- Food and Pharmaceutical Engineering Institute, Guiyang University, Guiyang, China
In this study, twenty novel pyrimidine derivatives bearing a 1,3,4-thiadiazole skeleton were designed and synthesized. Then their antifungal activity against Botrytis cinereal (B. cinereal), Botryosphaeria dothidea (B. dothidea), and Phomopsis sp. were determined using the poison plate technique. Biological test results showed that compound 6h revealed lower EC50 values (25.9 and 50.8 μg/ml) on Phompsis sp. than those of pyrimethanil (32.1 and 62.8 μg/ml).
1 Introduction
Due to their structure, which is similar to their alkaloid-like structure in living organisms, nitrogen-containing heterocyclic compounds have the characteristics of high target specificity and good environmental compatibility and have become the mainstream research field for the creation of new pesticides (Li et al., 2017; He et al., 2019). Among them, 1,3,4-thiadiazoles containing both N and S elements in the heterocyclic structure are important and lead molecules for designing biologically active compounds with various biological activities (Hu et al., 2014). For the past years, a large number of studies have shown that 1,3,4-thiadiazole and their derivatives had various biological activities including herbicidal (Sun et al., 2013), bactericidal (Li et al., 2015; Zhang et al., 2019; Wu Q. et al., 2020; Wu et al., 2021), fungicidal (Zou et al., 2002; Zine et al., 2016; Wu W. et al., 2020), antiviral (Wu et al., 2016a; Gan et al., 2017), insecticidal (Dai et al., 2016; Lv et al., 2018), anticancer (Chen et al., 2019), and so on. In the field of medicine and pesticides, especially in the field of fungicides, the products that have been successfully developed at present are thiabendazole, thiabendron copper, thiazole zinc, and thiazole.
Meanwhile, in the agricultural field, pyrimidine derivatives also have good biological activities such as antiviral (Wu, et al., 2015; Zan et al., 2020), insecticidal (Liu, et al., 2017; Wu, et al., 2019; Chen, et al., 2021; Liu, et al., 2021; Sun, et al., 2021), fungicidal (Guan et al., 2017; Yan et al., 2020; Yang, et al., 2020), bactericidal (Li et al., 2020), herbicidal (Chen et al., 2019; Li et al., 2020), and anticancer (Guo et al., 2020) properties. In the last few decades, some pyrimidine derivatives have been commercialized as pesticides for controlling plant diseases and insect pests. Therefore, pyrimidine was considered an active substructure to develop promising pesticides in recent years.
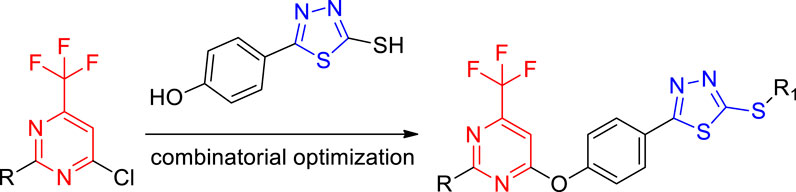
Based on the biological activity of 1,3,4-thiadiazole and the pyrimidine ring, in order to find new pyrimidine lead compounds with good biological activity, this work adopts the active substructure splicing method to design and synthesize a series of novel pyrimidine derivatives containing a 1,3,4-thiadiazole moiety (Figure 1), which were evaluated in vitro with regard to their antifungal activity against Botrytis cinereal (B. cinereal), Botryosphaeria dothidea (B. dothidea), and Phomopsis sp.
2 Materials and Methods
2.1 Chemistry
Melting points (m.p.) were obtained using a microscope apparatus (XT-4, Beijing Tech Instrument Co., China). Nuclear magnetic resonance (1H NMR and 13C NMR) was determined on a Bruker NMR spectrometer (Bruker, Germany). High-resolution mass spectrometry (HRMS) was performed on a Thermo Scientific Q Exactive Plus instrument (Thermo Fisher Scientific, United States).
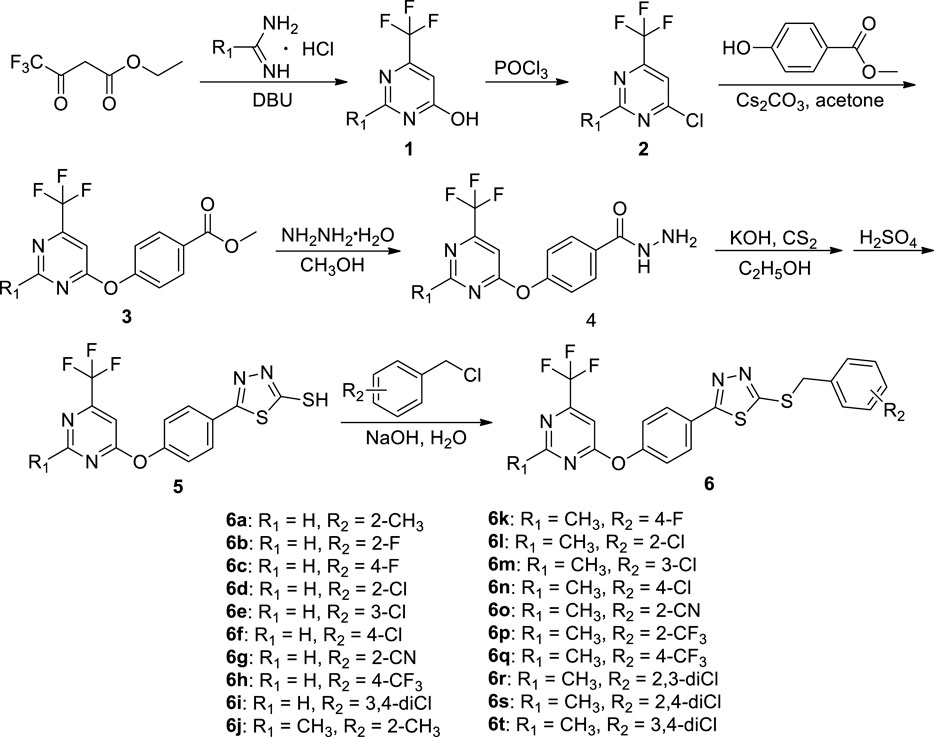
2.2 The Preparation Procedure of Intermediates 1–5
Intermediates 1 and 2 were obtained by referring to the previously reported methods (Wu W. et al., 2020).
To a 100-ml three round-bottom flask, intermediate 2 (0.01 mol), ethyl 4-hydroxybenzoate (0.012 mol), Cs2CO3 (0.02 mol), and acetone (50 ml) were added. After reacting for 2–4 h at room temperature, the solvent was vacuum evaporated. The residues were recrystallized from ethanol to give pure intermediate 3.
To a solution of intermediate 3 (20 mmol) in 40 ml absolute methanol, 80% hydrazine hydrate (60 mmol) was added dropwise. After reacting for 5–7 h under reflux conditions, the reaction was quenched to room temperature. The white solids precipitated from the reaction solution were filtrated and recrystallized from ethanol to give pure intermediate 4.
To a mixture of intermediate 4 (30 mmol), KOH (45 mmol), and ethanol (500 ml), carbon disulfide (36 mmol) was added dropwise. The white precipitates were filtered, dried under vacuum, and then added to 30 ml precooled concentrated H2SO4. After stirring for 2 h at 0°C, the mixture was poured into 1,000 ml ice water and neutralized with sodium bicarbonate saturated solution (Wu et al., 2016a; Wu et al., 2016b). The filtrate was acidified with 5% hydrochloric acid, and the produced solid was filtered and recrystallized from ethanol to give the key intermediate 5.
2.3 Preparation Procedure of the Target Compounds 6a−6t
Intermediate 5 (2 mmol), NaOH (2.2 mmol) dissolved in 15 ml water, and substituted benzyl chloride (2.1 mmol) were added in a 100-ml three round-bottom flask and stirred at room temperature for 2–4 h (Scheme 1). Upon completion of reaction, the residues were filtered and recrystallized from ethanol to produce the pure target compounds 6a–6t. The physical properties, NMR, and HRMS for title compounds are reported in Supplementary Data S1, and the spectral data of 6a are shown below. 2-((2-methylbenzyl)thio)-5-(4-((6-(trifluoromethyl)pyrimidin-4-yl)oxy)phenyl)-1,3,4-thiadiazole (6a). White solid; yield 65.24%; m. p. 104–107°C; 1H NMR (600 MHz, DMSO-d6, ppm) δ: 8.99 (s, 1H, pyrimidine-H), 8.04–8.02 (m, 2H, phenyl-H), 7.86 (s, 1H, pyrimidine-H), 7.50–7.48 (m, 4H, phenyl-H), 7.42 (d, 1H, J = 5.4 Hz, phenyl-H), 7.23–7.17 (m, 3H, phenyl-H), 4.65 (s, 2H, -SCH2-), 2.41 (s, 3H, pyrimidine-CH3); 13C NMR (150 MHz, DMSO-d6, ppm) δ: 170.32, 167.66, 165.34, 159.73, 156.22 (q, J = 35.1 Hz), 154.29, 137.37, 134.12, 130.98, 130.59, 129.74, 128.65, 127.66, 126.62, 123.27, 121.80 (q, J = 272.7 Hz), 116.13, 107.07, 36.66, 19.26; HRMS (ESI) calcd for C21H15ON4S2F3 [M+Na]+: 483.05249, found: 483.05316.
2.4 In vitro Antifungal Activity Test
The in vitro antifungal activity was determined according to the mycelial growth rate method (Zhang et al., 2018; Wang et al., 2019; Wu Q. et al., 2020). Each target compound (5 mg) was dissolved in DMSO (1 ml) and added to 9 ml H2O and 90 ml potato dextrose agar (PDA) medium to prepare 9 dishes of mixed PDA plates with a concentration of 50 μg/ml. After that, a 0.4-cm diameter of each test fungus was put onto the middle of mixed PDA plates and fostered in an incubator at 28°C for 3–4 days. After the mycelia diameter of the untreated PDA plate reached 5–6 cm, the inhibition rates I (%) are calculated using the following formula, where C (cm) and T (cm) represent the fungi diameters of the untreated and treated PDA plates, respectively.
3 Results and Discussion
3.1 Chemistry
In the 1H NMR data of compound 6a, a singlet appears at 4.65 ppm and indicates the presence of the -SCH2- group. The CH proton of the 6-trifluoromethylpyrimidine ring appeared as two singlets at 8.99 and 7.86 ppm. Meanwhile, in the 13C NMR data of compound 6a, two signals at 170.32 and 167.66 ppm indicated the presence of C proton in the 1,3,4-thiadiazole group. One quartet at 156.22 ppm indicated the presence of -CF3 in the pyrimidine fragment. In addition, compound 6a was confirmed correctly by combining HRMS data with the [M + Na]+ peaks.
3.2 In vitro Antifungal Activity
As shown in Table 1, compounds 6c, 6g, and 6h exhibited higher in vitro antifungal activity against Phomopsis sp., and the inhibition rates were 89.6%, 88.7%, and 89.2%, respectively, compared to that of pyrimethanil (85.1%). Meanwhile, Table 1 shows that the inhibitory activity values of compounds 6g, 6h, and 6q against B. cinerea were 86.1%, 90.7%, and 88.3%, respectively, which were superior to that of pyrimethanil (82.8%). In addition, compound 6h possessed similar bioactivity against B. dothidea (82.6%) to that of pyrimethanil (84.4%).
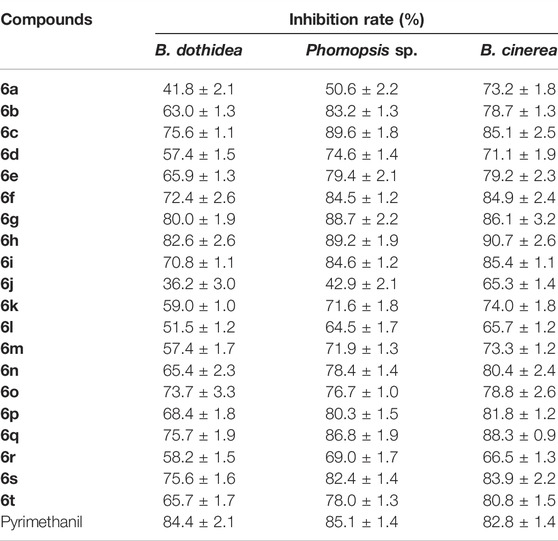
TABLE 1. Inhibition rates of compounds 6a−6t against B. cinereal, B. dothidea, and Phomopsis sp. at 50 µg/ml.
Table 2 shows that compounds 6c, 6g, and 6h had the EC50 values of 25.4, 28.8, and 25.9 μg/ml, respectively, which were better than that of pyrimethanil (32.1 μg/ml). Meanwhile, compounds 6g (EC50 = 57.5 μg/ml) and 6h (EC50 = 50.8 μg/ml) exhibited better in vitro bioactivity on B. cinerea than pyrimethanil (62.8 μg/ml). Meanwhile, compounds 6g (EC50 = 67.8 μg/ml) and 6h (EC50 = 63.6 μg/ml) exhibited lower in vitro bioactivity against B. dothidea than pyrimethanil (57.6 μg/ml).
Further structure–activity relationship analysis indicated that more than 80% of the title compounds showed excellent antifungal activity against Phomopsis sp. and B. cinerea. Meanwhile, changing R1 (H or CH3) did not significantly improve the antifungal activity of the compound. Only against Phomopsis sp., the number of compounds (R1 = H) with activity higher than 80% is twice that of compounds (R1 = CH3). In addition, the introduction of strong electron withdraw groups (CN and CF3) into R2 was able to enhance the activity of the compounds, while the introduction of an alkyl group (CH3) cannot obviously improve the antifungal activity of the compounds.
4 Conclusion
In conclusion, 20 novel 1,3,4-thiadiazole derivatives bearing a pyrimidine skeleton were synthesized and assessed for all compounds with regard to in vitro antifungal activities. Results of bioassays of the synthesized compounds showed excellent antifungal activity compared to that of pyrimethanil. Therefore, 1,3,4-thiadiazole derivatives bearing a pyrimidine skeleton can be used as candidate leading structures for discovering new fungicidal agents.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Author Contributions
NP, CL, and RW contributed to the synthesis, purification, and characterization of all compounds and the activity research and prepared the original manuscript. WW and QF designed and supervised the research and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the Science and Technology Fund Project of Guizhou (NO. (2020)1Z023) and disciplinary Talent Fund of of Guiyang University (NO. GYURC-12).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2022.922813/full#supplementary-material
References
Chen, S., Zhang, Y., Liu, Y., and Wang, Q. (2021). Highly Efficient Synthesis and Acaricidal and Insecticidal Activities of Novel Oxazolines with N-Heterocyclic Substituents. J. Agric. Food Chem. 69, 3601–3606. doi:10.1021/acs.jafc.0c05558
Chen, Z., Li, D., Xu, N., Fang, J., Yu, Y., Hou, W., et al. (2019). Novel 1,3,4-Selenadiazole-Containing Kidney-type Glutaminase Inhibitors Showed Improved Cellular Uptake and Antitumor Activity. J. Med. Chem. 62, 589–603. doi:10.1021/acs.jmedchem.8b01198
Dai, H., Li, G., Chen, J., Shi, Y., Ge, S., Fan, C., et al. (2016). Synthesis and Biological Activities of Novel 1,3,4-Thiadiazole-Containing Pyrazole Oxime Derivatives. Bioorg. Med. Chem. Lett. 26, 3818–3821. doi:10.1016/j.bmcl.2016.04.094
Gan, X., Hu, D., Chen, Z., Wang, Y., and Song, B. (2017). Synthesis and Antiviral Evaluation of Novel 1,3,4-Oxadiazole/thiadiazole-Chalcone Conjugates. Bioorg. Med. Chem. Lett. 27, 4298–4301. doi:10.1016/j.bmcl.2017.08.038
Guan, A., Wang, M., Yang, J., Wang, L., Xie, Y., Lan, J., et al. (2017). Discovery of a New Fungicide Candidate through Lead Optimization of Pyrimidinamine Derivatives and its Activity against Cucumber Downy Mildew. J. Agric. Food Chem. 65, 10829–10835. doi:10.1021/acs.jafc.7b03898
Guo, W., Xing, Y., Zhang, Q., Xie, J., Huang, D., Gu, H., et al. (2020). Synthesis and Biological Evaluation of B-Cell Lymphoma 6 Inhibitors of N-Phenyl-4-Pyrimidinamine Derivatives Bearing Potent Activities against Tumor Growth. J. Med. Chem. 63, 676–695. doi:10.1021/acs.jmedchem.9b01618
He, W., Liu, D., Gan, X., Zhang, J., Liu, Z., Yi, C., et al. (2019). Synthesis and Biological Activity of Novel 1,3,4-Thiadiazolo[3,2-A]pyrimidinone Mesoionic Derivatives. Chin. J. Org. Chem. 39, 2287–2294. doi:10.6023/cjoc201903023
Hu, Y., Li, C.-Y., Wang, X.-M., Yang, Y.-H., and Zhu, H.-L. (2014). 1,3,4-Thiadiazole: Synthesis, Reactions, and Applications in Medicinal, Agricultural, and Materials Chemistry. Chem. Rev. 114, 5572–5610. doi:10.1021/cr400131u
Li, J.-h., Wang, Y., Wu, Y.-p., Li, R.-h., Liang, S., Zhang, J., et al. (2021). Synthesis, Herbicidal Activity Study and Molecular Docking of Novel Pyrimidine Thiourea. Pesticide Biochem. Physiology 172, 104766. doi:10.1016/j.pestbp.2020.104766
Li, P., Shi, L., Gao, M.-N., Yang, X., Xue, W., Jin, L.-H., et al. (2015). Antibacterial Activities against Rice Bacterial Leaf Blight and Tomato Bacterial Wilt of 2-Mercapto-5-Substituted-1,3,4-Oxadiazole/thiadiazole Derivatives. Bioorg. Med. Chem. Lett. 25, 481–484. doi:10.1016/j.bmcl.2014.12.038
Li, Q., Pang, K., Zhao, J., Liu, X., and Weng, J. (2017). Synthesis and Biological Activity of Novel 1,3,4-thiadiazole Thioether Derivatives Containing Pyrimidine Moiety. Chin. J. Org. Chem. 37, 1009–1015. doi:10.6023/cjoc201610026
Liu, X.-H., Wang, Q., Sun, Z.-H., Wedge, D. E., Becnel, J. J., Estep, A. S., et al. (2017). Synthesis and Insecticidal Activity of Novel Pyrimidine Derivatives Containing Urea Pharmacophore againstAedes Aegypti. Pest. Manag. Sci. 73, 953–959. doi:10.1002/ps.4370
Liu, X.-H., Wen, Y.-H., Cheng, L., Xu, T.-M., and Wu, N.-J. (2021). Design, Synthesis, and Pesticidal Activities of Pyrimidin-4-Amine Derivatives Bearing a 5-(Trifluoromethyl)-1,2,4-Oxadiazole Moiety. J. Agric. Food Chem. 69, 6968–6980. doi:10.1021/acs.jafc.1c00236
Lv, M., Liu, G., Jia, M., and Xu, H. (2018). Synthesis of Matrinic Amide Derivatives Containing 1,3,4-thiadiazole Scaffold as Insecticidal/acaricidal Agents. Bioorg. Chem. 81, 88–92. doi:10.1016/j.bioorg.2018.07.034
Sun, C., Zhang, S., Qian, P., Li, Y., Ren, W., Deng, H., et al. (2021). Synthesis and Fungicidal Activity of Novel Benzimidazole Derivatives Bearing Pyrimidine‐thioether Moiety against Botrytis Cinerea. Pest. Manag. Sci. 77, 5529–5536. doi:10.1002/ps.6593
Sun, Z., Huang, W., Gong, Y., Lan, J., Liu, X., Weng, J., et al. (2013). Synthesis and Herbicidal Activity of New 1,3,4-thiadizols Sulfourea Derivative. Chin. J. Org. Chem. 33, 2612–2617. doi:10.6023/cjoc201306028
Wang, X., Fu, X., Yan, J., Wang, A., Wang, M., Chen, M., et al. (2019). Design and Synthesis of Novel 2-(6-Thioxo-1,3,5-Thiadiazinan-3-Yl)-N′-Phenylacethydrazide Derivatives as Potential Fungicides. Mol. Divers. 23, 573–583. doi:10.1007/s11030-018-9891-7
Wu, N., Cheng, L., Wang, J., Yu, J., Xing, J., Xu, T., et al. (2019). Synthesis and Insecticidal Activity of Novel 4-arylamino Pyrimidine Derivatives. Chin. J. Org. Chem. 39, 852–860. doi:10.6023/cjoc201807044
Wu, Q., Cai, H., Yuan, T., Li, S., Gan, X., and Song, B. (2020). Novel Vanillin Derivatives Containing a 1,3,4-thiadiazole Moiety as Potential Antibacterial Agents. Bioorg. Med. Chem. Lett. 30, 127113. doi:10.1016/j.bmcl.2020.127113
Wu, W.-N., Gao, M.-N., Tu, H., and Ouyang, G.-P. (2016a). Synthesis and Antibacterial Activity of Novel Substituted Purine Derivatives. J. Heterocycl. Chem. 53, 2042–2048. doi:10.1002/jhet.2527
Wu, W.-N., Tai, A.-Q., Chen, Q., and Ouyang, G.-P. (2016b). Synthesis and Antiviral Bioactivity of Novel 2-substituted Methlthio-5-(4-Amino-2-Methylpyrimidin-5-Yl)-1,3,4-Thiadiazole Derivatives. J. Heterocycl. Chem. 53, 626–632. doi:10.1002/jhet.2435
Wu, W., Chen, M., Fei, Q., Ge, Y., Zhu, Y., Chen, H., et al. (2020). Synthesis and Bioactivities Study of Novel Pyridylpyrazol Amide Derivatives Containing Pyrimidine Motifs. Front. Chem. 8, 522. doi:10.3389/fchem.2020.00522
Wu, W., Chen, Q., Tai, A., Jiang, G., and Ouyang, G. (2015). Synthesis and Antiviral Activity of 2-substituted Methylthio-5-(4-Amino-2-Methylpyrimidin-5-Yl)-1,3,4-Oxadiazole Derivatives. Bioorg. Med. Chem. Lett. 25, 2243–2246. doi:10.1016/j.bmcl.2015.02.069
Wu, Z., Shi, J., Chen, J., Hu, D., and Song, B. (2021). Design, Synthesis, Antibacterial Activity, and Mechanisms of Novel 1,3,4-thiadiazole Derivatives Containing an Amide Moiety. J. Agric. Food Chem. 69 (31), 8660–8670. doi:10.1021/acs.jafc.1c01626
Yan, Y., Cheng, W., Xiao, T., Zhang, G., Zhang, T., Lu, T., et al. (2020). Discovery of Novel 2,4,6-trisubstituted Pyrimidine Derivatives as Succinate Dehydrogenase Inhibitors. Chin. J. Org. Chem. 40, 4237–4248. doi:10.6023/cjoc202005057
Yang, J., Guan, A., Li, Z., Zhang, P., and Liu, C. (2020). Design, Synthesis, and Structure-Activity Relationship of Novel Spiropyrimidinamines as Fungicides against Pseudoperonospora Cubensis. J. Agric. Food Chem. 68, 6485–6492. doi:10.1021/acs.jafc.9b07055
Zan, N., Xie, D., Li, M., Jiang, D., and Song, B. (2020). Design, Synthesis, and Anti-ToCV Activity of Novel Pyrimidine Derivatives Bearing a Dithioacetal Moiety that Targets ToCV Coat Protein. J. Agric. Food Chem. 68, 6280–6285. doi:10.1021/acs.jafc.0c00987
Zhang, M., Xu, W., Wei, K., Liu, H., Yang, Q., Liu, Q., et al. (2019). Synthesis and Evaluation of 1,3,4‐Thiadiazole Derivatives Containing Cyclopentylpropionamide as Potential Antibacterial Agent. J. Heterocycl. Chem. 56, 1966–1977. doi:10.1002/jhet.3576
Zhang, Z.-J., Zeng, Y., Jiang, Z.-Y., Shu, B.-S., Sethuraman, V., and Zhong, G.-H. (2018). Design, Synthesis, Fungicidal Property and QSAR Studies of Novel β -carbolines Containing Urea, Benzoylthiourea and Benzoylurea for the Control of Rice Sheath Blight. Pest. Manag. Sci. 74, 1736–1746. doi:10.1002/ps.4873
Zine, H., Rifai, L. A., Faize, M., Bentiss, F., Guesmi, S., Laachir, A., et al. (2016). Induced Resistance in Tomato Plants against Verticillium Wilt by the Binuclear Nickel Coordination Complex of the Ligand 2,5-Bis(pyridin-2-Yl)-1,3,4-Thiadiazole. J. Agric. Food Chem. 64, 2661–2667. doi:10.1021/acs.jafc.6b00151
Keywords: 4-thiadiazole, pyrimidine, design, synthesis, antifungal activity
Citation: Pan N, Liu C, Wu R, Fei Q and Wu W (2022) Novel Pyrimidine Derivatives Bearing a 1,3,4-Thiadiazole Skeleton: Design, Synthesis, and Antifungal Activity. Front. Chem. 10:922813. doi: 10.3389/fchem.2022.922813
Received: 18 April 2022; Accepted: 25 April 2022;
Published: 08 June 2022.
Edited by:
Pei Li, Kaili University, ChinaCopyright © 2022 Pan, Liu, Wu, Fei and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenneng Wu, d3V3ZW5uZW5nMTIzQDEyNi5jb20=
†These authors have contributed equally to this work
 Nianjuan Pan†
Nianjuan Pan† Wenneng Wu
Wenneng Wu