
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ERRATUM article
Front. Bioeng. Biotechnol. , 03 November 2022
Sec. Tissue Engineering and Regenerative Medicine
Volume 10 - 2022 | https://doi.org/10.3389/fbioe.2022.1058241
This article is an erratum on:
Tips and Tricks and Clinical Outcome of Cryopreserved Human Amniotic Membrane Application for the Management of Medication-Related Osteonecrosis of the Jaw (MRONJ): A Pilot Study
by Odet S, Meyer C, Gaudet C, Weber E, Quenot J, Derruau S, Laurence S, Bompy L, Girodon M, Chatelain B, Mauprivez C, Brenet E, Kerdjoudj H, Zwetyenga N, Marchetti P, Hatzfeld A-S, Toubeau D, Pouthier F, Lafarge X, Redl H, Fenelon M, Fricain J-C, Di Pietro R, Ledouble C, Gualdi T, Parmentier A-L, Louvrier A and Gindraux F (2022). Front. Bioeng. Biotechnol. 10:936074. doi: 10.3389/fbioe.2022.936074
Due to a production error, there was a mismatch in Figures 4D, 5D, 5E as published. The corrected figures appear below.
Additionally, the inactive video link in the Introduction section (paragraph 7) has been replaced with the following link: https://youtu.be/GKy3I-n3NRQ.
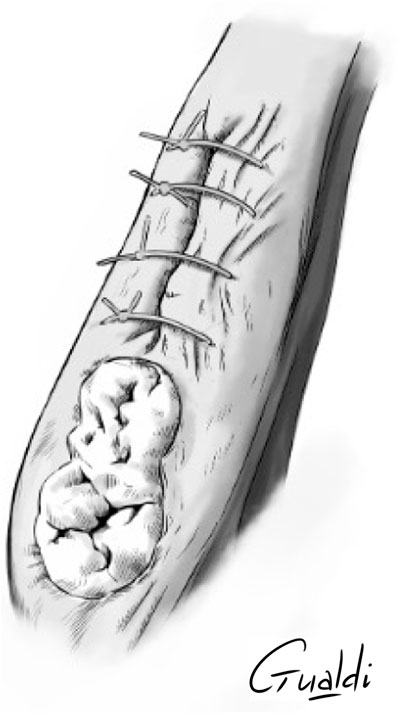
FIGURE 4. Patient 2 (A) Anterior mandibular stage 2 MRONJ. (B) hAM application. (C) Hermetical sutures from “hAM implantation with complete coverage” nomenclature (Odet et al., 2021). Here the sutures were done above the implanted hAM which was not visible. (D) Upper view and (E) Sagittal section illustrations of “hAM implantation with complete coverage” nomenclature.
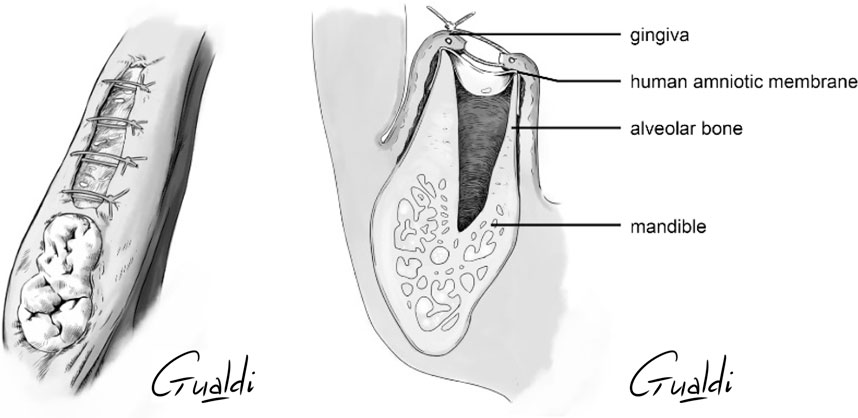
FIGURE 5. Patient 3 (A) Sector 3 posterior stage 2 MRONJ. (B) hAM application. (C) Non-hermetic sutures from “hAM implantation with partial coverage” nomenclature (Odet et al., 2021). Here the gingiva was sutured above the hAM, but leaving the hAM exposed in the oral cavity. (D) Upper view and (E) Sagittal section illustrations of “hAM implantation with partial coverage” nomenclature.
There were two typing errors in the Figure 3 legend, the correction appears below:
FIGURE 3
Patient 8 (A) hAM application, sutured on a collagen sponge. (B) Three days post-surgery. (C) Ten days post-surgery, with the reepithelialization on more than
Algorithm 1. of the surgical site.
The publisher apologizes for this mistake. The original version of this article has been updated.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: human amniotic membrane, osteonecrosis, oral mucosa, allograft, bisphosphonates, denosumab, antiangiogenic drugs
Citation: Frontiers Production Office (2022) Erratum: Tips and tricks and clinical outcome of cryopreserved human amniotic membrane application for the management of medication- related osteonecrosis of the jaw (MRONJ): A pilot study. Front. Bioeng. Biotechnol. 10:1058241. doi: 10.3389/fbioe.2022.1058241
Received: 30 September 2022; Accepted: 30 September 2022;
Published: 03 November 2022.
Approved by:
Frontiers in Editorial OfficeCopyright © 2022 Frontiers Production Office. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Frontiers Production Office, cHJvZHVjdGlvbi5vZmZpY2VAZnJvbnRpZXJzaW4ub3Jn
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.