- 1National R & D Center for Edible Fungus Processing Technology, Henan University, Kaifeng, China
- 2School of Chemistry, Sun Yat-sen University, Guangzhou, China
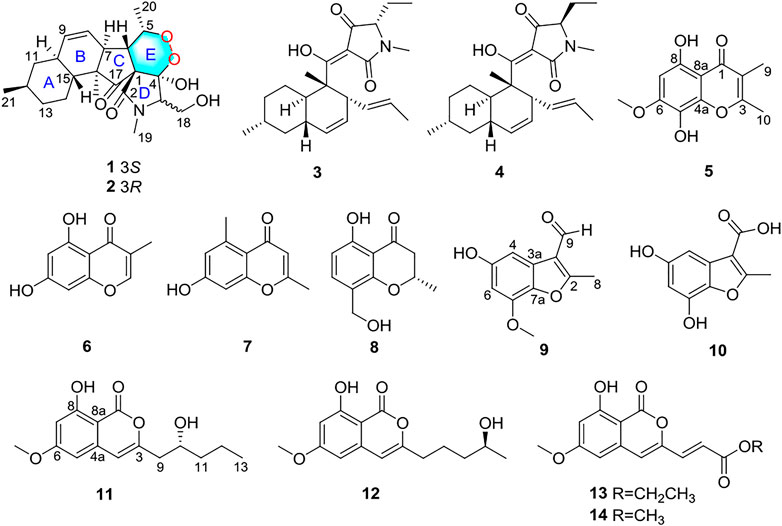
Two new 3-decalinoyltetramic acid derivatives with peroxide bridge fusarisetins E (1) and F (2), one new chromone fusarimone A (5), two new benzofurans fusarifurans A (9) and B (10), three new isocoumarins fusarimarins A–C (11–13), as well as five known analogues 3, 4, 6–8 and 14 were isolated from mangrove endophytic fungus Fusarium sp. 2ST2. Their structures and absolute configurations were established by spectroscopic analysis, density functional theory-gauge invariant atomic orbital NMR calculation with DP4+ statistical analysis, and electronic circular dichroism calculation. Compounds 1 and 2 showed significant cytotoxicity against human A549 cell lines with IC50 values of 8.7 and 4.3 μM, respectively.
Introduction
Endophytic fungi, inhabiting plants without any negative effects for the host, have been proven to be a promising source of novel structures and unique bioactivities (Liu et al., 2021; Viridiana et al., 2021). Fusarium spp. are endophytic fungi widely distributed in association with plants. It has attracted much attention due to their diverse bioactive secondary metabolites, including alkaloids, terpenes, cyclopeptide, anthraquinone, and lactones (Chen et al., 2019)—for example, indole alkaloids fusaindoterpenes A and B from Fusarium sp. showed antiviral activity (Guo et al., 2020), and fusarithioamide A from Fusarium chlamydosporium exhibited cytotoxic activity (Ibrahim et al., 2018).
Mangrove endophytic fungi, the second largest ecological group of marine fungi, have been reported to produce thousands of new metabolites until now (Chen et al., 2021; Chen et al., 2022). Over the past 2 decades, our group continues to explore bioactive novel structures from mangrove endophytic fungi (Huang et al., 2013; Xiao et al., 2013; Liu et al., 2016; Cui et al., 2017; Cai et al., 2019). In the course of our ongoing search for new antitumor active compounds from mangrove endophytic fungi, the strain Fusarium sp. 2ST2 attracted our attention because of the cytotoxicity of the crude extract. Then, eight new metabolites, including two alkaloids fusarisetin E (1) and F (2), one chromone fusarimone A (5), two benzofurans fusarifurans A (9) and B (10), three isocoumarins fusarimarins A–C (11–13), were obtained together with five analogues equisetin (3), epi-equisetin (4), takanechromone B (6), altechromone A (7), 4H-1-benzopyran-4-one-2,3-dihydro-5-hydroxy-8-(hydroxylmethyl)-2-methyl (8), and aspergisocoumrin A (14) (Figure 1). As expected, compounds 1 and 2 exhibited significant cytotoxicity against human A549 cell line, and compounds 8 and 14 showed potent cytotoxicity against A549 and MDA-MB-435 cell lines. The isolation, structure elucidation, and biological evaluation of these compounds were reported herein.
Materials and Methods
General Experimental Procedures
Optical rotations were measured on a PerkinElmer 341 instrument at 25°C. Melting points were recorded on a Fisher-Johns hot-stage apparatus. UV spectra were measured in MeOH using a Shimadzu UV-2700 spectrophotometer. Electronic circular dichroism (ECD) data were obtained on a Chirascan CD spectrometer (Applied Photophysics). A Bruker Avance 500 spectrometer (1H 500 MHz, 13C 125 MHz) was used for the 1D and 2D NMR data collection. All high-resolution electrospray ionization mass spectrometry (HRESIMS) data were obtained on an Agilent G6230 Q-TOF mass spectrometer. Silica gel (200–300 mesh, Qingdao Marine Chemical Factory) and Sephadex LH-20 (Amersham Pharmacia) were used in the column chromatography (CC). Silica gel plates (Qingdao Huang Hai Chemical Group Co., G60, F-254) were used for the thin-layer chromatography.
Fungal Material
The fungus Fusarium sp. 2ST2 was isolated from healthy leaves of Kandelia candel, which was collected in June 2015 from the South China Sea, Dong Zhai Harbor Mangrove Nature Reserve Area, Hainan Province, China. The strain was identified as Fusarium sp. (GenBank no. MZ801734) by a BLAST search which showed it to be 100% identical with the sequence of Fusarium sp. (GenBank no. KU296944.1).
Fermentation, Extraction, and Isolation
The fungus Fusarium sp. 2ST2 was cultivated on potato dextrose agar for 5 days. The mycelia of the strain were inoculated into 500 ml potato dextrose broth for 3 days to prepare the seed culture and then inoculated into the solid rice medium (70 g of rice, 3 g peptone, and 50 ml of distilled water, 60 flasks). It was incubated for 30 days at room temperature.
The medium was extracted with MeOH for three times, and the total residue of the strain (65.0 g) was obtained. The EtOAc extract was chromatographed by silica gel CC (200–300 mesh silica) and eluted with an increasing gradient of petroleum ether/EtOAc (9:1 to 1:9) to afford six fractions (Fr. A–F). Fraction B was applied to Sephadex LH-20 CC (CH2Cl2/MeOH v/v, 1:1) to give three fractions (Fr. B1–B3). Fraction B1 was subjected to silica gel CC (CH2Cl2/MeOH v/v, 98:2) to yield compounds 3 (5.8 mg) and 14 (2.5 mg). Fraction B2 was subjected to silica gel CC (CH2Cl2/MeOH v/v, 96:4) to yield compounds 9 (8.6 mg) and 13 (4.3 mg). Fraction C was eluted on Sephadex LH-20 CC (100% MeOH) to obtain compound 10 (7.5 mg) and two other fractions (Fr. C1–C2). Fraction C2 was separated using silica gel CC (CH2Cl2/MeOH v/v, 95:5) to yield compounds 11 (3.1 mg) and 12 (3.5 mg). Fraction D was eluted on Sephadex LH-20 CC (100% MeOH) to afford three fractions (Fr. D1–D3). Fraction D1 was purified by semipreparative UPLC (MeOH-H2O, 7:3) to give compounds 1 (3.6 mg) and 2 (4.0 mg). Fraction D2 was subjected to silica gel CC (CH2Cl2/MeOH v/v, 9:1) to give compounds 4 (2.5 mg) and 7 (6.5 mg). Fraction E was subjected to Sephadex LH-20 CC (CH2Cl2/MeOH v/v, 1:1) to give compound 6 (3.8 mg) and another fraction E1. Compounds 5 (3.0 mg) and 8 (2.8 mg) were obtained from fraction E1, which was subjected to UPLC (MeOH-H2O, 6:4).
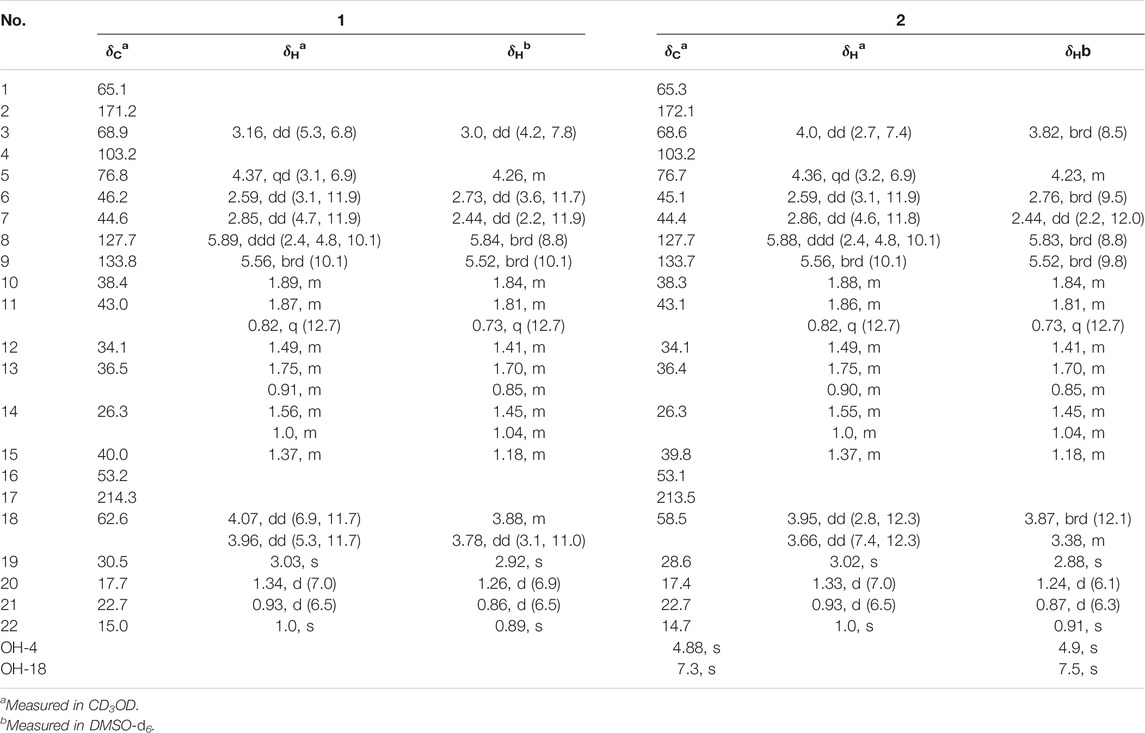
“Fusarisetin E (1): Colorless oil, [α] + 10.0 (c = 0.16, MeOH). UV (MeOH) λmax (log ε): 206 (3.02), 280 (2.16) nm. HRESIMS m/z 406.22293 [M + H]+ (calculated for C22H32NO6 406.22241). 1H and 13C NMR (CD3OD-d4) data, see Table 1.
Fusarisetin E (2): Colorless oil, [α] + 11.2 (c = 0.19, MeOH). UV (MeOH) λmax (log ε): 204 (3.0), 282 (2.56) nm. HRESIMS m/z 406.22274 [M + H]+ (calculated for C22H32NO6 406.22241). 1H and 13C NMR (CD3OD-d4) data, see Table 1.
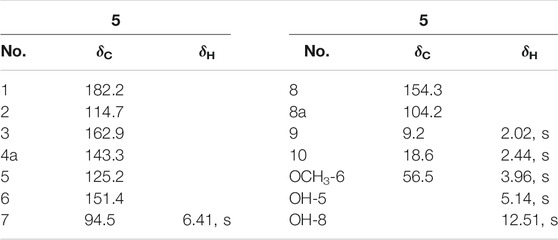
Fusarimone A (5): Yellow solid. HRESIMS m/z 237.07583 [M + H]+ (calculated for C12H13O5 237.07575). 1H and 13C NMR (CDCl3) data, see Table 2.
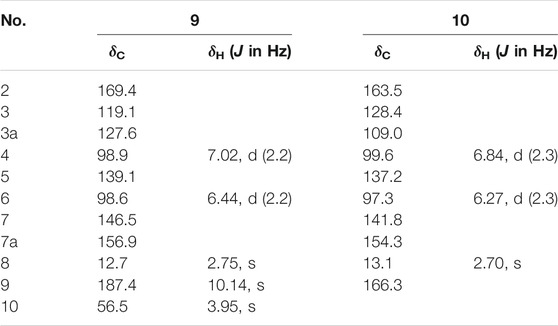
Fusarifuran A (9): White solid, HRESIMS m/z 205.05090 [M-H]- (calculated for C11H9O4 205.05063). 1H and 13C NMR (CD3OD-d4) data, see Table 3.
Fusarifuran B (10): White solid, HRESIMS m/z 207.03026 [M-H]‒ (calculated for C10H7O5 207.02990). 1H and 13C NMR (CD3OD-d4) data, see Table 3.
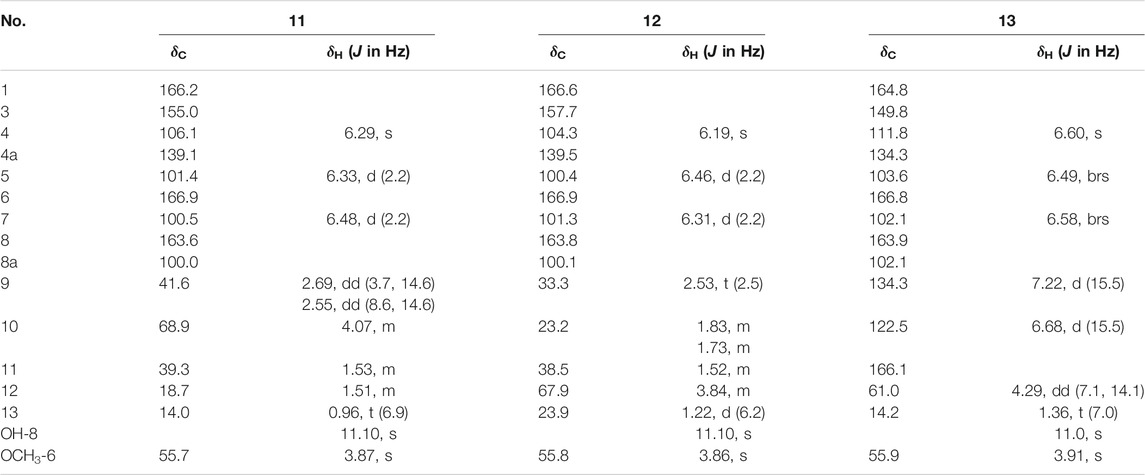
Fusarimarin A (11): Colorless oil, [α] −21.5 (с 0.06, MeOH). UV (MeOH) λmax (log ε): 219 (3.2), 238 (2.4), 318 (3.5) nm. HRESIMS m/z 279.12288 [M + H]+ (calculated for C15H19O5 279.12270). 1H and 13C NMR (CDCl3) data, see Table 4.
Fusarimarin B (12): Colorless oil, [α] +18.6 (с 0.07, MeOH). UV (MeOH) λmax (log ε): 220 (3.3), 252 (3.0), 316 (3.4) nm. HRESIMS m/z 279.12290 [M + H]+ (calculated for C15H19O5 279.12270). 1H and 13C NMR (CDCl3) data, see Table 4.
Fusarimarin C (13): Colorless oil, HRESIMS m/z 291.08639 [M + H]+ (calculated for C15H15O6 291.08631). 1H and 13C NMR (CDCl3) data, see Table 4.
NMR Calculations
In general, conformational analysis was carried out using Merck Molecular Field by Spartan’s 10 software. Conformers above 1% Boltzmann populations were optimized at the B3LYP/6-311+G (d, p) level in polarizable continuum model (PCM) methanol (Gaussian 09). Subsequently, NMR calculations were computed using the gauge invariant atomic orbital (GIAO) method at the mPWLPW91-SCRF/6-311+G (d, p) level using the PCM in methanol (Gaussian 09). Finally, the shielding constants were averaged by Boltzmann distribution theory for each stereoisomer, and their experimental and calculation data were analyzed by DP4+ probability.
ECD Calculations
The ECD calculations were performed as described previously (Chen et al., 2020). Geometric optimization of compounds 1 and 2 was carried out at the B3LYP/6-31+G(d) level in the liquid phase. Then, ECD calculations were performed using the TDDFT methodology at the WB97XD/CC-PVDZ and WB97XD/6-31G levels, respectively.
Cytotoxicity Assay
The cytotoxicity of all compounds against tumor cell lines was tested by the MTT assay as previously reported (Chen et al., 2019).
Results and Discussion
Compound 1 was isolated as a colorless oil. The molecular formula was determined as C22H32NO6 based on the HRESIMS data (m/z 406.22293 [M + H]+). The 1H NMR data of 1 (Table 1) showed three methyl signals at δH 0.93 (d, J = 6.5 Hz), 1.0 (s), and 1.34 (d, J = 7.0 Hz), one N-methyl proton at δH 3.03 (s), and two olefinic protons at δH 5.56 (brd, J = 10.1 Hz) and 5.89 (ddd, J = 2.4, 4.8, 10.1 Hz). The 13C NMR data of 1 (Table 1) displayed 22 carbon signals, including four methyls, four methylenes (one oxygenated), seven methines (two olefinic), and four quaternary carbons (one ketone carbonyl and one ester carbonyl carbon). The planar structure of 1 was a detailed analysis of the 1D and 2D NMR data. The spin system of H3-20/H-5/H-6/H-7/H-8/H-9/H-10/H2-11/H-12(/H3-21)/H2-13/H2-14/H-15(/H-10) from 1H-1H COSY data (Figure 2), together with the heteronuclear multiple-bond correlations (HMBC) (Figure 2) from H3-22 to C-7, C-15, C-16 and C-17 and from H-6 to C-1, established the partial ring system of A/B/C, while the HMBC correlations from H-6 to C-2 and C-4, from H-3 to C-1 and C-4, and from H3-19 to C-2 and C-3 indicated the presence of a γ-lactam moiety (ring D). In addition, a peroxide bridge between C-4 and C-5 was proposed according to two additional oxygen atoms in the molecular formula of 1, which constitute the ring E. Thus, the planar structure of 1 was deduced (Figure 1), which was similar to fusarisetin A (Jang et al., 2011), by comparing their NMR data.
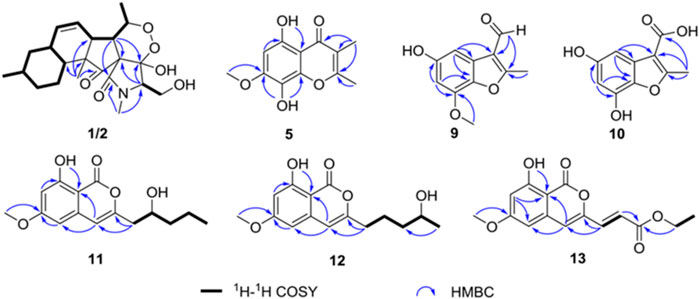
FIGURE 2. Key heteronuclear multiple-bond correlations and correlation spectroscopy of compounds 1, 2, 5 and 9–13.
Compound 2, with a molecular formula of C22H32NO6, the same as 1, was isolated as a colorless oil. The results of comparing the NMR data of 1 and 2 indicated that they shared a planar structure, and this was further confirmed by an extensive analysis of 1H-1H COSY and HMBC correlations (Figure 2), while the major difference of NMR shifts at H-3 (ΔδH +0.84), C-18 (ΔδC −4.1), and C-19 (ΔδC −1.9) suggested 1 and 2 to be 3-epimers.
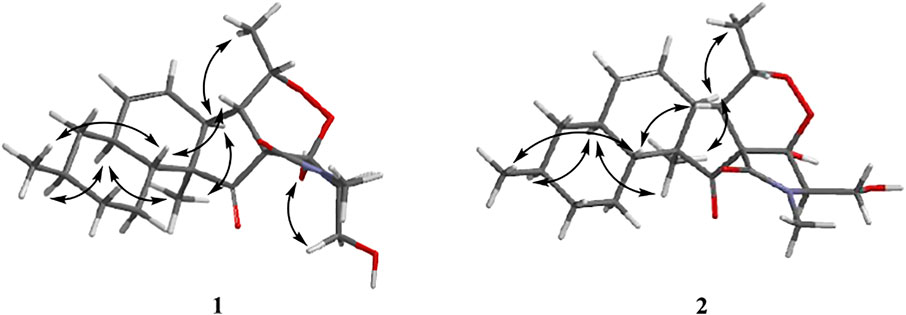
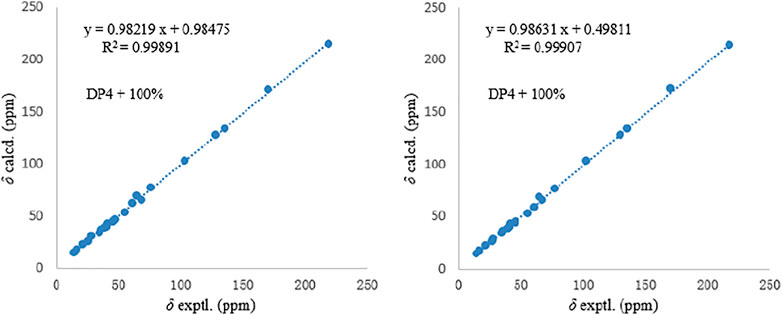
The relative configurations of 1 and 2 were determined by the NOESY correlations (Figure 3). The cross-peaks of H-12/H-10/H3-22/H-7/H3-20 suggested that these protons were co-facial, while the correlations of H3-21/H-15/H-6 showed that these protons were on the other face. Considering the absence of correlation from H2-18 and 4-OH to other protons, the NOESY spectrum of 1 and 2 were retested in DMSO-d6 reagent. Then, the correlation of 4-OH/H2-18 was only detected in 1, indicating that the protons of OH-4 and H2-18 were positioned on the same face in 1 and were opposite in 2. Thus, 1 and 2 were an epimer at C-3. Subsequently, the 13C NMR calculations of (1R*, 3S*, 4R*, 5S*, 6S*, 7S*, 10S*, 21R*, 15R*, 16S*)-1a and (1R*, 3R*, 4S*, 5S*, 6S*, 7S*, 10S*, 21R*, 15R*, 16S*)-1b were carried out using the GIAO method at mPW1PW91-SCRF/6–311+G (d, p)/PCM (MeOH). The results of the DP4+ probability analysis (Smith and Goodman, 2010; Kawazoe et al., 2020; Xu et al., 2021) showed that 1a was the most likely candidate structure, with a better correlation coefficient (R2 = 0.99891) and a high DP4+ probability of 100% (all data) probability (Figure 4). Similarly, 13C NMR calculations with the DP4+ probability analysis of the two isomers [(1R*, 3S*, 4S*, 5S*, 6S*, 7S*, 10S*, 21R*, 15R*, 16S*)-2a and (1R*, 3R*, 4R*, 5S*, 6S*, 7S*, 10S*, 21R*, 15R*, 16S*)-2b] of 2 were performed. The results showed that 2 gave the best match of 100% (all data) with the 2b isomer.
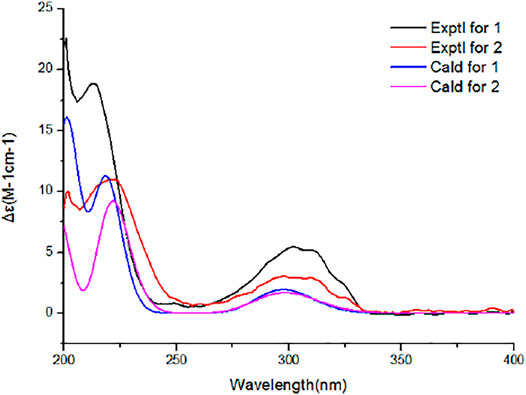
Aiming at determining the absolute configuration of 1, the ECD calculation was performed at the WB97XD/CC-PVDZ level. The results showed that the calculated ECD curve was in good agreement with the experimental one (Figure 5). Therefore, the absolute configuration of 1 was assigned as 1R, 3S, 4R, 5S, 6S, 7S, 10S, 21R, 15R, 16S. The absolute configuration of 2 was determined to be 1R, 3R, 4R, 5S, 6S, 7S, 10S, 21R, 15R, 16S by the identical experimental and calculated curves (Figure 5).
Compound 5 was obtained as a yellow solid. The molecular formula was determined to be C12H13O5 based on HRESIMS data (m/z 237.07583 [M + H]+). The 1H NMR spectrum (Table 2) of 5 showed two methyl groups at δH 2.02 (s), 2.44 (s), one methoxyl group at δH 3.96 (s), one olefinic proton at δH 6.41 (s), and one chelated hydroxyl group at δH 12.51 (s). The 13C NMR data (Table 2) of 5 highlighted the presence of 12 carbon resonances, including three methyls (one oxygenated), one olefinic carbon, and eight quaternary carbons (one carbonyl carbon and seven olefinic carbons). The 1H and 13C NMR data of 5 were similar to those of 6, indicating that 5 was one chromone. The structure of 5 was further established by the HMBC correlations (Figure 2) from H3-9 to C-1, C-2, and C-3 and from H3-10 to C-3.
Compound 9 was obtained as a white solid. The molecular formula was determined to be C11H9O4 based on HRESIMS data. The 1H NMR spectrum (Table 3) of 9 showed one methyl group at δH 2.75 (s), one methoxyl group at δH 3.95 (s), and two aromatic protons at δH 7.02 (d, J = 2.2 Hz),6.44 (d, J = 2.2 Hz). The 13C NMR data (Table 3) of 9 highlighted the presence of 11 carbon resonances, including two methyls, two sp2 methines, and seven quaternary carbons. These data suggest 9 to be a benzofuran derivate. The NMR data of 9 were closely similar to penicifuran C (Qi et al., 2013), except for the presence of a methoxyl group, The HMBC correlations (Figure 2) from H3-10 to C-7 indicated that the methoxyl group was located at C-7. Thus, the structure of 9 was determined as shown in Figure 1.
Compound 10 was obtained as a white solid. The molecular formula was determined to be C10H13O5 based on HRESIMS data. The 1H and 13C NMR data (Table 3) of 10 were similar to those of 9, except that the aldehyde group in 9 was oxidized to the carboxyl group, and there was an absence of the methoxyl group. The deduction was further confirmed by the HMBC correlations (Figure 2) from H3-8 to C-2, C-3, and C-9. Therefore, the structure of 10 was established as shown.
Compound 11 had the molecular formula of C15H18O5 by the HRESIMS data. The 1H NMR spectrum (Table 4) of 11 showed one chelated hydroxyl group at δH 11.10 (s), one methyl group at δH 0.96 (t, J = 6.9 Hz), one methoxyl group at δH 3.87 (s), and three olefinic protons at δH 6.29 (s), 6.33 (d, J = 2.2 Hz), and 6.48 (d, J = 2.2 Hz). The 13C NMR data (Table 4) of 11 revealed the presence of 15 carbon resonances, including two methyls, three methylenes, one sp3 and three sp2 methines, and six quaternary carbons. These data suggest 11 to be an isocoumarin class. The spin system of H2-9/H-10/H2-11/H2-12/H3-13 in the 1H-1H COSY spectrum (Figure 2) as well as the HMBC correlations (Figure 2) from H2-9 to C-3 and C-4 showed that the side chain was substituted at C-3. By comparing the specific rotation of 11 [[α]−21.5 (с 0.06, MeOH)] with (−)-citreoisocoumarin [[α] −29.8 (с 0.34, MeOH)] (Mallampudi et al., 2020), the 10R configuration at C-10 in 11 was indicated. Thus, the gross structure of 11 was defined as shown.
Compound 12 was isolated as a colorless oil. The molecular formula was determined to be C15H18O5 based on HRESIMS data. The comparison of the 1H and 13C NMR data (Table 4) with those of 11 revealed that they share the same isocoumarin structure, except that the hydroxyl group was substituted at C-12 in 12. The spin system of H2-9/H2-10/H2-11/H-12/H2-13 in the 1H-1H COSY spectrum (Figure 2), together with the HMBC correlations (Figure 2) from H2-9 to C-3 and C-4, further supported this possibility. The 12S configuration was confirmed by the positive specific rotation value of 12 [[α] +18.6 (с 0.07, MeOH)] when compared with peneciraistin D [[α] +21.1 (с 0.14, MeOH)] (Ma et al., 2012).
Compound 13 was isolated as a colorless oil with the molecular formula of C15H14O6 based on HRESIMS data. Upon comparing the 1H and 13C NMR data (Table 4) between 11 and 13, it was suggested that 13 also possessed the isocoumarin framework. The spin system of H-9/H-10 observed in the 1H-1H COSY spectrum (Figure 2) and the HMBC correlations (Figure 2) from H-9 to C-3 and C-4 from H-10 to C-11 made it possible to obtain the gross structure. Additionally, an ethyl group was linked with C-11 by the HMBC correlations from H-12 to C-11. The 9E configuration of the double bond was determined by the large coupling constant JH-9, H-10 = 15.5 Hz. Thus, the structure of 13 was confirmed as shown in Figure 1.
Five known analogues were characterized as equisetin (3) (Zhao et al., 2019), epi-equisetin (4) (Zhao et al., 2019), takanechromone B (6) (Qader et al., 2021), altechromone A (7) (Tanaka et al., 2009), 4H-1-benzopyran-4-one- 2,3-dihydro-5-hydroxy-8-(hydroxylmethyl)-2-methyl (8) (Sousa et al., 2016), and aspergisocoumrin A (14) (Wu et al., 2019) through a comparison of the spectroscopic data with the literature.
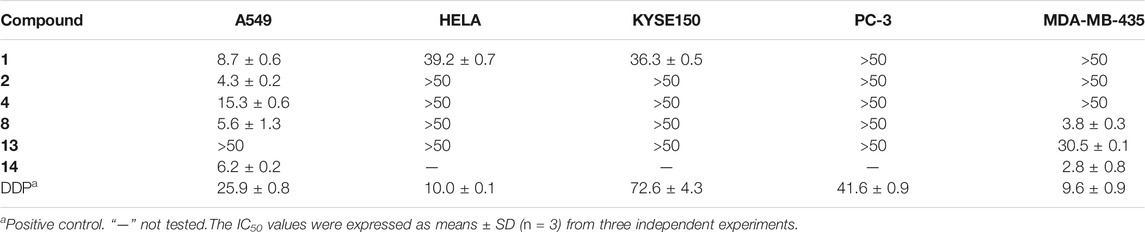
All compounds were evaluated for their cytotoxicity against the A549 (lung carcinoma), HELA (cervical carcinoma), KYSE150 (esophageal squamous carcinoma), PC-3 (pancreatic carcinoma), and MDA-MB-435 (breast carcinoma) human cancer cell lines (Table 5). As a result, compounds 1 and 2 showed selective cytotoxicity against A549 cell line with IC50 values of 8.7 and 4.3 μM, respectively. Compound 8 showed potent cytotoxicity against A549 and MDA-MB-435 cell lines with IC50 values of 5.6 and 3.8 μM, respectively. Compound 14 exhibited significant cytotoxicity against A549 and MDA-MB-435 cell lines with IC50 values of 6.2 and 2.8 μM, respectively, while the other compounds exhibited non-significant activity against the five cancer cell lines at the concentration of 50 μM.
Conclusion
In summary, two new 3-decalinoyltetramic acid (3DTA) derivatives, fusarisetins E (1) and F (2), with a peroxide bridge, were isolated from mangrove endophytic fungus Fusarium sp. 2ST2. The 3DTA derivatives showed various bioactivities, such as antimicrobial, anticancer, larvicidal, cytotoxic, and antiviral (Fan et al., 2020). The structure of fusarisetins E (1) and F (2) was similar to that of fusarisetin A, which was first isolated from the soil fungus Fusarium sp. FN080326 with inhibitory activity to acinar morphogenesis (Jang et al., 2011), while fusarisetin E (1) was identified as peroxyfusarisetin (Yin et al., 2012), a synthetic intermediate by mixture. Here fusarisetin E (1) was reported first as an optically pure new natural product with 1D and 2D NMR data (Supplementary Figures S1–S8). Moreover, natural peroxide compounds that usually have unique pharmacological activities, such as artemisinin with antimalarial activity (Zhao et al., 2018; Pandey et al., 1999), talaperoxides A-D with cytotoxicity (Li et al., 2011), phaeocaulisin M with anti-inflammatory activity (Ma et al., 2015), 1α,8α-epidioxy-4α-hydroxy-5αH-guai-7(11),9-dien-12,8-olide with antiviral activity (Dong et al., 2013), and plakinic acid M with antifungal activity (Matthew et al., 2016), were reported. Compounds 1 and 2 had selective cytotoxicity against A549 cell line with IC50 values of 8.7 and 4.38 μM, respectively. The cytotoxicity of fusarisetins was reported for the first time.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
YC performed the experiments, analyzed the data, and wrote the paper. GW and YY completed the biological activity test. GZ, WY, and QT participated in the experiments (fermentation and extraction of the strain). WK and ZS reviewed the manuscript. ZS designed and supervised the experiments. All authors have read and agreed to the published version of the manuscript.
Funding
This research received generous support and was funded by the National Natural Science Foundation of China (U20A2001, 21877133), Development Program of Guangdong Province (2020B1111030005), Key Project in Science and Technology Agency of Henan Province (212102311029), and Key Scientific Research Project in Colleges and Universities of Henan Province (22B350001).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2022.842405/full#supplementary-material
References
Cai, R., Jiang, H., Xiao, Z., Cao, W., Yan, T., Liu, Z., et al. (2019). (−)- and (+)-Asperginulin A, a Pair of Indole Diketopiperazine Alkaloid Dimers with a 6/5/4/5/6 Pentacyclic Skeleton from the Mangrove Endophytic Fungus Aspergillus Sp. SK-28. Org. Lett. 21, 9633–9636. doi:10.1021/acs.orglett.9b03796
Chen, J., Bai, X., Hua, Y., Zhang, H., and Wang, H. (2019a). Fusariumins C and D, Two Novel Antimicrobial Agents from Fusarium Oxysporum ZZP-R1 Symbiotic on Rumex Madaio Makino. Fitoterapia 134, 1–4. doi:10.1016/j.fitote.2019.01.016
Chen, S., Cai, R., Liu, Z., Cui, H., and She, Z. (2022). Secondary Metabolites from Mangrove-Associated Fungi: Source, Chemistry and Bioactivities. Nat. Prod. Rep. doi:10.1039/D1NP00041A
Chen, Y., Liu, Z., Huang, Y., Liu, L., He, J., Wang, L., et al. (2019b). Ascomylactams A-C, Cytotoxic 12- or 13-Membered-Ring Macrocyclic Alkaloids Isolated from the Mangrove Endophytic Fungus Didymella Sp. CYSK-4, and Structure Revisions of Phomapyrrolidones A and C. J. Nat. Prod. 82, 1752–1758. doi:10.1021/acs.jnatprod.8b00918
Chen, Y., Zhang, L., Zou, G., Li, C., Yang, W., Liu, H., et al. (2020). Anti-inflammatory Activities of Alkaloids from the Mangrove Endophytic Fungus Phomopsis Sp. SYSUQYP-23. Bioorg. Chem. 97, 103712. doi:10.1016/j.bioorg.2020.103712
Chen, Y., Zou, G., Yang, W., Zhao, Y., Tan, Q., Chen, L., et al. (2021). Metabolites with Anti-inflammatory Activity from the Mangrove Endophytic Fungus Diaporthe Sp. QYM12. Mar. Drugs 19, 56. doi:10.3390/md19020056
Cui, H., Lin, Y., Luo, M., Lu, Y., Huang, X., and She, Z. (2017). Diaporisoindoles A-C: Three Isoprenylisoindole Alkaloid Derivatives from the Mangrove Endophytic Fungus Diaporthe Sp. SYSU-HQ3. Org. Lett. 19, 5621–5624. doi:10.1021/acs.orglett.7b02748
Dong, J.-Y., Ma, X.-Y., Cai, X.-Q., Yan, P.-C., Yue, L., Lin, C., et al. (2013). Sesquiterpenoids from Curcuma Wenyujin with Anti-influenza Viral Activities. Phytochemistry 85, 122–128. doi:10.1016/j.phytochem.2012.09.008
Fan, B., Dewapriya, P., Li, F., Grauso, L., Blümel, M., Mangoni, A., et al. (2020). Pyrenosetin D, a New Pentacyclic Decalinoyltetramic Acid Derivative from the Algicolous Fungus Pyrenochaetopsis Sp. FVE-087. Mar. Drugs 18, 281. doi:10.3390/md18060281
Guo, Y.-W., Liu, X.-J., Yuan, J., Li, H.-J., Mahmud, T., Hong, M.-J., et al. (2020). l-Tryptophan Induces a Marine-Derived Fusarium Sp. To Produce Indole Alkaloids with Activity against the Zika Virus. J. Nat. Prod. 83, 3372–3380. doi:10.1021/acs.jnatprod.0c00717
Huang, X., Huang, H., Li, H., Sun, X., Huang, H., Lu, Y., et al. (2013). Asperterpenoid A, a New Sesterterpenoid as an Inhibitor of Mycobacterium tuberculosis Protein Tyrosine Phosphatase B from the Culture of Aspergillus Sp. 16-5c. Org. Lett. 15, 721–723. doi:10.1021/ol303549c
Ibrahim, S. R. M., Mohamed, G. A., Al Haidari, R. A., Zayed, M. F., El-Kholy, A. A., Elkhayat, E. S., et al. (2018). Fusarithioamide B, a New Benzamide Derivative from the Endophytic Fungus Fusarium Chlamydosporium with Potent Cytotoxic and Antimicrobial Activities. Bioorg. Med. Chem. 26, 786–790. doi:10.1016/j.bmc.2017.12.049
Jang, J.-H., Asami, Y., Jang, J.-P., Kim, S.-O., Moon, D. O., Shin, K.-S., et al. (2011). Fusarisetin A, an Acinar Morphogenesis Inhibitor from a Soil Fungus, Fusarium Sp. FN080326. J. Am. Chem. Soc. 133, 6865–6867. doi:10.1021/ja1110688
Kawazoe, R., Matsuo, Y., Saito, Y., and Tanaka, T. (2020). Computationally Assisted Structural Revision of Flavoalkaloids with a Seven-Membered Ring: Aquiledine, Isoaquiledine, and Cheliensisine. J. Nat. Prod. 83, 3347–3353. doi:10.1021/acs.jnatprod.0c00691
Li, H., Huang, H., Shao, C., Huang, H., Jiang, J., Zhu, X., et al. (2011). Cytotoxic Norsesquiterpene Peroxides from the Endophytic Fungus Talaromyces flavus Isolated from the Mangrove Plant Sonneratia Apetala. J. Nat. Prod. 74, 1230–1235. doi:10.1021/np200164k
Liu, G., Huo, R., Zhai, Y., and Liu, L. (2021). New Bioactive Sesquiterpeniods from the Plant Endophytic Fungus Pestalotiopsis theae. Front. Microbiol. 12, 641504. doi:10.3389/fmicb.2021.641504
Liu, Z., Chen, Y., Chen, S., Liu, Y., Lu, Y., Chen, D., et al. (2016). Aspterpenacids A and B, Two Sesterterpenoids from a Mangrove Endophytic Fungus Aspergillus terreus H010. Org. Lett. 18, 1406–1409. doi:10.1021/acs.orglett.6b00336
Ma, J.-H., Wang, Y., Liu, Y., Gao, S.-Y., Ding, L.-Q., Zhao, F., et al. (2015). Four New Sesquiterpenes from the Rhizomes ofCurcuma Phaeocaulisand Their iNOS Inhibitory Activities. J. Asian Nat. Prod. Res. 17, 532–540. doi:10.1080/10286020.2015.1046449
Ma, L.-Y., Liu, W.-Z., Shen, L., Huang, Y.-L., Rong, X.-G., Xu, Y.-Y., et al. (2012). Spiroketals, Isocoumarin, and Indoleformic Acid Derivatives from saline Soil Derived Fungus Penicillium raistrickii. Tetrahedron 68, 2276–2282. doi:10.1016/j.tet.2012.01.054
Mallampudi, N. A., Choudhury, U. M., and Mohapatra, D. K. (2020). Total Synthesis of (−)-Citreoisocoumarin, (−)-Citreoisocoumarinol, (−)-12-Epi-Citreoisocoumarinol, and (−)-Mucorisocoumarins A and B Using a Gold(I)-Catalyzed Cyclization Strategy. J. Org. Chem. 85, 4122–4129. doi:10.1021/acs.joc.9b03278
Matthew, T. J., Doralyn, S. D., and Tadeusz, F. M. (2016). Peroxide Natural Products from Plakortis Zyggompha and the Sponge Association Plakortis Halichondrioides-Xestospongia Deweerdtae: Antifungal Activity against Cryptococcus Gattii. J. Nat. Prod. 79, 555. doi:10.1021/acs.jnatprod.5b00951
Pandey, A. V., Tekwani, B. L., Singh, R. L., and Chauhan, V. S. (1999). Artemisinin, an Endoperoxide Antimalarial, Disrupts the Hemoglobin Catabolism and Heme Detoxification Systems in Malarial Parasite. J. Biol. Chem. 274, 19383–19388. doi:10.1074/jbc.274.27.19383
Qader, M. M., Hamed, A. A., Soldatou, S., Abdelraof, M., Elawady, M. E., Hassane, A. S. I., et al. (2021). Antimicrobial and Antibiofilm Activities of the Fungal Metabolites Isolated from the Marine Endophytes Epicoccum Nigrum M13 and Alternaria alternata 13A. Mar. Drugs 19, 232. doi:10.3390/md19040232
Qi, J., Shao, C.-L., Li, Z.-Y., Gan, L.-S., Fu, X.-M., Bian, W.-T., et al. (2013). Isocoumarin Derivatives and Benzofurans from a Sponge-Derived Penicillium Sp. Fungus. J. Nat. Prod. 76, 571–579. doi:10.1021/np3007556
Smith, S. G., and Goodman, J. M. (2010). Assigning Stereochemistry to Single Diastereoisomers by GIAO NMR Calculation: the DP4 Probability. J. Am. Chem. Soc. 132, 12946–12959. doi:10.1021/ja105035r
Sousa, J. P. B., Aguilar-Pérez, M. M., Arnold, A. E., Rios, N., Coley, P. D., Kursar, T. A., et al. (2016). Chemical Constituents and Their Antibacterial Activity from the Tropical Endophytic Fungus Diaporthe Sp. F2934. J. Appl. Microbiol. 120, 1501–1508. doi:10.1111/jam.13132
Tanaka, N., Kashiwada, Y., Nakano, T., Shibata, H., Higuchi, T., Sekiya, M., et al. (2009). Chromone and Chromanone Glucosides from Hypericum Sikokumontanum and Their Anti-Helicobacter pylori Activities. Phytochemistry 70, 141–146. doi:10.1016/j.phytochem.2008.11.006
Viridiana, M. S., Carmen, E. D., Olmeda, SA, Valcarcel, F, Elena, T., Azucena, G. C., et al. (2021). Bioactive Metabolites from the Endophytic Fungus Aspergillus Sp. SPH2. J. Fungi 7, 109. doi:10.3390/md13053091
Wu, Y., Chen, S., Liu, H., Huang, X., Liu, Y., Tao, Y., et al. (2019). Cytotoxic Isocoumarin Derivatives from the Mangrove Endophytic Fungus Aspergillus Sp. HN15-5D. Arch. Pharm. Res. 42, 326–331. doi:10.1007/s12272-018-1019-1
Xiao, Z. e., Huang, H., Shao, C., Xia, X., Ma, L., Huang, X., et al. (2013). Asperterpenols A and B, New Sesterterpenoids Isolated from a Mangrove Endophytic Fungus Aspergillus Sp. 085242. Org. Lett. 15, 2522–2525. doi:10.1021/ol401005j
Xu, M.-M., Zhou, J., Zeng, L., Xu, J., Onakpa, M. M., Duan, J.-A., et al. (2021). Pimarane-derived Diterpenoids with Anti-Helicobacter pylori Activity from the Tuber of Icacina Trichantha. Org. Chem. Front. 8, 3014–3022. doi:10.1039/d1qo00374g
Yin, J., Wang, C., Kong, L., Cai, S., and Gao, S. (2012). Asymmetric Synthesis and Biosynthetic Implications of (+)-Fusarisetin A. Angew. Chem. Int. Ed. 51, 7786–7789. doi:10.1002/anie.201202455
Zhao, D., Han, X., Wang, D., Liu, M., Gou, J., Peng, Y., et al. (2019). Bioactive 3-Decalinoyltetramic Acids Derivatives from a Marine-Derived Strain of the Fungus Fusarium Equiseti D39. Front. Microbiol. 10, 1285. doi:10.3389/fmicb.2019.01285
Keywords: mangrove, endophytic fungus, Fusarium sp., cytotoxicity, benzofuran, chromone
Citation: Chen Y, Wang G, Yuan Y, Zou G, Yang W, Tan Q, Kang W and She Z (2022) Metabolites With Cytotoxic Activities From the Mangrove Endophytic Fungus Fusarium sp. 2ST2. Front. Chem. 10:842405. doi: 10.3389/fchem.2022.842405
Received: 23 December 2021; Accepted: 12 January 2022;
Published: 15 February 2022.
Edited by:
Xiachang Wang, Nanjing University of Chinese Medicine, ChinaCopyright © 2022 Chen, Wang, Yuan, Zou, Yang, Tan, Kang and She. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenyi Kang, a2FuZ3dlbnlpQGhlbnUuZWR1LmNu; Zhigang She, Y2Vzc2h6aGdAbWFpbC5zeXN1LmVkdS5jbg==
 Yan Chen
Yan Chen Guisheng Wang1
Guisheng Wang1 Zhigang She
Zhigang She