
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Chem., 19 September 2023
Sec. Organic Chemistry
Volume 11 - 2023 | https://doi.org/10.3389/fchem.2023.1251299
This article is part of the Research TopicRecent Advances in Synthesizing and Utilizing Nitrogen-containing HeterocyclesView all 12 articles
Azetidine is a prevalent structural motif found in various biologically active compounds. In this research paper, we report La(OTf)3-catalyzed intramolecular regioselective aminolysis of cis-3,4-epoxy amines to afford azetidines. This reaction proceeded in high yields even in the presence of acid-sensitive and Lewis basic functional groups.
Arranging specific polar functional groups in three-dimensional space is a basic strategy for imparting a specific bioactive function to organic molecules and is universally found in nature and used in medicinal chemistry. The regioselective nucleophilic ring opening of epoxides is an efficient strategy for constructing contiguous chiral centers, and many methods have been developed to achieve this reaction (Caron and Sharpless, 1985; Faiz and Zahoor, 2016; Wang, 2017; Rodríguez-Berríos et al., 2023). Aminolysis of epoxides is a useful reaction for the synthesis of sterically complex nitrogen-containing compounds such as alkaloids. However, the regioselective aminolysis of epoxides poses a significant challenge, especially when Lewis and Brønsted acid promoters are used as catalysts. This is because the acid added to activate the epoxide is usually quenched by the high basicity of amine nucleophiles.
In the course of our study on the total synthesis of biologically active natural products (Uesugi et al., 2015), our research group discovered that lanthanoid (III) trifluoromethanesulfonate (Ln(OTf)3) functions as an excellent catalyst for the regioselective nucleophilic ring opening of epoxides, which led us to exploit the synthetic use of Ln(OTf)3 as a catalyst for epoxide ring-opening reactions. Thus, we have demonstrated that a catalytic amount of europium (III) trifluoromethanesulfonate (Eu(OTf)3) enables the introduction of alcohols and thiols, as well as aryl and aliphatic amines, onto the C3 position of 2,3-epoxy alcohols with high regioselectivity (Scheme 1A) (Uesugi et al., 2014). Eu(OTf)3 also efficiently catalyzed the C4-selective aminolysis of a 3,4-epoxy alcohol, the synthetic use of which was demonstrated by the efficient synthesis of the antipsychotic agent (+)-nemonapride (Uesugi et al., 2017). Furthermore, the lanthanum (III) trifluoromethanesulfonate (La(OTf)3) catalyst induced anti-Baldwin 5-endo-tet cyclization of trans-3,4-epoxy amines via C4-selective intramolecular epoxide aminolysis to give 3-hydroxypyrrolidines in high yields (Scheme 1B) (Kuriyama et al., 2021). Interestingly, the La(OTf)3 catalyst was found to promote the C3-selective intramolecular aminolysis of a cis-3,4-epoxy amine to give an azetidine in high yield, which led to the development of a novel synthetic route for azetidines, as reported herein.
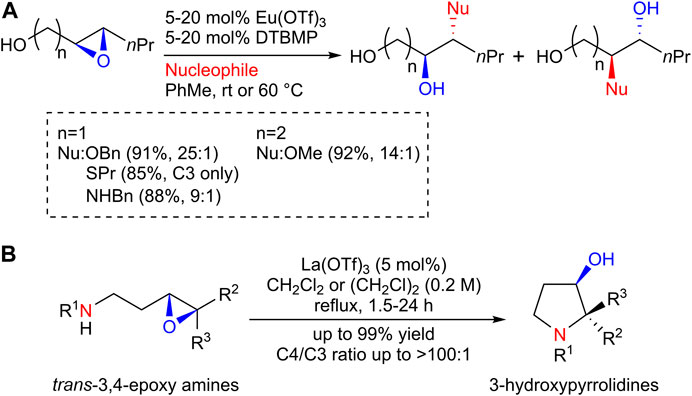
SCHEME 1. (A) Eu(OTf)3-catalyzed regioselective ring-opening reaction of epoxy alcohols; (B) La(OTf)3-catalyzed regioselective ring-opening reaction of trans-3,4-epoxy amines.
Azetidine is a structural motif found in various natural products and pharmaceuticals. This strained structure has encouraged synthetic chemists to develop strategies for the synthesis of azetidines (Hameed et al., 2017; Parmar et al., 2021; Yoda et al., 2011). Intramolecular SN2 reactions are often used to form azetidine rings in which a nitrogen atom attacks a carbon atom connected to a leaving group [halogen (Betz et al., 2019; Rowe et al., 2019; Dherange et al., 2022), mesylate (Bose et al., 1994), etc.]. The intramolecular aminolysis of 3,4-epoxy amines could be an alternative method for constructing an azetidine ring with a carbonyl group adjacent to the azetidine ring, which could be a useful scaffold for further functionalization. However, such reactions have rarely been reported, except the intramolecular aminolysis of 3,4-epoxy sulfonamide (Scheme 2A) (Moulines et al., 1993; Breternitz and Schaumann, 1999; Medjahdi et al., 2009; Faigl et al., 2012) and transannular aminolysis of 3,4-epoxy amine (Scheme 2B) (Shimokawa et al., 2011; Shing and So, 2011; Wang et al., 2018; Hocine et al., 2023). To the best of our knowledge, the intramolecular aminolysis of an unprotected linear 3,4-epoxy amine (rather than amide) has not been reported before. Herein, we describe further investigations to clarify the optimum conditions and substrate scope for the La(OTf)3-catalyzed highly regioselective 4-exo-tet cyclization of linear 3,4-epoxy amines to afford azetidines (Scheme 2C).
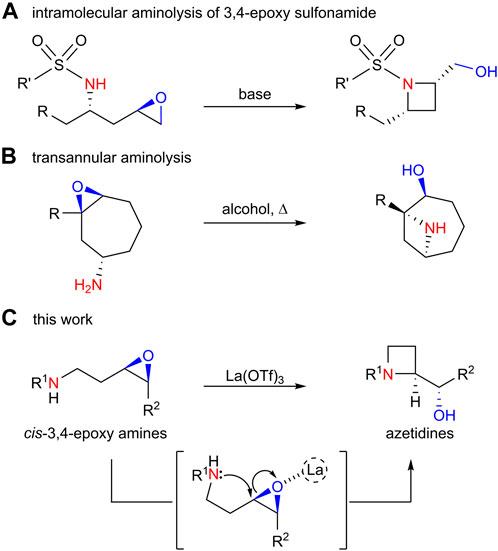
SCHEME 2. Azetidine syntheses by aminolysis reaction of epoxides; (A) intramolecular aminolysis of 3,4-epoxy sulfonamide; (B) transannular aminolysis; (C) this work.
All reactions were carried out in an argon atmosphere with dehydrated solvents under anhydrous conditions unless otherwise noted. Dehydrated THF and CH2Cl2 were purchased from Kanto Chemical Co., Inc., and the other solvents were dehydrated and distilled according to standard protocols. Reagents were obtained from commercial suppliers and used without further purification unless otherwise noted.
Reactions were monitored by thin-layer chromatography (TLC) on 0.25 mm Merck silica gel plates (60F-254). Column chromatography was performed using Silica Gel 60N (Kanto Chemical Co., Inc., spherical, neutral, particle size 63–210 mm) and NH-DM1020 (Fuji Silysia Chemical Ltd., spherical, particle size 100 μm); flash column chromatography was performed using Silica Gel 60N (Kanto Chemical Co., Inc., spherical, neutral, particle size 40–50 mm), unless otherwise noted.
Melting points were measured using a Yazawa BY-2 and Buchi M-565 and were uncorrected. Infrared (IR) spectra were obtained using a JASCO FT-IR-4600 instrument and are reported as wavenumbers. Proton nuclear magnetic resonance (1H-NMR) spectra were recorded using a JEOL JMN-AL400 (400 MHz) and a JEOL ECA-600 (600 MHz) spectrometer. Chemical shift (δ) is reported in parts per million (ppm) downfield relative to tetramethyl silane (TMS; 0.0 ppm) in CDCl3 and benzene (7.16 ppm) in C6D6. The coupling constants (J) are reported in Hz. Carbon-13 nuclear magnetic resonance (13C-NMR) spectra were recorded on a JEOL JMN-AL400 (100 MHz) spectrometer. Chemical shifts are reported in ppm relative to the centerline of the triplet of 13CDCl3 (77.0 ppm) and 13C6D6 (128.0 ppm). Low-resolution mass spectra (MS) were recorded on JEOL JMS-DX303, JMS-T100GC, and JEOL JMS-700 instruments. High-resolution mass spectra (HRMS) were recorded on JEOL JMS-T100GC and JEOL JMS-700 mass spectrometers using electron impact (EI) and on a Thermo Scientific Exactive Mass Spectrometer using electrospray ionization (ESI).
Et3N (2.5 eq) and MsCl (1.5 eq) were added to a solution of epoxy alcohol (1 eq) in CH2Cl2 (0.5 M) at 0°C, and the mixture was stirred for 10 min at room temperature. Then, saturated aqueous NaHCO3 was added to the mixture at 0°C, and the mixture was extracted thrice with CH2Cl2. The combined organic layers were dried over anhydrous MgSO4, filtered, and concentrated under reduced pressure. The resulting crude product was used immediately in the subsequent reaction without further purification.
Alkyl amine (3.0 eq) and NaI (10 mol%) were added to a solution of the crude product in DMSO (0.5 M) at room temperature (ca. 25°C), and the mixture was stirred for 2 days at ambient temperature. The mixture was diluted with H2O and extracted with Et2O. The combined organic layers were washed thrice with brine, dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure. The resulting residue was purified using column chromatography to yield the corresponding epoxy amines.
A pre-mixed solution of NaOCl·5H2O (1.5 eq) in saturated aqueous NaHCO was added dropwise to a cooled and well-stirred mixture of epoxy alcohol (1.0 eq) and TEMPO (1 mol%) in CH2Cl2 (0.2 M) and saturated aqueous NaHCO3 containing KBr (10 mol%), and the resulting mixture was stirred for 10 min at 0°C. Then, saturated aqueous Na2S2O3 was added at 0°C, and the mixture was extracted with CH2Cl2. The combined organic layers were washed with brine, dried over MgSO4, filtered, and concentrated under reduced pressure. The resulting crude product was used immediately in the subsequent reaction without purification (Lucio Anelli et al., 1987).
ArNH2 (1.0 eq) was added to a solution of the abovementioned crude product in CH2Cl2. After the mixture was stirred for 10 min at 0°C, NaBH(OAc)3 (1.2 eq) was added at 0°C and stirred at room temperature. Then saturated aqueous NaHCO3 was added, and the resulting mixture was extracted thrice with CH2Cl2. The combined organic layers were dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure. The resulting residue was purified using column chromatography to yield the corresponding epoxy anilines.
La(OTf)3 (5 mol%) was added to a solution of cis-3,4-epoxy amine (1 eq) in (CH2Cl)2 (0.2 M) at room temperature, and the mixture was stirred under reflux. Upon completion of the reaction, the mixture was cooled to 0°C, and saturated aqueous NaHCO3 was added. The mixture was extracted thrice with CH2Cl2. The combined organic layers were dried over Na2SO4, filtered, and then concentrated under reduced pressure. The resulting residue was purified using column chromatography to yield the corresponding azetidine.
The feasibility of azetidine synthesis via Ln(OTf)3-catalyzed intramolecular aminolysis of cis-3,4-epoxy amines was investigated using cis-3,4-epoxy amine 1aa, prepared from cis-3-hexen-1-ol in three steps, as a model substrate (Table 1). Preliminary experiments revealed the optimum conditions for the intramolecular aminolysis of trans-3,4-epoxy amines to yield pyrrolidine; catalytic La(OTf)3 in refluxing CH2Cl2 did not complete the reaction (Kuriyama et al., 2021). Therefore, 1,2-dichloroethane (DCE) was used for refluxing instead of CH2Cl2 for 2.5 h to afford azetidine 2aa in 81% yield, along with a trace amount of pyrrolidine 3aa (2aa/3aa = >20:1) (Table 1, entry 1). Solvents with almost the same boiling points similar to that of DCE were screened. The selectivity for benzene (PhH) was lower than that for DCE (Table 1, entry 2). Coordinative solvents such as MeCN and THF exhibited good selectivity, but some of the substrate 1aa remained (Table 1, entries 3 and 4). Subsequently, the acids were screened using DCE as the solvent. Using Sc(OTf)3 instead of La(OTf)3 required a longer reaction time and afforded 2aa in moderate yield (Table 1, entry 5). LiOTf afforded a complex mixture of products. Ni(ClO4)2·6H2O, the catalyst reported by Yamamoto for the intermolecular aminolysis of 3,4-epoxy alcohols (Wang and Yamamoto, 2015), and TfOH, a Brønsted acid, gave low yields of 2aa because some substrate 1aa remained; the reaction gave a complex mixture (Table 1, entries 7 and 8). In the absence of acids in refluxing DCE, no reaction occurred after 2.5 h (Table 1, entry 9). In contrast, although 1aa was completely consumed after 24 h, 2aa was not detected, and a complex mixture was obtained (Table 1, entry 10). Based on the aforementioned examination, the optimum conditions were identified as 5 mol% La(OTf)3 in refluxing DCE.
With the optimum conditions established, the effects of the substituents on the amino groups were evaluated (Figure 1). Azetidine formation proceeded smoothly in the presence of electron-rich and electron-deficient benzyl groups (2ba, 2ca). Substrates with an n-butyl amine moiety afforded azetidine 2da in high yield with high regioselectivity. A substrate with a bulky tert-butyl amine afforded azetidine 2ea in high yield. Substrates with π-basic allyl group also afforded the corresponding azetidine 2fa in moderate yield. Acid-prone functional groups, such as Boc, PMB, and TBS groups, were tolerated to afford azetidines (2ga–2ia) in high yields. Nitrile and sulfide functionalities hardly affected the yields of azetidines (2ja and 2ka). Interestingly, epoxy aniline 1la gave azetidine 2la in only 39% yield because of the competing formation of tetrahydroquinoline 4 via electrophilic aromatic substitution, whereas the corresponding trans-epoxy aniline efficiently underwent C4-selective intramolecular aminolysis to give pyrrolidine in high yield (Table 2) (Barvainiene et al., 2007; Wipf and Maciejewski, 2008; Mühlhaus et al., 2019). While epoxy aniline 1ma bearing an electron-donating methoxy group underwent aminolysis as did 1la, epoxy aniline 1na bearing an electron-withdrawing nitro group did not undergo the reaction. The effect of the functional groups adjacent to the epoxide was evaluated. Apart from the cation stabilization at the benzylic position, phenyl-substituted epoxide 1ab underwent aminolysis at the homobenzylic position, rather than the benzylic position, to afford azetidine 2ab in high yields (Scheme 3). Azetidine 2ac was also given in moderate yield from 4-fluorophenyl-substituted epoxide 1ac.
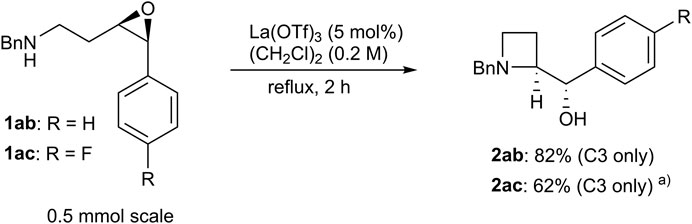
SCHEME 3. Aryl-substituted epoxy amine; a) A tiny amount of pyrrolidine was observed in the crude product (C3/C4 = >20:1).
Density functional theory (DFT) calculations were performed to gain insight into the opposite regioselectivity between trans- and cis-epoxy amines. Simplified trans- and cis-epoxy amines 5 and 6 were used as substrates to reduce computational costs (Figure 2). When naked lanthanum (III) is coordinated to trans-epoxy amine 5, the energy of the transition state that yields azetidine by C3-selective aminolysis is lower than that produced by C4-selective aminolysis, which indicates selectivity opposite to that of the experimental results (see Supplementary Material S1). Dimethylamine-coordinated lanthanum (III) was used as the activator. The calculations showed that the energy of the pyrrolidine transition state was lower than that of the azetidine transition state, which was consistent with the experimental results (Figure 3). Calculations of the transition states of cis-epoxy amines complexed with dimethylamine-coordinated lanthanum (III) showed that the transition state of azetidine was much smaller than that of pyrrolidine, which was consistent with the experimental results (Figure 4). These computational results suggest that lanthanum complexes coordinated by substrates and/or products are likely to contribute to inverse regioselectivity.
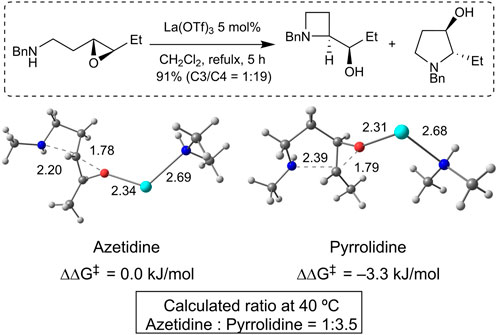
FIGURE 3. DFT studies of the selectivity for trans-epoxy amine (PCM (dichloromethane)/ωB97XD/6-311++G**, SDD (La)//PCM (dichloromethane)/ωB97XD/6-31G**, LanL2DZ (La)); lanthanum (light blue), oxygen (red), nitrogen (blue), carbon (gray), and hydrogen (white).

FIGURE 4. DFT studies of the selectivity for cis-epoxy amine (PCM (dichloroethane)/ωB97XD/6-311++G**, SDD (La)//PCM (dichloroethane)/ωB97XD/6-31G**, LanL2DZ (La)); lanthanum (light blue), oxygen (red), nitrogen (blue), carbon (gray), and hydrogen (white).
We have developed the La(OTf)3-catalyzed regioselective intramolecular aminolysis of cis-3,4-epoxy amines to afford azetidines. This reaction tolerated various functional groups, including coordinative and acid-prone functional groups. C3-selective aminolysis also proceeded with styrene oxide-type 3,4-epoxy amine, in which the C4 position was the benzylic position. Computational studies suggest that the difference in the regioselectivity of aminolysis between the cis- and trans-isomers was likely caused by lanthanum (III) coordinated with the substrate and/or product. Further investigations of the Lewis acid-promoted ring-opening reaction of strained heterocycles and its application to successive ring-opening reactions are currently underway. The reactions developed herein are expected to be applied to the synthesis of various highly functionalized bioactive compounds.
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author.
Investigation: YK, YS, and YI; experiment: YK; writing-original draft preparation: YK and YS; writing-review and editing: YS and YI; funding acquisition: YS and YI. All authors contributed to the article and approved the submitted version.
This research was partially supported by JSPS KAKENHI Grant Nos. 22H02739 and 21H05210 (Digitalization-driven Trans-formative Organic Synthesis (Digi-TOS)), and by Research Support Project for Life Science and Drug Discovery (Basis for Supporting Innovative Drug Discovery and Life Science Research (BINDS)) from AMED under Grant Number JP22ama121040.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2023.1251299/full#supplementary-material
Barvainiene, B., Stanisauskaite, A., and Getautis, V. (2007). Synthesis and thermal reactions of N-(2, 3-epoxypropyl) diphenylamine. Chem. Heterocycl. Compd. 43 (6), 718–721. doi:10.1007/s10593-007-0117-7
Betz, K. N., Chiappini, N. D., and Du Bois, J. (2019). Intermolecular sp3-C–H amination for the synthesis of saturated azacycles. Org. Lett. 22 (5), 1687–1691. doi:10.1021/acs.orglett.9b04096
Bose, A. K., Mathur, C., Wagle, D. R., Naqvi, R., Manhas, M. S., and Urbanczyk-Lipkowska, Z. (1994). Chiral β-lactams as synthons. Stereospecific synthesis of a 6-epi-lincosamine derivative. Heterocycles 2 (39), 491–496.
Breternitz, H.-J., and Schaumann, E. (1999). Ring-opening of N-tosylaziridines by heterosubstituted allyl anions. Application to the synthesis of azetidines and pyrrolidines. J. Chem. Soc. Perkin Trans. 1 (14), 1927–1932. doi:10.1039/a902117e
Caron, M., and Sharpless, K. (1985). Titanium isopropoxide-mediated nucleophilic openings of 2, 3-epoxy alcohols. A mild procedure for regioselective ring-opening. J. Org. Chem. 50 (9), 1557–1560. doi:10.1021/jo00209a047
Dherange, B. D., Yuan, M., Kelly, C. B., Reiher, C. A., Grosanu, C., Berger, K. J., et al. (2022). Direct deaminative functionalization. J. Am. Chem. Soc. 145 (1), 17–24. doi:10.1021/jacs.2c11453
Faigl, F., Kovács, E., Turczel, G., Szöllősy, Á., Mordini, A., Balázs, L., et al. (2012). Novel stereoselective synthesis of 1, 2, 3-trisubstituted azetidines. Tetrahedron Asymmetry 23 (22-23), 1607–1614. doi:10.1016/j.tetasy.2012.10.014
Faiz, S., and Zahoor, A. F. (2016). Ring opening of epoxides with C-nucleophiles. Mol. Divers. 20, 969–987. doi:10.1007/s11030-016-9686-7
Hameed, A., Javed, S., Noreen, R., Huma, T., Iqbal, S., Umbreen, H., et al. (2017). Facile and green synthesis of saturated cyclic amines. Molecules 22 (10), 1691. doi:10.3390/molecules22101691
Hocine, S., Duchamp, E., Mishra, A., Fourquez, J.-M., and Hanessian, S. (2023). Synthesis of aza-bridged perhydroazulene chimeras of tropanes and hederacine a. J. Org. Chem. 88 (7), 4675–4686. doi:10.1021/acs.joc.3c00169
Kuriyama, Y., Sasano, Y., Hoshino, Y., Uesugi, S.-i., Yamaichi, A., and Iwabuchi, Y. (2021). Highly regioselective 5-endo-tet cyclization of 3, 4-epoxy amines into 3-hydroxypyrrolidines catalyzed by La (OTf) 3. Chemistry–A Eur. J. 27 (6), 1961–1965. doi:10.1002/chem.202004455
Lucio Anelli, P., Biffi, C., Montanari, F., and Quici, S. (1987). Fast and selective oxidation of primary alcohols to aldehydes or to carboxylic acids and of secondary alcohols to ketones mediated by oxoammonium salts under two-phase conditions. J. Org. Chem. 52 (12), 2559–2562. doi:10.1021/jo00388a038
Medjahdi, M., Gonzalez-Gomez, J. C., Foubelo, F., and Yus, M. (2009). Stereoselective synthesis of azetidines and pyrrolidines from N-tert-butylsulfonyl (2-aminoalkyl) oxiranes. J. Org. Chem. 74 (20), 7859–7865. doi:10.1021/jo9016666
Moulines, J., Bats, J.-P., Hautefaye, P., Nuhrich, A., and Lamidey, A.-M. (1993). Substituent control in the synthesis of azetidines and pyrrolidines by N-tosyl-oxiraneethylamines base-mediated cyclization. Tetrahedron Lett. 34 (14), 2315–2318. doi:10.1016/s0040-4039(00)77602-7
Mühlhaus, F., Weißbarth, H., Dahmen, T., Schnakenburg, G., and Gansäuer, A. (2019). Merging regiodivergent catalysis with atom-economical radical arylation. Angew. Chem. 131 (40), 14346–14350. doi:10.1002/ange.201908860
Parmar, D. R., Soni, J. Y., Guduru, R., Rayani, R. H., Kusurkar, R. V., and Vala, A. G. (2021). Azetidines of pharmacological interest. Arch. Pharm. 354 (11), 2100062. doi:10.1002/ardp.202100062
Rodríguez-Berríos, R. R., Isbel, S. R., and Bugarin, A. (2023). Epoxide-based synthetic approaches toward polypropionates and related bioactive natural products. Int. J. Mol. Sci. 24 (7), 6195. doi:10.3390/ijms24076195
Rowe, E. A., Reisman, L., Jefcoat, J. A., and Rupar, P. A. (2019). Comparison of the anionic ring-opening polymerizations of N-(Alkylsulfonyl) azetidines. Macromolecules 52 (21), 8032–8039. doi:10.1021/acs.macromol.9b01436
Shimokawa, J., Harada, T., Yokoshima, S., and Fukuyama, T. (2011). Total synthesis of gelsemoxonine. J. Am. Chem. Soc. 133 (44), 17634–17637. doi:10.1021/ja208617c
Shing, T. K., and So, K. H. (2011). Facile and enantiospecific syntheses of (6 S, 7 R)-6-Chloro-7-benzyloxy-(7 S)-Halo-and (7 S)-Hydroxy-cocaine and natural (−)-Cocaine from d-(−)-ribose. Org. Lett. 13 (11), 2916–2919. doi:10.1021/ol2009686
Uesugi, S.-i., Sasano, Y., Matsui, S., Kanoh, N., and Iwabuchi, Y. (2017). Concise, Protecting-Group-Free Synthesis of (+)-Nemonapride via Eu(OTf)3-Catalyzed Aminolysis of 3,4-Epoxy Alcohol. Chem. Pharm. Bull. 65 (1), 22–24. doi:10.1248/cpb.c16-00568
Uesugi, S.-i., Watanabe, T., Imaizumi, T., Ota, Y., Yoshida, K., Ebisu, H., et al. (2015). Total synthesis and biological evaluation of irciniastatin a (aka psymberin) and irciniastatin b. J. Org. Chem. 80 (24), 12333–12350. doi:10.1021/acs.joc.5b02256
Uesugi, S.-i., Watanabe, T., Imaizumi, T., Shibuya, M., Kanoh, N., and Iwabuchi, Y. (2014). Eu (OTf) 3-catalyzed highly regioselective nucleophilic ring opening of 2, 3-epoxy alcohols: An efficient entry to 3-substituted 1, 2-diol derivatives. Org. Lett. 16 (17), 4408–4411. doi:10.1021/ol502264y
Wang, C. (2017). Electrophilic ring opening of small heterocycles. Synthesis 49 (24), 5307–5319. doi:10.1055/s-0036-1589102
Wang, C., and Yamamoto, H. (2015). Nickel-catalyzed regio-and enantioselective aminolysis of 3, 4-epoxy alcohols. J. Am. Chem. Soc. 137 (13), 4308–4311. doi:10.1021/jacs.5b01005
Wang, P., Gao, Y., and Ma, D. (2018). Divergent entry to gelsedine-type alkaloids: Total syntheses of (−)-gelsedilam,(−)-gelsenicine,(−)-gelsedine, and (−)-gelsemoxonine. J. Am. Chem. Soc. 140 (37), 11608–11612. doi:10.1021/jacs.8b08127
Keywords: azetidine, epoxide, catalytic reaction, regioselectivity, cyclization, Lewis acid, synthetic methods
Citation: Kuriyama Y, Sasano Y and Iwabuchi Y (2023) Azetidine synthesis by La(OTf)3-catalyzed intramolecular regioselective aminolysis of cis-3,4-epoxy amines. Front. Chem. 11:1251299. doi: 10.3389/fchem.2023.1251299
Received: 01 July 2023; Accepted: 22 August 2023;
Published: 19 September 2023.
Edited by:
Takashi Ohshima, Kyushu University, JapanReviewed by:
Rajendra Rohokale, University of Florida, United StatesCopyright © 2023 Kuriyama, Sasano and Iwabuchi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yoshiharu Iwabuchi, eS1pd2FidWNoaUB0b2hva3UuYWMuanA=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.