- 1Division of Gastroenterology, Hepatology, and Endoscopy, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, United States
- 2Department of Medicine, NYU Grossman School of Medicine, New York, NY, United States
- 3Department of Dermatology, University of Utah, Salt Lake City, UT, United States
- 4Division of Pathology-Lab Medicine Division, Department of Hematopathology, MD Anderson Cancer Center, Houston, TX, United States
- 5Division of Hematology and Medical Oncology, Mayo Clinic, Phoenix, AZ, United States
- 6Department of Malignant Hematology, H. Lee Moffitt Cancer Center, Tampa, FL, United States
- 7ARUP Laboratories, Department of Pathology, University of Utah School of Medicine, Salt Lake City, UT, United States
- 8Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, United States
Systemic mastocytosis (SM) is a rare hematologic condition characterized by the proliferation and accumulation in tissue of clonal mast cells in multiple organ systems. The release of mast cell mediators in the indolent disease type and the predominant mast cell infiltration of tissues in advanced disease contribute to the heterogeneous clinical presentation. The disease driver in >90% of adult cases is an activating KIT mutation, with D816V being the most frequent. Here we describe a case of a young adult male presenting with osteoporosis with associated symptoms of reflux and a history of bee sting anaphylaxis. A multidisciplinary approach to the diagnosis and management was required to minimize morbidities and prevent complications. Current best supportive care was inadequate to control the patient's disease, and a selective KIT D816V inhibitor (avapritinib) was initiated. Conventional, and advanced therapies, including those in the treatment pipeline for SM are discussed.
Introduction
Systemic mastocytosis (SM) is a rare hematologic disorder characterized by clonal proliferation and accumulation of mast cells (MC) in different organ systems, including bone marrow (BM), skin, gastrointestinal (GI) tract, liver, spleen, bone, and lymph nodes, resulting in a heterogeneous clinical presentation (1–14) (Table 1). Adult patients can present with or without cutaneous MC lesions and systemic symptoms, often necessitating consultation with a variety of medical specialists. Approximately half of patients with SM will experience systemic MC activation leading to anaphylaxis and need for emergency care.
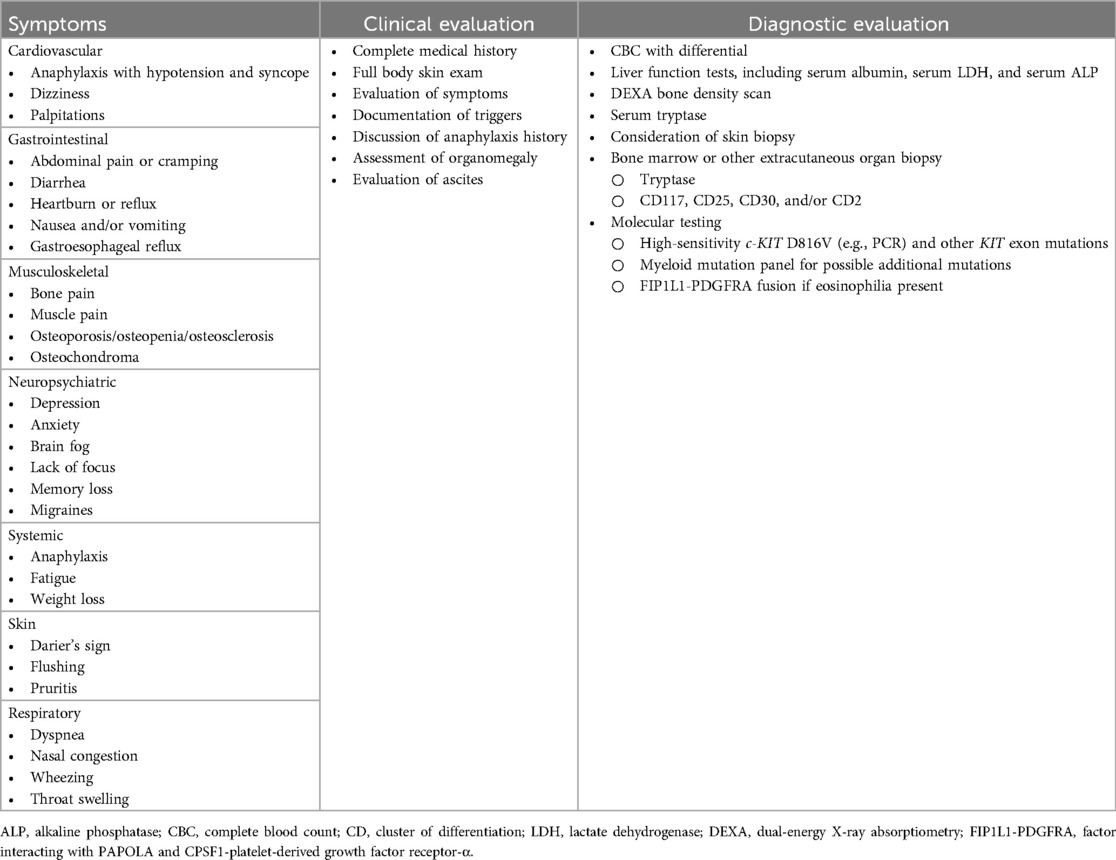
Table 1. Symptom presentation and clinical and laboratory evaluation of systemic mastocytosis (3–14).
Release of MC mediators leads to organ- and tissue-specific symptoms that can be episodic or chronic, with severe exacerbations, and result in poor quality of life. Misdiagnosis and underdiagnosis occur frequently due to the multi-organ involvement and range of systemic symptoms (8, 15–23). Eighty percent of all cases of SM are indolent, and the clinical case presented focuses on challenging diagnostic and treatment considerations (Table 1) (3–14). indolent systemic mastocytosis (ISM) may present with a range of signs and symptoms, including life-threatening acute episodes of mast cell activation (24).
Case description
A 44-year-old male truck driver slipped and fell on ice, landing on his back while at work. Over the next month, he developed lower back and calf muscle pain exacerbated by walking and standing. He sustained a lumbar spinal fracture 10 years prior during heavy lifting. The patient also reported reflux symptoms, right-sided abdominal pain, and generalized abdominal cramping with loose stools triggered by certain foods, heat, stress, and occasional alcohol use. He was regularly taking a proton-pump inhibitor (PPI) antacid for reflux and was not taking NSAIDs or narcotics.
The patient was initially seen at a spine clinic. Workup was unremarkable, but a second opinion with a neurologist for continued back pain revealed a vertebral body T3 “burst” compression fracture on MRI. Bone density exam showed osteoporosis in the spine (L1-L4 Z score −2.3, T score −2.5) and osteopenia in the hip (Z score −2.1, T score −2.4) and femoral head (Z score −1.2, T score −1.8), and bone imaging showed no evidence of osteolytic lesions. Rheumatologic workup revealed low serum vitamin D (16 ng/ml; normal range, 20–80 ng/ml) and normal thyroid, parathyroid, testosterone, and serum protein electrophoresis. Endocrinology was consulted to assess the diagnosis of osteoporosis in a young male. Laboratory testing revealed a normal celiac disease panel, normal collagen telopeptide for bone turnover (149 pg/ml, normal range 93–630 pg/ml), and an elevated serum tryptase (41.9 ng/ml; normal, <11.4 ng/ml), a minor criterion for SM. Teriparatide was prescribed for osteoporosis because of concern for exacerbation of his acid reflux with bisphosphonates.
The patient was referred to a mastocytosis center due to osteoporosis and elevated baseline serum tryptase (BST). Additional history revealed that he had anaphylaxis associated with loss of consciousness after two separate hornet sting episodes (at age 19 and 39). During the second sting, the patient required three doses of epinephrine for resuscitation. He had an additional episode of syncope when working outside on a hot day, wherein he felt the onset of flushing and dizziness before losing consciousness. He also described intermittent itching sensations on his chest and flushing associated with exercise, heat, and mental stress. In addition to back pain, he was most limited at this time by profound mental fog, with memory and concentration difficulties, finding it difficult to carry out routine tasks. He had a history of recurrent hives and allergic rhinitis.
On physical examination, he was alert and oriented and appeared healthy, with a blood pressure of 139/86 mmHg, heart rate of 81 bpm, and a body mass index of 28. Oropharynx and tympanic membranes were clear without lesions, and nostrils were mildly congested with pale and boggy mucosa with clear secretions and no polyps. Neck, heart, lungs, joints, and abdomen findings were normal. Skin was without hives, flushing, or lesions of cutaneous mastocytosis (i.e., urticaria pigmentosa). He had dermatographism with wheal and flare reactions upon skin stroking. Neurologic exam was grossly normal.
Further testing showed normal blood counts without eosinophilia (0.07 K/μl) and normal liver and kidney function tests. Abdominal CT scan revealed cholelithiasis, nonobstructing renal calculi in the left kidney, and “haziness” of the mesentery in the left upper quadrant with associated lymphadenopathy (largest lymph node, 16 × 13 mm). Upper endoscopy revealed no evidence of erosive esophagitis, Barrett's esophagus, or peptic ulcer disease. Blood tests showed elevated immunoglobulin E (IgE) level of 234 kU/L (upper limit, 100 kU/L) and negative serum-specific IgE against Hymenoptera venom antigens (test performed 5 years after last venom-induced anaphylaxis). Intradermal skin testing was positive to white-faced hornet and bee venoms. Repeat BST was 38.5 ng/ml, and 24-hour urine collection revealed elevated prostaglandin F2-α at 14,858 pg/mg (normal, <5,205 pg/mg) and N-methylhistamine at 1,905 mg/dl (normal, <1,800 mg/dl). He tested positive for KIT D816V mutation in peripheral blood (low level detected by droplet digital PCR (ddPCR), fractional abundance 0.08%). Due to the elevated prostaglandin and history of flushing, he began full-strength aspirin daily and was educated on the use of epinephrine. He was also prescribed daily oral cromolyn sodium and a nonsedating H1 antihistamine.
Bone marrow biopsy revealed a moderately hypocellular marrow (30% cellular), with approximately 5% of the cellularity composed of dense MC aggregates (>15 MC) with CD25-positive MC and spindled morphology in >25% of the MC population (Figure 1). Bone marrow aspirate demonstrated that 2% to 3% of cells were MC with elongated nuclei and abundant cytoplasm containing granules with cytoplasmic extensions and no other abnormalities. Genomic DNA extracted from unfractionated BM cells analyzed by ddPCR was KIT D816V-positive with a low-level allele fraction of 1.8%. BST at the time of BM biopsy was 40 ng/ml, and prostaglandin metabolites were 4,822 pg/mg (normal, <5,205 pg/mg) on 325 mg of aspirin daily. The patient met major and minor WHO criteria for ISM diagnosis; B or C findings, required for AdvSM diagnosis, were absent (Supplementary Table S1) (24, 25). He started daily oral compounded ketotifen and continued daily H1 antihistamines. Oral cromolyn sodium was discontinued due to equivocal efficacy and possible side effects of fatigue. Despite not having an anaphylactic event in 5 years, he was considered high risk and was therefore started on omalizumab. He additionally underwent ultra-rush desensitization immunotherapy to Hymenoptera venom, with the plan to continue immunotherapy to bee and white-faced hornet venoms.
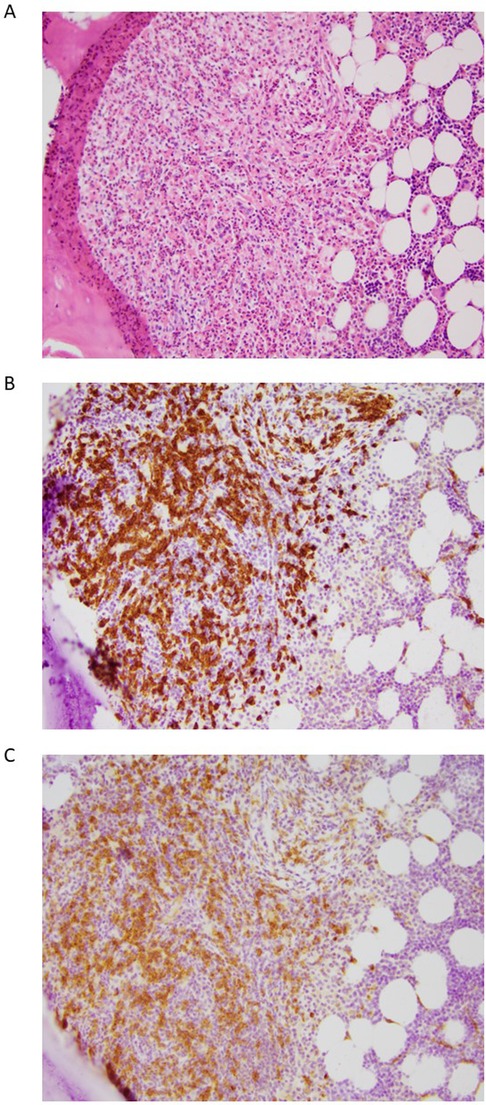
Figure 1. Representative histology images from a bone marrow biopsy of a patient with indolent systemic mastocytosis. The images show a mast cell aggregate in the paratrabecular space and many surrounding eosinophils. (A) Hematoxylin and eosin staining, (B) CD117 staining, (C) CD25 staining. All images presented at 20 × magnification.
Surgical consult determined that the abdominal lymph nodes were secondary to unrelated sclerosing mesenteritis, observed to be stable on serial imaging studies. Psychiatry was consulted for anxiety and depression, and he was started in mirtazapine and escitalopram. In follow-up with endocrinology, teriparatide was discontinued due to possible aggravation of joint pain and other side effects. Despite the original blood collagen telopeptide indicating relatively normal bone resorption, a single dose of zoledronic acid was prescribed (26). Follow-up bone density scan showed improvement in both hip (Z score −1.8) and spine (Z score −2.1).
The patient was able to return to work nearly 2 years following the vertebral fracture but continued exhibiting severe MC mediator symptoms, including flushing and mental fog, and remained limited due to ongoing mid-upper back pain attributed to paraspinal muscle spasms. It was therefore recommended that he enroll in a tyrosine kinase inhibitor clinical trial for symptomatic patients with ISM. He and his wife elected to defer the clinical trial due to their desire to start a family. He and his wife were successful in having a child, and while enjoying fatherhood, he continued experiencing ongoing symptoms of brain fog, fatigue, abdominal pain, and bone pain despite his medical regimen directed at MC mediators, including omalizumab. The patient elected to start a clinical trial with a KIT D816V-targeting tyrosine kinase inhibitor (avapritinib). To date, he has received 36 months of treatment and has noted improvement in many daily symptoms, with only occasional brain fog, fatigue, and bone pain persisting. He is no longer limited with his activities of daily living and can work full-time while parenting. His most recent BST was 7.1 ng/ml, and repeat BM biopsy showed low-level involvement with SM.
Discussion
The World Health Organization (WHO) defines 6 SM subtypes (Supplementary Table S1) (24, 25). Bone marrow–systemic mastocytosis (BMM), indolent SM (ISM), and smoldering SM (SSM) are chronic diseases with normal life expectancy, whereas aggressive SM (ASM), SM with an associated hematologic neoplasm (SM-AHN), and MC leukemia (MCL) are considered advanced diseases and have a reduced life expectancy that varies according to subtype. Across all types, the disease driver in >90% of adult cases is an activating KIT mutation, with D816V being the most frequent. The 2022 international consensus classification of SM was recently updated (27).
KIT, a tyrosine kinase surface receptor expressed by all MC, is involved in MC growth, proliferation, and activation. KIT mutations are acquired in SM, resulting in autophosphorylation and constitutive receptor activation independent of ligand binding. These lead to uncontrolled activation and accumulation of MC in the skin, BM, and other organs, which is a histologic hallmark of SM.
Osteoporosis in a 44-year-old male is unusual. SM can be associated with osteopenia, osteoporosis, and fracture and may be missed in a screening diagnostic workup if tryptase is not measured (27, 28). The trabecular bone of the spine is the most common location of ISM-associated bone disease, possibly because the trabecular bone's higher metabolic activity is suitable for abnormal MC proliferation. Notably, the patient's intake of PPI, which decreases stomach acid and impairs calcium absorption, further increases his osteoporosis risk.
Because this patient's collagen telopeptide was not elevated, an antiresorptive drug was chosen. Oral bisphosphonates (e.g., alendronate) often are poorly tolerated by patients with a history of acid reflux, which was a significant symptom for this patient (12, 29). Bone loss in SM may occur through the receptor activator of nuclear factor κ-B ligand (RANKL) system; therefore, denosumab is theoretically preferable to intravenous bisphosphonates based on mechanism and quick onset of action (26, 28). Other osteoporosis therapies include interferon-α combined with pamidronate, which was used in a small series of patients with ISM and improved bone density (30). Romosozumab, a drug that increases bone formation and decreases bone resorption, could also be used to treat osteoporosis in patients with ISM (31).
Up to 60% of patients with SM experience GI symptoms, although the prevalence varies in published reports (32). The effect of MC mediators most specific to patients with ISM (33, 34) include intermittent nausea, abdominal cramping, and loose stools, often triggered by a food or other exposure and commonly associated with flushing and pruritus. Involvement of acid-producing receptors in the stomach may result in peptic-type symptoms and an increased risk for peptic ulcer disease. Infiltration of abnormal MC underneath the epithelial surface of the intestine can cause chronic diarrhea and malabsorption symptoms, which is typically only observed in patients with advanced SM (AdvSM).
Endoscopy and colonoscopy with biopsy are important tools to establish a diagnosis of ISM in patients with GI symptoms who have not yet had a bone marrow biopsy, or to evaluate persistent or atypical GI symptoms in patients with an established diagnosis. Endoscopic findings in indolent disease can be normal, whereas patchy or diffusely congested intestinal mucosa appears with AdvSM. Random and targeted biopsies taken throughout the colon can reveal a patchy distribution of clonal MC by hematoxylin and eosin staining, KIT staining, and CD25 staining (35). Sheets and aggregates of clonal MC most often are distributed below the epithelial surface (36). Aggregates of ≥15 MC is a major WHO criterion for SM, and CD25-positive staining of MC within the aggregates is a minor criterion. Management may include PPIs for peptic ulcer disease and corticosteroids, such as oral budesonide, for chronic diarrhea and malabsorption secondary to MC infiltration in AdvSM. Sodium cromolyn has demonstrated anecdotal benefit in many patients; however, there are limited published studies demonstrating its efficacy (37).
A subset of patients with ISM may present initially with anaphylaxis with severe hypotension triggered by a Hymenoptera sting without classical cutaneous urticaria pigmentosa lesions (38) and may benefit from lifelong Hymenoptera venom immunotherapy (39). Skin testing should be done 4–6 weeks after the anaphylactic event since natural desensitization could lead to false negative results. In patients with positive skin test and/or positive specific IgE, “ultra-rush desensitization” is a novel and safe modality accomplished in one day with or without addition of omalizumab (40). Lifelong Hymenoptera venom immunotherapy is recommended, as several deaths have been reported from Hymenoptera stings in allergic ISM patients who discontinued immunotherapy (41). Omalizumab given concurrently with venom immunotherapy should also be considered because cases of anaphylaxis after a sting have occurred despite immunotherapy (42). Duration of treatment with omalizumab for patients on concomitant TKI has not been studied. The patient in this case was continued on omalizumab for 12 months to account for time to maximal clinical benefit of avapritinib and to ensure that there were no further anaphylactic events.
Serum tryptase should be evaluated in patients presenting with hypotension after a Hymenoptera sting. Two values should be obtained—one at the time of the reaction and one at least 24 h later which is considered baseline (BST). If the BST is elevated (>20 ng/ml), a BM biopsy is recommended to confirm the diagnosis of SM. If the BST is <20 ng/ml but >11.4 ng/ml, peripheral blood should be tested for the KIT D816V mutation, and a BM biopsy is recommended if positive. Allele burden for KIT D816V has prognostic value, with <2% allele burden present in patients with nonadvanced disease, whereas SSM and AdvSM present with >5% and >9% allele burden, respectively (43). In patients with a hypotensive episode after a Hymenoptera sting and BST >11.4 ng/ml and <20 ng/ml, a BM biopsy is recommended in the presence of negative KIT D816V mutation; lower sensitivity of the peripheral blood KIT D816V mutation is observed in patients with low mast cell burden.
All patients with a BST >8 ng/ml and signs and symptoms that suggest mast cell involvement should be evaluated for hereditary α-tryptasemia (HaT), a genetic trait present in 4% to 6% of the general population and defined by expression of extra copies of the MC tryptase gene TPSAB1 (44). An HaT diagnosis does not exclude SM, and patients with a BST >20 ng/ml are candidates for BM biopsy to rule out SM. Furthermore, because the risk of venom-induced anaphylaxis is increased in patients with ISM with coexisting HaT, it is recommended to test for KIT D816V if the baseline tryptase is >8 ng/ml and to perform BM biopsy if positive (45).
Cutaneous manifestations occur in the majority of adults with SM and are particularly common in the setting of ISM. Within a European registry of 1,510 adult patients, 79% presented with skin involvement (46). Typical lesions include diffuse red-brown macules and papules, 2–3 mm in diameter and monomorphic. A positive Darier's sign—erythema and wheal formation following mechanical stimulation—is often noted. In a minority of patients, telangiectatic lesions or fixed red macules may also affect the trunk and proximal extremities, although this is no longer favored as a distinct variant (1). Cutaneous manifestations in SM can be subtle or atypical (with either an uncharacteristic distribution (47–51) or morphology (52, 53)), necessitating a high index of suspicion in patients with additional compatible symptoms/signs. Patients may also present with nonspecific features, including isolated flushing, pruritus, and urticaria. Accurate identification of cutaneous lesions is essential given the implications of SM in the skin, including a high likelihood of systemic involvement in the adult population (1, 46, 54–56). Dermatology referral should be considered for appropriate characterization of disease, potential biopsy (to be interpreted by a pathologist/dermatopathologist familiar with the cutaneous histopathology of mastocytosis), and consideration of additional skin-directed therapies (57). Evolving evidence suggesting an increased risk of keratinocyte carcinoma and cutaneous melanoma in adult patients with SM further supports the importance of yearly skin surveillance (58, 59).
ISM treatment focuses on prevention and control of anaphylaxis and MC mediator–related symptoms, and treatment and prevention of osteoporosis. Available anti-mediator therapies include H1 and H2 histamine receptor blockers, leukotriene antagonists, aspirin, cromolyn sodium, and anti-IgE monoclonal antibody treatment (60, 61) (Table 2). To decrease disease burden in advanced diseases, cladribine, interferon-α, and hydroxyurea have shown efficacy. Tyrosine kinase inhibitors that target KIT include midostaurin and avapritinib, which were first approved for AdvSM (62, 63). In May 2023, the US Food and Drug Administration approved avapritinib for the treatment of adult patients with ISM (64, 65). Avapritinib is a selective KIT D816V inhibitor. The phase 2 PIONEER study (NCT03731260) evaluated the safety and efficacy of a low dose of avapritinib to reduce ISM symptoms and MC burden. This study met its primary endpoint—a significant reduction in total symptom scores at week 24 compared with placebo. Secondary endpoints included improved quality of life and decreased MC burden (66, 67). Midostaurin targets both KIT D816Y and D816V, and a phase 2 study (NCT01920204) in ISM demonstrated improvement in SM symptoms in 75% of patients at 12 weeks, 29% improvement in quality of life at 24 weeks, and reduction in MC burden (68).
Other tyrosine kinase inhibitors under investigation include elenestinib, a selective KIT D816V inhibitor with minimal central nervous system penetration; bezuclastinib, which targets KIT D816V and exon 17/18 loop mutations; masitinib, a selective inhibitor of wild-type KIT; and TL-895, a selective second-generation inhibitor of Bruton's tyrosine kinase. The safety and efficacy of each agent are currently being evaluated in ISM (elenestinib: HARBOR, NCT04910685; bezuclastinib: SUMMIT, NCT05186753; masitinib: NCT04333108; TL-895: NCT04655118) and AdvSM (elenestinib: AZURE, NCT05609942; bezuclastinib: APEX, NCT04996875).
ISM management and treatment are highly individualized and often require a multidisciplinary approach. An overall summary of this patient's case is presented in Table 3. Although symptoms can be effectively managed, some patients will have more refractory symptoms and complications, including life-threatening anaphylaxis and osteoporosis with fractures. Targeted, selective KIT D816V inhibitors have been shown to improve symptoms and survival with AdvSM and hold promise for patients with refractory non-AdvSM, including ISM. While studies support the use of current available therapies for ISM including TKIs, omalizumab, and venom immunotherapy with respect to safety and efficacy, the overall management is evolving, and additional considerations for treatment include cost and availability. Education and awareness for medical providers who treat these patients in collaboration with mast cell disorder centers are essential for proper diagnosis and management.
Patient perspective
It has been quite a journey to get to where I am today! Just the process of calling this mastocytosis was a challenge, especially when I was in a lot of pain. I had to tell my story and answer so many questions and do so many tests for what felt like years before I was on the right treatments. I was having so many other symptoms too, like brain fog, tired all the time, stomach pains that were not always treated. When we started the trial drug for mastocytosis I was nervous about side effects but have been thankful for it ever since. Things are not perfect, but I feel like I am getting my life back, which is a great thing.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Brigham and Women's Hospital Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
MH: Writing – original draft, Writing – review & editing. LG: Writing – review & editing. LM: Writing – review & editing. SW: Writing – review & editing. CAY: Writing – review & editing. AK: Writing – review & editing. TG: Writing – review & editing. MC: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The authors declare financial support was received for the publication fees for this article from Blueprint Medicines Corporation. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Acknowledgments
Editorial support was provided by Miranda Bader Goodman, PhD, of ProEd Communications, supported by Blueprint Medicines Corporation, according to Good Publication Practice guidelines.
Conflict of interest
MH reports serving on advisory boards for Blueprint Medicines Corporation and Allakos. LM reports serving on advisory boards for Blueprint Medicines Corporation and as a section editor for JAMA Dermatology. CAY reports serving on advisory boards for Blueprint Medicines Corporation and Cogent Biosciences. AK reports receiving research funding from Blueprint Medicines Corporation and honoraria from Blueprint Medicines Corporation and Novartis. TG reports receiving consulting fees and is a study steering committee member for Blueprint Medicines Corporation, BMS/Celgene, Cogent Biosciences, and Incyte. MC reports serving as a consultant and Principal Investigator on several clinical trials for Blueprint Medicines Corporation and has received author fees from UpToDate and the Editorial Board for Annals of Allergy, Asthma & Immunology.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/falgy.2024.1401187/full#supplementary-material
References
1. Hartmann K, Escribano L, Grattan C, Brockow K, Carter MC, Alvarez-Twose I, et al. Cutaneous manifestations in patients with mastocytosis: consensus report of the European Competence Network on Mastocytosis; the American Academy of Allergy, Asthma & Immunology; and the European Academy of Allergology and Clinical Immunology. J Allergy Clin Immunol. (2016) 137(1):35–45. doi: 10.1016/j.jaci.2015.08.034
2. Rossignol J, Polivka L, Maouche-Chretien L, Frenzel L, Dubreuil P, Hermine O. Recent advances in the understanding and therapeutic management of mastocytosis. F1000Res. (2019) 8:F1000 Faculty Rev-1961. doi: 10.12688/f1000research.19463.1
3. Amin K. The role of mast cells in allergic inflammation. Respir Med. (2012) 106(1):9–14. doi: 10.1016/j.rmed.2011.09.007
4. Gilreath JA, Tchertanov L, Deininger MW. Novel approaches to treating advanced systemic mastocytosis. Clin Pharmacol. (2019) 11:77–92. doi: 10.2147/CPAA.S206615
5. Gulen T, Hagglund H, Dahlen B, Nilsson G. Mastocytosis: the puzzling clinical spectrum and challenging diagnostic aspects of an enigmatic disease. J Intern Med. (2016) 279(3):211–28. doi: 10.1111/joim.12410
6. Jennings SV, Slee VM, Zack RM, Verstovsek S, George TI, Shi H, et al. Patient perceptions in mast cell disorders. Immunol Allergy Clin North Am. (2018) 38(3):505–25. doi: 10.1016/j.iac.2018.04.006
7. Mickys U, Barakauskiene A, De Wolf-Peeters C, Geboes K, De Hertogh G. Aggressive systemic mastocytosis complicated by protein-losing enteropathy. Dig Liver Dis. (2007) 39(7):693–7. doi: 10.1016/j.dld.2006.06.003
8. Theoharides TC, Valent P, Akin C. Mast cells, mastocytosis, and related disorders. N Engl J Med. (2015) 373(2):163–72. doi: 10.1056/NEJMra1409760
9. Arock M, Sotlar K, Akin C, Broesby-Olsen S, Hoermann G, Escribano L, et al. KIT mutation analysis in mast cell neoplasms: recommendations of the European Competence Network on Mastocytosis. Leukemia. (2015) 29(6):1223–32. doi: 10.1038/leu.2015.24
10. Hoermann G, Sotlar K, Jawhar M, Kristensen T, Bachelot G, Nedoszytko B, et al. Standards of genetic testing in the diagnosis and prognostication of systemic mastocytosis in 2022: recommendations of the EU-US Cooperative Group. J Allergy Clin Immunol Pract. (2022) 10(8):1953–63. doi: 10.1016/j.jaip.2022.03.001
11. Horny HP, Sotlar K, Valent P. Mastocytosis: immunophenotypical features of the transformed mast cells are unique among hematopoietic cells. Immunol Allergy Clin North Am. (2014) 34(2):315–21. doi: 10.1016/j.iac.2014.01.005
12. Lim KH, Tefferi A, Lasho TL, Finke C, Patnaik M, Butterfield JH, et al. Systemic mastocytosis in 342 consecutive adults: survival studies and prognostic factors. Blood. (2009) 113(23):5727–36. doi: 10.1182/blood-2009-02-205237
13. Metcalfe DD, Mekori YA. Pathogenesis and pathology of mastocytosis. Annu Rev Pathol. (2017) 12:487–514. doi: 10.1146/annurev-pathol-052016-100312
14. Pardanani A. Systemic mastocytosis in adults: 2019 update on diagnosis, risk stratification and management. Am J Hematol. (2019) 94(3):363–77. doi: 10.1002/ajh.25371
15. Jiang ZG, Vardeh H, Evenson A. A case of cryptogenic liver failure. Gastroenterology. (2018) 155(1):23–4.e1. doi: 10.1053/j.gastro.2017.12.029
16. Kirsch R, Geboes K, Shepherd NA, de Hertogh G, Di Nicola N, Lebel S, et al. Systemic mastocytosis involving the gastrointestinal tract: clinicopathologic and molecular study of five cases. Mod Pathol. (2008) 21(12):1508–16. doi: 10.1038/modpathol.2008.158
17. Kors JW, van Doormaal JJ, de Monchy JG. Anaphylactoid shock following Hymenoptera sting as a presenting symptom of systemic mastocytosis. J Intern Med. (1993) 233(3):255–8. doi: 10.1111/j.1365-2796.1993.tb00984.x
18. Mallya KP, Belurkar S, Kurian A, Rao L, Singhania B. Systemic mastocytosis: predominantly involving the bone, a case report. J Clin Diagn Res. (2013) 7(10):2276–7. doi: 10.7860/JCDR/2013/5669.3493
19. NORD. Rare Disease Database: Mastocytosis. Available online at: https://rarediseases.org/rare-diseases/mastocytosis/ (Accessed April 8, 2021).
20. Pardanani A. Systemic mastocytosis in adults: 2017 update on diagnosis, risk stratification and management. Am J Hematol. (2016) 91(11):1146–59. doi: 10.1002/ajh.24553
21. Schwaab J, Cabral do O Hartmann N, Naumann N, Jawhar M, Weiss C, Metzgeroth G, et al. Importance of adequate diagnostic workup for correct diagnosis of advanced systemic mastocytosis. J Allergy Clin Immunol Pract. (2020) 8(9):3121–7.e1. doi: 10.1016/j.jaip.2020.05.005
22. Vaughan ST, Jones GN. Systemic mastocytosis presenting as profound cardiovascular collapse during anaesthesia. Anaesthesia. (1998) 53(8):804–7. doi: 10.1046/j.1365-2044.1998.00536.x
23. Reiter N, Reiter M, Altrichter S, Becker S, Kristensen T, Broesby-Olsen S, et al. Anaphylaxis caused by mosquito allergy in systemic mastocytosis. Lancet. (2013) 382(9901):1380. doi: 10.1016/S0140-6736(13)61605-0
24. Valent P, Akin C, Metcalfe DD. Mastocytosis: 2016 updated WHO classification and novel emerging treatment concepts. Blood. (2017) 129(11):1420–7. doi: 10.1182/blood-2016-09-731893
25. Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia. (2022) 36(7):1703–19. doi: 10.1038/s41375-022-01613-1
26. Rossini M, Zanotti R, Viapiana O, Tripi G, Idolazzi L, Biondan M, et al. Zoledronic acid in osteoporosis secondary to mastocytosis. Am J Med. (2014) 127(11):1127.e1–e4. doi: 10.1016/j.amjmed.2014.06.015
27. Leguit RJ, Wang SA, George TI, Tzankov A, Orazi A. The international consensus classification of mastocytosis and related entities. Virchows Arch. (2023) 482(1):99–112. doi: 10.1007/s00428-022-03423-3
28. Greene LW, Asadipooya K, Corradi PF, Akin C. Endocrine manifestations of systemic mastocytosis in bone. Rev Endocr Metab Disord. (2016) 17(3):419–31. doi: 10.1007/s11154-016-9362-3
29. Kennel KA, Drake MT. Adverse effects of bisphosphonates: implications for osteoporosis management. Mayo Clin Proc. (2009) 84(7):632–7. quiz 8. doi: 10.1016/S0025-6196(11)60752-0
30. Laroche M, Livideanu C, Paul C, Cantagrel A. Interferon alpha and pamidronate in osteoporosis with fracture secondary to mastocytosis. Am J Med. (2011) 124(8):776–8. doi: 10.1016/j.amjmed.2011.02.038
31. Bolland M, Malaise O, Ribbens C. A breakthrough in the management of postmenopausal osteoporosis with very high fracture risk: romosozumab (evenity(R)), a humanized monoclonal anti-sclerostin antibody. Rev Med Liege. (2023) 78(4):239–44.37067842
32. Zanelli M, Pizzi M, Sanguedolce F, Zizzo M, Palicelli A, Soriano A, et al. Gastrointestinal manifestations in systemic mastocytosis: the need of a multidisciplinary approach. Cancers (Basel). (2021) 13(13):3316. doi: 10.3390/cancers13133316
33. Castells M, Austen KF. Mastocytosis: mediator-related signs and symptoms. Int Arch Allergy Immunol. (2002) 127(2):147–52. doi: 10.1159/000048188
34. Sokol H, Georgin-Lavialle S, Canioni D, Barete S, Damaj G, Soucie E, et al. Gastrointestinal manifestations in mastocytosis: a study of 83 patients. J Allergy Clin Immunol. (2013) 132(4):866–73.e1-3. doi: 10.1016/j.jaci.2013.05.026
35. Hahn HP, Hornick JL. Immunoreactivity for CD25 in gastrointestinal mucosal mast cells is specific for systemic mastocytosis. Am J Surg Pathol. (2007) 31(11):1669–76. doi: 10.1097/PAS.0b013e318078ce7a
36. Doyle LA, Sepehr GJ, Hamilton MJ, Akin C, Castells MC, Hornick JL. A clinicopathologic study of 24 cases of systemic mastocytosis involving the gastrointestinal tract and assessment of mucosal mast cell density in irritable bowel syndrome and asymptomatic patients. Am J Surg Pathol. (2014) 38(6):832–43. doi: 10.1097/PAS.0000000000000190
37. Wasserman SI, Soter NA, Austen KF. The efficacy of oral disodium cromoglycate in human mastocytosis. J Allergy Clin Immunol. (1979) 63(3):186.
38. Gonzalez-de-Olano D, Alvarez-Twose I, Vega A, Orfao A, Escribano L. Venom immunotherapy in patients with mastocytosis and Hymenoptera venom anaphylaxis. Immunotherapy. (2011) 3(5):637–51. doi: 10.2217/imt.11.44
39. Gonzalez-de-Olano D, Alvarez-Twose I, Esteban-Lopez MI, Sanchez-Munoz L, de Durana MD, Vega A, et al. Safety and effectiveness of immunotherapy in patients with indolent systemic mastocytosis presenting with Hymenoptera venom anaphylaxis. J Allergy Clin Immunol. (2008) 121(2):519–26. doi: 10.1016/j.jaci.2007.11.010
40. Giannetti M, Silver J, Hufdhi R, Castells M. One-day ultrarush desensitization for Hymenoptera venom anaphylaxis in patients with and without mast cell disorders with adjuvant omalizumab. J Allergy Clin Immunol Pract. (2020) 8(4):1431–5.e3. doi: 10.1016/j.jaip.2019.10.022
41. Bonadonna P, Bonifacio M, Lombardo C, Zanotti R. Hymenoptera allergy and mast cell activation syndromes. Curr Allergy Asthma Rep. (2016) 16(1):5. doi: 10.1007/s11882-015-0582-5
42. Carter MC, Maric I, Brittain EH, Bai Y, Lumbard K, Bolan H, et al. A randomized double-blind, placebo-controlled study of omalizumab for idiopathic anaphylaxis. J Allergy Clin Immunol. (2021) 147(3):1004–10.e2. doi: 10.1016/j.jaci.2020.11.005
43. Hoermann G, Gleixner KV, Dinu GE, Kundi M, Greiner G, Wimazal F, et al. The KIT D816V allele burden predicts survival in patients with mastocytosis and correlates with the WHO type of the disease. Allergy. (2014) 69(6):810–3. doi: 10.1111/all.12409
44. Lyons JJ, Yu X, Hughes JD, Le QT, Jamil A, Bai Y, et al. Elevated basal serum tryptase identifies a multisystem disorder associated with increased TPSAB1 copy number. Nat Genet. (2016) 48(12):1564–9. doi: 10.1038/ng.3696
45. Gonzalez-de-Olano D, Navarro-Navarro P, Munoz-Gonzalez JI, Sanchez-Munoz L, Henriques A, de-Andres-Martin A, et al. Clinical impact of the TPSAB1 genotype in mast cell diseases: a REMA study in a cohort of 959 individuals. Allergy. (2024) 79(3):711–23. doi: 10.1111/all.15911
46. Aberer E, Sperr WR, Bretterklieber A, Avian A, Hadzijusufovic E, Kluin-Nelemans HC, et al. Clinical impact of skin lesions in mastocytosis: a multicenter study of the European Competence Network on Mastocytosis. J Invest Dermatol. (2021) 141(7):1719–27. doi: 10.1016/j.jid.2020.12.030
47. Herrero-Moyano M, Capusan TM, Perez-Plaza A, Godoy A, Sanchez-Perez J. Intertriginous maculopapular mastocytosis in a patient with acute myeloid leukemia. JAAD Case Rep. (2017) 3(1):61–3. doi: 10.1016/j.jdcr.2016.11.004
48. Simmons BJ, LeBlanc RE, Glass JS. Intertriginous mastocytosis: a rare presentation of an uncommon disease. Int J Dermatol. (2019) 58(7):852–3. doi: 10.1111/ijd.14166
49. Bulai Livideanu C, Severino-Freire M, Jendoubi F, Tournier E, Commont T, Hermine O, et al. Image gallery: the vulva: an atypical localization of mastocytosis in adulthood. Br J Dermatol. (2019) 181(2):e33. doi: 10.1111/bjd.17897
50. Sammut J, Mercieca L, Boffa MM, Pisani D, Betts A, Boffa MJ. Palmoplantar maculopapular cutaneous mastocytosis. Int J Dermatol. (2019) 58(4):E79–80. doi: 10.1111/ijd.14385
51. Soilleux EJ, Brown VL, Bowling J. Cutaneous mastocytosis localized to a radiotherapy field. Clin Exp Dermatol. (2009) 34(1):111–2. doi: 10.1111/j.1365-2230.2008.02931.x
52. Griffiths WA, Daneshbod K. Pseudoxanthomatous mastocytosis. Br J Dermatol. (1975) 93(1):91–5. doi: 10.1111/j.1365-2133.1975.tb06482.x
53. Nabavi NS, Nejad MH, Feli S, Bakhshoodeh B, Layegh P. Adult onset of xanthelasmoid mastocytosis: report of a rare entity. Indian J Dermatol. (2016) 61(4):468. doi: 10.4103/0019-5154.185751
54. Berezowska S, Flaig MJ, Rueff F, Walz C, Haferlach T, Krokowski M, et al. Adult-onset mastocytosis in the skin is highly suggestive of systemic mastocytosis. Mod Pathol. (2014) 27(1):19–29. doi: 10.1038/modpathol.2013.117
55. Jendoubi F, Shourick J, Negretto M, Laurent C, Apoil PA, Evrard S, et al. Cutaneous mastocytosis in adults with a serum tryptase level <20 ng ml(-1): why we should investigate further. Br J Dermatol. (2021) 185(2):453–5. doi: 10.1111/bjd.20098
56. Tzankov A, Duncavage E, Craig FE, Kelemen K, King RL, Orazi A, et al. Mastocytosis. Am J Clin Pathol. (2021) 155(2):239–66. doi: 10.1093/ajcp/aqaa183
57. Brazzelli V, Grassi S, Merante S, Grasso V, Ciccocioppo R, Bossi G, et al. Narrow-band UVB phototherapy and psoralen-ultraviolet A photochemotherapy in the treatment of cutaneous mastocytosis: a study in 20 patients. Photodermatol Photoimmunol Photomed. (2016) 32(5-6):238–46. doi: 10.1111/phpp.12248
58. Hagglund H, Sander B, Gulen T, Lindelof B, Nilsson G. Increased risk of malignant melanoma in patients with systemic mastocytosis? Acta Derm Venereol. (2014) 94(5):583–4. doi: 10.2340/00015555-1788
59. Kaszuba A, Slawinska M, Zolkiewicz J, Sobjanek M, Nowicki RJ, Lange M. Mastocytosis and skin cancer: the current state of knowledge. Int J Mol Sci. (2023) 24(12):9840. doi: 10.3390/ijms24129840
60. Pardanani A. Systemic mastocytosis in adults: 2021 update on diagnosis, risk stratification and management. Am J Hematol. (2021) 96(4):508–25. doi: 10.1002/ajh.26118
61. Castells M, Butterfield J. Mast cell activation syndrome and mastocytosis: initial treatment options and long-term management. J Allergy Clin Immunol Pract. (2019) 7(4):1097–106. doi: 10.1016/j.jaip.2019.02.002
62. Castells M, Akin C. Finding the right KIT inhibitor for advanced systemic mastocytosis. Nat Med. (2021) 27(12):2081–2. doi: 10.1038/s41591-021-01588-z
63. Valent P, Akin C, Sperr WR, Escribano L, Arock M, Horny HP, et al. Aggressive systemic mastocytosis and related mast cell disorders: current treatment options and proposed response criteria. Leuk Res. (2003) 27(7):635–41. doi: 10.1016/S0145-2126(02)00168-6
64. Blueprint Medicines Coporation. FDA Approves AYVAKIT® (avapritinib) as the First and Only Treatment for Indolent Systemic Mastocytosis [press release]. Published May 22, 2023.
66. Akin C, Elberink HO, Gotlib J, Sabato V, Hartmann K, Broesby-Olsen S, et al. Pioneer part 2: a randomized, double-blind, placebo-controlled, phase 2 study to evaluate safety and efficacy of avapritinib in indolent systemic mastocytosis. Blood. (2020) 136(suppl 1):41–2. doi: 10.1182/blood-2020-136632
67. Gotlib J, Castells M, Elberink HO, Siebenhaar F, Hartmann K, Broesby-Olsen S, et al. Avapritinib versus placebo in indolent systemic mastocytosis. NEJM Evidence. (2023) 2(6):EVIDoa2200339. doi: 10.1056/EVIDoa2200339
Keywords: anaphylaxis, tryptase, tyrosine kinase, KIT mutation, case report, indolent systemic mastocytosis
Citation: Hamilton MJ, Greene LW, Madigan LM, Wang SA, Arana Yi C, Kuykendall A, George TI and Castells MC (2024) Case Report: Multidisciplinary management of a patient with indolent systemic mastocytosis and refractory symptoms. Front. Allergy 5:1401187. doi: 10.3389/falgy.2024.1401187
Received: 21 March 2024; Accepted: 26 September 2024;
Published: 18 October 2024.
Edited by:
Luisa Ricciardi, University of Messina, ItalyReviewed by:
Theo Gulen, Karolinska Institutet (KI), SwedenPolina Pyatilova, Charité - Universitätsmedizin Berlin, Germany
Copyright: © 2024 Hamilton, Greene, Madigan, Wang, Arana Yi, Kuykendall, George and Castells. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Matthew J. Hamilton, bWpoYW1pbHRvbkBid2guaGFydmFyZC5lZHU=
 Matthew J. Hamilton
Matthew J. Hamilton Loren W. Greene
Loren W. Greene Lauren M. Madigan3
Lauren M. Madigan3 Cecilia Arana Yi
Cecilia Arana Yi Tracy I. George
Tracy I. George Mariana C. Castells
Mariana C. Castells