
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Chem. , 17 May 2022
Sec. Organic Chemistry
Volume 10 - 2022 | https://doi.org/10.3389/fchem.2022.905108
This article is part of the Research Topic Bioactive Natural Products-Oriented Synthetic and Functional Studies View all 24 articles
A correction has been applied to this article in:
Corrigendum: New Sesquiterpenoids From Plant-Associated Irpex lacteus
Bacteria produce a large number of virulence factors through the quorum sensing (QS) mechanism. Inhibiting such QS system of the pathogens without disturbing their growth is a potential strategy to control multi-drug-resistant pathogens. To accomplish this, two new tremulane-type sesquiterpenoids, irpexolaceus H (1) and I (2), along with two known furan compounds, irpexlacte B (3) and C (4), were isolated from Orychophragmus violaceus (L.) OE Schulz endophytic fungus Irpex lacteus (Fr.) Fr. Their structures were elucidated by detailed spectroscopic data (NMR, HRESIMS, IR, and UV), single-crystal X-ray diffraction, and electronic circular dichroism (ECD) analysis. Furthermore, those compounds were evaluated for anti-quorum sensing (anti-QS) activity, and compound 3 was found contributing to the potential QS inhibitory activity.
Bacterial quorum sensing (QS) is a cell-density-dependent communication process by which cells measure population density and trigger appropriate responses and conduct behavioral regulation, such as luminescence, motility, secretion of virulence factors, and formation of biofilms (Papenfort and Bassler, 2016). QS inhibitor (QSI) inhibits the QS system without affecting bacteria’s growth and reduces its virulence production and biofilm formation; thus, the bacteria are in a low or non-toxicity state, the growth is not inhibited, and therefore it is difficult to cause drug resistance (Jiang and Li, 2013). However, the purpose of traditional antibacterial agents is to kill or inhibit the growth of bacteria, and it is difficult to avoid bacterial resistance (Kalia, 2013). Therefore, finding new QSIs to replace traditional antibacterial agents has become a new strategy in the antibacterial field.
Irpex lacteus (Fr.) Fr. (Phanerochaetaceae) is a basidiomycete that usually colonizes on the deadwood white (i.e., white rot), and it is often used as a traditional Chinese medicine for the treatment of chronic glomerulonephritis. In our previous study on this subject, seven sesquiterpenoids, irpexolaceus A–G, and two new furan derivatives, irpexonjust A–B, were isolated from I. lacteus OV38 (Luo et al., 2022). Furthermore, two new tremulane-type sesquiterpenoids, irpexolaceus H (1) and I (2), were isolated in this study from fungi, as well as two furan compounds, such as irpexlacte B (3) and C (4), were also obtained (Figure 1). These compounds were screened for QS inhibitory activity, and compound 3 exhibited the highest QS inhibitory activity among them. The details of the isolation, structure assignment, and QS inhibitory activities of 1–4 are presented.
Nuclear magnetic resonance (NMR) was obtained on a Bruker AV-400 spectrometer (Bruker Corporation, Karlsruhe, Germany). HRMSESI data were recorded on a Q-Exactive Orbitrap MS system (Thermo Fisher Scientific, Bremen, Germany). UV data were obtained on an Evolution 220 UV-vis spectrophotometer (Thermo Fisher Scientific, Madison, United States). Infrared spectroscopy (IR) spectra were obtained on a Nicolet™ iS10 FTIR spectrometer (Thermo Fisher Scientific, Madison, United States). Optical rotations were recorded on an Autopol VI automatic polarimeter (PerkinElmer, Waltham, MA, United States). The silica gel (100–200 and 200–300 mesh, Qingdao Marine Chemical Factory, Qingdao, China) and Sephadex LH-20 column (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) were used for open column chromatography (CC). Fractions were monitored by TLC (HSGF 254, Yantai Jiangyou Silica Gel Development Co., Yantai, China), and spots were visualized by heating silica gel plates after soaking in methanol supplemented with 10% H2SO4. The preparative HPLC was performed with UltiMate 300 HPLC (Thermo Fisher Scientific, Madison, United States) equipped with a YMC-Pack ODS-A column (250 × 10 mmI.D, S-5 μm, 12 nm, and flow speed = 2–3 ml/min, YMC Co., Ltd., Kyoto, Japan).
The strain Irpex lacteus was isolated from the healthy flowers and stems of Orychophragmus violaceus (L.) OE Schulz collected from Nanjing University of Science and Technology (NJUST) (Luo et al., 2021). The fungus I. lacteus was fermented in 250-ml Erlenmeyer flasks containing 100 ml Fungus No. 2 medium (2% sorbitol, 2% maltose, 1% glutamine, 1% glucose, 0.3% yeast extract, 0.05% tryptophan, 0.05% KH2PO4, and 0.03% MgSO4, pH 6.4) at 28°C and 140 rpm for 15 d (Zhou et al., 2017).
The supernatant (total 100 L) of I. lacteus fermentation was extracted three times by equal ethyl acetate (EtOAc) and concentrated under reduced pressure to give a crude extract (99.49 g), which was subjected to column chromatography (CC) over a silica gel (100–200 mesh) eluted with a gradient of dichloromethane/methyl alcohol (CH2Cl2/MeOH, 100:0-0:100) to obtain a fraction G (Fr. G) and other 13 fractions (Fr. A–F and H–M). Fr. A (19.61 g) was purified by CC over a silica gel (200–300 mesh) eluted with the gradient systems of petroleum ether/EtOAc (20:1–1:1) and EtOAc/MeOH (100:1–10:1) to yield six sub-fractions Fr. A1–A6. Fr. A6 (4.79 g) was subjected to CC over a silica gel eluted with a gradient system of CH2Cl2/MeOH (80:1–0:100) to yield five sub-fractions, that is, Fr. A6.1–A6.5. Fr. A6.1 (97.4 mg) was further purified by HPLC to give 3 (40.1 mg, 45% MeOH, flow speed = 2.5 ml/min, and tR = 31.0 min) and 4 (5.0 mg, 45% MeOH, flow speed = 2.5 ml/min, and tR = min). Fr. G (37.30 g) was continually separated by CC over a silica gel (200–300 mesh) eluted with a gradient system of CH2Cl2/MeOH (100:0–0:100) to give nine sub-fractions Fr. G1–G9. Fr. G2 (7.76 g) was repeatedly separated using CC over a silica gel, Sephadex LH-20 (100% MeOH), and finally purified by HPLC to give 1 (30.3 mg, 50% MeOH, flow speed = 3.0 ml/min, and tR = 14.1 min) and 2 (4.90 mg, 50% MeOH, flow speed = 3.0 ml/min, and tR = 25.6 min).
Irpexolaceus H (1). white amorphous powder; [α]18 D + 17.2 (c 0.19, MeOH); UV (MeOH) λmax (log ε) 203 (3.99) nm and 290 (2.65) nm; IR (KBr): υmax 3380.60, 2932.72, 2872.93, 1752.01, and 1027.87 cm−1; 1H NMR (400 MHz in MeOD) and 13C NMR (100 MHz in MeOD) data are shown in Table 1; and HRESIMS m/z 311.1502 [M + COOH]- (calcd for C16H23O6, 311.1500).
Irpexolaceus I (2). Yellow oil; [α]18 D + 21.6 (c 0.12, MeOH); UV (MeOH) λmax (log ε) 205 (3.57) nm; CD (MeOH) 195 (Δε—4.72) and 215 (Δε + 8.61) nm; IR (KBr): υmax 3359.87, 1564.95, 1397.66, and 1020.64 cm−1; 1H NMR (400 MHz in MeOD) and 13C NMR (100 MHz in MeOD) data are shown in Table 1; and HRESIMS m/z, 283.1551 [M-H]- (calcd for C15H23O5 and 283.1551).
Crystal data for Cu 1_0m. C15H22O4, M = 266.33, a = 6.6684 (4) Å, b = 12.1018 (5) Å, c = 8.4219 (4) Å, α = 90°, β = 93.164 (4)°, γ = 90°, V = 678.61 (6) Å3, Z = 2, T = 170.0 K, space group P212121, μ(Cu Kα) = 0.760 mm−1, Dcalc = 1.303 g/cm3, 10,065 reflections measured (10.520° ≤ 2θ ≤ 127.318°), and 2210 unique (Rint = 0.0504, Rsigma = 0.0357) which were used in all calculations. The final R1 was 0.0321 [I > 2σ(I)]. The final wR2 was 0.0752 [I > 2σ(I)]. The final R1 was 0.0346 (all data). The final wR2 was 0.0772 (all data). The goodness of fit on F2 was 1.091. Flack parameter = −0.02 (12), which was determined using 1101 quotients [(I+)−(I−)]/[(I+)+(I−)]. CCDC: 2133065 (www.ccdc.cam.ac.uk).
The experiment was performed as previously described (Luo et al., 2022). The conformers with Boltzmann population (over 1%) were initially optimized at B3LYP/6–31+G. The ECD spectra of all conformers were conducted by time-dependent density functional theory (TD-DFT) methodology at ωB97X/def2-TZVP, CAM-B3LYP/TZVP, and M062X/def2-TZVP using IEFPCM in MeOH (Neuhaus and Loesgen, 2020). ECD spectra were generated using the program SpecDis 1.71 from dipole-length rotational strength by applying Gaussian band shapes with sigma = 0.2 eV (Bruhn et al., 2013).
The efficacy of purified compounds on inhibiting QS mechanisms was screened using indicator organisms Chromobacterium violaceum CV026 and Agrobacterium tumefaciens A136. Overnight grown C. violaceum CV026 culture (1 ml) (OD600 ≈ 0.1) was added into 100 ml LB agar medium supplemented with kanamycin (20 μg/ml) and C6-HSL (5 μM), mixed, and poured into the plates (Kumar et al., 2021). After the medium was solidified, 2 μl of samples (1–4) (50 mg/ml) were dropped onto the plates and then incubated at 28°C for 24 h, recording the violacein changes in color. The absence of violacein production in CV026 represents compounds that inhibit the QS system. Furthermore, QS inhibitory activity-screened A. tumefaciens A136 was determined as described earlier (Zhu et al., 2018). X-gal (50 μg/ml) and C10-HSL (5 μM) were added into the LB agar medium supplemented with A. tumefaciens A136. Each experiment was repeated three times.
Irpexolaceus H (1), a white amorphous powder, was assigned the molecular formula of C15H24O4 with five degrees of unsaturation based on HRESIMS data at m/z 311.1502 [M + COOH]- (calcd for C16H23O6, 311.1500). The 1H and 13C NMR spectra of 1 (Table 1) revealed 15 carbon resonances, including two methyls at δC 11.8 and 23.9, three methylene carbons at δC 30.9, 40.4, and 42.1, two oxygenated methylene carbons at δC 70.8 and 68.6, three methine carbons at δC 38.7, 39.7, and 40.8, one oxygenated methine carbon at δC 73.3, three sp2 quaternary carbons at δC 126.8, 139.9, and 182.3, and one sp3 quaternary carbon at δC 44.9. These data demonstrated high similarity to irpexolaceus F (Luo et al., 2022), with the main difference being that the low field at δC 139.9 and 126.8 (1) shifted downfield to δC 165.9 and 128.5 in irpexolaceus F, indicating differences in the position of the carbon–carbon double bonds, which was confirmed by the HMBC correlations from H2-8 (δH 1.72, 1.40) and H2-10 (δH 2.17, 1.81) to C-1 (δC 126.8) and from H2-4 (δH 1.99, 1.84), H2-10 (δH 2.17, 1.81), and H2-11 (δH 4.82, 4.73) to C-2 (δC 139.9), in combination with the 1H–1H COSY correlations of H-3 (δH 3.62)/H2-4 (δH 1.99, 1.84)/H-5 (δH 3.96)/H-6 (δH 1.87)/H-7 (δH 3.39)/H2-8 (δH 1.72, 1.40) (Figure 2). The mentioned data combined with previously reported data (Luo et al., 2022) determined the planar structure of 1. In addition, the relative configuration of 1 was determined by the NOESY correlations of H-3 (δH 3.62)/H-6 (δH 1.87)/H-7 (δH 3.39) and Me-13 (δH 0.90)/H-5 (δH 3.96)/Me-14 (δH 1.11) (Figure 2). Combined with the single-crystal X-ray diffraction analysis (Figure 3), the absolute configuration of 1 was confirmed as 3S,5S,6S,7R,9S.
Irpexolaceus I (2), a yellow oil, was assigned the molecular formula of C15H24O5 with four degrees of unsaturation based on HRESIMS data at m/z 283.1551 [M-H]- (calcd for C15H23O5, 283.1551). The 1H and 13C NMR spectra of 2 (Table 1) revealed 15 carbon resonances, including one methyl at δC 24.5, four methylene carbons at δC 40.2, 43.8, 41.6, and 114.0, three oxygenated methylene carbons at δC 60.2, 66.4, and 69.5, three methine carbons at δC 42.1, 143.6, and 46.3, three sp2 quaternary carbons at δC 148.0, 134.1, and 181.5, and one sp3 quaternary carbon at δC 44.0. There were two C=C and one carbonyl group, and the remaining one unsaturation was a ring, which was confirmed by the 1H-1H COSY correlations of H2-8 (δH 1.93, 1.31)/H-7 (δH 3.43)/H-6 (δH 5.81)/H2-13 (δH 5.05, 4.94), and the HMBC correlations from H-7 (δH 3.43) to C-1 (δC 148.0) and C-10 (δC 41.6), from H2-8 (δH 1.93, 1.31) to C-1 (δC 148.0), C-7 (δC 46.3), C-9 (δC 44.0), and C-10 (δC 41.6), and from H2-10 (δH 2.57, 2.35) to C-1 (δC 148.0), C-7 (δC 46.3), C-8 (δC 43.8), and C-9 (δC 44.0). Moreover, a high-field quaternary carbon, C-9 (δC 44.0), was linked to C-14 (δC 24.5) and C-15 (δC 69.5). Combined with another set of 1H–1H COSY correlations of H-3 (δH 3.31)/H2-4 (δH 2.27, 2.11)/H2-12 (δH 3.60, 3.50), the planar structure of 2 was determined as shown in Figure 1, which was highly similar to ceriponol P (Ying et al., 2014), with a difference of a hydroxyl group binding to δC 69.5. In addition, the relative configuration of 2 was confirmed by the NOESY correlations of H-6 (δH 5.81)/H-3 (δH 3.31)/Me-14 (δH 1.07) (Figure 2). The calculated and experimental ECD spectra of 2 showed excellent fit (Figure 4), indicating that the absolute configuration of 2 was 2S,7S,9R.
In addition to the aforementioned compounds, two furans (3 and 4) were obtained and identified as irpexlacte B and C according to the previously reported data (Luo et al., 2022).
Tremulane (i) and (ii) were biosynthesized based on the relative structures of 1 and 2 by the cyclization and rearrangement of farnesyl pyrophosphate (FPP) (Ayer and Browne, 1981; Ayer and Cruz, 1993). After a multi-step reaction such as esterification, cyclization, and dehydration in microbes, tremulane (i) was possibly converted to irpexlaceus H (Figure 5). Moreover, lactarane skeletons ii-1 and ii-2 were produced by a series of methyl migrations of tremulane (ii)) (Ayer and Cruz, 1993), which differed from tremulane (i)) in the configuration of C-1 and C-7. Lactarane skeleton ii-2 was transformed to 5,6-secotremulane under the 5,6-cleavage (He et al., 2020) and then possibly converted to irpexlaceus I by a series of oxidation and dehydration (Figure 5).
Compounds 1–4 were evaluated for their QS inhibitory activities at 50 mg/ml against biomarker strains C. violaceum CV026 (Figure 6A) and A. tumefaciens A136 (Figure 6B). We assessed the inhibitory effect of the compound by assaying the inhibition of C6-HSL-induced violacein production by C. violaceum CV026, and C10-HSL-induced β-galactosidase expression (blue pigment) by A. tumefaciens A136. Compounds 1–2 showed no inhibitory activities against both QS systems of biomarker strains. However, compound 3, in which the binding position of hydroxyl was closer to the furan ring than 4, exhibited stronger inhibition activity against the production of violacein in C. violaceum CV026 than that of the latter but weaker inhibitory activity against the production of blue pigment in A. tumefaciens A136. The results demonstrated that the binding position of hydroxyl was vital for QS inhibitory activity.
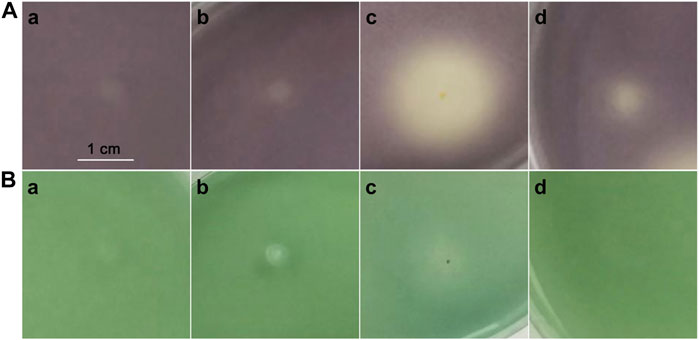
FIGURE 6. Screening of QS inhibitory activity by C. violaceum CV026 (A) and A. tumefaciens A136 (B). Compounds 1 (A), 2 (B), 3 (C), and 4 (D).
Two new tremulane-type sesquiterpenoids, irpexolaceus H (1) and I (2), were isolated from the liquid fermentation of I. lacteus. Their structures were established based on NMR, HRESIMS, IR, single-crystal X-ray diffraction, and ECD analysis. These compounds (1–4) were evaluated for QS inhibitory activities against C. violaceum CV026 and A. tumefaciens A136 at 50 mg/ml. The results found that compound 3 exhibited a significant QS inhibitory activity against C. violaceum CV026, and compound 4 showed a weaker activity. In addition, compound 3 also showed a weak QS inhibitory activity against A. tumefaciens A136. But interestingly, the hydroxyl binding to α-C in the furan ring showed a stronger QS inhibitory activity than that of 4 (hydroxyl binding to β-C in the furan ring), which suggested that the position of hydroxyl in the furan ring was possibly vital for QS inhibitory activity against C. violaceum CV026.
The original contributions presented in the study are publicly available. These data can be found at: https://www.ccdc.cam.ac.uk/, 2133065, 2095205, 2095201, and 2095200.
H-ZL contributed to the chemical and biological experiments and prepared the manuscript draft. HJ contributed to the spectra data analysis. X-SH contributed to the ECD calculation and analysis. A-QJ contributed to the manuscript revision and financial support. All authors approved this manuscript to submit and publish.
This work was supported by the Natural Science Foundation of Hainan Province (319QN165 and 221CXTD434) and the National Natural Science Foundation of China (82160664).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2022.905108/full#supplementary-material
Ayer, W. A., and Browne, L. M. (1981). Terpenoid Metabolites of Mushrooms and Related Basidiomycetes. Tetrahedron 37 (12), 2197–2248. doi:10.1016/S0040-4020(01)97979-7
Ayer, W. A., and Cruz, E. R. (1993). The Tremulanes, a New Group of Sesquiterpenes from the aspen Rotting Fungus Phellinus Tremulae. J. Org. Chem. 58 (26), 7529–7534. doi:10.1021/jo00078a035
Bruhn, T., Schaumlöffel, A., Hemberger, Y., and Bringmann, G. (2013). SpecDis: Quantifying the Comparison of Calculated and Experimental Electronic Circular Dichroism Spectra. Chirality 25 (4), 243–249. doi:10.1002/chir.22138
He, J., Pu, C.-J., Wang, M., Li, Z.-H., Feng, T., Zhao, D.-K., et al. (2020). Conosiligins A-D, Ring-Rearranged Tremulane Sesquiterpenoids from Conocybe Siliginea. J. Nat. Prod. 83 (9), 2743–2748. doi:10.1021/acs.jnatprod.0c00681
Jiang, T., and Li, M. (2013). Quorum sensing Inhibitors: a Patent Review. Expert Opin. Ther. Patents 23 (7), 867–894. doi:10.1517/13543776.2013.779674
Kalia, V. C. (2013). Quorum sensing Inhibitors: an Overview. Biotechnol. Adv. 31 (2), 224–245. doi:10.1016/j.biotechadv.2012.10.004
Kumar, L., Brenner, N., Brice, J., Klein-Seetharaman, J., and Sarkar, S. K. (2021). Cephalosporins Interfere with Quorum Sensing and Improve the Ability of Caenorhabditis elegans to Survive Pseudomonas aeruginosa Infection. Front. Microbiol. 12. doi:10.3389/fmicb.2021.598498
Luo, H.-Z., Jiang, H., Sun, B., Wang, Z.-N., and Jia, A.-Q. (2022). Sesquiterpenoids and Furan Derivatives from the Orychophragmus Violaceus (L.) O.E. Schulz Endophytic Fungus Irpex Lacteus OV38. Phytochemistry 194, 112996. doi:10.1016/j.phytochem.2021.112996
Luo, H.-Z., Zhou, J.-W., Sun, B., Jiang, H., Tang, S., and Jia, A.-Q. (2021). Inhibitory Effect of Norharmane on Serratia marcescens NJ01 Quorum Sensing-Mediated Virulence Factors and Biofilm Formation. Biofouling 37 (2), 145–160. doi:10.1080/08927014.2021.1874942
Neuhaus, G. F., and Loesgen, S. (2020). Antibacterial Drimane Sesquiterpenes from Aspergillus ustus. J. Nat. Prod. 84 (1), 37–45. doi:10.1021/acs.jnatprod.0c00910
Papenfort, K., and Bassler, B. L. (2016). Quorum sensing Signal-Response Systems in Gram-Negative Bacteria. Nat. Rev. Microbiol. 14 (9), 576–588. doi:10.1038/nrmicro.2016.89
Ying, Y.-M., Tong, C.-P., Wang, J.-W., Shan, W.-G., and Zhan, Z.-J. (2014). Ceriponol P, the First Example of Monocyclic Tremulane Sesquiterpene Produced by Ceriporia Lacerate a Fungal Endophyte of Huperzia Serrata. J. Chem. Res. 38 (5), 304–305. doi:10.3184/174751914X13975706150476
Zhou, J., Bi, S., Chen, H., Chen, T., Yang, R., Li, M., et al. (2017). Anti-biofilm and Antivirulence Activities of Metabolites from Plectosphaerella Cucumerina against Pseudomonas aeruginosa. Front. Microbiol. 8, 769. doi:10.3389/fmicb.2017.00769
Keywords: Orychophragmus violaceus (L.) O.E. Schulz, Irpex lacteus (Fr.) Fr, sesquiterpenoids, furan, quorum sensing
Citation: Luo H-Z, Jiang H, Huang X-S and Jia A-Q (2022) New Sesquiterpenoids From Plant-Associated Irpex lacteus. Front. Chem. 10:905108. doi: 10.3389/fchem.2022.905108
Received: 26 March 2022; Accepted: 11 April 2022;
Published: 17 May 2022.
Edited by:
Xuetao Xu, Wuyi University, ChinaReviewed by:
Gang Ding, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaCopyright © 2022 Luo, Jiang, Huang and Jia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ai-Qun Jia, YWppYUBoYWluYW51LmVkdS5jbg==, b3JjaWQub3JnLzAwMDAtMDAwMi04MDg5LTYyMDA=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.