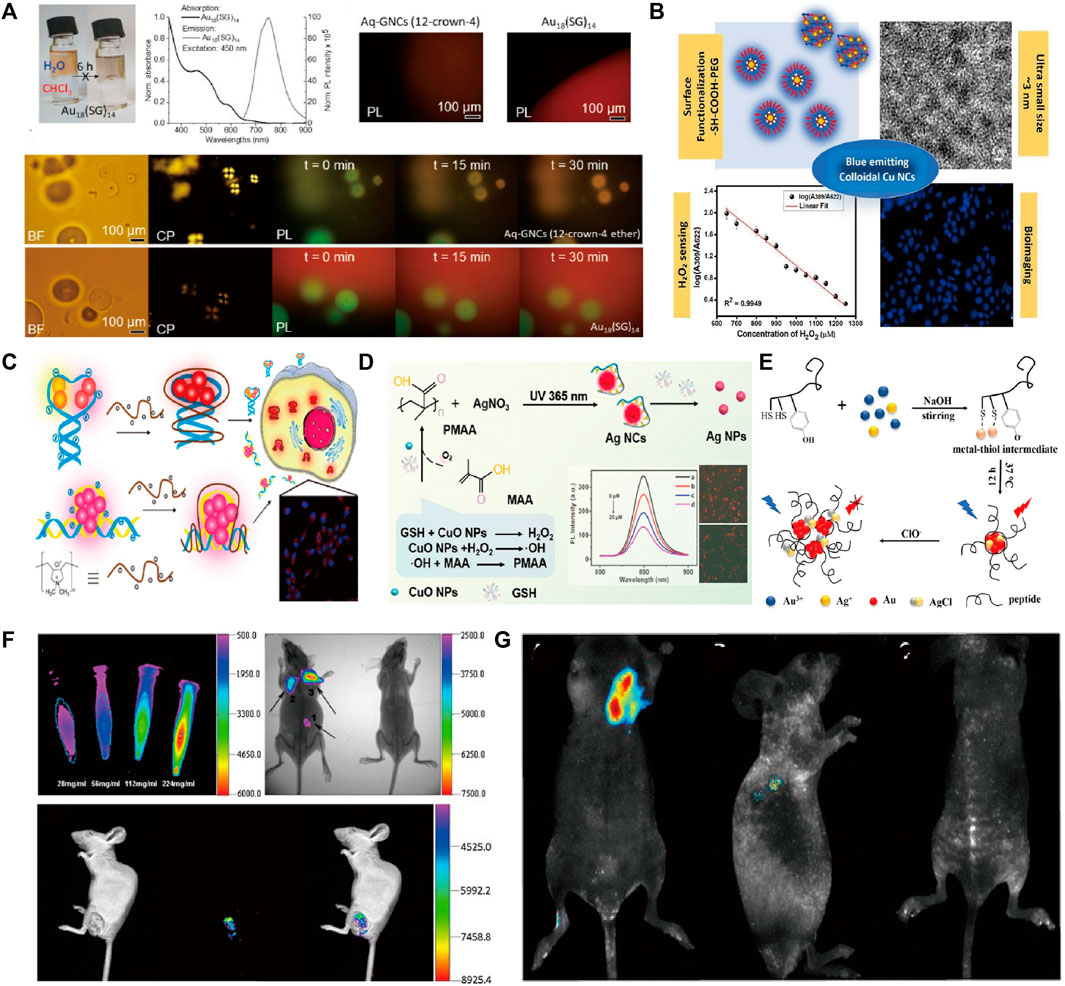
FIGURE 1. (A) Absorption and emission spectra and fluorescence microscopy images of Au18GNC in water; (B) PEGylated Cu nanoclusters: A nontoxic, multifunctional colloidal system for bioimaging and peroxide sensing; (C) The formation of FL DNA-Ag NC/cationic polyelectrolyte complexes for cell imaging; (D) Schem of self-Cascade Nanoenzyme of Cupric Oxide Nanoparticles (CuO NPs) Induced in Situ Catalysis Formation of Polyelectrolyte as Template for the Synthesis of Near-Infrared Fluorescent Silver Nanoclusters; (E) Schematic of synthesis of peptide@Ag/Au NCs and reaction mechanism of peptide@Ag/Au NCs with ClO−; (F) AuNCs@SiO2-FA nanoprobes for fluorescence imaging; (G) Representative xenograft tumor nude mice models of Cervical carcinoma in vivo imaging. Reproduced from Obstarczyk et al. (2023) with permission of ACS Omega. Reproduced from Chandran et al. (2023) with permission of Biochim Biophys Acta Gen Subj. Reproduced from Lyu et al. (2019) with permission of Anal Chem. Reproduced from Xu et al. (2022) with permission of Analytical Chemistry. Reproduced from Jia et al. (2020) with permission of Talanta. Reproduced from Zhou et al. (2013) with permission of J Nanobiotechnology. Reproduced from Ge et al. (2015) with permission of J Nanobiotechnology.
Copper (Cu), silver (Ag), and gold (Au) based clusters utilized in bioimaging systems
The work on photoluminescence characterization by utilizing Cu clusters in biomedical imaging works. Cu materials were thought to be one functional substrate material due to their abundance and relatively low cost (Lai et al., 2020; Akhuli et al., 2021; Saraf et al., 2021) in nature. In addition, as one typical application of biosensors, it was modified by different surfactants to form functional agents. As in bioimaging works, Chandran et al. (2023) developed blue-emitting colloids, Cu NCs, using different functional groups (-SH and -COOH). Some characteristics (size, cytotoxicity, and emission properties of Cu NCs) were controlled due to surface functionalization. It protected the particle surface from aggregation and oxidation, mainly through the binding of polymer molecules with thiol and carboxyl groups. The bright blue fluorescence emitted by HeLa cells treated with acetic acid (sample code: CAGP) (Figure 1B) showed excellent bioimaging properties. The functional group in molecules was used as a simple and cost-effective method to synthesize glutathione-coated copper nanoclusters (Cu-GSH NCs) with strong, bright red fluorescence (625 nm) (Chandran et al., 2020). It was used as an effective pH-based bio-imaging probe for the detection of cancerous cells and had the potential to be used for label-free subcellular organelles for tracking and labeling. Also, Ag clusters received tremendous attention for biomedical applications due to their low toxicity, size-dependent, and emission properties (Liu et al., 2017). Despite the relatively low photo-oxidative stability of Ag, it has been shown that very strong fluorescence signals can also be generated by using different ligands in the formatting process (Tao et al., 2015; Song et al., 2016). In a recent study, Lyu et al. (2019) utilized cationic polyelectrolytes to modify fluorescent DNA-Ag NCs through electrostatic interactions between the positive polymer backbone and the negatively charged phosphate groups of the DNA strand. The experimental results showed a 3-fold enhancement of fluorescence emission from Ag NCs for rapid cellular imaging, increased stability in the internal environment, and enhanced cellular uptake of DNA-Ag NCs (Figure 1C). Xu et al. (2022) prepared Ag clusters with near-red fluorescence by using polymethacrylates (PMAA) as a template. Which was used as a glutathione (GSH) sensing and bioimaging probe (Figure 1D). Yu et al. (2021) stabilized the Ag cluster (BSA-Ag) by using bovine serum albumin (BSA) with near-infrared electrochemiluminescence (ECL) properties. It exhibited a strong anodic ECL spectral peak at 904 nm in aqueous media with excellent bioimaging properties. As one acceptable approach, the integration procedure should be one effective path to construct heterogeneous composites because of their superiority over single metals in terms of electronic, optical, and catalytic properties (Tian et al., 2017; Zhai et al., 2017). Jia et al. (2020) developed peptide-capped Au-Ag clusters for lysosome-targeted imaging of hypochlorite with high fluorescence quantum yield (Figure 1E). Zhou et al. (2013) utilized folic acid (FA)-coupled silica-coated Au clusters in forming AuNCs@SiO2-FA probes with biocompatible properties and applied them to fluorescence imaging in mice (Figure 1F). Ge et al. (2015) explored a new strategy for the synthesis of fluorescent Au-Ce NCs by doping trivalent cerium into the crystal seed growth process of Au clusters. Its fluorescent property can be used to achieve highly sensitive in vitro or in vivo bioimaging of tumor targets (Figure 1G). In addition, Yang et al. (2020) developed a therapeutic nanomedicine (AuNCs-Pt) based on nanocarrier gold nanoclusters (AuNCs), which has the dual function of NIR-I/NIR-II imaging and glutathione scavenging ability. The long emission wavelength of AuNCs-Pt allows deep penetration, enabling high-resolution NIR-II tumor imaging and improved visualization of platinum transport in deep tissues. Moreover, alloy structures such as Ag-Pt (Bootharaju et al., 2017) and Au-Cu (Shellaiah et al., 2019; Shan et al., 2022) have a wide range of applications in the field of bio-imaging, where they are characterized by good light stabilization, strong NIR-II absorption, and better imaging depths. In summary precious metal clusters with good biocompatibility, stable optical properties, and high luminescence efficiency have a wide range of development prospects in the field of bioimaging.
Rare earth element-doped fluorescent materials in imaging systems
Rare earth-doped nanoparticles (RENPs) have become the preferred candidate for NIR-II imaging due to their attractive features such as a narrow emission spectrum, long fluorescence lifetime, and absence of photobleaching (Qu et al., 2020; Gu et al., 2022; Song et al., 2022). Zhang et al. (2021) prepared two RENPs, NaYF4:Yb20Er2@NaYF4 and NaYF4:Nd5@NaYF4, and modified them with poly (ethylene glycol) (PEG) to explore simultaneous imaging in NIR-IIb (1,530 nm, under 980 nm laser excitation) and NIR-IIb. It showed that RENP’s NIR-II fluorescence has a highly synergistic imaging capability in versatile biomedical applications with higher temporal and spatial resolution, respectively. Tan et al. (2018) reported a synthesis of long-lived rare-earth-doped fluoride nanoparticles using a different strategy: core/shell and dopant engineering, which showed intense infrared emission in a second biological window with a luminescence lifetime of close to 1 m. Karthickraja et al. (2021) synthesized calcium fluoride (CaF2) nanoparticles by doping them with optimal concentrations of Nd3+ and Yb3+ as sensitizers and activators. It performed ex vivo fluorescence imaging experiments on chicken breast tissues of different thicknesses with a maximum theoretical depth of penetration of 14 mm for near-infrared light. There is a process of surface modification in doped materials, lanthanide-doped materials can be carried out to realize luminescence through energy level jumps with high luminescence efficiency (Li et al., 2022). Alternatively, the loading of fluorescent molecules into organic nanomaterial structures via covalent bonding or electrostatic/hydrophobic interactions to generate fluorescent organic nanoparticles (FON) can also enable efficient bioimaging. FON is another classical material with bioimaging properties that is mainly composed of natural or synthetic organic polymers. The related advantages involved luminescence, biocompatibility, and a high signal-to-noise ratio (Asahi et al., 2008; Sinha et al., 2022). In imaging usability, FONs generally showed stable photo-induced characterizations (Blasi et al., 2017), but they also conveyed that small molecules contributed to higher fluorophore density and spatial resolution in whole fabrications (Vargas-Nadal et al., 2022).
Conclusion and outlooks
This mini-review, mainly describes the recent advancements in fluorescent precious metal clusters, which are used in biomedical imaging applications such as fluorescence imaging and near-infrared region applications. Based on recent research, precious metal clusters could be considered an acceptable unit for fabricating multifunctional constructions. But also, its particular morphology is a fundamental agent in further designing optical characterization modifications. Using precious metal nanoclusters as the basic building blocks can improve the photostability and durability of bio-imaging and achieve better imaging results. In addition, referring to the heterogeneous structure, precious metals can dope with or be doped with different materials for fabricating imaging substitutes. Meanwhile, their optical properties and biocompatibility are mainly represented by precious metal clusters and hetero composites. Doped with rare-earth elements, they can be used as highly sensitive and selective molecular probes for precise imaging at the cellular and tissue levels, providing more accurate diagnostic and therapeutic tools for clinical medicine. By recognizing the fundamental property, these different precious metal clusters played an important role in the early diagnosis of diseases, individualized therapy, and life-accessible research for further challenges.
Author contributions
XHe: Formal Analysis, Investigation, Writing–original draft. SL: Data curation, Formal Analysis, Writing–review and editing. XHu: Investigation, Project administration, Resources, Writing–review and editing. XHa: Data curation, Formal Analysis, Writing–original draft. HZ: Formal Analysis, Methodology, Writing–original draft. XM: Conceptualization, Funding acquisition, Supervision, Writing–review and editing, Project administration, Resources.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Chongqing Municipal Natural Science Foundation: CSTB2022NSCQ-MSX0113; Scientific Foundation Project of Chongqing Education Commission: KJQN201900436.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Akhuli, A., Chakraborty, D., Agrawal, A. K., and Sarkar, M. (2021). Probing the interaction of bovine serum albumin with copper nanoclusters: realization of binding pathway different from protein corona. Langmuir 37, 1823–1837. doi:10.1021/acs.langmuir.0c03176
An, Y., Ren, Y., Bick, M., Dudek, A., Hong-Wang, W. E., Tang, J., et al. (2020). Highly fluorescent copper nanoclusters for sensing and bioimaging. Biosens. Bioelectron. 154, 112078. doi:10.1016/j.bios.2020.112078
Asahi, T., Sugiyama, T., and Masuhara, H. (2008). Laser fabrication and spectroscopy of organic nanoparticles. Acc. Chem. Res. 41, 1790–1798. doi:10.1021/ar800125s
Babu Busi, K., Palanivel, M., Kanta Ghosh, K., Basu Ball, W., Gulyás, B., Padmanabhan, P., et al. (2022). The multifarious applications of copper nanoclusters in biosensing and bioimaging and their translational role in early disease detection. Nanomaterials 12, 301. doi:10.3390/nano12030301
Blasi, D., Nikolaidou, D. M., Terenziani, F., Ratera, I., and Veciana, J. (2017). Excimers from stable and persistent supramolecular radical-pairs in red/NIR-emitting organic nanoparticles and polymeric films. Phys. Chem. Chem. Phys. 19, 9313–9319. doi:10.1039/c7cp00623c
Bootharaju, M. S., Kozlov, S. M., Cao, Z., Harb, M., Maity, N., Shkurenko, A., et al. (2017). Doping-induced anisotropic self-assembly of silver icosahedra in [Pt2Ag23Cl7(PPh3)10] nanoclusters. J. Am. Chem. Soc. 139, 1053–1056. doi:10.1021/jacs.6b11875
Cai, H., Zhu, Y., Xu, H., Chu, H., Zhang, D., and Li, J. (2021). Fabrication of fluorescent hybrid nanomaterials based on carbon dots and its applications for improving the selective detection of Fe (III) in different matrices and cellular imaging. Spectrochimica Acta. Part A, Mol. Biomol. Spectrosc. 246, 119033. doi:10.1016/j.saa.2020.119033
Chakraborty, I., and Pradeep, T. (2017). Atomically precise clusters of noble metals: emerging link between atoms and nanoparticles. Chem. Rev. 117, 8208–8271. doi:10.1021/acs.chemrev.6b00769
Chandran, N., Janardhanan, P., Bayal, M., Pilankatta, R., and Nair, S. S. (2023). Development of PEGylated Cu nanoclusters: a nontoxic, multifunctional colloidal system for bioimaging and peroxide sensing. Biochim. Biophys. Acta Gen. Subj. 1867, 130372. doi:10.1016/j.bbagen.2023.130372
Chandran, N., Janardhanan, P., Bayal, M., Unniyampurath, U., Pilankatta, R., and Nair, S. S. (2020). Label free, nontoxic Cu-GSH NCs as a nanoplatform for cancer cell imaging and subcellular pH monitoring modulated by a specific inhibitor: bafilomycin A1. ACS Appl. Bio Mater 3, 1245–1257. doi:10.1021/acsabm.9b01036
Chen, L.-Y., Wang, C.-W., Yuan, Z., and Chang, H.-T. (2015). Fluorescent gold nanoclusters: recent advances in sensing and imaging. Anal. Chem. 87, 216–229. doi:10.1021/ac503636j
Collot, M. (2021). Recent advances in dioxaborine-based fluorescent materials for bioimaging applications. Mater Horiz. 8, 501–514. doi:10.1039/d0mh01186j
Drozdov, A. S., Komarova, K. S., Mochalova, E. N., Komedchikova, E. N., Shipunova, V. O., and Nikitin, M. P. (2022). Fluorescent magnetic nanoparticles for bioimaging through biomimetic surface modification. Int. J. Mol. Sci. 24, 134. doi:10.3390/ijms24010134
Ge, W., Zhang, Y., Ye, J., Chen, D., Rehman, F. U., Li, Q., et al. (2015). Facile synthesis of fluorescent Au/Ce nanoclusters for high-sensitive bioimaging. J. Nanobiotechnology 13, 8. doi:10.1186/s12951-015-0071-y
Gu, M., Li, W., Jiang, L., and Li, X. (2022). Recent progress of rare earth doped hydroxyapatite nanoparticles: luminescence properties, synthesis and biomedical applications. Acta Biomater. 148, 22–43. doi:10.1016/j.actbio.2022.06.006
Guo, Y., Amunyela, H. T. N. N., Cheng, Y., Xie, Y., Yu, H., Yao, W., et al. (2021). Natural protein-templated fluorescent gold nanoclusters: syntheses and applications. Food Chem. 335, 127657. doi:10.1016/j.foodchem.2020.127657
Jia, M., Mi, W., Guo, S., Yang, Q.-Z., Jin, Y., and Shao, N. (2020). Peptide-capped functionalized Ag/Au bimetal nanoclusters with enhanced red fluorescence for lysosome-targeted imaging of hypochlorite in living cells. Talanta 216, 120926. doi:10.1016/j.talanta.2020.120926
Ju, J., Xu, D., Mo, X., Miao, J., Xu, L., Ge, G., et al. (2023). Multifunctional polysaccharide nanoprobes for biological imaging. Carbohydr. Polym. 317, 121048. doi:10.1016/j.carbpol.2023.121048
Karlas, A., Pleitez, M. A., Aguirre, J., and Ntziachristos, V. (2021). Optoacoustic imaging in endocrinology and metabolism. Nat. Rev. Endocrinol. 17, 323–335. doi:10.1038/s41574-021-00482-5
Karthickraja, D., Kumar, G. A., Sardar, D. K., Karthi, S., Dannangoda, G. C., Martirosyan, K. S., et al. (2021). Fabrication of Nd3+ and Yb3+ doped NIR emitting nano fluorescent probe: a candidate for bioimaging applications. Mater. Sci. Eng. C, Mater. Biol. Appl. 125, 112095. doi:10.1016/j.msec.2021.112095
Klymchenko, A. S., Liu, F., Collot, M., and Anton, N. (2021). Dye-loaded nanoemulsions: biomimetic fluorescent nanocarriers for bioimaging and nanomedicine. Adv. Healthc. Mater. 10, 2001289. doi:10.1002/adhm.202001289
Lai, W.-F., Wong, W.-T., and Rogach, A. L. (2020). Development of copper nanoclusters for in vitro and in vivo theranostic applications. Adv. Mater. 32, 1906872. doi:10.1002/adma.201906872
Li, Y., Chen, C., Liu, F., and Liu, J. (2022). Engineered lanthanide-doped upconversion nanoparticles for biosensing and bioimaging application. Mikrochim. Acta 189, 109. doi:10.1007/s00604-022-05180-1
Liu, H., Hong, G., Luo, Z., Chen, J., Chang, J., Gong, M., et al. (2019). Atomic-precision gold clusters for NIR-II imaging. Adv. Mater. 31, 1901015. doi:10.1002/adma.201901015
Liu, X.-L., Niu, L.-Y., Chen, Y.-Z., Yang, Y., and Yang, Q.-Z. (2017). A multi-emissive fluorescent probe for the discrimination of glutathione and cysteine. Biosens. Bioelectron. 90, 403–409. doi:10.1016/j.bios.2016.06.076
Lyu, D., Li, J., Wang, X., Guo, W., and Wang, E. (2019). Cationic-polyelectrolyte-modified fluorescent DNA-silver nanoclusters with enhanced emission and higher stability for rapid bioimaging. Anal. Chem. 91, 2050–2057. doi:10.1021/acs.analchem.8b04493
Mazumder, A., Mozammal, M., and Talukder, M. A. (2022). Three-dimensional imaging of biological cells using surface plasmon coupled emission. J. Biomed. Opt. 27, 106002–106005. doi:10.1117/1.JBO.27.10.106002
Obstarczyk, P., Pniakowska, A., Nonappa, G. M. P., Olesiak-Bańska, J., and Olesiak-Bańska, J. (2023). Crown ether-capped gold nanoclusters as a multimodal platform for bioimaging. ACS Omega 8, 11503–11511. doi:10.1021/acsomega.3c00426
Qu, Z., Shen, J., Li, Q., Xu, F., Wang, F., Zhang, X., et al. (2020). Near-IR emissive rare-earth nanoparticles for guided surgery. Theranostics 10, 2631–2644. doi:10.7150/thno.40808
Saraf, M., Tavakkoli Yaraki, M., Prateek, T. Y. N., and Gupta, R. K. (2021). Insights and perspectives regarding nanostructured fluorescent materials toward tackling COVID-19 and future pandemics. ACS Appl. Nano Mater 4, 911–948. doi:10.1021/acsanm.0c02945
Shan, B., Liu, H., Li, L., Lu, Y., and Li, M. (2022). Near-infrared II plasmonic phototheranostics with glutathione depletion for multimodal imaging-guided hypoxia-tolerant chemodynamic-photocatalytic-photothermal cancer therapy triggered by a single laser. Small 18, 2105638. doi:10.1002/smll.202105638
Shellaiah, M., Simon, T., Thirumalaivasan, N., Sun, K. W., Ko, F.-H., and Wu, S.-P. (2019). Cysteamine-capped gold-copper nanoclusters for fluorometric determination and imaging of chromium(VI) and dopamine. Mikrochim. Acta 186, 788. doi:10.1007/s00604-019-3974-8
Sinha, S., Kelemen, Z., Hümpfner, E., Ratera, I., Malval, J.-P., Jurado, J. P., et al. (2022). o-Carborane-based fluorophores as efficient luminescent systems both as solids and as water-dispersible nanoparticles. Chem. Commun. 58, 4016–4019. doi:10.1039/d1cc07211k
Sobhanan, J., Anas, A., and Biju, V. (2023). Nanomaterials for fluorescence and multimodal bioimaging. Chem. Rec. 23, 202200253. doi:10.1002/tcr.202200253
Song, X.-R., Goswami, N., Yang, H.-H., and Xie, J. (2016). Functionalization of metal nanoclusters for biomedical applications. Analyst 141, 3126–3140. doi:10.1039/c6an00773b
Song, Y., Zhu, Y., Jiang, K., Liu, X., Dong, L., Li, D., et al. (2022). Self-assembling ferrimagnetic fluorescent micelles for bioimaging guided efficient magnetic hyperthermia therapy. Nanoscale 15, 365–375. doi:10.1039/d2nr02059a
Tan, M., Del Rosal, B., Zhang, Y., Martín Rodríguez, E., Hu, J., Zhou, Z., et al. (2018). Rare-earth-doped fluoride nanoparticles with engineered long luminescence lifetime for time-gated in vivo optical imaging in the second biological window. Nanoscale 10, 17771–17780. doi:10.1039/c8nr02382d
Tao, Y., Li, M., Ren, J., and Qu, X. (2015). Metal nanoclusters: novel probes for diagnostic and therapeutic applications. Chem. Soc. Rev. 44, 8636–8663. doi:10.1039/c5cs00607d
Tian, L., Li, Y., Ren, T., Tong, Y., Yang, B., and Li, Y. (2017). Novel bimetallic gold-silver nanoclusters with "Synergy"-enhanced fluorescence for cyanide sensing, cell imaging and temperature sensing. Talanta 170, 530–539. doi:10.1016/j.talanta.2017.03.107
Vargas-Nadal, G., Köber, M., Nsamela, A., Terenziani, F., Sissa, C., Pescina, S., et al. (2022). Fluorescent multifunctional organic nanoparticles for drug delivery and bioimaging: a tutorial review. Pharmaceutics 14, 2498. doi:10.3390/pharmaceutics14112498
Wang, G., Qian, K., and Mei, X. (2018). A theranostic nanoplatform: magneto-gold@fluorescence polymer nanoparticles for tumor targeting T1&T2-MRI/CT/NIR fluorescence imaging and induction of genuine autophagy mediated chemotherapy. Nanoscale 10, 10467–10478. doi:10.1039/c8nr02429d
Wang, M., Chen, Y., Cai, W., Feng, H., Du, T., Liu, W., et al. (2019). In situ self-assembling Au-DNA complexes for targeted cancer bioimaging and inhibition. Proc. Natl. Acad. Sci. U. S. A. 117, 308–316. doi:10.1073/pnas.1915512116
Wang, Y., Xia, K., Wang, L., Wu, M., Sang, X., Wan, K., et al. (2021). Peptide-Engineered fluorescent nanomaterials: structure design, function tailoring, and biomedical applications. Small 17, 2005578. doi:10.1002/smll.202005578
Wen, L., Wen, C., Zhang, F., Wang, K., Yuan, H., and Hu, F. (2020). siRNA and chemotherapeutic molecules entrapped into a redox-responsive platform for targeted synergistic combination therapy of glioma. Nanomedicine 28, 102218. doi:10.1016/j.nano.2020.102218
Xu, Y., Yan, J., Zhu, Y., Chen, H., Wu, C., Zhu, X., et al. (2022). Self-cascade Nanoenzyme of cupric Oxide nanoparticles (CuO NPs) induced in situ Catalysis Formation of polyelectrolyte as template for the synthesis of near-infrared fluorescent silver nanoclusters and the application in glutathione detection and bioimaging. Anal. Chem. 94, 14642–14651. doi:10.1021/acs.analchem.2c02832
Yang, Y., Gao, F., Wang, Y., Li, H., Zhang, J., Sun, Z., et al. (2022). Fluorescent organic small molecule probes for bioimaging and detection applications. Molecules 27, 8421. doi:10.3390/molecules27238421
Yang, Y., Yu, Y., Chen, H., Meng, X., Ma, W., Yu, M., et al. (2020). Illuminating platinum transportation while maximizing therapeutic efficacy by gold nanoclusters via simultaneous near-infrared-I/II imaging and glutathione scavenging. ACS Nano 14, 13536–13547. doi:10.1021/acsnano.0c05541
Yu, L., Li, M., Kang, Q., Fu, L., Zou, G., and Shen, D. (2021). Bovine serum albumin-stabilized silver nanoclusters with anodic electrochemiluminescence peak at 904 nm in aqueous medium and applications in spectrum-resolved multiplexing immunoassay. Biosens. Bioelectron. 176, 112934. doi:10.1016/j.bios.2020.112934
Zhai, Q., Xing, H., Zhang, X., Li, J., and Wang, E. (2017). Enhanced electrochemiluminescence behavior of gold-silver bimetallic nanoclusters and its sensing application for mercury(II). Anal. Chem. 89, 7788–7794. doi:10.1021/acs.analchem.7b01897
Zhang, X., He, S., Ding, B., Qu, C., Chen, H., Sun, Y., et al. (2021). Synergistic strategy of rare-earth doped nanoparticles for NIR-II biomedical imaging. J. Mater. Chem. B 9, 9116–9122. doi:10.1039/d1tb01640g
Zhang, Z., Liu, G., Li, X., Zhang, S., Lü, X., and Wang, Y. (2020). Design and synthesis of fluorescent nanocelluloses for sensing and bioimaging applications. Chempluschem 85, 487–502. doi:10.1002/cplu.201900746
Keywords: precious metals, clusters, fluorescent property, optical stability, bio-imaging
Citation: He X, Liu S, Hu X, Huang X, Zhang H and Mao X (2023) Precious metal clusters as fundamental agents in bioimaging usability. Front. Chem. 11:1296036. doi: 10.3389/fchem.2023.1296036
Received: 18 September 2023; Accepted: 18 October 2023;
Published: 09 November 2023.
Edited by:
Liangcan He, Harbin Institute of Technology, ChinaCopyright © 2023 He, Liu, Hu, Huang, Zhang and Mao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoxiao He, aHh4MjI0MDA5MzUzN0AxNjMuY29t; Xiang Mao, bWFveEBjcW11LmVkdS5jbg==
 Xiaoxiao He1*
Xiaoxiao He1* Hehua Zhang
Hehua Zhang Xiang Mao
Xiang Mao