- 1Teaching and Research Office of Chinese Medicines authentication, College of Pharmacy, Shandong University of Traditional Chinese Medicine, Jinan, China
- 2Department of Pharmaceutical Preparation Technology, Department of Pharmaceutical Engineering, Shandong Drug and Food Vocational College, Weihai, China
- 3Grade Three Laboratory of Traditional Chinese Medicine Preparation, Department of Pharmacy, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan, China
- 4Lunan Pharmaceutical Group Co., Ltd., State Key Laboratory of Generic Manufacture Technology of Chinese Traditional Medicine, Linyi, China
Chicoric acid has been widely used in food, medicine, animal husbandry, and other commercial products because of its significant pharmacological activities. However, the shortage of chicoric acid limits its further development and utilization. Currently, Echinacea purpurea (L.) Moench serves as the primary natural resource of chicoric acid, while other sources of it are poorly known. Extracting chicoric acid from plants is the most common approach. Meanwhile, chicoric acid levels vary in different plants as well as in the same plant from different areas and different medicinal parts, and different extraction methods. We comprehensively reviewed the information regarding the sources of chicoric acid from plant extracts, its chemical synthesis, biosynthesis, and bioactive effects.
1 Introduction
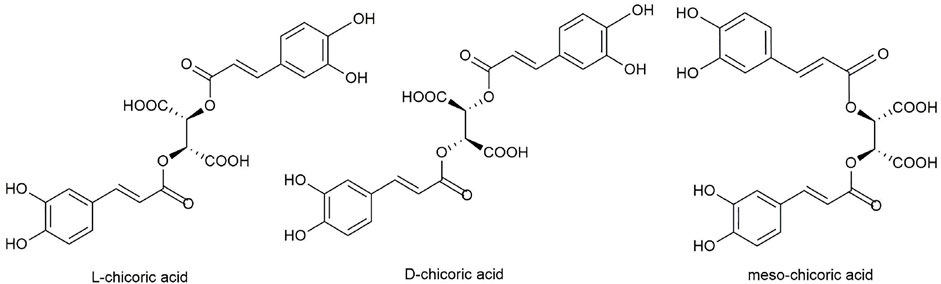
Current research on chicoric acid focuses primarily on medicinal, chemical, natural agricultural production, and food and accounts for 50%, 18%, 13%, and 18%, respectively. Chicoric acid belongs to caffeic acid derivatives and its molecular formula is C22H18O12. Chicoric acid is soluble in ethanol, methanol, dioxane, acetone, and hot water; slightly soluble in ethyl acetate and ether; and insoluble in ligroin, benzene, and chloroform (Scarpati and Oriente, 1958). There are two chiral carbon atoms in this structure, so chicoric acid is divided into levorotatory-chicoric acid (L-chicoric acid), dextrorotatory-chicoric acid (D-chicoric acid), and meso-chicoric acid (meso-chicoric acid) (Figure 1).
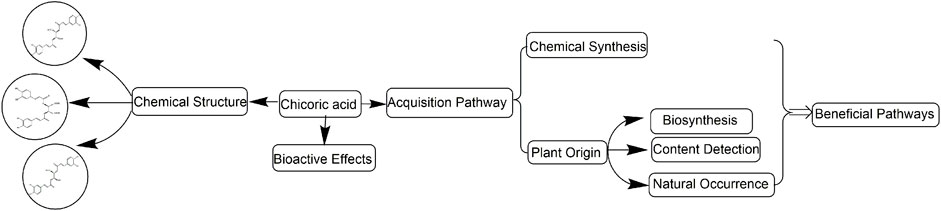
Chicoric acid is a rare and valuable functional food ingredient with no obvious dose dependence, no overdose side effects, and no contraindications and drug interactions. Chicoric acid has been widely used in medicines, nutritional supplements, and health foods due to its promising pharmacological effects in regulating glucose and lipid metabolism; anti-inflammatory, antioxidant, and anti-aging properties, and against digestive system diseases (Peng et al., 2019). Echinacea purpurea (L.) Moench, the main plant material of chicoric acid, has a 400-year history of use in Europe and America. However, the shortage of chicoric acid limits its further development and utilization. So, this article focuses on systematically summarizing chicoric acid sources, such as chemical synthesis and biosynthesis, and further elaborates on resource distribution and content of chicoric acid in different plants, aiming to find a beneficial pathway of chicoric acid production for further development and utilization (Figure 2).
2 Natural Occurrence of Chicoric Acid
Plants containing chicoric acid are rich in resources and widely distributed, so chicoric acid has been utilized as an alternative medicine or a food supplement for quite some time (Street et al., 2013). Chicoric acid levels in different plants and different parts of the same plant often differ significantly. However, there are few reports on chicoric acid levels and different plant resource distributions. At present, E. purpurea is the main crude material for the extraction of chicoric acid, but the shortage of crude material limits further development and utilization. In order to solve that problem, it is necessary to analyze the resource distribution and chicoric acid levels in different plants.
2.1 Plants the Principal Sources of Chicoric Acid
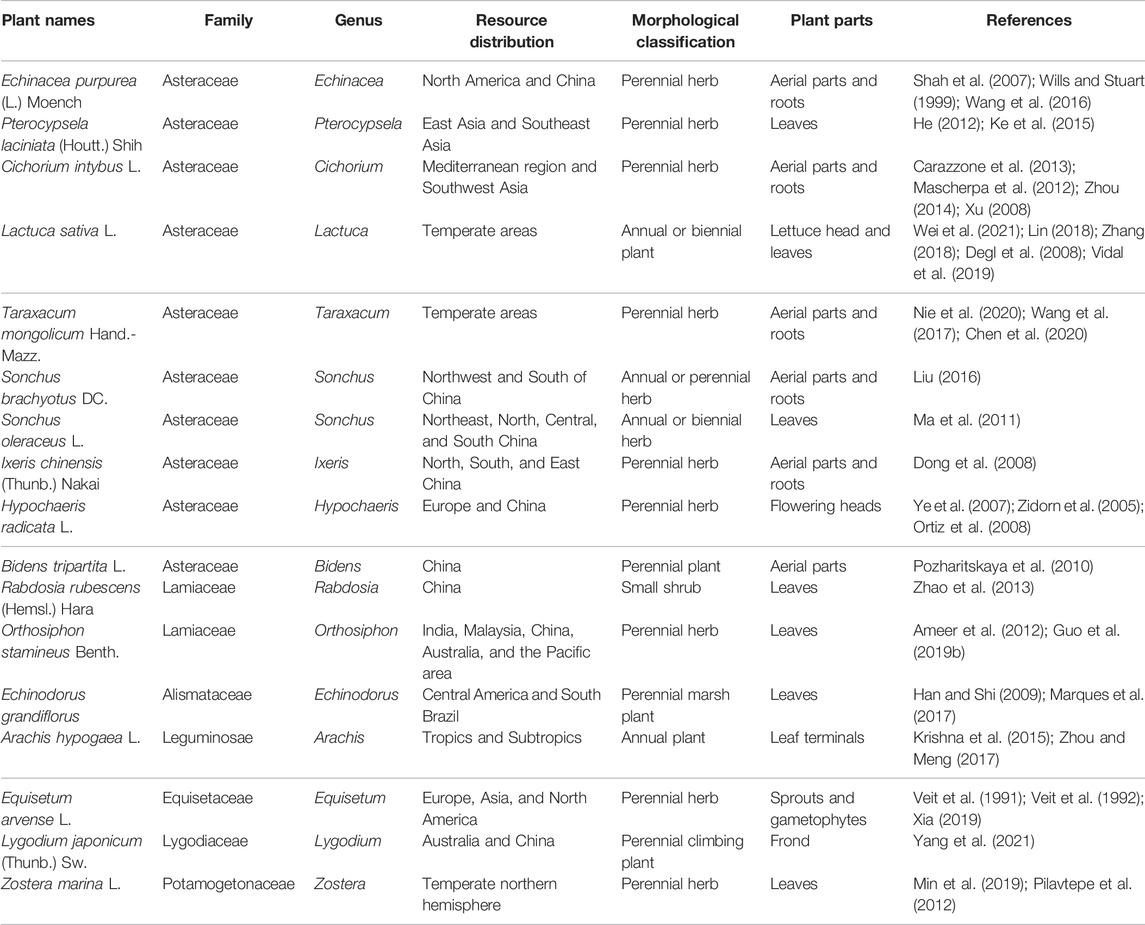
At least 25 families, 63 genera and species, in the plant kingdom contain chicoric acid (Grignon-Dubois and Rezzonico, 2013), there is no substantial guidance due to the lack of detailed information on plant resources. For the convenience of discussion, this article has divided chicoric acid–containing plants into angiosperms, ferns, and other categories (Table 1) and discussed the resource distribution, historical changes, and usage of chicoric acid.
2.1.1 Angiospermae
Chicoric acid is widely distributed in dicotyledons of Angiospermae, namely, Asteraceae, Lamiaceae, Rosaceae, Alismataceae, Cucurbitaceae, and others. Many reports have focused on the study of E. purpurea, Pterocypsela laciniata, and Cichorium intybus of the Asteraceae family.
2.1.1.1 Asteraceae
There are eight species and several varieties of the Echinacea, among which E. purpurea, Echinacea angustifolia (DC) Hell., and Echinacea pallida (Nutt.) are widely used in medicine. E. purpurea is a perennial herb with a medical history dating back more than 300 years (Shah et al., 2007) and was introduced in China as a flower in the 1970s. It is currently cultivated on a large scale in China as a medicinal plant. E. purpurea is widely used in drug materials, nutritional supplements, and health foods. Chicoric acid is often used as an indicator component in the quality evaluation of materials and preparations (Wills and Stuart, 1999; Xu et al., 2006; Sun, 2011; Chen et al., 2012; Han et al., 2013; Han et al., 2014; Wang et al., 2016).
There are eight species and one variety species of Pterocypsela, located primarily in East Asia and Southeast Asia. China is rich in resources, most of which are distributed in the east. As a common weed, Pterocypsela has strong fecundity and adaptability to harsh environments (He, 2012; Ke et al., 2015). P. laciniata is a perennial herb of Pterocypsela with abundant plant resources and chicoric acid.
Cichorium originated in ancient Rome and Greece and was distributed in the Mediterranean and Southwest Asia. Of the eight total species, three exist in China. C. intybus, a perennial herb of Cichorium, was cultivated as a high-grade vegetable in the 19th century. It can be cooked into lettuce (Carazzone et al., 2013) and used for health care. Chicoric acid is an important index for the quality evaluation of C. intybus (Mascherpa et al., 2012; Zhou, 2014), and the amount of chicoric acid differs significantly in different regions and in different areas of the same plant (Xu, 2008).
Lactuca sativa, an annual or biennial vegetable crop, is widely cultivated in temperate areas around the globe. An in-depth analysis did not reveal the origin of L. sativa, but it was first domesticated near the Caucasus (Wei et al., 2021). A variety of cultivation types gradually formed after long-term directional selection and cultivation (Lin, 2018), which were divided into six cultivation types (Zhang, 2018). The content of chicoric acid varied significantly in response to different storage environments (Degl et al., 2008; Oh et al., 2009; Becker et al., 2015; Vidal et al., 2019).
There are more than 2,000 species of the Taraxacum, widely distributed from the temperate areas in the northern hemisphere to the central subtropical regions and South America (Gong et al., 2001). Dandelion is a perennial herb of Taraxacum, and there are 20 species used as medicinal plants in China. Large-scale artificial cultivation has continued in China because of its low growing environment requirements, simple management techniques, and high planting yield (Chen et al., 2020). Taraxacum mongolicum and Taraxacum sinicm Kitag reportedly contain chicoric acid, but there are few studies on the topic (Wang et al., 2017; Nie et al., 2020).
There are 50 species of Sonchus worldwide, located mostly in Europe, Asia, Africa, and the Mediterranean/Atlantic islands (Liu, 2016). Sonchus brachyotus, an annual or perennial herb of Sonchus, sees extensive use in northwest and southern China. S. oleraceus, distributed in Northeast, North, Central, and Southern China (Ma et al., 2011), is not only easy to cultivate but also rich in nutrition. Sonchus asper (L.) Hill and S. oleraceus also contain chicoric acid, but the amount remains unclear.
There are 20 species of Ixeris in North, South, and East China. Ixeris chinensis and Ixeris sonchifolia Hance belong to Ixeris. The perennial herb I. chinensis has been used as a traditional Chinese herbal medicine for thousands of years. I. sonchifolia has been cultivated artificially recently in many areas. Chicoric acid is often used as one of the quality control indexes of these plants (Dong et al., 2008; Zhao and Jiang, 2016).
Hypochaeris radicata is a perennial herb, and studies have indicated that the oldest populations of H. radicata originated in Europe and expanded via at least three migratory routes to other countries (Ortiz et al., 2008) and have been found in Zhejiang and Guizhou of China (Ye et al., 2007). The species are largely used for both food and medicine in Italy. One study has shown a positive correlation between the altitude of the growth environment and the content of chicoric acid (Zidorn et al., 2005). Bidens tripartita, a perennial plant, is widely distributed throughout China. Previous studies on B. tripartita confirmed the presence of chicoric acid in this plant (Pozharitskaya et al., 2010).
2.1.1.2 Lamiaceae
Rabdosia rubescens, a small shrub of Rabdosia, is native to the valley of the Yellow River and the Yangtze River, as well as the Jiyuan Taihang Mountain and Wangwu Mountain in Henan province. The growing environment is characterized by hillsides and woodlands. Recently, the artificial planting of R. rubescens in Jiyuan City has expanded, with yields accounting for 95% of the total Chinese output. R. rubescens in Jiyuan City is a “national geographic product.” In China, R. rubescens is consumed as a famous traditional medicinal herb and tea (Zhao et al., 2013). Although the plant contains chicoric acid, not much research exists on this.
Ocimum basilicum L., a perennial herb of the Ocimum, has more than 150 species around the world. O. basilicum, originated in the warm tropical climates of India, Africa, and southern Asia and has been cultivated worldwide as an aromatic crop and ornamental plant. Some research reported that levels of chicoric acid varied from 0.09 to 0.16 mg/g in dried samples (Kwee and Niemeyer, 2011).
Orthosiphon stamineus belongs to a perennial Orthosiphon herb, occurs widely in India, Malaysia, China, Australia, and the Pacific area. O. stamineus is a valued medicinal plant in traditional folk medicine (Ameer et al., 2012). Leaves of this plant find use in tea, and the rest of the dried plant is used for medicine. Chicoric acid is also the most important bioactive component of this plant (Guo et al., 2019a).
2.1.1.3 Other Genera
Chicoric acid has been detected in other plants from different families, namely, Echinodorus grandiflorus, Cucurbita pepo L. (Iswaldi et al., 2013), Arachis hypogaea, Pulsatilla chinensis (Bge.) Regel (Zhang et al., 2008), and Pyracantha fortuneana (Maxim.) Li. However, acid levels in these plants are unknown and require additional research.
E. grandiflorus, a perennial marsh plant of the Alismataceae, originates from Central America and southern Brazil (Han and Shi, 2009) and has medicinal uses (Marques et al., 2017). C. pepo is a trailing annual herb of the Cucurbitaceae. A. hypogaea, an annual plant of the Leguminosae, is widely distributed in the tropics and subtropics. A. hypogaea plays an important role in the world agricultural economy not only for vegetable oil but also as a source of proteins, minerals, and vitamins (Krishna et al., 2015). A. hypogaea is the highest yielding oil crop in China (Zhou and Meng, 2017).
P. fortuneana is an evergreen shrub or small tree of the Rosaceae. Because of its strong adaptability, P. fortuneana thrives with high yields and is widely distributed in Asia and Europe. Chicoric acid was also detected in the fruit of P. fortuneana. P. chinensis, belonging to the genus Pulsatilla of the buttercup family, is also widely distributed in Europe and Asia. Eleven of the 43 species of this plant have been found in Liaoning, Hebei, and Henan.
2.1.2 Pteridophyta
Chicoric acid may be a specific chemical component in Pteridophyta, because chicoric acid has been detected in 23 of 29 species (Hasegawa and Taneyama, 1973). Pteridaceae, Dryopteridaceae, Equisetaceae, and other Pteridophyta (Cao et al., 2013) have been cultivated for health care.
Equisetum arvense, a perennial herb of Equisetaceae, is native to Europe, Asia, and North America and widely distributed in Heilongjiang (Xia, 2019). Meso-chicoric acid was isolated from the sprouts (fertile) and gametophytes of E. arvense (Veit et al., 1991; Veit et al., 1992), but little reports about the content.
Pteris cretica L. and Onychium japonicum (Thumb.) Kze. belong to Pteridaceae. P. cretica is a perennial evergreen herb. O. japonicum occurs primarily in Taiwan, Japan, Korea, and other Asian countries and is used to treat enteritis, jaundice, flu, chronic gastritis, and fever. Dryopteris erythrosora (D.C. Eaton) Kuntze is a species of Dryopteridaceae native to China and Japan and distributed throughout East Asia. Chicoric acid was detected in the frond of these plants.
Lygodium japonicum, a perennial climbing plant of Lygodiaceae, mainly distributed in Australia and the southwestern area of China (Yang et al., 2021). The entire L. japonicum plant is used to treat various inflammatory diseases. Pteridium aquilinum (L.) Kuhn is a serious invasive weed of upland and marginal lands in many parts of the world (Stewart et al., 2008); however, chicoric acid has been detected in it.
2.1.3 Other Categories
The ocean is a potential source of various raw materials for food and drugs. Compared with terrestrial plants, marine plants with large biomass have obvious advantages as a source of chemical raw materials. Cymodocea nodosa, Syringodium fifiliforme Kütz, and Posidonia oceanica (L.) Delile also contain chicoric acid.
P. oceanica occurs primarily in the Mediterranean Sea. Phenolic compounds are the major metabolites and chicoric acid accounts for 80–89% of the total phenolics. Due to the significant content of chicoric acid, more and more investigations on this plant have taken place (Haznedaroglu and Zeybek, 2007). However, this species is endangered because of anthropogenic effects. C. nodosa is one of the most important macrophytes in the Mediterranean Sea and eastern Atlantic coasts. Some studies have shown that the content of chicoric acid varies in different parts of the plant (Grignon-Dubois and Rezzonico, 2013).
Zostera marina, the most widespread seagrass species of Potamogetonaceae throughout the temperate northern hemisphere (Min et al., 2019), is the largest seagrass meadow in the Bohai Sea and Yellow Sea areas in China. Although chicoric acid has been detected in the leaves of Z. marina, the data are incomplete (Pilavtepe et al., 2012). S. fifiliforme is distributed across the Caribbean Sea and the Gulf of Mexico as seagrass, and it has been reported that S. fifiliforme contains chicoric acid, but no exact statistics are available (Nuissier et al., 2010).
2.2 Methods of Chicoric Acid Extractions and Its Contents in Plants
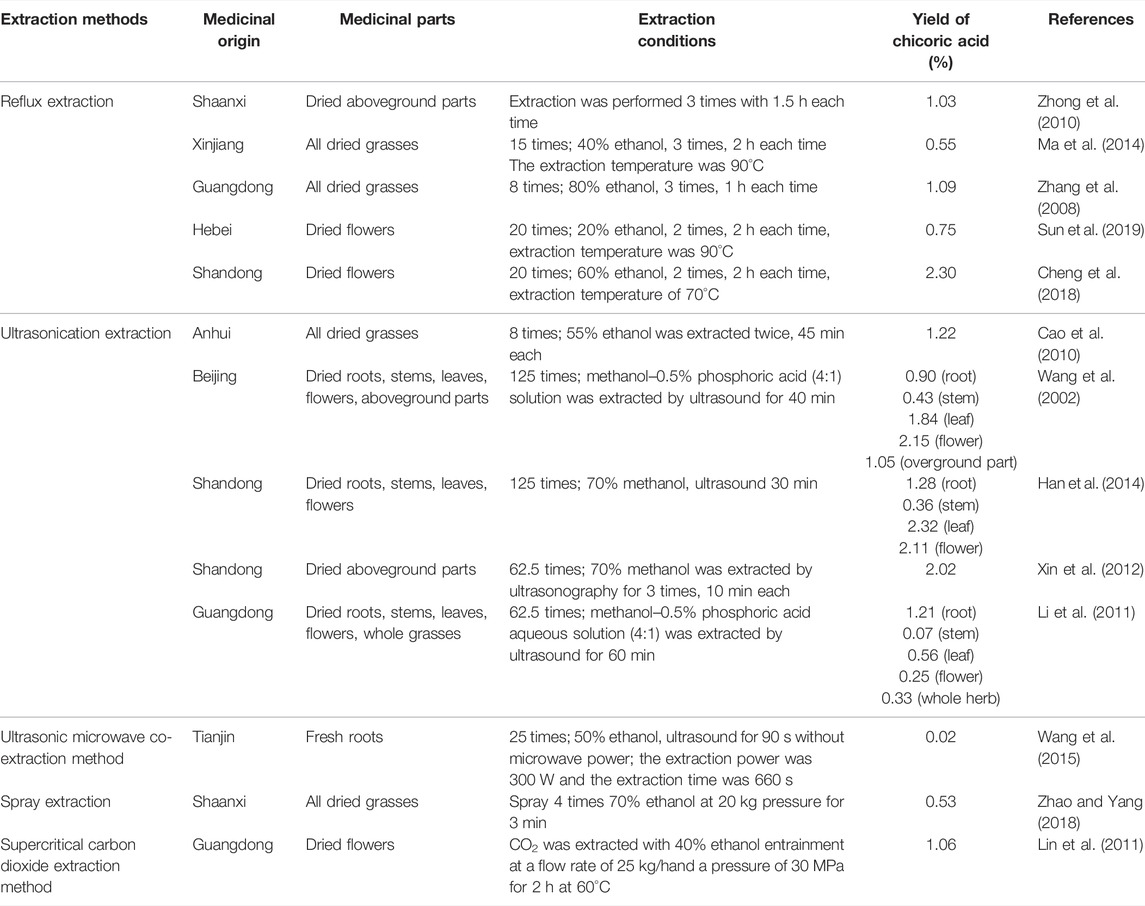
The amount of chicoric acid is closely related to the plant source, medicinal parts, harvest period, processing, and extraction methods. So far, a systematic study on chicoric acid levels has not been found. Although chicoric acid comes from a variety of plants, the selection of safe and economical plant sources requires further research. By analyzing the relationship between factors and the content of chicoric acid, the distribution of chicoric acid in the plant can be preliminarily predicted, which provides a basis for further development of chicoric acid.
2.2.1 Content of Chicoric Acid in Echinacea purpurea
E. purpurea is the raw material for chicoric acid extraction in most studies. Several factors impact chicoric acid levels (Table 2). For 2-year old E. purpurea, the content of chicoric acid is higher in flowers than in other parts during flowering (Wang et al., 2002). The content of chicoric acid in the stems, leaves, and flowers were 9.7%, 44.7%, and 23.6%, respectively (Li et al., 2011). The chicoric acid levels maximized during flowering and provided the best harvest period for E. purpurea (Innocenti et al., 2005).
2.2.2 Chicoric Acid Levels in Other Plant Resources
Related references have reported that the content of chicoric acid in P. laciniata is 26.1 mg/g, 2.2 times higher than in E. purpurea (Ke, 2015). Chicoric acid levels varied with different plant areas and medicinal parts in C. intybus, but chicoric acid levels above ground from Jiangsu were higher (Innocenti et al., 2005). The content of chicoric acid in I. chinensis and S. brachyotus varied greatly, the reason for which may be related to medicinal parts and picking time (Liu, 2016), drying process and preparation method (Sun, 2011), and extraction conditions and other factors (Xin et al., 2012). A summary of these studies appears in Table 3.
3 Chemical Synthesis of Chicoric Acid
E. purpurea is often used as a crude material for chicoric acid production, but a large-scale preparation of high purity chicoric acid has not been reported, which limits its further development and utilization. This requires a synthetic method as an alternative to complement natural plant extraction. In this article, synthetic methods to produce chicoric acid are summarized and the characteristics of each method are compared to provide new synthetic routes.
3.1 Chicoric Acid Synthesis Using (E)-3-(2-Oxo-3a,7a-Dihydrobenzo[d][1,3]Dioxol-5-yl)Acryloyl Chloride and (2R,3S)-2,3-Dihydroxysuccinic Acid
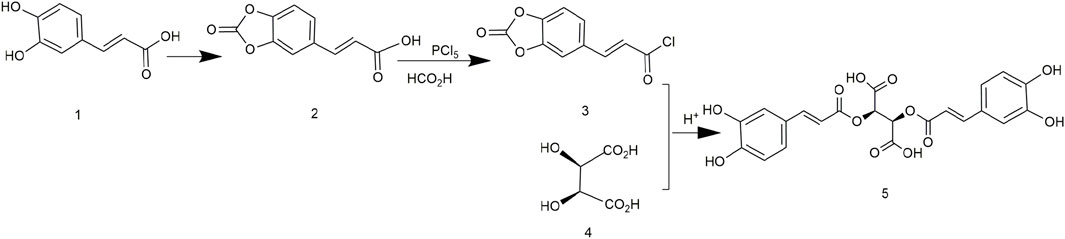
In 1958, chicoric acid was first extracted from C. intybus and its different configurations were chemically synthesized. L-chicoric acid was synthesized via caffeoyl chloride and D-tartaric acid and deprotection of acetic acid (Figure 3). D-chicoric acid, L-chicoric acid, and meso-chicoric acid were synthesized by reactions with L-tartaric acid, D-tartaric acid, and meso-tartaric acid, respectively. These syntheses were simple, but the purity of chicoric acid was too low. In addition, the use of the unstable caffeoyl chloride led to low yields of chicoric acid (Scarpati and Oriente, 1958).
3.2 Chicoric Acid Synthesis Using Di-Tert-Butyl (2R,3S)-2,3-Dihydroxysuccinate and (E)-4-(3-Chloro-3-Oxoprop-1-En-1-yl)-1,2-Phenylene Diacetate
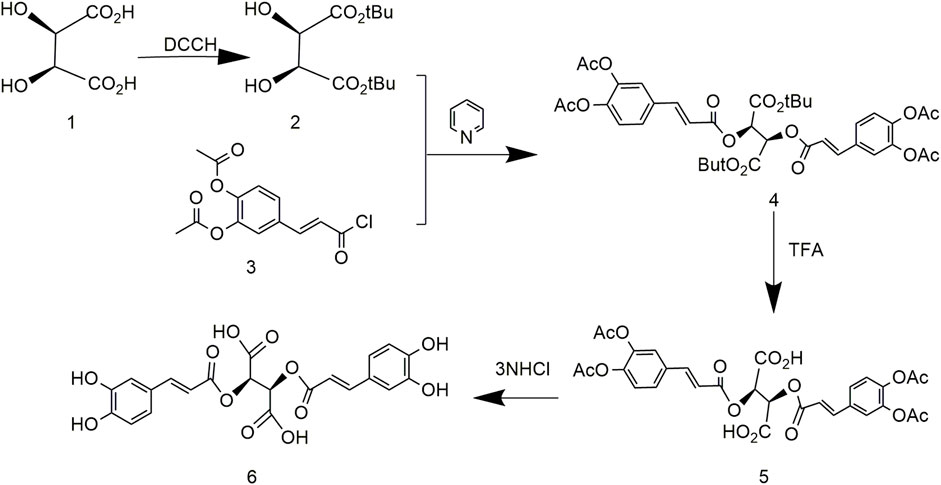
Based on previous research, the optimized process simplified production and occurred under mild reaction conditions (Kulangiappar et al., 2014). First, tert-butyl tartrate was obtained by protecting the carboxyl group of tartaric acid with a tert-butyl group followed by protecting the phenolic hydroxyl of caffeic acid with an acetyl group to produce diacetyl caffeic acid. Then, compound 2 reacted with compound 3, and finally the protective group of previous reaction was removed to obtain L-chicoric acid (Figure 4). The reaction conditions of this method are mild, but L-tert-butyl tartrate is difficult to synthesize. Many by-products and low yields limit large-scale production.
3.3 Chicoric Acid Synthesis Using Dibenzhydryl (2R,3R)-2,3-Dihydroxysuccinate and (Z)-4-(3-Chloro-3-Oxoprop-1-En-1-yl)-1,2-Phenylene Dimethyl Bis(Carbonate)
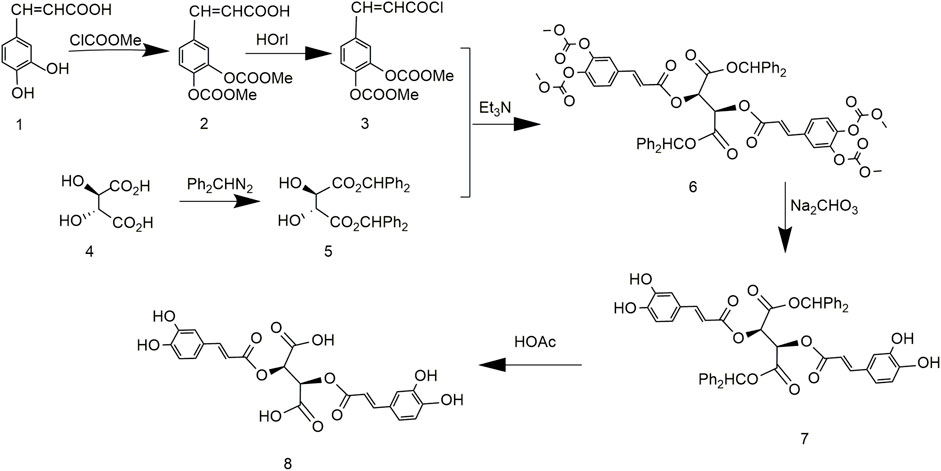
King et al. (1999) reported a method for synthesizing chicoric acid with different configurations by protecting tartaric acid with diphenylmethyl. The synthesis of chicoric acid was mainly carried out in two directions (Figure 5). Diphenylmethane protected the carboxyl group of L-tartaric acid, and the phenolic hydroxyl group of caffeic acid was protected by ClCOOMe to obtain 3,4-cyclocarbonate of caffeoyl chloride. The two protected components reacted, and the protecting groups were removed to obtain L-chicoric acid. Too many steps lead to an overall yield of 33.3%. In addition, the carboxyl-protected crude material of tartaric acid diphenyl diazomethane was unstable to the point of explosion during synthesis, which seriously curtails industrial adaptation.
3.4 Chicoric Acid Synthesis Using Dibenzyl (2R,3R)-2,3-Dihydroxysuccinate and (E)-3-(3,4-Bis(Benzyloxy)Phenyl)Acrylic Acid
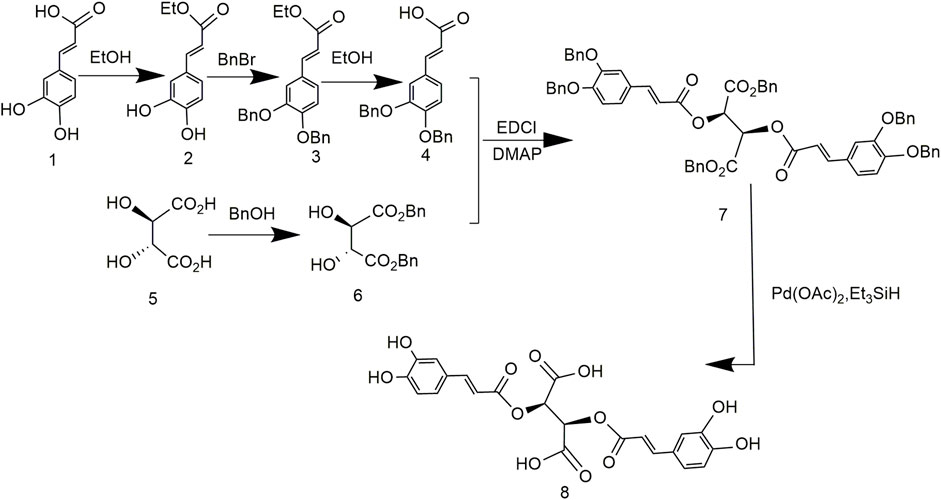
To address problems caused by expensive tartaric acid derivatives and poor reproducibility, the synthesis of chicoric acid was further optimized. Both carboxyl groups of tartaric acid and the phenolic hydroxyl group of caffeic acid were protected by benzylation, followed by esterification and reduction to obtain L-chicoric acid (Figure 6). This synthetic route used heavy metal salts, which increased the production cost and led to heavy metal residues. In addition, the by-products affected drug quality (Lamidey et al., 2002).
3.5 Chicoric Acid Synthesis Using (E)-3-(3,4-Diacetoxyphenyl)Acrylic Acid and (2R,3R)-2,3-Dihydroxysuccinic Acid
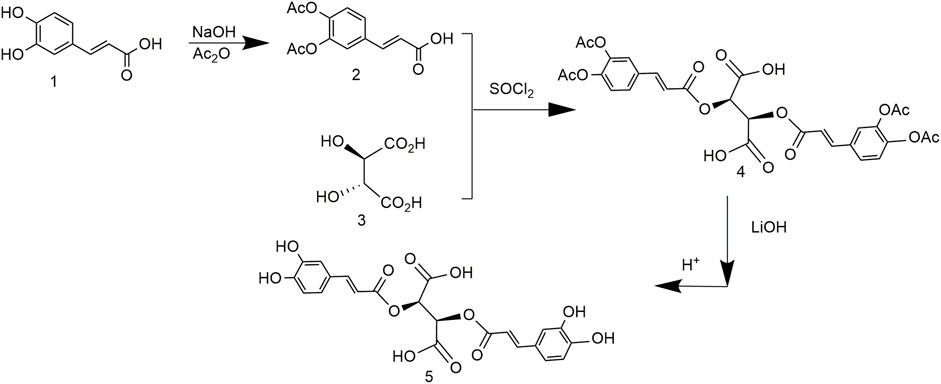
Chicoric acid synthesis was improved based on a previous work to simplify the route and improve the purity (Zhou, 2014). The phenolic hydroxyl of caffeic acid was protected by an acetyl group and reacted with L-tartaric acid to obtain (2R,3R)-2,3-bis(((E)-3-(3,4-diacetoxyphenyl)acryloyl)oxy)succinic acid. LiOH hydrolysis removed the protecting group; the metal ions and chicoric acid formed a stable complex and were hydrolyzed to obtain relatively pure chicoric acid (Figure 7). Although the purity of the product was 99.7%, it did not maximize the yield or reduce the cost.
3.6 Chicoric Acid Synthesis Using (E)-Hypochlorous (E)-3-(2-Oxidobenzo[d][1,3,2]Aioxathiol-5-yl)Acrylic Anhydride and (2R,3R)-2,3-Dihydroxysuccinic Acid
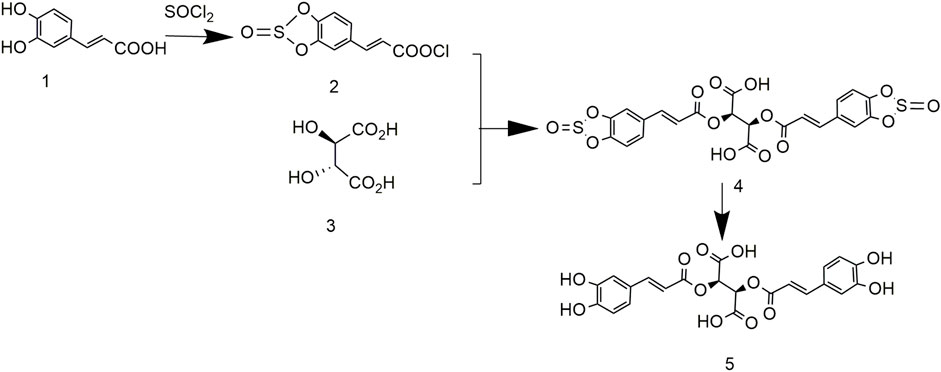
To solve the problem of high cost and low yield, the synthesis of chicoric acid was optimized, and its crystal shape studied (Tian et al., 2021). The product of caffeic acid and sulfoxide chloride reacted with L-tartaric acid to yield L-gesnerate sulfonate. After removing the protecting group in an alkaline solution, L-chicoric acid was obtained (Hunan Normal University, Changsha, 2021; Figure 8). After further crystallization, the yield and purity of chicoric acid were 80.5% and 98.5%, respectively. Although the purity and yield of the product increased, the process was still cumbersome.
The structure of chicoric acid was obtained by intermolecular esterification of tartaric acid and two molecules of caffeic acid. Because both tartaric acid and caffeic acid contained hydroxyl and carboxyl groups, side reactions readily occurred. Therefore, the most common synthetic pathway utilized protective groups to protect phenolic hydroxyl groups of caffeic acid and carboxyl groups of tartaric acid before esterification. The difference lies in the selection of raw materials and the different reagents to protect phenolic hydroxyl groups of tartaric acid. These protecting groups include tert-butyl, benzyl, or carboxyl benzyl to protect tartaric acid, while the caffeic acid phenol hydroxyl was protected by acetyl, benzyl ethyl, oxygen acyl, or metal ions. Despite the myriad synthetic methods, none brought higher quality chicoric acid in high purity, yield, and economic and environmental protection.
4 Biosynthesis of Chicoric Acid
Chicoric acid is a phenolic acid and generally forms via the shikimic acid‐phenylpropanoid pathway (Kuhnl et al., 1987; Petersen et al., 2009). Understanding what factors regulate the distribution of chicoric acid in plants may help regulate chicoric acid accumulation by altering certain conditions. Although there are many studies on the biosynthetic pathway of chicoric acid, its mechanism remains unclear.
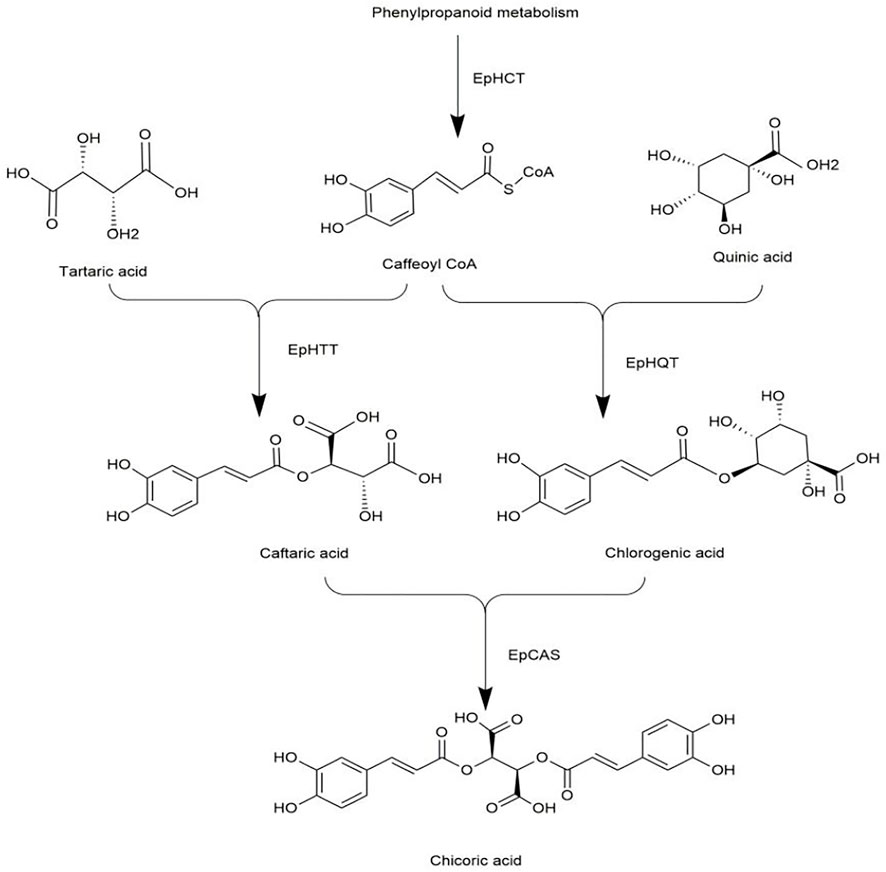
Recently, a study filled this gap by reporting the biosynthetic pathway of chicoric acid in Echinacea (Fu et al., 2021). The biosynthesis of chicoric acid occurs in three main stages. Firstly, phenylpropanoid reacts to form caffeoyl CoA via the enzyme EpHCT. Secondly, EpHTT and EpHQT were responsible for the biosynthesis of caftaric acid and chlorogenic acid in the cytosol, respectively. Finally, caftaric acid and chlorogenic acid were transferred from the cytosol into vacuole, and chicoric acid was synthesized via EpCAS (Figure 9). The biosynthetic pathway of chicoric acid production has been reprogrammed in tobacco, but its applicability in other species needs additional study.
5 Bioactive Effects
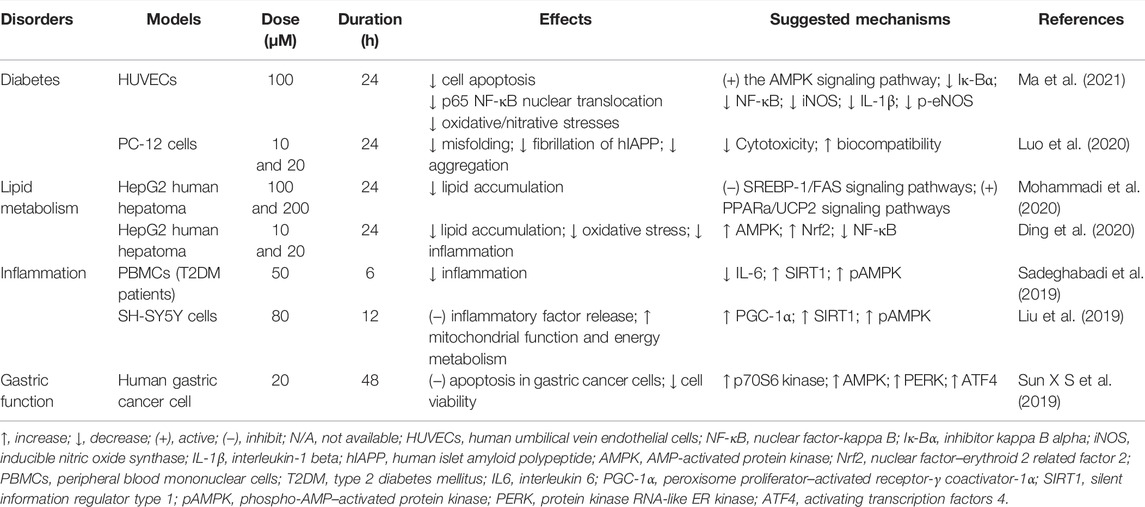
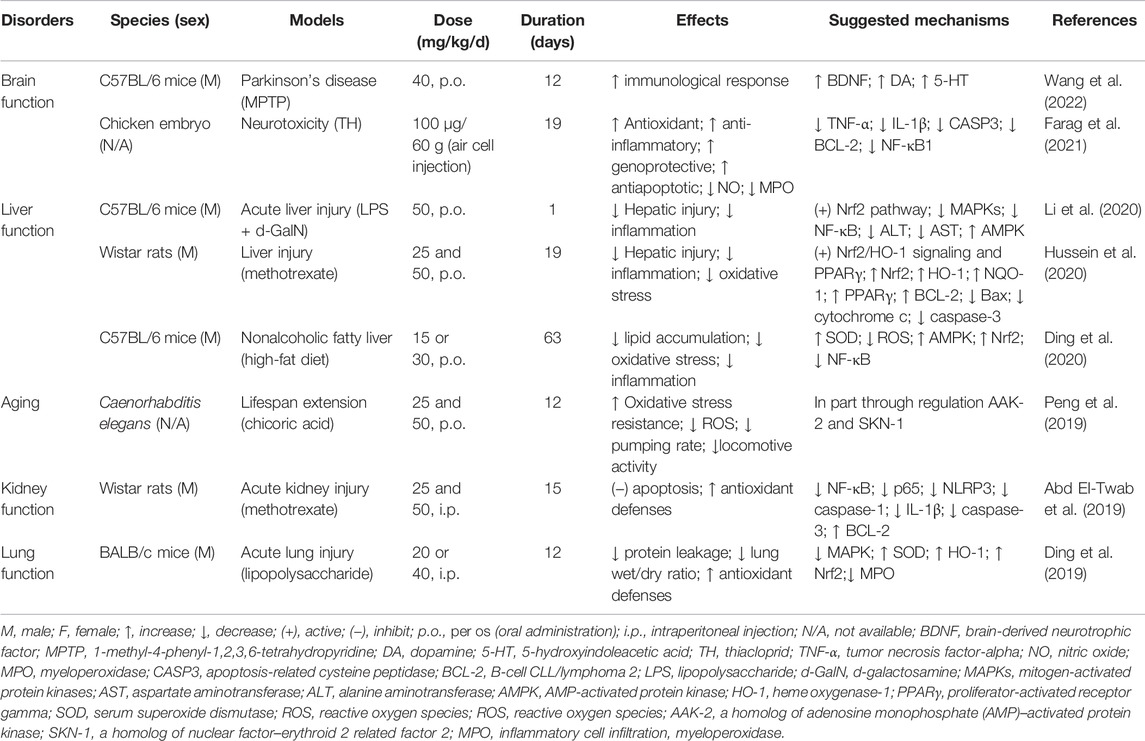
Chicoric acid has a long history of clinical use and can effectively treat a variety of diseases. Because of this, numerous studies that have focused on chicoric acid have been conducted on the biological activities of both in vitro and in vivo models. Chicoric acid has long attracted attention as a medication and nutraceutical to improve health based on its anti-inflammation (Liu, 2016; Liu et al., 2017a; Liu et al., 2017b; Tsai et al., 2017; Liu et al., 2019; Li et al., 2020), glucose and lipid homeostasis (Kim et al., 2017; Lipchock et al., 2017), neuroprotection effects (Bekinschtein et al., 2008; Kour and Bani, 2011a), anti-aging effects (De Winter, 2015; Peng et al., 2019), and antioxidant and immune-stimulating properties (Kour and Bani, 2011b; Schlernitzauer et al., 2013; Chen et al., 2017; Wang et al., 2017; Jia et al., 2018; Ma et al., 2018). In addition, its antivirus properties, such as immunodeficiency viruses (Healy et al., 2009; Crosby et al., 2010; Nobela et al., 2018), herpes simplex viruses (Langland et al., 2018), and respiratory syncytial virus (Zhang et al., 2021) are particularly significant. Tables 4 and 5 summarize these studies.
6 Conclusion and Perspectives
Although there are many chemical synthetic methods, the environmentally friendly and economic synthesis method for bulk preparation of chicoric acid with high purity and high yield needs optimization. Chicoric acid is unstable and the solubility of chicoric acid varies greatly in different solvents. Therefore, the stability and solubility of chicoric acid can be improved by a structural modification to preserve the activity of chicoric acid to meet the needs of different dosages. The biosynthesis of chicoric acid in E. purpurea is still at the preliminary research stage, and the mechanism also requires additional study to clarify. The enrichment of chicoric acid in different plants, parts, and stages can be further explained via an in-depth study of its biosynthesis to regulate and improve the content of chicoric acid by modern biotechnology. Even though there are an increasing number of studies reporting bioactivities of chicoric acid, there are still limitations in arriving at a concrete conclusion due to differences in models, doses, and treatment durations used. Thus, research on chicoric acid requires additional study, and in-depth studies on the pharmacodynamic mechanism are needed to guide clinical medication.
L-chicoric acid was isolated from E. purpurea and P. chinensis (Zhang et al., 2008), D-chicoric acid from C. intybus, and meso-chicoric acid from E. arvense (Veit et al., 1991; Veit et al., 1992), but the optical isomers of chicoric acid from different plants have not been systematically summarized and their pharmacological activities of different optical isomers need to be further studied. C. intybus, L. sativa, E. purpurea, and other herbs belong to medicinal and food homologous plants. The amount of chicoric acid in P. laciniata was significantly higher than in E. purpurea. Some marine resources with large biomasses require more study and utilization. New medicinal resources of chicoric acid should be expanded using resource survey, biological relationships, pharmacological activity, and similarity of the growing environment. It is necessary to systematically study the related factors affecting chicoric acid levels, look for dominant species, and formulate a good scientific agriculture practice based on the biosynthesis and accumulation mechanism of chicoric acid. At the same time, a combination of modern technology and new methods such as tissue culture and biotechnology should help optimize the synthesis of chicoric acid.
Author Contributions
Data collection: XL, FJ, LQ, TZ, CW, and GL; design, analysis, and interpretation of the data: CW, TZ, LS, JL, and HL; critical revision of the manuscript: SF and FL. All authors contributed to writing and formatting of the final version.
Funding
This study was supported by the National Science and Technology Major Project for Key New Drug Development of China (No. 2017ZX09301058); National Natural Science Foundation of China (No. 82004233); and Project of Medical and Health Technology Development Program in Shandong Province (No. 202013030996).
Conflict of Interest
Author HL was employed by the company Lunan Pharmaceutical Group Co., Ltd
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2022.888673/full#supplementary-material
References
Abd El-Twab, S. M., Hussein, O. E., Hozayen, W. G., Bin-Jumah, M., and Mahmoud, A. M. (2019). Chicoric Acid Prevents Methotrexate-Induced Kidney Injury by Suppressing NF-Κb/nlrp3 Inflammasome Activation and Up-Regulating Nrf2/ARE/HO-1 Signaling. Inflamm. Res. 68, 511–523. doi:10.1007/s00011-019-01241-z
Ameer, O. Z., Salman, I. M., Asmawi, M. Z., Ibraheem, Z. O., and Yam, M. F. (2012). Orthosiphon Stamineus: Traditional Uses, Phytochemistry, Pharmacology, and Toxicology. J. Med. Food 15, 678–690. doi:10.1089/jmf.2011.1973
Becker, C., Urlić, B., Jukić Špika, M., Kläring, H. P., Krumbein, A., Baldermann, S., et al. (2015). Nitrogen Limited Red and Green Leaf Lettuce Accumulate Flavonoid Glycosides, Caffeic Acid Derivatives, and Sucrose while Losing Chlorophylls, Β-Carotene and Xanthophylls. PLoS One 10, e0142867. doi:10.1371/journal.pone.0142867
Bekinschtein, P., Cammarota, M., Katche, C., Slipczuk, L., Rossato, J. I., Goldin, A., et al. (2008). BDNF Is Essential to Promote Persistence of Long-Term Memory Storage. Proc. Natl. Acad. Sci. U.S.A. 105, 2711–2716. doi:10.1073/pnas.071186310510.1073/pnas.0711863105
Cao, J., Xia, X., Chen, X., Xiao, J., and Wang, Q. (2013). Characterization of Flavonoids from Dryopteris Erythrosora and Evaluation of Their Antioxidant, Anticancer and Acetylcholinesterase Inhibition Activities. Food Chem. Toxicol. 51, 242–250. doi:10.1016/j.fct.2012.09.039
Cao, L., Hao, Z. H., Sun, J., and Wang, C. Y. (2010). Study on the Optimization of Uitrasoni Wave Extraction Cichoric Acid from Echinacea Purpurea. Chin. J. Veterinary Med. 44, 35–37. CNKI: SUN: ZSYY.0.2010-05-017. https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD2010&filename=ZSYY201005017&uniplatform=NZKPT&v=U8GakrH3eo7DiiaNV3JTqHZuzGNxW_inEnpPpBHp0ePYKkofWX7A2z2ycDd-tI0s.
Carazzone, C., Mascherpa, D., Gazzani, G., and Papetti, A. (2013). Identification of Phenolic Constituents in Red Chicory Salads (Cichorium Intybus) by High-Performance Liquid Chromatography with Diode Array Detection and Electrospray Ionisation Tandem Mass Spectrometry. Food Chem. 138, 1062–1071. doi:10.1016/j.foodchem.2012.11.060
Chen, L., Huang, G., Gao, M., Shen, X., Gong, W., Xu, Z., et al. (2017). Chicoric Acid Suppresses BAFF Expression in B Lymphocytes by Inhibiting NF-Κb Activity. Int. Immunopharmacol. 44, 211–215. doi:10.1016/j.intimp.2017.01.021
Chen, M. G. N., Wang, H., Li, Y. S., Fan, Q. L., and Qin, X. (2020). Research Progress on Cultivation Techniques and Application of Medicinal and Edible Dandelion. J. Shanxi Agric. Sci. 48, 2007–2011. CNKI: SUN: SXLX.0.2020-12-037. https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFDLAST2020&filename=SXLX202012037&uniplatform=NZKPT&v=sAxHpliTfBVr29GyS7p2Y7ET6rD5rv_hlyp5UrDWMeKx0_FY7NMv71rNLiLufvFk.
Chen, S., Pan, S., Zhao, W., Yang, Z., Wu, H., and Yang, Y. (2012). Synthesis, Crystal Structure and Characterization of a New Compound, Li3NaBaB6O12. Solid State Sci. 14, 1186–1190. CNKI: SUN: ZCYO.0.2012-06-043. doi:10.1016/j.solidstatesciences.2012.05.026 https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD2012&filename=ZCYO201206043&uniplatform=NZKPT&v=Rhr96zUc9WoK_QLcCWWurdOCoHZiC9TmJjDKvKasaWQ2k6OoOwSuuYO_XgBLGDvy.
Cheng, Y. X., Liu, Y. F., Sun, Q. X., and Guo, S. F. (2018). Study on the Extraction Technology and Anti-inflammatory Activity of the Effective Components in Echinacea Purpurea Flower. J. Pharm. Res. 37, 39–141+145. doi:10.13506/j.cnki.jpr.2018.03.003
Crosby, D. C., Lei, X., Gibbs, C. G., McDougall, B. R., Robinson, W. E., and Reinecke, M. G. (2010). Design, Synthesis, and Biological Evaluation of Novel Hybrid Dicaffeoyltartaric/diketo Acid and Tetrazole-Substituted L-Chicoric Acid Analogue Inhibitors of Human Immunodeficiency Virus Type 1 Integrase. J. Med. Chem. 53, 8161–8175. doi:10.1021/jm1010594
De Winter, G. (2015). Aging as Disease. Med Health Care Philos 18, 237–243. doi:10.1007/s11019-014-9600-y
Degl, E. I., Pardossi, A., Tattini, M., and Guidi, L. (2008). Phenolic Compounds and Antioxidant Power in Minimally Processed Salad. J. Food Biochem. 32, 642–653. doi:10.1111/j.1745-4514.2008.00188.x
Ding, H., Ci, X., Cheng, H., Yu, Q., and Li, D. (2019). Chicoric Acid Alleviates Lipopolysaccharide-Induced Acute Lung Injury in Mice through Anti-inflammatory and Anti-oxidant Activities. Int. Immunopharmacol. 66, 169–176. doi:10.1016/j.intimp.2018.10.042
Ding, X., Jian, T., Li, J., Lv, H., Tong, B., Li, J., et al. (2020). Chicoric Acid Ameliorates Nonalcoholic Fatty Liver Disease via the AMPK/Nrf2/NFκB Signaling Pathway and Restores Gut Microbiota in High-Fat-Diet-Fed Mice. Oxidative Med. Cell. Longev. 2020, 1–20. doi:10.1155/2020/9734560
Dong, J. P. (2008). Isolation of Chemical Composition in Ixeris Sonchifolia (Bge.) Hance and Assaying Collected at Different Time. [Jilin (JL)]: Jilin Agricultural University. [dissertation].
Farag, M. R., Khalil, S. R., Zaglool, A. W., Hendam, B. M., Moustafa, A. A., Cocco, R., et al. (2021). Thiacloprid Induced Developmental Neurotoxicity via ROS-Oxidative Injury and Inflammation in Chicken Embryo: The Possible Attenuating Role of Chicoric and Rosmarinic Acids. Biology 10, 1100. doi:10.3390/biology10111100
Fu, R., Zhang, P., Jin, G., Wang, L., Qi, S., Cao, Y., et al. (2021). Versatility in Acyltransferase Activity Completes Chicoric Acid Biosynthesis in Purple Coneflower. Nat. Commun. 12. doi:10.1038/s41467-021-21853-6
Gong, Z. N., Zhang, W. M., Liu, C. H., He, B., and Tan, R. X. (2001). Taraxacum Plant Resources in China. China's Wild Plant Resour. 3, 9–14+5. CNKI: SUN: ZYSZ.0.2001-03-003. https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD2001&filename=ZYSZ200103003&uniplatform=NZKPT&v=Jj1LfyIr3XIY0gklnrP1hhkO3P0sw7j5upxP_Tw53J0OwqRbRuxOXrl2EOT34fBb.
Grignon-Dubois, M., and Rezzonico, B. (2013). The Economic Potential of Beach-Cast Seagrass - Cymodocea Nodosa: a Promising Renewable Source of Chicoric Acid. Bot. Mar. 56, 303–311. doi:10.1515/bot-2013-0029
Guo, Z., Li, B., Gu, J., Zhu, P., Su, F., and Bai, R. (2019a). Simultaneous Quantification and Pharmacokinetic Study of Nine Bioactive Components of Orthosiphon Stamineus Benth. Extract in Rat Plasma by UHPLC-MS/MS. Molecules 24, 3057. doi:10.3390/molecules24173057
Guo, Z., Liang, X., and Xie, Y. (2019b). Qualitative and Quantitative Analysis on the Chemical Constituents in Orthosiphon Stamineus Benth. Using Ultra High-Performance Liquid Chromatography Coupled with Electrospray Ionization Tandem Mass Spectrometry. J. Pharm. Biomed. Analysis 164, 135–147. doi:10.1016/j.jpba.2018.10.023
Han, L. N., Sun, J. Y., and Guo, Q. M. (2014). Dynamic Determination of Active Ingredients in Introduced Echinacea Purpurea. World Sci. Technol. TCM Mat. Med. 16, 1858–1861. CNKI: SUN: SJKX.0.2014-08-033.
Han, L. N., Sun, J. Y., Guo, Q. M., and Zhou, F. Q. (2013). Study on Drying Methods of Introduced Echinacea Purpurea. J. Tradit. Chin. Med. 40, 2549–2551. doi:10.13192/j.issn.1000-1719.2013.12.062
Han, S. Q., and Shi, W. F. (2009). Study on the Cultivation Methods of Echinodorus Grandiflorus. J. Lands. Res. 1, 1054–1055. doi:10.13989/j.cnki.0517-6611.2009.03.107
Hasegawa, M., and Taneyama, M. (1973). Chicoric Acid from Onychium Japonicum and its Distribution in the Ferns. Bot. Mag. Tokyo 86, 315–317. doi:10.1007/bf02488787
Haznedaroglu, M. Z., and Zeybek, U. (2007). HPLC Determination of Chicoric Acid in Leaves ofPosidonia Oceanica. Pharm. Biol. 45, 745–748. doi:10.1080/1388020070158571710.1080/13880200701585717
He, W. Q. (2012). A Taxonomic Study on Pterocypsela Shih (Lactuceae-Compositae). [Zhengzhou (HA)]: Zhengzhou University. [dissertation].
Healy, E. F., Sanders, J., King, P. J., and Robinson, W. E. (2009). A Docking Study of L-Chicoric Acid with HIV-1 Integrase. J. Mol. Graph. Model. 27, 584–589. doi:10.1016/j.jmgm.2008.09.011
Hua, C., Li, J. L., Zhou, F., Zhou, Q. C., Zhang, B. J., Wang, R. L., et al. (2011). Process Optimization for Cichoric Acid Extraction from Cichorium Lntybus L. Shoots. Food Sci. 32, 126–129. CNKI: SUN: SPKX.0.2011-20-027. https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD2011&filename=SPKX201120027&uniplatform=NZKPT&v=BYZSl-Ec_IkNMeWiUlunMoviCLluBYN9Mg6uzPawPNDn5iVODCvPNYLXq8cq6Xuu.
Hussein, O. E., Hozayen, W. G., Bin-Jumah, M. N., Germoush, M. O., Abd El-Twab, S. M., and Mahmoud, A. M. (2020). Chicoric Acid Prevents Methotrexate Hepatotoxicity via Attenuation of Oxidative Stress and Inflammation and Up-Regulation of PPARγ and Nrf2/HO-1 Signaling. Environ. Sci. Pollut. Res. 27, 20725–20735. doi:10.1007/s11356-020-08557-y
Innocenti, M., Gallori, S., Giaccherini, C., Ieri, F., Vincieri, F. F., and Mulinacci, N. (2005). Evaluation of the Phenolic Content in the Aerial Parts of Different Varieties of Cichorium Intybus L. J. Agric. Food Chem. 53, 6497–6502. doi:10.1021/jf050541d
Iswaldi, I., Gómez-Caravaca, A. M., Lozano-Sánchez, J., Arráez-Román, D., Segura-Carretero, A., and Fernández-Gutiérrez, A. (2013). Profiling of Phenolic and Other Polar Compounds in Zucchini (Cucurbita Pepo L.) by Reverse-phase High-Performance Liquid Chromatography Coupled to Quadrupole Time-Of-Flight Mass Spectrometry. Food Res. Int. 50, 77–84. doi:10.1016/j.foodres.2012.09.030
Jia, L., Chen, Y., Tian, Y. H., and Zhang, G. (2018). MAPK Pathway Mediates the Anti-oxidative E-ffect of C-hicoric A-cid against C-erebral I-schemia-reperfusion I-njury I-n�vivo. Exp. Ther. Med. 15, 1640–1646. doi:10.3892/etm.2017.5598
Ke, X. H. (2015). Separation, Purification and Microbial Transformation of Cichoric Acid from Pterocarpus Multilobate. Nanjing (China): Nanjing Normal University.
Ke, X. H., Chen, Y. R., Zhao, W. W., Qian, Y. Y., Dai, K. W., and Tang, C. K. (2015). Separation and Purification of Cichoric Acid in Pterocypsela Laciniata (Houtt.) Shih Extracts. J. Nanjing Norm. Univ. 15, 88–92. CNKI:SUN: NJSE. 0.2015-02-016.
Kim, M., Yoo, G., Randy, A., Kim, H. S., and Nho, C. W. (2017). Chicoric Acid Attenuate a Nonalcoholic Steatohepatitis by Inhibiting Key Regulators of Lipid Metabolism, Fibrosis, Oxidation, and Inflammation in Mice with Methionine and Choline Deficiency. Mol.Nutr.Food Res. 65. doi:10.1002/mnfr.201600632
King, P.J., Ma, G., Miao, W., Jia, Q., McDougall, B. R., and Reinecke, M. G. (1999). Structure‐Activity Relationships: Analogues of the Dicaffeoylquinic and Dicaffeoyltartaric Acids as Potent Inhibitors of Human Immunodeficiency Virus Type 1 Integrase and Replication. J. Med. Chem. 42, 497–509. doi:10.1021/jm9804735
Kour, K., and Bani, S. (2011b). Augmentation of Immune Response by Chicoric Acid through the Modulation of CD28/CTLA-4 and Th1 Pathway in Chronically Stressed Mice. Neuropharmacology 60, 852–860. doi:10.1016/j.neuropharm.2011.01.001
Kour, K., and Bani, S. (2011a). Chicoric Acid Regulates Behavioral and Biochemical Alterations Induced by Chronic Stress in Experimental Swiss Albino Mice. Pharmacol. Biochem. Behav. 99, 342–348. doi:10.1016/j.pbb.2011.05.008
Krishna, G., Singh, B. K., Kim, E.-K., Morya, V. K., and Ramteke, P. W. (2015). Progress in Genetic Engineering of Peanut (Arachis hypogaea L.)-A Review. Plant Biotechnol. J. 13, 147–162. doi:10.1111/pbi.12339
Kühnl, T., Koch, U., Heller, W., and Wellmann, E. (1987). Chlorogenic Acid Biosynthesis: Characterization of a Light-Induced Microsomal 5-O-(4-Coumaroyl)-D-Quinate/shikimate 3′-hydroxylase from Carrot (Daucus Carota L.) Cell Suspension Cultures. Archives Biochem. Biophysics 258, 226–232. doi:10.1016/0003-9861(87)90339-0
Kulangiappar, K., Anbukulandainathan, M., and Raju, T. (2014). Nuclear versus Side-Chain Bromination of 4-Methoxy Toluene by an Electrochemical Method. Synth. Commun. 44, 2494–2502. doi:10.1080/00397911.2014.905599
Kwee, E. M., and Niemeyer, E. D. (2011). Variations in Phenolic Composition and Antioxidant Properties Among 15 Basil (Ocimum Basilicum L.) Cultivars. Food Chem. 128, 1044–1050. doi:10.1016/j.foodchem.2011.04.011
Lamidey, A.-M., Fernon, L., Pouységu, L., Delattre, C., Quideau, S., and Pardon, P. (2002). A Convenient Synthesis of the Echinacea-Derived Immunostimulator and HIV-1 Integrase Inhibitor (−)-(2r,3r)-Chicoric Acid. Hca 85, 2328–2334. doi:10.1002/1522-2675(200208)85:8<2328:aid-hlca2328>3.0.co;2-x
Langland, J., Jacobs, B., Wagner, C. E., Ruiz, G., and Cahill, T. M. (2018). Antiviral Activity of Metal Chelates of Caffeic Acid and Similar Compounds towards Herpes Simplex, VSV-Ebola Pseudotyped and Vaccinia Viruses. Antivir. Res. 160, 143–150. doi:10.1016/j.antiviral.2018.10.021
Li, J., Nie, B., Wu, L., and Zeng, Z. L. (2011). Determination of Cichoric Acid in Introducing Plant of Echinacea Purpurea. Chin. J. Pharm. Anal. 31, 1804–1807. doi:10.16155/j.0254-1793.2011.09.025
Li, Z., Feng, H., Han, L., Ding, L., Shen, B., Tian, Y., et al. (2020). Chicoric Acid Ameliorate Inflammation and Oxidative Stress in Lipopolysaccharide and D ‐galactosamine Induced Acute Liver Injury. J. Cell Mol. Med. 24, 3022–3033. doi:10.1111/jcmm.14935
Lin, J., Chen, R. X., and Ceng, W. S. (2011). Extraction of Cichoric Acid from Echinacea Purpurea by Supercritical CO2 Fliud. China Food Addit. 3, 60–64. CNKI: SUN: ZSTJ.0.2011-03-00.
Lin, K. (2018). “Investigation of Suitable Lighting Modes for Lactuca sativa L,” in Protected Hydroponic Cultivation ([Fujian (FJ)]: Fujian Agriculture and Forestry University). [dissertation].
Lipchock, J. M., Hendrickson, H. P., Douglas, B. B., Bird, K. E., Ginther, P. S., Rivalta, I., et al. (2017). Characterization of Protein Tyrosine Phosphatase 1B Inhibition by Chlorogenic Acid and Cichoric Acid. Biochemistry 56, 96–106. doi:10.1021/acs.biochem.6b01025
Liu, H. X. (2016). Comparative Study on Major or Chemical Constituents and Anti-inflammatory and Hepatoprotective Activities of Ixeris Chinensis (Thunb.) Nakai and Sonchus Brachyotus DC. [Shanxi (SX)]: Shanxi University of Chinese Medicine. [dissertation].
Liu, Q., Chen, Y., Shen, C., Xiao, Y., Wang, Y., Liu, Z., et al. (2017a). Chicoric Acid Supplementation Prevents Systemic Inflammation‐induced Memory Impairment and Amyloidogenesis via Inhibition of NF‐κB. FASEB J. 31, 1494–1507. doi:10.1096/fj.201601071R
Liu, Q., Fang, J., Chen, P., Die, Y., Wang, J., Liu, Z., et al. (2019). Chicoric Acid Improves Neuron Survival against Inflammation by Promoting Mitochondrial Function and Energy Metabolism. Food Funct. 10, 6157–6169. doi:10.1039/c9fo01417a
Liu, Q., Hu, Y., Cao, Y., Song, G., Liu, Z., and Liu, X. (2017b). Chicoric Acid Ameliorates Lipopolysaccharide-Induced Oxidative Stress via Promoting the Keap1/Nrf2 Transcriptional Signaling Pathway in BV-2 Microglial Cells and Mouse Brain. J. Agric. Food Chem. 65, 338–347. doi:10.1021/acs.jafc.6b04873
Luo, Z., Gao, G., Ma, Z., Liu, Q., Gao, X., Tang, X., et al. (2020). Cichoric Acid from Witloof Inhibit Misfolding Aggregation and Fibrillation of hIAPP. Int. J. Biol. Macromol. 148, 1272–1279. doi:10.1016/j.ijbiomac.2019.10.100
Ma, J., Li, M., Kalavagunta, P. K., Li, J., He, Q., Zhang, Y., et al. (2018). Protective Effects of Cichoric Acid on H2O2-Induced Oxidative Injury in Hepatocytes and Larval Zebrafish Models. Biomed. Pharmacother. 104, 679–685. doi:10.1016/j.biopha.2018.05.081
Ma, X. L., Wei, H. Y., Feng, W. Y., and Fei, F. (2014). Study on Optimization of Extraction Process of Echinacea by Orthogonal Design. Xinjiang J. TCM. 32, 52–54. CNKI: SUN: XJZY.0.2014-03-025. https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD2014&filename=XJZY201403025&uniplatform=NZKPT&v=szAESMCry96YpidvMkLV9333TC-tz2104KQwnE8WGcQ62mL4XuYOtYzID_mM_Fx6.
Ma, X., Zhang, J., Wu, Z., and Wang, X. (2021). Chicoric Acid Attenuates Hyperglycemia-Induced Endothelial Dysfunction through AMPK-dependent Inhibition of Oxidative/nitrative Stresses. J. Recept. Signal Transduct. 41, 1–15. doi:10.1080/10799893.2020.1817076
Ma, X. Z., Zeng, Y., Wu, Y., and Li, P. Y. (2011). Review on the Chemical Constituents and Pharmcological Activities of Sonchus L. J. Qinghai Norm. Univ. 27 (02), 49–52. doi:10.16229/j.cnki.issn1001-7542.2011.02.009
Marques, A. M., Provance, D. W., Kaplan, M. A. C., and Figueiredo, M. R. (2017). Echinodorus Grandiflorus : Ethnobotanical, Phytochemical and Pharmacological Overview of a Medicinal Plant Used in Brazil. Food Chem. Toxicol. 109, 1032–1047. doi:10.1016/j.fct.2017.03.026
Mascherpa, D., Carazzone, C., Marrubini, G., Gazzani, G., and Papetti, A. (2012). Identification of Phenolic Constituents inCichorium endiviaVar.Crispumand Var.latifoliumSalads by High-Performance Liquid Chromatography with Diode Array Detection and Electrospray Ioniziation Tandem Mass Spectrometry. J. Agric. Food Chem. 60, 12142–12150. doi:10.1021/jf3034754
Meng, C. G., Fu, H. F., Chen, X. W., Wang, Y. Y., and Zhang, Y. D. (2015). Study on Extraction and Antioxidant Activity of Cichoric Acid from Cichorium Lntybus L. J. Food Saf. Qual. 6, 4460–4467. doi:10.19812/j.cnki.jfsq11-5956/ts.2015.11.036
Meng, C. G. (2016). Study on the Technology of Extraction and Separation and Physiological Activity of Cichoric Acid from Cichoriuh Intybus L. [Shanxi (SX)]: Northwest Agriculture and Forestry University.
Min, X., Yi, Z., Xiao-Jing, S., Yun-Ling, Z., and Hai-Peng, Z. (2019). The Distribution of Large Floating Seagrass (Zostera Marina) Aggregations in Northern Temperate Zones of Bohai Bay in the Bohai Sea, China. PLoS One 14, e0201574. doi:10.1371/journal.pone.0201574
Mohammadi, M., Abbasalipourkabir, R., and Ziamajidi, N. (2020). Fish Oil and Chicoric Acid Combination Protects Better against Palmitate-Induced Lipid Accumulation via Regulating AMPK-Mediated SREBP-1/FAS and PPARα/UCP2 Pathways. Archives Physiology Biochem., 1–9. doi:10.1080/13813455.2020.1789881
Nie, W. J., Xu, S. S., and Zhang, Y. M. (2020). Advances in the Study of Effective Components and Pharmacological Action of Dandelion. J. Liaoning Univ. TCM. 22, 140–145. doi:10.13194/j.issn.1673-842x.2020.07.034
Nobela, O., Renslow, R. S., Thomas, D. G., Colby, S. M., Sitha, S., Njobeh, P. B., et al. (2018). Efficient Discrimination of Natural Stereoisomers of Chicoric Acid, an HIV-1 Integrase Inhibitor. J. Photochem. Photobiol. B Biol. 189, 258–266. doi:10.1016/j.jphotobiol.2018.10.025
Nuissier, G., Rezzonico, B., and Grignon-Dubois, M. (2010). Chicoric Acid from Syringodium Filiforme. Food Chem. 120, 783–788. doi:10.1016/j.foodchem.2009.11.010
Oh, M.-M., Carey, E. E., and Rajashekar, C. B. (2009). Environmental Stresses Induce Health-Promoting Phytochemicals in Lettuce. Plant Physiology Biochem. 47, 578–583. doi:10.1016/j.plaphy.2009.02.008
Ortiz, M. Á., Tremetsberger, K., Terrab, A., Stuessy, T. F., García-castaño, J. L., Urtubey, E., et al. (2008). Phylogeography of the Invasive weed Hypochaeris radicata(Asteraceae): from Moroccan Origin to Worldwide Introduced Populations. Mol. Ecol. 17, 3654–3667. doi:10.1111/j.1365-294X.2008.03835.x
Peng, Y., Sun, Q., Gao, R., and Park, Y. (2019). AAK-2 and SKN-1 Are Involved in Chicoric-Acid-Induced Lifespan Extension in caenorhabditis Elegans. J. Agric. Food Chem. 67, 9178–9186. doi:10.1021/acs.jafc.9b00705
Petersen, M., Abdullah, Y., Benner, J., Eberle, D., Gehlen, K., Hücherig, S., et al. (2009). Evolution of Rosmarinic Acid Biosynthesis. Phytochemistry 70, 1663–1679. doi:10.1016/j.phytochem.2009.05.010
Pilavtepe, M., Yucel, M., Helvaci, S. S., Demircioglu, M., and Yesil-Celiktas, O. (2012). Optimization and Mathematical Modeling of Mass Transfer between Zostera Marina Residues and Supercritical CO2 Modified with Ethanol. J. Supercrit. Fluids 68, 87–93. doi:10.1016/j.supflu.2012.04.013
Pozharitskaya, O. N., Shikov, A. N., Makarova, M. N., Kosman, V. M., Faustova, N. M., Tesakova, S. V., et al. (2010). Anti-inflammatory Activity of a HPLC-Fingerprinted Aqueous Infusion of Aerial Part of Bidens Tripartita L. Phytomedicine 17, 463–468. doi:10.1016/j.phymed.2009.08.001
Sadeghabadi, Z. A., Ziamajidi, N., Abbasalipourkabir, R., Mohseni, R., and Borzouei, S. (2019). Palmitate-induced IL6 Expression Ameliorated by Chicoric Acid through AMPK and SIRT1-Mediated Pathway in the PBMCs of Newly Diagnosed Type 2 Diabetes Patients and Healthy Subjects. Cytokine 116, 106–114. doi:10.1016/j.cyto.2018.12.012
Scarpati, M. L., and Oriente, G. (1958). Chicoric Acid (Dicaffeyltartic Acid): Its Isolation from Chicory (Chicorium Intybus) and Synthesis. Tetrahedron 4, 43–48. doi:10.1016/0040-4020(58)88005-9
Schlernitzauer, A., Oiry, C., Hamad, R., Galas, S., Cortade, F., Chabi, B., et al. (2013). Chicoric Acid Is an Antioxidant Molecule that Stimulates AMP Kinase Pathway in L6 Myotubes and Extends Lifespan in Caenorhabditis elegans. PLoS One 8, e78788. doi:10.1371/journal.pone.0078788
Shah, S. A., Sander, S., White, C. M., Rinaldi, M., and Coleman, C. I. (2007). Evaluation of Echinacea for the Prevention and Treatment of the Common Cold: A Meta-Analysis. Lancet Infect. Dis. 7, 473–480. doi:10.1016/s1473-3099(07)70160-3
Stewart, G., Cox, E., Le Duc, M., Pakeman, R., Pullin, A., and Marrs, R. (2008). Control of pteridium Aquilinum: Meta-Analysis of a Multi-Site Study in the UK. Ann. Bot. 101, 957–970. doi:10.1093/aob/mcn020
Street, R. A., Sidana, J., and Prinsloo, G. (2013). Cichorium Intybus: Traditional Uses, Phytochemistry, Pharmacology, and Toxicology. Evidence-Based Complementary Altern. Med. 2013, 1–13. doi:10.1155/2013/579319
Sun, J. Y. (2011). The Preliminary Study on Morphology and Active Components Dynamical Accumulation of Echinacea Purpurea (L.) Moench Cultivated. [Shandong (SD)]: Shandong University of Traditional Chinese Medicine. [dissertation].
Sun, X. S., Zhu, L. Z., Zhu, L. P., Zhang, W. C., and Yan, S. G. (2019). Optimization of Reflux Extraction Conditions of Chicory Acid in Echinacea Purpurea. J. qilu Univ. Technol. 33, 35–40. doi:10.16442/j.cnki.qlgydxxb.2019.01.006
Sun, X., Zhang, X., Zhai, H., Zhang, D., and Ma, S. (2019). Chicoric Acid (CA) Induces Autophagy in Gastric Cancer through Promoting Endoplasmic Reticulum (ER) Stress Regulated by AMPK. Biomed. Pharmacother. 118, 109144–144. doi:10.1016/j.biopha.2019.109144
Tian, S. X., Li, H. L., Xu, G. Y., and Chen, B. (2021). A Synthetic Method of Cichoric Acid and Preparation of L-Cichoric Acid Crystal Form. China. Patent No CN109678706B. Beijing: China: State Intellectual Property Office Patent Office.
Tsai, K.-L., Kao, C.-L., Hung, C.-H., Cheng, Y.-H., Lin, H.-C., and Chu, P.-M. (2017). Chicoric Acid Is a Potent Anti-atherosclerotic Ingredient by Anti-oxidant Action and Anti-inflammation Capacity. Oncotarget 8, 29600–29612. doi:10.18632/oncotarget.16768
Veit, M., Strack, D., Czygan, F.-C., Wray, V., and Witte, L. (1991). Di-E-caffeoyl-meso-tartaric Acid in the Barren Sprouts of Equisetum Arvense. Phytochemistry 30, 527–529. doi:10.1016/0031-9422(91)83720-6
Veit, M., Weidner, C., Strack, D., Wray, V., Witte, L., and Czygan, F.-C. (1992). The Distribution of Caffeic Acid Conjugates in the Equisetaceae and Some Ferns. Phytochemistry 31, 3483–3485. doi:10.1016/0031-9422(92)83711-7
Vidal, V., Laurent, S., Charles, F., and Sallanon, H. (2019). Fine Monitoring of Major Phenolic Compounds in Lettuce and Escarole Leaves during Storage. J. Food Biochem. 43, e12726. doi:10.1111/jfbc.12726
Wang, H., Liu, W. Z., Lu, X. L., Chen, S. Z., and Al, T. M. (2002). Determination of Cichoric Acid in Echinacea Purpuea. Zhongguo Zhong Yao Za Zhi 27, 418–420. CNKI: SUN: ZGZY.0.2002-06-004. https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD2002&filename=ZGZY200206004&uniplatform=NZKPT&v=V2C53YkAq4Qo3-JfQoHPMalPR2zQqpVIisWLwznTDIn-TbEZggQS8QARaKrSt6zS.
Wang, N., Li, R., Feng, B., Cheng, Y., Guo, Y., and Qian, H. (2022). Chicoric Acid Prevents Neuroinflammation and Neurodegeneration in a Mouse Parkinson's Disease Model: Immune Response and Transcriptome Profile of the Spleen and Colon. Ijms 23, 2031. doi:10.3390/ijms23042031
Wang, Y. C., Wang, L., Jin, H., Li, Q. C., and Yang, X. L. (2015). Study on Extraction Conditions of Ultrasonic Microwave Synergistic Extraction Method of Cichoric Acid. Food Res. Evelopment 36, 28–31. CNKI: SUN: SPYK.0.2015-15-011.
Wang, Y., Diao, Z., Li, J., Ren, B., Zhu, D., Liu, Q., et al. (2017). Chicoric Acid Supplementation Ameliorates Cognitive Impairment Induced by Oxidative Stress via Promotion of Antioxidant Defense System. RSC Adv. 7, 36149–36162. doi:10.1039/c7ra06325c
Wang Y.R, Y. R., Li, Y. M., Yang, N., Zhou, B. S., Chen, F., Liu, J. P., et al. (2017). Research Progress on Chemical Compositions and Pharmacological Actions of Taraxacum. Special Wild Econ. Animal Plant Res 39, 67–75. doi:10.16720/j.cnki.tcyj.2017.04.015
Wang, Y. Y., Sun, Y. B., Wang, H. S., Yang, H., He, Z. M., and Li, Z. J. (2016). Determination of Chicoric Acid from Different Medicinal Part of E. Purpurea Moench by HPLC. Ginseng Res. 28, 23–25. doi:10.19403/j.cnki.1671-1521.2016.02.007
Wei, T., van Treuren, R., Liu, X., Zhang, Z., Chen, J., Liu, Y., et al. (2021). Whole-genome Resequencing of 445 Lactuca Accessions Reveals the Domestication History of Cultivated Lettuce. Nat. Genet. 53, 752–760. doi:10.1038/s41588-021-00831-01
Wills, R. B. H., and Stuart, D. L. (1999). Alkylamide and Cichoric Acid Levels in Echinacea Purpurea Grown in Australia. Food Chem. 67, 385–388. doi:10.1016/S0308-8146(99)00129-6
Xia, Y. (2019). Discussion on the Distribution Law of Horsetail Economic Plants in Heilongjiang Province. J. For. Surv. Des. 3, 81–82. CNKI: SUN: LYKC.0.2019-03-034. https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFDLAST2019&filename=LYKC201903034&uniplatform=NZKPT&v=fpzZ6Ov6TRzaaGhjrI4GNGRJHkXYkvSDdxjZwqWN2HtpzpiXxn_QSe3xuPd_LoUU.
Xin, J., Han, L. N., and Zhou, F. Q. (2012). Optimization of Extraction Technology of Cichoric Acid from Echinacea Purpurea by Orthogonal Test. J. Shandong Univ. TCM 36, 539–541. doi:10.16294/j.cnki.1007-659x.2012.06.032
Xu, D., Tang, Y. W., and Zhu, W. (2006). Determ Ination of 2 Caffeic Acid Derivatives in Echinacea Extracts by RP-HPLC. Chin. Tradit. Pat. Med. 28, 1493–1497. CNKI: SUN: ZCYA.0.2006-10-030. https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD2006&filename=ZCYA200610030&uniplatform=NZKPT&v=znJyWvpZrrrr-qckmtgxztC_9qJIfGohFGRp6le3ytMaydtiIDYmpDHkc7bD5RCt.
Xu, J. M. (2008). Study on Extracting Cichoric Acid from Cichory and Fingerprint. [Beijing (BJ)]: Beijing University of Chemical Technology. [dissertation].
Yang, L., Wang, X. H., Cheng, H. Y., Chen, J. W., Zhan, Z. L., and Bai, J. Q. (2021). Textual Research on the Materia Medica of Hai Jin Sha. J. Shaanxi Univ. Chin. Med. 44, 55–62. doi:10.1038/s41440-020-00605-x
Ye, X. Y., Li, G. Y., Ma, D. D., Xie, W. Y., and Wang, Y. P. (2007). A Newly Escaped Plant Species in Zhejiang Province:Hypochaeris Radicata. J. Zhejiang For. Coll. 5, 647–648. CNKI: SUN: ZJLX.0.2007-05-028. https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD2007&filename=ZJLX200705028&uniplatform=NZKPT&v=NUvY6BnY4B2Jhu_4US60MdjqLeh7dCBGMF298bHaUyWC-mahzS98_pv6I34s9XB2.
Zhang, L. (2018). Studies on the Lettuce Genomic Evolution and Genetic Cloning of a Major Generesponsible for Leaf Color Variation in Lettuce. [WuHan (HB)]: Hua Zhong Agricultural University. [dissertation].
Zhang, L., Zou, R. X., Deng, Y., Zhang, Q. Y., and Bi, X. L. (2008). Study on Extraction Technology of Effective Parts of Echinacea Purpurea. Chin. Pat. Med. 4, 603–605.
Zhang, T., Li, J., Shi, L., Feng, S., and Li, F. (2021). Anti-RSV Activities of Chicoric Acid from Echinacea Purpurea In Vitro. Minerva. Surg. 77. doi:10.23736/S2724-5691.21.08925-5
Zhang, X. Q., Shi, B. J., Li, Y. L., Hiroshi, K., and Ye, W. C. (2008). Chemical Constituents in Aerial Parts of Pulsatilla Chinensis. Chin. Traditional Herb. Drugs 39, 651–653. CNKI: SUN: ZCYO.0.2008-05-005. https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD2008&filename=ZCYO200805005&uniplatform=NZKPT&v=3PosB37mGFB_h-yoaGna7kVepPcjinlLHGKeXxzGfc1uaJ6-VjBNLPRfC1sfOuLr.
Zhao, C. Y., and Jiang, M. Y. (2016). The Chemical Constituents and Antioxidant Activity of Ixeris Sonchifolia. J. Pharm. Pract. 34, 24–27+51. CNKI: SUN: YXSJ.0.2016-01-008. https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFDLAST2016&filename=YXSJ201601008&uniplatform=NZKPT&v=HDTjppNTJcz5TFALG6JzYyu5clF9KpH_nDPtPsYURq-6D-l8NSlW1fV7_V75wJY4.
Zhao, J., Deng, J. W., Chen, Y. W., and Li, S. P. (2013). Advanced Phytochemical Analysis of Herbal Tea in China. J. Chromatogr. A 1313, 2–23. doi:10.1016/j.chroma.2013.07.039
Zhao, Y. M., and Yang, L. (2018). Study on the Spray Extraction Process of Cichoric Acid from Echinacea Purpurea. J. Shaanxi Agric. Sci. 64, 32–35. CNKI: SUN: SNKX.0.2018-10-010.
Zhong, Y. J., Li, L., and Xu, F. L. (2010). Studies on Extraction Process of Cichoric Acid in Echinacea Purpurea. Chin. J. Exp. Tradit. Med. Formulae 16, 1–4. doi:10.13422/j.cnki.syfjx.2010.04.011
Zhou, S., and Meng, H. K. (2017). Factors Affecting Changes in Planting Areas of Main Peanut Producing Areas in China. Jiangsu Agric. Sci. 45, 250–253. doi:10.15889/j.issn.1002-1302.2017.13.065
Zhou, X. J. (2014). Comprehensive Evaluation on the Quality of Aerial Parts of Chicory. [Beijing (BJ)]: Beijing University of Chinese Medicine. [dissertation].
Keywords: chicoric acid, biosynthesis, chemical synthesis, natural occurrence, content detection, bioactive effects
Citation: Yang M, Wu C, Zhang T, Shi L, Li J, Liang H, Lv X, Jing F, Qin L, Zhao T, Wang C, Liu G, Feng S and Li F (2022) Chicoric Acid: Natural Occurrence, Chemical Synthesis, Biosynthesis, and Their Bioactive Effects. Front. Chem. 10:888673. doi: 10.3389/fchem.2022.888673
Received: 04 March 2022; Accepted: 09 May 2022;
Published: 23 June 2022.
Edited by:
Kamaldeep Paul, Thapar Institute of Engineering & Technology, IndiaReviewed by:
Viduranga Y. Waisundara, Australian College of Business and Technology, Sri LankaAmit Jaisi, Walailak University, Thailand
Copyright © 2022 Yang, Wu, Zhang, Shi, Li, Liang, Lv, Jing, Qin, Zhao, Wang, Liu, Feng and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuai Feng, ZmVuZ3NodWFpaGFwcHlAMTYzLmNvbQ==; Feng Li, MTM5NjkxNDE3OTZAMTYzLmNvbQ==
†ORCID: Min Yang, orcid.org/0000-0002-0264-5123
 Min Yang
Min Yang Chao Wu
Chao Wu Tianxi Zhang1
Tianxi Zhang1 Jian Li
Jian Li