
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Allergy, 06 June 2024
Sec. Food Allergy
Volume 5 - 2024 | https://doi.org/10.3389/falgy.2024.1360073
Background: Birch pollen–related food allergy (BPFA) is the most common type of food allergy in birch-endemic areas such as Western and Central Europe. Currently, there is no treatment available for BPFA. Due to the cross-reactivity between birch pollen and a range of implicated plant foods, birch pollen allergen immunotherapy (AIT) may be effective in the treatment of BPFA. In this study, we systematically evaluate the effectiveness of birch pollen–specific subcutaneous or sublingual immunotherapy in treating BPFA.
Methods: A search was performed in the PubMed, Embase, and Cochrane libraries. Studies were independently screened by two reviewers against predefined eligibility criteria. The outcomes of interest were changes in (1) severity of symptoms during food challenge, (2) eliciting dose (ED), and (3) food allergy quality of life (FA-QoL). The validity of the selected articles was assessed using the revised Cochrane risk of bias tool. We focused on studies with the lowest risk of bias and considered studies with a high risk of bias as supportive. Data were descriptively summarized.
Results: Ten studies were selected that included 475 patients in total. Seven studies were categorized into “high risk of bias” and three into “moderate risk of bias.” The three moderate risk of bias studies, with a total of 98 patients, reported on severity of symptoms during challenge and on the ED. All three studies had a control group. Compared to the control group, improvement in severity of symptoms was observed during challenge in two out of the three studies and on the eliciting dose in one out of three. Only one study investigated the effect of birch pollen AIT on FA-QoL, showing that there was no significant difference between patients receiving subcutaneous immunotherapy or a placebo. Of the seven supportive studies, four had a control group and of those, three showed improvement on both severity of symptoms and ED. None of the supportive studies investigated the effect of the therapy on FA-QoL.
Conclusion: This systematic review shows that there is not enough evidence to draw firm conclusions about the effect of AIT on BPFA. Future research is warranted that uses robust clinical studies that include long-term effects, QoL, and multiple BPFA-related foods.
In Europe, the rate of prevalence of birch pollen sensitization ranges from approximately 8% to 16%, and climate change is likely to cause this number to increase over time (1, 2). Pollen derived from the Betulaceae and Fagaceae family constitutes the most prominent source of tree pollen in Western and Central Europe (2). The major birch pollen allergen, Bet v 1, is a PR-10 protein whose homologous structures are present in a large number of plant foods (3). Due to cross-reactivity between Bet v 1 and these homologs in foods, approximately 70% of birch pollen–allergic patients report allergic reactions to foods, commonly referred to as birch pollen–related food allergy (BPFA) (3). BPFA is the most common type of food allergy in Western and Central Europe involving many different foods and food groups, for example, Rosaceae fruits such as apples and peaches, tree nuts such as hazelnuts and walnuts, and vegetables such as carrots, celeriac, and soy (2, 4).
Symptoms of BPFA are usually mild and restricted to the oral cavity; hence, they are often referred to as oral allergy syndrome (OAS). However, sometimes more severe allergic reactions with cardiovascular symptoms, or even anaphylaxis, can occur involving some foods, for example, soy protein–containing food (2, 5).
To date, no treatment is available for BPFA. The evidence for the effectiveness of oral immunotherapy to foods relating to BPFA is sparse (6). Because birch pollen is the primary sensitizer in BPFA, birch pollen AIT has often been considered possibly effective also in the treatment of BPFA (3). However, there is no evidence supporting this, and it is even hypothesized that due to insufficient homology between Bet v 1 and plant food allergens, it is not possible to alleviate BPFA symptoms (7). Both subcutaneous (SCIT) and sublingual (SLIT) immunotherapy are available for birch pollen allergy, but their effectiveness for treating associated food allergies remains a matter of debate (8, 9).
The aim of this review was to systematically evaluate the effect of birch pollen–specific SCIT and SLIT on BPFA with regard to severity of symptoms during challenge, eliciting dose (ED), and food allergy-related quality of life (FA-QoL).
A systemic search strategy (Supplementary Material S1) was developed by combining synonyms for the patient population and intervention using both keywords and medical subject headings. The patient population consisted of those with birch pollen allergy and birch pollen–related food allergy; subcutaneous or sublingual birch pollen–specific immunotherapy was used as an intervention. Our search was performed in the PubMed, Embase, and Cochrane libraries on 3 November 2022.
Citations from the PubMed, Embase, and the Cochrane libraries were imported into the Rayyan tool for removing duplicates and for screening. Two authors (JL and EK) independently screened the titles and abstracts. When a paper was deemed possibly relevant, the full text was also independently screened by these two authors. Selection was based on consensus, and discrepancies were resolved by two other authors (TL and PW). English language articles that met the following criteria were included: (1) subjects with a birch pollen allergy, (2) subjects with BPFA for at least one food, (3) those with either birch pollen–specific SCIT or birch pollen–specific SLIT as an intervention, and (4) studies in which the effectiveness of this treatment was evaluated in terms of food challenge. Studies focusing on food allergy immunotherapy, non-original studies (editorials and expert opinions), conference abstracts, case studies, and animal studies were excluded. Reviews were also excluded, but they were used to obtain additional articles of interest based on reference checking.
Two authors (JL and EK) independently recorded the characteristics of the selected studies using a predefined checklist, comprising the following items: (1) study information (first author, year of publication, and country in which the study was performed); (2) study design [randomized controlled trial (RCT) or comparative/single-arm prospective cohort]; (3) type of food challenge [double-blind, placebo-controlled food challenge (DBPCFC) or open food challenge (OFC)]; (4) type of food; (5) treatment group characteristics (number of patients and type of immunotherapy), if applicable; (6) control group characteristics (number of patients and type of control); (7) timepoint when the outcome was measured; and (8) type of reported outcomes. The extracted outcome measurements were changes in (1) severity of symptoms during challenge, (2) eliciting dose, and (3) food allergy–related quality of life. Improvement in the eliciting dose was defined as the percentage of patients who could tolerate at least one higher dose without symptoms during the last-performed food challenge compared with baseline.
The validity of the selected studies was assessed using the revised Cochrane risk of bias tool (RoB2) (10), which evaluated five domains of bias: D1, the randomization process; D2, deviations from intended interventions; D3, missing outcome data; D4, measurement of the outcome; and D5, selection of the reported result. The following information was assessed: D1, performance of randomization, observed baseline differences in patient characteristics, and concealment of allocation sequence; D2, awareness of the assigned intervention, deviations from the intended intervention due to the trial context, and whether the analysis used to estimate the effect of assignment to the intervention was appropriate; D3, availability of outcome data; D4, whether the method of measuring the outcome was appropriate, comparable between intervention groups, and insensitive to awareness of the received intervention; and D5, whether data analysis was prespecified. Each of the questions in the domains could be answered with “yes,” “probably yes,” “probably no,” “no,” and “no information, which led to a risk of bias per domain classified as ‘low’, ‘some concerns’, or ‘high’”. Furthermore, the overall risk of bias was determined. Single-arm studies scored high for domains 1 and 2 because there was no control group/randomization (treatment effect estimates concerned pre/post-treatment differences) and patients and caregivers were aware of the received intervention.
Due to evident heterogeneity between the studies in terms of design, timepoint when outcome was measured, type of food, type of immunotherapy, type of control group, and availability and measurement of the five outcomes, it was considered inappropriate to pool the results. Therefore, a qualitative synthesis of the available results was performed without producing a formal statistical summary. Studies with the lowest risk of bias with a control group were considered the most important, while studies with the highest risk of bias without a control group were considered only supportive. Furthermore, a distinction was made between the direction of the effect (positive, no effect, and negative) and the size of the effect in case of an effect (large or small). Based on clinical interpretation, the size of the effect was considered large when there was an improvement of at least 20%. In the summary of the effect of birch pollen AIT on BPFA, only objective results are shown when a study reported on both subjective and objective results, because these are more reliable.
Our search yielded 3,652 unique articles (Figure 1). After screening the articles by title, abstract, full text, and reference checking, 10 articles were included.
Details of the 10 selected studies can be found in Table 1. All the studies were conducted in Europe. In total, there were five RCTs (of which one was a sub-study of an RCT) and five prospective cohort studies. A control group was used in all RCTs and in two prospective cohorts. In four out of five RCTs, the control group was a placebo group. In the other studies, the control group consisted of patients without AIT. In the studies without a control group, a pre/post-AIT comparison of the outcome(s) was made.
Altogether, 475 patients were analyzed, of whom 320 received AIT and 152 did not. Of the 320 patients who received AIT, 127 served as their own controls. Six studies focused on SCIT, three on SLIT, and one on either SCIT or SLIT. The last study was a three-arm study, in which patients receiving SCIT or SLIT or a placebo were compared. An OFC was performed in six studies, a DBPCFC in three studies, and both OFC and DBPCFC in one study.
During treatment, outcomes were reported at timepoints between 6.5 and 48 months after the start of treatment, but in 6 out of 10 studies, they were reported at 12 months.
The overall risk of bias was moderate in three studies and high in seven. Therefore, we focused on the three studies with the lowest risk of bias; the remaining studies were considered to be supportive. The overall risk of bias was mainly high because of issues with domain 2, “Deviations from the intended interventions,” which were attributed to no correction of prognostic factors in non-randomized studies. Details of the assessment per outcome reported are presented in Table 2.
A summary of the study results regarding the outcomes of severity of symptoms during challenge and ED is provided in Tables 3 and 4. In both tables, a distinction is made between studies with the lowest and the highest risk of bias and those with or without a control group. In studies with a control group, treatment effects pertained to the comparison between treatment and control groups, and in studies without a control group, treatment effects related to changes from baseline. Henceforth, for the three studies with the lowest risk of bias, only the comparison between the treatment and the control groups is discussed subsequently. Within these studies, a total of 61 patients received AIT and 37 did not.
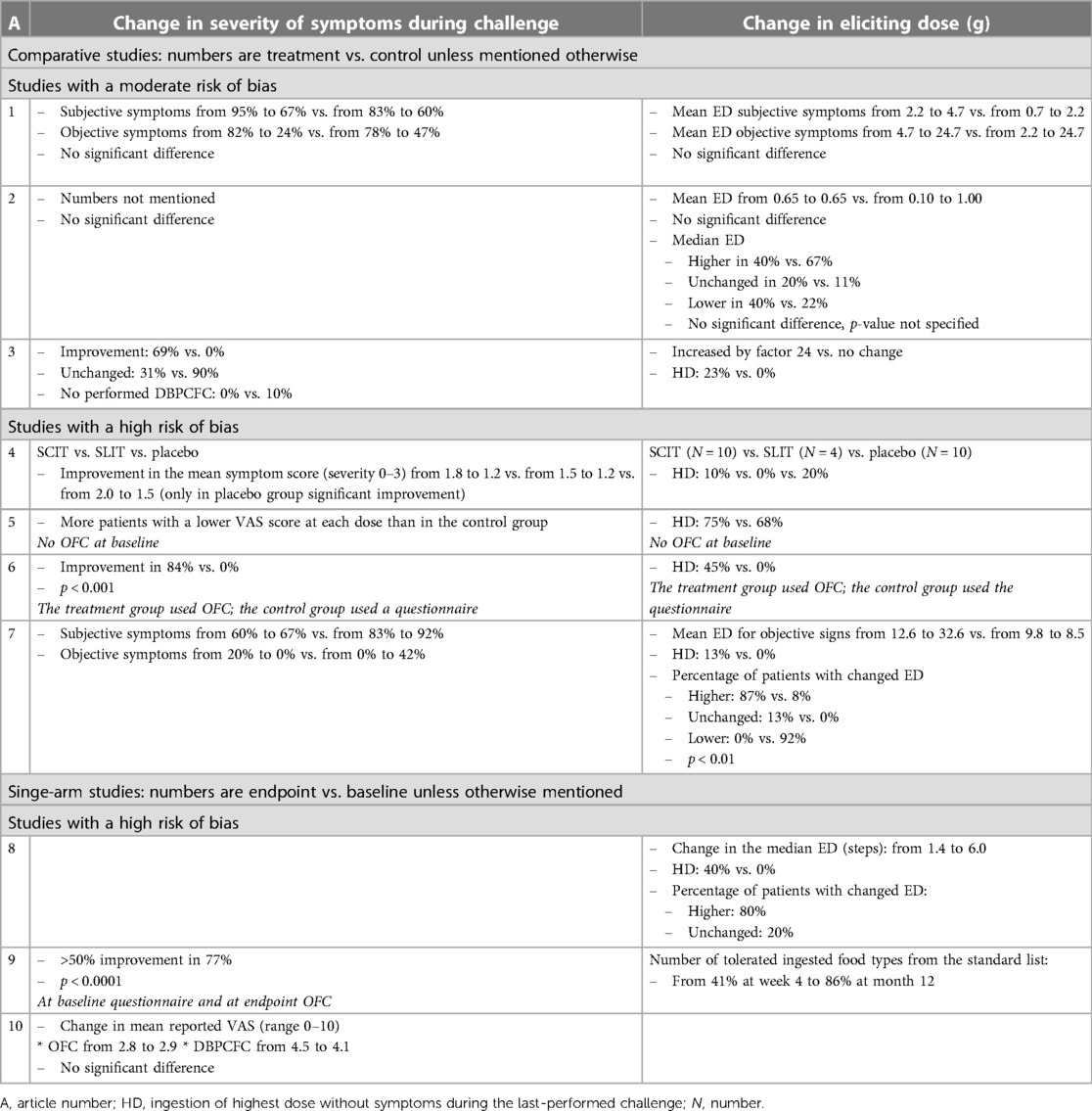
Table 3. Overview of the effect of birch pollen AIT on severity of symptoms during challenge and eliciting dose.
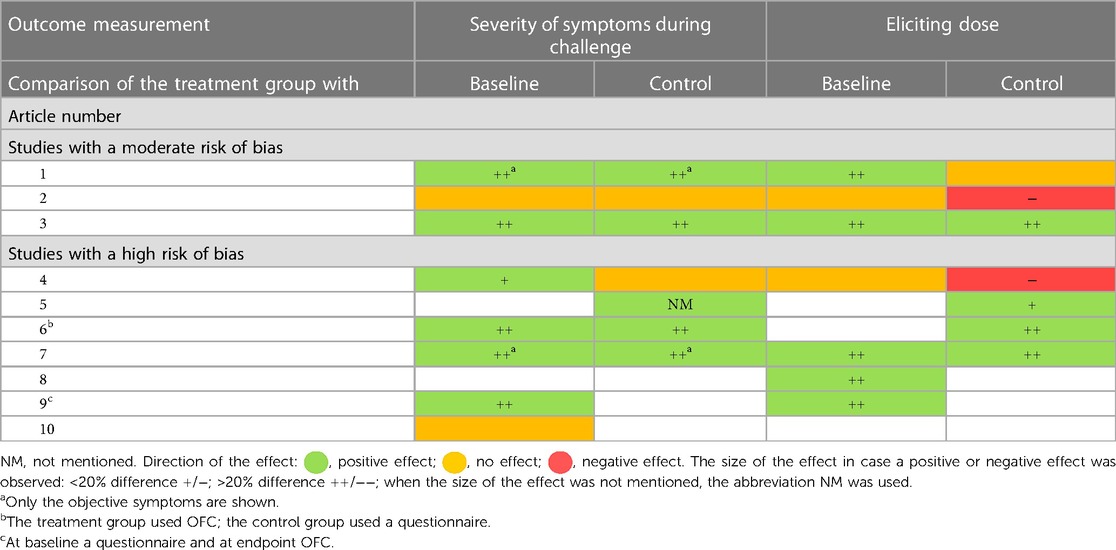
Table 4. Summary of the effect of birch pollen AIT on severity of symptoms during challenge and eliciting dose within the treatment group (change from baseline) and between the treatment and the control group.
Birch pollen AIT seems to have had a positive effect on severity of symptoms during challenge in the three studies with a moderate risk of bias; two showed a positive effect (11, 13) on severity of symptoms and one no effect (12) (Tables 3, 4). The first study distinguished between objective and subjective symptoms and showed a decrease in both objective and subjective symptoms in both the treatment and the control groups. Although there was no significant difference in the decrease, the patients in the treatment group tended to have a higher numerical decrease in objective symptoms than those in the control group (11). The second study with a positive effect showed a rate of reduction in symptoms of 69% in the treatment group vs. 0% in the control group (13).
The study with no effect only mentioned that there was no significant difference in symptoms during challenge; no numbers were mentioned (12).
The effect of birch pollen AIT on the eliciting dose is unclear. Of the three studies with a moderate risk of bias, one showed a positive effect (13), one no effect (11), and one a negative effect (12) on the ED (Tables 3, 4). The study with a positive effect showed a 24-fold increase in the eliciting dose in the treatment group vs. no change in the control group. Furthermore, 23% of the patients in the treatment group vs. 0% of the patients in the control group reached ingestion of the highest dose without symptoms during the last challenge (13). The study with a negative effect showed a baseline mean ED that did not change in the treatment group but increased from 0.10 to 1.00 g in the control group (12).
Further research is needed to assess the effect of birch pollen AIT on FA-QoL. Only one study with a moderate risk of bias investigated the effect of birch pollen AIT on FA-QoL (11). This study used the validated food allergy quality of life questionnaire—adult form (FAQLQ-AF) and showed that there was no significant difference between patients receiving SCIT or a placebo. No studies with a high risk of bias investigated the effect of birch pollen AIT on FA-QoL
Supporting studies showed mostly positive effects. The seven studies with a high risk of bias were considered supportive, of which four (14–17) included a control group and three (18–20) did not (Table 4). When there was a positive effect on severity of symptoms during challenge, there was also a positive effect seen on the ED. Of the studies with a control group, three (15–17) showed a positive effect on severity of symptoms and the ED with mostly a large effect size and one (14) no effect on severity of symptoms during challenge and a negative effect with a small effect size on the eliciting dose. Of the studies without a control group, two (18, 19) showed a positive effect with a large effect size and one (20) no effect.
Overall, these high risk of bias studies supported the moderately positive effect of birch pollen AIT on severity of symptoms during challenge and indicated that the effect on the eliciting dose was more likely to be positive.
Due to the small number of included studies and the moderate to high risk of bias in these studies, this systematic review primarily shows that there is not enough evidence to conclude that AIT reduces BPFA. There may be a positive effect on the severity of symptoms during challenge. The effect on the eliciting dose is, however, unclear, and there is not enough information to draw a conclusion about the effect of birch pollen AIT on FA-QoL.
As mentioned previously, 7 (14–20) of the 10 studies had a high risk of bias, mostly due to domain 2, “Deviations from the intended interventions,” followed by domain 4, “Measurement of the outcome.” The high risk of bias in domain 2 was due to studies without a control group and studies with a control group but not adjusted for prognostic factors when no randomization was performed. The high risk of bias in domain 4 was attributed to the fact that, among others, studies performed an OFC only at the end of the study and not at the start (15, 19) or used a method in the treatment group that was different from that in the control group (16). Because most studies had a high risk of bias, the quality of evidence was low. To obtain the best possible estimation of effectiveness, we decided to use the three studies with the lowest risk of bias for the assessment and the studies with a high risk of bias only as supporting evidence.
Birch pollen AIT seems to have a positive effect on BPFA as evidenced by an alleviation of symptoms during challenge, and it remains unclear whether there is also a positive effect on the ED. That some studies found no or even a negative effect could be attributed to the fact that the included patients might not have had a pure BPFA but also a primary food allergy that did not reduce or even worsen during treatment and/or to the fact that there was an imbalance in the groups in this respect. Nowadays, by measuring both the PR-10 and the non-PR-10 components, it has become possible to differentiate between a pure BPFA and a primary food allergy (21). The study that showed that there was no effect on symptoms during challenge and a negative effect on the eliciting dose measured only Cor a 1 and Cor a 8 but not Cor a 9 and 14 (12). Therefore, it was unclear whether only patients with pure BPFA were included.
Another reason could be that the follow-up period was too short. In general, the effect of immunotherapy increases with a longer duration (22, 23). All three studies with the lowest risk of bias reported their results only after 1 year of AIT use.
Patient-reported outcome measures (PROMs) are often the best way of measuring patient symptoms and quality of life and can help reduce observer bias (24). Unfortunately, only one study investigated the effect of birch pollen AIT on the patient-reported outcome “FA-QoL” using the validated FAQLQ-AF questionnaire and showed that there was no significant difference between patients receiving SCIT or placebo at endpoint (11). As the primary burden on patients living with food allergy is a reduced QoL, treatment success in trials should also be defined by an improved QoL (25, 26). Future studies, investigating the effect of birch pollen AIT on BPFA, should therefore include QoL as an outcome.
In total, six studies investigated the effect of birch pollen SCIT (11–13, 16–18), three that of birch pollen SLIT (15, 19, 20), and one that of both birch pollen SCIT and SLIT (14) on BPFA. Because of the small number of studies and the high risk of bias, there is too little evidence to draw a conclusion.
In our systematic review, we found that almost all studies investigated the effect of AIT with either hazelnut or apple as a type of food. This is not surprising, as hazelnut and apple allergies are among the top three birch pollen–related food allergies reported in birch pollen–endemic areas (27). However, patients with BPFA are mostly allergic to multiple types of fruits, vegetables, and nuts. The effect on other foods remains unknown. Therefore, it is important to evaluate the effect of AIT on a broader range of BPFA foods.
We expected a better therapeutic effect of birch pollen AIT on foods with PR-10 components that are more homologous to Bet v 1. However, although the PR-10 components of apple and hazelnut are more homologous to Bet v 1 than those of soy (28), this review showed a better effect of birch pollen AIT on soy (11) than on hazelnut allergy (12). To confirm this hypothesis, more studies are needed that compare the effect of birch pollen AIT on multiple foods related to BPFA.
It is unknown how long the effects last after one discontinues AIT. Most of the included studies measured the outcome after 12 months of the start of birch pollen AIT, but none showed results after discontinuation of AIT. In 2003, Asero conducted a prospective cohort study to evaluate the long-term effect of birch pollen–specific SCIT on apple allergy after treatment cessation (29). In this study, 21 BPFA patients who discontinued birch pollen SCIT could tolerate apple. However, the effect appeared to decrease over time, since after 30 months of discontinuation, only 52% of the patients remained symptom-free. Further studies are needed to investigate the long-term effects of birch pollen AIT on BPFA so that clinicians can advise patients appropriately.
Apart from the effect of birch pollen AIT on BPFA, several studies showed the effect of other immunotherapies on plant food allergy. Two studies reported about an effective treatment of birch pollen–related apple allergy. Kinacyian et al. (30) showed that patients receiving SLIT with rMal d 1 required a significantly higher dose of rMal d 1 to induce OAS compared with the group that received rBet v 1 and placebo (p = 0.001), and Kopac et al. (31) showed that apple consumption induced a transient tolerance. Furthermore, studies from Japan (32) and Italy (33) reported the positive effects of Japanese cedar pollen–based SCIT and grass pollen SLIT, respectively, on plant food allergy. All of the above studies showed promising approaches for the effective treatment of plant food allergy, but these results should be confirmed before they are used in clinical practice.
Due to the high risk of bias and the heterogeneity of the included studies in terms of, among other elements, the study design, type of food, type and dose of immunotherapy, and method of assessing response to treatment, it was not possible to pool the results. Furthermore, symptoms were often not specified, and none of the studies reported the minimal clinically important difference (MCID) to define improvement, which made it difficult to interpret whether the differences found were clinically relevant (34). To provide the most reliable results, we focused on the three studies with the lowest risk of bias. Because of this selection, the total number of patients was small, with the total number of patients receiving AIT being 61 and those not receiving AIT being 37. However, the strengths of this review included its comprehensive search and methodological rigor, which also took into account patient-reported outcomes. This factor made this review the first systematic one to show the effect of BPFA on clinical and patient-reported outcomes.
To our knowledge, this is the first systematic review that evaluates the effect of birch pollen AIT on BPFA. Due to the low number of studies that fulfilled the inclusion criteria, a moderate to high risk of bias in these studies, and the low number of included patients per study, the level of evidence is low. The three studies with the lowest risk of bias showed that there might be a positive effect on severity of symptoms during challenge, but there was an unclear effect on the eliciting dose, and there was not enough information available to draw a conclusion about the effect of birch pollen AIT on FA-QoL. Taken together, no firm conclusions can be drawn, and future research is warranted that uses robust clinical studies that take into account the abovementioned aspects, including the long-term effects.
EK: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Visualization, Writing – original draft, Writing – review & editing. PW: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Visualization, Writing – original draft, Writing – review & editing. JL: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing. RVR: Conceptualization, Investigation, Supervision, Visualization, Writing – original draft, Writing – review & editing. AK: Conceptualization, Investigation, Methodology, Supervision, Visualization, Writing – original draft, Writing – review & editing. TL: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Visualization, Writing – original draft, Writing – review & editing.
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
RVR reports consultancies for HAL Allergy BV, Citeq BV, Angany Inc., Reacta Healthcare Ltd., Mission MightyMe, AB Enzymes, The Protein Brewery, Unilever India; speaker fees for HAL Allergy BV, Thermofisher Scientific, ALK; stock option of Angany Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/falgy.2024.1360073/full#supplementary-material
AIT, allergen immunotherapy; BPFA, birch pollen-related food allergy; DBPCFC, double-blind, placebo-controlled food challenge; ED, eliciting dose; FA-QoL, food allergy-related quality of life; FAQLQ-AF, food allergy quality of life questionnaire—adult form; OFC, open food challenge; QoL, quality of life; RCT, randomized controlled trial; RoB2, revised Cochrane risk of bias tool; SCIT, subcutaneous immunotherapy; SLIT, sublingual immunotherapy; VAS, visual analog scale; vs, versus.
1. Lake IR, Jones NR, Agnew M, Goodess CM, Giorgi F, Hamaoui-Laguel L, et al. Climate change and future pollen allergy in Europe. Environ Health Perspect. (2017) 125(3):385. doi: 10.1289/EHP173
2. Biedermann T, Winther L, Till SJ, Panzner P, Knulst A, Valovirta E. Birch pollen allergy in Europe. Allergy. (2019) 74(7):1237–48. doi: 10.1111/all.13758
3. Matricardi PM, Kleine-Tebbe J, Hoffmann HJ, Valenta R, Hilger C, Hofmaier S, et al. EAACI Molecular allergology user’s guide. Pediatr Allergy Immunol. (2016) 27(Suppl 23):1–250. doi: 10.1111/pai.12563
4. Werfel T, Asero R, Ballmer-Weber BK, Beyer K, Enrique E, Knulst AC, et al. Position paper of the EAACI: food allergy due to immunological cross-reactions with common inhalant allergens. Allergy. (2015) 70(9):1079–90. doi: 10.1111/all.12666
5. Kleine-Tebbe J, Wangorsch A, Vogel L, Crowell DN, Haustein UF, Vieths S. Severe oral allergy syndrome and anaphylactic reactions caused by a bet v 1-related PR-10 protein in soybean, SAM22. J Allergy Clin Immunol. (2002) 110(5):797–804. doi: 10.1067/mai.2002.128946
6. Skypala IJ, Hunter H, Krishna MT, Rey-Garcia H, Till SJ, du Toit G, et al. BSACI guideline for the diagnosis and management of pollen food syndrome in the UK. Clin Exp Allergy. (2022) 52(9):1018–34. doi: 10.1111/cea.14208
7. Clayton J, Skypala I. Late breaking poster session LB TPS 10–18. Allergy. (2016) 71:592–633. doi: 10.1111/all.12979
8. Boonpiyathad T, Lao-Araya M, Chiewchalermsri C, Sangkanjanavanich S, Morita H. Allergic rhinitis: what do we know about allergen-specific immunotherapy? Front Allergy. (2021) 2:1–22. doi: 10.3389/falgy.2021.747323
9. Pavón-Romero GF, Parra-Vargas MI, Ramírez-Jiménez F, Melgoza-Ruiz E, Serrano-Pérez NH, Teran LM. Allergen immunotherapy: current and future trends. Cells. (2022) 11:1–22. doi: 10.3390/cells11020212
10. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. Rob 2: a revised tool for assessing risk of bias in randomised trials. Br Med J. (2019) 366(I4898):1–8. doi: 10.1136/bmj.l4898
11. Treudler R, Franke A, Schmiedeknecht A, Ballmer-Weber B, Worm M, Werfel T, et al. BASALIT trial: double-blind placebo-controlled allergen immunotherapy with rBet v 1-FV in birch-related soya allergy. Allergy. (2017) 72(8):1243–53. doi: 10.1111/all.13112
12. Van Hoffen E, Peeters KABM, Van Neerven RJJ, Van Der Tas CWH, Zuidmeer L, Van Ieperen-Van Dijk AG, et al. Effect of birch pollen-specific immunotherapy on birch pollen-related hazelnut allergy. J Allergy Clin Immunol. (2011) 127(1):100–1.e3. doi: 10.1016/j.jaci.2010.08.021
13. Bolhaar STHP, Tiemessen MM, Zuidmeer L, Van Leeuwen A, Hoffmann-Sommergruber K, Bruijnzeel-Koomen CAFM, et al. Efficacy of birch-pollen immunotherapy on cross-reactive food allergy confirmed by skin tests and double-blind food challenges. Clin Exp Allergy. (2004) 34(5):761–9. doi: 10.1111/j.1365-2222.2004.1939.x
14. Hansen KS, Khinchi MS, Skov PS, Bindslev-Jensen C, Poulsen LK, Malling HJ. Food allergy to apple and specific immunotherapy with birch pollen. Mol Nutr Food Res. (2004) 48(6):441–8. doi: 10.1002/mnfr.200400037
15. Till SJ, Stage BS, Skypala I, Biedermann T. Potential treatment effect of the SQ tree SLIT-tablet on pollen food syndrome caused by apple. Allergy. (2020) 75:2059–61. doi: 10.1111/all.14242
16. Asero R. Effects of birch pollen-specific immunotherapy on apple allergy in birch pollen-hypersensitive patients. Clin Exp Allergy. (1998) 28(11):1368–73. doi: 10.1046/j.1365-2222.1998.00399.x
17. Bucher X, Fichier WJ, Dahinden CA, Helbling A. Effect of tree pollen specific, subcutaneous immunotherapy on the oral allergy syndrome to apple and hazelnut. Allergy. (2004) 59(12):1272–6. doi: 10.1111/j.1398-9995.2004.00626.x
18. van der Valk J, Nagl B, van Wljk RG, Bohle B, de Jong N. The effect of birch pollen immunotherapy on apple and rmal d 1 challenges in adults with apple allergy. Nutrients. (2020) 12(2):1–11. doi: 10.3390/nu12020519
19. Bergmann KC, Wolf H, Schnitker J. Effect of pollen-specific sublingual immunotherapy on oral allergy syndrome: an observational study. World Allergy Organ J. (2008) 1(5):79–84. doi: 10.1097/WOX.0b013e3181752d1c
20. Kinaciyan T, Jahn-Schmid B, Radakovics A, Zwölfer B, Schreiber C, Francis JN, et al. Successful sublingual immunotherapy with birch pollen has limited effects on concomitant food allergy to apple and the immune response to the bet v 1 homolog mal d 1. J Allergy Clin Immunol. (2007) 119(4):937–43. doi: 10.1016/j.jaci.2006.11.010
21. Dodig S, Čepelak I. The potential of component-resolved diagnosis in laboratory diagnostics of allergy. Biochem Med (Zagreb). (2018) 28:1–9. doi: 10.11613/BM.2018.020501
22. Roberts G, Pfaar O, Akdis CA, Ansotegui IJ, Durham SR, Gerth van Wijk R, et al. EAACI guidelines on allergen immunotherapy: allergic rhinoconjunctivitis. Allergy. (2018) 73(4):765–98. doi: 10.1111/all.13317
23. Penagos M, Eifan AO, Durham SR, Scadding GW. Duration of allergen immunotherapy for long-term efficacy in allergic rhinoconjunctivitis. Curr Treat Options Allergy. (2018) 5(3):275–90. doi: 10.1007/s40521-018-0176-2
24. McGee RG. How to include patient-reported outcome measures in clinical trials. Curr Osteoporos Rep. (2020) 18:480–5. doi: 10.1007/s11914-020-00611-5
25. Sim K, Mijakoski D, Stoleski S, del Rio PR, Sammut P, Le TM, et al. Outcomes for clinical trials of food allergy treatments. Ann Allergy Asthma Immunol. (2020) 125:35–42. doi: 10.1016/j.anai.2020.06.023
26. Lloyd M, Dunn Galvin A, Tang MLK. Measuring the impact of food immunotherapy on health-related quality of life in clinical trials. Front Allergy. (2022) 3:1–7. doi: 10.3389/falgy.2022.941020
27. Lyons SA, Burney PGJ, Ballmer-Weber BK, Fernandez-Rivas M, Barreales L, Clausen M, et al. Food allergy in adults: substantial variation in prevalence and causative foods across Europe. J Allergy Clin Immunol Pract. (2019) 7(6):1920–28.e11. doi: 10.1016/j.jaip.2019.02.044
28. Hoffmann-Sommergruber K, Hilger C, Santos A, De Las Vecillas L, Dramburg S. Molecular Allergology User’s Guide 2.0. Zurich, Switzerland: European Academy of Allergy and Clinical Immunology (2022).
29. Asero R. How long does the effect of birch pollen injection SIT on apple allergy last? Allergy. (2003) 58(5):435–8. doi: 10.1034/j.1398-9995.2003.00139.x
30. Kinaciyan T, Nagl B, Faustmann S, Frommlet F, Kopp S, Wolkersdorfer M, et al. Efficacy and safety of 4 months of sublingual immunotherapy with recombinant mal d 1 and bet v 1 in patients with birch pollen–related apple allergy. J Allergy Clin Immunol. (2018) 141(3):1002–8. doi: 10.1016/j.jaci.2017.07.036
31. Kopac P, Rudin M, Gentinetta T, Gerber R, Pichler C, Hausmann O, et al. Continuous apple consumption induces oral tolerance in birch-pollen- associated apple allergy. Allergy. (2012) 67(2):280–5. doi: 10.1111/j.1398-9995.2011.02744.x
32. Inuo C, Kondo Y, Tanaka K, Nakajima Y, Nomura T, Ando H, et al. Japanese cedar pollen-based subcutaneous immunotherapy decreases tomato fruit-specific basophil activation. Int Arch Allergy Immunol. (2015) 167(2):137–45. doi: 10.1159/000437325
33. Furci F, Ricciardi L. Plant food allergy improvement after grass pollen sublingual immunotherapy: a case series. Pathogens. (2021) 10(11):1–6. doi: 10.3390/pathogens10111412
Keywords: food allergy, pollen food allergy syndrome (PFAS), BPFA, birch pollen allergy, sublingual immunotherapy (SLIT), subcutaneous immunotherapy (SCIT), allergen immune therapy (AIT), systematic review
Citation: Kallen EJJ, Welsing PMJ, Löwik JM, Van Ree R, Knulst AC and Le TM (2024) The effect of subcutaneous and sublingual birch pollen immunotherapy on birch pollen–related food allergy: a systematic review. Front. Allergy 5:1360073. doi: 10.3389/falgy.2024.1360073
Received: 22 December 2023; Accepted: 15 May 2024;
Published: 6 June 2024.
Edited by:
Anna Nowak Wegrzyn, New York University, United StatesReviewed by:
Jose Luis Subiza, Inmunotek SL, Spain© 2024 Kallen, Welsing, Löwik, Van Ree, Knulst and Le. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: E. J. J. Kallen, ZS5qLmoua2FsbGVuQHVtY3V0cmVjaHQubmw=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.