
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Chem. , 18 September 2019
Sec. Organic Chemistry
Volume 7 - 2019 | https://doi.org/10.3389/fchem.2019.00623
This article is part of the Research Topic Isocyanide-based Multicomponent Reactions View all 10 articles
A novel, efficient and environmentally friendly approach has been developed for the synthesis of biologically important bis-heterocyclic oxazepine-quinazolinone derivatives. The structurally interesting compounds of high purity were synthesized by a one-pot three-component reaction of 2-(2-formylphenoxy) acetic acid and 2-aminobenzamide as bifunctional reagents and an isocyanide without using any catalyst, with excellent overall yields.
To date, the development of new methods for the synthesis of heterocyclic compounds has been and remains a hot topic in organic chemistry, due to their importance in biologically active natural products and synthetic materials (Armstrong and Collins, 2010; Kaur et al., 2016). Remarkably, seven out of the top ten pharmaceutical products according to worldwide sales in 2009 contain a heterocyclic motif as their core structure (Chen et al., 2014). Seven-membered heterocyclic rings have been the object of deep investigation owing to their prevalence in molecules with biological activities (Goutham et al., 2015; Voigt et al., 2015; Xu, 2016).
Oxazepines, a privileged scaffold in medicinal chemistry, are a well-known class of seven-membered heterocycles with two heteroatoms and have been receiving continuing attention due to the wide range of biological activities. Among these activities, it is worth mentioning anti-inflammatory (Chakrabarti and Hicks, 1987; Verma et al., 2008), antifungal (Serrano-Wu et al., 2002), antithrombotic (Mishra et al., 2010; Agirbas et al., 2011), anti-epileptic (Pekcec et al., 2009), anti-convulsant (Sharma et al., 2008), progesterone agonist (Dols et al., 2008), antagonist and analgesic (Hallinan et al., 1994), anti-histaminic (Sleevi et al., 1991), anti-psychotic (Liegeois et al., 1994; Liao et al., 1999), anxiolytics (Effland et al., 1982), anti-aggregating (Aono et al., 1991), and epidermal growth factor receptor (EGFR) tyrosine kinase inhibitory (Smith et al., 2006) activities. Compounds containing oxazepine motif, sintamil (Nagarajan et al., 1986) and loxapine (Liao et al., 1999) were reported, due to their antidepressant and potential clozapine-like properties, respectively (Figure 1) (Samet et al., 2005; Liu et al., 2011) Considering the structural characteristics of the benzoxazepine-3-ones, the existence of seven-membered heterocyclic ring system, fused aromatic group and the group –N–C(= O)–, similar to protein amide bond, it is reasonable to expect inherent physiological activities (Agirbas et al., 2011).
Nitrogen heterocycles are the most important structural units in natural products and synthetic drugs. Thus, tremendous efforts have been made to develop new strategies and technologies for their synthesis (Tietze, 1996; Tietze and Modi, 2000; D'Souza and Mueller, 2007; Priebbenow et al., 2011; Rixson et al., 2012). Typically, quinazolinone derivatives widely occur in natural products (Yoshida et al., 1991; Wattanapiromsakul et al., 2003), and they show various biological and pharmacological activities, such as anti-inflammatory, antioxidant, antimicrobial, antipsychotic, and antihypertensive activity, strong analgesic activity, and many effects on the central nervous system (CNS) (Khalil et al., 1994; Bartoli et al., 1998; Liverton et al., 1998; Malecki et al., 2004; Arora et al., 2011; Chawla and Batra, 2013; Nepali et al., 2013). A quinazolinone motif is present in the structure of numerous drugs, e.g., the hypnotic methaqualone, the muscle relaxant afloqualone, the diuretic quinethazone, the antineoplastic agents trimetrexate and raltitrexed, and the serotonin antagonist ketanserin (Figure 2) (Kleemann et al., 1999; Abraham, 2003).
Combination of a molecule with several heterocyclic compounds with different pharmacological activities due to the synergism effect is a useful strategy to assign and discover new biological compounds. The Ugi four-component reaction (U-4CR) is one of the most commonly used multicomponent reactions (MCRs), in which a carboxylic acid, an amine, a carbonyl compound, and an isocyanide are reacting to result in peptide-like heterocyclic products (Hebach and Kazmaier, 2003; Dömling, 2006; Giovenzana et al., 2006; Ngouansavanh and Zhu, 2007; Hartweg and Becer, 2016; Yugandhar et al., 2016). Although a large diversity can be quickly achieved through the U-4CR, the scaffolds that are accessible through it are limited. The replacement of two participants in this reaction with a single bifunctional reagent is a fruitful strategy to broaden the scope of structures that are accessible by the U-4CR and toward various drug-like heterocycles (Hulme and Dietrich, 2009). 2-(2-formylphenoxy) acetic acid 1 has previously been employed to provide various derivatives of oxazepines (Zhang et al., 1999; Ilyin et al., 2006; Tsaloev et al., 2011; Hajishaabanha and Shaabani, 2014). As a part of our ongoing research program on the isocyanide-based MCRs (Shaabani et al., 2007, 2008a,b, 2009, 2011, 2014, 2016; Hajishaabanha and Shaabani, 2014), a novel strategy was designed to explore the Ugi one-pot three-component four-center reaction with two bifunctional starting materials, 2-(2-formylphenoxy)acetic acid 1 and 2-aminobenzamide 2 for the synthesis of bis-heterocyclic oxazepine-benzodiazepine 4 (Scheme 1, cyclization path A) or oxazepine-quinazolinone 5 (Scheme 1, cyclization path B) derivatives. The results show the reaction proceeded via the pathway B affording a new interesting class of oxazepine-quinazolinone 5 in high yields.
In a pilot experiment, 2-(2-formylphenoxy)acetic acid 1, 2-aminobenzamide 2, and tert-butyl isocyanide 3a were refluxed in ethanol. The progress of reaction was monitored by TLC. After 24 h, the reaction was completed and N-(tert-butyl)-5-oxo-5,7-dihydro-13H-benzo[6,7][1,4]oxazepino[4,3-a]quinazoline-13-carboxamide 5a (Scheme 1, cyclization path B) was obtained in 94% yield (Scheme 2). It is worth mentioning that in the course of this reaction, one C-C bond, several C-N bonds, one amide group, a benzoxazepine ring and a quinazolinone ring are newly formed. These new structures broaden the scaffolds that are accessible through Ugi reaction and may represent interesting pharmacophores.
In view of the success of the above reaction, we explored its scope and limitations, by extending the procedure to various isocyanides 3a-c. As indicated in Figure 3, the reactions proceed very efficiently in EtOH and led to the formation of novel oxazepine-quinazolinone bis-heterocyclic scaffolds 5a-c in excellent yields. The reaction did not require any optimization.
The structures of products 5 were deduced from their IR, 1H NMR, 13C NMR, mass spectra and CHN analysis data. The 1H NMR spectrum of 5c consisted of a multiplet for the methylene protons of the cyclohexyl ring (δ = 1.03-1.63 ppm, 10H), a broad singlet for the NH–CH cyclohexyl (δ = 3.58 ppm, 1H), two doublets for two non-equivalent methylene protons of the oxazepine ring (δ = 4.83 and 5.37 ppm, J = 14.5 Hz), a singlet for CH (δ = 6.65 ppm, 1H), a multiplet for aromatic protons and NH (7.03–8.11 ppm, 9H). Also, the 1H decoupled 13C NMR spectrum of 5c is completely consistent with the suggested structure. The mass spectra of these compounds displayed molecular ion peaks at the appropriate m/z values. Finally, the structure of the product 5c was confirmed unambiguously by single-crystal X-ray analysis (Figure 4) (Petríček et al., 2014).
A possible mechanism for the formation of products 5 is shown in Scheme 3. It is conceivable that the initial event in this reaction is the nucleophilic attack of amine 2 to formyl group to afford the iminium intermediate 6. The addition of the carbenoid C-atom of the isocyanides 3 onto the iminium group followed by the addition of the carboxylate ion onto the C-atom of the nitrilium ion leads to the formation of the adduct 7, which undergoes an intramolecular acylation known as Mumm rearrangement to give the Ugi adduct 8. Finally, Ugi adduct 8 undergoes an amide-amide cyclocondensation through pathway A (instead of pathway B) to give the oxazepine-quinazolinone bis-heterocyclic products 5 (Scheme 3).
It is worth mentioning that to expand the structure diversity accessible through this type of Ugi 3-component reaction, the reaction between 2-formylbenzoic acid 8, 2-aminobenzamide 2, and cyclohexyl isocyanide under the previously mentioned conditions was also investigated. However, Ugi adduct 9 does not undergo an intramolecular cyclization to give the expected quinazolinone-isoindoline bis-heterocyclic product 10. The analytical data obtained on the final material support the preparation and isolation of 9 as the product (Scheme 4).
The fused benzodiazepine-quinazolinone is a unique tetracyclic scaffold in several respects. A SciFinder and ChEMBL database search revealed no other example (Figure S1). Known substructures are benzoxazepines and quinazolinones. The parent scaffold benzoxazepine-quinazolinone is non planar through the introduction of the seven-membered aliphatic oxazepine ring in the center of the tetracycle, comprising a butterfly shape and showing an interesting combination of pharmacophores (Figure 5). The quinazolinone bicycle and the phenyl group are planar and can potentially undergo pi stacking interactions with the receptor amino acids. The quinazolinone also comprise a rare vicinal hydrogen bond acceptor hydrogen bond acceptor moiety. The other nitrogen atom is fully encapsulated in the ring systems and involved in the aromatic bicycle and cannot undergo hydrogen bonding interactions. The ether oxygen of the seven-membered oxazepane ring can act as another hydrogen bond acceptor.
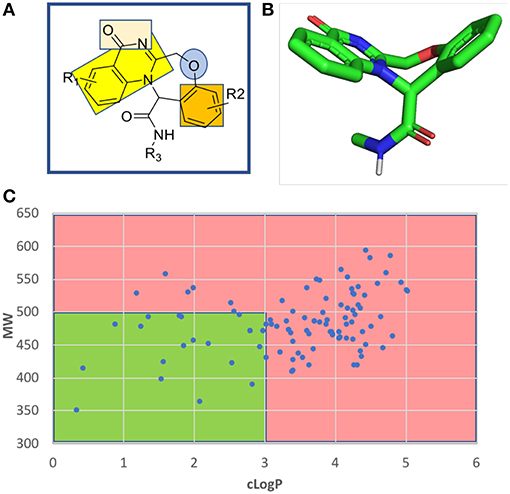
Figure 5. Chemoinformatic analysis of the unprecedented benzoxazepine-quinazolinone scaffold. (A) 2D structure and pharmacophores. (B) 3D energy minimized butterfly structure of the scaffold induced by the central seven-membered oxazepine ring. (C) Molecular weight over lipophilicity of a randomly generated a 100 compound library highlighting the drug-like preferred and non-preferred area in green and red, respectively.
A randomly generated library of benzoxazepine-quinazolinones reveals a good fraction of compounds with an attractive MW and cLogP thus rendering the scaffold interesting for receptor ligand interactions (Figure 5 and Supplementary Information).
In conclusion, we have successfully developed a one-pot three-component four-center reaction strategy leading to novel bis-heterocyclic oxazepine-quinazolinones which are two important pharmacological and biological scaffolds, starting from simple and readily available inputs. To the best of our knowledge, it is the first report of using two bifunctional starting materials in Ugi reaction to obtain fused oxazepine-quinazolinone heterocycles. Moreover, it is a new isocyanide based bicyclization reaction (Gao et al., 2015, 2016; Hao et al., 2016; Tang et al., 2016). The reaction is high-yielding and product isolation is very straightforward. Moreover, it is noteworthy that this operationally friendly and scalable manner allows C–C bond, C-O and C–N bond formation with excellent scope. The potential uses of this route in synthetic and medicinal chemistry may be significant, since the products share structural and functional group properties of the biologically active molecules. Structural diversity and biological activity of the synthesized compounds will be tested and results of these tests will be reported in due course.
All datasets generated for this study are included in the manuscript and/or the Supplementary Files.
SS did the design, synthesis, and wrote the manuscript. AS directed the project and co-wrote the manuscript. MK and MD did the crystallographic part.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We gratefully acknowledge financial support from the Iran National Science Foundation (INSF) and Research Council of Shahid Beheshti University. The crystallographic part was supported by the project 14-03276S of the Czech Science Foundation.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2019.00623/full#supplementary-material
Abraham, D. J. (2003). Burger's Medicinal Chemistry and Drug Discovery. Hoboken, NJ: Wiley Online Library. doi: 10.1002/0471266949
Agirbas, H., Kemal, B., and Budak, F. (2011). Synthesis and structure–antibacterial activity relationship studies of 4-substituted phenyl-4, 5-dihydrobenzo [f][1, 4] oxazepin-3 (2H)-thiones. Med. Chem. Res. 20, 1170–1180. doi: 10.1007/s00044-010-9457-4
Aono, J., Sugawa, M., Koide, T., and Takato, M. (1991). The role of cGMP in the anti-aggregating properties of BY-1949, a novel dibenzoxazepine derivative. Eur. J. Pharmacol. 195, 225–231. doi: 10.1016/0014-2999(91)90539-3
Armstrong, A., and Collins, J. C. (2010). Direct azole amination: C-H functionalization as a new approach to biologically important heterocycles. Angew. Chem. Int. Ed. 49, 2282–2285. doi: 10.1002/anie.200906750
Arora, D., Kumar, H., Malhotra, D., and Malhotra, M. (2011). Current trends in anticonvulsant 4 (3H)-quinazolinone: a review. Pharmacologyonline 3, 659–668.
Bartoli, J., Turmo, E., Alguero, M., Boncompte, E., Vericat, M., Conte, L., et al. (1998). New azole antifungals. 3. Synthesis and antifungal activity of 3-substituted-4 (3H)-quinazolinone derivatives of 3-amino-2-aryl-1-azolyl-2-butanol. J. Med. Chem. 41, 1869–1882. doi: 10.1021/jm9707277
Chakrabarti, J. K., and Hicks, T. A. (1987). 2-[10, 11-Dihydro-11-oxodibenz [b, f][1, 4] oxazepin-7 or 8-yl] propanoic acids as potential anti-inflammatory agents. Eur. J. Med. Chem. 22, 161–163. doi: 10.1016/0223-5234(87)90013-4
Chawla, A., and Batra, C. (2013). Recent advances of quinazolinone derivatives as marker for various biological activities. Int. Res. J. Pharm. 4, 49–58. doi: 10.7897/2230-8407.04309
Chen, J., Natte, K., Neumann, H., and Wu, X. F. (2014). Palladium-catalyzed carbonylative reactions of 1-bromo-2-fluorobenzenes with various nucleophiles: effective combination of carbonylation and nucleophilic substitution. Chem. Eur. J. 20, 16107–16110. doi: 10.1002/chem.201405221
Dols, P. P., Folmer, B. J., Hamersma, H., Kuil, C. W., Lucas, H., Ollero, L., et al. (2008). SAR study of 2, 3, 4, 14b-tetrahydro-1H-dibenzo [b, f] pyrido [1, 2-d][1, 4] oxazepines as progesterone receptor agonists. Bioorg. Med. Chem. Lett. 18, 1461–1467. doi: 10.1016/j.bmcl.2007.12.065
Dömling, A. (2006). Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem. Rev. 106, 17–89. doi: 10.1021/cr0505728
D'Souza, D. M., and Mueller, T. J. (2007). Multi-component syntheses of heterocycles by transition-metal catalysis. Chem. Soc. Rev. 36, 1095–1108. doi: 10.1039/B608235C
Effland, R. C., Helsley, G. C., and Tegeler, J. J. (1982). Synthesis of 1, 4-benzodiazepino [4, 5-d][1, 4] benzoxazepines. J. Heterocycl. Chem. 19, 537–539. doi: 10.1002/jhet.5570190317
Gao, Q., Hao, W. J., Liu, F., Tu, S. J., Wang, S. L., and Jiang, B. (2016). Unexpected isocyanide-based three-component bicyclization for the stereoselective synthesis of densely functionalized pyrano [3, 4-c] pyrroles. Chem. Commun. 52, 900–903. doi: 10.1039/C5CC08071A
Gao, Q., Zhou, P., Liu, F., Hao, W. J., Yao, C., and Tu, J. (2015). Cobalt (II)/silver relay catalytic isocyanide insertion/cycloaddition cascades: a new access to pyrrolo [2, 3-b] indoles. Chem. Commun. 51, 9519–9522. doi: 10.1039/C5CC02754C
Giovenzana, G. B., Tron, G. C., Di Paola, S., Menegotto, I. G., and Pirali, T. (2006). A mimicry of primary amines by bis-secondary diamines as components in the ugi four-component reaction. Angew. Chem. Int. Ed. 45, 1099–1102. doi: 10.1002/anie.200503095
Goutham, K., Ashok Kumar, D., Suresh, S., Sridhar, B., Narender, R., and Karunakar, G. V. (2015). Gold-catalyzed intramolecular cyclization of N-propargylic β-enaminones for the synthesis of 1, 4-oxazepine derivatives. J. Org. Chem. 80, 11162–11168. doi: 10.1021/acs.joc.5b01733
Hajishaabanha, F., and Shaabani, A. (2014). Synthesis of oxazepin-quinoxaline bis-heterocyclic scaffolds via an efficient three component synthetic protocol. RSC Adv. 4, 46844–46850. doi: 10.1039/C4RA08486A
Hallinan, E. A., Stapelfeld, A., Savage, M. A., and Reichman, M. (1994). 8-chlorodibenz [b, f][1, 4] oxazepine-10 (11H)-carboxylic acid, 2-[3-[2-(furanylmethyl) thio]-1-oxopropyl] hydrazide (SC-51322): a potent PGE 2 antagonist and analgesic. Bioorg. Med. Chem. Lett. 4, 509–514. doi: 10.1016/0960-894X(94)80027-8
Hao, W. J., Gao, Q., Jiang, B., Liu, F., Wang, S. L., Tu, S. J., et al. (2016). Base-promoted [4+ 1]/[3+ 1+ 1] bicyclization for accessing functionalized indeno [1, 2-c] furans. J. Org. Chem. 81, 11276–11281. doi: 10.1021/acs.joc.6b02249
Hartweg, M., and Becer, C. R. (2016). Direct polymerization of levulinic acid via Ugi multicomponent reaction. Green Chem. 18, 3272–3277. doi: 10.1039/C6GC00372A
Hebach, C., and Kazmaier, U. (2003). Via Ugi reactions to conformationally fixed cyclic peptides. Chem. Commun. 596–597. doi: 10.1039/b210952b
Hulme, C., and Dietrich, J. (2009). Emerging molecular diversity from the intra-molecular Ugi reaction: iterative efficiency in medicinal chemistry. Mol. Divers. 13, 195–207. doi: 10.1007/s11030-009-9111-6
Ilyin, A. P., Parchinski, V. Z., Peregudova, J. N., Trifilenkov, A. S., Poutsykina, E. B., Tkachenko, S. E., et al. (2006). One-step assembly of carbamoyl substituted annulated 1,4-oxazepines. Tetrahedron Lett. 47, 2649–2653. doi: 10.1016/j.tetlet.2006.01.158
Kaur, T., Wadhwa, P., Bagchi, S., and Sharma, A. (2016). Isocyanide based [4+1] cycloaddition reactions: an indispensable tool in multi-component reactions (MCRs). Chem. Commun. 52, 6958–6976. doi: 10.1039/C6CC01562J
Khalil, M., Soliman, R., Farghaly, A., and Bekhit, A. (1994). Non-steroidal anti-inflammatory agents: novel pyrazolyl-, 1, 2-oxazolyl-, and 1, 3-diazinyl derivatives of 4 (3H)-quinazolinones. Arch. Pharm. 327, 27–30. doi: 10.1002/ardp.19943270105
Kleemann, A., Engel, J., Kutscher, B., and Reichert, D. (1999). Pharmaceutical Substances: Syntheses, Patents, Applications, Hoboken, NJ: Thieme Stuttgart.
Liao, Y., Venhuis, B. J., Rodenhuis, N., Timmerman, W., Wikström, H., Meier, E., et al. (1999). New (sulfonyloxy) piperazinyldibenzazepines as potential atypical antipsychotics: chemistry and pharmacological evaluation. J. Med. Chem. 42, 2235–2244. doi: 10.1021/jm991005d
Liegeois, J. F. F., Rogister, F. A., Bruhwyler, J., Damas, J., Nguyen, T. P., and Delarge, J. E. (1994). Pyridobenzoxazepine and pyridobenzothiazepine derivatives as potential central nervous system agents: synthesis and neurochemical study. J. Med. Chem. 37, 519–525. doi: 10.1021/jm00030a011
Liu, Y., Chu, C., Huang, A., Zhan, C., Ma, Y., and Ma, C. (2011). Regioselective synthesis of fused oxazepinone scaffolds through one-pot Smiles rearrangement tandem reaction. ACS Comb. Sci. 13, 547–553. doi: 10.1021/co2001058
Liverton, N. J., Armstrong, D. J., Claremon, D. A., Remy, D. C., Baldvin, J. J., Lynch, R. J., et al. (1998). Nonpeptide glycoprotein IIb/IIIa inhibitors: substituted quinazolinediones and quinazolinones as potent fibrinogen receptor antagonists. Bioorg. Med. Chem. Lett. 8, 483–486. doi: 10.1016/S0960-894X(98)00047-X
Malecki, N., Carato, P., Rigo, B., Goossens, J. F., Houssin, R., Bailly, C., et al. (2004). Synthesis of condensed quinolines and quinazolines as DNA ligands. Biorg. Med. Chem. 12, 641–647. doi: 10.1016/j.bmc.2003.10.014
Mishra, J. K., Samanta, K., Jain, M., Dikshit, M., and Panda, G. (2010). Amino acid based enantiomerically pure 3-substituted benzofused heterocycles: a new class of antithrombotic agents. Bioorg. Med. Chem. Lett. 20, 244–247. doi: 10.1016/j.bmcl.2009.10.126
Nagarajan, K., David, J., Kulkarni, Y., Hendi, S., Shenoy, S., and Upadhyaya, P. (1986). Piperazinylbenzonaphthoxazepines with CNS depressant properties. Eur. J. Med. Chem. 21, 21–26. doi: 10.1002/chin.198627336
Nepali, K., Sharma, S., Ojha, R., and Dhar, K. L. (2013). Vasicine and structurally related quinazolines. Med. Chem. Res. 22, 1–15. doi: 10.1007/s00044-012-0002-5
Ngouansavanh, T., and Zhu, J. (2007). IBX-mediated oxidative ugi-type multicomponent reactions: application to the N and C1 functionalization of tetrahydroisoquinoline. Angew. Chem. Int. Ed. 46, 5775–5778. doi: 10.1002/anie.200701603
Pekcec, A., Unkrüer, B., Schlichtiger, J., Soerensen, J., Hartz, A. M., Bauer, B., et al. (2009). Targeting prostaglandin E2 EP1 receptors prevents seizure-associated P-glycoprotein up-regulation. J. Pharmacol. Exp. Ther. 330, 939–947. doi: 10.1124/jpet.109.152520
Petríček, V., Dušek, M., and Palatinus, L. (2014). Crystallographic computing system JANA2006: general features. Z. Kristallogr. 229, 345–352. doi: 10.1515/zkri-2014-1737
Priebbenow, D. L., Stewart, S. G., and Pfeffer, F. M. (2011). A general approach to N-heterocyclic scaffolds using domino Heck–aza-Michael reactions. Org. Biomol. Chem. 9, 1508–1515. doi: 10.1039/c0ob00835d
Rixson, J. E., Chaloner, T., Heath, C. H., Tietze, L. F., and Stewart, S. G. (2012). The development of domino reactions incorporating the heck reaction: the formation of n-heterocycles. Eur. J. Org. Chem. 2012, 544–558. doi: 10.1002/ejoc.201101305
Samet, A. V., Marshalkin, V. N., Kislyi, K. A., Chernysheva, N. B., Strelenko, Y. A., and Semenov, V. V. (2005). Synthetic utilization of polynitroaromatic compounds. 3. Preparation of substituted dibenz [b, f][1, 4] oxazepine-11 (10 H)-ones from 2, 4, 6-trinitrobenzoic acid via nucleophilic displacement of nitro groups. J. Org. Chem. 70, 9371–9376. doi: 10.1021/jo051425c
Serrano-Wu, M. H., Laurent, D. R. S., Chen, Y., Huang, S., Lam, K. R., and Wong, H. S. (2002). Sordarin oxazepine derivatives as potent antifungal agents. Bioorg. Med. Chem. Lett. 12, 2757–2760. doi: 10.1016/S0960-894X(02)00529-2
Shaabani, A., Hooshmand, S. E., and Tabatabaei, A. T. (2016). Synthesis of fully substituted naphthyridines: a novel domino four-component reaction in a deep eutectic solvent system based on choline chloride/urea. Tetrahedron Lett. 57, 351–353. doi: 10.1016/j.tetlet.2015.12.017
Shaabani, A., Maleki, A., Mofakham, H., and Moghimi-Rad, J. (2008a). A novel one-pot pseudo-five-component synthesis of 4,5,6,7-tetrahydro-1H-1,4-diazepine-5-carboxamide derivatives. J. Org. Chem. 73, 3925–3927. doi: 10.1021/jo8002612
Shaabani, A., Maleki, A., and Moghimi-Rad, J. (2007). A novel isocyanide-based three-component reaction: synthesis of highly substituted 1,6-dihydropyrazine-2,3-dicarbonitrile derivatives. J. Org. Chem. 72, 6309–6311. doi: 10.1021/jo0707131
Shaabani, A., Rezayan, A. H., Keshipour, S., Sarvary, A., and Ng, S. W. (2009). A novel one-pot three-(in situ five-)component condensation reaction: an unexpected approach for the synthesis of tetrahydro-2,4-dioxo-1H-benzo[b][1,5]diazepine-3-yl-2-methylpropanamide derivatives. Org. Lett. 11, 3342–3345. doi: 10.1021/ol901196z
Shaabani, A., Sarvary, A., Ghasemi, S., Rezayan, A. H., Ghadari, R., and Ng, S. W. (2011). An environmentally benign approach for the synthesis of bifunctional sulfonamide-amide compounds via isocyanide-based multicomponent reactions. Green Chem. 13, 582–585. doi: 10.1039/c0gc00442a
Shaabani, A., Soleimani, E., Rezayan, A. H., Sarvary, A., and Khavasi, H. R. (2008b). Novel isocyanide-based four-component reaction: a facile synthesis of fully substituted 3,4-dihydrocoumarin derivatives. Org. Lett. 10, 2581–2584. doi: 10.1021/ol800856e
Shaabani, S., Shaabani, A., and Ng, S. W. (2014). One-pot synthesis of coumarin-3-carboxamides containing a triazole ring via an isocyanide-based six-component reaction. ACS Comb. Sci. 16, 176–183. doi: 10.1021/co4001259
Sharma, G., Park, J. Y., and Park, M. S. (2008). Synthesis and anticonvulsant evaluation of 6-amino-1, 4-oxazepane-3, 5-dione derivatives. Arch. Pharmacal Res. 31, 838–842. doi: 10.1007/s12272-001-1235-0
Sleevi, M. C., Cale, A. D. Jr., Gero, T. W., Jaques, L. W., Welstead, W. J., Johnson, A. F., et al. (1991). Optical isomers of rocastine and close analogs: synthesis and H1 antihistaminic activity of its enantiomers and their structural relationship to the classical antihistamines. J. Med. Chem. 34, 1314–1328. doi: 10.1021/jm00108a012
Smith, L., Wong, W. C., Kiselyov, A. S., Burdzovic-Wizemann, S., Mao, Y., Xu, Y., et al. (2006). Novel tricyclic azepine derivatives: biological evaluation of pyrimido [4, 5-b]-1, 4-benzoxazepines, thiazepines, and diazepines as inhibitors of the epidermal growth factor receptor tyrosine kinase. Bioorg. Med. Chem. Lett. 16, 5102–5106. doi: 10.1016/j.bmcl.2006.07.031
Tang, Z., Liu, Z., An, Y., Jiang, R., Zhang, X., Li, C., et al. (2016). Isocyanide-based multicomponent bicyclization with substituted allenoate and isatin: synthesis of unusual spirooxindole containing [5.5]-fused heterocycle. J. Org. Chem. 81, 9158–9166. doi: 10.1021/acs.joc.6b01711
Tietze, L. F. (1996). Domino reactions in organic synthesis. Chem. Rev. 96, 115–136. doi: 10.1021/cr950027e
Tietze, L. F., and Modi, A. (2000). Multicomponent domino reactions for the synthesis of biologically active natural products and drugs. Med. Res. Rev. 20, 304–322. doi: 10.1002/1098-1128(200007)20:4<304::AID-MED3>3.0.CO;2-8
Tsaloev, A., Ilyin, A., Tkachenko, S., Ivachtchenko, A., Kravchenko, D., and Krasavin, M. (2011). Cyclic products of the Ugi reaction of aldehydo and keto carboxylic acids: chemoselective modification. Tetrahedron Lett. 52, 1800–1803. doi: 10.1016/j.tetlet.2011.02.028
Verma, N. K., Dempsey, E., Conroy, J., Olwell, P., Mcelligott, A. M., Davies, A. M., et al. (2008). A new microtubule-targeting compound PBOX-15 inhibits T-cell migration via post-translational modifications of tubulin. J. Mol. Med. 86, 457–469. doi: 10.1007/s00109-008-0312-8
Voigt, B., Linke, M., and Mahrwald, R. (2015). Multicomponent cascade reactions of unprotected carbohydrates and amino acids. Org. Lett. 17, 2606–2609. doi: 10.1021/acs.orglett.5b00887
Wattanapiromsakul, C., Forster, P. I., and Waterman, P. G. (2003). Alkaloids and limonoids from Bouchardatia neurococca: systematic significance. Phytochemistry 64, 609–615. doi: 10.1016/S0031-9422(03)00205-X
Xu, Y. (2016). Recent progress on bile acid receptor modulators for treatment of metabolic diseases. J. Med. Chem. 59, 6553–6579. doi: 10.1021/acs.jmedchem.5b00342
Yoshida, S., Aoyagi, T., Harada, S., Matsuda, N., Ikeda, T., Naganawa, H., et al. (1991). Production of 2-methyl-4 (3H)-quinazolinone, an inhibitor of poly (ADP-ribose) synthetase, by bacterium. J. Antibiot. 44, 111–112. doi: 10.7164/antibiotics.44.111
Yugandhar, D., Kuriakose, S., Nanubolu, J. B., and Srivastava, A. K. (2016). Synthesis of alkaloid-mimicking tricyclic skeletons by diastereo- and regioselective Ugi/ipso-cyclization/Aza-Michael Cascade reaction in one-pot. Org. Lett. 18, 1040–1043. doi: 10.1021/acs.orglett.6b00164
Keywords: oxazepine, quinazolinone, Ugi reaction, multicomponent reaction, isocyanide
Citation: Shaabani S, Shaabani A, Kucerakova M and Dusek M (2019) A One-Pot Synthesis of Oxazepine-Quinazolinone bis-Heterocyclic Scaffolds via Isocyanide-Based Three-Component Reactions. Front. Chem. 7:623. doi: 10.3389/fchem.2019.00623
Received: 06 April 2019; Accepted: 29 August 2019;
Published: 18 September 2019.
Edited by:
Ramon Rios, University of Southampton, United KingdomReviewed by:
Bo Jiang, Jiangsu Normal University, ChinaCopyright © 2019 Shaabani, Shaabani, Kucerakova and Dusek. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ahmad Shaabani, YS1zaGFhYmFuaUBzYnUuYWMuaXI=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.