
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Cell Dev. Biol. , 05 October 2022
Sec. Molecular and Cellular Pathology
Volume 10 - 2022 | https://doi.org/10.3389/fcell.2022.950682
This article is part of the Research Topic Dynamic and Diverse Functions of Oligodendrocytes and Myelin in the Central Nervous System View all 6 articles
Tau is abundantly expressed in neurons, however previous reports and our recent study showed tau also exist in oligodendrocytes. Also the expression levels of tau are dramatical changed in hypomyelination model rat and in demyelination region of stroke model mice. The review demonstrate microtubule and its binding partner Tau might be necessary for oligodendrocyte function based on previous reports.
Microtubules and the binding partners essentially control cellular functions during cell proliferation, differentiation, and migration in mammalian cells (Brouhard and Rice, 2018). Microtubules are polymers of alpha-tubulin/beta-tubulin subunits, and the stability and/or instability of microtubules (microtubule dynamics) are controlled by several microtubule-associated protein family proteins.
Among them, Tau is expressed mainly in neurons in the brain and has been used as a neuronal (and axonal) marker. However, its expression in oligodendrocytes has also been implicated (LoPresti et al., 1995; Bonetto et al., 2021). Recent studies unambiguously showed that tau is expressed in olig2-positive oligodendrocytes of mouse brain (Kubo et al., 2019) (Figure 1B) and in myelin basic protein (MBP)-positive cells of adult rat brain (Kanaan and Grabinski, 2020) using well-validated antibodies. These studies also confirmed that Tau does not present in NG2-positive oligodendrocyte precursor cells (OPCs), astrocytes, and microglia. Based on these findings, Tau is recognized to exists in mature oligodendrocytes as well as in neurons of central nervous system (CNS) (Figure 1). However, the physiological roles of Tau in oligodendrocytes is not well-understood, as the normal myelination observed in Tau conventional knockout mice (Takei et al., 2000) implicates that Tau is not indispensable for OPC migration, differentiation, and myelination.
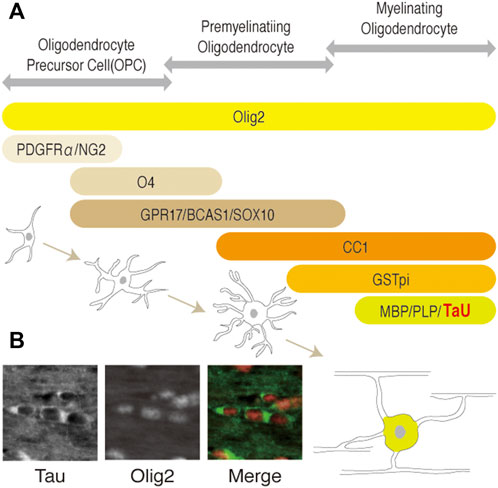
FIGURE 1. (A) Schematic representation of the developmental stages and stage-specific markers of the oligodendrocytes lineage. OPC, pre-myelinating oligodendrocytes, or myelinating oligodendrocytes (mature oligodendrocytes) are identified by each marker as shown. Olig2 is expressed in all cells of the lineage during development. O4, GPR17, BCAS1, and SOX10 (Ulloa-Navas et al., 2021) are pre-myelinating oligodendrocyte markers. Platelet-derived growth factorα: PDGFRα; neuron-glial antigen 2: NG2; Oligodendrocyte marker O4: O4; G-protein coupled receptor 17: GPR17; breast carcinoma amplified sequence 1: BCAS1; myelin basic protein: MBP; proteolipid protein 1: PLP1; kallikrein related peptidase: Klk6. (B) Representation of Tau and olig2 distribution in oligodendrocytes of adult mice brain. Cytoplasmic localization of Tau in mature oligodendrocytes of corpus callosum are shown, respectively.
In contrast, insights into the pathological roles of Tau in oligodendrocytes have emerged from a number of studies using human patient postmortem tissues and animal models. Aggregation of the phosphorylated tau, that is detected with the anti-AT8 antibody, is observed in astrocytes as well as in oligodendrocytes in the brains of patients with globular glial tauopathy (GGT) (Ahmed et al., 2013). Also, rats harboring mutation in the Tubulin beta 4a gene (Tubb4a), which exhibit hypo- and/or de-myelination, also show elevated Tau expression in oligodendrocytes (in culture) (Song et al., 1999). The Tubb4a mutation has been shown to affect microtubule dynamics such as its elongation, length, duration, and the frequency of them in oligodendrocytes of dystonia or hypomyelination with atrophy of the basal ganglia and cerebellum (H-ABC) (Figure 2B (Krajka et al., 2022). Similarly, a Tubb4a mutagenesis result in abnormal myelination and microtubule accumulation in oligodendrocytes (not in axon of neurons) (Duncan et al., 1992) (Figure 2A). Tau isoform with three repeats of the microtubule-binding motif (3R-Tau) has also been shown to be up-regulated or accumulated in damaged area (demyelination lesion) of stroke model mice (Villa González et al., 2020) (Figure 2C). Similarly, a FTDP-17 mutation, delK280, in the tau gene enhances the expression of 3R-Tau and also is associated with tau inclusions in oligodendrocytes (van Swieten et al., 2007).
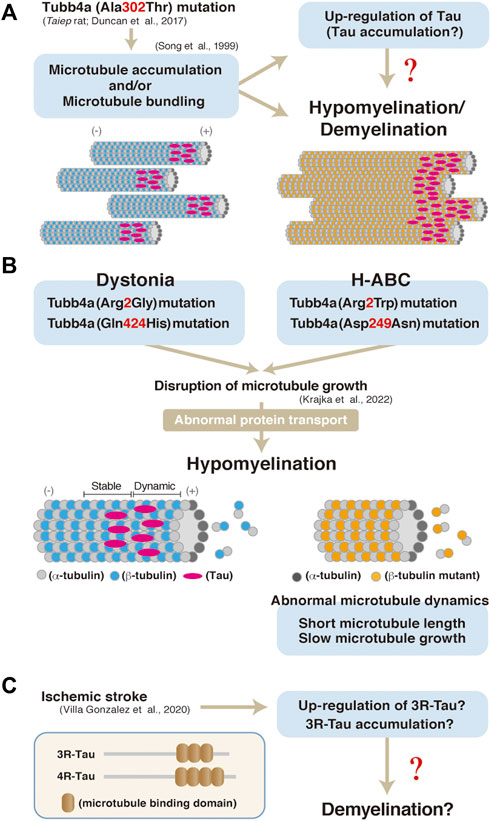
FIGURE 2. (A) A model of hypomyelination and/or demyelination through Tubb4a and possibly Tau in Taubb4a mutant mice. (B) A possibly molecular pathogenesis through Tubb4a mutation in oligodendrocytes (Krajka et al., 2022). (C) Up-regulation or accumulation of 3R-Tau in ischemic stroke model mice and a possible model of pathogenesis of demyelination through Tau in the mice. Representation of 3R and 4R Tau isoform structure (Villa González et al., 2020).
These findings implicate that 1) microtubule-stability and dynamics, and 2) abnormal expression, phosphorylation, and aggregation of Tau are associated with pathological dysfunction of oligodendrocytes. Tau might be needed to compensate the dysfunctions of microtubules in oligodendrocytes, or excess tau possibly induces hypomyelination and/or demyelination. Therefore, studying Tau in various models of oligodendrocyte disorders would benefit the understanding of the pathophysiology, which might identify tau as a new therapeutic target for these diseases, and also may provide insights into the physiological roles of Tau in oligodendrocytes.
TT and HM wrote the manuscript and drew figure. TM provided scientific content of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ahmed, Z., Bigio, E. H., Budka, H., Dickson, D. W., Ferrer, I., Ghetti, B., et al. (2013). Globular glial tauopathies (GGT): Consensus recommendations. Acta Neuropathol. 126, 537–544. doi:10.1007/s00401-013-1171-0
Bonetto, G., Belin, D., and Káradóttir, R. T. (2021). Myelin: A gatekeeper of activity-dependent circuit plasticity? Science 374, eaba6905. doi:10.1126/science.aba6905
Brouhard, G. J., and Rice, L. M. (2018). Microtubule dynamics: An interplay of biochemistry and mechanics. Nat. Rev. Mol. Cell Biol. 19, 451–463. doi:10.1038/s41580-018-0009-y
Duncan, I. D., Lunn, K. F., Holmgren, B., Urba-Holmgren, R., and Brignolo-Holmes, L. (1992). The taiep rat: A myelin mutant with an associated oligodendrocyte microtubular defect. J. Neurocytol. 21, 870–884. doi:10.1007/BF01191684
Kanaan, N. M., and Grabinski, T. (2021). Neuronal and glial distribution of tau protein in the adult rat and monkey. Front. Mol. Neurosci. 14, 607303. doi:10.3389/fnmol.2021.607303
Krajka, V., Vulinovic, F., Genova, M., Tanzer, K., Jijumon, A. S., Bodakuntla, S., et al. (2022). H-ABC- and dystonia-casing TUBB4A mutations show distinct pathogenis effects. Sci. Adv. 8, eabj9229. doi:10.1126/sciadv.abj9229
Kubo, A., Misonou, H., Matsuyama, M., Nomori, A., Wada-Kakuda, S., Takashima, A., et al. (2019). Distribution of endogenous normal tau in the mouse brain. J. Comp. Neurol. 527, 985–998. doi:10.1002/cne.24577
LoPresti, P., Szuchet, S., Papasozomenos, S. C., Zinkowski, R. P., and Binder, L. I. (1995). Functional implications for the microtubule-associated protein tau: Localization in oligodendrocytes. Proc. Natl. Acad. Sci. U. S. A. 92, 10369–10373. doi:10.1073/pnas.92.22.10369
Song, J., Oconnor, L. T., Yu, W., Baas, P. W., and Duncan, I. D. (1999). Microtubule alterations in cultured taiep rat oligodendrocytes lead to deficits in myelin membrane formation. J. Neurocytol. 28, 671–683. doi:10.1023/a:1007060832459
Takei, Y., Teng, J., Harada, A., and Hirokawa, N. (2000). Defects in axonal elongation and neuronal migration in mice with disrupted tau and map1b genes. J. Cell Biol. 150, 989–1000. doi:10.1083/jcb.150.5.989
Ulloa-Navas, M. J., Pérez-Borredá, P., Morales-Gallel, R., Saurí-Tamarit, A., García-Tárraga, P., Gutiérrez-Martín, A. J., et al. (2021). Ultrastructural characterization of human oligodendrocytes and their progenitor cells by pre-embedding immunogold. Front. Neuroanat. 15, 696376. doi:10.3389/fnana.2021.696376
van Swieten, J. C., Bronner, I. F., Azmani, A., Severijnen, L. A., Kamphorst, W., Ravid, R., et al. (2007). The DeltaK280 mutation in MAP tau favors exon 10 skipping in vivo. J. Neuropathol. Exp. Neurol. 66, 17–25. doi:10.1097/nen.0b013e31802c39a4
Keywords: tau, myelin, oligodendrocyte, myelination, demyelination
Citation: Torii T, Miyasaka T and Misonou H (2022) The organization of microtubules and Tau in oligodendrocytes: Tau pathology in damaged oligodendrocytes. Front. Cell Dev. Biol. 10:950682. doi: 10.3389/fcell.2022.950682
Received: 23 May 2022; Accepted: 05 September 2022;
Published: 05 October 2022.
Edited by:
Nobuharu Suzuki, Tokyo Medical and Dental University, JapanReviewed by:
Hahn Young Kim, Konkuk University Medical Center, South KoreaCopyright © 2022 Torii, Miyasaka and Misonou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tomohiro Torii, dHRvcmlpQG1haWwuZG9zaGlzaGEuYWMuanA=; Hiroaki Misonou, aG1pc29ub0BtYWlsLmRvc2hpc2hhLmFjLmpw
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.