
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Bioeng. Biotechnol. , 04 March 2025
Sec. Biomechanics
Volume 13 - 2025 | https://doi.org/10.3389/fbioe.2025.1529545
This article is part of the Research Topic Biomechanical and Biomaterial Advances in Degenerative Diseases of Bone and Joint View all 16 articles
 Shuai Zhou1,2,3,4†
Shuai Zhou1,2,3,4† Zexiang Liu1,2,3†
Zexiang Liu1,2,3† Haoge Huang1,2,3†
Haoge Huang1,2,3† Hanxu Xi5
Hanxu Xi5 Xiao Fan1,2,3
Xiao Fan1,2,3 Yanbin Zhao1,2,3
Yanbin Zhao1,2,3 Xin Chen1,2,3
Xin Chen1,2,3 Yinze Diao1,2,3
Yinze Diao1,2,3 Yu Sun1,2,3
Yu Sun1,2,3 Hong Ji5*
Hong Ji5* Feifei Zhou1,2,3*
Feifei Zhou1,2,3*Introduction: This study aimed to develop machine learning models to predict neurological outcomes in patients with degenerative cervical myelopathy (DCM) after surgical decompression and identify key factors that contribute to a better outcome, providing a reference for patient consultation and surgical decision-making.
Methods: This retrospective study reviewed 1,895 patients who underwent cervical decompression surgery for DCM at Peking University Third Hospital from 2011 to 2020, with 672 patients included in the final analysis. Five machine learning methods, namely, linear regression (LR), support vector machines (SVM), random forest (RF), XGBoost, and Light Gradient Boosting Machine (LightGBM), were used to predict whether patients achieved the minimal clinically important difference (MCID) in the improvement in the Japanese Orthopedic Association (JOA) score, which was based on basic information, symptoms, physical examination signs, intramedullary high signals on T2-weighted (T2WI) magnetic resonance imaging (MRI), and various scale scores. After training and optimizing multiple ML algorithms, we generated a model with the highest area under the receiver operating characteristic curve (AUROC) to predict short-term outcomes following DCM surgery. We evaluated the importance of the features and created a feature-reduced model. The model’s performance was assessed using an external dataset.
Results: The LightGBM algorithm performed the best in predicting short-term neurological outcomes in the testing dataset, achieving an AUROC value of 0.745 and an area under the precision–recall curve (AUPRC) value of 0.810. The important features influencing performance in the short-term model included the preoperative JOA score, age, SF-36-GH, SF-36-BP, and SF-36-PF. The feature-reduced LightGBM model, which achieved an AUROC value of 0.734, also showed favorable performance. Moreover, the feature-reduced model showed an AUROC value of 0.785 for predicting the MCID of postoperative JOA in the external dataset, which included 58 patients from other hospitals.
Conclusion: We developed models based on machine learning to predict postoperative neurological outcomes. The LightGBM model presented the best predictive power regarding the surgical outcomes of DCM patients. Feature importance analysis revealed that variables, including age, preoperative JOA score, SF-36-PF, SF-36-GH, and SF-36-BP, were essential factors in the model. The feature-reduced LightGBM model, designed for ease of application, achieved nearly the same predictive power with fewer variables.
Degenerative cervical myelopathy (DCM) is a progressive, non-traumatic degenerative disease that leads to the compression of the cervical spinal cord (Fehlings et al., 2013), resulting in the loss of manual dexterity; gait and balance disturbances; sensory loss in the hands or feet; arm or hand weakness; and defecatory or urinary frequency, urgency, or hesitancy (Okada et al., 2009). With the aging society, DCM has become an urgent clinical and public health concern (GBD, 2017 Disease and Injury Incidence and Prevalence Collaborators et al., 2018).
For moderate and severe DCM, as well as for patients with progressive disease, surgical decompression is recommended; it has been proved to improve neurological function and quality of life for patients. However, studies indicate that 5%–30% of patients did not achieve a satisfactory outcome after surgery (Fejer et al., 2006). In previous studies, the factors affecting surgical outcomes included age, baseline severity score, duration of preoperative symptoms, signs and symptoms, comorbidities, and high signal intensity (SI) on T2-weighted (T2-WI) magnetic resonance imaging (MRI) (Gembruch et al., 2021; Tetreault L. A. et al., 2015). However, predicting outcomes for individual patients is a multivariable and chaotic system of interactions. The influence of various variables and their interactions on postoperative outcomes cannot be accurately assessed by traditional statistical methods (Combi, 2017). The significance of different features in predicting surgical outcomes for patients with DCM remains undetermined (Tetreault et al., 2013; Tetreault et al., 2014; Tetreault et al., 2016).
Machine learning is a developing method that can be applied to clinical datasets for the purpose of developing robust risk models and redefining patient classes (Shah et al., 2023). Previous studies have used machine learning methods to establish models to predict postoperative neurological function in patients with better predictive power than traditional statistical models. These models have achieved good performance, with area under the receiver operating characteristic curve values (AUROC) ranging from 0.7 to 0.9 (Khan et al., 2021; Merali et al., 2019; Song et al., 2024). However, a large-scale study and prospective external validation are still lacking. A tradeoff exists in machine learning between model complexity and generalizability to new datasets. One solution is to build a model with fewer features and appropriate performance (Shah et al., 2023).
In this study, we aimed to develop machine learning models to predict individual DCM patients’ neurological outcomes after surgery and select the model with the best performance. Additionally, we aimed to identify influential features and create a model with fewer features and good performance in external validation, which would benefit the clinical practice of spine surgeons.
This study was approved by our hospital’s Medical Research Ethics Committee (LM2021299). This retrospective study reviewed 1,895 patients who underwent DCM surgical decompression, including laminoplasty, laminectomy and fusion, anterior cervical decompression and fusion, and anterior cervical corpectomy and fusion from 2011 to 2020 at Peking University Third Hospital. The data were obtained from the Electronic Data Capture (EDC) system at the Peking University Third Hospital Information Center. All private information was masked.
The inclusion criteria were as follows: 1) age ≥18; 2) diagnosed with DCM; 3) underwent surgical decompression for DCM including laminoplasty, laminectomy and fusion, anterior cervical decompression and fusion, and anterior cervical corpectomy and fusion; and 4) had at least one follow-up record between 3 and 6-month follow-up. Exclusion criteria included a history of spinal tumor, active infection, rheumatoid arthritis, cervical trauma, ankylosing spondylitis, and previous cervical spine surgery. Patients with missing scale scores and invalid follow-up records were also excluded. Patients with JOA scores ≥15 were excluded to avoid a ceiling effect as they could not achieve the minimal clinically important difference (MCID). We standardized surgical indications and approach selection based on guidelines and our cervical spine professional group’s recommendations. A total of 672 patients were included in the final analysis.
The baseline data for the training models included demographics (age, sex, and profession), personal history (number of comorbidities, history of tobacco and alcohol intake, etc.), symptoms, signs, imaging examination (intramedullary high signals on T2WI MRI), and preoperative scale scores (JOA and SF-36). We applied a natural language processing (NLP) algorithm to extract symptom data from unstructured electronic medical records. Personal history, symptoms, and signs recorded as binary variables were selected based on the standardized medical records for orthopedics.
We examined patients’ JOA scores 3–6 months after surgery to evaluate neurological outcomes. The primary outcome was whether patients achieved MCID in JOA scores. According to previous research, the MCID for the JOA score in DCM patients was 2.5 (Maki et al., 2021). Categorical features such as professions were transferred to one-hot coding.
In the baseline dataset, all the demographic data were valid. Based on word segmentation of symptoms, signs, diseases, and other information using natural language processing technology, synonym information of the standard terminology database is introduced for standardization and level normalization. By “document category prediction” and “chapter prediction in the document,” different medical records and chapter contents are distinguished. Based on this, a combined approach using a bidirectional long short-term memory conditional random field (BiLSTM-CRF) network along with rule matching was employed to extract clinical data information. The NLP algorithm would leave blanks in the dataset if there were no matched descriptions in electronic medical history records. Therefore, we filled all the missing data in binary features (personal history, symptoms, and signs) with “normal” or “negative.”
We randomly split the dataset into a training set (70%) and a test set (30%). Five machine learning algorithms, namely, linear regression (LR), support vector machine (SVM), random forest (RF), extreme gradient boosting (XGBoost), and light gradient boosting machine (LightGBM), were trained using 3-fold cross-validation on the training set with default hyperparameters. Model performance was evaluated using five metrics, namely, balanced accuracy, weighted precision, weighted recall, weighted area under the precision–recall curve (AUPRC, Supplementary Document), and area under the receiver operating characteristic curve (AUROC). Based on these metrics, we selected the optimal model for further refinement. We employed a bootstrap approach to obtain robust estimates and compute confidence intervals (CIs). Specifically, we repeated the cross-validation procedure 1,000 times with varied splits of the training data. The 95% CI for each metric was approximated using the 2.5th and 97.5th percentiles of the resulting performance distributions.
Hyperparameter optimization was performed on the selected model using a grid search algorithm. We first predefined a set of candidate values for each hyperparameter and then exhaustively evaluated all combinations via 3-fold cross-validation on the training set. The optimal hyperparameter set was chosen based on the highest AUROC.
Using the five aforementioned metrics, the fine-tuned model was subsequently evaluated on the test set. To calculate CIs, we again applied the bootstrap method by randomly splitting the dataset 1,000 times and retraining the model (with fixed best hyperparameters) on each new training split. The resulting performance distributions were used to derive the 95% CIs (2.5th and 97.5th percentiles).
All machine learning models were implemented in Python 3.8.5 using the scikit-learn, XGBoost, and LightGBM library. We adhered to the transparent reporting of multivariable prediction models for individual prognosis or diagnosis (TRIPOD) guidelines throughout model development.
The machine learning module used to train the model offers built-in methods for assessing feature importance. For linear models (LR and SVM), importance is determined by the absolute value of the learned coefficients. For tree-based models (RF, XGBoost, and LightGBM), importance is calculated based on the frequency of each feature used for splitting nodes across all trees. Additionally, the Shapley additive explanation (SHAP) value of a feature is calculated by determining its marginal contribution across all possible feature subsets, and these contributions are then averaged to yield the feature’s overall impact on the prediction.
To improve the model’s compatibility across different healthcare systems, we aimed to reduce the number of features. According to the importance of features in the final model and based on clinical experience, we selected a subset of features to build a feature-reduced machine learning model. We then extracted the relevant feature data from the previous training and testing sets to train this model. The outcome measures remained consistent with those aforementioned.
Another dataset containing 31 patients from West China Hospital and 27 patients from Shanghai Changzheng Hospital was used as the external validation set. The dataset includes patients’ demographics, JOA scores, and SF-36 scores, which were used to validate the feature-reduced model. We analyzed AUROC values and decision curve analysis (DCA) to evaluate the performance of the feature-reduced model in the external dataset.
Of the 1,895 patients, 298 were excluded because they were diagnosed with cervical spondylotic radiculopathy or cervical spondylosis with sympathetic symptoms; 143 patients were excluded due to previous cervical spine surgery history; 498 patients’ records were invalid or missing; and 284 patients had preoperative JOA scores ≥15. Finally, a dataset with 672 patients and 63 features was generated (Figure 1). The baseline characteristics are shown in Table 1. In addition, 369 patients reached the MCID of JOA score improvement at the short-term follow-up postoperatively.
Based on the training set, we generated five models (LR, SVM, RF, XGBoost, and LightGBM). The ROC curves and AUROC values produced by the three-fold cross-validation algorithms are presented in Figure 2. Figure 3 presents the comparisons of the balanced accuracy, weighted precision, weighted recall, AUPRC, and AUROC values in the training set. The decision tree algorithms (RF, XGBoost, and LightGBM) demonstrated superior performance among the five selected machine learning models. The LightGBM model achieved the highest AUROC value of 0.745. We used the testing set to evaluate the five trained models. The AUROC values of the training and testing sets are summarized in Table 2. The LightGBM model demonstrated the best performance in training and testing sets, with a slight decrease in the AUROC value, indicating no overfitting (Figure 4).
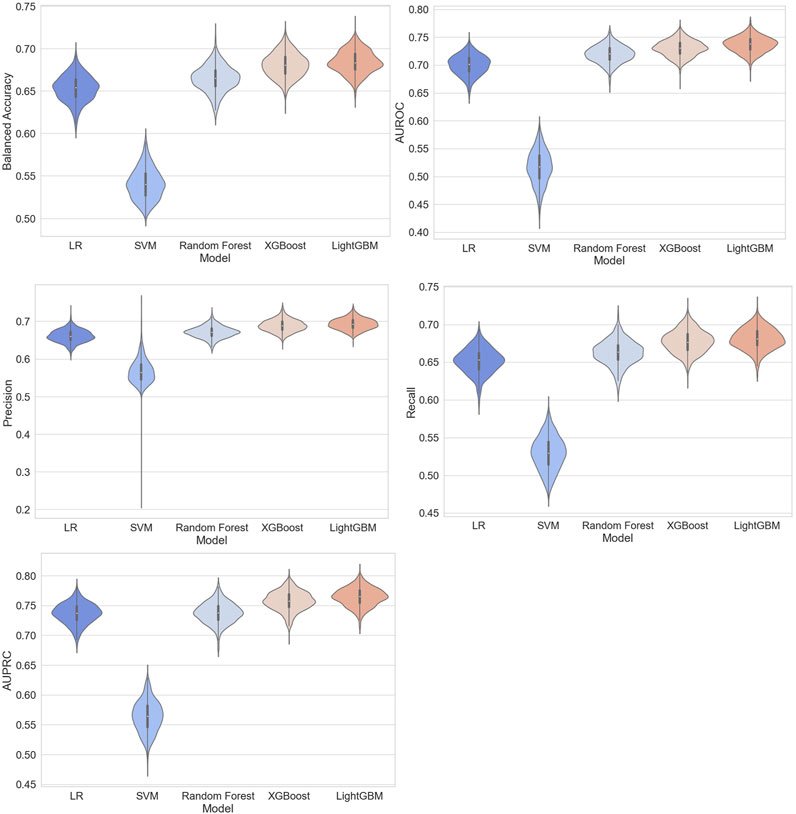
Figure 3. Comparisons of balanced accuracy, weighted area under the precision–recall curve (AUPRC), weighted precision, weighted recall, and AUPRC of the five models in the training set.
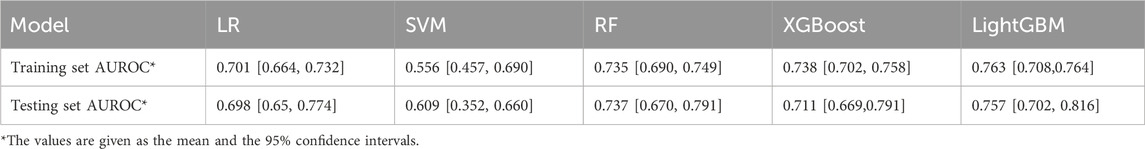
Table 2. AUROC values were generated by 3-fold cross-validation algorithms in the training and testing sets.
The grid search strategy was used to optimize the hyperparameters of LightGBM. We tuned six hyperparameters, including “max_depth,” “num_leaves,” “subsample,” “colsample_bytree,” “reg_alpha,” and “reg_lambda.” The search spaces and best values are provided in Table 3. The AUROC value of the tuned LightGBM model increased to 0.763 in the training set (Figure 5).
We evaluated the feature importance of the LightGBM model. Preoperative JOA scores, SF-36-BP, age, SF-36-SF, SF-36-PF, SF-36-MH, body pain, and SF-36-GH are the eight most critical predictors for outcome prediction (Figure 6). We selected the top 10 features based on feature importance, and the SHAP values were calculated to evaluate the importance of features at the individual level (Figure 7). A single-sample SHAP force plot for a patient is shown in Figure 8.
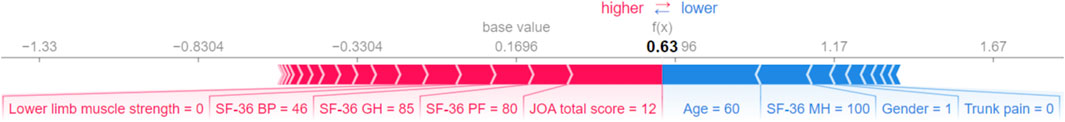
Figure 8. Single-sample SHAP force plot. Blue indicates that the feature has a negative effect on the prediction (arrow to the left, SHAP value decreases), and red indicates that the feature has a positive effect on the prediction (arrow to the right, SHAP value increases). On the number line, 0.1696 is the base SHAP value, which is the average predicted by the model. In this sample, the total JOA score = 12 had the most significant positive effect and age = 60 years had the most significant negative effect, resulting in a SHAP value of 0.63 for this patient.
According to the feature importance and the accessibility of input data, we chose age, sex, preoperative JOA scores, and preoperative SF-36 scores as features to build the feature-reduced model. We applied the same training set to construct the feature-reduced model. Then, the grid-search algorithm was used to optimize the hyperparameter with the same search spaces. The best value is presented in Table 4. The feature-reduced model was also tested with the same dataset. The ROC curve in the testing set is shown in Figure 9. The feature-reduced LightGBM model had an AUROC value of 0.734, which showed almost the same performance as the initial model (Figure 9).
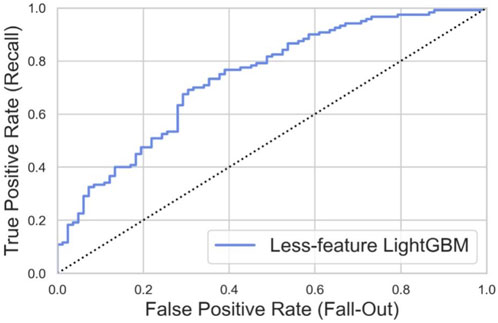
Figure 9. ROC curve of the feature-reduced model in the testing set with an area under curve (AUROC) value of 0.734.
The external dataset included 31 patients from West China Hospital and 27 patients from Shanghai Changzheng Hospital. To generate the external testing set, we extracted the data according to the reduced features (age, sex, preoperative JOA scores, and preoperative SF-36 scores). The outcome measure was whether patients achieved the MCID of the JOA score at 3–6 months after the DCM decompression surgery. The ROC curve is presented in Figure 10, with an AUROC value of 0.785. This indicated that our model also performed well in extrapolation.
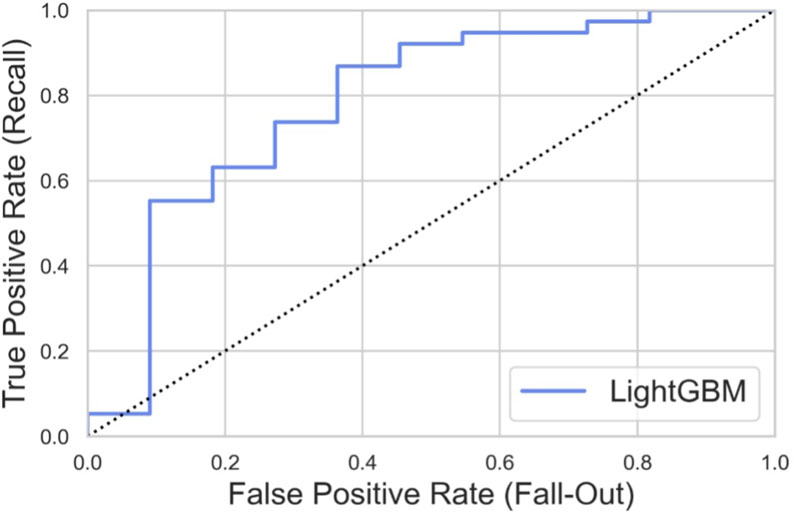
Figure 10. ROC curve of the feature-reduced model in the external dataset with an area under curve (AUROC) value of 0.785.
Surgical decompression is recommended for the treatment of DCM and has shown long-term improvement in patients. However, the degree of patient recovery can vary widely (Gulati et al., 2021). In previous studies, several machine learning models have been developed to predict improvements in neurological function using the preoperative clinical characteristics of patients with DCM (Khan et al., 2021; Merali et al., 2019; Song et al., 2024). In this study, we applied a natural language processing algorithm to extract symptom data from unstructured medical history records, which could include more features than previous studies. We compared the performance of each model and identified that the LightGBM model demonstrated the best predictive performance. To the best of our knowledge, we were the first to build a feature-reduced model based on LightGBM to predict surgery outcomes for DCM patients, which showed good performance.
We built machine learning models using five classic algorithms. The decision tree algorithms (RF, XGBoost, and LightGBM) showed better performance with an AUROC value of more than 0.7, and the LightGBM model showed the best performance. Decision tree algorithms correlate features with outcomes and have been effectively utilized across various learning disciplines. At each node or branch point, training examples are partitioned based on the value of a particular feature (Shah et al., 2023). Maki et al. reported that XGBoost showed the highest AUROC value (0.72) for predicting the MCID of the JOA score in patients with cervical ossification of the posterior longitudinal ligament (OPLL) 1 year after surgical treatment, whereas RF demonstrated the highest AUROC value (0.75) for predicting MCID at 2 years (Maki et al., 2021). Merali et al. (2019) reported that the best-performing predictive model used a random forest structure with an average area under the curve (AUROC) value of 0.70 to predict postoperative MCID of SF-6D and mJOA score in DCM patients at 6-, 12-, and 24-month follow-up. Our results showed that decision tree algorithms have a good predictive ability for surgical outcomes in DCM patients, which was consistent with prior studies.
The feature importance of the LightGBM model was calculated, and the preoperative JOA scores, SF-36-BP, age, SF-36-SF, SF-36-PF, SF-36-MH, body pain, and SF-36-GH are the top eight most crucial predictors for the prediction of neurological outcomes. Previous studies have demonstrated that preoperative clinical features of patients with DCM were associated with neurological function improvement after surgery. It was reported that gender, age, and preoperative functional scores were related to the surgical prognosis of patients with DCM (Tetreault et al., 2018; Nakashima et al., 2012). We found that body pain correlated to the trunk sensation and was a crucial predictor of surgery outcome. Previous studies showed that positive pathologic signs of lower limbs, finger numbness, and hyperreflexia are associated with unsatisfactory improvement of nerve function measured by JOA after surgery (Tetreault et al., 2013; Tetreault et al., 2016; Tetreault L. et al., 2015). The body pain could also be a sign of more serious neurological impairment and predict worse improvement.
We demonstrated that patients with lower preoperative JOA scores were more likely to achieve MCID in their recovery of neurological function at 3–6 months after surgery. This may be because higher preoperative JOA scores have less room for improvement due to ceiling effects, similar to previous studies for the prediction of MCID (Khan et al., 2021; Maki et al., 2021). Patients with lower preoperative JOA scores could be more likely to benefit from surgery. This insight is crucial for understanding the prognosis of DCM patients after surgery.
We built a feature-reduced model and showed that the feature-reduced model could get the same prediction accuracy by only paying attention to age, sex, preoperative JOA scores, and preoperative SF-36 scores. As far as we know, this is the first study to build a feature-reduced machine learning model to predict DCM patient outcomes after surgical treatment. However, the feature-reduced LightGBM model showed slightly worse performance in the testing set, which might be attributed to fewer features. Reducing the number of features could enhance the model’s generalizability. In external validation using an independent patient cohort from two hospitals, our model performed well with an AUROC value of 0.785. This indicated that developing a feature-reduced model could be an effective strategy for the generalization of the prediction model of DCM patient outcomes.
Our study still has some limitations. First, our follow-up data are limited to the short-term period of 3–6 months. Second, preoperative clinical variables only include intramedullary high signals on T2WI MRI. More preoperative imaging parameters of patients with DCM may improve the predictive efficacy of the model (Naruse et al., 2009; Hamburger et al., 1997). Third, the amount of data we have is not particularly large. Although machine learning offers a powerful way to build complex models and generate predictions, compared to traditional statistical methods, machine learning models require relatively large datasets to achieve optimal performance.
We established models to predict postoperative neurological outcomes based on machine learning. The LightGBM model presented the best predictive power with an AUROC value of 0.745. Feature importance analysis showed that age, preoperative JOA score, SF-36-PF, SF-36-GH, and SF-36-BP were crucial factors for prediction. The feature-reduced model could achieve almost the same prediction accuracy by only paying attention to age, sex, preoperative JOA scores, and preoperative SF-36 scores, making it more practical for clinical application. Machine learning could help spine surgeons make more precise predictions of DCM patient outcomes after cervical decompression surgery.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Peking University Third Hospital Medical Science Research Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because this project is a retrospective study, only involving data collection and analysis, and will not endanger the safety of patients. We will restrict access to patients’ data at work, shield patients’ personal information when sorting data, and protect patients’ personal privacy when publishing articles.
SZ: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, software, validation, writing–original draft, and writing–review and editing. ZL: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, validation, writing–review and editing, and writing–original draft. HH: data curation, formal analysis, investigation, methodology, software, validation, writing–original draft, and writing–review and editing. HX: data curation, formal analysis, software, validation, methodology, and writing–review and editing. XF: data curation, funding acquisition, investigation, methodology, resources, and writing–review and editing. YZ: data curation, funding acquisition, resources, and writing–review and editing. XC: conceptualization, funding acquisition, resources, and writing–review and editing. YD: conceptualization, funding acquisition, resources, and writing–review and editing. YS: conceptualization, data curation, funding acquisition, resources, and writing–review and editing. HJ: writing–review and editing, writing–original draft, data curation, formal analysis, methodology, project administration, resources, supervision, investigation, conceptualization, and validation. FZ: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, software, supervision, validation, visualization, and writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was funded by Peking University Third Hospital (BYSYZD2021007).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbioe.2025.1529545/full#supplementary-material
Combi, C. (2017). Editorial from the new editor-in-chief: artificial intelligence in medicine and the forthcoming challenges. Artif. Intell. Med. 76, 37–39. doi:10.1016/j.artmed.2017.01.003
Fehlings, M. G., Wilson, J. R., Kopjar, B., Yoon, S. T., Arnold, P. M., Massicotte, E. M., et al. (2013). Efficacy and safety of surgical decompression in patients with cervical spondylotic myelopathy: results of the AOSpine North America prospective multi-center study. J. Bone Jt. Surg. Am. 95 (18), 1651–1658. doi:10.2106/jbjs.l.00589
Fejer, R., Kyvik, K. O., and Hartvigsen, J. (2006). The prevalence of neck pain in the world population: a systematic critical review of the literature. Eur. Spine J. 15 (6), 834–848. doi:10.1007/s00586-004-0864-4
GBD 2017 Disease and Injury Incidence and Prevalence Collaborators Abate, D., Abate, K. H., Abay, S. M., Abbafati, C., Abbasi, N., et al. (2018). Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392 (10159), 1789–1858. doi:10.1016/s0140-6736(18)32279-7
Gembruch, O., Jabbarli, R., Rashidi, A., Chihi, M., Hetze, S., Barthel, L., et al. (2021). Surgery for degenerative cervical myelopathy: what really counts? Spine (Phila Pa 1976) 46 (5), 294–299. doi:10.1097/brs.0000000000003750
Gulati, S., Vangen-Lønne, V., Nygaard, Ø. P., Gulati, A. M., Hammer, T. A., Johansen, T. O., et al. (2021). Surgery for degenerative cervical myelopathy: a nationwide registry-based observational study with patient-reported outcomes. Neurosurgery 89 (4), 704–711. doi:10.1093/neuros/nyab259
Hamburger, C., Büttner, A., and Uhl, E. (1997). The cross-sectional area of the cervical spinal canal in patients with cervical spondylotic myelopathy. Correlation of preoperative and postoperative area with clinical symptoms. Spine (Phila Pa 1976) 22 (17), 1990–1994. doi:10.1097/00007632-199709010-00009
Khan, O., Badhiwala, J. H., Witiw, C. D., Wilson, J. R., and Fehlings, M. G. (2021). Machine learning algorithms for prediction of health-related quality-of-life after surgery for mild degenerative cervical myelopathy. Spine J. 21 (10), 1659–1669. doi:10.1016/j.spinee.2020.02.003
Maki, S., Furuya, T., Yoshii, T., Egawa, S., Sakai, K., Kusano, K., et al. (2021). Machine learning approach in predicting clinically significant improvements after surgery in patients with cervical ossification of the posterior longitudinal ligament. Spine (Phila Pa 1976) 46 (24), 1683–1689. doi:10.1097/brs.0000000000004125
Merali, Z. G., Witiw, C. D., Badhiwala, J. H., Wilson, J. R., and Fehlings, M. G. (2019). Using a machine learning approach to predict outcome after surgery for degenerative cervical myelopathy. PLoS One 14 (4), e0215133. doi:10.1371/journal.pone.0215133
Nakashima, H., Yukawa, Y., Ito, K., Machino, M., Kanbara, S., Morita, D., et al. (2012). Prediction of lower limb functional recovery after laminoplasty for cervical myelopathy: focusing on the 10-s step test. Eur. Spine J. 21 (7), 1389–1395. doi:10.1007/s00586-012-2241-z
Naruse, T., Yanase, M., Takahashi, H., Horie, Y., Ito, M., Imaizumi, T., et al. (2009). Prediction of clinical results of laminoplasty for cervical myelopathy focusing on spinal cord motion in intraoperative ultrasonography and postoperative magnetic resonance imaging. Spine (Phila Pa 1976) 34 (24), 2634–2641. doi:10.1097/brs.0b013e3181b46c00
Okada, E., Matsumoto, M., Ichihara, D., Chiba, K., Toyama, Y., Fujiwara, H., et al. (2009). Aging of the cervical spine in healthy volunteers: a 10-year longitudinal magnetic resonance imaging study. Spine (Phila Pa 1976) 34 (7), 706–712. doi:10.1097/brs.0b013e31819c2003
Shah, A. A., Devana, S. K., Lee, C., Olson, T. E., Upfill-Brown, A., Sheppard, W. L., et al. (2023). Development and external validation of a risk calculator for prediction of major complications and readmission after anterior cervical discectomy and fusion. Spine (Phila Pa 1976) 48 (7), 460–467. doi:10.1097/brs.0000000000004531
Song, J., Li, J., Zhao, R., and Chu, X. (2024). Developing predictive models for surgical outcomes in patients with degenerative cervical myelopathy: a comparison of statistical and machine learning approaches. Spine J. 24 (1), 57–67. doi:10.1016/j.spinee.2023.07.021
Tetreault, L., Kopjar, B., Côté, P., Arnold, P., and Fehlings, M. G. (2015b). A clinical prediction rule for functional outcomes in patients undergoing surgery for degenerative cervical myelopathy: analysis of an international prospective multicenter data set of 757 subjects. J. Bone Jt. Surg. Am. 97 (24), 2038–2046. doi:10.2106/jbjs.o.00189
Tetreault, L., Palubiski, L. M., Kryshtalskyj, M., Idler, R. K., Martin, A. R., Ganau, M., et al. (2018). Significant predictors of outcome following surgery for the treatment of degenerative cervical myelopathy: a systematic review of the literature. Neurosurg. Clin. N. Am. 29 (1), 115–127.e35. doi:10.1016/j.nec.2017.09.020
Tetreault, L., Wilson, J. R., Kotter, M. R., Nouri, A., Côté, P., Kopjar, B., et al. (2016). Predicting the minimum clinically important difference in patients undergoing surgery for the treatment of degenerative cervical myelopathy. Neurosurg. Focus 40 (6), E14. doi:10.3171/2016.3.focus1665
Tetreault, L. A., Karpova, A., and Fehlings, M. G. (2015a). Predictors of outcome in patients with degenerative cervical spondylotic myelopathy undergoing surgical treatment: results of a systematic review. Eur. Spine J. 24 (Suppl. 2), 236–251. doi:10.1007/s00586-013-2658-z
Tetreault, L. A., Kopjar, B., Vaccaro, A., Yoon, S. T., Arnold, P. M., Massicotte, E. M., et al. (2013). A clinical prediction model to determine outcomes in patients with cervical spondylotic myelopathy undergoing surgical treatment: data from the prospective, multi-center AOSpine North America study. J. Bone Jt. Surg. Am. 95 (18), 1659–1666. doi:10.2106/jbjs.l.01323
Keywords: degenerative cervical myelopathy, spine surgery, machine learning, outcome, prediction model
Citation: Zhou S, Liu Z, Huang H, Xi H, Fan X, Zhao Y, Chen X, Diao Y, Sun Y, Ji H and Zhou F (2025) Predicting postoperative neurological outcomes of degenerative cervical myelopathy based on machine learning. Front. Bioeng. Biotechnol. 13:1529545. doi: 10.3389/fbioe.2025.1529545
Received: 17 November 2024; Accepted: 30 January 2025;
Published: 04 March 2025.
Edited by:
Lianlei Wang, Shandong University, ChinaReviewed by:
Takashi Kaito, Osaka University, JapanCopyright © 2025 Zhou, Liu, Huang, Xi, Fan, Zhao, Chen, Diao, Sun, Ji and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Ji, amlfaG9uZ0AxMjYuY29t; Feifei Zhou, b3J0aG96aG91QDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.