Abstract
We report herein a facile Hiyama cross-coupling reaction of arylsilanes with thiuram reagents (tetraalkylthiuram disulfides or tetraalkylthiuram monosulfide) enabled by copper fluoride. Compared to our previous work, this protocol is an alternative protocol for the generation of S-aryl dithiocarbamates. It features low toxic and readily available substrates, cost-effective promoter, easy performance, and provides good yields.
Introduction
Transition-metal-catalyzed cross-coupling reactions have been found broad applications for the construction of carbon-carbon and carbon-heteroatom bonds enable the facile preparation of more complex molecules (Miyaura, 2002; Magano and Dunetz, 2011; Negishi, 2011; Suzuki, 2011; Guo and Rueping, 2018; Zhang et al., 2020). In 1972, Kumada and Tamao (Tamao et al., 1972) reported the cross-coupling reaction of Grignard reagents (RMgX) with organic halides (R’X) catalyzed by nickel/phosphine system. From then on, a wide range of organometallic reagents such as lithium (Yamamura et al., 1975; Murahashi et al., 1979), aluminum (Negishi et al., 1978), zinc (Sekiya and Ishikawa, 1976; King et al., 1977; Negishi and Van Horn, 1977; Negishi, 2011), zirconium (Negishi et al., 1977; Okukado et al., 1978) and tin (Milstein and Stille, 1979a; Milstein et al., 1979b) have emerged and exerted a ubiquitous influence on the synthesis community. However, their instability, air and moisture sensitivity and the production of corrosive halogen wastes are disadvantageous from both synthetic and environmental points of view. In addition to these well-established organometallic reagents, the silicon reagent which was developed by Hiyama and co-workers, is an alternative and attractive coupling partner for cross-coupling reactions (the so-called Hiyama cross-coupling) (Nakao and Hiyama, 2011; Sore et al., 2012; Denmark and Ambrosi, 2015; Komiyama et al., 2016). Generally, organosilicon reagents exhibit some remarkable advantages such as non-toxicity, high stability, good tolerance toward various functional groups and natural abundance of silicon. In the overpast several decades, significant advances on transition-metal-catalyzed Hiyama cross-coupling have been achieved (Nareddy et al., 2017; Nareddy et al., 2018; González et al., 2019; Han et al., 2019; Zhang et al., 2019; Idris and Lee, 2020; Lu et al., 2020; Wu et al., 2021), nevertheless, the diverse applications of this methodology are still less explored and worthy of in-depth exploration under the concept of green chemistry.
Thiuram reagents (tetraalkylthiuram disulfides TMTD, or tetraalkylthiuram monosulfide TMTM) are cheap and stable organosulfur compounds which can be widely used in biologically active compounds, agricultural pesticides and vulcanization accelerators (Enders et al., 2010), and also act as readily available sulfur reagents in organic synthesis. (Zeng et al., 2017a; Zeng et al., 2017b; Wu and Yan, 2019). Among them, organic dithiocarbamates have been extensively investigated for their outstanding biological activities (Hou et al., 2006; Zou et al., 2014; Liénard et al., 2008; Horita et al., 2011) and synthetic value (Boas et al., 2004; Derouet et al., 2009; Wults and Greene, 2007; Zhang et al., 2005). Hence, much attention has been paid to the development of highly efficient and convenient methods for the construction of such scaffolds. Traditionally, the portion of S-aryl dithiocarbamates was prepared through the reactions of classical organometallic reagents with tetramethyllitium disulfide (Jen and Cava, 1982; Knochel et al., 2006) (Scheme 1A). The reactions of sodium dialkyldithiocarbamates with diaryliodonium salts (Chen et al., 1987), aryl halide (Liu and Bao, 2007) or aryl boronic acid (Gao et al., 2018) were also proved to be an effective strategy (Scheme 1B). Recently, the three-component reactions of amines, carbon disulfide, and diverse electrophiles including alkyl halides (Azizi et al., 2006), aryl halides (Bhadra et al., 2008), aryldiazonium fluoroborates (Chatterjee et al., 2011), pentafluorobenzonitrile (Yin et al., 2015), and phenylboronic acid (Qi et al., 2016) (Scheme 1C) have been achieved by some research groups. Moreover, the cross-coupling reactions of tetraalkylthiuram disulfide with aryl iodide (Dong et al., 2017; Cao et al., 2018; Wu and Yan, 2019), phenylboronic acid (Xu et al., 2018), diaryl disulfides (Peng et al., 2019), or diaryliodonium salts (Zeng et al., 2017b) were also successively established by some chemists (Scheme 1D). However, these methods always suffer from one or more disadvantages such as toxic reagents, multiple reaction steps or flammable and explosive substrates, which limit their applications. To our knowledge, the synthesis of S-aryl dithiocarbamates using thiuram reagents (tetraalkylthiuram disulfides (TATD), or tetraalkylthiuram monosulfide (TATM)) and arylsilanes as the coupling partners has not been documented so far. As a continuation of our interest in the cross-coupling of tetraalkylthiuram disulfide (Wu et al., 2018; Lai et al., 2019a; Cheng et al., 2019; Hu et al., 2020), herein we wish to report the first example of copper-mediated C-S bond construction by cross-coupling of arylsilanes with thiuram reagents (TATD or TMTM) in the presence of CuF2 and N ligand (Scheme 1E), which would be an alternative way for the synthesis of S-aryl dithiocarbamates.
SCHEME 1
Result and Discussion
Initially, the reaction parameters were optimized using trimethoxy (phenyl)silane (1a) and tetramethylthiuram disulfide (TMTD, 2a), and the results were summarized in Table 1. Firstly, the reaction of 1a (0.1 mmol) and 2a (0.2 mmol) was performed in the presence of CuF2 (3 equiv.) together with 20 mol% of CoCl2 in Toluene at 120°C. To our delight, the initial reaction conditions provided the desired product 3a (phenyl dimethylcarbamodithioate) in 22% yield (Table 1, entry 1). The exact structure of 3a was confirmed by NMR and HRMS spectra. When the reaction was carried out in the absence of CuF2, it didn’t produce any products (Table 1, entries 2 and 4). However, the reaction gave 23% yield of product 3a when CoCl2 was removed from the reaction system (Table 1, entry 3). The control experiment clearly indicated that CuF2 was indispensable for this reaction. Inspired by the reported literature (Clarke., 2005; McManus et al., 2006; Fihri et al., 2007; Hachiya et al., 2010; Wu et al., 2016; Sahani et al., 2018; Luo et al., 2020), some nitrogen and phosphorus ligands were screened (Table 1, entries 5-15, 0–78%), and 1, 10-phenanthroline was proved to be the optimized N ligand, affording the product 3a in 82% yield (Table 1, entry 6). Subsequently, other fluoride activators for the C-Si bond cleavage were evaluated in this reaction (Table 1, entries 16-17), but all of them turned out to be invalid. The effect of solvents such as Xylene, Mesitylene, 1,4-Dioxane, Acetonitrile, DMF and DMSO were also examined, and the experimental results showed that Toluene was the most suitable candidate with remarkably higher yields (Table 1, entry 6 vs entries 18-23, 82% vs 0–43%). Furthermore, the effect of CuF2 and N ligand loading was investigated (Table 1, entries 24-28, 26–74%). The obtained results revealed that a relatively lower reaction efficiency was detected in these reactions. Further optimization indicated that the temperature also played an important role in this transformation, and 80°C was identified as the ideal reaction temperature (Table 1, entry 6 and entries 29-31, 88% vs 59–85%). Meanwhile, the reaction time was also examined (Table 1, entries 32-33, 54–84%), and 16 h was found to be the best choice. Thus, the reaction efficiently proceeded when 3 equiv. of CuF2 was used in combination with 1,10-phenanthroline (2 equiv.) in Toluene at 80°C for 16 h. Noteworthily, the combination CuF2/phenantroline acted as the activator of C-Si bond, and also acted as the promoter on the formation of C-S bond.
TABLE 1
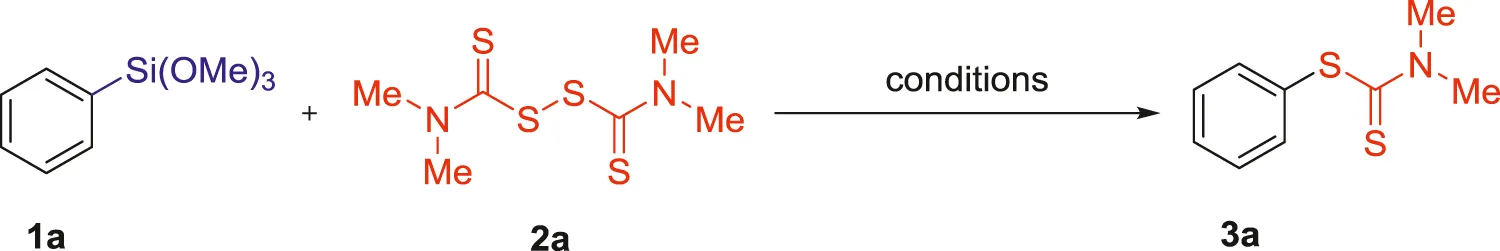 | |||||
|---|---|---|---|---|---|
| Entry | Promoter | Ligand (Equiv.) | Solvent | T (°C) | Yields of 3a (%)b |
| 1c | CuF2 | - | Toluene | 120 | 22 |
| 2 | - | - | Toluene | 120 | 0 |
| 3 | CuF2 | - | Toluene | 120 | 23 |
| 4 | CoCl2 | - | Toluene | 120 | 0 |
| 5 | CuF2 | bipyridine (2) | Toluene | 120 | 61 |
| 6 | CuF2 | 1,10-phenanthroline (2) | Toluene | 120 | 82 |
| 7 | CuF2 | pyridine (2) | Toluene | 120 | 78 |
| 8 | CuF2 | N,N,N′,N′-tetramethylethylenediamine (2) | Toluene | 120 | 0 |
| 9 | CuF2 | 2,2’:6′,2’’-terpyridine (2) | Toluene | 120 | 43 |
| 10 | CuF2 | (R,R)-2,2’-(2,6-pyridinediyl)bis (4-isopropyl-2-oxazoline (2) | Toluene | 120 | 39 |
| 11 | CuF2 | 8-benzoylaminoquinoline (2) | Toluene | 120 | 76 |
| 12 | CuF2 | 1,2-bis(diphenylphosphino)ethane (2) | Toluene | 120 | <5 |
| 13 | CuF2 | 2,2′-bis(diphenylphosphino)-1,1′-biphenyl (2) | Toluene | 120 | <5 |
| 14 | CuF2 | 1,1′-bis(diphenylphosphino)ferrocene (2) | Toluene | 120 | <5 |
| 15 | CuF2 | (R)-(+)-2,2′-bis(diphenylphosphino)-1,1′-binaphthyl (2) | Toluene | 120 | 11 |
| 16 | AgF | 1,10-phenanthroline (2) | Toluene | 120 | 0 |
| 17 | CsF | 1,10-phenanthroline (2) | Toluene | 120 | 0 |
| 18 | CuF2 | 1,10-phenanthroline (2) | Xylene | 120 | 38 |
| 19 | CuF2 | 1,10-phenanthroline (2) | Msitylene | 120 | 43 |
| 20 | CuF2 | 1,10-phenanthroline (2) | 1,4-Dioxane | 120 | 20 |
| 21 | CuF2 | 1,10-phenanthroline (2) | Acetonitrile | 120 | 42 |
| 22 | CuF2 | 1,10-phenanthroline (2) | DMF | 120 | 0 |
| 23 | CuF2 | 1,10-phenanthroline (2) | DMSO | 120 | 0 |
| 24 | CuF2 | 1,10-phenanthroline (3) | Toluene | 120 | 58 |
| 25 | CuF2 | 1,10-phenanthroline (0.5) | Toluene | 120 | 74 |
| 26 | CuF2 | 1,10-phenanthroline (2) | Toluene | 120 | 37 |
| 27d | CuF2 | 1,10-phenanthroline (2) | Toluene | 120 | 55 |
| 28e | CuF2 | 1,10-phenanthroline (2) | Toluene | 120 | 26 |
| 29 | CuF2 | 1,10-phenanthroline (2) | Toluene | 100 | 85 |
| 30 | CuF2 | 1,10-phenanthroline (2) | Toluene | 80 | 88 |
| 31 | CuF2 | 1,10-phenanthroline (2) | Toluene | 60 | 59 |
| 32f | CuF2 | 1,10-phenanthroline (2) | Toluene | 120 | 84 |
| 33g | CuF2 | 1,10-phenanthroline (2) | Toluene | 120 | 54 |
Optimization of reaction conditions a.
Trimethoxy (phenyl)silane 1a (0.10 mmol), Tetramethylthiuram disulfide 2a (0.20 mmol), promoter (3.0 equiv.), and Toluene (1 ml) for 16 h, under air.
Isolated yields.
20 mol% of CoCl2 was added.
Promoter (4.0 equiv.).
Promoter (2.0 equiv.).
24 h.
48 h.
Having the optimized conditions in hand, we then proceeded to explore the scope of the reaction with respect to both the organosilane reagents and the thiuram disulfides (Table 2 and Table 3). Generally, phenylsilanes bearing diverse substituents such as methyl, methoxyl, tert-butyl, chloro and fluoro groups offered the desired products in moderate to good yields. Notably, this reaction tolerated the electron-rich arylsilanes (Table 2, 3b-g), as (4-methylphenyl) trimethoxylsilane, (4-methoxyphenyl) trimethoxylsilane and (4-(tert-butyl)phenyl) trimethoxylsilane coupled efficiently with tetramethylthiuram disulfide 2a to give 3b-d in 65–72% yields. When methyl, methoxyl, were introduced into the meta position of phenylsiloxanes, slight lower yields were obtained (3e-g, 43–55%), which may be caused by steric hindrance effect. Excellent yields were got for electron-deficient arylsilanes such as (4-chlorophenyl)trimethoxysilane and (4-fluorophenyl) trimethoxysilane. Compared with electron-rich substituents, arylsilanes with electron-withdrawing groups on the aromatic ring presented relatively higher reactivity (3b-c vs 3h-i, 65–72% vs 78–93%). This result makes the said cross-coupling reaction particularly attractive for further transformation by transition-metal-catalyzed coupling reactions. Pleasingly, these reaction conditions were also compatible with trimethoxy (4-vinylphenyl)silane, 1-(trimethoxysilyl)naphthalene and 2-furan-trimethoxysilane, which provided the corresponding products 3l-n in 52–72 yields.
TABLE 2
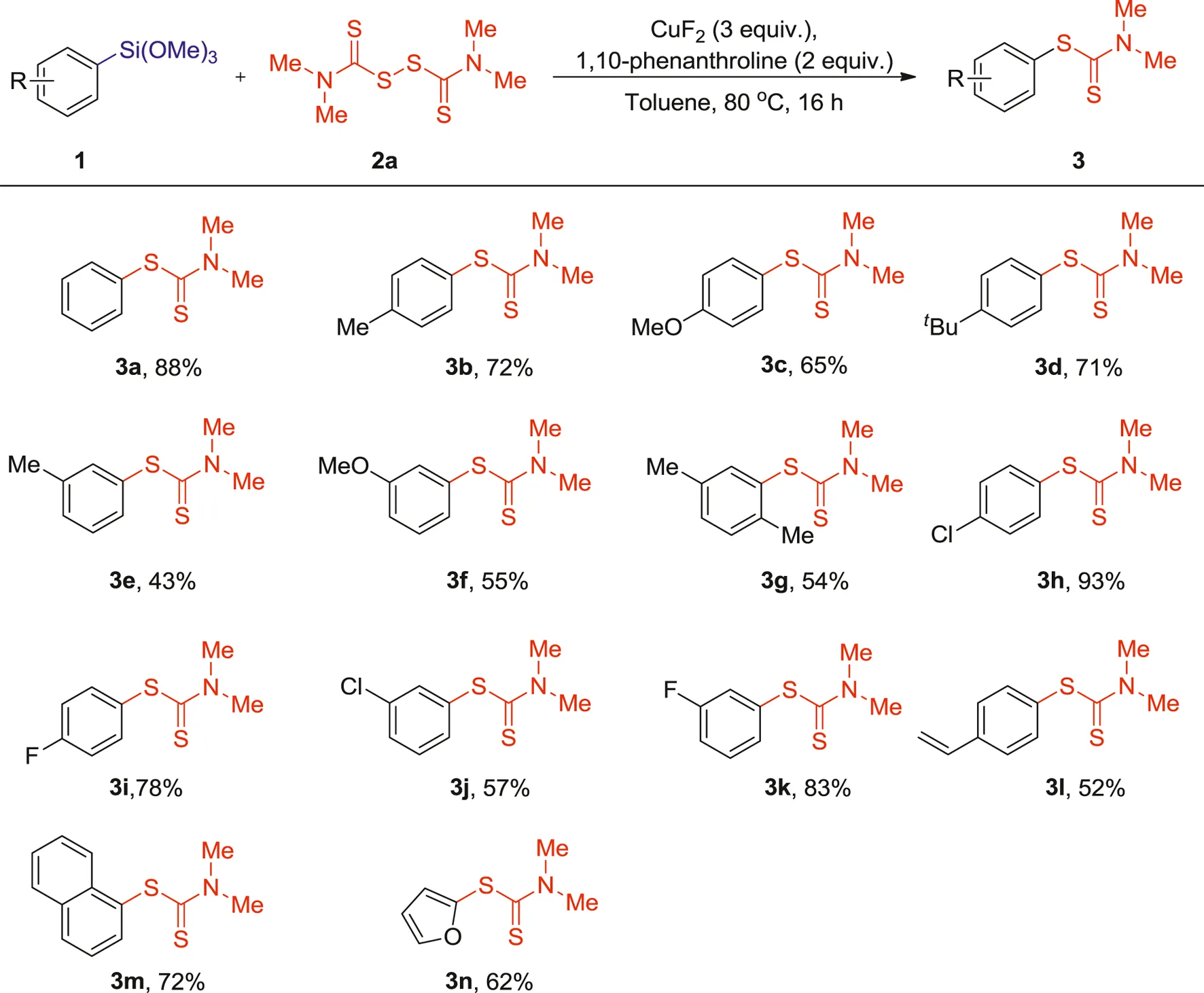 |
1 (0.1 mmol), 2a (0.2 mmol), CuF2 (3 equiv.), 1,10-phenanthroline (2 equiv.), Toluene (1 ml), 80°C, 16 h, under air.
Isolated yields.
TABLE 3
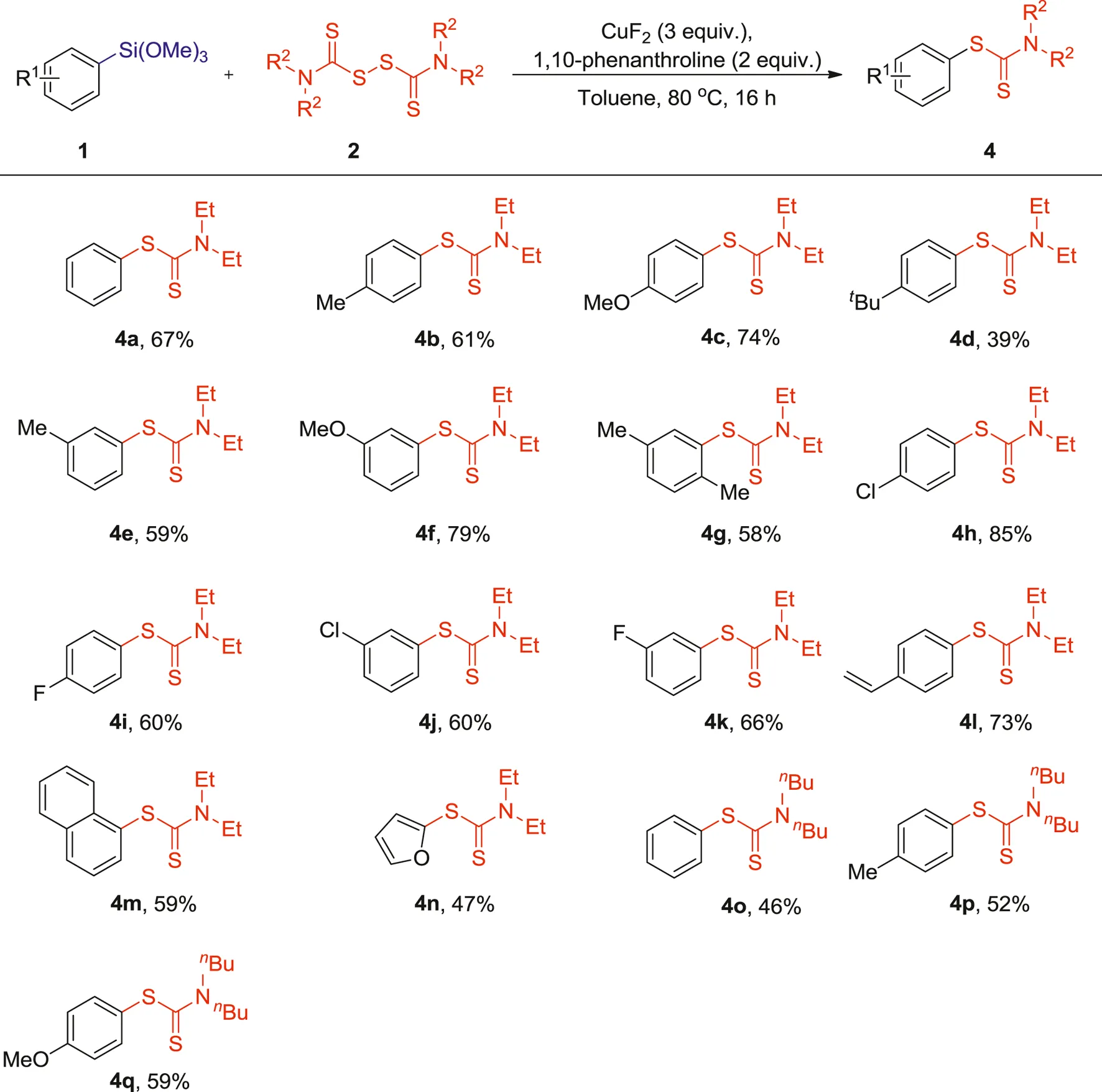 |
1 (0.10 mmol), 2 (0.20 mmol), CuF2 (3 equiv.), 1,10-phenanthroline (2 equiv.), Toluene (1 ml), 80°C, 16 h.
Isolated yields.
This cross-coupling reaction also demonstrated a good tolerance toward other N,N,N′,N′-tetraalkylthiuram disulfides as shown in Table 2. The N,N,N′,N′-tetraethylthiuram disulfide (TETD, 2b) showed a good reactivity and furnished the corresponding S-aryl dithiocarbamates products in moderate to good yields (4a-n, 39–85%). Comparatively, the reaction of N,N,N′,N′-tetrabutylthiuram (TBTD, 2c) and arylsilanes showed relatively lower reactivity, and afforded lower yields of the corresponding products (4o-q, 46–59%). It is worth noting that the yields of the resulting products were modulated by the presence of different alkyl substituents on the tetraalkylthiuram disulfides. Slightly lower yields were obtained when longer chain-substituted tetraalkylthiuram disulfides were used in these reactions (3a vs 4a and 4°).
To further evaluate the applicability of this reaction, the reactivity of trimethoxy (phenyl)silane (1a) was investigated using tetramethylthiuram monosulfide (TMTM, 5) as the coupling partner (Wu et al., 2020). As expected, the cross-coupling reaction occurred smoothly, and the phenyl dimethylcarbamodithioate 3a was formed in 46% yield (Scheme 2).
SCHEME 2
In order to find the appropriate conditions to achieve an ideal yield, we spent a bit more time on the optimization of reaction conditons. Some bidentate, tridentate N ligands as well as diphoshines ligands and their loading to this reaction were tested, and a summative result of the optimization was presented in Supplementary Table S1. After the simple optimization, we found that 1 equiv. of 2, 2′-bipyridine increased the yield to 68% (Supplementary Table S1, entry 3), N,N,N′,N′-tetramethylethylenediamine, 2,2’:6′,2’’-terpyridine; (R,R)-2,2’-(2,6-pyridinediyl)bis (4-isopropyl-2-oxazoline, 8-benzoylaminoquinoline, 1,2-bis(diphenylphosphino)ethane, 2,2′-bis(diphenylphosphino)-1,1′-biphenyl, 1,1′-bis(diphenylphosphino)ferrocene and (R)-(+)-2,2′-bis(diphenylphosphino)-1,1′-binaphthyl resulted in a relatively lower yields (Supplementary Table S1, entries 4-11). It probably because of the coordination of 2, 2′-bipyridine with copper, which provided a more stable and active copper intermediate for the said cross-coupling reaction. After the simple optimization, we found that 1 equiv. of 2, 2′-bipyridine acted as the suitable N ligand. With the new optimized reaction conditions in hand, some more substituted arylsilanes were subjected to this reaction and the results were summarized in Table 4.
TABLE 4
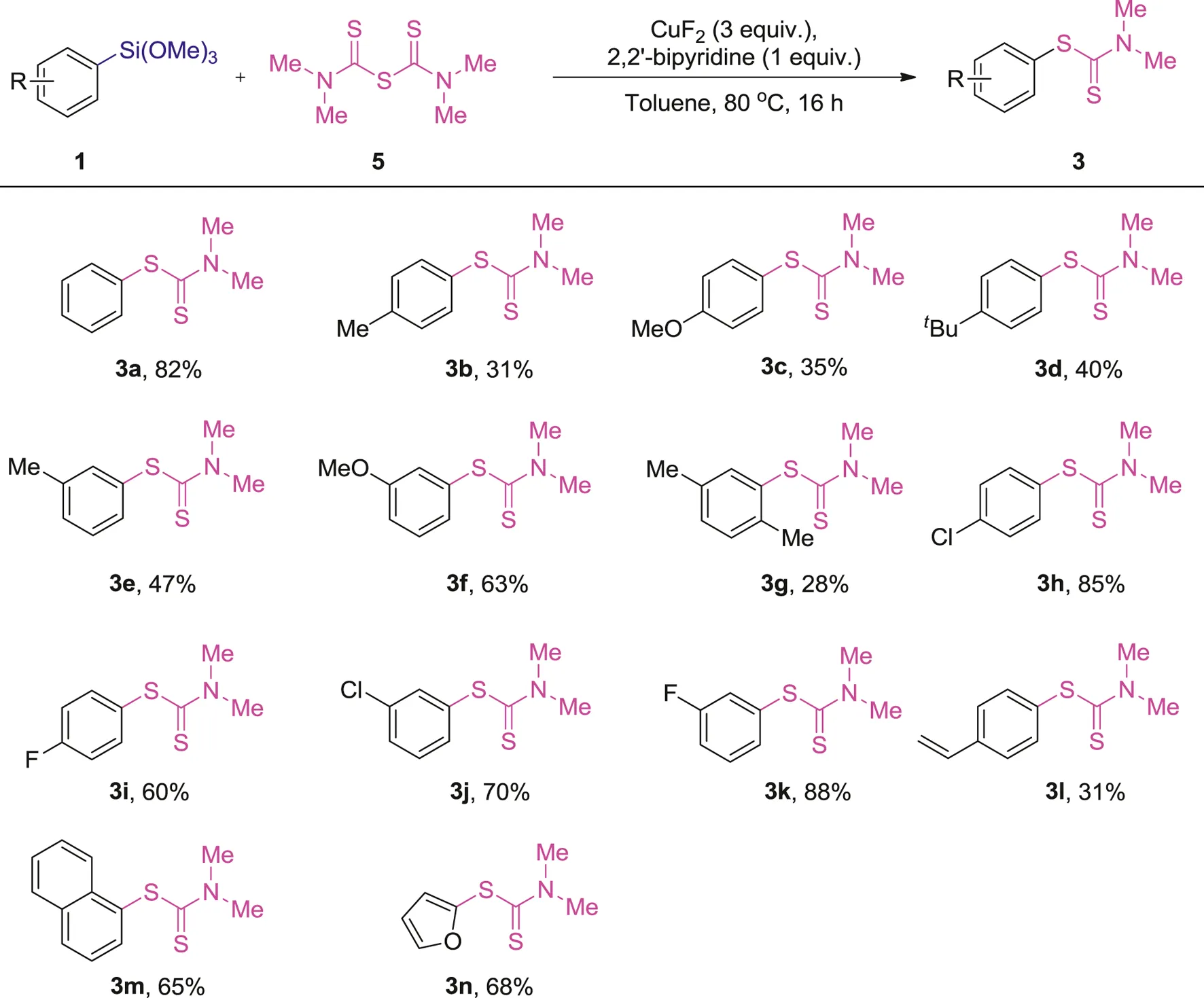 |
1 (0.10 mmol), 5 (0.20 mmol), CuF2 (3 equiv.), 2, 2′-bipyridine (1 equiv.), Toluene (1 ml), 80°C, 16 h.
Isolated yields.
In general, the results obtained from the cross-coupling reaction of arylsilanes 1) with tetramethylthiuram monosulfide (TMTM, 5) are different from the reaction with tetramethylthiuram disulfide (TMTD, 2a), in which the electron-rich arylsilanes are less active (3b-g, 28–63%). With regard to the electron-deficient arylsilanes, they showed a similar efficiency as the reaction with tetramethylthiuram disulfide (TMTD, 2a), and the products (3h-k) were provided in 60–88% yields. The 1-(trimethoxysilyl)naphthalene and 2-furan-trimethoxysilane also participated in this reaction to give the corresponding products (3m-n) in 65–68% yields, which are nearly the same results compared with the reaction with TMTD (2a). In contrast, the trimethoxy (4-vinylphenyl)silane exhibited a less activity in this reaction and displayed lower yield (3l, 31%).
In order to ascertain the mechanism, some control experiments were conducted and the results were exhibited in Scheme 3. When 2 equiv. of radical scavenger 2,2,6,6-tetramethyl-1-piperidinyloxyl (TEMPO), butylated hydroxyl toluene (BHT), galvinoxyl free radical or 1,1-diphenylethylene were added to the reaction of 1a and 2a under the standard conditions, a substantial decrease of the reaction efficiency was observed (Scheme 3A). Subsequently, the radical quencher 1,1-diphenylethylene was added to the tetraalkylthiuram disulfides participated reaction system, the thiuram radical was captured to give the corresponding product six in 29% yield (Scheme 3B). The above mentioned results illustrating that a radical process may be exist in the reaction of 1a and 2a. In sharp contrast, when the reactions were occurred between 1a and 5 in the presence of radical inhibitors (2.0 equiv of TEMPO, BHT) or 1,1-diphenylethylene, which gave the desired product 3a in 77, 71, and 75% yields, respectively (Scheme 3C). Furthermore, no desired product six was observed when 1,1-diphenylethylene react with 5 (TMTM) under the standard conditions (Scheme 3D). These results suggesting that the reaction of 1a and 5 is more likely to be an ionic-type pathway.
SCHEME 3
Considering the experimental evidence as well the previous reports (Lu et al., 2020; Dong et al., 2017; Luo et al., 2020; Hao et al., 2020; Lai et al., 2019b), a plausible reaction mechanism was tentatively proposed and described in Scheme 4. Firstly, the coordination of 1, 10-phenanthroline with copper salts to produce the copper complex A. Simultaneously, the C-Si cleavage process occurred lead to the intermediate B, which activated by fluoride ion (Sugiyama et al., 2008). In step ii, the reaction of intermediate B with copper complex A generates the Cu(II) complex C. Subsequently, thiuram radical D may be formed through the homolysis of tetramethylthiuram disulfide at 80°C probably assisted by Cu(II). Then, the interreaction of Cu(II) complex C with thiuram radical D to provide the intermediate E, which undergoes reductive elimination to yield the desired product three along with the release of Cu(II) species.
SCHEME 4
With regard to the reaction pathway between 1a and 5 (TMTM), a plausible ionic-type reaction mechanism was tentatively proposed according to the obtained results as well as the reported literatures (Dong et al., 2017; Luo et al., 2020; Wu et al., 2020) and described in Scheme 5. Analogously, the initial coordination of bipyridine with copper salts to produce the copper complex F. Concurrently, the intermediate G is generated by the C-Si cleavage manner, which activated by fluoride ion (Sugiyama et al., 2008). Then, the intereaction of intermediate G with copper complex F to generate the Cu(II) complex H. In the meantime, nucleophile F probably produces by the intereaction of copper ion with 5 at 80°C. Subsequently, the interreaction of Cu(II) complex H with nucleophile I to provide the intermediate J, which undergoes reductive elimination lead to the desired product three along with the release of Cu(II) species.
SCHEME 5
Conclusion
In summary, we have developed an interesting methodology on the copper-promoted cross-coupling of arylsilanes and thiuram reagents (TATD or TMTM), affording the valuable S-aryl dithiocarbamates in moderate to good yields. This facile strategy allows practical and friendly reaction conditions, which significantly broadens the substrate scope, improves the functional group compatibility, and emphasizes the synthetic application in complex molecules. It offers not only a protocol for the streamlined synthesis of S-aryl dithiocarbamates from cheap and stable substrates, but also a new example for the application of Hiyama cross-coupling in biological interesting molecules’ construction.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
MC, WS and ZW contributed to the conception and design of the study. The synthetic work and data collection were carried out by YW, HS, JQ and LW. FB, MZ and HW contributed to the article revision. All authors read and approved the submitted version.
Funding
The authors greatly acknowledge the financial support by Natural Science Foundation of Henan Province (212300410163), the Education Department of Henan Province (20A210023), Henan Agricultural University (30500567) and China Tobacco Henan Industrial Co., Ltd. (2021410001300070, 2021410001300071, YN201805).
Conflict of interest
Authors HS, JQ, MC, WS, FB and HW are employed by China Tobacco Henan Industrial Co. Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that this study received funding from China Tobacco Henan Industrial Co., Ltd. The funder had the following involvement in the study: study design, data collection and decision to publish.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2022.867806/full#supplementary-material
References
1
AziziN.AryanasabF.SaidiM. R. (2006). Straightforward and Highly Efficient Catalyst-free One-Pot Synthesis of Dithiocarbamates under Solvent-free Conditions. Org. Lett.8, 5275–5277. 10.1021/ol0620141
2
BhadraS.SahaA.RanuB. C. (2008). One-pot Copper Nanoparticle-Catalyzed Synthesis of S-Aryl- and S-Vinyl Dithiocarbamates in Water: High Diastereoselectivity Achieved for Vinyl Dithiocarbamates. Green. Chem.10, 1224–1230. 10.1039/b809200a
3
BoasU.GertzH.ChristensenJ. B.HeegaardP. M. H. (2004). Facile Synthesis of Aliphatic Isothiocyanates and Thioureas on Solid Phase Using Peptide Coupling Reagents. Tetrahedron Lett.45, 269–272. 10.1016/j.tetlet.2003.10.182
4
ChatterjeeT.BhadraS.RanuB. C. (2011). Transition Metal-free Procedure for the Synthesis of S-Aryl Dithiocarbamates Using Aryl Diazonium Fluoroborate in Water at Room Temperature. Green. Chem.13, 1837–1842. 10.1039/c1gc00001b
5
ChenZ.JinY.StangP. J. (1987). Polyvalent Iodine in Synthesis. 2. A New Method for the Preparation of Aryl Esters of Dithiocarbamic Acids. J. Org. Chem.52, 4117–4118. 10.1021/jo00227a032
6
ChengC.ZhaoM.LaiM.ZhaiK.ShiB.WangS.et al (2019). Synthesis of Aza-Heteroaromatic Dithiocarbamates via Cross-Coupling Reactions of Aza-Heteroaromatic Bromides with Tetraalkylthiuram Disulfides. Eur. J. Org. Chem.2019, 2941–2949. 10.1002/ejoc.201900475
7
ClarkeM. L. (2005). First Microwave-Accelerated Hiyama Coupling of Aryl- and Vinylsiloxane Derivatives: Clean Cross-Coupling of Aryl Chlorides within Minutes. Adv. Synth. Catal.347, 303–307. 10.1002/adsc.200404196
8
DenmarkS. E.AmbrosiA. (2015). Why You Really Should Consider Using Palladium-Catalyzed Cross-Coupling of Silanols and Silanolates. Org. Process. Res. Dev.19, 982–994. 10.1021/acs.oprd.5b00201
9
DerouetD.TranQ. N.ThucH. H. (2009). Synthesis of Polymer-Grafted Natural Rubbers by Radical Photopolymerization of Vinyl Monomers Initiated from the Rubber Chains. J. Appl. Polym. Sci.114, 2149–2160. 10.1002/app.30266
10
DongZ.-B.CaoQ.PengH.-Y.ChengY. (2018). A Highly Efficient CuCl2-Catalyzed C-S Coupling of Aryl Iodides with Tetraalkylthiuram Disulfides: Synthesis of Aryl Dithiocarbamates. Synthesis50, 1527–1534. 10.1055/s-0036-1589166
11
DongZ.-B.LiuX.BolmC. (2017). Copper-Catalyzed C(sp2)-S Coupling Reactions for the Synthesis of Aryl Dithiocarbamates with Thiuram Disulfide Reagents. Org. Lett.19, 5916–5919. 10.1021/acs.orglett.7b02911
12
DongZ.-B.WuY.-X.PengK.LiJ.-H. (2020). An Efficient Copper-Catalyzed C(sp2)-S Formation Starting from Aryl Iodides and Tetramethylthiuram Monosulfide (TMTM). Synthesis52, 3001–3006. 10.1055/s-0040-1707899
13
EndersD.RembiakA.LiebichJ. (2010). Direct Organocatalytic α-Sulfenylation of Aldehydes and Ketones with Tetramethylthiuram Disulfide. Synthesis2011, 281–286. 10.1055/s-0030-1258359
14
FihriA.MeunierP.HiersoJ.-C. (2007). Performances of Symmetrical Achiral Ferrocenylphosphine Ligands in Palladium-Catalyzed Cross-Coupling Reactions: A Review of Syntheses, Catalytic Applications and Structural Properties. Coord. Chem. Rev.251, 2017–2055. 10.1016/j.ccr.2007.03.020
15
GaoM.-Y.XuW.ZhangS.-B.LiY.-S.DongZ.-B. (2018). Synthesis of Phenyl Dithiocarbamates Starting from Sodium Dialkyldithiocarbamates and Aryl Boronic Acids: a Copper Catalyzed S -Arylation. Eur. J. Org. Chem.2018, 6693–6698. 10.1002/ejoc.201801271
16
GonzálezJ.SchäferP.FletcherS. P. (2019). Highly Enantioselective Hiyama Cross-Coupling via Rh-Catalyzed Allylic Arylation of Racemic Allyl Chlorides. Organometallics38, 3991–3995. 10.1021/acs.organomet.9b00197
17
GuoL.RuepingM. (2018). Transition-Metal-Catalyzed Decarbonylative Coupling Reactions: Concepts, Classifications, and Applications. Chem. Eur. J.24, 7794–7809. 10.1002/chem.201704670
18
HachiyaH.HiranoK.SatohT.MiuraM. (2010). Nickel-Catalyzed Direct CH Arylation and Alkenylation of Heteroarenes with Organosilicon Reagents. Angew. Chem.122, 2248–2251. 10.1002/ange.200906996
19
HanC.ZhangZ.XuS.WangK.ChenK.ZhaoJ. (2019). Palladium-Catalyzed Hiyama Coupling of Benzylic Ammonium Salts via C-N Bond Cleavage. J. Org. Chem.84, 16308–16313. 10.1021/acs.joc.9b02554
20
HaoS.YeX.ZhaoM.HuJ.WangN.LiJ.et al (2020). Synthesis of 2‐Aryl‐2‐hydroxyethyl Dithiocarbamates via Regioselective Addition of Tetraalkylthiuram Disulfides to Styrenes under Transition‐Metal‐Free Conditions. Adv. Synth. Catal.362, 5014–5019. 10.1002/adsc.202000729
21
HoritaY.TakiiT.KuroishiR.ChibaT.OgawaK.KremerL.et al (2011). Synthesis and Evaluation of Anti-tubercular Activity of New Dithiocarbamate Sugar Derivatives. Bioorg. Med. Chem. Lett.21, 899–903. 10.1016/j.bmcl.2010.12.084
22
HouX.GeZ.WangT.GuoW.CuiJ.ChengT.et al (2006). Dithiocarbamic Acid Esters as Anticancer Agent. Part 1: 4-Substituted-Piperazine-1-Carbodithioic Acid 3-Cyano-3,3-Diphenyl-Propyl Esters. Bioorg. Med. Chem. Lett.16, 4214–4219. 10.1016/j.bmcl.2006.05.085
23
HuJ.YeX.HaoS.ZhaoQ.ZhaoM.WeiY.et al (2020). Amidation Reaction of Quinoline‐3‐carboxylic Acids with Tetraalkylthiuram Disulfides under Simple Conditions: A Facile Synthesis of Quinoline‐3‐carboxamides. Asian J. Org. Chem.9, 2191–2195. 10.1002/ajoc.202000462
24
IdrisM. A.LeeS. (2020). Palladium-Catalyzed Amide N-C Hiyama Cross-Coupling: Synthesis of Ketones. Org. Lett.22, 9190–9195. 10.1021/acs.orglett.0c03260
25
JenK.-Y.CavaM. P. (1982). A New Synthesis of Aromatic Thiolis. Tetrahedron Lett.23, 2001–2004. 10.1016/s0040-4039(00)87244-5
26
KingA. O.OkukadoN.NegishiE.-i. (1977). Highly General Stereo-, Regio-, and Chemo-Selective Synthesis of Terminal and Internal Conjugated Enynes by the Pd-Catalysed Reaction of Alkynylzinc Reagents with Alkenyl Halides. J. Chem. Soc. Chem. Commun., 683–684. 10.1039/c39770000683
27
KnochelP.KrasovskiyA.GavryushinA. (2006). Highly Stereoselective Access to Sulfur Derivatives Starting from Zinc -Organometallics. Synlett, 0792–0794. 10.1055/s-2006-933117
28
KomiyamaT.MinamiY.HiyamaT. (2016). Recent Advances in Transition-Metal-Catalyzed Synthetic Transformations of Organosilicon Reagents. ACS Catal.7, 631–651. 10.1021/acscatal.6b02374
29
LaiM.WuZ.LiS.-J.WeiD.ZhaoM. (2019b). Regioselective Synthesis of Sulfonyl-Containing Benzyl Dithiocarbamates through Copper-Catalyzed Thiosulfonylation of Styrenes. J. Org. Chem.84, 11135–11149. 10.1021/acs.joc.9b01829
30
LaiM.WuZ.WangY.ZhengY.ZhaoM. (2019a). Selective Synthesis of Aryl Thioamides and Aryl-α-Ketoamides from α-oxocarboxylic Acids and Tetraalkylthiuram Disulfides: an Unexpected Chemoselectivity from Aryl Sulfonyl Chlorides. Org. Chem. Front.6, 506–511. 10.1039/c8qo01127c
31
LiénardB. M. R.GarauG.HorsfallL.KarsisiotisA. I.DamblonC.LassauxP.et al (2008). Structural Basis for the Broad-Spectrum Inhibition of Metallo-β-Lactamases by Thiols. Org. Biomol. Chem.6, 2282–2294. 10.1039/b802311e
32
LiuY.BaoW. (2007). A New Method for the Synthesis of Dithiocarbamates by CuI-Catalyzed Coupling Reaction. Tetrahedron Lett.48, 4785–4788. 10.1016/j.tetlet.2007.03.168
33
LuM.-Z.DingX.ShaoC.HuZ.LuoH.ZhiS.et al (2020). Direct Hiyama Cross-Coupling of (Hetero)arylsilanes with C(sp2)-H Bonds Enabled by Cobalt Catalysis. Org. Lett.22, 2663–2668. 10.1021/acs.orglett.0c00631
34
LuoH.SunK.XieQ.LiX.ZhangX.LuoX. (2020). Copper‐Mediated Phosphorylation of Arylsilanes with H‐Phosphonate Diesters. Asian J. Org. Chem.9, 2083–2086. 10.1002/ajoc.202000529
35
MaganoJ.DunetzJ. R. (2011). Large-Scale Applications of Transition Metal-Catalyzed Couplings for the Synthesis of Pharmaceuticals. Chem. Rev.111, 2177–2250. 10.1021/cr100346g
36
McManusH. A.CozziP. G.GuiryP. J. (2006). Application of Tridentate Bis(oxazoline) Ligands in Catalytic Asymmetric Nozaki-Hiyama Allylation and Crotylation: An Example of High Enantioselection with a Non-symmetric Bis(oxazoline) Ligand. Adv. Synth. Catal.348, 551–558. 10.1002/adsc.200505332
37
MilsteinD.StilleJ. K. (1979a). Mechanism of Reductive Elimination. Reaction of Alkylpalladium(II) Complexes with Tetraorganotin, Organolithium, and Grignard Reagents. Evidence for Palladium(IV) Intermediacy. J. Am. Chem. Soc.101, 4981–4991. 10.1021/ja00511a031
38
MilsteinD.StilleJ. K. (1979b). Palladium-catalyzed Coupling of Tetraorganotin Compounds with Aryl and Benzyl Halides. Synthetic Utility and Mechanism. J. Am. Chem. Soc.101, 4992–4998. 10.1021/ja00511a032
39
MurahashiS.YamamuraM.YanagisawaK.MitaN.KondoK. (1979). Stereoselective Synthesis of Alkenes and Alkenyl Sulfides from Alkenyl Halides Using Palladium and Ruthenium Catalysts. J. Org. Chem.44, 2408–2417. 10.1021/jo01328a016
40
MiyauraN. (Editor) (2002). “Cross-coupling Reactions: a Practical Guide,” In Series Top. Curr. Chem. Vol. 219 (Berlin: Springer). 10.1007/3-540-45313-x
41
NakaoY.HiyamaT. (2011). Silicon-based Cross-Coupling Reaction: an Environmentally Benign Version. Chem. Soc. Rev.40, 4893–4901. 10.1039/c1cs15122c
42
NareddyP.JordanF.SzostakM. (2017). Highly Chemoselective Ruthenium(ii)-Catalyzed Direct Arylation of Cyclic and N,N-dialkyl Benzamides with Aryl Silanes. Chem. Sci.8, 3204–3210. 10.1039/c7sc00156h
43
NareddyP.JordanF.SzostakM. (2018). Ruthenium(II)-Catalyzed Direct C-H Arylation of Indoles with Arylsilanes in Water. Org. Lett.20, 341–344. 10.1021/acs.orglett.7b03567
44
NegishiE.-i. (2011). Magical Power of Transition Metals: Past, Present, and Future (Nobel Lecture). Angew. Chem. Int. Ed.50, 6738–6764. 10.1002/anie.201101380
45
NegishiE.KingA. O.OkukadoN. (1977). Selective Carbon-Carbon Bond Formation via Transition Metal Catalysis. 3. A Highly Selective Synthesis of Unsymmetrical Biaryls and Diarylmethanes by the Nickel- or Palladium-Catalyzed Reaction of Aryl- and Benzylzinc Derivatives with Aryl Halides. J. Org. Chem.42, 1821–1823. 10.1021/jo00430a041
46
NegishiE.OkukadoN.KingA. O.Van HornD. E.SpiegelB. I. (1978). Selective Carbon-Carbon Bond Formation via Transition Metal Catalysts. 9. Double Metal Catalysis in the Cross-Coupling Reaction and its Application to the Stereo- and Regioselective Synthesis of Trisubstituted Olefins. J. Am. Chem. Soc.100, 2254–2256. 10.1021/ja00475a059
47
NegishiE.Van HornD. E. (1977). Selective Carbon-Carbon Bond Formation via Transition Metal Catalysis. 4. A Novel Approach to Cross-Coupling Exemplified by the Nickel-Catalyzed Reaction of Alkenylzirconium Derivatives with Aryl Halides. J. Am. Chem. Soc.99, 3168–3170. 10.1021/ja00451a0550
48
OkukadoN.Van HornD. E.KlimaW. L.NegishiE. (1978). A Highly Stereo-, Regio-, and Chemoselective Synthesis of Conjugated Dienes by the Palladium-Catalyzed Reaction of (E)-1-alkenylzirconium Derivatives with Alkenyl Halides. Tetrahedron Lett.1027–1030. 10.1016/s0040-4039(01)85443-5
49
PengK.ZhuH.LiuX.PengH.-Y.ChenJ.-Q.DongZ.-B. (2019). Chemoselective C-S/S-S Formation between Diaryl Disulfides and Tetraalkylthiuram Disulfides. Eur. J. Org. Chem.2019, 7629–7634. 10.1002/ejoc.201901401
50
QiC.GuoT.XiongW. (2016). Copper-Mediated Coupling of Boronic Acids, Amines, and Carbon Disulfide: An Approach to Organic Dithiocarbamates. Synlett27, 2626–2630. 10.1055/s-0035-1560561
51
SahaniA. J.BurangeA. S.NarasimhanS.JayaramR. V. (2018). Cross‐Coupling Reactions of Aryltriethoxysilanes and Diaryldiselenides ‐ A New Route for the Synthesis of Diarylselenides. ChemistrySelect3, 12291–12296. 10.1002/slct.201802442
52
SekiyaA.IshikawaN. (1976). The Cross-Coupling of Aryl Halides with Grignard Reagents Catalyzed by iodo(phenyl)bis(triphenylphosphine)Palladium(II). J. Organomet. Chem.118, 349–354. 10.1016/s0022-328x(00)93215-7
53
SoreH. F.GallowayW. R. J. D.SpringD. R. (2012). Palladium-catalysed Cross-Coupling of Organosilicon Reagents. Chem. Soc. Rev.41, 1845–1866. 10.1039/c1cs15181a
54
SugiyamaA.OhnishiY.-y.NakaokaM.NakaoY.SatoH.SakakiS.et al (2008). Why Does Fluoride Anion Accelerate Transmetalation between Vinylsilane and Palladium(II)−Vinyl Complex? Theoretical Study. J. Am. Chem. Soc.130, 12975–12985. 10.1021/ja801362e
55
SuzukiA. (2011). Cross-Coupling Reactions of Organoboranes: An Easy Way to Construct CC Bonds (Nobel Lecture). Angew. Chem. Int. Ed.50, 6722–6737. 10.1002/anie.201101379
56
TamaoK.SumitaniK.KumadaM. (1972). Selective Carbon-Carbon Bond Formation by Cross-Coupling of Grignard Reagents with Organic Halides. Catalysis by Nickel-Phosphine Complexes. J. Am. Chem. Soc.94, 4374–4376. 10.1021/ja00767a075
57
WuX.-m.YanG.-b. (2019). Copper-Catalyzed Synthesis of S-Aryl Dithiocarbamates from Tetraalkylthiuram Disulfides and Aryl Iodides in Water. Synlett30, 610–614. 10.1055/s-0037-1612086
58
WuX.-X.YeH.JiangG.HuL. (2021). Domino Heck/Hiyama Cross-Coupling: Trapping of the σ-alkylpalladium Intermediate with Arylsilanes. Org. Biomol. Chem.19, 4254–4257. 10.1039/d1ob00595b
59
WuY.ZhangH.-R.CaoY.-X.LanQ.WangX.-S. (2016). Nickel-Catalyzed Monofluoroalkylation of Arylsilanes via Hiyama Cross-Coupling. Org. Lett.18, 5564–5567. 10.1021/acs.orglett.6b02803
60
WuZ.LaiM.ZhangS.ZhongX.SongH.ZhaoM. (2018). An Efficient Synthesis of Benzyl Dithiocarbamates by Base-Promoted Cross-Coupling Reactions of Benzyl Chlorides with Tetraalkylthiuram Disulfides at Room Temperature. Eur. J. Org. Chem.2018, 7033–7036. 10.1002/ejoc.201801449
61
WutsP. G. M.GreeneT. W. (2006). Greene's Protective Groups in Organic Synthesis. John Wiley & Sons. 10.1002/0470053488
62
XuW.GaoF.DongZ.-B. (2018). Copper-CatalyzedS-Arylation Starting from Arylboronic Acids and Tetraalkylthiuram Disulfide. Eur. J. Org. Chem.2018, 821–828. 10.1002/ejoc.201701757
63
YamamuraM.MoritaniI.MurahashiS.-I. (1975). The Reaction of σ-vinylpalladium Complexes with Alkyllithiums. Stereospecific Syntheses of Olefins from Vinyl Halides and Alkyllithiums. J. Organomet. Chem.91, 39–42. 10.1016/s0022-328x(00)89636-9
64
YinX.GuoY.LiuC.WangZ.ZhangB. (2015). One-pot Two-step Facile Synthesis of 2,3,5,6-Tetrafluorobenzonitrile-Containing Dithiocarbamic Acid Esters. Tetrahedron Lett.56, 5135–5139. 10.1016/j.tetlet.2015.07.009
65
ZengM.-T.XuW.LiuM.LiuX.ChangC.-Z.ZhuH.et al (2017a). Copper-catalyzed Amidation of Benzoic Acids Using Tetraalkylthiuram Disulfides as Amine Sources. Synth. Commun.47, 1434–1440. 10.1080/00397911.2017.1330960
66
ZengM.-T.XuW.LiuX.ChangC.-Z.ZhuH.DongZ.-B. (2017b). Copper-CatalyzedS-Arylation of Tetraalkylthiuram Disulfides by Using Diaryliodonium Salts. Eur. J. Org. Chem.2017, 6060–6066. 10.1002/ejoc.201701056
67
ZhangD.ChenJ.LiangY.ZhouH. (2005). Facile Synthesis of Novel Ionic Liquids Containing Dithiocarbamate. Synth. Commun.35, 521–526. 10.1081/scc-200049773
68
ZhangJ.HouY.MaY.SzostakM. (2019). Synthesis of Amides by Mild Palladium-Catalyzed Aminocarbonylation of Arylsilanes with Amines Enabled by Copper(II) Fluoride. J. Org. Chem.84, 338–345. 10.1021/acs.joc.8b02874
69
ZhangJ.WangS.ZhangY.FengZ. (2020). Iron‐Catalyzed Cross‐Coupling Reactions for the Construction of Carbon‐Heteroatom Bonds. Asian J. Org. Chem.9, 1519–1531. 10.1002/ajoc.202000334
70
ZouY.YuS.LiR.ZhaoQ.LiX.WuM.et al (2014). Synthesis, Antifungal Activities and Molecular Docking Studies of Novel 2-(2,4-Difluorophenyl)-2-Hydroxy-3-(1h-1,2,4-Triazol-1-Yl)propyl Dithiocarbamates. Eur. J. Med. Chem.74, 366–374. 10.1016/j.ejmech.2014.01.009
Summary
Keywords
Hiyama cross-coupling, arylsilanes, thiuram reagents, C-S bond formation, aryl dithiocarbamates
Citation
Wang Y, Shen H, Qiu J, Chen M, Song W, Zhao M, Wang L, Bai F, Wang H and Wu Z (2022) Copper-Promoted Hiyama Cross-Coupling of Arylsilanes With Thiuram Reagents: A Facile Synthesis of Aryl Dithiocarbamates. Front. Chem. 10:867806. doi: 10.3389/fchem.2022.867806
Received
01 February 2022
Accepted
09 March 2022
Published
26 April 2022
Volume
10 - 2022
Edited by
Simone Brogi, University of Pisa, Italy
Reviewed by
David Morales-Morales, Instituto de Química, Universidad Nacional Autónoma de México, Mexico
Kevin Alan Lobb, Rhodes University, South Africa
Michal Szostak, Rutgers University, United States
Updates
Copyright
© 2022 Wang, Shen, Qiu, Chen, Song, Zhao, Wang, Bai, Wang and Wu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mengqi Chen, 479820476@qq.com; Weimin Song, gongyishi@126.com; Zhiyong Wu, zhiyongwu@henau.edu.cn
This article was submitted to Organic Chemistry, a section of the journal Frontiers in Chemistry
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.