- Department of Pharmacognosy, Kyoto Pharmaceutical University, Kyoto, Japan
Five new cyclic organosulfur compounds, foliogarlic disulfanes A1 (1), A2 (2), and A3 (3) and foliogarlic trisulfane A1 (4) and A2 (5), were isolated from the leaves of Allium sativum (garlic). The chemical structures of these compounds were elucidated on the basis of physicochemical evidence including Nuclear Magnetic Resonance (NMR) and Mass Spectrometry (MS). Compounds 1–5 were obtained as complex compounds with disulfane or trisulfane and tetrahydro-2H-difuro[3,2-b:2′,3′-c]furan-5(5aH)-one. In addition, the hypothetical biosynthetic pathways of these compounds were suggested.
Introduction
Allium plants (Allieae), such as garlic, onion, and chives, have been cultivated as not only foodstuffs but also medicinal plants in the worldwide from ancient. For example, the extract of Allium plants, such as garlic, has shown anticancer, antidiabetic, and antibacterial effects. In addition, the National Cancer Institute in the United States had focused on Allium species as expecting cancer prevention (Theisen, 2001). Allium plants are well-known to have various cysteine sulfoxide derivatives such as alliin, methiin, and propiin (Rose et al., 2005). The type and contents of cysteine sulfoxides were also known to be different among Allium species (Fritsch and Keusgen, 2006). The cysteine sulfoxides change to thiosulfinates, such as allicin (Cavallito and Bailey, 1944; Cavallito et al., 1944), by the reaction with enzyme called alliinase (Ellmore and Feldberg, 1994) when the tissues of Allium plants are broken. Allicin has been reported to have several biological effects (Gebhardt et al., 1994; Briggs et al., 2000; Cañizares et al., 2004; Oommen et al., 2004; Arditti et al., 2005). However, unstable thiosulfinates including allicin are changed to organosulfur compounds, such as ajoene (Block et al., 1984), methyl 1-(methylthio)ethyl disulfane, and 5,7-diethyl-1,2,3,4,6-pentathiepane (Kuo et al., 1990). These compounds are also comparatively unstable and volatile. Although ajoene was known to have significantly anticancer effect, the application as medicines is difficult. On the other hand, several cyclic organosulfur compounds with anticancer effects isolated from the bulbs of the Allium sativum (garlic) have been reported by Nohara et al. (2012, 2013, 2014). Thus, cyclic organosulfur compounds are important on the development of medicines including anticancer effect. On the basis of this background, we have isolated several comparatively stable organosulfur compounds from Allium fistulosum (green onion and welsh onion) (Fukaya et al., 2018, 2019a) and Allium schoenoprasum var. foliosum (Japanese chive) (Fukaya et al., 2019b). In the course of our ongoing research program for discovery of bioactive organosulfur compounds, the constituents from the leaves of Allium sativum were examined. In this article, we discuss the isolation and the structure elucidation of cyclic organosulfur compounds, foliogarlic disulfanes A1 (1), A2 (2), and A3 (3) and foliogarlic trisulfane A1(4) and A2 (5), from the leaves of A. sativum and the biosynthetic pathways.
Results and Discussions
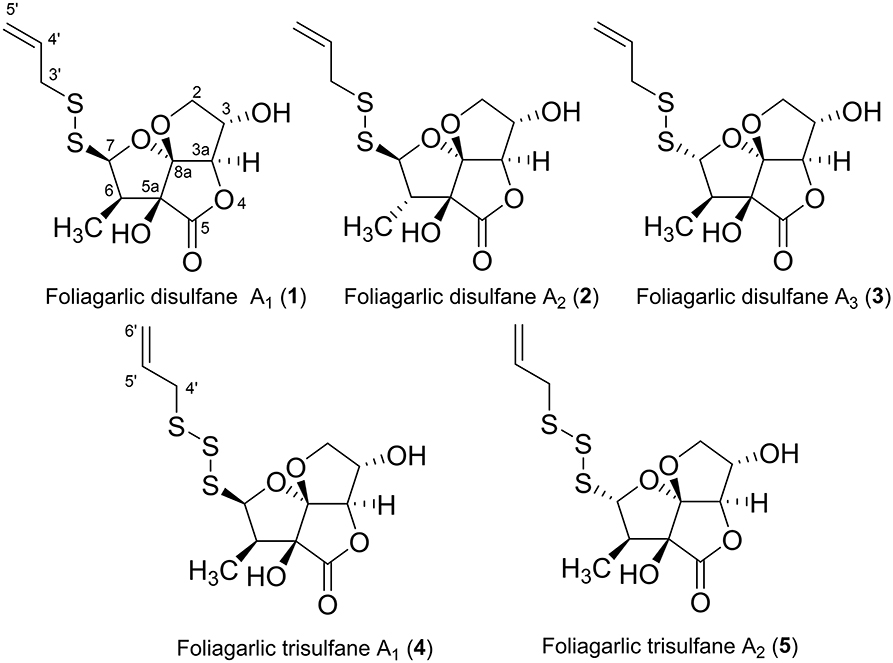
The fresh leaves of A. sativum (15.0 kg) were mixed with water. Then, acetone was added into the mixture to be 80% acetone solution. The solution was concentrated after standing for 4 days (96 h) at room temperature. The acetone extract was portioned between ethyl acetate (EtOAc) and water. The organic fraction was evaporated in vacuo and obtained EtOAc fraction as syrup (41.98 g, 0.27% from the plant). The EtOAc fraction was also subjected with the normal and reversed-phase column chromatography and high-performance liquid chromatography (HPLC) to give foliogarlic disulfanes A1 (1, 0.00013%), A2 (2, 0.00021%), and A3 (3, 0.00009%) and foliogarlic trisulfanes A1 (4, 0.00015%) and A2 (5, 0.00008%) (Figure 1).
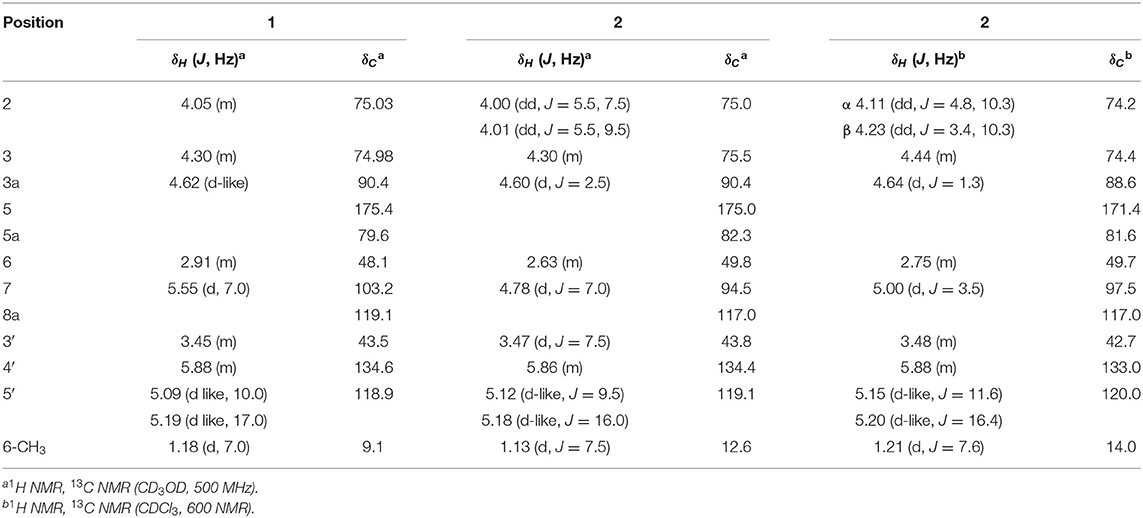
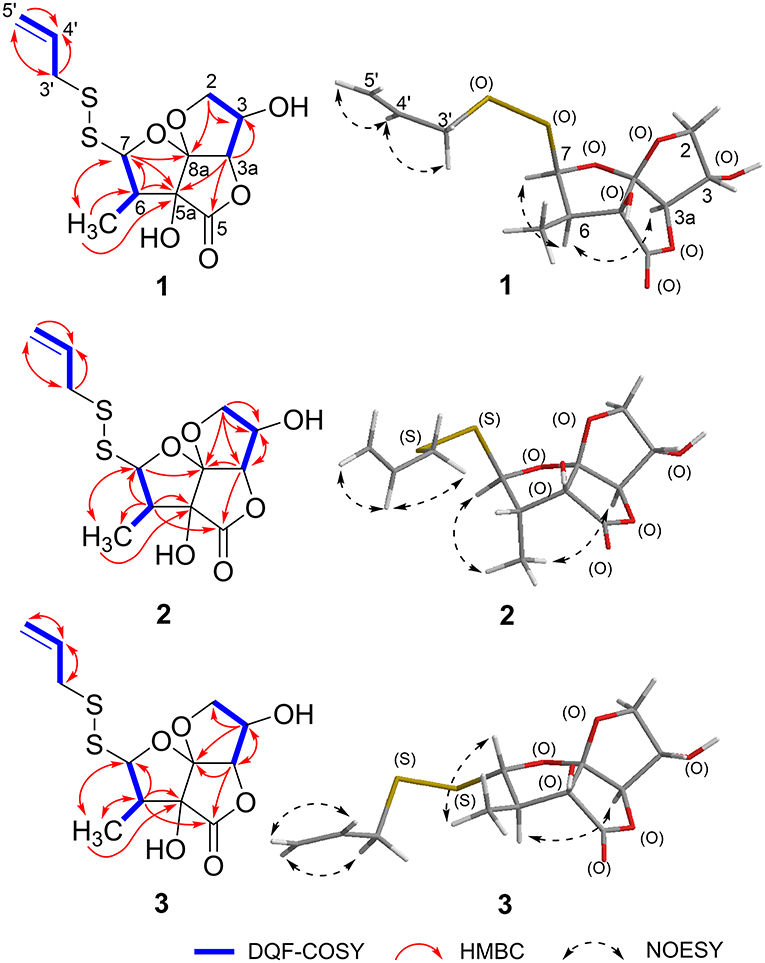
Foliogarlic disulfanes A1 (1) was obtained as yellow oil and showed positive optical rotation (+160.9). In the Electrospray Ionization MS (ESIMS) measurement of 1, a pseudomolecular ion peak [M + Na]+ was observed at m/z 343.0282, and the molecular formula was determined as C12H16O6S2 on the basis of the High Resolution ESIMS (HRESIMS) peak and the 13C NMR data. The 13C NMR spectra of 1 showed signals corresponding to a secondary methyl group at δC 9.1 (6-CH3), a methine at δC 48.1 (C-6), a diastereotopic oxygen-bearing methylene at δC 74.98 (C-3); an oxygen-bearing methine at δC 75.03 (C-2); two methines neighboring the electron-withdrawing atom at δC 90.4 (C-3a) and δC 103.2 (C-7); an oxygen-bearing quaternary carbon at δC 79.6 (C-5a); a two-oxygen–bearing quaternary carbon at δC 119.1 (C-8a); and a lactone carbonyl carbon at δC 175.4 (C-5) (Table 1, Figure 2, and Supplementary Material). The correlations of COSY double-quantum filter (DQF COSY) NMR spectroscopy were observed between 6-CH3, H-6, and H-7 and between H-2, H-3, and H-3a (Figure 2). The heteronuclear multiple-bond correlation (HMBC) spectrum of 1 is shown in Figure 2. Namely, the correlation of H-2 to C-8a, H-7 to C-5a and H-3a to C-5a indicates acetal structure, the correlation of H-3a to C-5 and C-5a indicates a lactone, and the correlations of H-6 to C-5a and C-7, H-7 to C-5 and 6-CH3, 6-CH3 to C-5a, C-6, and C-7 indicate a secondary methyl moiety. These evidences indicate that compound 1 had a tetrahydro-2H-difuro[3,2-b:2′,3′-c]furan-5(5aH)-one skeleton. In addition, 1-propenyl disulfane structure at the side chain was confirmed by High Resolution MS (HRMS) and NMR (Nuclear Magnetic Resonance) data. Next, the NOESY spectrum of 1 showed key correlations between H-3a and H-6; and H-6 and H-7 (Figure 2). The results prove that the relative configurations among H-3, H-6, and H-7 were of the same orientation, respectively. Furthermore, the 1H and 13C NMR signals of 1 assigned to tetrahydro-2H-difuro[3,2-b:2′,3′-c]furan-5(5aH)-one skeleton were superimposable on those of known compound, kujounin A3, except for 1-propenyl disulfane moiety (Fukaya et al., 2019a). All the evidences support that the chemical structure of 1 was (3S*,3aR*,5aS*,6R*,7R*,8aR*)-3,5a-dihydroxy-6-methyl-7-(allyldisulfanyl)tetrahydro-2H-difuro[3,2-b:2′,3′-c]furan-5(5aH)-one.
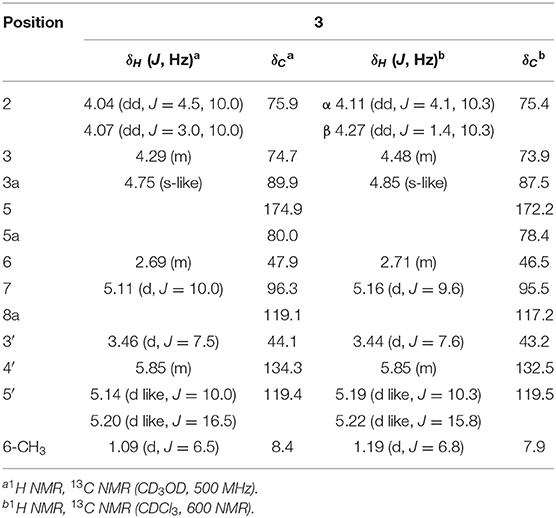
Foliogarlic disulfanes A2 (2) and A3 (3) were isolated as yellow oil with positive specific rotations (2: + 139.0° in MeOH) and negative specific rotations (3: – 213.6° in MeOH). In the ESIMS spectra of 2 and 3, the same quasi-molecular ion peaks (2 and 3: [M+Na]+) were observed at m/z 343. The molecular formulas (2 and 3: C12H16O6S2) were determined on the basis of HRESIMS peaks at [2: m/z 343.0277, 3: m/z 343.0282 (calcd. 343.0281)] and the 13C NMR data. The 1H and 13C NMR spectrum of 2 and 3 showed signals corresponding to a secondary methyl group, a methine, a diastereotopic oxygen-bearing methylene, and an oxygen-bearing methine (Tables 1, 2 and Figure 2). On the basis of this evidence and detailed examination of DQF COSY and HMBC experiments, the planner structures of 2 and 3 were found to be the same as that of 1. Next, the relative configurations of 2 and 3 were characterized by the detailed NOESY experiments. The NOESY spectrum of 2 showed key correlations between H-3a and 6-CH3; and H-7 and 6-CH3 (Figure 2). The NOESY spectrum of 3 showed key correlations between H-3a and H-6; and H-7 and 6-CH3 (Figure 2). In addition, the 1H and 13C NMR signals of 2 and 3 were superimposable on those of known compounds, kujounin A1 and A2, respectively, except for 1-propenyl disulfane structure (Fukaya et al., 2018). Consequently, the chemical structures of foliogarlic disulfanes A2 (2) and A3 (3) were determined as (3S*,3aR*,5aS*,6S*,7R*,8aR*)-3,5a-dihydroxy-6-methyl-7-(allyldisulfanyl)tetrahydro-2H-difuro[3,2-b:2′,3′-c]furan-5(5aH)-one and (3S*,3aR*,5aS*,6R*,7S*,8aR*)-3,5a-dihydroxy-6-methyl-7-(allyldisulfanyl)tetrahydro-2H-difuro[3,2-b:2′,3′-c]furan-5(5aH)-one, respectively.
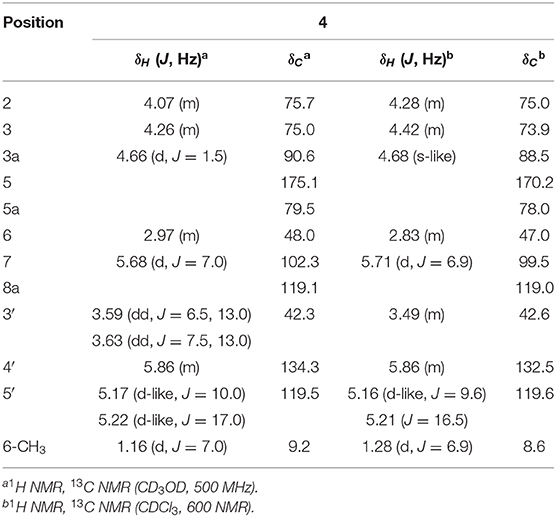
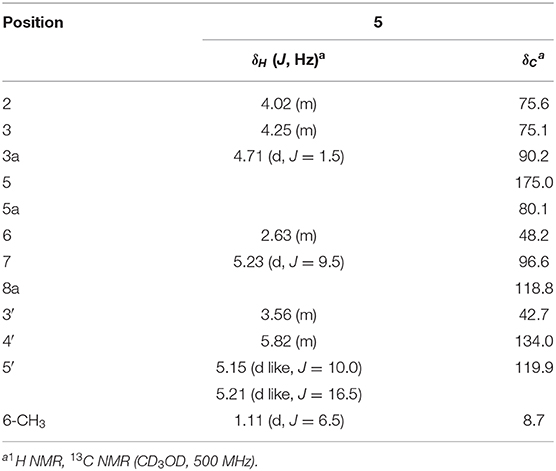
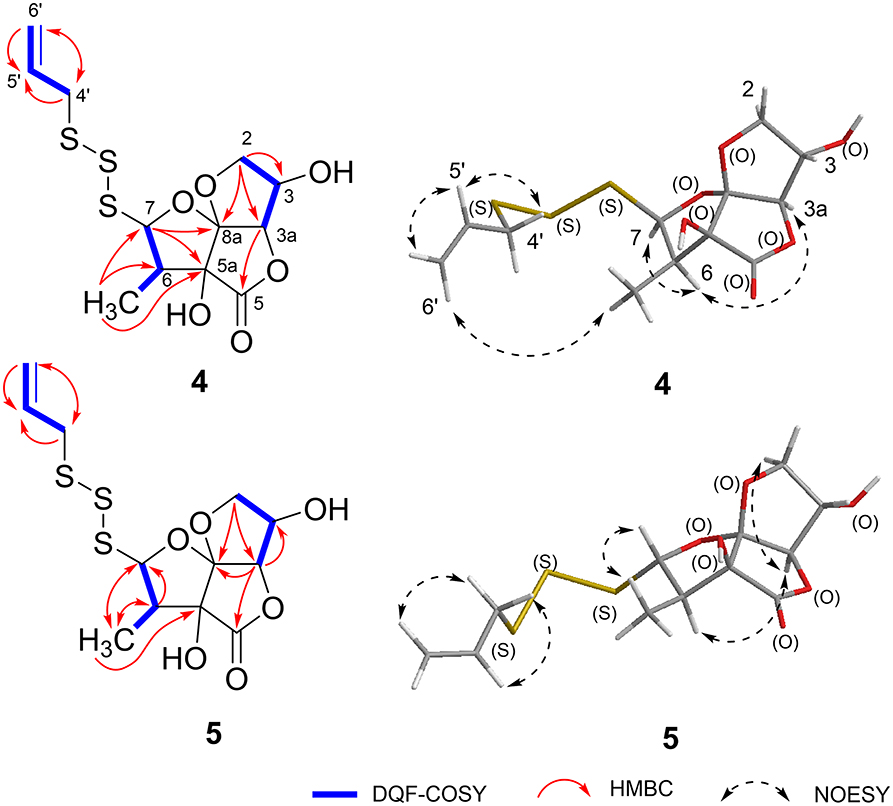
Foliogarlic trisulfanes A1 (4) and A2 (5) were isolated as yellow oil with positive specific rotations (4: +124.6° in MeOH) and negative specific rotations (5: −119.8° in MeOH). In the ESIMS spectra of 4 and 5, the same quasi-molecular ion peaks (4 and 5: [M+Na]+) were observed at m/z 375. The molecular formulas (4 and 5: C12H16O6S3) were determined on the basis of HRESIMS peaks at [4: m/z 374.9998, 3: m/z 374.0003 (calcd. 374.0001)], and the 13C NMR data. On the basis of the detailed analysis of the 1H and 13C NMR, 2D-NMR (DQF COSY, HMBC, NOESY) spectrum of 4 and 5, the relative structures of tetrahydro-2H-difuro[3,2-b:2′,3′-c]furan-5(5aH)-one skeleton on 4 and 5 were found to be the same as those of 1 and 3, respectively (Tables 3, 4 and Figure 3). Next, the 1H and 13C NMR spectrum at the side chain showed signals corresponding to an allyl group, as well as those of compounds 1–3. The determination of the sulfur linkage was confirmed by the HRMS spectrum. Namely, the pseudomolecular formula was established as C12H16O6S3Na. Therefore, compounds 4 and 5 were found to have a trisulfane bridge. Finally, the relative configurations of 4 and 5 were characterized by the comparison of 13C NMR data with 1 and 3 and the NOESY experiments. The 13C NMR signals of 4 and 5 were superimposable on those of 1 and 3. All the evidences supported that the chemical structures of 4 and 5 were (3S*,3aR*,5aS*,6R*,7R*,8aR*)-3,5a-dihydroxy-6-methyl-7-(allyltrisulfanyl)tetrahydro-2H-difuro[3,2-b:2′,3′-c]furan-5(5aH)-one and (3S*,3aR*,5aS*,6R*,7S*,8aR*)-3,5a-dihydroxy-6-methyl-7-(allyltrisulfanyl)tetrahydro-2H-difuro[3,2-b:2′,3′-c]furan-5(5aH)-one, respectively.
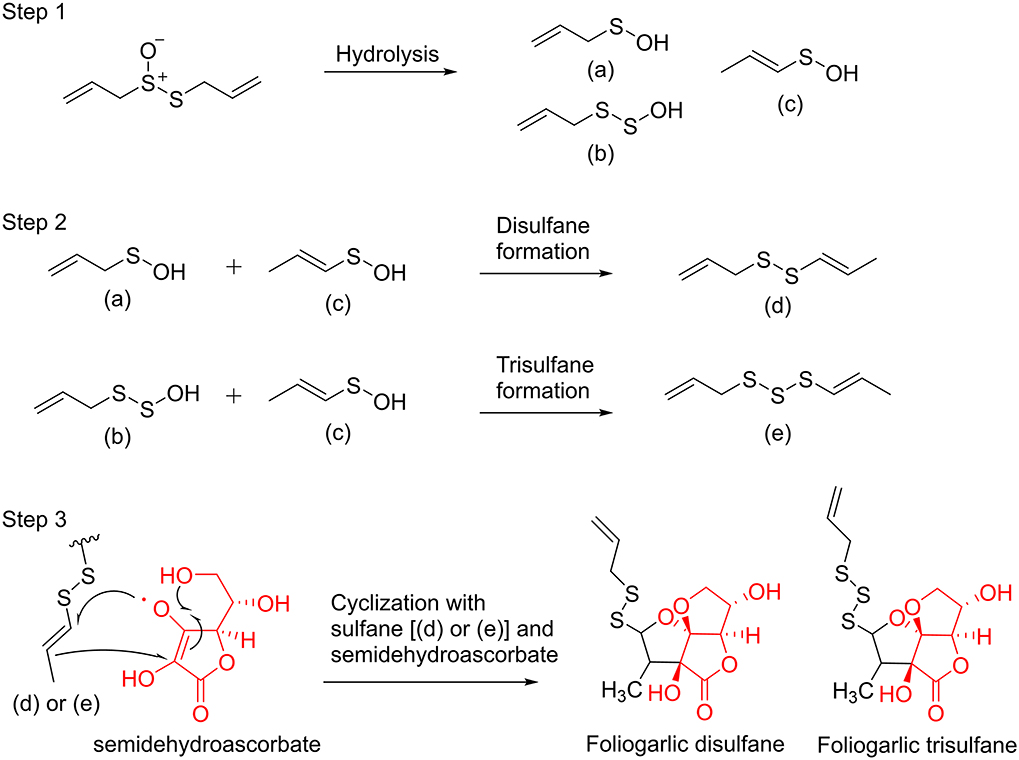
The biological synthetic pathways for compounds 1–5 are presumed. At first, allicin is generated from alliin by alliinase when plant tissues of A. sativum are broken. Next, allicin is decomposed into intermediates (a), (b), and (c) by hydrolysis and is reconstructed to disulfane (d) and trisulfane (e) (Jacob, 2006). Finally, the structure of tetrahydro-2H-difuro[3,2-b:2′,3′-c]furan-5(5aH)-one skeleton is formed from semidehydroascorbate by cyclization and sulfane formation with the intermediates d and e. Consequently, compounds 1–5 were presumed to be obtained (Figure 4).
Conclusion
Five new organosulfur compounds, foliogarlic disulfanes 1–3 and foliogarlic trisulfanes 4 and 5, were isolated from the leaves of A. sativum. These compounds 1–5 have a tetrahydro-2H-difuro[3,2-b:2′,3′-c]furan-5(5aH)-one skeleton with methyl group at 6-position and 2-propenyl disulfane or 2-propenyl trisulfane group at 7-position. Particularly, foliogarlic trisulfanes 4 and 5 with a trisulfane moiety are a rare compound derived from medicinal plants. The biological effects of these cyclic organosulfur compounds should be studied further.
Experimental
General
The following instruments were used to obtain physical data: specific rotations, a Horiba (Kyoto, Japan) SEPA-300 digital polarimeter (l = 5 cm); IR spectra, JASCO (Tokyo, Japan) FT/IR-4600 Fourier Transform Infrared Spectrometer; ESIMS, Agilent Technologies (CA, US) Quadrupole LC/MS 6130; HRESIMS, SHIMADZU LCMS-IT-TOF; 1H NMR spectra, JEOL (Tokyo, Japan) JNM-LA 500 (500 MHz) spectrometer; 13C-NMR spectra, JEOL JNM-LA 500 (125 MHz) spectrometer; NOESY spectra, JNM-ECA 600 (600 MHz) spectrometer; HPLC, a Shimadzu (Kyoto, Japan) SPD-20AVP UV-VIS detector. YMC-triart C18 (250 × 4.6 mm i.d. and 250 × 10 mm i.d.) and YMC-triart PFP (250 × 4.6 mm i.d. and 250 × 10 mm i.d.) columns were used for analytical and preparative purposes. The following experimental materials were used for chromatography: normal-phase silica gel column chromatography, silica gel BW-200 (Fuji Silysia Chemical, Ltd. (Aichi, Japan, 150–350 mesh); reversed-phase silica gel column chromatography, Cosmosil 140C18-OPN [Nacalai Tesque (Kyoto, Japan)], TLC, precoated TLC plates with silica gel 60F254 [Merck (NJ, US), 0.25 mm] (ordinary phase), and silica gel RP-18 F254S (Merck, 0.25 mm) (reversed phase); reversed-phase HPTLC, precoated TLC plates with silica gel RP-18 WF254S. Detection was achieved by spraying with 1% Ce (SO4) 2–10% aqueous H2SO4 followed by heating.
Plant Material
Fresh leaves of A. sativum cultivated in Kochi prefecture, Japan, were obtained as commercial products purchased from Japan Agricultural Cooperatives (JA) farmers' market (Kochi, Japan) in April 2017. The plants were identified by the authors (H.M. and S.N.).
Extraction and Isolation
The fresh leaves of A. sativum (15.2 kg) were chopped and mixed with water, and then acetone was added to the mixture to be 80% acetone solution. The mixture was soaked for 4 days (96 h) at room temperature. Evaporation of the filtrate under reduced pressure provided acetone extract (1,500.37 g, 9.87%). The extract was partitioned between EtOAc and H2O (1:1, vol/vol) to obtain EtOAc fraction (41.98 g, 0.27%) and aqueous phase. The EtOAc-soluble fraction (41.98 g) was subjected to normal phase silica gel column chromatography [1,260 g, CHCl3-MeOH (1:0 → 100:1 → 50:1 → 30:1 → 10:1 → 0:1, vol/vol)] to give nine fractions {Fr.1 (1,471.6 mg), Fr.2 (715.5 mg), Fr.3 (7,193.2 mg), Fr.4 (8,339.2 mg), Fr.5 (4,085.8 mg), Fr.6 (1,334.9 mg), Fr.7 (4,367.0 mg), Fr.8 (617.7 mg), Fr.9 (5,841.2 mg)}. r. 5 (4,085.8 mg) was further separated by reversed-phase silica gel column chromatography [200 g, MeOH-H2O (2:8 → 4:6 → 6:4 → 8:2 → 1:0, vol/vol)] to give 13 fractions {Fr.5-1 (52.3 mg), Fr.5-2 (21.0 mg), Fr.5-3 (31.0 mg), Fr.5-4 (19.1 mg), Fr.5-5 (84.4 mg), Fr.5-6 (281.7 mg), Fr.5-7 (43.5 mg), Fr.5-8 (26.3 mg), Fr.5-9 (67.9 mg), Fr.5-10 (482.9 mg), Fr.5-11 (2,637.7 mg), Fr.5-12 (178.7 mg), Fr.5-13 (16.9 mg)}. Fr.5-5 (84.4 mg) was purified by HPLC {mobile phase: MeOH-H2O (35:65, vol/vol) [YMC-triart PFP (250 × 10 mm i.d.)]} to give 1 (6.0 mg) and 2 (16.3 mg). Fr.5-6 (281.7 mg) was purified by HPLC {mobile phase: MeOH-H2O (50:50, vol/vol) [YMC-triart C18 (250 × 10 mm i.d.)]} to give 1 (14.6 mg), 2 (16.0 mg), 4 (24.2 mg), and 5 (12.3 mg). Fr.5-6-5 (37.1 mg) was purified by HPLC {mobile phase: MeOH-H2O (45:55, vol/vol) [YMC-triart C18 (250 × 10 mm i.d.)]} to give 3 (14.7 mg) and 4 (4.5 mg).
Foliogarlic Disulfane A1 (1)
Yellow oil; +160.9 (MeOH); HRESIMS: calcd for C12H16O6S2Na (M+Na)+: 343.0281, found: 343.0282; IR(ATR): 3,400, 2,975, 1,782 cm−1; 1H NMR (CD3OD), 13C NMR (CD3OD, 500 MHz): given in Table 1.
Foliogarlic Disulfane A2 (2)
Yellow oil; +139.0 (MeOH); HRESIMS: calcd for C12H16O6S2Na (M+Na)+: 343.0281, found: 343.0277; IR(ATR): 3,400, 2,970, 1,785 cm−1; 1H NMR (CD3OD, CDCl3), 13C NMR (CD3OD, CDCl3): given in Table 1.
Foliogarlic Disulfane A3 (3)
Yellow oil; 213.6 (MeOH); HRESIMS: calcd for C12H16O6S2Na (M+Na)+: 343.0281, found: 343.0282; IR(ATR): 3,400, 2,981, 1,785 cm−1; 1H NMR (CD3OD, CDCl3), 13C NMR (CD3OD, CDCl3): given in Table 2.
Foliogarlic Trisulfane A1 (4)
Yellow oil; +124.6 (MeOH); HRESIMS: calcd for C12H16O6S3Na (M+Na)+: 375.0001, found: 374.9998; IR(ATR): 3,400, 2,975, 1,780 cm−1; 1H NMR (CD3OD, CDCl3), 13C NMR (CD3OD, CDCl3): given in Table 3.
Foliogarlic Trisulfane A2 (5)
Yellow oil; 119.8 (MeOH); HRESIMS: calcd for C12H16O6S3Na (M+Na)+: 375.0001, found: 375.0003; IR(ATR): 3,400, 2,931, 1,789 cm−1; 1H NMR (CD3OD), 13C NMR (CD3OD): given in Table 4.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Author Contributions
MF: isolation of constituents and Structure elucidation of new compounds. SeiN: Structure elucidation of new compounds and overall supervision in this study. HH and DN: isolation of constituents. SouN: Preparation of plant extracts and overall supervision in this study. TY: Structure elucidation of new compounds. HM: overall supervision in this study.
Funding
This research was supported in part by a Ministry of Education, Culture, Sports, Science and Technology (MEXT)-Supported Program for the Strategic Research Foundation at Private Universities 2015–2019. This work was supported by JSPS KAKENHI Grant Number 17K08354 (SeiN).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2020.00282/full#supplementary-material
References
Arditti, F. D., Rabinkov, A., Miron, T., Reisner, Y., Berrebi, A., Wilcheck, M., et al. (2005). Apoptotic killing of B-chronic lymphocytic leukemia tumor cells by allicin generated in situ using a rituximab-alliinase conjugate. Mol. Cancer Ther. 4, 325–331.
Block, E., Ahmad, S., Jain, M. K., Crecely, R. W., Apitz-Castro, R., and Cruz, M. R. (1984). The chemistry of alkyl thiosulfate esters. 8. (E,Z)-Ajoene: a potent antithrombotic agent from garlic. J. Am. Chem. Soc. 106, 8295–8296. doi: 10.1021/ja00338a049
Briggs, W. H., Xiao, H., Parkin, K. L., Shen, C., and Goldman, I. L. (2000). Differential inhibition of human platelet aggregation by selected allium thiosulfinates. J. Agric. Food Chem. 48, 5731–5735. doi: 10.1021/jf0004412
Cañizares, P., Gracia, I., Gómez, L. A., Argila, C. M., Boixeda, D., García, A., et al. (2004). Allyl-thiosulfinates, the bacteriostatic compounds of garlic against Helicobacter pylori. Biotechnol. Prog. 20, 397–401. doi: 10.1021/bp034143b
Cavallito, C. J., and Bailey, J. H. (1944). Allicin, the antibacterial principle of Allium sativum. I. isolation, physical properties and antibacterial action. J. Am. Chem. Soc. 66, 1950–1951. doi: 10.1021/ja01239a048
Cavallito, C. J., Buck, J. S., and Suter, C. M. (1944). Allicin, the antibacterial principle of Allium sativum. II. determination of the chemical structure. J. Am. Chem. Soc. 66, 1952–1954. doi: 10.1021/ja01239a049
Ellmore, G. S., and Feldberg, R. S. (1994). Alliin lyase localization in bundle sheaths of the garlic clove (Allium sativum). Am. J. Bot. 81, 89–94. doi: 10.1002/j.1537-2197.1994.tb15413.x
Fritsch, M. R., and Keusgen, M. (2006). Occurrence and taxonomic significance of cysteine sulphoxides in the genus Allium L. (alliaceae). Phytochemistry 67, 1127–1135. doi: 10.1016/j.phytochem.2006.03.006
Fukaya, M., Nakamura, S., Kyoku, Y., Nakashima, S., Yoneda, T., and Matsuda, H. (2019b). Cyclic sulfur metabolites from Allium schoenoprasum var. foliosum. Phytochem. Lett. 29, 125–128. doi: 10.1016/j.phytol.2018.11.018
Fukaya, M., Nakamura, S., Nakagawa, R., Kinka, M., Nakashima, S., and Matsuda, H. (2019a). Cyclic sulfur-containing compounds from Allium fistulosum ‘Kujou’. J. Nat. Med. 73, 397–403. doi: 10.1007/s11418-018-1272-0
Fukaya, M., Nakamura, S., Nakagawa, R., Nakashima, S., Yamashita, M., and Matsuda, H. (2018). Rare sulfur-containing compounds, kujounins A1 and A2 and allium sulfoxide A1, from Allium fistulosum 'Kujou'. Org. Lett. 20, 28–31. doi: 10.1021/acs.orglett.7b03234
Gebhardt, R., Beck, H., and Wagner, K. G. (1994). Inhibition of cholesterol biosynthesis by allicin and ajoene in rat hepatocytes and HepG2 cells. Biochim. Biophys. Acta Lipids Lipid. Metab. 1213, 57–62. doi: 10.1016/0005-2760(94)90222-4
Jacob, C. (2006). A scent of therapy: pharmacological implications of natural products containing redox-active sulfur atoms. Nat. Prod. Rep. 23, 851–863. doi: 10.1039/b609523m
Kuo, M. C., Chien, M., and Ho, C. T. (1990). Novel polysulfides identified in the volatile components from welsh onions (Allium fistulosum L. var. maichuon) and scallions (Allium fistulosum L. var. caespitosum). J. Agric. Food Chem. 38, 1378–1381. doi: 10.1021/jf00096a017
Nohara, T., Fujiwara, Y., Ikeda, T., Murakami, K., Ono, M., Nakano, D., et al. (2013). Cyclic sulfoxides garlicnins B2, B3, B4, C2, and C3 from Allium sativum. Chem. Pharm. Bull. 61, 695–699. doi: 10.1248/cpb.c13-00082
Nohara, T., Fujiwara, Y., Ikeda, T., Yamaguchi, K., Manabe, H., Murakami, K., et al. (2014). Acyclic sulfides, garlicnins L-1–L-4, E, and F, from Allium sativum. Chem. Pharm. Bull. 62, 477–482. doi: 10.1248/cpb.c14-00003
Nohara, T., Kitaya, Y., Sakamoto, T., Manabe, H., Ono, M., Ikeda, T., et al. (2012). Garlicnins B1, C1, and D, from the fraction regulating macrophage activation of Allium sativum. Chem. Pharm. Bull. 60, 747–751. doi: 10.1248/cpb.60.747
Oommen, S., Anto, R. J., Srinivas, G., and Karunagaran, D. (2004). Allicin (from garlic) induces caspase-mediated apoptosis in cancer cells. Eur. J. Pharmacol. 485, 97–103. doi: 10.1016/j.ejphar.2003.11.059
Rose, P., Whiteman, M., Moore, P. K., and Zhu, Y. Z. (2005). Bioactive S-alk(en)yl cysteine sulfoxide metabolites in the genus allium: the chemistry of potential therapeutic agents. Nat. Prod. Rep. 22, 351–368. doi: 10.1039/b417639c
Keywords: Allium sativum L., garlic, organosulfur compound, foliogarlic disulfane, foliogarlic trisulfane
Citation: Fukaya M, Nakamura S, Hayashida H, Noguchi D, Nakashima S, Yoneda T and Matsuda H (2020) Structures of Cyclic Organosulfur Compounds From Garlic (Allium sativum L.) Leaves. Front. Chem. 8:282. doi: 10.3389/fchem.2020.00282
Received: 23 December 2019; Accepted: 23 March 2020;
Published: 30 April 2020.
Edited by:
Tao Wang, Tianjin University of Traditional Chinese Medicine, ChinaReviewed by:
Florenci Vicent González, University of Jaume I, SpainHitendra M. Patel, Sardar Patel University, India
Copyright © 2020 Fukaya, Nakamura, Hayashida, Noguchi, Nakashima, Yoneda and Matsuda. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hisashi Matsuda, bWF0c3VkYUBtYi5reW90by1waHUuYWMuanA=
 Masashi Fukaya
Masashi Fukaya Seikou Nakamura
Seikou Nakamura Hisashi Matsuda
Hisashi Matsuda