- 1Laboratoire de Synthèse Organique, CNRS, Ecole Polytechnique, ENSTA ParisTech, UMR 7652, Université Paris-Saclay, Palaiseau, France
- 2Chemistry Department, Faculty of Science, Ain Shams University, Cairo, Egypt
We report here selective Tsuji-Trost type allylation of Ugi adducts using a strategy based on the enhanced nucleophilicity of amide dianions. Ugi adducts derived from aromatic aldehydes were easily allylated at their peptidyl position with allyl acetate in the presence of palladium catalysts. These substitutions were compared to more classical transition metal free allylations using allyl bromides.
Introduction
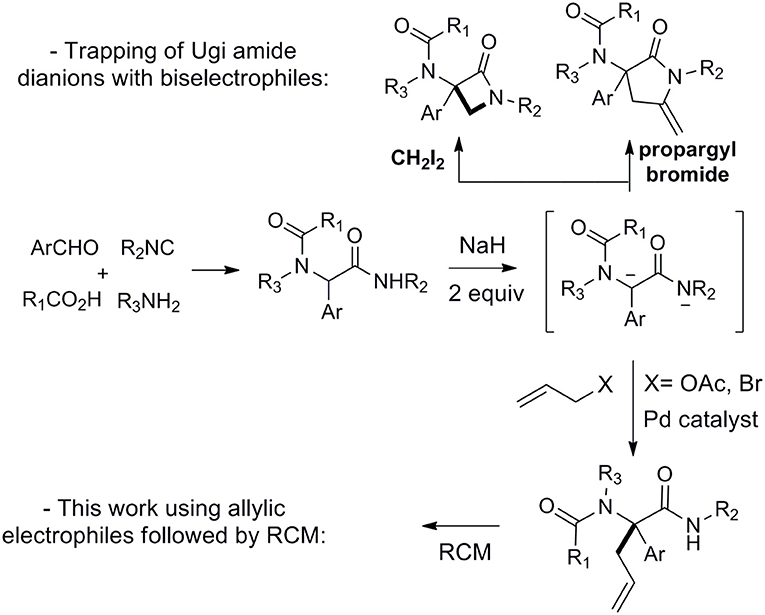
Since its discovery and even more after the 1980's, the Ugi reaction has fascinated chemists with the high diversity brought by its four components nature together with its impressive functional tolerance (Dömling and Ugi, 2000; Hulme and Gore, 2003; Orru and De Greef, 2003; Zhu and Bienaymé, 2005; Dömling, 2006; Dömling et al., 2012; Zhu et al., 2014; Boyarskiy et al., 2015; Váradi et al., 2016; Lei et al., 2018). Besides important efforts devoted to the preparation of libraries of heterocycles through cyclization of properly functionalized Ugi adducts (Tempest, 2005; Sunderhaus and Martin, 2009; Ivachtchenko et al., 2010; Orru and Ruijter, 2010a,b; Sadjadi and Heravi, 2011; Eckert, 2012; Sharma et al., 2015), a number of studies have focused on raising the diversity by letting Ugi adducts react in further intermolecular couplings (Elders et al., 2009; Brauch et al., 2010; Zarganes-Tzitzikas et al., 2015a,b; Kaur et al., 2016). In contrast with the previous intramolecular couplings, these strategies (such as the combination of MCRs) are much more sensitive to steric hindrance and require more attention in selecting the functionalities required for further couplings. For this reason, most transformations involving the peptidyl position of Ugi adducts are limited to intramolecular reactions (Bossio et al., 1997; Trifilenkov et al., 2007; Salcedo et al., 2008; El Kaïm et al., 2011; Tyagi et al., 2013; Zhang et al., 2013; Ben Abdessalem et al., 2015; Ghandi et al., 2015; Vachhani et al., 2015; Li et al., 2016). We recently proposed a dianionic amide strategy to raise the nucleophilic behavior of Ugi adducts derived from aromatic aldehydes and demonstrated the interest of this approach using bis-electrophilic derivatives prone to trap the dianions and form heterocycles (Scheme 1) (Zidan et al., 2017, 2018). Following the high yielding cyclizations observed in these studies, we decided to explore more thoroughly the synthetic potential of these dianions (Thompson, 1994; Langer and Freiberg, 2004) toward more simple electrophiles. We now wish to report further applications of this chemistry in Tsuji-Trost reactions as well as metal-free alkylations with bromide derivatives (Scheme 1).
Results and Discussion
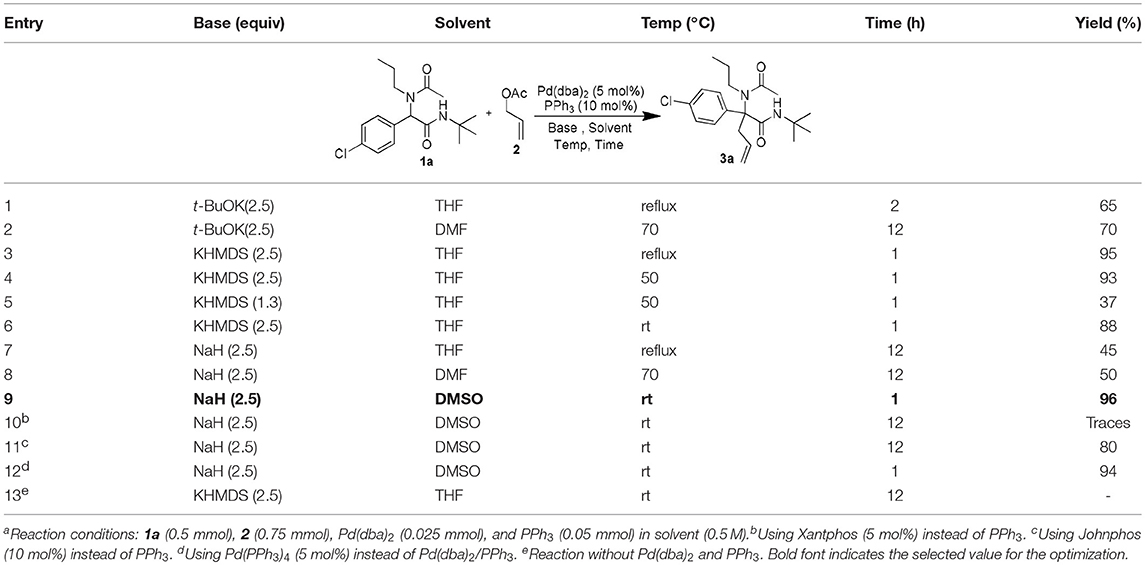
Following our interest in Tsuji-Trost reactions involving isocyanide based MCRs (Dos Santos and El Kaïm, 2014; Cordier et al., 2015; El Mamouni et al., 2016), we decided to explore the behavior of Ugi adducts dianions toward allyl acetate. Besides the efficiency and regioselectivity issues of the reaction of these dianions with π-allyl palladium complexes, such study might bring interesting pathways for further enantioselective approaches. For this study, Ugi adduct 1a was selected due to its good behavior in our previous studies with propargyl bromide and diiodomethane. It was prepared in 89% yield from 4-chloro-benzaldehyde, propylamine, acetic acid and tert-butylisocyanide (Table 1). When 1a was heated with allyl acetate in THF using 2.5 equivalents of potassium tert-butoxide together with a Pd(dba)2/PPh3 catalytical couple, we were delighted to observe a selective C-allylation of 1a giving 3a in 65% isolated yield after 2 h refluxing (entry 1, Table 1).
Different bases in various solvents were then evaluated using the same palladium/phosphine couple. The enhanced nucleophilicty of the 1,3-amide dianion toward the π-allyl palladium cationic complex was confirmed experimentally by comparing the use of 2.5 and 1.3 equiv of KHMDS (affording respectively 93 and 37% isolated yields: entries 4 and 5, Table 1). NaH in DMSO, gave the best conditions affording to our delight a nearly quantitative yield of 3a in only 1 h at room temperature (entry 9, Table 1). Further modifications of the catalyst using more complex phosphines led to longer reaction time and lower yields (entries 10–12, Table 1), while in absence of palladium source, no product was observed (entry 13, Table 1).
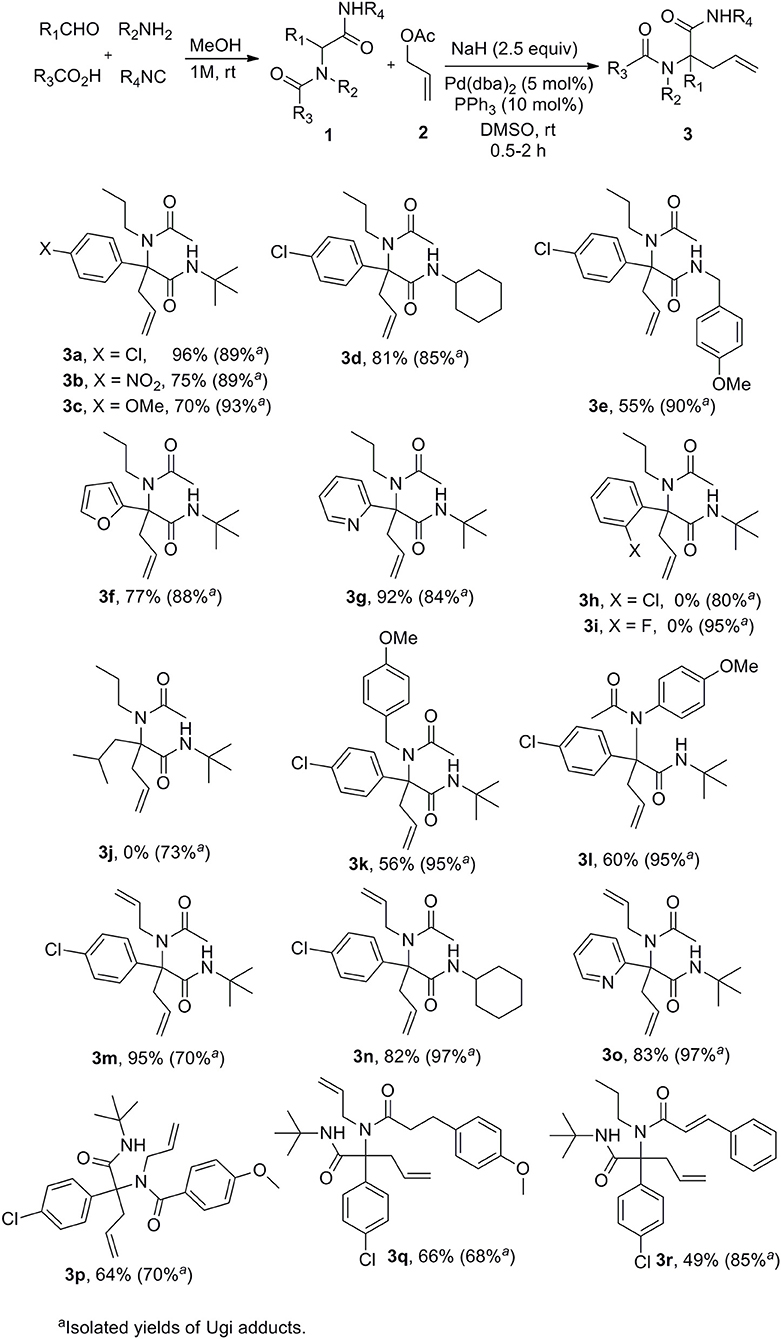
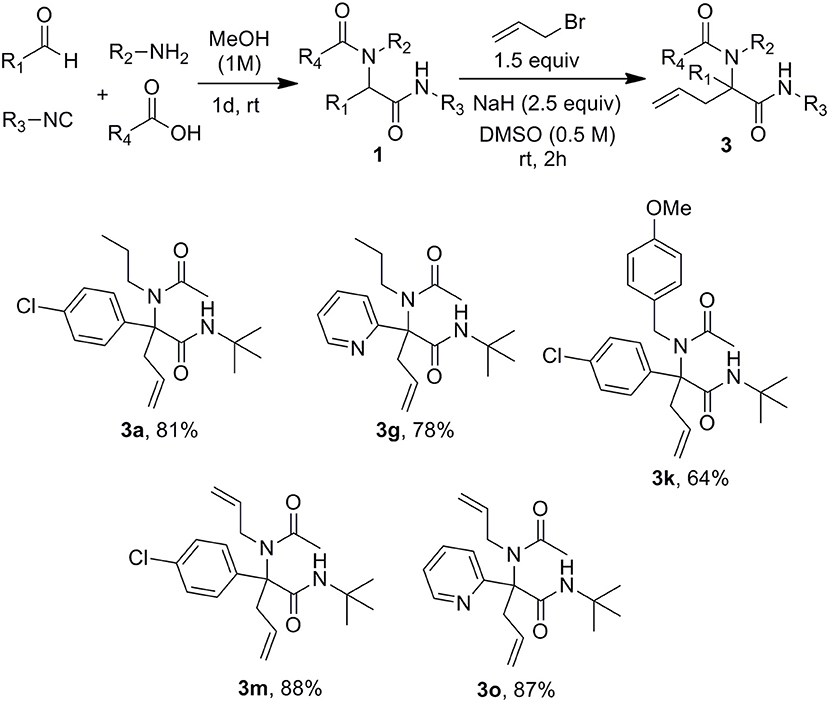
A set of Ugi adducts were then prepared in methanol and submitted to these optimized allylation conditions. The results are gathered in Scheme 2. As observed in our previous studies (Zidan et al., 2017, 2018), an aryl group tethering the peptidyl position is required for efficient allylation. Indeed, Ugi adduct 1j prepared from isovaleraldehyde failed to form the expected 3j probably due to the inability to form the dianionic intermediate with this less acidic substrate. Surprisingly, we didn't observe much correlation between yields and the electronic nature of the aldehyde as shown by the similar yields obtained with both 4-nitro or 4-methoxy substituted derivatives 3b and 3c. A much stronger effect of the substitution partner of the aromatic moiety was observed with 2-chloro substituted Ugi adduct 1h which failed to give any adduct under these conditions as observed with 1j. The same behavior was observed for the attempted synthesis of the fluoro analog 3i. This lack of reactivity can probably be explained by the steric hindrance brought by substituents at the ortho position preventing to reach the planar geometry required for benzylic anion stabilization. Initial trials on enantioselective allylations were rather deceiving. When triphenylphosphine was replaced by BINAP (5mol %) for the reaction of 1a with 2a, 3a could be formed rapidly in good yield (89%) but poor enantioselectivity (5%) whereas the use of Trost ligand (DACH-phenyl) failed to give any allylation reaction. A wider scope of chiral ligands as well as alternative solvents allowing lower temperatures will have to be evaluated to reach an enantioselective version. The reaction could not be extended to more substituted allylic acetate derivatives such as cinnamyl acetate. The latter failed to react with Ugi adduct 1a even under prolonged heating, leading only to saponification of the ester and isolation of cinnamyl alcohol.
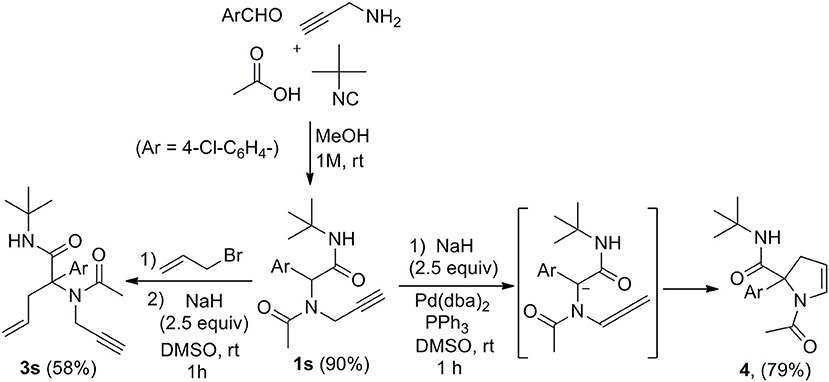
Submitting propargylamine-derived Ugi adduct 1s to the same reaction conditions didn't afford any allylated product but resulted in the clean formation of 2,3-dihydropyrrole 4 (Scheme 3), a reaction already reported by Miranda et al. (Polindara-García and Miranda, 2012). They could isolate the same dihydropyrrole 4 in 36% yield treating 1s with t-BuOK (2.5 equiv) in THF, while we could obtain 79% yield with our conditions. This selectivity was not very surprising when considering the respective intra and intermolecular properties of the two pathways. However, the ability to form enyne derivatives from Ugi adducts together with the interest of reversing a preferred selectivity were challenging enough to explore different set of conditions. Indeed, the dihydropyrrole formation probably involves a first isomerization into allene followed by further 5-endo-trig cyclization. This could leave some space for a previous intermolecular allylation if the lifetime of the dianion could be reduced by increasing the kinetic of the allylation step. Introducing the allyl acetate together with the palladium catalyst and the Ugi adduct in DMSO followed by the addition of the base resulted in a lower 54% yield of 4 together with a complex mixture of allylated products. We next explored the use of allyl bromide as a potential electrophilic species in the absence of palladium. When adding the latter to Ugi adduct 1s followed by NaH, we were delighted to observe the expected enyne 3s obtained in 58% isolated yield without any trace of dihydropyrrole (Scheme 3). This interesting control of the selectivity offers an attractive access to enyne derivatives for further cyclization studies.
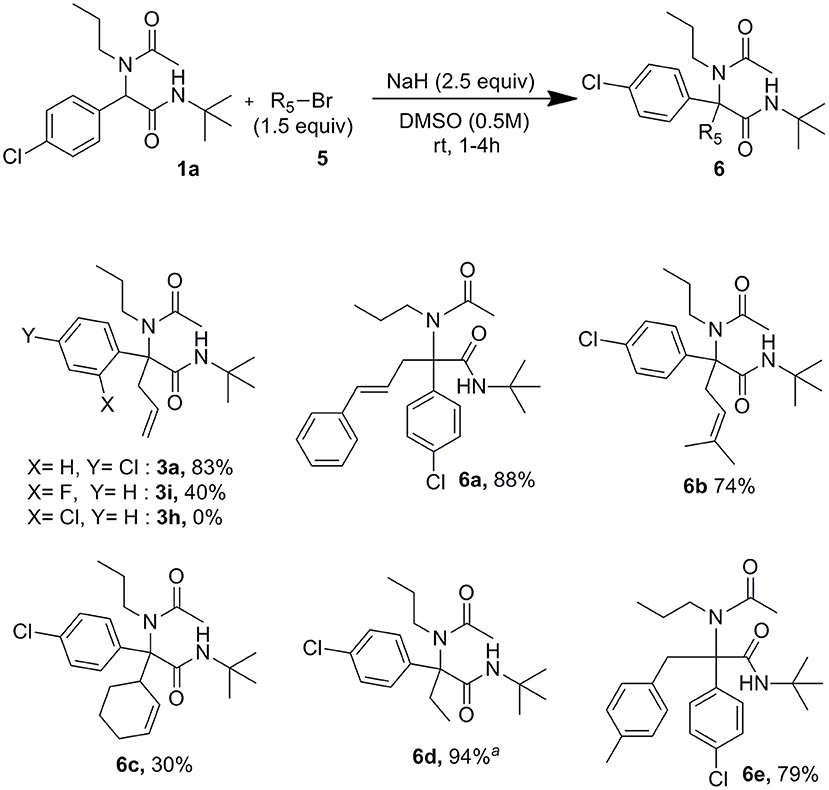
These conditions settled with allyl bromide proved useful as well for the reaction of other alkyl bromides and iodides as shown in Scheme 4. The lowest 30% yield of 6c obtained using 3-bromocyclohex-1-ene may be explained by the use of secondary halides together with the high steric hindrance around the peptidyl position of the Ugi adduct. In order to confirm further the importance of forming dianionic intermediates, the formation of 3a was attempted using allyl bromide in excess (1.5 equiv) but reducing the amount of sodium hydride to 0.9 equiv. In this case, only traces of allylated compound could be identified after 2 h at rt whereas the reaction was completed after the same time when using 2.5 equiv of sodium hydride. Interestingly, whereas under Tsuji-Trost type conditions we couldn't observe any allylation of both 2-chloro and 2-fluoro substituted derivatives 1h and 1i, the latter gave us a moderate 40% isolated yields of 3i when using just sodium hydride with allyl bromide.
A further interest of the use of palladium free conditions may be found in the ability to carry the reaction starting directly from the four Ugi components. Indeed remaining isocyanides after the Ugi reaction are potentially inhibitor for most classical palladium catalyzed processes making thus one-pot processes difficult to achieve (El Kaim et al., 2008)1. After completion of the Ugi adduct, the methanol was evaporated under reduced pressure. The solvent was then replaced by DMSO. Addition of sodium hydride followed by allyl bromide afforded the final allylated Ugi adducts in good overall yields (Scheme 5).
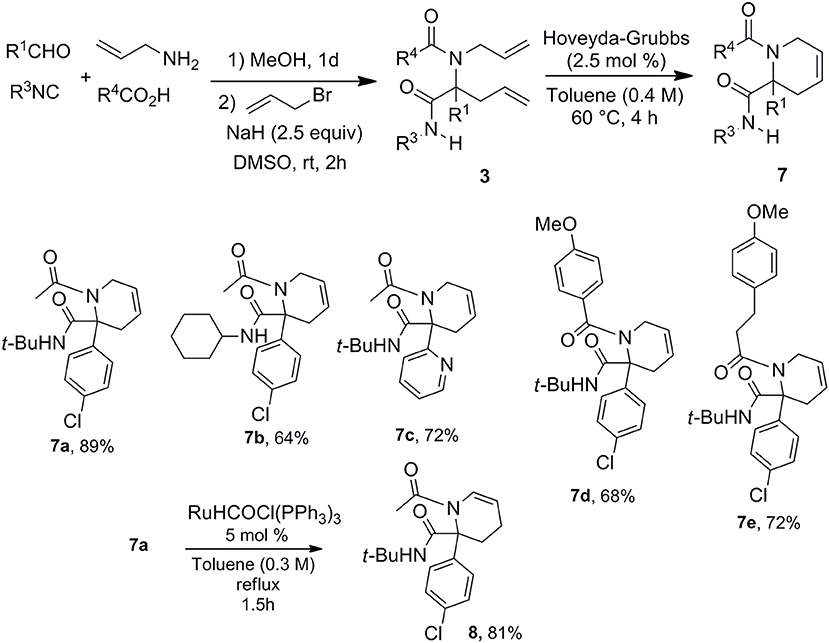
With this easy allylation of Ugi adducts in hand, we decided to take advantage of the diversity offered by the latter coupling to explore Ugi/allylation/Ring Closure Methathesis (RCM) strategies toward various nitrogen based heterocycles. The use of RCM as Ugi post-condensation has already demonstrated its power for the formation of 5, 6-membered heterocycles as well as macrocyclic derivatives (Banfi et al., 2003; Beck et al., 2003; Hebach and Kazmaier, 2003; Ribelin et al., 2007; Ku et al., 2011). However, the synthetic potential of these strategies is in a way limited by the need of introducing the two allylated moieties at an early stage which reduces the diversity offered by the two-step process. The late stage allylation we propose is highly versatile allowing to settle the strategy with a single alkenyl moiety in the starting components. Thus using allyl amine, we could easily prepare a library of piperidines with five points of diversity (Scheme 6).
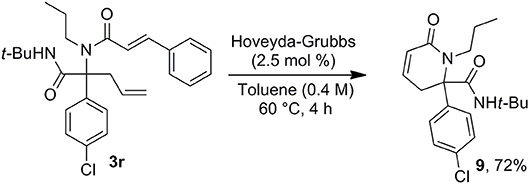
This access to 3,4-dehydropiperidine derivatives 7 could be further enriched by a potential isomerization into cyclic enamines as demonstrated by the ruthenium hydride catalyzed conversion of 7a into 8 (Scheme 6). The versatility of the method can be pictured by the alternative choice of the alkenyl moiety on the acidic component such as in cinnamic acid offering now a very simple access to 3,4-dehydropiperidine-2-one 9 (Scheme 7).
Conclusion
In summary, we have extended the potential of the Ugi reaction using 1,3-amide dianionic species as intermediates for efficient allylations at the peptidyl moiety of Ugi adducts. The procedure raises the diversity of Ugi adducts and offers unique opportunities for the preparation of nitrogen based heterocycles through association with ring closure metathesis (Supplementary Data Sheet 1). The power of the latter strategy has been demonstrated by the preparation of various piperidines which are important scaffolds for medicinal applications.
Author Contributions
AZ was responsible for designing and performing the experiments. AZ, AE-N, NA, AA, and LE discussed the evolution of the project and revised the manuscript together. LE and AA directed the project and wrote the publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We dedicate this work to our colleague Mohamed Ali Hassan who passed away few months before the end of this study. We gratefully acknowledge a Scholarship from the Egyptian Government, which was awarded to AZ.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fchem.2019.00020/full#supplementary-material
Footnotes
1. ^However, one-pot processes may be envisioned if the remaining isocyanide is destroyed by an acidic hydrolysis prior palladium addition.
References
Banfi, L., Basso, A., Guanti, G., and Riva, R. (2003). Application of tandem Ugi reaction/ring-closing metathesis in multicomponent synthesis of unsaturated nine-membered lactams. Tetrahedron Lett. 44, 7655–7658. doi: 10.1016/j.tetlet.2003.08.027
Beck, B., Larbig, G., Mejat, B., Magnin-Lachaux, M., Picard, A., Herdtweck, E., et al. (2003). Short and diverse route toward complex natural product-like macrocycles. Org. Lett. 5, 1047–1050. doi: 10.1021/ol034077e
Ben Abdessalem, A., Abderrahim, R., Agrebie, A., Dos Santos, A., El Kaïm, L., and Komesky, A. (2015). Formal [3+2] cycloaddition of Ugi adducts towards pyrrolines. Chem. Commun. 51, 1116–1119. doi: 10.1039/C4CC08282F
Bossio, R., Marcos, C. F., Marcaccini, S., and Pepino, R. (1997). Studies on isocyanides and related compounds. An unusual synthesis of functionalized succinimides. Synthesis 1997, 1389–1390. doi: 10.1055/s-1997-1367
Boyarskiy, V., Bokach, N., Luzyanin, K. V., and Kukushkin, V. (2015). Metal-mediated and Metal-catalyzed reactions of isocyanides. Chem. Rev. 115, 2698–2779. doi: 10.1021/cr500380d
Brauch, S., Gabriel, L., and Westermann, B. (2010). Seven-component reactions by sequential chemoselective Ugi–Mumm/Ugi–Smiles reactions. Chem. Commun. 46, 3387–3389. doi: 10.1039/B927388C
Cordier, M., Dos Santos, A., El Kaïm, L., and Narboni, N. (2015). Passerini/Tsuji–Trost strategies towards achieving lactams and cyclopentane derivatives. Chem. Commun. 51, 6411–6414. doi: 10.1039/C5CC00584A
Dömling, A. (2006). Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem. Rev. 106, 17–89. doi: 10.1021/cr0505728
Dömling, A., and Ugi, I. (2000). Multicomponent reactions with isocyanides. Angew. Chem. Int. Ed. 39, 3168–3210. doi: 10.1002/1521-3773(20000915)39:18<3168::AID-ANIE3168>3.0.CO;2-U
Dömling, A., Wang, K., and Wang, W. (2012). Chemistry and Biology of multicomponent reactions. Chem. Rev. 112, 3083–3135. doi: 10.1021/cr100233r
Dos Santos, A., and El Kaïm, L. (2014). Reductive passerini/tsuji–trost strategy towards β,γ-unsaturated amides. Synlett 25, 1901–1903. doi: 10.1055/s-0033-1340187
Eckert, H. (2012). Diversity oriented syntheses of conventional heterocycles by smart Multi Component Reactions (MCRs) of the last decade. Molecules 17, 1074. doi: 10.3390/molecules17011074
El Kaim, L., Gizzi, M., and Grimaud, L. (2008). New MCR–Heck–isomerization cascade toward indoles. Org. Lett. 10, 3417–3419. doi: 10.1021/ol801217a
El Kaïm, L., Grimaud, L., Le Goff, X.-F., Menes-Arzate, M., and Miranda, L. D. (2011). Straightforward four-component access to spiroindolines. Chem. Commun. 47, 8145–8147. doi: 10.1039/C1CC12236C
El Mamouni, E. H., Cattoen, M., Cordier, M., Cossy, J., Arseniyadis, S., Ilitki, H., et al. (2016). Selective Tsuji–Trost type C-allylation of hydrazones: a straightforward entry into 4,5-dihydropyrazoles. Chem. Commun. 52, 14490–14493. doi: 10.1039/C6CC08171A
Elders, N., Van Der Born, D., Hendrickx, L. J. D., Timmer, B. J. J., Krause, A., Janssen, E., et al. (2009). The efficient One-Pot reaction of up to eight components by the union of Multicomponent reactions. Angew. Chem. Int. Ed. 48, 5856–5859. doi: 10.1002/anie.200902683
Ghandi, M., Zarezadeh, N., and Abbasi, A. (2015). One-pot synthesis of spiropyrroloquinoline-isoindolinone and their aza-analogs via the Ugi-4CR/metal-free intramolecular bis-annulation process. Org. Biomol. Chem. 13, 8211–8220. doi: 10.1039/C5OB01095K
Hebach, C., and Kazmaier, U. (2003). Via Ugi reactions to conformationally fixed cyclic peptides. Chem. Commun. 596–597. doi: 10.1039/B210952B
Hulme, C., and Gore, V. (2003). Multi-component reactions: emerging chemistry in drug discovery from xylocain to crixivan. Curr. Med. Chem. 10, 51–80. doi: 10.2174/0929867033368600
Ivachtchenko, A. V., Ivanenkov, Y. A., Kysil, V. M., Krasavin, M. Y., and Ilyin, A. P. (2010). Multicomponent reactions of isocyanides in the synthesis of heterocycles. Russ. Chem. Rev. 79, 787–817. doi: 10.1070/RC2010v079n09ABEH004086
Kaur, T., Gautam, R. N., and Sharma, A. (2016). Assembly of new heterocycles through an effective use of bisaldehydes by using a sequential GBB/Ugi reaction. Chem. Asian J. 11, 2938–2945. doi: 10.1002/asia.201601009
Ku, I. W., Kang, S. B., Keum, G., and Kim, Y. (2011). Synthesis of α,β-unsaturated 2-Oxopyrrolidinyl acetamide derivatives by application of the Ugi/RCM reaction sequence. Bull. Korean Chem. Soc. 32, 3167–3170. doi: 10.5012/bkcs.2011.32.8.3167
Langer, P., and Freiberg, W. (2004). Cyclization reactions of dianions in organic synthesis. Chem. Rev. 104, 4125–4150. doi: 10.1021/cr010203l
Lei, J., Meng, J. P., Tang, D. Y., Frett, B., Chen, Z. Z., and Xu, Z. G. (2018). Recent advances in the development of polycyclic skeletons via Ugi reaction cascades. Mol. Divers. 22, 503–516. doi: 10.1007/s11030-017-9811-2
Li, Z., Kumar, A., Peshkov, A., and Van Der Eycken, E. V. (2016). A domino Ugi/Michael approach for the synthesis of α,β-unsaturated γ-lactams. Tetrahedron Lett. 57, 754–756. doi: 10.1016/j.tetlet.2016.01.014
Orru, R., and De Greef, M. (2003). Recent advances in Solution-Phase multicomponent methodology for the synthesis of heterocyclic compounds. Synthesis 10, 1471–1499. doi: 10.1055/s-2003-40507
Orru, R. V. A., and Ruijter, E. (2010a). Synthesis of Heterocycles via Multicomponent Reactions I. Berlin; Heidelberg: Springer Verlag.
Orru, R. V. A., and Ruijter, E. (2010b). Synthesis of Heterocycles via Multicomponent Reactions II. Berlin; Heidelberg: Springer Verlag.
Polindara-García, L. A., and Miranda, L. D. (2012). Two-step synthesis of 2,3-dihydropyrroles via a formal 5-endo cycloisomerization of Ugi 4-CR/propargyl adducts. Org. Lett. 14, 5408–5411. doi: 10.1021/ol3024727
Ribelin, T. P., Judd, A. S., Akritopoulou-Zanze, I., Henry, R. F., Cross, J. L., Whittern, D. N., et al. (2007). Concise construction of novel bridged bicyclic lactams by sequenced Ugi/RCM/Heck reactions. Org. Lett. 9, 5119–5122. doi: 10.1021/ol7023373
Sadjadi, S., and Heravi, M. M. (2011). Recent application of isocyanides in synthesis of heterocycles. Tetrahedron 67, 2707–2752. doi: 10.1016/j.tet.2011.01.086
Salcedo, A., Neuville, L., and Zhu, J. (2008). Palladium-catalyzed intramolecular C-arylation of benzylic carbon: synthesis of 3-benzoxazolylisoindolinones by a sequence of Ugi-4CR/postfunctionalization. J. Org. Chem. 73, 3600–3603. doi: 10.1021/jo800266y
Sharma, U. K., Sharma, N., Vachhani, D. D., and Van Der Eycken, E. V. (2015). Metal-mediated post-Ugi transformations for the construction of diverse heterocyclic scaffolds. Chem. Soc. Rev. 44, 1836–1860. doi: 10.1039/C4CS00253A
Sunderhaus, J. D., and Martin, S. F. (2009). Applications of multicomponent reactions to the synthesis of diverse heterocyclic scaffolds. Chem. Eur. J. 15, 1300–1308. doi: 10.1002/chem.200802140
Tempest, P. A. (2005). Recent advances in heterocycle generation using the efficient Ugi multiple-component condensation reaction. Curr. Opin. Drug Discov. Dev. 8, 776–788. doi: 10.1080/17518253.2012.691183
Trifilenkov, A. S., Ilyin, A. P., Kysil, V. M., Sandulenko, Y. B., and Ivachtchenko, A. V. (2007). One-pot tandem complexity-generating reaction based on Ugi four component condensation and intramolecular cyclization. Tetrahedron Lett. 48, 2563–2567. doi: 10.1016/j.tetlet.2007.02.015
Tyagi, V., Khan, S., and Chauhan, P. M. S. (2013). Facile synthesis of diverse isoindolinone derivatives via Ugi-4CR followed by Cu-catalyzed deamidative C(sp2)–C(sp3) coupling. Tetrahedron Lett. 54, 1279–1284. doi: 10.1016/j.tetlet.2012.12.091
Vachhani, D. D., Butani, H. H., Sharma, N., Bhoya, U. C., Shah, A. K., and Van Der Eycken, E. V. (2015). Domino Heck/borylation sequence towards indolinone-3-methyl boronic esters: trapping of the σ-alkylpalladium intermediate with boron. Chem. Commun. 51, 14862–14865. doi: 10.1039/C5CC05193B
Váradi, A., Palmer, T., Dardashti, R., and Majumdar, S. (2016). Isocyanide-based multicomponent reactions for the synthesis of heterocycles. Molecules 21:19. doi: 10.3390/molecules21010019
Zarganes-Tzitzikas, T., Chandgude, A. L., and Dömling, A. (2015a). Multicomponent reactions, union of MCRs and beyond. Chem. Rec. 15, 981–996. doi: 10.1002/tcr.201500201
Zarganes-Tzitzikas, T., Patil, P., Khoury, K., Herdtweck, E., and Dömling, A. (2015b). Concise synthesis of tetrazole-keto-piperazines by two consecutive Ugi reactions. Eur. J. Org. Chem. 1, 51–55. doi: 10.1002/ejoc.201403401
Zhang, L., Zhao, F., Zheng, M., Zhai, Y., and Liu, H. (2013). Rapid and selective access to three distinct sets of indole-based heterocycles from a single set of Ugi-adducts under microwave heating. Chem. Commun. 49, 2894–2896. doi: 10.1039/C3CC00111C
Zhu, J., Wang, Q., and Wang, M.-X. (2014). Multicomponent Reactions in Organic Synthesis. Weinheim: Wiley-VCH.
Zidan, A., Cordier, M., El-Naggar, A. M., Abd El-Sattar, N. E. A., Hassan, M. A., Ali, A. K., et al. (2018). Propargylation of Ugi amide dianion: an entry into pyrrolidinone and benzoindolizidine alkaloid analogues. Org. Lett. 20, 2568–2571. doi: 10.1021/acs.orglett.8b00687
Keywords: Ugi reaction, allylation, palladium, Tsuji-Trost reaction, metathesis, piperidines
Citation: Zidan A, El-Naggar AM, Abd El-Sattar NEA, Ali AK and El Kaïm L (2019) Raising the Diversity of Ugi Reactions Through Selective Alkylations and Allylations of Ugi Adducts. Front. Chem. 7:20. doi: 10.3389/fchem.2019.00020
Received: 30 October 2018; Accepted: 10 January 2019;
Published: 29 January 2019.
Edited by:
Alexander Dömling, University of Groningen, NetherlandsReviewed by:
Thomas J. J. Müller, Heinrich-Heine-Universität Düsseldorf, GermanyJavier Adrio, Universidad Autónoma de Madrid, Spain
Copyright © 2019 Zidan, El-Naggar, Abd El-Sattar, Ali and El Kaïm. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ali Khalil Ali, YWxpa2hhbGlsQHNjaS5hc3UuZWR1LmVn
Laurent El Kaïm, bGF1cmVudC5lbGthaW1AZW5zdGEtcGFyaXN0ZWNoLmZy
 Alaa Zidan
Alaa Zidan Abeer M. El-Naggar
Abeer M. El-Naggar Nour E. A. Abd El-Sattar
Nour E. A. Abd El-Sattar Ali Khalil Ali
Ali Khalil Ali Laurent El Kaïm
Laurent El Kaïm