
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Bioeng. Biotechnol., 13 April 2021
Sec. Synthetic Biology
Volume 9 - 2021 | https://doi.org/10.3389/fbioe.2021.662432
This article is part of the Research TopicNovel Biological Synthesis of Nutrients for Chronic Diseases InterventionView all 10 articles
In this study, acenaphthylene was used as the raw material, and a series of novel 1,8-naphthalimide-1,2,3-triazole derivatives was obtained through oxidation, acylation, alkylation, and click reactions, and subsequently, their anti-tumor activities were tested. After screening, we found that Compound 5e showed good activity against H1975 lung cancer cells, with the half maximal inhibitory concentration (IC50) reaching 16.56 μM.
Cancer is the leading cause of death worldwide. At present, surgery is still the first choice of treatment for many cancers, but easy recurrence and metastasis after surgery greatly affect the efficacy and prognosis (Gaitanis et al., 2018). For patients with local advanced tumors or distant metastases that are not suitable for surgery, traditional chemotherapy and radiotherapy show poor efficacy, and finding active and effective cancer treatments has become a main focus for researchers, especially the development of safe anti-cancer drugs. Based on DNA intercalators, small molecule drugs are being developed as anti-tumor drugs. Due to differences between the DNA of cancerous cells and normal cells, DNA intercalators play a significant role in treating tumors (Jiang, 2013). Naphthalimide derivatives have a special rigid planar structure, which gives them a strong ability to intercalate into DNA (Finney, 2006; Takahashi et al., 2007), so they have attracted extensive attention in the field of anti-tumor drug research and development. Some mononaphthalimides, such as amonafide (Wang et al., 2017; Johnson et al., 2019) and mitonafide (Sinha et al., 1985; Rosell et al., 1992), and bisnaphthalimides have entered the clinical research stage. Amonafide and mitonafide can not only intercalate into DNA and inhibit the synthesis of DNA and RNA, but also inhibit the activity of topoisomerase II, thereby inhibiting tumor cell division (Nazari and Ghandi, 2015). In addition, bisnaphthalimide compounds can bis-intercalate into DNA, have specificity to G-C bases, and exhibit stronger DNA binding and greater cytotoxicity than mononaphthalimides (Laquindanum et al., 1996; Wang and Yu, 2003; Langhals, 2004; Li and Wonneberger, 2012; Verma et al., 2013). For example, DMP-840 is a novel DNA-interactive chemical entity with excellent activityagainst human tumor colony-formingunits and its IC50 to Leukemia and other cell lines is 2.3–53 nM (Kirshenbaum et al., 1994; Nitiss et al., 1998). LU-79553, a bisnaphthalimides, exhibited dramatic antitumor activity in a variety of xenograft models and activity over a broad spectrum of tumor types made it an excellent candidate (Bousquet et al., 1995). For example, IC50 of LU-79553 is 18.0 μM to ovarian cell (Braña et al., 2004; Gonzalez-Bulnes and Gallego, 2009; Ferri et al., 2011; González-Bulnes and Gallego, 2012). Yet, the activity of naphthalimide derivatives on lung cancer is rarely reported (Figure 1).
Herein, a series of novel 1,8-naphthalimide-linked 1,2,3-triazole compounds were synthesized and tested for their antilung-cancer activity. From the raw material acenaphthylene (Compound 1), 1,8-naphthalic anhydride (Compound 2) was obtained by an oxidation reaction, and then the intermediate (Compound 3) was obtained by an acylation reaction. Compound 3 underwent alkylation with 4-bromo-1-butyne to yield Compound 4, which underwent a 1,3-dipolar cycloaddition reaction with different substituted azides to obtain target Compound 5 with a 1,2,3-triazole structure. The structures of the synthesized compounds Scheme 1) were confirmed by nuclear magnetic resonance (1H NMR and 13C NMR).
The key to this reaction is to use a strong oxidant to oxidize the carbon–carbon double bond to yield acid anhydride. We chose sodium dichromate as the oxidant and screened the amount of added sodium dichromate (Table 1). It can be seen from the table that under the condition that the reaction conditions remained unchanged, the reaction yield gradually increased with increasing amount of sodium dichromate. When the molar ratio of compound 1 to sodium dichromate is 1:2, the yield reached 80%. With the increase of the oxidant, the yield did not increase obviously. Therefore, the ratio of 1:2 was chosen as the optimal ratio to prepare compound 2.
This reaction is an acylation reaction, and its temperature has a great influence on the yield of the reaction product. If the reaction is not complete, the raw materials will precipitate with the product during the cooling and crystallization process, which will affect the purity of the product. Therefore, we maintained the other reaction conditions unchanged and studied the reaction temperature (Table 2). With the increase of temperature, the yield increased. But the yield remained unchanged with the increase of temperature until 70°C. So we chose 70°C as the optimal reaction temperature.
The inhibitory effect of Compounds 5a–5o on H1975 lung cancer cells is shown in Table 3. Most of the compounds in this series had poor inhibitory effects on this cell type. Only compounds 5e, 5g, 5h, and 5j had a certain activity. Among them, 5e showed the strongest effect with an IC50 of 16.56 μM. We then studied the structure-activity relationship. By comparing compounds 5a and 5j, 5c and 5f, 5k and 5o, and 5e and 5m, we clearly found that compounds with substituents at the ortho position of the benzene ring were more active than those with substituents at the para position. For compounds 5j and 5k, and 5h and 5m, we found that the activity of 1,2,3-triazole linked with the phenyl structure was generally stronger than those with 1,2,3-triazole linked with the benzyl structure. The results of 5e and 5h showed that when an electron withdrawing group with steric hindrance is at the para position of the benzene ring, the activity was obviously improved. The reason is worthy of further exploring.
In summary, we designed and synthesized a series of novel 1,8-naphthalimide-1,2,3-triazole derivatives through simple and efficient methods, conducted anti-tumor activity studies on them, and found that compound 5e had a good inhibitory effect on H1975 lung cancer cells. The results showed that compounds 5e deserves further research about its anti-tumor activity and mechanism of action in order to find better compounds. Meanwhile, because the introduced substituents of our compounds are lipophilic, the solubility in water of our compounds is less than DMP. Our next step is to improve the water solubility of 5e.
1,8-naphthalimide-1,2,3-triazole derivatives were in-house synthesized. All reagents were obtained from commercial sources and used without further purification. All reactions were monitored by thin-layer chromatography (TLC). 1H and 13C spectra were recorded on a Bruker Avance 400 or 600 MHz spectrometer, respectively. NMR spectra were recorded in CDCl3 or DMSO-d6 at room temperature (20 ± 2 °C). 1H and 13C chemical shifts are quoted in parts per million downfield from TMS. H1975 lung cancer cell line, DMEM medium and fetal bovine serum were purchased from ATCC (Virginia, United States). MTT powder and Dimethyl sulfoxide (DMSO) was purchased from Acros Organics (Morris Plains, NJ, United States).
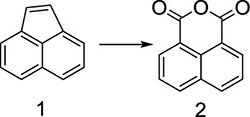
In a reaction flask, acenaphthylene (Compound 1, 15 g, 0.1 mol) was added into 500 mL of glacial acetic acid, followed by adding 55 g (0.2 mol) of sodium dichromate. The mixture was stirred evenly at room temperature, and then the temperature was slowly increased to 80 °C, after which the reaction was carried out for 6 h. Thin layer chromatography (TLC) was used to monitor the progress of the reaction. When the reaction was complete, the reaction mixture was poured into 2,000 mL of ice water while it was hot. Solid precipitates appeared, and the reaction mixture was filtered, and the filter cake was dried to obtain 1,8-naphthalic anhydride (Compound 2, 16 g), and the yield was 80%; 1H NMR (400 MHz, DMSO-d6): δ 8.55 (dd, J1 = 8.0 Hz, J1 = 4.0 Hz, 4H), 7.93 (t, J1 = 4.0 Hz, J1 = 8.0 Hz, 2H); 13C NMR (100 MHz, DMSO-d6): 161.19, 135.86, 132.93, 130.22, 128.03, 119.54 ppm.
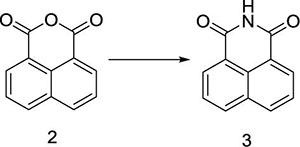
In a reaction flask, 1,8-naphthalic anhydride (Compound 2, 50 g, 0.25 mol) was added to 1,000 mL of saturated ammonia water, and the mixture was stirred at room temperature for 10 min to obtain a yellow mixed liquid, which was slowly heated to 70°C. At this temperature, the reaction was carried out for 90 min. TLC was used to monitor the progress of the reaction. When the reaction was complete, heating was stopped. The flask was slowly cooled down to room temperature, solid precipitates appeared, and the reaction mixture was filtered. The filter cake was washed with 500 mL of water to neutralize it, and then dried at 60 °C to obtain 1,8-naphthalimide (Compound 3,44 g), with a yield of 88%; 1H NMR (400 MHz, DMSO-d6): δ 8.42 (d, J = 4.0 Hz, 4H), 7.83 (t, J1 = 8.0 Hz, J2 = 4.0 Hz, 2H); 13C NMR (100 MHz, DMSO-d6): 164.56, 134.78, 132.03, 130.42, 127.53, 122.93ppm.
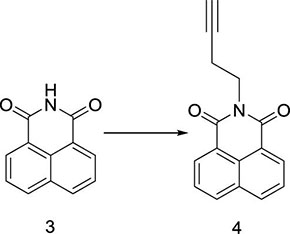
Compound 3 (10.00 g, 50.71 mmol) was added to a reaction flask and dissolved in N,N-dimethylformamide (DMF) (150 mL), and 4-bromo-1-butyne (7.42 g, 55.78 mmol) and potassium carbonate (21.02 g, 152.13 mmol) were added successively. Under nitrogen protection, the reaction was carried out at 100 °C overnight, and TLC was used to monitor the progress of the reaction. When the reaction was completed, the reaction mixture was filtered while it was hot, the filtrate was cooled at room temperature, and solid precipitates appeared. The filter cake was washed with a small amount of DMF and dried to obtain a white solid Compound 4 (9.65 g, 38.71 mmol), with a yield of 76.3%; 1H NMR (600 MHz, DMSO-d6) δ 8.50 (d, J = 7.2 Hz, 2H), 8.46 (d, J = 7.8 Hz, 2H), 7.86 (t, J = 7.8 Hz, 2H), 4.45 (t, J = 7.2 Hz, 2H), 3.18 (t, J = 7.2 Hz, 2H), 2.89 (s, 1H). 13C NMR (150 MHz, DMSO-d6) δ 163.85, 143.96, 134.65, 131.68, 128.63, 127.84, 124.26, 80.95, 56.53, 44.18, 24.92.
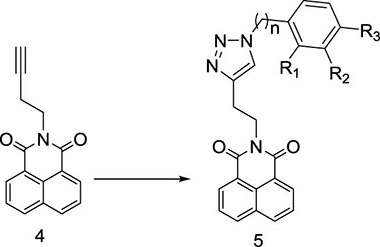
Aryl-azido (1.2 mmol) and compound 4 (1.0 mmol) were added to 15 mL mixed solvent (water/tert-butanol = 2:1). The reaction was catalyzed with CuI (0.1 mmol) at 80 °C. After completion of the reaction (monitored by TLC), the mixture was extracted with dichloromethane (15 mL × 3). The combined organic phase was washed successively with water and brine, dried over sodium sulfate and concentrated in vacuo. The residue was purified by through column chromatography (CH2Cl2/MeOH) to obtain the desired compound 5 as a crystalline powder.
1H NMR (600 MHz, DMSO-d6) δ 8.50 (d, J = 7.2 Hz, 2H), 8.49 (d, J = 1.8 Hz, 1H), 8.47 (d, J = 7.8 Hz, 2H), 7.88 (t, J = 7.8 Hz, 2H), 7.80 (td, J1 = 7.8 Hz, J2 = 1.2Hz, 1H), 7.61–7.58 (m, 1H), 7.57–7.53 (m, 1H), 7.43 (t, J = 8.4 Hz, 1H), 4.40 (t, J = 7.2 Hz, 2H), 3.14 (t, J = 7.8 Hz, 2H). 13C NMR (150 MHz, DMSO-d6) δ 163.88, 144.94, 134.83, 131.80, 131.55, 131.49, 131.21, 127.90, 127.71, 126.28, 126.00, 124.60, 124.57, 122.56, 117.68, 117.55, 24.00.
ield:69.5%; 1H NMR (400 MHz, DMSO-d6) δ 8.78 (s, 1H), 8.48 (dd, J = 7.2, 1.2 Hz, 2H), 8.45 (dd, J1 = 8.0 Hz, J2 = 1.2Hz, 2H), 7.88–7.84 (m, 2H), 7.81–7.74 (m, 2H), 7.66–7.59 (m, 1H), 7.35–7.28 (m, 1H), 4.38 (t, J = 8.0 Hz, 2H), 3.11 (t, J = 7.6 Hz, 2H). 13C NMR (100 MHz, DMSO-d6) δ 163.86, 161.68, 145.63, 134.81, 132.33, 132.24, 131.78, 131.19, 127.88, 127.67, 122.52, 121.49, 116.14, 115.68, 115.47, 107.82, 107.55, 39.70, 24.10.
ield: 35.9%; 1H NMR (400 MHz, DMSO-d6) δ 8.70 (s, 1H), 8.48 (dd, J1 = 7.2 Hz, J2 = 1.2 Hz, 2H), 8.45 (dd, J1 = 8.4 Hz, J2 = 1.2 Hz, 2H), 7.93–7.88 (m, 2H), 7.86 (m, 2H), 7.43 (t, J = 8.8 Hz, 2H), 4.38 (t, J = 7.6 Hz, 2H), 3.14–3.07 (t, J = 7.6 Hz, 2H). 13C NMR (100 MHz, DMSO-d6) δ 163.89, 145.47, 134.81, 133.79, 131.77, 131.22, 127.71, 122.65, 122.61, 122.57, 122.53, 121.55, 117.27, 117.07, 24.13.
ield:36.1%; 1H NMR (600 MHz, DMSO-d6) δ 8.50 (dd, J1 = 7.2 Hz, J2 = 1.2Hz, 2H), 8.48–8.45 (m, 2H), 8.37 (s, 1H), 7.90–7.86 (m, 3H), 7.62–7.59 (m, 1H), 7.58 (dd, J1 = 7.8 Hz, J2 = 1.8Hz, 1H), 7.54–7.52 (m, 1H), 4.41 (t, J = 7.2 Hz, 2H), 3.14 (t, J = 7.2 Hz, 2H). 13C NMR (150 MHz, DMSO-d6) δ 163.84, 144.16, 136.84, 134.82, 134.02, 132.27, 131.80, 131.23, 129.38, 129.09, 127.91, 127.70, 125.30, 122.59, 119.28, 39.65, 23.95.
ield29.2%; 1H NMR (400 MHz, DMSO-d6) δ 8.76 (s, 1H), 8.49 (d, J = 7.2 Hz, 2H), 8.46 (d, J = 8.4 Hz, 2H), 7.89–7.83 (m, 4H), 7.78 (d, J = 8.8 Hz, 2H), 4.38 (d, J = 7.6 Hz, 2H), 3.11 (d, J = 7.6 Hz, 2H). 13C NMR (100 MHz, DMSO-d6) δ 163.91, 145.65, 136.38, 134.83, 133.22, 131.79, 131.20, 127.89, 127.69, 122.53, 122.20, 122.17, 121.48, 121.39, 24.11.
Yield17.4%; 1H NMR (600 MHz, DMSO-d6) δ 8.54 (d, J = 7.2 Hz, 2H), 8.51 (d, J = 8.0 Hz, 2H), 8.44 (s, 1H), 7.93 (t, J = 7.8 Hz, 2H), 7.78 (d, J = 8.4 Hz, 1H), 7.68–7.64 (m, 2H), 7.62 (t, J = 7.8 Hz, 1H), 4.46 (t, J = 7.2 Hz, 2H), 3.20 (t, J = 7.2 Hz, 2H). 13C NMR (150 MHz, DMSO-d6) δ 163.84, 144.27, 135.16, 134.81, 131.92, 131.79, 131.21, 130.95, 128.96, 128.87, 128.76, 127.90, 127.68, 125.30, 122.57, 39.65, 23.97.
ield7.5%; 1H NMR (600 MHz, DMSO-d6) δ 8.51 (d, J = 7.2 Hz, 2H), 8.47 (d, J = 8.4 Hz, 2H), 8.31 (s, 1H), 7.89 (t, J = 7.8 Hz, 2H), 7.58 (dd, J1 = 7.8 Hz, J2 = 1.8 Hz, 1H), 7.53–7.48 (m, 1H), 7.30 (d, J = 8.3 Hz, 1H), 7.13 (t, J = 7.6 Hz, 1H), 4.40 (t, J = 7.8 Hz, 2H), 3.78 (s, 3H), 3.12 (t, J = 7.2 Hz, 2H). 13C NMR (151 MHz, DMSO-d6) δ 163.87, 152.00, 144.01, 134.82, 131.80, 131.22, 130.95, 127.90, 127.71, 126.34, 126.07, 124.91, 122.58, 121.32, 113.47, 56.48, 24.05.
ield30.5%; 1H NMR (600 MHz, DMSO-d6) δ 8.97 (s, 1H), 8.52 (d, J = 7.2 Hz, 2H), 8.49 (d, J = 8.4 Hz, 2H), 8.45 (d, J = 8.4 Hz, 2H), 8.20 (d, J = 8.4 Hz, 2H), 7.90 (t, J = 7.8 Hz, 2H), 4.42 (t, J = 7.8 Hz, 2H), 3.15 (t, J = 7.8 Hz, 2H). 13C NMR (150 MHz, DMSO-d6) δ 163.94, 147.01, 146.19, 141.42, 134.88, 131.84, 131.26, 127.96, 127.74, 126.10, 122.60, 121.84, 120.77, 40.53, 24.09.
1H NMR (600 MHz, DMSO-d6) δ 8.46 (t, J = 7.2 Hz, 4H), 8.02 (s, 1H), 7.87 (t, J = 7.8 Hz, 2H), 7.34 (t, J = 7.2 Hz, 2H), 7.31 (t, J = 6.6 Hz, 1H), 7.21 (d, J = 6.6 Hz, 2H), 5.54 (s, 2H), 4.31 (t, J = 7.2 Hz, 2H), 3.03 (t, J = 7.2 Hz, 2H). 13C NMR (150 MHz, DMSO-d6) δ 163.81, 136.77, 134.80, 131.78, 131.20, 129.13, 128.39, 128.00, 127.85, 127.69, 123.38, 122.50, 53.04, 24.06.
1H NMR (600 MHz, DMSO-d6) δ 8.49–8.41 (m, 4H), 8.00 (s, 1H), 7.86 (t, J = 7.2 Hz, 2H), 7.43–7.38 (m, 1H), 7.23 (t, J = 9.0 Hz, 1H), 7.20–7.17 (m, 2H), 5.60 (s, 2H), 4.31 (t, J = 7.2 Hz, 2H), 3.03 (t, J = 7.2 Hz, 2H). 13C NMR (150 MHz, DMSO-d6) δ 163.79, 134.77, 131.76, 131.17, 130.96, 130.91, 130.66, 130.64, 127.66, 125.24, 125.22, 123.63, 123.53, 123.43, 122.47, 116.03, 115.89, 47.11, 24.03.
1H NMR (600 MHz, DMSO-d6) δ 8.46 (t, J = 7.8 Hz, 4H), 7.99 (s, 1H), 7.87 (t, J = 7.8 Hz, 2H), 7.65 (d, J = 7.8 Hz, 1H), 7.37 (t, J = 7.2 Hz, 1H), 7.29 (t, J = 7.8 Hz, 1H), 6.95 (d, J = 7.8 Hz, 1H), 5.60 (s, 2H), 4.33 (t, J = 7.2 Hz, 2H), 3.05 (t, J = 7.2 Hz, 2H). 13C NMR (150 MHz, DMSO-d6) δ 163.84, 144.40, 135.67, 134.81, 133.21, 131.78, 131.21, 130.61, 130.15, 128.67, 127.86, 127.68, 123.74, 122.89, 122.50, 53.16, 39.80, 24.02.
1H NMR (600 MHz, DMSO-d6) δ 8.46 (t, J = 7.2 Hz, 4H), 8.05 (s, 1H), 7.86 (t, J = 7.8 Hz, 2H), 7.53 (d, J = 8.4 Hz, 1H), 7.49 (s, 1H), 7.31 (t, J = 7.8 Hz, 1H), 7.20 (d, J = 7.8 Hz, 1H), 5.55 (s, 2H), 4.31 (t, J = 7.2 Hz, 2H), 3.03 (t, J = 7.2 Hz, 2H). 13C NMR (150 MHz, DMSO-d6) δ 163.81, 144.61, 139.39, 134.79, 131.78, 131.34, 131.18, 130.89, 127.85, 127.67, 127.15, 123.47, 122.50, 122.28, 52.22, 39.81, 24.06.
1H NMR (600 MHz, DMSO-d6) δ 8.46 (d, J = 1.8 Hz, 2H), 8.45 (d, J = 3.0 Hz, 2H), 7.87 (t, J = 7.8 Hz, 3H), 7.22 (t, J = 7.2 Hz, 1H), 7.18 (d, J = 7.2 Hz, 1H), 7.14 (t, J = 7.2 Hz, 1H), 6.94 (d, J = 7.2 Hz, 1H), 5.52 (s, 2H), 4.31 (t, J = 7.2 Hz, 2H), 3.03 (t, J = 7.2 Hz, 2H), 2.24 (s, 3H). 13C NMR (151 MHz, DMSO-d6) δ 163.81, 144.36, 136.55, 134.78, 131.78, 131.18, 130.73, 128.64, 128.55, 127.84, 127.67, 126.63, 123.28, 122.49, 51.27, 39.79, 24.02, 19.02.
1H NMR (600 MHz, DMSO-d6) δ 8.45 (dd, J1 = 7.2 Hz, J2 = 4.8 Hz, 4H), 7.97 (s, 1H), 7.87 (t, J = 7.8 Hz, 2H), 7.14–7.10 (m, 4H), 5.47 (s, 2H), 4.30 (t, J = 7.2 Hz, 2H), 3.01 (t, J = 7.2 Hz, 2H), 2.28 (s, 3H). 13C NMR (150 MHz, DMSO-d6) δ 163.79, 137.68, 134.78, 133.71, 131.77, 131.19, 129.65, 128.09, 127.84, 127.66, 123.12, 122.49, 52.87, 39.78, 24.05, 21.16.
1H NMR (600 MHz, DMSO-d6) δ 8.67 (s, 1H), 8.50 (d, J = 7.2 Hz, 2H), 8.47 (d, J = 8.4 Hz, 2H), 7.88 (t, J = 7.8 Hz, 2H), 7.42 (d, J = 2.4 Hz, 1H), 7.37 (dd, J1 = 8.4 Hz, J2 = 2.4 Hz, 1H), 7.11 (d, J = 9.0 Hz, 1H), 4.39 (t, J = 7.8 Hz, 2H), 3.85 (s, 3H), 3.82 (s, 3H), 3.10 (t, J = 7.8 Hz, 2H). 13C NMR (150 MHz, DMSO-d6) δ 163.88, 149.76, 149.14, 145.12, 134.83, 131.80, 131.21, 130.69, 127.91, 127.70, 122.58, 121.36, 112.49, 112.29, 104.88, 56.31, 56.27, 39.79, 24.15.
Cells (approximately 3,000–5,000 cells/well) were seeded in 96-well plates and cultured for 16 h until the cells were adherent. The concentration of test compounds was varied between 2, 4, 8, 16, and 32 μM, 0.1% DMSO was added to cells as control five multiple wells. Plates were cultured at 37 °C in 5% CO2 environment for 72 h. After treatment, 10 μL of MTT (5 mg/mL) was added to each well and the plates were incubated at 37 °C and 5% CO2 for 4 h. The reaction mixture containing 10% SDS and 0.1 mM of HCL was then added to each well before plates were incubated at 37 °C for another 4 h. The absorbance was measured on a microplate reader at a wavelength of 570 nm (Wang et al., 2020).
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
L-FM and WL conceived the study, designed the experiments, and supervised all research. Z-JX prepared the draft of the manuscript. Z-JX and Y-JZ synthesized all compounds. J-HW carried out the experiments and analyzed the data. G-QX contributed to the preliminary activity test and article collation of this paper. All authors reviewed the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This study was supported by the Scientific and Technological Project of Henan Province (No. 192102310142) and Key scientific research projects of colleges and universities in Henan Province (No. 21B180010).
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fbioe.2021.662432/full#supplementary-material
Bousquet, P. F., Braña, M. F., Conlon, D., Fitzgerald, K. M., Perron, D., Cocchiaro, C., et al. (1995). Preclinical evaluation of LU 79553: a novel bis-naphthalimide with potent antitumor activity. Cancer Res. 55, 1176–1180. doi: 10.1007/BF01517352
Braña, M. F., Cacho, M., García, M., Pascual-Teresa, B. D., Ramos, A., Domínguez, M., et al. (2004). New analogues of amonafide and elinafide, containing aromatic heterocycles: synthesis, antitumor activity, molecular modeling, and DNA binding properties. J. Med. Chem. 47, 1391–1399. doi: 10.1021/jm0308850
Ferri, N., Radice, T., Antonino, M., Beccalli, E. M., Tinelli, S., Zunino, F., et al. (2011). Synthesis, structural, and biological evaluation of bis-heteroarylmaleimides and bis-heterofused imides. Bioorg. Med. Chem. 19, 5291–5299. doi: 10.1016/j.bmc.2011.08.016
Finney, N. S. (2006). Combinatorial discovery of fluorophores and fluorescent probes. Curr. Opin. Chem. Biol. 10, 238–245. doi: 10.1016/j.cbpa.2006.04.025
Gaitanis, G., Magiatis, P., Velegraki, A., and Bassukas, I. D. (2018). A traditional Chinese remedy points to a natural skin habitat: indirubin (indigo naturalis) for psoriasis and the Malassezia metabolome. Br. J. Dermatol. 179:800. doi: 10.1111/bjd.16807
González-Bulnes, L., and Gallego, J. (2012). Analysis of mixed DNA-bisnaphthalimide interactions involving groove association and intercalation with surface-based and solution methodologies. Biopolymers 97, 974–987. doi: 10.1002/bip.22114
Gonzalez-Bulnes, L., and Gallego, J. (2009). Indirect effects modulating the interaction between DNA and a cytotoxic bisnaphthalimide reveal a two-step binding process. J. Am. Chem. Soc. 131:7781. doi: 10.1021/ja901505p
Jiang, Z. C. (2013). Wavelength conversion perylene diester chromophores and luminescent films. U.S.Pat. Appl. Publ. 20130284265. vol,Google Scholar
Johnson, A. D., Zammit, R., Vella, J., Valentino, M., Buhagiar, J. A., and Magri, D. C. (2019). Aminonaphthalimide hybrids of mitoxantrone and amonafide as anticancer and fluorescent cellular imaging agents. Bioorg. Chem. 93:103287. doi: 10.1016/j.bioorg.2019.103287
Kirshenbaum, M. R., Chen, S. F., Behrens, C. H., Papp, L. M., Stafford, M. M., Sun, J. H., et al. (1994). (R,R)-2,2’-[1,2-ethanediylbis[imino(1-methyl-2,1-ethanediyl)]]-bis[5-nitro-1Hbenz[de] isoquinoline-1,342H)-dione] dimethanesulfonate (DMP 840), a novel Bis-naphthalimide with potent nonselective tumoricidal activity in Vitro. Cancer Res. 54, 2199–2206.
Langhals, H. (2004). Properties and applications of organic dyes and pigments. Angew. Chem. Int. Ed. 43, 5291–5293. doi: 10.1002/anie.200385122
Laquindanum, J. G., Katz, H. E., Dodabalapur, A., and Lovinger, A. J. (1996). N-channel organic transistor materials based on naphthalene frameworks. J. Am. Chem. Soc. 45, 11331–11332. doi: 10.1021/ja962461j
Li, C., and Wonneberger, H. (2012). Perylene imides for organic photovoltaics: yesterday, today, and tomorrow. Adv. Mater. 24, 613–636. doi: 10.1002/adma.201104447
Nazari, S., and Ghandi, K. (2015). Solvent and microwave effects on oxidation of aromatic α-diketones. J. Ind. Eng. Chem. 21, 198–205. doi: 10.1016/j.jiec.2014.02.025
Nitiss, J. L., Zhou, J., Rose, A., Hsiung, Y., Gale, K. C., and Osheroff, N. (1998). The bis(naphthalimide) DMP-840 causes cytotoxicity by its action against eukaryotic topoisomerase II. Biochemistry 37, 3078–3085. doi: 10.1021/bi9723257
Rosell, R., Carles, J., Abad, A., Ribelles, N., Barnadas, A., Benavides, A., et al. (1992). Phase i study of mitonafide in 120 hour continuous infusion in non-small cell lung cancer. Invest. New Drugs 10, 171–175. doi: 10.1007/bf00877242
Sinha, B. K., Strong, J., Gibson, N. W., and Kalyanaraman, B. (1985). Mechanism of DNA strand breaks by mitonafide, an imide derivative of 3-nitro-1,8-naphthalic acid. Biochem. Pharmacol. 34, 3845–3852. doi: 10.1016/0006-2952(85)90433-2
Takahashi, M., Suzuki, Y., Ichihashi, Y., Yamashita, M., and Kawai, H. (2007). 1,3,8,10-tetrahydro-2,9-diazadibenzo[cd, lm]perylenes: synthesis of reduced perylene bisimide analogues. Tetrahedron. Lett. 3, 357–359. doi: 10.1016/j.tetlet.2006.11.100
Verma, M., Luxami, V., and Paul, K. (2013). Synthesis, in vitro evaluation and molecular modelling of naphthalimide analogue as anticancer agents. Eur. J. Med. Chem. 68, 352–360. doi: 10.1016/j.ejmech.2013.07.027
Wang, H., and Yu, G. (2003). Copolymers having tunable energy levels and color of emission. PCT Int. Appl. 2003103070. vol,Google Scholar
Wang, W. J., Mao, L. F., Lai, H. L., Wang, Y. W., Jiang, Z. B., Li, W., et al. (2020). Dolutegravir derivative inhibits proliferation and induces apoptosis of non-small cell lung cancer cells via calcium signaling pathway. Pharmacol. Res. 161:105129. doi: 10.1016/j.phrs.2020.105129
Keywords: acenaphthylene, synthesis, 1,8-naphthalimide-1,2,3-triazole derivatives, anti-proliferative activities, H1975
Citation: Xu Z-j, Zhou Y-j, Wang J-h, Mao L-f, Li W and Xu G-q (2021) The Synthesis and Antitumor Activity of 1,8-Naphthalimide Derivatives Linked 1,2,3-Triazole. Front. Bioeng. Biotechnol. 9:662432. doi: 10.3389/fbioe.2021.662432
Received: 01 February 2021; Accepted: 22 March 2021;
Published: 13 April 2021.
Edited by:
Wenzhen Liao, Southern Medical University, ChinaReviewed by:
Ana Margarida Goncalves Carvalho Dias, New University of Lisbon, PortugalCopyright © 2021 Xu, Zhou, Wang, Mao, Li and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Li, bGl3ZWlncUAxNjMuY29t; Longfei Mao, bG9uZ2ZlaW1hbzE5ODhAMTYzLmNvbQ==; Gui-qing Xu, Z3VpcWluZ3h1QDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.