
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Chem. , 24 June 2021
Sec. Medicinal and Pharmaceutical Chemistry
Volume 9 - 2021 | https://doi.org/10.3389/fchem.2021.698700
This article is part of the Research Topic Isolation, Structural Elucidation, and Biological Evaluation of Bioactive Products from Traditional Medicine View all 21 articles
Four new guaiane-type sesquiterpenes, argyin H–K (1–4), and two known analogues (5 and 6) were isolated from the leaves of Artemisia argyi Lévl et Vant. The new compounds were characterized by the basic analysis of the spectroscopic data obtained (1H NMR, 13C NMR, HMBC, and NOESY experiments), and their absolute configurations were determined by empirical approaches, combined with the exciton chirality method and electronic circular dichroism calculations. To further understand the antitumor effects of A. argyi, the antiproliferative activities of these compounds against A549, MCF-7, and HepG2 cell lines were tested in vitro using CCK-8 assays. The results showed that these compounds had significant antiproliferative effects on MCF-7, with IC50 values of 15.13–18.63 μM, which were superior to that of oxaliplatin (i.e., IC50 22.20 μM).
Artemisia argyi Levl. et Vant, an important species in the genus of Compositae, is distributed in China, Japan, Korea, Far East of Russia, etc. (Doh et al., 2016; Ozek et al., 2014). A. argyi often appears in people's life in various forms. As a common medicinal resource for curing eczema, diarrhea, hemostasis, and irregular menstruation in Chinese history, it has advantages of low price, easy access, wide application, and low toxicity (Li et al., 2018; Li et al., 2018; Wang et al., 2013). Pharmacological studies have shown that A. argyi is rich in terpenoids, flavonoids, and tannins (Zan et al., 2012; Tan and Jia, 1992; Dahae et al., 2018). In vitro experiments indicated that terpenoids exhibited various promising biological activities, including antibacterial, antivirus, and antitumor activities (Merfort, 2011; Zhang et al., 2005). In an effort to explore the structural diversity and biological activities of sesquiterpenes in A. argyi, a comprehensive phytochemical investigation was carried out, and all the compounds isolated were evaluated for their antiproliferative activities in A549, MCF-7, and HepG2 cell lines.
Compound 1 gave a molecular formula of C20H28O7 (HR-ESI-MS m/z 403.1728 [M + Na]+, calcd for 403.1733), which suggested seven unsaturation degrees. The 1H NMR signals at δ 6.04 (1H, d, J = 3.3 Hz) and 5.68 (1H, d, J = 3.3 Hz) indicated an exocyclic methylene group. In the 13C NMR spectrum, apart from five characteristic carbon signals for a 3-methylbutyryl group, 15 carbon resonances were observed and indicated a sesquiterpene structure.
In the HMBC spectrum (Figure 1A), the cross-peak among H-6/C-8; H-5/C-4, C-7, and C-15; H2-2/C-3, C-4, and C-5; and H2-14/C-1, C-9, and C-10 established a guaiane-type sesquiterpene skeleton with a ∆10,14 double bond. The hydroxyl substitutions at C1 (δ 5.13, 1H, s), C3 (δ 4.80, 1H, d, J = 4.6 Hz), and C4 (δ 4.31, 1H, s) were confirmed by the correlations of 1-OH/C-2, C-5, and C-10; 3-OH/C-2; 4-OH/C-3 and C-5, respectively. The position of 3-methylbutyryl was secured by the key HMBC correlation of H-8 (δH 4.85)/C-1′ (δC 172.1). Thus, the planar framework of one was established (Figure 2; Table 1).

TABLE 1. 1H NMR (DMSO-d6, 600 MHz) and 13C NMR (DMSO-d6, 150 MHz) spectroscopic data for compounds one to four.
The trans disposition of H-5 and H-6 was deduced from the large vicinal coupling constants (J5,6 = 11.6 Hz) (Wang et al., 2014). The strong NOE correlation between H-6/H3-15 and H-8, H3-15/H-3 revealed their cis relationship; H-5/H-7, C1-OH, and C4-OH indicated that they have co-facial orientations and assigned as α-oriented (Figure 1B). The overall pattern of the experimentally CD of one well matched the calculated ECD curve, which elucidated the absolute configuration of one was 1S, 3R, 4S, 5R, 6S, 7R, 8S, and named argyin H (Figure 3A).
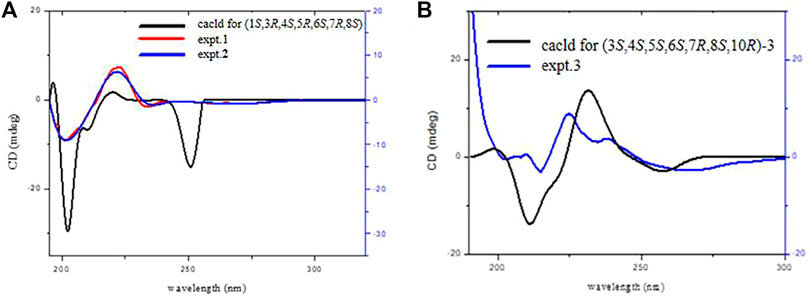
FIGURE 3. ECD spectra of compounds 1, 2 (A), and 3 (B) (data calculated using the TDDFT method at the B3LYP/6–31 + g(d,p) level.).
The exciton chirality method in CD spectra is a very useful method to determine the absolute configuration of organic molecules. It can be used to determine not only the spatial relationship between two identical chromophores but also their absolute configuration according to the interaction of two different conjugated systems (Ying et al., 1988; Luo et al., 2011). Due to the existences of two conjugate systems in the isolated compounds, the absolute configurations could be further studied by the exciton chirality method. The ECD spectrum of one showed negative exciton chirality around the UV maximum of 234 nm; the anticlockwise array of two coupling chromophores in space (Figure 4A) and absolute configurations of two bridgehead stereogenic centers (6S, 8S) were thus determined. This result was confirmed by the unambiguous match of its experimental and calculated ECD curves.
Compound 2 gave a molecular formula of C20H28O7 (HR-ESI-MS m/z 403.1730 [M + Na]+, calcd for 403.1733), which also suggested seven unsaturation degrees. Apart from the resonances attributed to 2-methylbutyryloxy (δC 175.4, 41.1, 26.3, 12.0, and 17.3) instead of 3-methylbutyryloxy group, 1H NMR, 13C NMR, and HMBC spectra were almost similar to those of 1, and the planar framework of two was also established by 1D NMR along with HMBC data (Figures 2, 5; Table 1).
The large vicinal coupling constants of H-5/6 (J5,6 = 11.5 Hz) indicated they were trans disposition (Wang et al., 2014). The strong NOE correlation between H-7/H-5 and H-9α, H-5/4-OH, and 1-OH revealed their cis α-orientation. In addition, the correlations of H3-15/H-3, H-6, and H-8/H-9β indicated the β-oriented of H3-15, H-3, and H-8 (Figure 5B). The experimental ECD curve of two resembled that of 1 and led to the assignment of the absolute configuration 1S, 3R, 4S, 5R, 6S, 7R, and 8S (Figure 3A). The negative exciton chirality around the UV maximum of 236 nm (Figure 4B) also illustrated the absolute configurations of 6S, 8S, and two was finally named argyin I.
Compound 3 gave a molecular formula of C20H26O7 with an HR-ESI-MS ion at m/z 401.1553 [M + Na]+ (calcd for 401.1576), which suggested eight unsaturation degrees. The 1H NMR signals at δ 5.98 (1H, d, J = 3.4 Hz) and 5.40 (1H, d, J = 3.4 Hz) indicated an exocyclic methylene group. The 1D NMR and HMBC data were similar to 3α,4α,10β-trihydroxy-8α-acetoxyguai-1,11 (13)-dien-6α,12-olide (Ahmed et al., 2004). The difference was the angeloyloxy substitution at C-8 instead of acetoxyl in the known compound (Figure 2; Table 1).
In the HMBC spectrum (Figure 6A), the cross-peak between H-5/C-1, C-2; H3-14/C-1 determined the location of 1,2-double bond. The HMBC correlation of H-8 (δH 5.13)/C-1′ (δC 167.2) confirmed the substituent group at C-8. J5,6 = 11.2 Hz and J6,7 = 9.6 Hz indicated that H-5, H-6, and H-7 were reciprocal trans oriented (Ahmed et al., 2004). The NOESY correlations (Figure 6B) between H-3/H-5 suggested that they were α-oriented. Additionally, H-6/H3-14 and H3-15, H3-14/H-8 correlations and the lack of NOE cross-peak between H-5/H3-15 indicated that H-6, H-8, H3-14, and H3-15 were on the same side. The negative exciton chirality of its ECD spectrum (Figure 4C) combined with the experimental and calculated ECD curves (Figure 3B) led to the assignment of the absolute configuration 3,4,5,6S,7R,8S,10R of 3, which was named as argyin J.
Compound 4 had a molecular formula of C20H26O7 (HR-ESI-MS m/z 401.1571 [M + Na]+, calcd for 401.1576), which suggested eight unsaturation degrees. 1H NMR signals at δ 6.04 and 5.48 (each 1H, d, J = 3.0 Hz) indicated the presence of an exocyclic methylene group. In 13C NMR, apart from five characteristic carbon signals (angeloyloxy group) at C8, other 15 carbon resonances were found to be similar to those of the previously published compound (Reinhardt et al., 2019), argyinolide N (5) and argyinolide M (6), indicating their similar structures (Figure 2; Table 1).
The HMBC cross-peak among H-6/C-4, C-5, and C-8; H3-15/C-3 and C-5; and H3-14/C-1, C-9, and C-10 confirmed the above planar structure deduction. H-8 (δH 5.31)/C-1′ (δC 167.0) revealed the presence of the angeloyloxy group at C-8 (Figure 7A).
The relative configuration of H-5 and H-6 was assigned to be α-, β-oriented based on their coupling constants of J5,6 = 9.6 Hz (Reinhardt et al., 2019). H-7 and OH-1 were α-oriented owing to the NOESY correlations of OH-1/H-5 and H-5/H-7, while H-6/H-8 correlation indicated the α-orientation for the 8-angeloyloxy group. Besides, H3-14/H-2β and H-3, H-3/H3-15 suggested H-3 and H3-15 were β-oriented (Figure 7B). In addition, the consistency of the experimental CD curve of 4 at 196 nm (+), 209 nm (−), 218 (+), and 232 nm (−) with those of argyinolide M and argyinolide N were reported previously (Reinhardt et al., 2019). Around the UV maximum of 232 nm, the ECD spectrum of 4 showed negative exciton chirality, the two carbon atoms (6, 8) were anticlockwise array, and the absolute configurations of these two carbon atoms (6S, 8S) were thus determined (Figure 7D). This result was confirmed by the match of its experimental and calculated ECD curves. Thus, 4 was established as (1S,3R,4S,5R,6S,7R,8S)-8-angeloyloxy-1,3,4-trihydroxy-guai-9 (10)-en-6,12-olide and named as argyin K.
In order to test the cytotoxic activities of compounds 1–6 isolated from A. argyi, CCK-8 assay was used to evaluate the inhibitory effects against HepG2, A549, and MCF-7 cell lines (Table 2). These six guaianolide sesquiterpenes showed strong inhibitory effect in a dose-dependent manner against three cell lines, and the most sensitive cell line is MCF-7, in which the IC50 of 1–6 (15.13–21.62 μM) was lower than that of the positive control, oxaliplatin (22.20 μM). It can be seen that the angeloyloxy substitution could strengthen the inhibitory effects compared with others.
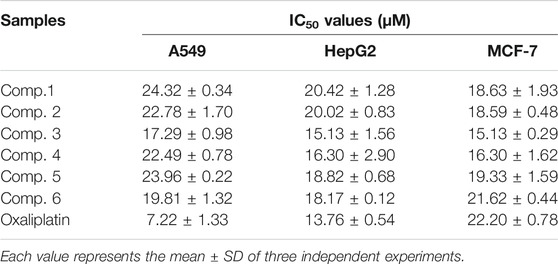
TABLE 2. Antiproliferative activities of compounds one to six against A549, HepG2, and MCF-7 cell lines.
In our continuing investigation on A. argyi, four undescribed guaiane-type sesquiterpenes were isolated from the Artemisia argyi Lévl. et Van. The antiproliferative activities of these compounds against A549, MCF-7, and HepG2 cell lines were tested in vitro using CCK-8 assays. Notably, these compounds could induce more cell death than positive control (oxaliplatin). Among them, compounds 3 and 4 with angeloyloxy substitution displayed the most potent antiproliferative effects, which could offer a promising lead structure with anticancer activity.
HPLC separation was performed on SHIMADZU LC-20AR pump and a SHIMADZU SPD-20A detector (Tokyo, Japan), using the COSMOSIL C18 preparative column (250 × 20 mm) and YMC-pack Prep-ODS column (250 × 20 mm). HR-ESI-MS spectra (Agilent 6200 series Q-TOF spectrometer, United States) were in the m/z mode. Column chromatography: silica gel (SiO2, 200–300 meshes), Sephadex LH-20 (Qingdao, China), and reversed-phase ODS (Kyoto, Japan). The 1D- and 2D-NMR were texted on a Bruker ARX-600 spectrometer (Bremen, Germany) in DMSO-d6 (Sigma-Aldrich Company).
Artemisia argyi (the name Artemisia argyi Levl. et Vant. was recorded in Chinese Pharmacopoeia for 2015) was collected in Qizhou, Hubei Province in May (spring) 2014. Prof. Jincai Lu identified this plant as Artemisia argyi Lévl et Vant. A voucher specimen (No. AY-1405) was deposited in the herbarium of Shenyang Pharmaceutical University.
A. argyi (10 kg) were extracted under the heat with 70% ethanol (3*25 L). The extraction of crude extract was as mentioned before (Sun et al., 2019). Briefly, the ethanol extracts were partitioned successively with petroleum ether (PE, 4.0 g), dichloromethane (CH2Cl2, 125.0 g), ethyl acetate (EtOAc, 60.0 g), and n-butyl alcohol (n-BuOH, 8.0 g).
The CH2Cl2 extract was chromatographed on silica gel, eluting with CH2Cl2-CH3OH to obtain Fr. A1–Fr. A6 (100:2 to 1:1). Combinations (Fr. A2 and Fr. A3) were then applied on reversed-phase ODS washing with aqueous MeOH (25–100%). The subfraction Fr. A2,3 was further separated by Sephadex LH-20 to obtain Fr. A3-1 to Fr. A3-5. Fr. A3-2 was isolated by HPLC (27% CH3CN/H2O) to afford compound 1 (4.2 mg) and 2 (2.2 mg). Fr.C3-5 was further fractionated by HPLC (32% CH3CN/H2O) to give compound 3 (2.6 mg). Fr. A4 was isolated by HPLC with MeOH/H2O (58: 42) as an eluent to afford 4 (1.8 mg), 5 (3.2 mg), and 6 (5.0 mg).
Compound 1: white powder; UV (MeOH) λ max (log ε) 206 (0.883) nm, ECD (MeOH) λ max (Δε): 201 (Δε −9.07), 223 (Δε +7.31), 234 (Δε −1.54); 1H NMR and 13C NMR (DMSO-d6, 600/150 MHz) data, see Table 1; HR-ES-IMS m/z 403.1728 [M + Na]+, calcd for 403.1733.
Compound 2: white powder; UV (MeOH) λ max (log ε) 211 (2.695) nm, ECD (MeOH) λ max (Δε): 202 (Δε −9.07), 222 (Δε +6.29), 236 (Δε −1.20); 1H NMR and 13C NMR (DMSO-d6, 600/150 MHz) data, see Table 1; HR-ES-IMS m/z 403.1730 [M + Na]+, calcd for 403.1733.
Compound 3: white powder; UV (MeOH) λ max (log ε) 208 (1.879) nm, ECD (MeOH) λ max (Δε): 215 (Δε −3.00), 225 (Δε +8.86), 266 (Δε −2.71); 1H NMR and 13C NMR (DMSO-d6, 600/150 MHz) data, see Table 1; HR-ES-IMS m/z 401.1553 [M + Na]+, calcd for 401.1576.
Compound 4: white powder; UV (MeOH) λ max (log ε) 211 (2.647) nm, ECD (MeOH) λ max (Δε): 209 (Δε −13.74), 232 (Δε −15.78); 261 (Δε +1.87); 1H NMR and 13C NMR (DMSO-d6, 600/150 MHz) data, see Table 1; HR-ES-IMS m/z 401.1571 [M + Na]+, calcd for 401.1576.
The cell proliferation assays were measured using the CCK-8 method (Du et al., 2020). HepG2 (liver hepatocellular cells), MCF-7 (breast cancer cells), and A549 (lung adenocarcinoma cells) were cultured at 37°C with 5% CO2. HepG2 and A549 cell lines were cultured in RPMI-1640 medium (10% fetal bovine); MCF-7 were cultured in DMEM medium (10% fetal bovine). The tumor cells (106 cells/ml, 96-well plate) were treated in various concentrations (6.25, 12.5, 25, 50, and 100 μM) for 24 h and measured in a microplate reader (540 nm) after adding 10 μl CCK-8 (Dalian Meilun Biotechnology Co., Ltd.). Besides, oxaliplatin was used as a positive control reagent for HepG2, MCF-7, and A549 cells.
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
WM, YZ, and YS contributed to conception and design of the study. GB and JS organized the database. YZ and ZS performed the statistical analysis. WM and YS wrote the first draft of the manuscript. WM, DM, YZ, and GB wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (Grant No. 81573694), and the National Drug Standard Improvement Project in 2021 (General Technology) (Grant No. 2021Z01). The project was also sponsored by “Liaoning BaiQianWan Talents Program in 2018.”
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2021.698700/full#supplementary-material
Ahmed, A. A., El-Moghazy, S. A., El-Shanawany, M. A., Abdel-Ghani, H. F., Karchesy, J., Sturtz, G., et al. (2004). Polyol Monoterpenes and Sesquiterpene Lactones from the Pacific Northwest PlantArtemisiasuksdorfii. J. Nat. Prod. 67, 1705–1710. doi:10.1021/np049954j
Doh, E. J., Paek, S. H., Lee, G., Lee, M. Y., and Oh, S. E., (2016). Application of Partial Internal Transcribed Spacer Sequences for the Discrimination of Artemisia Capillaris from Other Artemisia Species. Evid. Based Complement. Alternat Med. 2016, 7043436–7043602. doi:10.1155/2016/7043436
Du, K., Yang, X., Li, J., and Meng, D., (2020). Antiproliferative Diterpenoids and Acetophenone Glycoside from the Roots of euphorbia Fischeriana. Phytochemistry. 177, 112437. doi:10.1016/j.phytochem.2020.112437
Lee, D., Kim, C. E., Park, S. Y., Kim, K. O., Hiep, N. T., Lee, D., et al. (2018). Protective Effect of Artemisia Argyi and its Flavonoid Constituents against Contrast-Induced Cytotoxicity by Iodixanol in LLC-PK1 Cells. Int. J. Mol. Sci. 19, 1387–1405. doi:10.3390/ijms19051387
Li, S., Zhou, S., Yang, W., and Meng, D., (2018). Gastro-protective Effect of Edible Plant Artemisia Argyi in Ethanol-Induced Rats via Normalizing Inflammatory Responses and Oxidative Stress. J. Ethnopharmacology 214, 207–217. doi:10.1016/j.jep.2017.12.023
Luo, J., Wang, J.-S., Wang, X.-B., Luo, J.-G., and Kong, L.-Y. (2011). Phragmalin-Type Limonoid Orthoesters from Chukrasia Tabularis Var. Velutina. Chem. Pharm. Bull. 59, 225–230. doi:10.1248/cpb.59.225
Merfort, I., (2011). Perspectives on Sesquiterpene Lactones in Inflammation and Cancer. Cdt 12, 1560–1573. doi:10.2174/138945011798109437
Ozek, G., Suleimen, Y., Tabanca, N., Doudkin, R., Gorovoy, P. G., and Goger, F., (2014). Chemical Diversity and Biological Activity of the Volatiles of Five Artemisia Species from Far East Russia. Rec. Nat. Prod. 3, 242–261.
Reinhardt, J. K., Klemd, A. M., Danton, O., De Mieri, M., Smieško, M., Huber, R., et al. (2019). Sesquiterpene Lactones from Artemisia Argyi: Absolute Configuration and Immunosuppressant Activity. J. Nat. Prod. 82, 1424–1433. doi:10.1021/acs.jnatprod.8b00791
Sun, Y. W., Ju, Y., Liu, C. H., Du, K. C., and Meng, D. L., (2019). Polyhydroxyl Guaianolide Terpenoids as Potential NF‐kB Inhibitors Induced Cytotoxicity in Human Gastric Adenocarcinoma Cell Line. Bioorg. Chem. 95, 103551. doi:10.1016/j.bioorg.2019.103551
Tan, R., and Jia, Z., (1992). Eudesmanolides and Other Constituents from Artemisia Argyi. Planta Med. 58, 370–372. doi:10.1055/s-2006-961488
Wang, S., Li, J., Sun, J., Zeng, K.-w., Cui, J.-r., Jiang, Y., et al. (2013). NO Inhibitory Guaianolide-Derived Terpenoids from Artemisia Argyi. Fitoterapia. 85, 169–175. doi:10.1016/j.fitote.2012.12.005
Wang, S., Sun, J., Zeng, K., Chen, X., Zhou, W., Zhang, C., et al. (2014). Sesquiterpenes from Artemisia Argyi: Absolute Configurations and Biological Activities. Eur. J. Org. Chem. 2014, 973–983. doi:10.1002/ejoc.201301445
Ying, B. P., Xu, R. S., Mi, J. F., and Han, J., (1988). The Absolute Configuration of Pseudolaric Acid B. J. Chem. 1, 87–88.
Zan, K., Chai, X.-Y., Chen, X.-Q., Wu, Q., Fu, Q., Zhou, S.-X., et al. (2012). Artanomadimers A-F: Six New Dimeric Guaianolides from Artemisia Anomala. Tetrahedron. 68, 5060–5065. doi:10.1016/j.tet.2012.04.046
Keywords: Artemisia argyi, guaiane-type sesquiterpenoids, antiproliferative, configuration determination, antitumor
Citation: Ming W, Zhang Y, Sun Y, Bi G, Su J, Shao Z and Meng D (2021) Guaianolide Sesquiterpenes With Significant Antiproliferative Activities From the Leaves of Artemisia argyi. Front. Chem. 9:698700. doi: 10.3389/fchem.2021.698700
Received: 22 April 2021; Accepted: 28 May 2021;
Published: 24 June 2021.
Edited by:
Liqin Ding, Tianjin University of Traditional Chinese Medicine, ChinaReviewed by:
Wen-Yu Zhao, Dalian Medical University, ChinaCopyright © 2021 Ming, Zhang, Sun, Bi, Su, Shao and Meng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dali Meng, bWVuZ2RsQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.