
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Allergy, 21 February 2024
Sec. Mechanisms in Allergy
Volume 5 - 2024 | https://doi.org/10.3389/falgy.2024.1357901
This article is part of the Research TopicT and B Cell Immune Dynamics in Allergic ResponseView all 5 articles
 Rebecca R. Meredith1
Rebecca R. Meredith1 Pooja Patel2
Pooja Patel2 Polly Huang2
Polly Huang2 Chinelo Pamela Onyenekwu3
Chinelo Pamela Onyenekwu3 Herleen Rai3
Herleen Rai3 Jody Tversky2
Jody Tversky2 Santiago Alvarez-Arango2,4*
Santiago Alvarez-Arango2,4*
Insulin-induced type III hypersensitivity reactions (HSRs) are exceedingly rare and pose complex diagnostic and management challenges. We describe a case of a 43-year-old woman with type 1 diabetes mellitus (DM), severe insulin resistance, and subcutaneous nodules at injection sites, accompanied by elevated anti-insulin IgG autoantibodies. Treatment involved therapeutic plasma exchange (TPE) and intravenous immunoglobulin (IVIg) as bridge therapy, followed by long-term immunosuppression, which reduced autoantibody levels and improved insulin tolerance. Given the limited treatment guidelines, we conducted a comprehensive literature review, identifying 16 similar cases. Most patients were females with a median age of 36.5 years; 63% had type 1 DM, and 44% had concurrent insulin resistance (56% with elevated autoantibodies). Treatment approaches varied, with glucocorticoids used in 67% of cases. Patients with type 1 DM were less responsive to steroids than those with type 2 DM, and had a more severe course. Of those patients with severe disease necessitating immunosuppression, 66% had poor responses or experienced relapses. The underlying mechanism of insulin-induced type III HSRs remains poorly understood. Immunosuppressive therapy reduces anti-insulin IgG autoantibodies, leading to short-term clinical improvement and improved insulin resistance, emphasizing their crucial role in the condition. However, the long-term efficacy of immunosuppression remains uncertain and necessitates continuous evaluation and further research.
Hypersensitivity reactions (HSRs) to human and analog insulins are rare and can be categorized as immediate or delayed. Immunoglobulin E (IgE) mediated HSRs, known as type I HSRs, generally develop within minutes after injection and can vary from local erythema or a pruritic wheal at the injection site to anaphylaxis. In contrast, delayed T-cell-mediated reactions, type IV HSRs, tend to appear within days as contact dermatitis, marked by eczematous areas. Such reactions are frequently attributed to additives found in insulin formulations (1–3). Infrequently, insulin hypersensitivity can arise from the formation of antigen-antibody immune complexes (ICs), resulting in type III HSRs. These reactions are often characterized by the development of painful subcutaneous nodules, commonly referred to as “Arthus’ reactions”, occurring at the insulin injection sites within 24 h of the subcutaneous injection (2, 4).
We report a unique case involving the concurrent presence of increasing insulin resistance and severely painful subcutaneous nodules at the insulin subcutaneous injection sites in a 43-year-old female with type 1 diabetes mellitus (DM) and high anti-insulin IgG autoantibodies. We hypothesize this presentation to be triggered by the high-titer anti-insulin IgG autoantibodies, resulting in the formation of ICs. These ICs may deposit at the injection sites, causing localized skin reactions as well as potentially inducing insulin resistance through a consumptive process. The pathogenesis of insulin-induced type III HSRs remains poorly understood, and the prevalence of coexisting insulin resistance remains limited, presenting substantial challenges in terms of management.
We conducted a thorough literature review to identify similar cases involving insulin-induced type III HSRs, either in conjunction with or independently of insulin resistance. Within this review, we identified 16 cases with confirmed or suspected type III HSRs, among which 7 exhibited concurrent evidence of insulin resistance. In the cases we reviewed, various immunosuppressive strategies were employed with varying degrees of success. In our case, due to the severity of injection site reactions and insulin resistance, we employed a novel treatment strategy involving therapeutic plasma exchange (TPE) followed by intravenous immunoglobulin (IVIg) as bridge therapy. This was then followed by long-term immunosuppression with rituximab and mycophenolate mofetil (MMF). This treatment resulted in a significant reduction in insulin autoantibody levels, allowing for the successful reintroduction of subcutaneous insulin.
The scarce case reports we identified in our literature review emphasize the exceptional rarity and likely underreporting of insulin-induced type III HSRs with or without insulin resistance. Managing this condition presents a significant challenge due to the absence of clear guidelines. The significant improvement in our patient's reactions and insulin requirements, along with a decrease in anti-insulin IgG autoantibody levels, aligns with the observations in other cases we reviewed. This pattern suggests a potential role for anti-insulin IgG autoantibodies in the pathogenesis of insulin-induced type III HSRs and concurrent insulin resistance. Nonetheless, further research is needed.
A 43-year-old woman with previously well-controlled type 1 DM, celiac disease, and hypothyroidism presented with a month-long escalation in insulin requirements, uncontrolled hyperglycemia, and painful skin lesions at insulin subcutaneous injection sites. The patient had used an insulin pump for over 30 years without incident. However, one month prior to presentation, her daily insulin requirements began to increase, ultimately doubling from 60 to 120 units/day, with no changes in diet or weight. About a week later, she developed painful skin reactions at injection sites occurring more than 6 h post-injection. Despite multiple attempts with various insulin formulations, her condition failed to improve, resulting in an inability to administer insulin subcutaneously and necessitating hospitalization due to diabetic ketoacidosis (DKA).
Her physical examination revealed tender, erythematous subcutaneous nodules on the bilateral flanks, lower abdomen, and arms at insulin injection sites (Figures 1A,B). Laboratories revealed glucose >500 mg/dl, bicarbonate 16 mmol/L, anion gap 16 mmol/L, and moderate ketones on urinalysis. Furthermore, laboratories were notable for anti-insulin IgE <0.10 kUA/L and elevated anti-insulin IgG of 27.8 U/ml, subsequently rising to >50 U/ml (Figure 2). Skin biopsies showed mixed dermal infiltrates with prominent lymphocytes and eosinophils and granulomatous subcutaneous infiltrate (Figures 1C–E). Direct immunofluorescence (DIF) showed patchy deposition of C3 in a granular pattern in the superficial dermal papillae with negative IgM and IgG (Figure 1F).
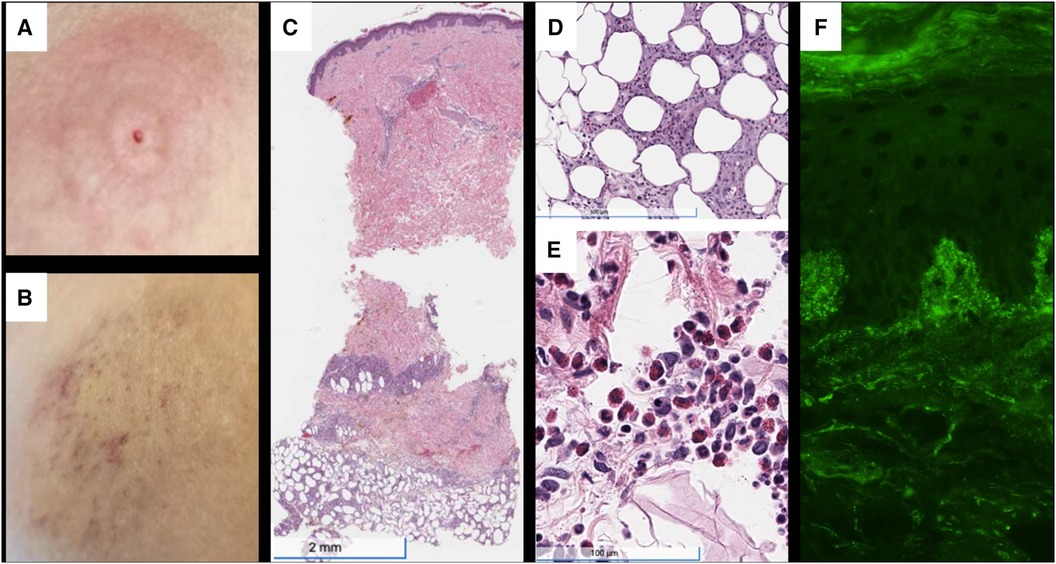
Figure 1. (A) Painful, erythematous skin rash at the insulin pump injection site on the upper abdomen, just prior to presentation. (B) Area of induration at previous insulin injection site on admission, with evolving erythema, ecchymosis, and petechiae. (C) H&E staining demonstrating subcutaneous granulomatous infiltrate with numerous eosinophils. (D) 10.5× magnification showing granuloma and eosinophils. (E) Superficial and deep perivascular and interstitial infiltrate, including lymphocytes and numerous eosinophils. (F) Direct immunofluorescence with C3 deposition in dermal papillae and basement membrane. H&E, hematoxylin and eosin stain.

Figure 2. Timeline of the case report of insulin-induced type III HSR and co-existing insulin resistance, illustrating the changes in anti-insulin IgG autoantibody titers, treatment interventions, and the evolution of symptoms over time. HSR, hypersensitivity reaction; IgG, immunoglobulin G; IVIG, intravenous immunoglobulin; MTX, methotrexate; SC, subcutaneous; TPE, therapeutic plasma exchange.
The patient was admitted to the intermediate care unit due to DKA. She responded well to intravenous (IV) insulin therapy without any complications or evidence of an allergic reaction, ruling out a type I IgE-mediated hypersensitivity. Attempts to use various subcutaneous insulin formulations with protamine and metacresol to investigate a potential HSR secondary to additives were unsuccessful. Moreover, a subcutaneous insulin dose escalation protocol was attempted, but painful localized skin reactions persisted at doses ≥4 units.
Given the concern for an IC-mediated HSR, immunosuppression was pursued, initially with methotrexate and prednisone. However, the patient needed escalating IV insulin doses, up to 10 units/h, along with frequent boluses (total daily doses of up to 230–240 units/24 h), to sustain euglycemia. Based on previously reported clinical responses to IVIg and TPE, a combination of both was pursued (5–8). She completed 5 sessions of TPE followed by 2 g/kg IVIg administered over two days. Treatment was complicated by severe headache after completion of IVIg therapy, with cerebrospinal fluid studies concerning for aseptic meningitis, which was successfully treated with a short course of oral glucocorticoids. Following IVIg and TPE, anti-insulin IgG titer significantly declined, from >50 U/ml to 2.7 U/ml (Figure 2). Treatment was then followed with one dose of rituximab, with a planned second dose two weeks later, and the initiation of mycophenolate mofetil (MMF) for long-term immunosuppression. Subcutaneous insulin was slowly reintroduced and effectively titrated to therapeutic doses, leading to the patient's discharge after a 42-day hospitalization.
We thoroughly searched the PubMed electronic database, covering articles published from 1959 to 2023. Our search utilized Medical Subject Headings (MESH) Terms, “insulin” and (“allergy” or “hypersensitivity”). We refined the search by filtering for case reports and systematic reviews. Articles without full-text availability were excluded from our study. The initial abstract screening phase involved two independent reviewers (RM and SAA), who assessed the relevance of the identified articles. Subsequently, articles that met our inclusion criteria underwent a thorough full-text screening conducted by three independent reviewers (RM, PP, and SAA). Articles meeting our inclusion criteria proceeded to full-text extraction and in-depth analysis. Lastly, we examined the reference lists of each retrieved article to identify any potential additional cases that met the criteria for our literature review.
We implemented a structured data abstraction form that included country of origin, year of publication, first author, patient demographics, presence of co-morbid autoimmune disease, diabetes type (type 1 vs. type 2), presence of anti-insulin IgG autoantibodies, biopsy results, evidence of insulin resistance, administered medications, utilization of bridge therapy (including TPE or IVIg), immunosuppressive medications administered, use of systemic glucocorticoids, and detailed outcomes and any recorded adverse events (Table 1).
To ensure the quality and consistency of our data collection process, we employed the Covidence software (Covidence Pty Ltd. in Melbourne, Australia). This software facilitated search result management, application of inclusion criteria, conflict resolution, and review tracking. Additionally, it supported quality assessments and ensured standardized data extraction.
The inclusion criteria included the presence of subcutaneous nodules and/or indurations within 24 h following insulin administration, the occurrence of painful nodules, cases where continuous subcutaneous insulin dose escalation proved ineffective, as well as the report of type III HSRs, antigen-antibody immune complex-mediated reactions, and/or Arthur's reactions.
Conversely, the studies were excluded if they reported immediate local reactions within less than an hour, urticaria-like lesions without providing a detailed account of subcutaneous nodules or indurations, systemic allergic symptoms (such as generalized urticaria, angioedema, bronchospasm, anaphylaxis), responses to continuous subcutaneous insulin dose escalation, or lesions that occurred more than 24 h after the insulin injection.
Our search using MESH Terms, “insulin” and (“allergy” or “hypersensitivity”), with a filter for case reports and systematic reviews, initially yielded 367 articles, of which 248 had full text available. After screening the abstracts, we identified 28 articles for further full-text review. Among these, 15 met the inclusion criteria. Furthermore, we identified one additional article through the references of the selected articles, bringing the total to 16 articles that reported suspected or confirmed type III HSR to insulin (Table 1).
Out of the 16 cases we examined, 12 (75%) were female. The median age was 36.5 years, with an interquartile range (IQR) of 32.5 years. Race or ethnicity information was unavailable in 11 out of the 16 cases. Among those with reported race data, there were 3 White patients and 2 Asian patients. Additionally, 10 patients (62.5%) had type 1 DM. Anti-insulin IgG autoantibodies were investigated in 9 (56%) cases and were elevated in all 9. Insulin resistance co-occurred in 7 out of the 16 cases (44%). Lastly, comorbid autoimmune disease was reported in 2 cases, both in patients with type 1 DM (Table 2).
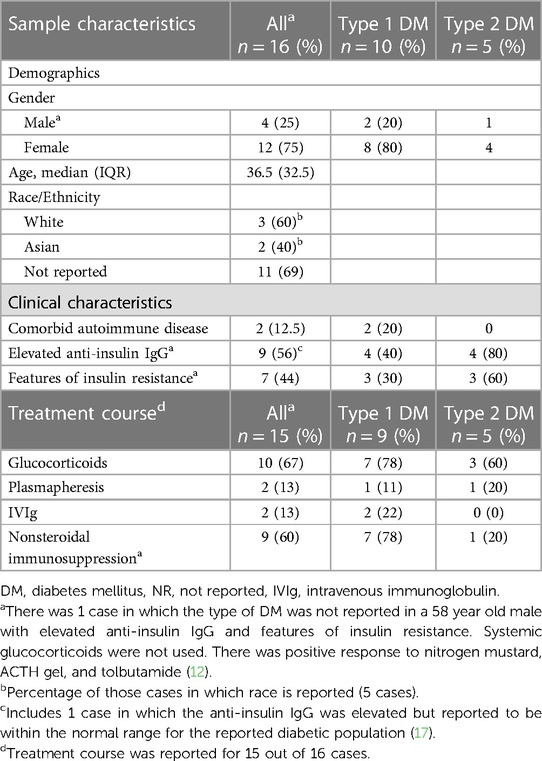
Table 2. Demographics, characteristics, and treatment interventions in patients with type 1 vs. Type 2 DM.
The treatment course was reported for 15 of the cases. Among those, 10 patients (67%) received systemic glucocorticoid treatment. Out of those patients, 4 (36%) had a positive clinical response, defined as clinical improvement following glucocorticoid therapy as described by the authors, and did not require additional immunosuppression. Among the 7 patients with type 1 DM who were treated with systemic glucocorticoids, a positive response was reported in 4 (57%) cases, while a poor response was reported in 3 (43%) cases. Among the 3 patients with type 2 DM treated with systemic glucocorticoids, all 3 (100%) were steroid responsive. Of note, glucocorticoids were discontinued in 3 out of the 10 patients (30%) due to side effects including hyperglycemia and GI symptoms. Among the 15 patients for whom treatment was reported, 9 (60%) had severe diseases that necessitated treatment with steroid-sparing immunosuppression, either alone or in combination with glucocorticoids. Of these patients, 7 (78%) had type 1 DM. In 2 out of the 15 cases (13%), TPE was employed, with positive short-term outcomes in both instances, and 2 (13%) patients received IVIg, with mixed short-term outcomes (Tables 1, 2).
Among the 9 patients treated with immunosuppression, 6 (67%) either had a poor clinical response or eventually experienced a relapse of their condition after an initial positive clinical response. Notable outcomes also included one patient who showed no initial positive response to immunosuppression and required a pancreas transplant, another patient who did not respond initially and underwent an Islet of Langerhans cell transplant, one patient who died from anaphylaxis during TPE while awaiting a pancreas transplant, and another who experienced a relapse in glycemic control after discontinuation of immunosuppressive therapy due to sepsis, ultimately resulting in poor glycemic control and a fatal cardiac event (Table 1).
Other outcomes include one patient who experienced symptom remission upon switching to a different insulin formulation, one patient who had remission with the use of subcutaneous glucocorticoids alone, two patients in whom insulin was discontinued in favor of oral hypoglycemics, and one patient who did not receive treatment because the injection site reactions were considered tolerable. Notably, in all of those instances, the patients had type 2 DM (Table 1).
Insulin-induced type III HSRs are exceptionally rare and pose distinctive diagnostic and management challenges. Key indicators involve the development of a delayed-onset, non-urticarial, and painful rash at insulin injection sites. While definitive tests are not available, the presence of elevated anti-insulin IgG autoantibodies and skin biopsy findings, such as subcutaneous granulomas and DIF showing the presence of complement or IgG deposits, should prompt suspicion. Other biopsy findings that may be associated with insulin-induced type III HSRs include red cell extravasation (indicating leukocytoclastic vasculitis) and panniculitis, although it is important to note that several cases reported biopsy findings of nonspecific inflammatory infiltrates (Table 1). Moreover, DIF sensitivity varies among diseases, with higher rates seen in conditions like vesiculobullous diseases and small-vessel vasculitis (20). In certain cases, like ours, insulin-induced type III HSRs coincide with autoimmune insulin resistance, adding complexity to management, especially for type 1 DM patients. Among these patients, many prove to be resistant to steroids, and while some exhibit partial acute responses to bridge therapies involving TPE and/or IVIg, the majority do not respond to immunosuppression or experience relapses after an initial positive response. Long-term management of these patients necessitates multimodal immunosuppression strategies. However, the long-term effectiveness of this treatment approach remains to be determined and requires ongoing follow-up assessment.
Since the introduction of purified insulins and human insulin, the prevalence of anti-insulin autoantibodies in patients previously treated with insulin has declined; however, prevalence remains high. Wredling et al. found that among individuals with prior insulin treatment, up to 78% had insulin autoantibodies, particularly prevalent in type 1 DM patients and those with prolonged insulin use (21). Although rarely clinically significant, anti-insulin IgG autoantibodies can bind to exogenous insulin and form ICs that can deposit in various tissues, triggering the classical complement pathway and causing inflammation. When this process occurs in the skin, it presents with granulomatous lesions and painful nodules at the insulin injection sites (22, 23). Furthermore, immunologic insulin resistance may develop due to the formation of ICs; however, its exact prevalence remains unknown, with only isolated case reports available (22, 24). Berson et al. demonstrated that in individuals with suspected immunologic insulin resistance caused by anti-insulin autoantibodies, these antibodies bound to insulin, forming ICs that neutralized insulin effects (25). In several cases, including ours, immunosuppressive therapy reduced anti-insulin IgG autoantibodies, leading to clinical improvement and a decrease in insulin resistance, highlighting the significance of these autoantibodies.
Treatment for insulin-induced type III HSRs is challenging due to their rarity and reliance on individual case reports, resulting in varying approaches and outcomes. This complexity is amplified in patients with type 1 DM because of their insulin dependence. Glucocorticoids, although offering temporary relief, often prove ineffective in preventing skin reactions and may worsen hyperglycemia. Notably, most cases, including ours, involved individuals with type 1 DM, and a significant portion showed a weaker response to glucocorticoids, requiring alternative forms of immunosuppression compared to those with type 2 DM. Additionally, 57% of the patients with type 1 DM did not respond to immunosuppression and among those who did respond, most experienced relapses after an initial positive response (Table 2). Interestingly, there were 3 cases, including ours, with reported comorbid autoimmune disease, all occurring in patients with type 1 DM. The increased occurrence of type III HSRs in individuals with type 1 DM may be linked to their heightened susceptibility to other autoimmune conditions and autoantibody formation, highlighting the importance of further research in this field.
Both TPE and IVIg have been utilized in the treatment of insulin-induced type III HSRs. TPE was effective in 3 cases, including ours, while IVIg's effectiveness varied, with an initial positive response reported in one case (4–8). Immunosuppressive agents targeting B and T cells, such as rituximab, methotrexate, azathioprine, and MMF, have yielded varying effectiveness results (Table 1). In our case, due to the severity of the clinical course, with ongoing high IV insulin requirements, we adopted a novel approach that combined TPE and IVIg, which resulted in a rapid improvement in clinical symptoms and subcutaneous insulin tolerance, as well as a reduction in anti-insulin IgG autoantibody titers. Compared to using TPE alone, the effectiveness of combining TPE and IVIg on the clinical course and auto-antibody levels remains uncertain. We withheld reintroducing subcutaneous insulin during TPE due to persistently high IV insulin requirements to avoid further burdening the system until tolerance was assured.
Determining whether the observed effects resulted from TPE, IVIg, or their combination is inconclusive. Mechanistically, the reduction in autoantibody titers attributed to TPE is a plausible hypothesis, while the precise impact of IVIg on autoantibody titers is less evident. TPE operates by eliminating intravascular antibodies, whereas the immunomodulatory actions of IVIg include interference with the autoantibody-antigen complex, disruption of complement activation, and modulation of T and B cell activation (26). Nevertheless, what we can confirm is that about three days after IVIg treatment was completed, the patient's IV insulin requirements significantly decreased, facilitating the successful reintroduction of subcutaneous insulin and leading to her discharge from the hospital. Subsequent long-term immunosuppression with rituximab and MMF initially maintained a low anti-insulin IgG level (0.3 U/ml) with optimal glycemic control and sustained subcutaneous insulin tolerance at short-term hospital follow-up.
Upon discharge, her allergy/immunology team planned a second Rituximab infusion two weeks after the first, but it was delayed due to insurance issues. She received the second dose 32 days after the first infusion. Around the same time, insulin requirements increased again, accompanied by burning sensations at injection sites and an elevated anti-insulin IgG level to 25.1 U/ml. An acute intervention involving a four-day steroid pulse regimen led to a temporary reduction of IgG levels to 12.8 U/ml and an improvement in symptoms. Since then, she has received two additional doses of Rituximab. While localized reactions have significantly decreased in frequency, they continue to occur intermittently, up to 2–3 times per week. Insulin requirements have decreased by approximately 45% since hospital discharge, indicating some improvement in insulin resistance with immunosuppression, and she has not re-required hospitalization. However, due to persistent localized reactions and insulin requirements still above baseline, introducing an alternative immunosuppressive medication into the treatment plan and considering a potential pancreatic transplant are actively being explored. Our case and literature review highlight the complexities in managing insulin-induced type III HSRs, especially when they co-occur with insulin resistance, particularly in patients with type 1 DM. This emphasizes the critical need for research in this field.
Delayed non-urticarial skin reactions to subcutaneous insulin, accompanied by an elevated anti-insulin IgG autoantibody titer, should raise suspicion of insulin-induced type III HSR. In rare instances, coexisting insulin resistance may result from anti-insulin IgG autoantibodies. Managing this condition poses significant challenges due to its exceptional rarity, likely underreporting, and the absence of clear guidelines. There is a high rate of treatment failure and relapse, especially among those with type 1 DM. Treatment should involve bridge therapy with TPE and/or IVIg to promptly lower autoantibody levels, followed by systemic multimodal immunosuppression. Long-term effectiveness remains uncertain and requires ongoing assessment.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
RM: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. PP: Conceptualization, Investigation, Methodology, Validation, Writing – review & editing. PH: Conceptualization, Investigation, Visualization, Writing – review & editing. CO: Conceptualization, Investigation, Writing – review & editing. HR: Conceptualization, Investigation, Writing – review & editing. JT: Conceptualization, Investigation, Supervision, Writing – review & editing. SA-A: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article.
SA-A receives support from the NIH-NCATS (KL2TR003099) and the American Academy of Allergy Asthma and Immunology Foundation. Departmental funding from the Department of Medicine will be provided for publication fees should the manuscript be accepted for publication.
We thank Grant Anhalt, dermatopathologist, Marchalik, dermatologist, and Robert Hamilton, immunologist, for their critical guidance.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
DIF, direct immunofluorescence; DKA, diabetic ketoacidosis; HSR(s), hypersensitivity reaction(s); DM, diabetes mellitus; IC, immune complex; IV, intravenous; MMF, mycophenolate mofetil; IVIg, intravenous immunoglobulin; TPE, therapeutic plasma exchange; MTX, methotrexate; HCQ, hydroxychloroquine.
1. Teo CB, Tan PY, Lee SX, Khoo J, Tan JG, Ang SF, et al. Insulin allergy to detemir followed by rapid onset of diabetic ketoacidosis: a case report and literature review. Front Endocrinol (Lausanne). (2022) 13:844040. doi: 10.3389/fendo.2022.844040
2. Ghazavi MK, Johnston GA. Insulin allergy. Clin Dermatol. (2011) 29(3):300–5. doi: 10.1016/j.clindermatol.2010.11.009
3. Heinzerling L, Raile K, Rochlitz H, Zuberbier T, Worm M. Insulin allergy: clinical manifestations and management strategies. Allergy. (2008) 63(2):148–55. doi: 10.1111/j.1398-9995.2007.01567.x
4. Ahmed M, Subbalaxmi MVS, Anne B, Deme S. Recurrent diabetic ketoacidosis and extreme insulin resistance due to anti-insulin antibodies: response to immunosuppression and plasma exchange. Diabetes Technol Ther. (2021) 23(3):227–9. doi: 10.1089/dia.2020.0438
5. Alkhatib EH, Grundman JB, Adamusiak AM, Bellin MD, Brooks JP, Buckley KS, et al. Case report: insulin hypersensitivity in youth with type 1 diabetes. Front Endocrinol (Lausanne). (2023) 14:1226231. doi: 10.3389/fendo.2023.1226231
6. Bayraktar F, Akinci B, Demirkan F, Yener S, Yesil S, Kirmaz C, et al. Serum sickness-like reactions associated with type III insulin allergy responding to plasmapheresis. Diabet Med. (2009) 26(6):659–60. doi: 10.1111/j.1464-5491.2009.02733.x
7. Greenfield JR, Tuthill A, Soos MA, Semple RK, Halsall DJ, Chaudhry A, et al. Severe insulin resistance due to anti-insulin antibodies: response to plasma exchange and immunosuppressive therapy. Diabet Med. (2009) 26(1):79–82. doi: 10.1111/j.1464-5491.2008.02621.x
8. Harvey JN, Cronin M, Arkwright P. Insulin hypersensitivity in type 1 diabetes: investigation and treatment with immunodepletion. Pract Diab. (2020) 37:59–61a. doi: 10.1002/pdi.2265
9. Clarke B, Loudovaris T, Radford T, Drogemuller C, Coates PT, Torpy D. Ambulatory intravenous insulin and islet cell transplantation to treat severe type III insulin hypersensitivity in a patient with type 1 diabetes mellitus. Clin Case Rep. (2020) 8(12):2759–62. doi: 10.1002/ccr3.3200
10. Darmon P, Castera V, Koeppel MC, Petitjean C, Dutour A. Type III allergy to insulin detemir. Diabetes Care. (2005) 28(12):2980. doi: 10.2337/diacare.28.12.2980
11. Edwards M, Liy-Wong C, Byrne A, Cowan KN, Ahmet A. Insulin reactions: what do you do when your treatment’s the trigger? Can J Diabetes. (2023) 47(2):190–2. doi: 10.1016/j.jcjd.2022.08.006
12. Friedlander EO, Bryant MD Jr. Idiopathic insulin-resistant diabetes mellitus; report of a case associated with insulin allergy. Am J Med. (1959) 26(1):139–45. doi: 10.1016/0002-9343(59)90334-1
13. Mandrup-Poulsen T, Mølvig J, Pildal J, Rasmussen AKAK, Andersen L, Skov BG, et al. Leukocytoclastic vasculitis induced by subcutaneous injection of human insulin in a patient with type 1 diabetes and essential thrombocytemia. Diabetes Care. (2002) 25(1):242–3. doi: 10.2337/diacare.25.1.242
14. Müller CSL, Bourg C, Schweitzer LF, Friesenhahn-Ochs B, Pföhler C. Injection site reaction to Various insulins as type III allergy with urticarial vasculitis in a patient with type I diabetes Mellitus. Am J Dermatopathol. (2023) 45(2):86–9. doi: 10.1097/DAD.0000000000002356
15. Murray BR, Jewell JR, Jackson KJ, Agboola O, Alexander BR, Sharma P. Type III hypersensitivity reaction to subcutaneous insulin preparations in a type 1 diabetic. J Gen Intern Med. (2017) 32(7):841–5. doi: 10.1007/s11606-017-4037-7
16. Rachid B, Rabelo-Santos M, Mansour E, de Lima Zollner R, Velloso LA. Type III hypersensitivity to insulin leading to leukocytoclastic vasculitis. Diabetes Res Clin Pract. (2010) 89(3):e39–40. doi: 10.1016/j.diabres.2010.05.019
17. Silva ME, Mendes MJ, Ursich MJ, Rocha DM, Brito AH, Fukui RT, et al. Human insulin allergy-immediate and late type III reactions in a long-standing IDDM patient. Diabetes Res Clin Pract. (1997) 36(2):67–7. doi: 10.1016/S0168-8227(97)00031-4
18. Takahashi K, Anno T, Takenouchi H, Iwamoto H, Horiya M, Kimura Y, et al. Serious diabetic ketoacidosis induced by insulin allergy and anti-insulin antibody in an individual with type 2 diabetes mellitus. J Diabetes Investig. (2022) 13(10):1788–92. doi: 10.1111/jdi.13838
19. Winocour PH, Haeney M. Persistent local insulin allergy in a diabetic with chronic lymphatic leukaemia. Postgrad Med J. (1986) 62(731):865–8. doi: 10.1136/pgmj.62.731.865
20. Mysorekar VV, Sumathy TK, Shyam Prasad AL. Role of direct immunofluorescence in dermatological disorders. Indian Dermatol Online J. (2015) 6(3):172–80. doi: 10.4103/2229-5178.156386
21. Wredling R, Lins P, Adamson U. Prevalence of anti-insulin antibodies and its relation to severe hypoglycaemia in insulin-treated diabetic patients. Scand J Clin Lab Invest. (1990) 50(5):551–7. doi: 10.1080/00365519009089170
22. Van Haeften TW. Clinical significance of insulin antibodies in insulin-treated diabetic patients. Diabetes Care. (1989) 12(9):641–8. doi: 10.2337/diacare.12.9.641
23. Usman N, Annamaraju P. Type III Hypersensitivity Reaction. In: StatPearls [Internet]. Treasure Island: StatPearls Publishing (2023).
24. Koyama R, Kato M, Yamashita S, Nakanishi K, Kuwahara H, Katori H. Hypoglycemia and hyperglycemia due to insulin antibodies against therapeutic human insulin: treatment with double filtration plasmapheresis and prednisolone. Am J Med Sci. (2005) 329(5):259–64. doi: 10.1097/00000441-200505000-00007
25. Berson SA, Yalow RS, Bauman A, Rothschild MA, Newerly K. Insulin-I131 metabolism in human subjects: demonstration of insulin binding globulin in the circulation of insulin treated subjects. J Clin Invest. (1956) 35(2):170–90. doi: 10.1172/JCI103262
Keywords: insulin hypersensitivity, immune complex-mediated hypersensitivity, insulin allergy, IgG-mediated hypersensitivity reactions, type III hypersensitivity reaction, insulin resistance, insulin autoantibody
Citation: Meredith RR, Patel P, Huang P, Onyenekwu CP, Rai H, Tversky J and Alvarez-Arango S (2024) A case report and systematic literature review: insulin-induced type III hypersensitivity reaction. Front. Allergy 5:1357901. doi: 10.3389/falgy.2024.1357901
Received: 18 December 2023; Accepted: 9 February 2024;
Published: 21 February 2024.
Edited by:
Tarun Keswani, Harvard Medical School, United StatesReviewed by:
Mahinder Paul, Albert Einstein College of Medicine, United States© 2024 Meredith, Patel, Huang, Onyenekwu, Rai, Tversky and Alvarez-Arango. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Santiago Alvarez-Arango c2FsdmFyZXpAamhtaS5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.