
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Chem. , 30 October 2019
Sec. Organic Chemistry
Volume 7 - 2019 | https://doi.org/10.3389/fchem.2019.00692
 Chun-Lan Xie1,2
Chun-Lan Xie1,2 Jin-Mei Xia1
Jin-Mei Xia1 Ting Lin2
Ting Lin2 Ying-Jie Lin1
Ying-Jie Lin1 Yu-Kun Lin1
Yu-Kun Lin1 Man-Li Xia1
Man-Li Xia1 Hai-Feng Chen2
Hai-Feng Chen2 Zhu-Hua Luo1
Zhu-Hua Luo1 Zong-Ze Shao1
Zong-Ze Shao1 Xian-Wen Yang1*
Xian-Wen Yang1*Two new (1, 2) and one known (3) meroterpenoids were isolated from the deep-sea-derived fungus Penicillium allii-sativi. The relative structures of new compounds were determined on the basis of an extensive analysis of the NMR and MS data, and the absolute configurations were established by ECD calculations. Andrastone A (1) is a rare andrastin bearing an unusual cyclopentan-1,3-dione. It shows a selectively antiproliferative effect against HepG2 tumor cells with an IC50 value of 7.8 μM. Mechanism study showed that apoptosis via Caspase and RXRα pathways are responsible for the inhibitory effect.
The ocean yields an impressive array of novel compounds with exquisite structures and potent bioactivities (Blunt et al., 2017, 2018). Interestingly, more and more evidence shows that marine natural compounds are mainly produced by marine microorganisms. In 2017, 57% of the total new marine natural products were reported from marine microorganisms (Carroll et al., 2019). As our research investigating bioactive secondary metabolites from the deep-sea-derived microorganisms continues, a series of interesting compounds were obtained (Yang et al., 2013; Niu et al., 2017; Xie et al., 2019). The crude extract of Penicillium allii-sativi, a fungus isolated from the deep-sea water of the western Pacific, showed significant antiproliferative activities against several cancer cell lines. Therefore, a chemical investigation was conducted, which led to the discovery of two new and one known meroterpenoids (Figure 1), among which compound 1 showed potent effects. Herein, we report the isolation, structure, and bioactivities of these compounds.
The NMR spectra were recorded on a Bruker 400 MHz spectrometer using TMS as an internal standard. The HRESIMS spectra were measured on a Waters Xevo G2 Q-TOF (Waters) mass spectrometer. Optical rotations were measured with an Anton Paar MCP100 polarimeter. CD spectra were measured on a JASCO J-715 spectropolarimeter. The semipreparative HPLC was carried out on an Agilent technologies 1260 infinity instrument equipped with DAD detector (Agilent, USA) using a reversed-phase C18 column (5 μm, 10 × 250 mm; Cosmosil, Japan). Column chromatography (CC) was performed on silica gel, Sephadex LH-20 (Amersham Pharmacia Biotech AB), and ODS (50 μm, Daiso, Japan).
Penicillium allii-sativi was isolated from the deep-sea water of the western Pacific at a depth of −4,302 m, in 2012. The voucher strain is preserved at the Marine Culture Collection of China (Xiamen, China) with the accession number of MCCC 3A00580.
The fungus was cultured on a PDA plate at 25°C for 3 days. The fresh mycelia were then cultured in 50 × 1 L Erlenmeyer flasks under static conditions at 28°C, each containing 80 g rice and 120 mL distilled water. After 30 days, the fermented broth was extracted with EtOAc for three times. The organic solvent was evaporated under reduced pressure to afford an organic extract (120 g). The extract was partitioned by MeOH and then extracted with petroleum ether (PE). The MeOH layer was concentrated to provide a defatted extract (60.4 g).
The extract was subjected to CC on silica gel eluted with gradient CH2Cl2-MeOH to get 8 fractions (Fr.1–Fr.8). Fractions Fr.3 (5.5 g) was CC over ODS using a gradient H2O-MeOH, followed by CC on silica gel (PE-acetone, 5:1) to give 1 (5.0 mg). The other fraction Fr.5 (6.7 g) was subsequently subjected to CC over ODS and Sephadex LH-20 (MeOH). Final purification by preparative HPLC using H2O-MeOH (20 → 80%) provided 2 (3.5 mg), and by preparative TLC (CH2Cl2-MeOH, 10:1) afforded 3 (4.3 mg), respectively.
Andrastone A (1): colorless oil; 0.32 (c 0.50, CHCl3); −7.2 (c 0.50, MeOH); UV (MeOH) λmax (logε) 206 (3.34) nm; CD (MeOH) Δε214 +0.48, Δε231 −0.08, Δε260 +1.36, Δε315 −2.03; 1H and 13C NMR data (see Table 1), HRESIMS m/z 511.2672 [M + Na]+ (calcd for C28H40O7Na, 511.2666).
16-epi-Citreohybriddione A (2): colorless oil; −166.3 (c 1.0, CHCl3); UV (MeOH) λmax (logε) 205 (4.06) nm; CD (MeOH) Δε215 +5.55, Δε222 +2.67, Δε254 +14.18, Δε310 −36.39; 1H and 13C NMR data (see Table 1); HRESIMS m/z 539.2257 [M + Na]+ (calcd for C28H36O9Na, 539. 2252).
Citreohybriddione A (3): non-merohedral twin crystal; −134.5 (c 0.3, MeOH); 1H and 13C NMR data (see Table 1); HRESIMS m/z 539.2260 [M + Na]+ (calcd for C28H36O9Na, 539. 2259).
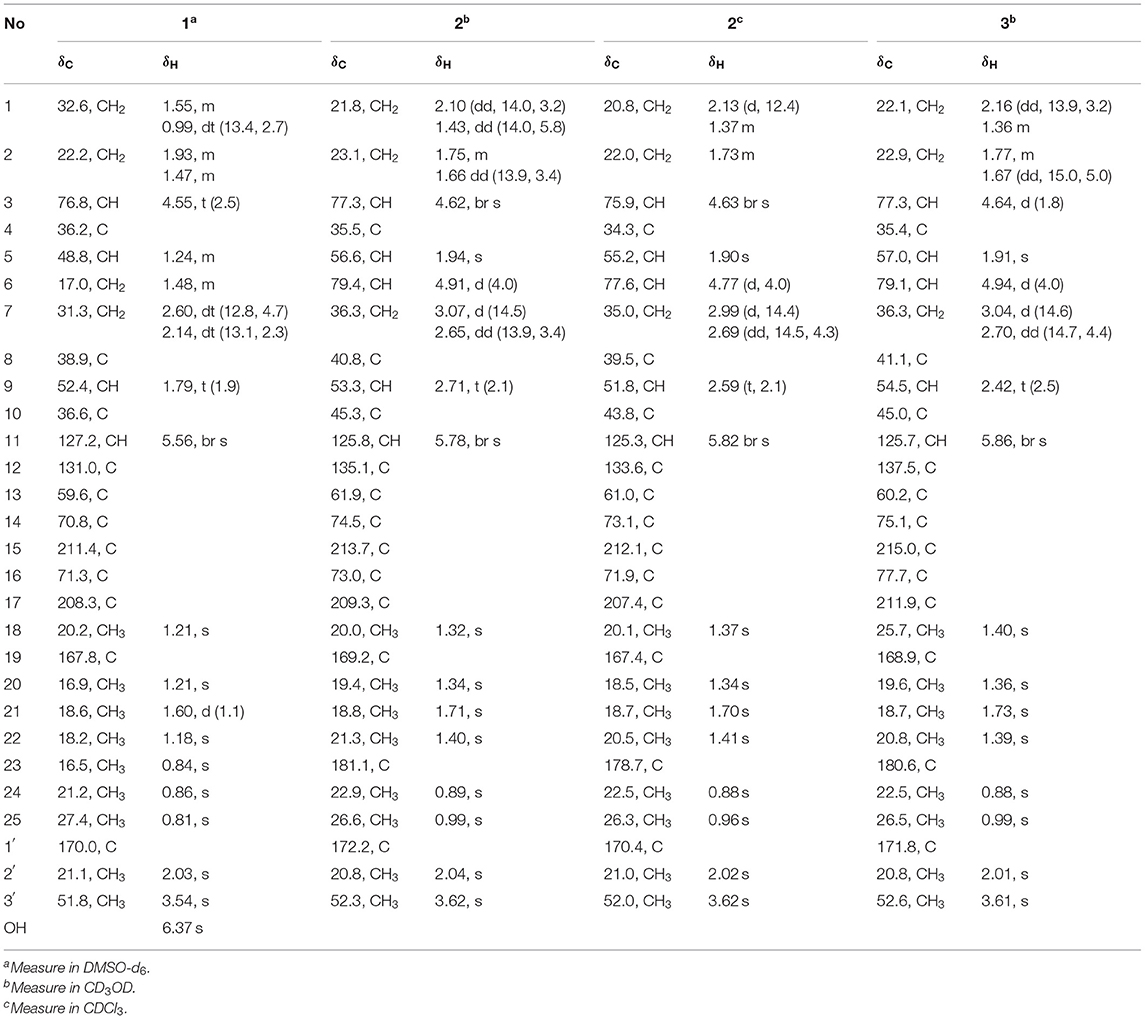
Table 1. 1H (400 MHz) and 13C (100 MHz) NMR spectroscopic data of compounds 1–3 (δ in ppm, J in Hz within the parenthesis).
Compound 3 was obtained as a colorless non-merohedral twin crystal. The crystal data were recorded with an Xcalibur Eos Gemini single-crystal diffractometer with Cu Kα radiation (λ = 1.54184 Å). Space group C2(5), a = 16.6584(6) Å, b = 9.2116(4) Å, c = 18.8507(10) Å, α = 90°, β = 109.545°, γ = 90°, V = 2725.97 Å3, Z = 4, Dcalcd = 1.259 g/cm3; μ = 0.830 mm−1, F (000) = 1,154; The final R indices [I > 2sigma(I)] R1 = 0.0691, wR2 = 0. 2044. The absolute structure parameter was 0.2 (2). Crystallographic data of 3 have been deposited in the Cambridge Crystallographic Data Center, with deposition number CCDC 1936654. Copies of the data can be obtained, free of charge, on application to CCDC, 12 Union Road, Cambridge CB21EZ, U.K. [fax +44(0)-1233-336033; email: ZGVwb3NpdEBjY2RjLmNhbS5hYy51aw==].
Cytotoxic activities of all three compounds were conducted on seven human cancer cell lines of HepG2, A549, BIU-87, BEL-7402, ECA-109, Hela-S3, and PANC-1 by MTT method as reported previously (Yang et al., 2008).
After treated with 1 (5 μM) for 24 h, HepG2 cells were stained with 5 μL propidium iodide (PI) by adding 5 μL Annexin V-APC, in darkness for 15 min. The apoptosis results were analyzed using a FAC scan flow cytometer (Becton Dickinson, Sparks, Maryland, USA), as described previously (Xie et al., 2019). Paclitaxel was used as the positive control.
HepG2 cells were treated with different concentrations of compound 1 (5, 10, 20 μM) for 12 h and caspase activation was investigated using a Caspase-Glo 3/8/9 assay (Promega) following the directions provided by the kit's manufacturer. The resulting luminescence was read using a Multimode plate reader (Envision, Perkin Elmer, USA).
As reported previously (Duan et al., 2010), the two target plasmids (30 ng pBind RXRα LBD and 60 ng PG5 LUC) were transfected by Liposome 2000 (Invitrogen) in the cell. The cells were then exposed to 1 (10, 20, 40 μM) for 16 h. According to the introduction of the Dual-Luciferase Reporter Assay System kit (promega), the activities of Firefly luciferase (FL) and Rellina luciferase (RL) were checked. The fold activities were calculated as the relative luciferase activities ratio between sample and blank control.
Compound 1 was obtained as colorless oil. The molecular formula of C28H40O7 was determined according to the sodium adduct ion peak at m/z 511.2672 [M + Na]+ in its HRESIMS spectrum, indicating nine degrees of unsaturation. The 1H and 13C NMR spectra (Supplementary Material) showed 28 carbon signals including eight methyls, one methoxyl, four methylenes, four methines (one olefinic, one oxygenated, and two aliphatic), and 11 non-protonated carbons (two ketones, two carboxyls, one olefinic, one oxygenated, and five aliphatic). Except for the typical signals of one methoxyl group [δH 3.54 (3H, s); δC 51.8 q] and one acetoxyl moiety [δH 2.03 (3H, s); δC 21.1 q, 170.0 s] group, the remaining signals indicated a 25-carbon skeleton of 1. Since two ketones, two carboxyls, and one olefinic moiety accounted for five degrees of unsaturation, compound 1 should be a tetracyclic molecule.
In the COSY spectrum, three fragments were easily deduced according to the correlations of H-3/H-2/H-1, H-5/H-6/H-7, and H-9/H-11/H-21. In the HMBC spectrum, the long-range cross peaks originated from Me-18, Me-20, and Me-22–Me-25 established a tetracyclic skeleton of 1. Further HMBC correlations of H-3 and H-3′ to the carboxyl groups at δC 170.0 and 167.8 confirmed the attachment of the acetoxyl and methoxyl at C-3 and C-19, respectively. Accordingly, the planar structure of 1 was established as shown in Figure 2.
The relative configuration of 1 was assigned mainly by the NOESY spectrum. Correlations were found of H-5 to H-9/Me-25/H-7a (δH 2.60, td, J = 12.8, 4.7 Hz), H-9 to 16-OH (δH 6.37, s), Me-25 to 3-OAc/H-2a (δH 1.47 m), H-2b (δH 1.93 m) to Me-24, H-7b (δH 2.14, td, J = 13.1, 2.3 Hz) to Me-22/Me-23/3′-OMe. Therefore, H-5/H-9/Me-25/3-OAc/16-OH was supposed to be in the same plane (tentatively assumed as β-orientation), which is the opposite to Me-23/H-3/Me-24/Me-22/Me-18 (regarded as α-configuration).
To further assign its absolute stereochemistry, the theoretical calculation of the electronic circular dichroism (ECD) was adopted. As shown in Figure 3, the calculated ECD of (3S,5S,8S,9S,10R,13R, 14R,16R)-1 (a) showed the same Cotton effects as those of the experimental ones. On the basis of the above evidence, 1 was therefore assigned and given the trivial name of Andrastone A.
Compound 2 was also obtained as colorless oil. The molecular formula was established as C28H36O9 on the basis of the sodium adduct at m/z 539.2257 [M + Na]+ (calcd for C28H36O9Na, 539.2252) in its HRESIMS spectrum. The 1H and 13C NMR spectra showed 28 carbons consisting of seven methyls, one methoxyl, three methylenes, five methines (one olefinic, two oxygenated, and two aliphatic), and 12 non-protonated carbons (two ketones, three carboxyls, one olefinic, one oxygenated, and five aliphatic). These signals were very similar to those of compound 3, a meroterpene that was established as citreohybriddione A by single X-ray crystallography (Figure 4) (Kosemura et al., 1991a). However, clear differences were found in 2 showing that C-16, Me-18 were upshifted up to 4.7 and 5.7 ppm, respectively, suggesting the hydroxy moiety at the C-16 position should be the β-configuration in 2, instead of α in 3. This was confirmed by the NOESY correlations of 3′-Me to Me-18. By comparison of the experimental and theoretical calculation of the ECD spectra (Figure 3), 2 was therefore assigned to be 16-epi-citreohybriddione A.
Andrastone A (1) and 16-epi-citreohybriddione A (2) are two meroterpenoids biosynthesized from a sesquiterpene and a tetraketide. In fact, compounds possessing the same scaffold are rarely found in nature, including citreohybridones A and B (Kosemura et al., 1991b), Andrastins A–D (Shiomi et al., 1996; Uchida et al., 1996), atlantinones A and B (Wang et al., 2010), citreohybriddiones A–C (Kosemura, 2003), citreohybridones A–G and J–L (Kosemura, 2002), citreohybridonol (Oezkaya et al., 2018), 3-deacetylcitreohybridonol (Gao et al., 2012), 15-deacetylated citreohybridone E (Cheng et al., 2019), isocitreohybridones A–C and H–J (Kosemura, 2002, 2003), and isopenicins A–C (Tang et al., 2019). Among them, 18 possess a lactone moiety at the C-23 position. For the other 13 compounds, 10 have the keto-enol tautomerism at the cyclopentane ring. Interestingly, compound 1 is a rare meroterpene without the lactone moiety but possesses the cyclopentan-1,3-dione group.
Compounds 1–3 were evaluated for their antiproliferative effects against HepG2, A549, BIU-87, BEL-7402, ECA-109, Hela-S3, and PANC-1 tumor cells. However, only compound 1 exhibited significant inhibitory activity. Interestingly, it showed a selective effect only on HepG2 cells with an IC50 value of 7.8 μM. To uncover its inhibition mechanism, 1 was first subjected to flow cytometry. According to a previous study, Paclitaxel can induce apoptosis via arresting cells mainly in the G2/M phase of the cell cycle (Jelinek et al., 2015; Maushagen et al., 2016). Therefore, it was used as the positive control. As shown in Figure 5, Paclitaxel and 1 could obviously induce apoptosis in HepG2 cells.
Since caspase is a key pathway to induce apoptosis (Porter and Janicke, 1999; Li and Yuan, 2008), we measured the activities of the caspase-3, 8, and 9. As a result, 1 could significantly increase the activities of caspase-3 and caspase-8, but had almost no effect on caspase-9 (Figure 6), suggesting that the apoptosis was caused by direct caspase-8-mediated caspase-3 activation (Kaufmann et al., 2008).
Moreover, a dual luciferase reporter gene assay was also carried out to investigate the RXRα transcriptional activity. Interestingly, 1 not only increased the reporter transcriptional activation of RXRα, but also reduced the transactivity of RXRα induced by 9-cis-RA (Figure 7).
In summary, from the deep-sea-derived fungus Penicillium allii-sativi, two new and one known meroterpenes were obtained. Andrastone A (1) showed significant inhibitory effect selectively against HepG2 tumor cells by activating caspase-3 and regulating the transcriptional activation function of RXRα.
All datasets for this study are included in the manuscript/Supplementary Files.
C-LX conducted chemical and biological experiments. J-MX, Y-JL, Y-KL, and M-LX assisted C-LX's chemical experiments. TL and H-FC assisted C-LX's bioactive experiments. Z-HL isolated the fungus and Z-ZS deposited it to MCCC. X-WY initiated and oversaw all research.
This work was financially supported by the Scientific Research Foundation of the Third Institute of Oceanography (2017035), the COMRA program (DY135-B2-08), and the Xiamen Southern Oceanographic Center Project (17GYY026NF05).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Mr. Tian-Hua Zhong for the measurement of the NMR data.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2019.00692/full#supplementary-material
Blunt, J. W., Carroll, A. R., Copp, B. R., Davis, R. A., Keyzers, R. A., and Prinsep, M. R. (2018). Marine natural products. Nat. Prod. Rep. 35, 8–53. doi: 10.1039/C7NP00052A
Blunt, J. W., Copp, B. R., Keyzers, R. A., Munro, M. H., and Prinsep, M. R. (2017). Marine natural products. Nat. Prod. Rep. 34, 235–294. doi: 10.1039/C6NP00124F
Carroll, A. R., Copp, B. R., Davis, R. A., Keyzers, R. A., and Prinsep, M. R. (2019). Marine natural products. Nat. Prod. Rep. 36, 122–173. doi: 10.1039/C8NP00092A
Cheng, Z., Xu, W., Wang, Y., Bai, S., Liu, L., Luo, Z., et al. (2019). Two new meroterpenoids and two new monoterpenoids from the deep sea-derived fungus Penicillium sp. YPGA11. Fitoterapia 133, 120–124. doi: 10.1016/j.fitote.2018.12.022
Duan, Y. H., Yi, D., Wang, G. H., Xue, Z., Chen, H. F., Chen, J. B., et al. (2010). Bioactive xanthones from the stems of Cratoxylum formosum ssp. pruniflorum. J. Nat. Prod. 73, 1283–1287. doi: 10.1021/np1001797
Gao, S. S., Shang, Z., Li, X. M., Li, C. S., Cui, C. M., and Wang, B. G. (2012). Secondary metabolites produced by solid fermentation of the marine-derived fungus Penicillium commune QSD-17. Biosci. Biotechnol. Biochem. 76, 358–360. doi: 10.1271/bbb.110332
Jelinek, M., Balusikova, K., Schmiedlova, M., Nemcova-Furstova, V., Sramek, J., Stancikova, J., et al. (2015). The role of individual caspases in cell death induction by taxanes in breast cancer cells. Cancer Cell Int. 15, 1–26. doi: 10.1186/s12935-015-0155-7
Kaufmann, S. H., Lee, S. H., Meng, X. W., Loegering, D. A., Kottke, T. J., Henzing, A. J., et al. (2008). Apoptosis-associated caspase activation assays. Methods 44, 262–272. doi: 10.1016/j.ymeth.2007.11.005
Kosemura, S. (2002). Structure and absolute configuration of citreohybridones isolated from Penicillium species. Tetrahedron Lett. 43, 1253–1256. doi: 10.1016/S0040-4039(01)02345-0
Kosemura, S. (2003). Meroterpenoids from Penicillium citreo-viride B. IFO 4692 and 6200 hybrid. Tetrahedron 59, 5055–5072. doi: 10.1016/S0040-4020(03)00739-7
Kosemura, S., Matsunaga, K., and Yamamura, S. (1991a). Citreohybriddiones A and B and related terpenoids, new metabolites of a hybrid strain KO 0031 derived from Penicillium citreo-viride B. IFO 6200 and 4692. Chem. Lett. 20, 1811–1814. doi: 10.1246/cl.1991.1811
Kosemura, S., Matsunaga, K., Yamamura, S., Kubota, M., and Ohba, S. (1991b). The structures of citreohybridone A and B novel sesterterpenoid-type metabolites of a hybrid strain KO 0031 derived from Penicillium citreo-viride B. IFO 6200 and 4692. Tetrahedron Lett. 32, 3543–3546. doi: 10.1016/0040-4039(91)80828-T
Li, J., and Yuan, J. (2008). Caspases in apoptosis and beyond. Oncogene 27, 6194–6206. doi: 10.1038/onc.2008.297
Maushagen, R., Reers, S., Pfannerstill, A.-C., Hahlbrock, A., Stauber, R., Rahmanzadeh, R., et al. (2016). Effects of paclitaxel on permanent head and neck squamous cell carcinoma cell lines and identification of anti-apoptotic caspase 9b. J. Cancer Res. Clin. Oncol. 142, 1261–1271. doi: 10.1007/s00432-016-2150-3
Niu, S., Fan, Z. W., Xie, C. L., Liu, Q., Luo, Z. H., Liu, G., et al. (2017). Spirograterpene A, a tetracyclic spiro-diterpene with a fused 5/5/5/5 ring system from the deep-sea-derived fungus Penicillium granulatum MCCC 3A00475. J. Nat. Prod. 80, 2174–2177. doi: 10.1021/acs.jnatprod.7b00475
Oezkaya, F. C., Ebrahim, W., Klopotowski, M., Liu, Z., Janiak, C., and Proksch, P. (2018). Isolation and X-ray structure analysis of citreohybridonol from marine-derived Penicillium atrovenetum. Nat. Prod. Res. 32, 840–843. doi: 10.1080/14786419.2017.1311893
Porter, A. G., and Janicke, R. U. (1999). Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 6, 99–104. doi: 10.1038/sj.cdd.4400476
Shiomi, K., Uchida, R., Inokoshi, J., Tanaka, H., Iwai, Y., and Omura, S. (1996). Andrastins A–C, new protein farnesyltransferase inhibitors, produced by Penicillium sp. FO-3929. Tetrahedron Lett. 37, 1265–1268. doi: 10.1016/0040-4039(95)02412-3
Tang, J. W., Kong, L. M., Zu, W. Y., Hu, K., Li, X. N., Yan, B. C., et al. (2019). Isopenicins A–C: Two types of antitumor meroterpenoids from the plant endophytic fungus Penicillium sp. sh18. Org. Lett. 21, 771–775. doi: 10.1021/acs.orglett.8b04020
Uchida, R., Shiomi, K., Inokoshi, J., Tanaka, H., Iwai, Y., and Omura, S. (1996). Andrastin D, novel protein farnesyltransferase inhibitor produced by Penicillium sp. FO-3929. J. Antibiot. 49, 1278–1280. doi: 10.7164/antibiotics.49.1278
Wang, X. R., Sena Filho, J. G., Hoover, A. R., King, J. B., Ellis, T. K., Powell, D. R., et al. (2010). Chemical epigenetics alters the secondary metabolite composition of guttate excreted by an Atlantic forest soil-derived Penicillium citreonigrum. J. Nat. Prod. 73, 942–948. doi: 10.1021/np100142h
Xie, C. L., Zhang, D., Xia, J. M., Hu, C. C., Lin, T., Lin, Y. K., et al. (2019). Steroids from the deep-sea-derived fungus Penicillium granulatum MCCC 3A00475 induced apoptosis via retinoid X receptor (RXR)-α pathway. Mar. Drugs 17:178. doi: 10.3390/md17030178
Yang, X. W., Peng, K., Liu, Z., Zhang, G. Y., Li, J., Wang, N., et al. (2013). Strepsesquitriol, a rearranged zizaane-type sesquiterpenoid from the deep-sea-derived actinomycete streptomyces sp. SCSIO 10355. J. Nat. Prod. 76, 2360–2363. doi: 10.1021/np400923c
Keywords: deep-sea, meroterpenoids, microorganisms, anti-tumor, nuclear receptors
Citation: Xie C-L, Xia J-M, Lin T, Lin Y-J, Lin Y-K, Xia M-L, Chen H-F, Luo Z-H, Shao Z-Z and Yang X-W (2019) Andrastone A From the Deep-Sea-Derived Fungus Penicillium allii-sativi Acts as an Inducer of Caspase and RXRα-Dependent Apoptosis. Front. Chem. 7:692. doi: 10.3389/fchem.2019.00692
Received: 16 July 2019; Accepted: 07 October 2019;
Published: 30 October 2019.
Edited by:
Guigen Li, Texas Tech University, United StatesReviewed by:
Dong Liu, Peking University, ChinaCopyright © 2019 Xie, Xia, Lin, Lin, Lin, Xia, Chen, Luo, Shao and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xian-Wen Yang, eWFuZ3hpYW53ZW5AdGlvLm9yZy5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.