- 1Paediatric Department, Maternal and Child Health Hospital of Guangxi Zhuang Autonomous Region, Guangxi Clinical Research Center for Pediatric Diseases, Nanning, China
- 2Institute of Translational Medicine, Medical College, Yangzhou University, Yangzhou, China
- 3Guangxi Key Laboratory of Reproductive Health and Birth Defects Prevention, Maternal and Child Health Hospital of Guangxi Zhuang Autonomous Region, Nanning, China
- 4Jiangsu Key Laboratory of Experimental & Translational Non-Coding RNA Research, Yangzhou University Medical College, Yangzhou, China
Context: Type I hypersensitivity affects approximately one-third of the global population. As the pathophysiology underlying the development of type I hypersensitivity (asthma, food allergy, and anaphylactic shock, etc.) is complex and heterogeneous, animal model studies continue to be the key to identifying novel molecular pathways and providing therapeutic strategies.
Objective: Selection of the animal model should be done with careful consideration of the protocol variables, animal species, and strains to accurately reflect the clinical symptoms typical of humans.
Methods: The following databases were searched: PubMed and Web of Science.
Results and conclusion: Foreign allergens include allergenic proteins and chemical haptens. This review summarizes the various methods used for designing animal models of common allergenic protein-induced type I hypersensitivity, namely, passive anaphylaxis model, active systemic anaphylaxis/anaphylaxis shock model, food allergy model, asthma model, and IgE-mediated cell models. Additionally, we summarize shrimp tropomyosin-induced type I hypersensitivity models from our previous studies and discuss their advantages and limitations compared with that of ovalbumin-induced models.
1 Introduction
Hypersensitivity reactions—often referred to as allergies or allergic reactions—constitute over-reactions of the immune system. The four primary classification of hypersensitivity reactions are types I, II, III, and IV; despite some modifications and extensions, this classification is still used today (1). The type I hypersensitivity reactions are the most commonly occurring ones, and they manifest as physiological dysfunction or tissue or cell damage upon subsequent exposure to the same allergen after an initial response. These reactions are driven by allergen-specific IgE antibodies that activate mast cells to produce specific effects (2). Type II and III hypersensitivity reactions are mediated by IgG or IgM antibodies, which activate the complement system. Type II is a cytotoxic reaction in which antibodies predominantly bind to cell-surface antigens on their own cells, followed by phagocytosis or destruction. In type III reactions, the antibodies bind to soluble antigens and form immune complexes through crosslinking, which results in tissue damage. Type IV reactions are delayed reactions mediated by T lymphocytes, where macrophages are activated by Th1 cells, followed by Th2-associated eosinophilic inflammation and direct damage to tissues by cytotoxic T cells (3).
Type I hypersensitivity leads to atopic and allergic diseases, which are immediate reactions to foreign allergen challenge. A wide range of allergens exist. For example, respiratory allergens include dust mites, fungal elements, pollen, and cockroach extracts; they are pivotal for the development of both allergic rhinitis and asthma (4). Dietary allergenic antigens include milk, peanuts, and shellfish, leading to protein-induced enterocolitis syndrome (5). Insect venoms contain a wide range of allergens (6). IgE-mediated drug hypersensitivity is most commonly induced by precipitating drugs such as antibiotics (particularly beta-lactams). In other cases drug may act as haptens that form covalent hapten–carrier links and initiate during sensitization (7). Generally, anaphylactic shock is triggered by drug, food, or venom and represents the most severe systemic hypersensitivity reaction (8).
Type I hypersensitivity is characterized by an IgE-mediated response. Generally, IgE is a protective antibody, particularly in the immune response against parasitic infections. Upon initial exposure to an allergen, native CD4+ T cells are converted into Th2 cells, which secrete IL-4 and IL-13. The engagement of CD40 on B cells with the surface CD40 ligand expressed on CD4+ T cells triggers IL-4-driven IgE isotype switching (9). IgE binds to specific receptors (FcεRI) on mast cells and basophils. Upon re-exposure to the same allergen, FcεRI on mast cells and basophils cross-links with the IgE and antigen, which initiates cascades leading to cell degranulation, synthesis and secretion of lipid mediators and cytokines (IL-4 and IL-13), and CD40 ligand expression, which further amplifies IgE-mediated responses (10).
Thus, Type I hypersensitivity is induced by allergenic proteins or chemical haptens. Various types of in vivo and in vitro models have been used in laboratory experiments to explore the molecular mechanism underlying type I hypersensitivity and test novel therapeutic approaches. This review summarizes various methods used to establish animal models of common allergenic protein-induced type I hypersensitivity and IgE-mediated cell models and discusses their advantages and limitations. However, hapten-induced dermatitis, asthma, and drug anaphylaxis models have not been discussed in this review.
2 Animal models of type I hypersensitivity
2.1 Animals
Animal models of allergic reactions are commonly established in guinea pigs, rats, and mice. Guinea pigs have been used for asthma research for several decades owing to the high anatomical and physiological similarities between the airways of humans and guinea pigs. The important similarity is that the acute bronchoconstriction response to allergens is largely mediated by histamine and leukotrienes. Guinea pigs are recommended for preclinical research on allergen-induced airway hyper-responsiveness (11). However, allergen-induced IgG1 is associated with a high degree of early airway smooth muscle contraction in the guinea pig, leading to a higher level of histamine release by the mast cells (12). Thus, guinea pig models are suited for investigating mast cell-dependent reactions. Among rat strains, Sprague–Dawley (SD) rats are typically used for studying passive cutaneous anaphylaxis (PCA) reactions (13). Brown Norway (BN) rats are used for exploring the allergenicity of food proteins (14) and asthma studies because of their high sensitivity (15). Mice have also been used as allergy models, and different species or strains show variability in terms of physiology and immunology. BALB/c mice are the most commonly used mouse strain in antigen challenge models because they develop a Th2-biased immunological response (16). C57BL/6 and A/J mice have been successfully used as experimental models for allergic asthma (17). C57BL/6 mice are commonly used to establish transgenic or knockout models that provide information about specific genes involved in human diseases. In the OVA-induced asthma model, C57BL/6 mice display a higher eosinophil percentage in the bronchoalveolar lavage fluid (BALF) than that of BALB/c mice, whereas BALB/c mice show an increased lung mast cell count and airway hyperresponsiveness (AHR) than that observed in C57BL/6 mice (18). A/J mice exhibit higher levels of AHR and reactivity to methacholine than do BALB/C and C57BL/6 mice (19).
Humanized mice, that is, mice reconstituted with human immune cells, are a novel tool for studying the immune response during type I hypersensitivity. Eschborn M developed a humanized mouse model of allergen-induced IgE-dependent gut inflammation in PBMC-engrafted immunodeficient mice (20). Another humanized mouse model of immunodeficient γc-deficient mice expressing transgenes for human stem cell factor, granulocyte-macrophage colony-stimulating factor, and IL-3 developed mature functional human mast cells. Human mast cells in mice were sensitized with patient-derived IgE monoclonal antibodies specific for peanut (Arachis hypogaea) allergen 2, which induced fatal anaphylaxis on exposure to the peanut allergen (21). These humanized mice are useful for recapitulating human IgE-mediated or mast cell-induced anaphylaxis.
2.2 Allergens
The classical antigens used for Type I allergic reaction models include ovalbumin (OVA) and 2,4-dinitrophenol (DNP). DNP is a low-molecular-weight chemical hapten that is conjugated to carrier proteins such as human serum albumin or bovine serum albumin. Anti-DNP-IgE and anti-DNP-human serum albumin (or anti-DNP-bovine serum albumin) antibodies have been used in PCA or mast cell models (22). Nevertheless, OVA is most commonly used, primarily for practical reasons such as low costs, high purity, and commercial availability of antibodies (OVA-specific IgE/IgG) and transgenic mice (OVA-specific transgenic mice). OVA is an allergenic protein that is commonly used to induce allergic bronchospasm in guinea pigs and PCA, asthma, and food allergies in rodent models. However, immunization with OVA in mice does not always induce high levels of IgE and may require re-exposure to allergens. In fact, OVA-induced allergy requires the use of both OVA and Th2-adjuvant aluminum hydroxide to sensitize the mice (23).
Antigens that cause food allergies are typically protein antigens such as milk, eggs, wheat, fish, shellfish, peanuts, walnuts, and soybeans (24). To better mimic the gastrointestinal lesions observed in human food allergies, animals are often sensitized via the oral route. Due to their oral tolerance, cholera toxin and staphylococcal enterotoxin B (SEB) are generally used as mucosal adjuvants to enhance the induction of antigen-specific IgE. Subcutaneous sensitization has been proposed as an alternative approach for inducing gastrointestinal symptoms of food allergies in animals (25).
The allergen inhalation challenge is a useful clinical model for evaluating allergic airway diseases that are triggered by the inhalation of food allergens or airborne allergens (26). Although OVA is widely used in asthma experiments, it is not a relevant aeroallergen for human asthma. The clinical relevance of aeroallergens such as house dust mites (HDM) has been investigated. Additionally, HDM sensitize animals via the airways and do not require adjuvants (27) similar to that of the human sensitization route.
2.3 Passive anaphylaxis model
Passive anaphylactic models are broadly categorized into local cutaneous and systemic reactions. The principle of PCA is the same as that of anti-allergen IgE sensitization and allergen re-exposure in mast cells. The model is generated by administering a subcutaneous injection of mouse IgE-rich antiserum or anti-allergen-IgE monoclonal antibodies (28) at the back or dorsal portion of either ears in rats (29) or paw of mice (30). After 24–48 h, Evans blue dye containing the antigen is administered via the tail vein. As the local vascular permeability increases owing to the allergic reaction, the degree of the reaction is assessed by measuring the diameter of the local blue skin spots or observing the extent of blue skin staining. However, this parameter is non-quantitative and may not reflect a dose-response relationship for evaluating drug efficacy; hence, some researchers have collected local skin samples and quantitatively determined Evans blue absorbance using formamide (22) or acetone–saline extraction (28). Allergen-dependent paw swelling is confirmed by measuring changes in hind paw width using digital calipers (31).
In the passive systemic anaphylaxis (PSA) model, sensitized animals are injected with anti-allergen IgE monoclonal antibodies at the tail vein, followed by administration of the antigen via the tail vein after 24 h, which causes a decline in body temperature and an increase in symptom scores with respect to antigen-specific IgE-dependent PSA reactions (32). In our previous study, mice were intravenously sensitized with anti-shrimp tropomyosin monoclonal IgE. After 24 h, the mice were challenged intravenously with shrimp tropomyosin. After 30 min, rectal temperature decreased by 1.6℃ (33). The degree of PSA was determined by measuring histamine, leukotrienes, and prostaglandins levels in the blood, which were collected through cardiac puncture 5 min after allergen challenge (34).
2.4 Active systemic anaphylaxis (ASA)/anaphylaxis shock model
The ASA is commonly used to evaluate drug safety in guinea pigs. The drug is administered via multi-point injection into the muscles three times every alternate day. On days 14 and 21, an intravenous drug challenge is administered to evaluate the degree of systemic reactions (35). Additionally, ASA is elicited by injecting a protein antigen into the tail vein of mice immunized with the antigen and adjuvant for to 2–4 weeks. Similar systemic symptoms, including shock, develops during ASA therapy. Antigens and adjuvants induce IgG1/2 and IgE in ASA mice. IgG-class antibodies recognize and bind to Fc gamma receptors (FcγRs) that are expressed on mast cells, neutrophils, monocytes and macrophages in humans (36). In mouse models, antigens induce basophils (37), macrophages, and neutrophils (38) to release PAF by activating FcγRs, accounting for IgG-induced anaphylaxis. A higher mortality rate is observed during ASA testing than during PSA testing. Moreover, clinical surrogate measurements such as rectal temperature measurements and behavior scales were used similar to that used for PCA. The central temperature variation (ΔT) observed during the 30 min following the challenge was classified according to the standard described by Jonsson F et al. (39) as Grade 1: no shock, 1°C > ΔT ≥ −1°C, no mortality; Grade 2: mild shock, −1°C > ΔT ≥ −4°C, no mortality; Grade 3: severe shock, −4°C > ΔT, and/or mortality.
2.5 Food allergy models
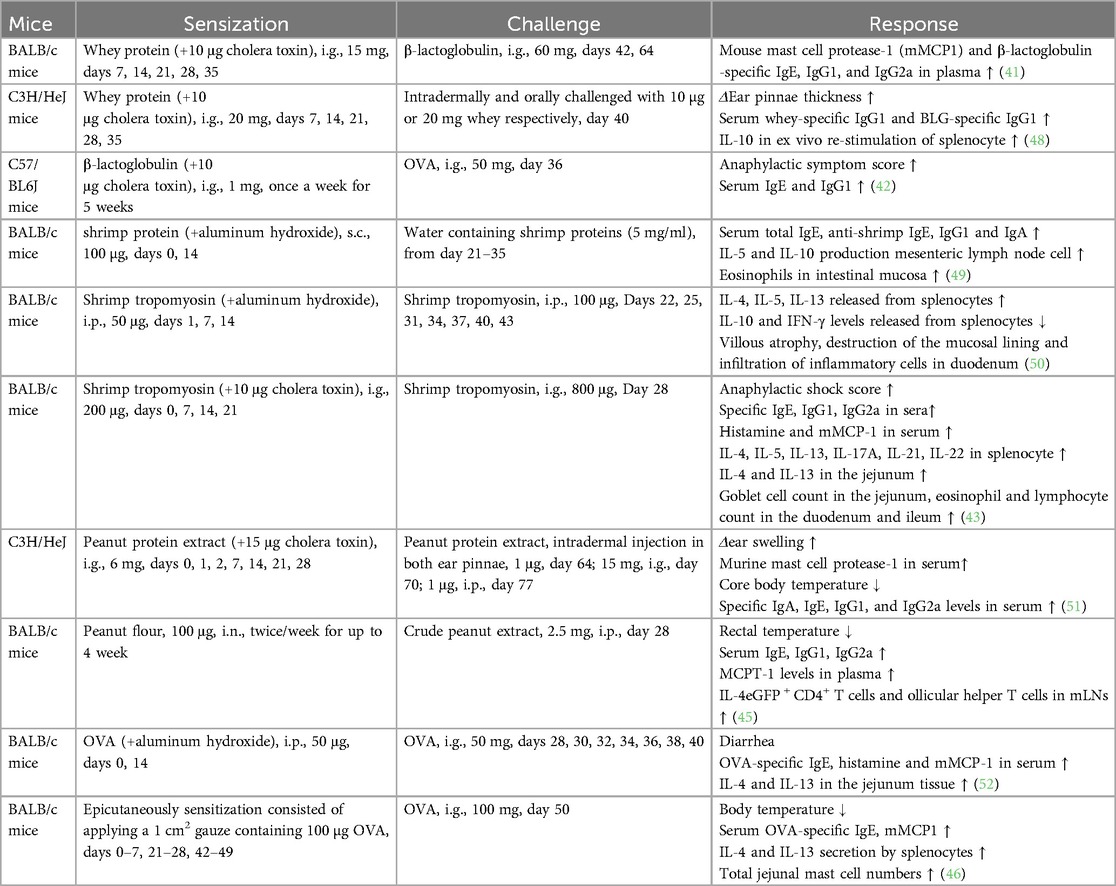
Skin prick tests and serum allergen-specific IgE levels are routinely used for clinical diagnosis of food allergies. Alternatively, basophil activation test is performed, where flow cytometry is used to measure the expression of both CD63 and CD203c on the surface of basophils following stimulation with food allergens (40). In animal models, the sensitization routes may be intragastric, epicutaneous, subcutaneous, intraperitoneal, or inhalational. However, oral sensitization is the primary route in food allergy models as it is similar to the natural oral route in humans. As an adjuvant, cholera toxin disrupts the intestinal barrier and promotes mucosal immune reactions. The combination of whey protein (15–20 mg) or β-lactoglobulin (1 mg) (to represent cow milk allergen) and 10 μg of cholera toxin is often administered for 5 weeks for sensitization to generate a mouse model of cow milk allergy. Notably, a whey-free diet was provided to the mice for at least 1 week prior to initiating the sensitization procedure (41, 42). Shrimp could trigger severe food allergies with tropomyosin as the major cross-reactive allergen to other shellfish. The quantity of shrimp tropomyosin required for sensitization varies from 30 to 200 μg (43, 44). Peanut is another common food allergen. In addition to using peanut allergen with cholera toxin for sensitization, Joseph J. Dolence developed a mouse model for inhalation-based peanut allergen sensitization. A large number of follicular helper T cells have been detected in the mediastinal lymph nodes of allergen-sensitized mice, and the IL-1 pathway is involved in the Tfh response to peanut allergen (45). Although OVA-specific IgE levels were elevated in mice that were orally immunized with OVA–cholera toxin combination, high serum IL-4 levels, increased mast cell counts in the jejunum, and anaphylaxis were not detected following oral challenge. Upon epicutaneous sensitization with OVA, mice exhibited IgE-mediated mast cell expansion, intestinal allergy, and anaphylaxis, as evidenced by decreased core temperature and increased serum mMCP-1 levels (46). Keiko Kameda developed a food allergy mouse model using 1 mg of chicken egg ovomucoid or cow milk casein in epicutaneous sensitization process without adjuvants (47), indicating that the skin may be an efficient route of sensitization to food antigens. Thus, the protocol design for a mouse food allergy model including the allergen, adjuvant, route, dose, timing of sensitization and challenge exposures, endpoint selected, and outcome of the study is described in Table 1.
2.6 Allergic asthma models
Human allergic asthma and rhinitis are triggered by aeroallergens and foodborne allergens. Most animal models of asthma and rhinitis are based on initial sensitization to antigens, followed by a local challenge. None of the currently used animal models emulate all the characteristics of allergic airway diseases. Guinea pigs are commonly used to induce immediate mast cell-dependent hypersensitivity reactions. Bronchial circulation, number of mast cells and mucus glands, and airway neural control in the lungs of guinea pig lungs are significantly similar to those described in humans. Notably, the immediate hypersensitivity reaction in the lung mimics that observed in humans and involves the activation of H1 and cysLT1 receptors (53). However, large doses of antigens induce severe immediate hypersensitivity and favor IgG production, whereas low doses favor mixed IgE and IgG production (54). Moreover, few protocols have been suggested for airway remodeling in guinea pigs. In rats, strains such as Wistar, SD, Fisher, and Lewis do not always develop an allergic response to IgE production, whereas Brown Norway (BN) rats have a high IgE response (55). Airway smooth muscle contraction elicited by allergens in rats is relatively weak and primarily mediated by serotonin, which differs between guinea pigs and humans (54).
BALB/c mice are commonly used to generate mouse models to evaluate Type 2 eosinophilic inflammation or airway remodeling. T2 asthma responds to steroid treatment. Briefly, 10–100 μg of OVA is used to elicit systemic sensitization through intraperitoneal administration for 2–3 weeks, primarily in combination with aluminum hydroxide. In the challenge phase, the animal is exposed to 1%–5% aerosolized OVA inhalation or OVA intranasal instillation (3–7 days for acute asthma or 4–6 weeks for airway remodeling). Large doses of OVA (500 μg) (56) or long-term aerosolized OVA inhalation challenge (57, 58) induce more severe subepithelial fibrosis, whereas low dose (5 μg) challenges favor bronchial epithelial thickening and mucus accumulation in the epithelium (56). Similarly, in our recent studies, shrimp tropomyosin was used as an alternative to OVA, and it elicited high IgE production, AHR, eosinophilic inflammation, Th2 response, basophil and M2 macrophage activation, and airway remodeling in mice (23, 59, 60). Complete Freund's adjuvant (CFA) favors IgG2a activation, indicating a Th1 type immune response (61). OVA/CFA-sensitized mice were developed to induce a non-T2 asthma phenotype, which is characterized by airway neutrophil-dominant inflammation and glucocorticoid insensitivity (62, 63). However, most foodborne allergens, including OVA and shrimp tropomyosin, do not induce airway inflammation or AHR in humans.
Worldwide, Dermatophagoides pteronyssinus (Der p) and Dermatophagoides farina (Der f) are the most common HDM allergens inhaled by humans. Blomia tropicalis is a mite species prevalent in both tropical and subtropical regions (64). Unlike OVA, HDM sensitization and challenge were achieved in mice without the use of adjuvants. Murine eosinophilic asthma is induced by intranasal exposure to Blomia tropicalis allergen without an adjuvant (65). Three different asthma phenotypes, namely, eosinophilic, mixed, and neutrophilic were induced in mice via different doses and routes of HDM (a mixture of Der p and Der f allergen extracts) sensitization and challenge. Mice were sensitized with three intraperitoneal injections of low-dose HDM extract and aluminum hydroxide and challenged with low-dose HDM for eosinophilic airway inflammation. To induce the mixed or neutrophilic phenotype, mice were intranasally sensitized and challenged with low or high doses of HDM, respectively (66). Wang et al. compared asthma induction in mice treated with Der p or Der f extracts. When compared with the Der f-challenged group, Der p-exposed mice exhibited higher BALF neutrophil counts and increased levels of IL-17A and MCPT-1 in lung homogenates, whereas the mice challenged with Der f exhibited a higher expression of MMP-9 and MMP-12 (67). Der p1 and Der f1 are the major allergen proteins in HDM. Recombinant purified Der p1 or Der f1 has been used to develop a capture IgE-ELISA to detect allergen-specific IgE responses in mite-allergic subjects (68). In addition, Der p1 or Der f1 challenged mice exhibited a similar phenotype of airway eosinophilic inflammation, as observed in mice induced with HDM. These recombinant proteins are often used to investigate the molecular mechanisms underlying allergen sensitization. CD163 is a Der p1-binding protein. Der p1 increases CCL24 secretion from bone marrow-derived macrophages in Cd163−/− mice when compared to WT mice (69). Der p 2 drives airway Th2 inflammation via TLR4/Der p 2 interaction. Der p 2 and low-dose lipopolysaccharide (LPS) promote airway inflammation in WT mice, but not in TLR4−/− mice (70).
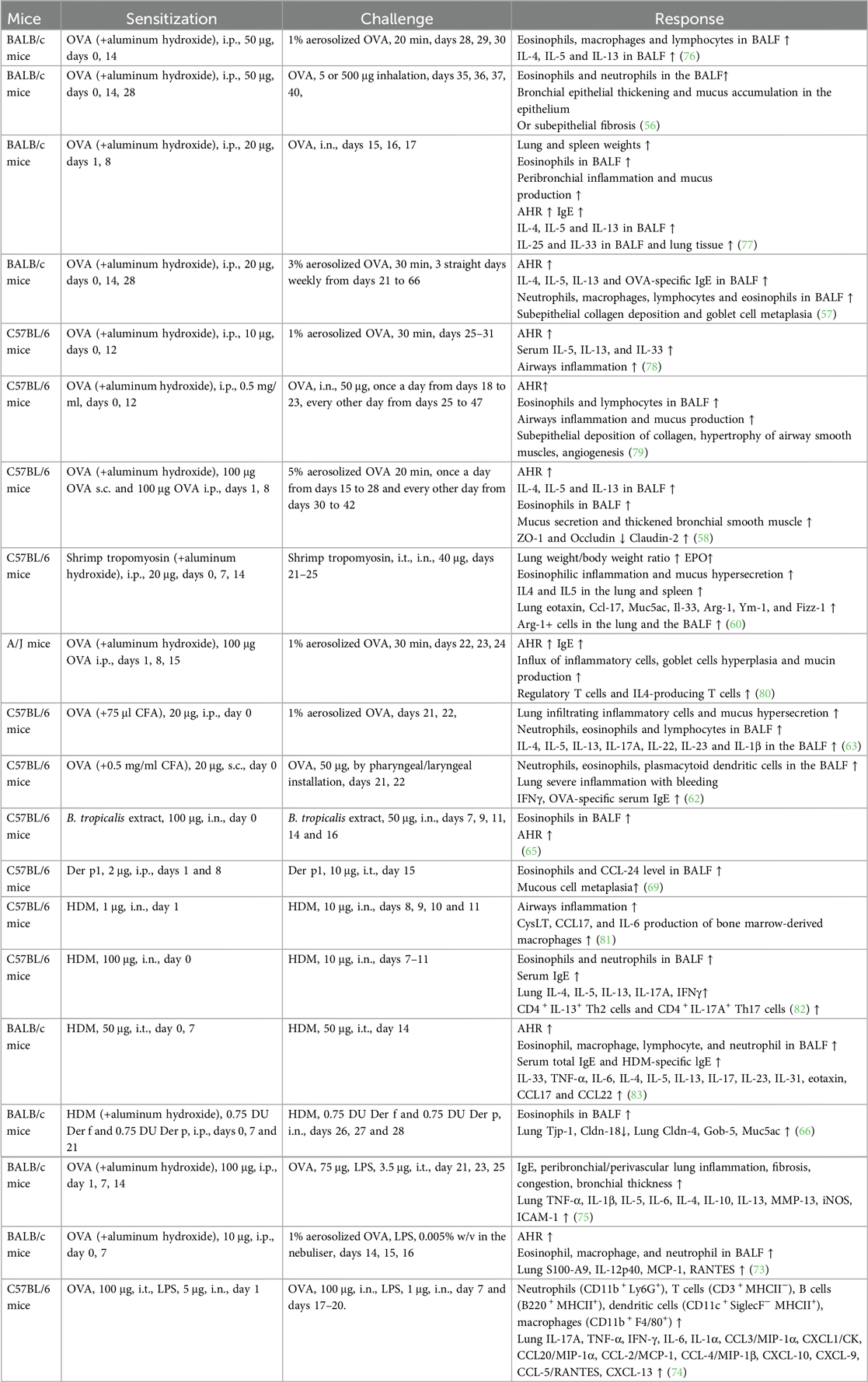
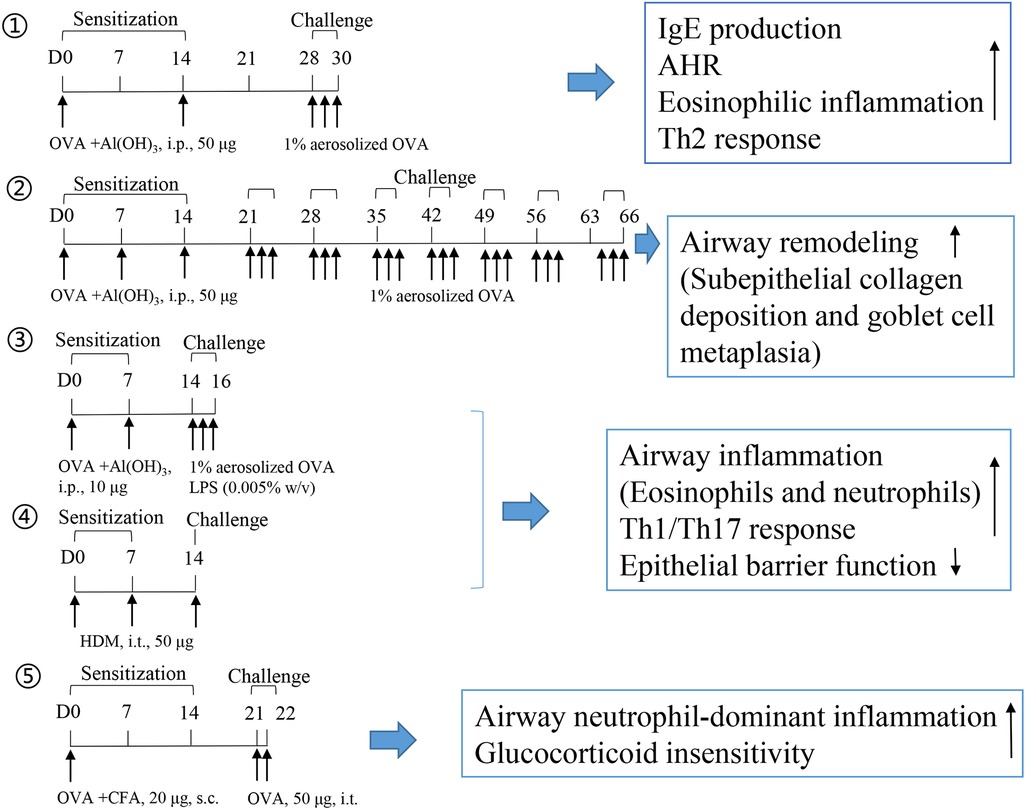
LPS is a known risk factor for asthma exacerbation in humans. A combination of allergens and LPS was used to generate a severe asthma model. However, successfully establishing a severe asthma phenotype considerably depends on the allergen (OVA or HDM), dose of LPS (100 ng, 1 μg, or 10 μg), the starting time of LPS administration (in the sensitization or challenge phase), and LPS administration duration. A single dose of LPS (100 ng) in HDM-treated mice induces the accumulation of NET-releasing CXCR4hi neutrophils in the lungs and promotes HDM-induced type 2 allergic airway inflammation (71). In OVA-induced asthma mice, LPS (1 μg) was intraperitonially injected before each OVA/alum sensitization. LPS treatment significantly alleviates Th2 allergic airway inflammation after OVA challenge (72). In our recent experiment, LPS (250 ng) was intranasally instilled to mice in the shrimp tropomyosin/alum sensitization phase, which reduced the eosinophil count in the BALF. A modified LPS/shrimp tropomyosin-induced severe asthma model was used in this study. Mice were intraperitonially injected with 20 μg of shrimp tropomyosin mixed with aluminum hydroxide on days 0, 7, 14, and 21. The combination of shrimp tropomyosin (20 μg) and LPS (1 μg) was intranasally instilled to mice on days 7, 14, and 21. In the challenge phase, the mice were intratracheally instilled with 50 μg of shrimp tropomyosin once on day 28 and exposed to an intranasal challenge with shrimp tropomyosin from days 29–31. Shrimp tropomyosin combined with LPS induced significant increase in eosinophil and neutrophil counts in the BALF, excessive mucus secretion, increase in IL-17A and IL-1β levels, and decrease in E-cadherin level, which are partially improved by dexamethasone treatment (unpublished data). Additionally, LPS has been used in the OVA challenge phase for determining the neutrophilic phenotype, which is characterized by increased neutrophil count in the BALF (>30% of the total cell count) (73, 74). However, LPS/allergen-induced peribronchial inflammation has often been complicated by the development of alveolitis (75), which differ from the airway lesions in asthmatic humans. Protocol design for mouse asthma model including allergen, adjuvant, route, dose, timing of sensitization and challenge exposures, endpoint selected, and outcomes of the study are described in Table 2. The experimental protocol, including OVA-induced eosinophilic asthma model (76) and airway remodeling model (57), OVA/LPS (73), or HDM-induced mix phenotype model (83), and OVA/CFA-induced neutrophilic asthma model (63), is shown in Figure 1.
3 IgE-mediated cell models
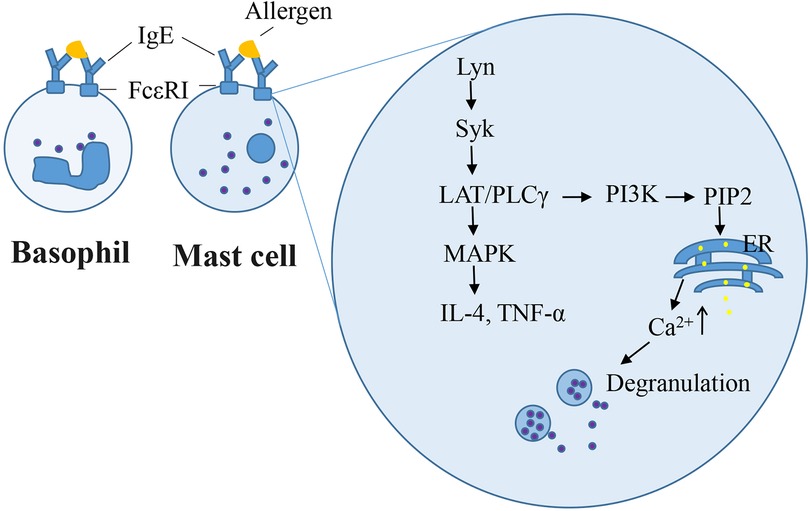
The rat basophilic leukemia (RBL-2H3) cell line has been widely used as a mast cell model for in vitro studies because it mimics the mechanism of IgE-mediated degranulation of human mast cells. RBL-2H3 cell surface has abundant high-affinity IgE receptors (FcεRI), which bind with mouse/rat IgE (IgE-rich antiserum or anti-allergen IgE monoclonal antibodies) in in vitro experiments. Zhongcheng Liu constructed a stable hFcεRIα (α-chain of human FcεRI)/RBL-2H3 cell line, which exhibits the species specificity in the interaction between IgE and FcεRI as that observed in humans (84). IgE-mediated RBL cell model is used to study of IgE–FcεRI interactions, intracellular signaling for degranulation and pro-inflammatory mediators, and novel anti-allergic drug screening. Degranulation and other mast cell responses were observed when cells were incubated overnight with anti-allergen IgE and challenged with allergens (59, 85, 86). Once IgE-antigen stimulation occurred on the cell surface, the level of phosphorylated Lyn, which is a Src family kinase, is enhanced, followed by the recruitment and activation of the tyrosine kinase Syk (87). This induces the LAT/PLCγ signaling pathway cascade and Ca2+ flux, which culminates in the exocytotic release of secretory granules (86). Other protein kinases, namely, Fyn and Fgr are required for the regulation of phospholipase D2 activation and degranulation (88). Furthermore, degranulation is a hallmark of immediate hypersensitivity reactions. The β-hexosaminidase activity is widely measured as a degranulation marker instead of histamine in IgE-sensitized RBL-2H3 cells. Late-phase Type I hypersensitivity reactions typically involve the formation of inflammatory mediators. Syk/LAT activation induces downstream MAPKs, which are involved in the production of IL-4 and TNF-α (89). In our previous study, RBL-2H3 cells were sensitized overnight using 2% anti-shrimp tropomyosin mouse serum and 100 ng/ml ST challenge for 1.5 h or 6 h, respectively, β-hexosaminidase release (33), or elevating the IL-4 level (59) in cells. Other cell lines, such as human mast cell line HMC-1, human peripheral blood basophilic leukemia cells KU812, and mouse mastocytoma P815 cell, lack FcεRI. The human leukemic mast cell line LAD2 possess FcεRI but still exhibits a very slow growth rate (90). Compound 48/80 or calcium ionophore induced-HMC1 or LAD2 cell degranulation model do not involve the initial events of FcεRI signaling upstream of Ca2+ influx. Primary cells such as rat peritoneal mast cells and mouse bone marrow-derived mast cells closely mimic in vivo biological responses. However, the quality of rat peritoneal mast cells remains insufficient. Mouse bone marrow-derived mast cells require 4–6 weeks for differentiation, maturation, and senescence after a brief culture period (91). IgE-dependent mast cell degranulation is shown in Figure 2.
4 Perspectives
Foreign allergens trigger Type I hypersensitivity, and few preventive therapies have been established apart from strict dietary/environmental exposure or allergen-specific immunotherapy. Thus, animal and cellular models are helpful for exploring potential molecular targets and screening drugs. An ideal animal model would require the same amount of allergen via the same route to induce sensitization and duration of exposure and present the same Th2 response as that in humans (92). Some biological agents that block Th2 response, such as anti-IL-4Rα mAb, anti-IL-5/IL-5R mAb, anti-IgE mAb, are used for patients with allergic diseases (93). However, not all findings obtained from animal models may be replicated in human diseases. For example, although IL-17 blocking is effective in mouse models, a clinical trial using an anti-IL-17-receptor antibody failed to improve asthma exacerbation (94). Accordingly, the limitations of mouse models should be considered in the protocol design and interpretation of results.
In our previous studies, shrimp tropomyosin without alum adjuvant elicited a higher increase in total IgE levels in mouse sera compared with that of OVA, indicating greater allergenic potential. Various models, including shrimp tropomyosin-induced PCA, PSA, ASA, rhinitis/asthma mouse model, and RBL-2H3 cell degranulation model, have been established. Moreover, shrimp tropomyosin-induced asthma without adjuvants exhibited several hallmarks of human asthma, including high IgE production, AHR, eosinophilic inflammation, Th2 response, and airway remodeling. Owing to the advantage of sensitization without requiring an adjuvant, shrimp tropomyosin may be suitable for local sensitization when administered by intranasal or intratracheal instillation without an adjuvant, similar to that of HDM. In a future study, we plan to explore a modified protocol for establishing shrimp tropomyosin-induced asthma model and compare the allergenic potency between shrimp tropomyosin and HDM.
Author contributions
YF: Investigation, Writing – original draft, Writing – review & editing. LX: Investigation, Writing – original draft. JZ: Investigation, Writing – original draft. JB: Investigation, Project administration, Writing – original draft. XP: Investigation, Writing – original draft. SH: Funding acquisition, Project administration, Writing – original draft, Writing – review & editing. LF: Conceptualization, Funding acquisition, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Opening Topic Fund of Guangxi Key Laboratory of Reproductive Health and Birth Defects Prevention (GXWCH-ZDKF-2023-03), the Postdoctoral Innovation Practice Base Fund of Maternal and Child Health Hospital of Guangxi Zhuang Autonomous Region (GXFYKYQD-07), and the General Program of Jiangsu Provincial Health Commission (M2022034).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Descotes J, Choquet-Kastylevsky G. Gell and Coombs’s classification: is it still valid? Toxicology. (2001) 158(1–2):43–9. doi: 10.1016/s0300-483x(00)00400-5
2. Nakamura T. The roles of lipid mediators in type I hypersensitivity. J Pharmacol Sci. (2021) 147(1):126–31. doi: 10.1016/j.jphs.2021.06.001
3. Knol EF, Gilles S. Allergy: type I, II, III, and IV. Handb Exp Pharmacol. (2022) 268:31–41. doi: 10.1007/164_2021_510
4. Nappi E, Paoletti G, Malvezzi L, Ferri S, Racca F, Messina MR, et al. Comorbid allergic rhinitis and asthma: important clinical considerations. Expert Rev Clin Immunol. (2022) 18(7):747–58. doi: 10.1080/1744666X.2022.2089654
5. Guarnieri KM, Saba NK, Schwartz JT, Devonshire AL, Bufford J, Casale TB, et al. Food allergy characteristics associated with coexisting eosinophilic esophagitis in FARE registry participants. J Allergy Clin Immunol Pract. (2023) 11(5):1509–1521.e6. doi: 10.1016/j.jaip.2023.02.008
6. Incorvaia C, Mauro M, Gritti BL, Makri E, Ridolo E. Venom immunotherapy in patients with allergic reactions to insect stings. Expert Rev Clin Immunol. (2018) 14(1):53–9. doi: 10.1080/1744666X.2018.1413350
7. Pichler WJ. Immune pathomechanism and classification of drug hypersensitivity. Allergy. (2019) 74(8):1457–71. doi: 10.1111/all.13765
8. Bilò MB, Martini M, Tontini C, Corsi A, Antonicelli L. Anaphylaxis. Eur Ann Allergy Clin Immunol. (2021) 53(1):4–17. doi: 10.23822/EurAnnACI.1764-1489.158
9. Kim EY, Sturgill JL, Hait NC, Avni D, Valencia EC, Maceyka M, et al. Role of sphingosine kinase 1 and sphingosine-1-phosphate in CD40 signaling and IgE class switching. FASEB J. (2014) 28(10):4347–58. doi: 10.1096/fj.14-251611
10. Shamji MH, Valenta R, Jardetzky T, Verhasselt V, Durham SR, Würtzen PA, et al. The role of allergen-specific IgE, IgG and IgA in allergic disease. Allergy. (2021) 76(12):3627–41. doi: 10.1111/all.14908
11. Ramos-Ramírez P, Noreby M, Liu JL, Ji J, Abdillahi SM, Olsson H, et al. A new house dust mite-driven and mast cell-activated model of asthma in the guinea pig. Clin Exp Allergy. (2020) 50(10):1184–95. doi: 10.1111/cea.13713
12. Riley JP, Fuchs B, Sjöberg L, Nilsson GP, Karlsson L, Dahlén SE, et al. Mast cell mediators cause early allergic bronchoconstriction in guinea-pigs: a model of relevance to asthma. Clin Sci. (2013) 125(11-12):533–42. doi: 10.1042/Cs20130092
13. Jung HS, Kim MH, Gwak NG, Im YS, Lee KY, Sohn Y, et al. Antiallergic effects of on inflammation in vivo and in vitro. J Ethnopharmacol. (2012) 141(1):345–9. doi: 10.1016/j.jep.2012.02.044
14. Knippels LMJ, Penninks AH. Assessment of protein allergenicity—studies in Brown Norway rats. Ann N Y Acad Sci. (2002) 964:151–61. doi: 10.1111/j.1749-6632.2002.tb04140.x
15. Gustafsson Å, Jonasson S, Sandström T, Lorentzen JC, Bucht A. Genetic variation influences immune responses in sensitive rats following exposure to TiO nanoparticles. Toxicology. (2014) 326:74–85. doi: 10.1016/j.tox.2014.10.004
16. Aun MV, Bonamichi-Santos R, Arantes-Costa FM, Kalil J, Giavina-Bianchi P. Animal models of asthma: utility and limitations. J Asthma Allergy. (2017) 10:293–301. doi: 10.2147/Jaa.S121092
17. Serra MF, Cotias AC, Pao CRR, Daleprane JB, Jurgilas PB, Couto GC, et al. Repeated allergen exposure in A/J mice causes steroid-insensitive asthma via a defect in glucocorticoid receptor bioavailability. J Immunol. (2018) 201(3):851–60. doi: 10.4049/jimmunol.1700933
18. Gueders MM, Paulissen G, Crahay C, Quesada-Calvo F, Hacha J, Van Hove C, et al. Mouse models of asthma: a comparison between C57BL/6 and BALB/c strains regarding bronchial responsiveness, inflammation, and cytokine production. Inflammation Res. (2009) 58(12):845–54. doi: 10.1007/s00011-009-0054-2
19. Ewart SL, Kuperman D, Schadt E, Tankersley C, Grupe A, Shubitowski DM, et al. Quantitative trait loci controlling allergen-induced airway hyperresponsiveness in inbred mice. Am J Respir Cell Mol Biol. (2000) 23(4):537–45. doi: 10.1165/ajrcmb.23.4.4199
20. Eschborn M, Weigmann B, Reissig S, Waisman A, Saloga J, Bellinghausen I. Activated glycoprotein A repetitions predominant (GARP)-expressing regulatory T cells inhibit allergen-induced intestinal inflammation in humanized mice. J Allergy Clin Immunol. (2015) 136(1):159–68. doi: 10.1016/j.jaci.2015.04.020
21. Alakhras NS, Shin J, Smith SA, Sinn AL, Zhang WW, Hwang G, et al. Peanut allergen inhibition prevents anaphylaxis in a humanized mouse model. Sci Transl Med. (2023) 15(682):eadd6373. doi: 10.1126/scitranslmed.add6373
22. Han SY, Bae JY, Park SH, Kim YH, Park JHY, Kang YH. Resveratrol inhibits IgE-mediated basophilic mast cell degranulation and passive cutaneous anaphylaxis in mice. J Nutr. (2013) 143(5):632–9. doi: 10.3945/jn.112.173302
23. Fang L, Zhou FC, Wu F, Yan Y, He ZP, Yuan XL, et al. A mouse allergic asthma model induced by shrimp tropomyosin. Int Immunopharmacol. (2021) 91:107289. doi: 10.1016/j.intimp.2020.107289
24. De Martinis M, Sirufo MM, Suppa M, Ginaldi L. New perspectives in food allergy. Int J Mol Sci. (2020) 21(4):1474. doi: 10.3390/ijms21041474
25. Oyoshi MK, Oettgen HC, Chatila TA, Geha RS, Bryce PJ. Food allergy: insights into etiology, prevention, and treatment provided by murine models. J Allergy Clin Immunol. (2014) 133(2):309–17. doi: 10.1016/j.jaci.2013.12.1045
26. Hesse L, Elberink JNGO, van Oosterhout AJM, Nawijn MC. Allergen immunotherapy for allergic airway diseases: use lessons from the past to design a brighter future. Pharmacol Ther. (2022) 237:108115. doi: 10.1016/j.pharmthera.2022.108115
27. Krishnamoorthy N, Douda DN, Brüggemann TR, Ricklefs I, Duvall MG, Abdulnour REE, et al. Neutrophil cytoplasts induce T17 differentiation and skew inflammation toward neutrophilia in severe asthma. Sci Immunol. (2018) 3(26):eaao4747. doi: 10.1126/sciimmunol.aao4747
28. Gao XY, Qin LL, Zhang ZX, Zhao HZ, Zhou WJ, Xie ZY, et al. Deciphering biochemical basis of qingkailing injection-induced anaphylaxis in a rat model by time-dependent metabolomic profiling based on metabolite polarity-oriented analysis. J Ethnopharmacol. (2018) 225:287–96. doi: 10.1016/j.jep.2018.07.013
29. Choi KS, Shin TS, Chun J, Ahn G, Han EJ, Kim MJ, et al. Sargahydroquinoic acid isolated from Sargassum serratifolium as inhibitor of cellular basophils activation and passive cutaneous anaphylaxis in mice. Int Immunopharmacol. (2022) 105:108567. doi: 10.1016/j.intimp.2022.108567
30. Cao J, Li CM, Ma PY, Ding YY, Gao JP, Jia QQ, et al. Effect of kaempferol on IgE-mediated anaphylaxis in C57BL/6 mice and LAD2 cells. Phytomedicine. (2020) 79:153346. doi: 10.1016/j.phymed.2020.153346
31. Mackl M, Tonc E, Ashbaugh A, Wetzel A, Sykes A, Engblom C, et al. Clonal differences in IgE antibodies affect cutaneous anaphylaxis-associated thermal sensitivity in mice. Immunol Lett. (2014) 162(1):149–58. doi: 10.1016/j.imlet.2014.08.007
32. Bryce PJ, Falahati R, Kenney LL, Leung J, Bebbington C, Tomasevic N, et al. Humanized mouse model of mast cell-mediated passive cutaneous anaphylaxis and passive systemic anaphylaxis. J Allergy Clin Immunol. (2016) 138(3):769–79. doi: 10.1016/j.jaci.2016.01.049
33. Gao Y, Hou R, Fei QL, Fang L, Han YX, Cai RL, et al. The Three-Herb Formula Shuang-Huang-Lian stabilizes mast cells through activation of mitochondrial calcium uniporter. Sci Rep. (2017) 7:38736. doi: 10.1038/srep38736
34. Lu Y, Yang JH, Li X, Hwangbo K, Hwang SL, Taketomi Y, et al. Emodin, a naturally occurring anthraquinone derivative, suppresses IgE-mediated anaphylactic reaction and mast cell activation. Biochem Pharmacol. (2011) 82(11):1700–8. doi: 10.1016/j.bcp.2011.08.022
35. Huang ZQ, Li Y, Yi HK, Wu ZC, Li C, Du TF, et al. Absence of active systemic anaphylaxis in guinea pigs upon intramuscular injection of inactivated SARS-CoV-2 vaccine (vero cells). Immunopharmacol Immunotoxicol. (2022) 44(5):633–40. doi: 10.1080/08923973.2022.2073889
36. Cianferoni A. Non-IgE-mediated anaphylaxis. J Allergy Clin Immunol. (2021) 147(4):1123–31. doi: 10.1016/j.jaci.2021.02.012
37. Tsujimura Y, Obata K, Mukai K, Shindou H, Yoshida M, Nishikado H, et al. Basophils play a pivotal role in immunoglobulin-G-mediated but not immunoglobulin-E-mediated systemic anaphylaxis. Immunity. (2008) 28(4):581–9. doi: 10.1016/j.immuni.2008.02.008
38. Jönsson F, de Chaisemartin L, Granger V, Gouel-Chéron A, Gillis CM, Zhu QQ, et al. An IgG-induced neutrophil activation pathway contributes to human drug-induced anaphylaxis. Sci Transl Med. (2019) 11(500): eaat1479. doi: 10.1126/scitranslmed.aat1479
39. Jönsson F, Mancardi DA, Kita Y, Karasuyama H, Iannascoli B, Van Rooijen N, et al. Mouse and human neutrophils induce anaphylaxis. J Clin Invest. (2011) 121(4):1484–96. doi: 10.1172/Jci45232
40. Peters RL, Krawiec M, Koplin JJ, F A. Santos: update on food allergy. Pediatr Allergy Immunol. (2021) 32(4):647–57. doi: 10.1111/pai.13443
41. Esber N, Mauras A, Delannoy J, Labellie C, Mayeur C, Caillaud MA, et al. Three candidate probiotic strains impact gut Microbiota and induce anergy in mice with Cow’s milk allergy. Appl Environ Microbiol. (2020) 86(21):e01203–20. doi: 10.1128/AEM.01203-20
42. Smith NA, Nagamoto-Combs K. Induction of hypersensitivity with purified beta-lactoglobulin as a mouse model of Cow’s milk allergy. Methods Mol Biol. (2021) 2223:67–78. doi: 10.1007/978-1-0716-1001-5_5
43. Xie Y, Shao H, Hu X, Hua X, Meng X, Chen H. Characterization of systemic allergenicity of tropomyosin from shrimp (macrobrachium nipponense) and anaphylactic reactions in digestive tract. J Sci Food Agric. (2021) 101(7):2940–9. doi: 10.1002/jsfa.10926
44. Capobianco F, Butteroni C, Barletta B, Corinti S, Afferni C, Tinghino R, et al. Oral sensitization with shrimp tropomyosin induces in mice allergen-specific IgE, T cell response and systemic anaphylactic reactions. Int Immunol. (2008) 20(8):1077–86. doi: 10.1093/intimm/dxn065
45. Dolence JJ, Kobayashi T, Iijima K, Krempski J, Drake LY, Dent AL, et al. Airway exposure initiates peanut allergy by involving the IL-1 pathway and T follicular helper cells in mice. J Allergy Clin Immunol. (2018) 142(4):1144–1158.e8. doi: 10.1016/j.jaci.2017.11.020
46. Bartnikas LM, Gurish MF, Burton OT, Leisten S, Janssen E, Oettgen HC, et al. Epicutaneous sensitization results in IgE-dependent intestinal mast cell expansion and food-induced anaphylaxis. J Allergy Clin Immunol. (2013) 131(2):451–60.e1-6. doi: 10.1016/j.jaci.2012.11.032
47. Kameda K, Takahashi E, Kimoto T, Morita R, Sakai S, Nagao M, et al. A murine model of food allergy by epicutaneous adjuvant-free allergen sensitization followed by oral allergen challenge combined with aspirin for enhanced detection of hypersensitivity manifestations and immunotherapy monitoring. Nutrients. (2023) 15(3):757. doi: 10.3390/nu15030757
48. Liu M, Thijssen S, van Nostrum CF, Hennink WE, Garssen J, Willemsen LEM. Inhibition of cow’s milk allergy development in mice by oral delivery of beta-lactoglobulin-derived peptides loaded PLGA nanoparticles is associated with systemic whey-specific immune silencing. Clin Exp Allergy. (2022) 52(1):137–48. doi: 10.1111/cea.13967
49. Nunes IV, Andrade CM, Guerra PV, Khouri MI, Galantini MPL, da Silva RAA, et al. A new experimental model to study shrimp allergy. Immunol Lett. (2023) 260:73–80. doi: 10.1016/j.imlet.2023.06.007
50. Li Xu L, Wei Zhang H, Lin H, Mei Zhang X, Qi Wen Y, Long Zhao J, et al. SWATH-MS-based proteomics reveals functional biomarkers of Th1/Th2 responses of tropomyosin allergy in mouse models. Food Chem. (2022) 383:132474. doi: 10.1016/j.foodchem.2022.132474
51. Wagenaar L, Bol-Schoenmakers M, Giustarini G, Vonk MM, van Esch B, Knippels LMJ, et al. Dietary supplementation with nondigestible oligosaccharides reduces allergic symptoms and supports low dose oral immunotherapy in a peanut allergy mouse model. Mol Nutr Food Res. (2018) 62(20):e1800369. doi: 10.1002/mnfr.201800369
52. Shi J, Dong P, Liu C, Xu Y, Zheng M, Cheng L, et al. Lactobacillus rhamnosus Probio-M9 alleviates OVA-sensitized food allergy through modulating gut microbiota and its metabolism. Food Funct. (2023) 14(24):10784–95. doi: 10.1039/d3fo03321j
53. Blume C, Davies DE. In vitro and ex vivo and models of human asthma. Eur J Pharm Biopharm. (2013) 84(2):394–400. doi: 10.1016/j.ejpb.2012.12.014
54. Pauluhn M, Mohr U. Experimental approaches to evaluate respiratory allergy in animal models. Exp Toxicol Pathol. (2005) 56(4-5):203–34. doi: 10.1016/j.etp.2004.10.002
55. Périz M, Pérez-Cano FJ, Rodríguez-Lagunas MJ, Cambras T, Pastor-Soplin S, Best I, et al. Development and characterization of an allergic asthma rat model for interventional studies. Int J Mol Sci. (2020) 21(11):3841. doi: 10.3390/ijms21113841
56. Matsuda M, Tanaka Y, Shimora H, Takemoto N, Nomura M, Terakawa R, et al. Pathogenic changes in group 2 innate lymphoid cells (ILC2s) in a steroid-insensitive asthma model of mice. Eur J Pharmacol. (2022) 916: 174732. doi: 10.1016/j.ejphar.2021.174732
57. Yi L, Zhou YL, Song JR, Tang WF, Yu H, Huang X, et al. A novel iridoid glycoside leonuride (ajugol) attenuates airway inflammation and remodeling through inhibiting type-2 high cytokine/chemokine activity in OVA-induced asthmatic mice. Phytomedicine. (2022) 105:154345. doi: 10.1016/j.phymed.2022.154345
58. Sun YB, Liu M, Fan XS, Zhou LP, Li MW, Hu FY, et al. Effects of cigarette smoke on the aggravation of ovalbumin-induced asthma and the expressions of TRPA1 and tight junctions in mice. Mol Immunol. (2021) 135:62–72. doi: 10.1016/j.molimm.2021.04.006
59. Fang L, Yan Y, Xu ZX, He ZP, Zhou ST, Jiang X, et al. Tectochrysin ameliorates murine allergic airway inflammation by suppressing Th2 response and oxidative stress. Eur J Pharmacol. (2021) 902:174100. doi: 10.1016/j.ejphar.2021.174100
60. Li XR, Hou RT, Ding H, Gao X, Wei ZC, Qi T, et al. Mollugin ameliorates murine allergic airway inflammation by inhibiting Th2 response and M2 macrophage activation. Eur J Pharmacol. (2023) 946:175630. doi: 10.1016/j.ejphar.2023.175630
61. Habjanec L, Frkanec R, Halassy B, Tomasic J. Effect of liposomal formulations and immunostimulating peptidoglycan monomer (PGM) on the immune reaction to ovalbumin in mice. J Liposome Res. (2006) 16(1):1–16. doi: 10.1080/08982100500528537
62. Özkan M, Eskiocak YC, Wingender G. Macrophage and dendritic cell subset composition can distinguish endotypes in adjuvant-induced asthma mouse models. PLoS One. (2021) 16(6):e0250533. doi: 10.1371/journal.pone.0250533
63. Xu WJ, Wang YM, Ma Y, Yang J. MiR-223 plays a protecting role in neutrophilic asthmatic mice through the inhibition of NLRP3 inflammasome. Respir Res. (2020) 21(1):116. doi: 10.1186/s12931-020-01374-4
64. Santos da Silva E, Asam C, Lackner P, Hofer H, Wallner M, Silva Pinheiro C, et al. Allergens of blomia tropicalis: an overview of recombinant molecules. Int Arch Allergy Immunol. (2017) 172(4):203–14. doi: 10.1159/000464325
65. Belkadi A, Dietrich C, Machavoine F, Victor JR, Leite-de-Moraes M. Gammadelta T cells amplify blomia tropicalis-induced allergic airway disease. Allergy. (2019) 74(2):395–8. doi: 10.1111/all.13618
66. Tan HTT, Hagner S, Ruchti F, Radzikowska U, Tan G, Altunbulakli C, et al. Tight junction, mucin, and inflammasome-related molecules are differentially expressed in eosinophilic, mixed, and neutrophilic experimental asthma in mice. Allergy. (2019) 74(2):294–307. doi: 10.1111/all.13619
67. Wang W, An G, Li Y, Corrigan CJ, Wang W, Ying S, et al. Similarities and differences in the effects of sensitisation and challenge with Dermatophagoides farinae and Dermatophagoides pteronyssinus extracts in a murine asthma surrogate. Cell Immunol. (2020) 348:104038. doi: 10.1016/j.cellimm.2020.104038
68. Zhang Z, Cai Z, Hou Y, Hu J, He Y, Chen J, et al. Enhanced sensitivity of capture IgE-ELISA based on a recombinant Der f 1/2 fusion protein for the detection of IgE antibodies targeting house dust mite allergens. Mol Med Rep. (2019) 19(5):3497–504. doi: 10.3892/mmr.2019.10050
69. Dai C, Yao X, Gordon EM, Barochia A, Cuento RA, Kaler M, et al. A CCL24-dependent pathway augments eosinophilic airway inflammation in house dust mite-challenged Cd163(−/−) mice. Mucosal Immunol. (2016) 9(3):702–17. doi: 10.1038/mi.2015.94
70. Trompette A, Divanovic S, Visintin A, Blanchard C, Hegde RS, Madan R, et al. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature. (2009) 457(7229):585–8. doi: 10.1038/nature07548
71. Radermecker C, Sabatel C, Vanwinge C, Ruscitti C, Maréchal P, Perin F, et al. Locally instructed CXCR4 neutrophils trigger environment-driven allergic asthma through the release of neutrophil extracellular traps. Nat Immunol. (2019) 20(11):1444. doi: 10.1038/s41590-019-0496-9
72. Gao LC, Wu M, Liu HY, He M, Jiang H, Shang RS, et al. Neonatal LPS administered before sensitization reduced the number of inflammatory monocytes and abrogated the development of OVA-induced Th2 allergic airway inflammation. Front Immunol. (2021) 12:725906. doi: 10.3389/fimmu.2021.725906
73. Bergquist M, Jonasson S, Hjoberg J, Hedenstierna G, Hanrieder J. Comprehensive multiplexed protein quantitation delineates eosinophilic and neutrophilic experimental asthma. BMC Pulm Med. (2014) 14:110. doi: 10.1186/1471-2466-14-110
74. James BN, Weigel C, Green CD, Brown RDR, Palladino END, Tharakan A, et al. Neutrophilia in severe asthma is reduced in Ormdl3 overexpressing mice. FASEB J. (2023) 37(3):e22799. doi: 10.1096/fj.202201821R
75. Tirpude NV, Sharma A, Joshi R, Kumari M, Acharya V. Vitex negundo Linn. extract alleviates inflammatory aggravation and lung injury by modulating AMPK/PI3K/Akt/p38-NF-κB and TGF-β/Smad/Bcl2/caspase/LC3 cascade and macrophages activation in murine model of OVA-LPS induced allergic asthma. J Ethnopharmacol. (2021) 271:113894. doi: 10.1016/j.jep.2021.113894
76. Park SJ, Im DS. Blockage of sphingosine-1-phosphate receptor 2 attenuates allergic asthma in mice. Br J Pharmacol. (2019) 176(7):938–49. doi: 10.1111/bph.14597
77. Kim DI, Song MK, Lee K. Comparison of asthma phenotypes in OVA-induced mice challenged via inhaled and intranasal routes. BMC Pulm Med. (2019) 19(1):241. doi: 10.1186/s12890-019-1001-9
78. Xiang QW, Yan XM, Lin XX, Zheng H, Wang LK, Wan JY, et al. Intestinal microflora altered by vancomycin exposure in early life up-regulates type 2 innate lymphocyte and aggravates airway inflammation in asthmatic mice. Inflammation. (2023) 46(2):509–21. doi: 10.1007/s10753-022-01748-4
79. Cui LL, Qin XF, Fu TT, Li CD, Wang D, Hu Y, et al. Attenuated airways inflammation and remodeling in IL-37a and IL-37b transgenic mice with an ovalbumin-induced chronic asthma. Cell Immunol. (2023) 391:104759. doi: 10.1016/j.cellimm.2023.104759
80. Youssef M, De Sanctis JB, Kanagaratham C, Tao S, Ahmed E, Radzioch D. Efficacy of optimized treatment protocol using LAU-7b formulation against ovalbumin (OVA) and house dust mite (HDM) -induced allergic asthma in atopic hyperresponsive A/J mice. Pharm Res. (2020) 37(2):31. doi: 10.1007/s11095-019-2743-z
81. Lechner A, Henkel FDR, Hartung F, Bohnacker S, Alessandrini F, Gubernatorova EO, et al. Macrophages acquire a TNF-dependent inflammatory memory in allergic asthma. J Allergy Clin Immunol. (2022) 149(6):2078–90. doi: 10.1016/j.jaci.2021.11.026
82. Zhang M, Yu QY, Tang W, Wu YJ, Lv JJ, Sun L, et al. Epithelial exosomal contactin-1 promotes monocyte-derived dendritic cell-dominant T-cell responses in asthma. J Allergy Clin Immunol. (2021) 148(6):1545–58. doi: 10.1016/j.jaci.2021.04.025
83. Liao WP, Foo HYC, Tran TNQ, Chai CLL, Wong WSF. Calcaratarin D, a labdane diterpenoid, attenuates mouse asthma via modulating alveolar macrophage function. Br J Pharmacol. (2023) 180(8):1056–71. doi: 10.1111/bph.15993
84. Liu ZC, Hao LF, Wang NN, Zhang S, Zhang N, Xu ZZ, et al. Construction and identification of the recombinant hFcεRIα/RBL-2H3 cells. Plasmid. (2018) 98:31–6. doi: 10.1016/j.plasmid.2018.09.004
85. Waritani T, Lomax S, Cutler D, Chang JSC. Development and evaluation of mouse anti-Ara h 1 and Ara h 3 IgE monoclonal antibodies for advancing peanut allergy research. Methodsx. (2023) 11:102470. doi: 10.1016/j.mex.2023.102470
86. Xu C, Li LC, Wang CY, Jiang JZ, Li L, Zhu LH, et al. Effects of G-Rh2 on mast cell-mediated anaphylaxis via AKT-Nrf2/NF-?B and MAPK-Nrf2/NF-?B pathways. J Ginseng Res. (2022) 46(4):550–60. doi: 10.1016/j.jgr.2021.10.001
87. Kanagy WK, Cleyrat C, Fazel M, Lucero SR, Bruchez MP, Lidke KA, et al. Docking of Syk to FcepsilonRI is enhanced by Lyn but limited in duration by SHIP1. Mol Biol Cell. (2022) 33(10):ar89. doi: 10.1091/mbc.E21-12-0603
88. Choi WS, Hiragun T, Lee JH, Kim YM, Kim HP, Chahdi A, et al. Activation of RBL-2H3 mast cells is dependent on tyrosine phosphorylation of phospholipase D2 by Fyn and Fgr. Mol Cell Biol. (2004) 24(16):6980–92. doi: 10.1128/Mcb.24.16.6980-6992.2004
89. Kim JK, Seo YK, Park S, Park SA, Lim S, Lee S, et al. Spiraeoside inhibits mast cells activation and IgE-mediated allergic responses by suppressing phospholipase C-γ-mediated signaling. Biochem Cell Biol. (2015) 93(3):227–35. doi: 10.1139/bcb-2014-0055
90. Hermans MAW, van Stigt AC, van de Meerendonk S, Schrijver B, van Daele PLA, van Hagen PM, et al. Human mast cell line HMC1 expresses functional mas-related G-protein coupled receptor 2. Front Immunol. (2021) 12:625284. doi: 10.3389/fimmu.2021.625284
91. Shim JK, Kennedy RH, Weatherly LM, Abovian AV, Hashmi HN, Rajaei A, et al. Searching for tryptase in the RBL-2H3 mast cell model: preparation for comparative mast cell toxicology studies with zebrafish. J Appl Toxicol. (2019) 39(3):473–84. doi: 10.1002/jat.3738
92. Gouel-Cheron A, Dejoux A, Lamanna E, Bruhns P. Animal models of IgE anaphylaxis. Biology. (2023) 12(7):931. doi: 10.3390/biology12070931
93. Tan R, Liew MF, Lim HF, Leung BP, Wong WSF. Promises and challenges of biologics for severe asthma. Biochem Pharmacol. (2020) 179:114012. doi: 10.1016/j.bcp.2020.114012
Keywords: type I hypersensitivity, allergy, IgE, ovalbumin, RBL-2H3
Citation: Feng Y, Xu L, Zhang J, Bin J, Pang X, He S and Fang L (2024) Allergenic protein-induced type I hypersensitivity models: a review. Front. Allergy 5:1481011. doi: 10.3389/falgy.2024.1481011
Received: 15 August 2024; Accepted: 4 October 2024;
Published: 17 October 2024.
Edited by:
Wai Tuck Soh, Max Planck Institute for Multidisciplinary Sciences, GermanyReviewed by:
Alexis Labrada, National Center of Bioproducts (BIOCEN), CubaVanesa Esteban, Health Research Institute Foundation Jimenez Diaz (IIS-FJD), Spain
Copyright: © 2024 Feng, Xu, Zhang, Bin, Pang, He and Fang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Fang, ZmFuZ2xlaUB5enUuZWR1LmNu; Sheng He, aGVzaGVuZ2Jpb2xAMTYzLmNvbQ==
 Yanhua Feng
Yanhua Feng Liangyu Xu2
Liangyu Xu2 Sheng He
Sheng He Lei Fang
Lei Fang