
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Allergy, 30 January 2024
Sec. Asthma
Volume 4 - 2023 | https://doi.org/10.3389/falgy.2023.1291185
This article is part of the Research TopicHypotheses Explaining the Allergy EpidemicView all 11 articles
 G. K. Scadding1,2
G. K. Scadding1,2 M. McDonald3
M. McDonald3 V. Backer4
V. Backer4 G. Scadding5
G. Scadding5 M. Bernal-Sprekelsen6,7
M. Bernal-Sprekelsen6,7 D. M. Conti8*
D. M. Conti8* E. De Corso9
E. De Corso9 Z. Diamant10,11,12,13
Z. Diamant10,11,12,13 C. Gray14,15
C. Gray14,15 C. Hopkins16
C. Hopkins16 M. Jesenak17,18,19
M. Jesenak17,18,19 P. Johansen20,21
P. Johansen20,21 J. Kappen22
J. Kappen22 J. Mullol23
J. Mullol23 D. Price24,25
D. Price24,25 S. Quirce26
S. Quirce26 S. Reitsma27
S. Reitsma27 S. Salmi28,29
S. Salmi28,29 B. Senior30
B. Senior30 J. P. Thyssen31
J. P. Thyssen31 U. Wahn32
U. Wahn32 P. W. Hellings33,34,35
P. W. Hellings33,34,35
Asthma, which affects some 300 million people worldwide and caused 455,000 deaths in 2019, is a significant burden to suffers and to society. It is the most common chronic disease in children and represents one of the major causes for years lived with disability. Significant efforts are made by organizations such as WHO in improving the diagnosis, treatment and monitoring of asthma. However asthma prevention has been less studied. Currently there is a concept of pre- diabetes which allows a reduction in full blown diabetes if diet and exercise are undertaken. Similar predictive states are found in Alzheimer's and Parkinson's diseases. In this paper we explore the possibilities for asthma prevention, both at population level and also investigate the possibility of defining a state of pre-asthma, in which intensive treatment could reduce progression to asthma. Since asthma is a heterogeneous condition, this paper is concerned with allergic asthma. A subsequent one will deal with late onset eosinophilic asthma.
Several chronic diseases only become symptomatic from the moment a certain threshold of inflammation or volume of tissue damage has been reached (1–4). By this stage the disease may be irreversible with no possibility of cure (1, 5). Recently the idea of earlier pre-emptive diagnosis has evolved. For example, the concept of pre-diabetes has led to identification of likely future diabetics and provision of measures to reduce disease progression. Similarly, the pre-diagnostic phase of Alzheimer's and Parkinson's diseases are being explored in population-based studies.
In this paper we explore the possibilities for similar action in asthma.
The concept of pre- diabetes, a state of intermediate hyperglycemia of which the sufferer is unaware, with a significant risk for diabetes development and associated adverse health outcomes, has proved useful. Lifestyle advice has been shown in studies to lead to a 58% risk reduction in diabetes, improving health, reducing some morbidities and costs (6). Weight loss was the biggest determinant of risk reduction: with a 16% decrease in diabetes development for every kilogram lost (7). As pre- diabetes affects some 352 million adults between the ages of 20 and 79 (7.3% of that population) and health costs for those with diabetes are double those of people without it, this is cost-effective preventive medicine (8, 9).
The soluble oligomer binding assay (SOBA), detects toxic (alpha sheet) Aβ oligomers in serum long before symptoms of Alzheimer's disease emerge. This should enable earlier diagnosis and raises the possibility of prevention of full -blown neurodegeneration (10).
Parkinson's, a disease caused by a lack of dopamine, is often present for several years before the diagnosis is made. Machine learning applied to voice recordings can be used to detect Parkinson's disease automatically and early, allowing earlier treatment and better quality of life for sufferers (11).
Asthma is a highly prevalent disease of modern societies, affecting 1–29% of the population of different countries, some 3–400 million individuals being affected worldwide (12, 13).
Asthma is occasionally fatal; in most patients it reduces quality of life (QoL), requires long term daily treatment and is costly to the individuals, their families and to society. A significant number of people with severe asthma receive long-term oral corticosteroid (OCS) treatment, which can result in adverse outcomes and increase long-term healthcare costs (14). Recently more widely available monoclonal antibodies provide an alternative option for some severely affected patients but are extremely expensive.
At present, several risk factors for asthma are universally recognised, yet there is a lack of routinely applied screening programmes for asthma in individuals at risk. A recent systematic review of childhood asthma predictive tools concluded that they have” poor predictive accuracy with performance variation in sensitivity and positive predictive value” (15).
Is there a possibility of defining a state of pre- asthma whereby preventive measures could be taken to reduce asthma development? In this paper we consider what is currently known about the origins and pathophysiology of asthma in order to explore the potential for its prevention both at population level and if pre-asthma could be identified.
Search terms including” asthma” together with” causes”, “phenotypes”, “endotypes”, “genomics”, “origins”, “natural history”, “pathogenesis”,” predisposition “, “provoking factors” were used, plus “allergic march” together with the Global Initiative for Asthma (GINA) and the British Thoracic Society/Scottish Intercollegiate Guidelines Network (BTS/SIGN) guidelines, references contained within the articles found plus articles in the collections of the authors.
Asthma is a chronic lung disease affecting people of all ages. It is caused by inflammation and muscle tightening around the airways, which makes it harder to breathe (16). Clinically asthma is characterized by a symptom complex of wheeze, shortness of breath, cough and chest tightness together with reduction in expiratory outflow which is initially variable but later may become fixed.
Asthma is a heterogeneous condition with various phenotypes and endotypes usually associated with chronic airways inflammation and hyperresponsiveness, though rarer non-inflammatory subtypes exist (17). It is a variable disease in which symptoms and signs can be precipitated by physical and environmental triggers, including stress and exercise, weather or temperature changes, smoke, airborne particles and chemicals, microbes (mainly viruses), and allergens; several of these acting initially on the respiratory epithelium (18, 19) (Figure 1). The genetic background of the individual affects the response which may also be modified by exercise (20), thus both hereditary and epigenetic factors are at play in asthma pathogenesis.
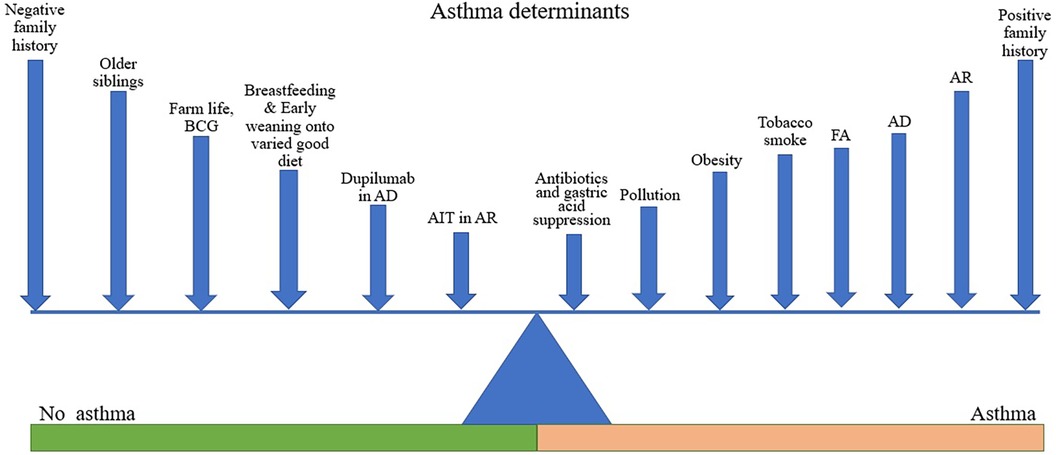
Figure 1. Asthma determinants. Figure shows various factors acting to promote or reduce the likelihood of asthma development. The length of line is not intended to denote the magnitude of effect but is varied to facilitate labelling. Different factors may be more or less relevant in different individuals depending on their genetic background.
The main drivers of inflammation include allergens, viruses and pollutants in allergic asthma, in other forms they are less well identified. Based on inflammatory profile we can distinguish two major inflammatory forms of asthma, both of which are usually associated with Type 2 eosinophilic inflammation: allergic asthma and late-onset (adult) asthma, which is mainly non- allergic. In children allergic asthma predominates and asthma incidence and prevalence are higher; morbidity and mortality are higher in adults (21).
This paper will focus on allergic asthma in childhood since this form of asthma has a well—described pathophysiology and known triggers.
A fundamental property of the immune system is its ability to distinguish between “self” and “non-self”, that is, between molecules produced by the “self”s” own genes and those that are not. In atopic asthma, allergens release proteases, which disrupt the epithelial barrier and induce secretion of alarmins such as interleukin-25 (IL-25), interleukin-33 (IL-33), and thymic stromal lymphopoietin (TSLP) from epithelial cells. IL-33 and TSLP activate dendritic cells (DCs) which then present antigens to naïve T cells, which are activated to differentiate into Th2 cells. Th2 cells, together with alarmin-stimulated IL-C2 innate immune cells which are largely located at epithelial surfaces (22), are the key cellular mediators of allergic airway inflammation.
People who smoke tobacco are exposed to nicotine and a range of combustion products such as carbon particles, partially combusted hydrocarbons, lipopolysaccharides, and a range of gases. Smokers with existing asthma have increased morbidity, more severe asthma symptoms, accelerated decline in lung function, reduced lung growth, an altered inflammatory phenotype, and reduced corticosteroid responsiveness with increasing neutrophilic inflammation. Furthermore, smoking introduces several changes into the airways including mucus hyperproduction, chronic airway inflammation and microbiome dysbiosis. Hence, smoking may induce airway remodelling resulting in fixed airflow limitation (23–27). The risk is accelerated especially in case of positive family history for atopy (28).
Epidemiologically, smoking is highly prevalent among young adults (29) and adult patients with asthma. Unfortunately, tobacco consumption is important in asthma induction not only for the smokers, but also for the unborn infant of smokers for many years to come (30–34).
Maternal smoking during pregnancy is associated with the cumulative incidence of asthma in offspring between the ages of 31 and 46 years (35).
New phenomena are the use of non-tobacco nicotine in different ways of delivery, such as vapes and packages (chewing bags). These products have also been shown to lead to small airways illness and asthma so a switch to them is unlikely to play any role in asthma prevention (36, 37).
Nitrogen dioxide fumes from gas cookers is associated with increased asthma with a summary odds ratio of 1.32 [95% confidential interval (CI) 1.18–1.48] (38).
The prevalence of asthma and allergies have increased markedly with modern living (39). It appears that this may in part be due to our exposure to environmental chemicals. Around 91% of the world's population are living in areas where the levels of air pollutants exceed the World Health Organization (WHO) limits. There are many different types of airborne pollution, but simplistically these can be divided into gases and particulate matter. The latter is considered as particularly dangerous as respirable particles can remain airborne over large distances.
Air pollution as a risk factor for asthma can also be considered from the perspective of indoor or outdoor sources of pollution. High numbers of domestic cleaning agents are associated with an increased risk of asthma. A Canadian cohort study noted an association between use of cleaning agents early in life and risk of childhood wheeze and asthma at age 3 years (40). Further evidence shows that this association holds across age groups (41). Likely mechanisms are (a) that chemicals increase epithelial permeability and thus allow allergens and pathogens to access the immune cells within the underlying mucosa and (b) that combination with, or altering the structure of, allergens may increase their allergenicity (27). Subsequently, pro-inflammatory immune responses and complex interactions with structural cells and sensory nerves cause chronic airway inflammation (42).
Obesity is becoming increasingly common, and it is associated with an increased risk of asthma (43). There are several different mechanisms through which obesity could predispose to or worsen asthma. Obesity may induce pro-inflammatory changes such as increased production of adipokines and pro-inflammatory cytokines, e.g., IL-6 and TNF-α, by adipose tissue. These cytokines cause a low-grade systemic inflammatory response and can induce a non-eosinophilic or neutrophilic airway inflammatory response (44, 45).
Obesity is also associated with co-morbidities such as depression and gastroesophageal reflux disease which can further worsen the clinical manifestations of asthma (43, 46).
When obesity complicates existing asthma, patients report increased symptoms, poorer asthma control, more frequent exacerbations and a poorer quality of life, whereas weight loss increases asthma control. Recent randomized controlled trial (RCT) studies have shown an association between weight-loss and asthma control, as well as increased QoL (47, 48). However, whether weight loss can prevent asthma development is an open question (49).
Recent data suggests not only a bidirectional association between mood and anxiety disorders and asthma but that asthma can predispose to neurodegeneration, another reason for its prevention (50).
Mendelian randomization studies, which distinguish between association and causation, show a causal relationship between major depressive disorder and asthma with a relative risk of 1.23 (1.13–1.33) (51).
Allergic asthma often begins in childhood as part of a collection of atopic disorders (atopic dermatitis, food allergy, allergic rhinitis) mediated by IgE, the allergy antibody. There is often a family history of allergic disease with multiple associated genes predisposing to allergy and to asthma (52, 53). However environmental factors are also relevant (54, 55).
The Allergic March is one recognised pattern starting in infancy with atopic dermatitis, with subsequent development of asthma and allergic rhinitis (AR). In a recent meta-analysis, some 30% of children with atopic dermatitis (AD) also have asthma, with a figure of 60% in severe AD (56). There is emerging evidence that AD can be predicted through biomarkers measured in non-invasively collected skin tape strips (57), and it is possible that these infants with type 2 skewed skin immune response may also have a higher risk of subsequent asthma development.
Other patterns associated with asthma development may occur, one of which is initial AR, starting in childhood or young adulthood, with subsequent development of asthma (58). Allergic asthma can also begin in adult life. Sometimes this is related to occupational exposures (59, 60).
Pre- asthma could be defined as a state in which the subject is experiencing mild, probably intermittent lower airways inflammation/ hypersensitivity which is insufficient to cause symptoms, but which is capable of progression to asthma. Therefore in children with AD or AR or both there is the possibility of identifying those most liable to develop asthma. These are groups where pre-asthma could possibly be identified using cohort studies and sensitive airway assays, such as FeNO, sputum eosinophils or bronchial hyper-reactivity.
Table 1 lists major known risk factors for allergic asthma, some of which could serve to identify those at risk of pre-asthma.
Both genetic predisposition and environmental factors and triggers are implicated in asthma (94). There is interaction between them, for example in children who grow up on a farm and consume raw milk, the protective effect of raw milk is enhanced with CUD14/1721 A allele than with the homozygous G allele (95).
A risk level for future asthma could be explored. Persons with no predisposing factors could be considered as risk level 0, those with a family history only or one risk factor from Table 1 as risk level 1, and persons with both a predisposing factor and a family history of asthma level 2. A family history of asthma plus two or more risk factors, such as existing FA, AD or AR could be level 3. If there is evidence of lower airway inflammation they could be graded as level 4. Examination of existing clinical data from cohort studies could be mined to validate the appropriateness or applicability of this classification. In fact some such studies already exist (96). Development of childhood asthma prediction models using machine learning approaches (97). The latter improved on regression-based model predictions by utilizing machine- learning approaches.
If a classification proves sufficiently clinically valid, then medical interventions to endangered subjects should then be devised according to level of risk.
Several factors could be addressed, both pre- and post-natally. Genes cannot be altered as yet, but their identification could suggest possible protective environmental measures, such as altering skin pH in AD which reduces asthma development in AD mice (53, 98).
Table 2 shows possible routes of asthma prevention. Many sensible measures, such as avoidance of pollution and good diet are suitable for use at a population level, i.e., primary prevention of asthma in those at all levels, rather than a secondary prevention in those more predisposed to develop asthma. Each area is expanded in the text.
Maternal obesity increases the risk of developing asthma in offspring. In mothers who were obese, each added 1 kg/m2 was associated with a 2–3% increased risk of the infants developing asthma at an age between 14 months and 16 years (99). However, the GINA guidelines, an initiative with the goals of disseminating information about asthma management, and providing a mechanism to translate scientific evidence into improved asthma care, do not recommend unguided weight loss in pregnancy (14).
A healthy gut microbiome in early infancy can be influenced by maternal diet during pregnancy as well as the maternal environment. It can affect the development of immunity and alter the predisposition to later immune- mediated diseases, such as asthma (100). The protective effect against allergies of having older siblings may be mediated via the gut microbiota (101).
Whereas there is little evidence in favour of dietary allergen avoidance in pregnancy (14), non—allergenic food intake during pregnancy was assessed in a systematic review of studies investigating any association between it and all allergic outcomes in the offspring. A Mediterranean diet rich in vegetables, legumes and high on fish/fatty fish and Vitamin D appeared protective from developing allergies and asthma. Because of the heterogeneity of the studies and lack of clarity on intervals and measurement of intake no clear recommendations could be made (102, 103).
More comprehensive work in the Healthy Start study (104) was on a cohort of over 1,200 mothers and had a reliable maternal diet index. This index weighted measures of intake of vegetables, yogurt, fried potatoes, rice/grains, red meats, pure fruit juice, and cold cereals. A prenatal diet that included cooked vegetables and yoghurt reduced the risk of allergic disease at 4 years. In adjusted models, a one-unit increase in the index was significantly associated with reduced odds of offspring allergic rhinitis [odds ratio (CI) 0.82 (0.72–0.94)], atopic dermatitis [0.77 (0.69–0.86)], asthma [0.84 (0.74–0.96)], and wheeze [0.80 (0.71–0.90)], but not food allergy [0.84 (0.66–1.08)]. A diet consisting mainly of fried potato, red meat and undiluted fruit juice had an increased risk of allergic disease. As the authors state: “This is the first study that has shown associations between an index of maternal dietary intake during pregnancy and multiple offspring allergic diseases. The results give hope for prevention of allergic diseases in utero.”
The sensitization capacity of acid suppressing drugs was confirmed in a recent study performed as an investigation of three human databases in Sweden, which found a positive correlation with such medication taken by the mother during pregnancy and the induction of allergy and asthma in the child (105).
Pre-natal antibiotics have been shown to lead to increased risk for asthma. In a cohort of 213,661 mothers from 1996 to 2012, of the 38.6% of mothers who received antibiotics 10.8% of their infants developed asthma. Maternal antibiotics nine months prior to pregnancy and nine months postpartum also gave an increased risk of developing asthma (106).
These results could not be confirmed in a similar study in a Danish population (107). From 2005 to 2011, 22.3% of 407,804 women received antibiotics during pregnancy Antibiotic use during pregnancy was associated with childhood asthma in cohort analyses (HR 1.21, 95% CI 1.18–1.24), but not in sibling analyses (HR 0.96, 95% CI 0.90–1.03). This suggests that previous studies showing increased risk may have had confounders such as differences in genetics and in history of environmental exposure.
As mentioned earlier, maternal smoking carries a very high risk of children developing asthma. In a review of 70 publications, citing 71 studies, the risk of developing wheeze with maternal smoking was 52% with a 20% increased risk of developing asthma between 5 and 18 years. There was an increased risk for maternal passive smoking as well as for household smoke. Longitudinal studies show that postnatal altered indices of airway function are not only a risk factor for subsequent asthma or wheezing illness development but also predict adult airway dimensions. Vitamin C supplementation to pregnant smokers might ameliorate the effects of in utero smoke on offspring lung function. Interventions in well-phenotyped longitudinal birth cohorts with early airway function assessments monitored through to adulthood are needed (79, 80).
Birth by caesarean section (CS) is associated with adverse immune effects including predisposition to infections, allergies, and inflammatory disorders, probably related to failure to collect the vaginal microbiota. The lower success of breastfeeding after CS adds to alterations in normal microbiota development (74–77).
Caesarean section was associated with an increased risk of asthma at age 8 in a birth cohort study of 2,917 children (OR 1.79; 95% CI 1.27–2.51). The risk was higher with two allergic parents: OR 2.91; 95% CI 1.20–7.05; compared to one: OR 1.86; 95% CI 1.12–3.09). In those with non-allergic parents the OR was 1.36; 95% CI 0.77–2.42) (78).
Breastfeeding is protective of asthma/wheeze from 0 to 2 years at which time the protective effect seems to wane (92). There was however a protective effect from developing asthma from 5 to 18 years based on meta- analysis by Lodge (108).
Breastfeeding data in weaker- designed studies might have a recall bias or difference in breastfeeding data might be attributable to the duration of breastfeeding. Breastfeeding helps establish the infant's gut microbiome that takes up to 1,000 days to stabilize (Pregnancy to 2 years). The Oligosaccharides (HMO), IgA, protein, free amino acids, vitamins, cytokines and antibacterial peptides in breastmilk play a role not only in establishing the gut microbiome of infants as well as their innate and adaptive immune system. Breastfed infants have 90% Bifidobacteria colonisation and formula fed infants have 40% Bifidobacteria and 60% Lactobacillus (109).
Increased food diversity post-natally in the first year of life had an inverse relationship to developing asthma and with each new food introduced there was a relative risk reduction of developing asthma with a dose-response effect [adjusted odds ratio with each additional food item introduced, 0.74 (95% CI, 0.61–0.89)]. A similar effect was observed for food allergy and food sensitization. Non- pasteurised milk, butter, and yoghurt as well as fruit and vegetables gave the greatest protection. Increased dietary diversity was associated with an increase expression of Foxp3 on T regulatory cells, indicating a possible inhibition of class switch to IgE at 6 years (87, 88).
The timing of food introduction has been shown to have a crucial effect upon the development of food allergies in predisposed infants (89, 90). Since food allergy predisposes to asthma development this is an important area for identification and management of those at risk, i.e., those with pre- asthma.
The association between vitamin D and development of allergy is contradictory with some evidence of a U-shaped curveA publication reviewed pre- natal vitamin D supplementation and concluded that it probably prevented transient wheezing, but not allergic asthma (110). A recent large Japanese study of almost 74,000 mother—infant pairs showed no association between maternal vitamin D levels and asthma or atopic dermatitis at age 3. However there was an inverse relationship between maternal dietary vitamin D intake and the risk of developing allergic rhinitis symptoms at the age of 3 years (111). Since rhinitis predisposes to asthma further follow up is necessary. Large, well-planned randomized controlled trials on vitamin D supplementation are needed.
A higher dietary intake of long chain omega-3 fatty acids in childhood may reduce the risk of developing subsequent asthma, but only in children carrying a common gene variant in the fatty acid desaturase (FADS) gene which is associated with lower blood levels of long chain omega-3 fatty acids (112).
Probiotic supplementation may be the next step in correcting gut dysbiosis and preventing asthma. Heterogeneity of study design as well as of probiotic strains used contribute to the complexity of evaluating the available data. Most probiotics evaluated failed the primary outcome of preventing asthma. Individual probiotics seem to have different effects, some more beneficial than others. Lactobacillus rhamnosus GG was studied in infants 6–24 months for 6 months. The supplement had no effect on development of asthma or eczema. The treatment group had mild effect on decreased sensitisations and less severe asthma, 6 months after discontinuation of the supplementation (113). The answer may be to use pre- or synbiotics (114). Studies are needed.
Micronutritional supplementation with a holoBLG-based food for special medical purposes (FSMP) lozenge alleviates allergic symptoms in BALB/c mice, imitating the protective effect of farm living (vide infra) (115, 116). It appears to act by inserting complexed iron into immune cells. Clinical studies have shown that holo beta-lactoglobulin (holoBLG) can restore micronutritional deficits in atopic immune cells and alleviate allergic symptoms in a completely allergen-nonspecific manner (117). A recent study demonstrates protection against cat challenge in cat allergic human subjects (118). The effects of providing such micronutrition on a large scale to pre-atopic children is unknown, as yet.
Microbiota colonise our skin, gut and respiratory system and play a regulatory role between inflammation and immune tolerance. In children and infants establishing a stable microbiome is essential for immune homeostasis. Children who grew up on a farm have a risk reduction of developing asthma between 32% and 78%. In the GABRIEL and PARSIFAL studies, children who grew up on a farm had an increased exposure to endotoxins and an inverse relationship to asthma and allergic rhinitis (119, 120).
The protective effect in pre-school -aged children in the Genetic and Environmental Causes of Asthma in the European Community (GABRIEL) study found an inverse relationship of allergy to exposure to cows that live in shed and their fodder. The timing of exposure has an enhanced protective effect with pre- natal exposure to farm animals offering protection from developing atopic dermatitis in infancy and from asthma and allergic rhinitis at school age (121, 122).
Consuming dairy products that are not pasteurised had an inverse relationship with developing respiratory allergies, hay fever and asthma. This relationship was found in four large European studies. The increased levels of whey in farm milk might be the explanation as there was an inverse relationship between bovine serum albumin (BSA), lactoglobulin and lactalbumin. Higher levels of polyunsaturated fats and TGF -beta in farm milk were inversely related to asthma as well as positively to increased regulatory T-cell levels in children at age six.
Endotoxin—bearing dust from homes of Amish families, who farm traditionally with animals rather than machinery, administered nasally to mice inhibited eosinophilia and airway hyperreactivity, unlike dust from genetically similar Huttite communities who use modern farming methods (123). The protective effects failed in mice deficient in innate immune signalling molecules MyD88 and Trif.
If there are particular organisms related to this farm protective effect it might be possible to use them, or molecules derived from them, in future to prevent allergic diseases, including asthma.
Respiratory viruses are known to precipitate asthma attacks, but also appear to invoke the development of asthma (81). Although 30%–50% of children will wheeze before 1 year, and 30%–40% will have recurrent wheezing, the prevalence of asthma is 5%–10% in children. Respiratory syncytial virus (RSV) is the main cause of bronchiolitis; Rhinovirus (RV) is usually detected in wheezing children thereafter. Severe respiratory illness with either virus is associated with subsequent asthma. The risk is greatest for young children who wheeze with RV infections. They could be labelled as having pre- asthma. Better knowledge of factors promoting more severe viral illnesses might lead to new prevention strategies, possibly reducing the subsequent risk for asthma.
RSV typically causes bronchiolitis in the 3–6 month age group, by the age of 2 most children have had RSV. Risk factors for severe RSV infection is age below 3 months, chronic lung disease, prematurity and immune deficiency. RSV infection is associated with recurrent wheezing episodes in childhood. Infants who avoided infection with respiratory syncytial virus, or RSV, during the first year of life had a 26% lower risk of developing asthma by age 5 (82).
The anti-RSV monoclonal antibody Palivizumab decreases the risk of severe RSV-induced illness and subsequent recurrent wheeze, but not asthma. In a systematic review live attenuated RSV vaccine in the 6–24 month-old group resulted in a 4-fold increase in anti-F antibodies in 90%–95% of children. Nirsevimab, a long-acting monoclonal antibody that lasts 5 months, reduces hospitalisation and severe disease in healthy premature infants (83). Whether RSV vaccination prevents asthma development is as yet unknown.
Indirect immunological protection is also being explored, reducing the age for the measles vaccine from 6 to 4 months to protect high risk infants (84, 85).
RV-A and RV-C are associated with more viral asthma exacerbations than RV-B. RV-C is associated with more hospitalisations. The effects of viral exacerbations in allergic asthma are dose related. Higher allergen exposure and higher IgE levels lead to more severe viral exacerbations of allergic asthma. Human rhinovirus type C receptor CDHR3 polymorphisms have been shown to affect receptor epithelial expression, activation, and asthma development and exacerbation severity in children (86). Anti-inflammatory treatments have efficacy for RV-induced wheezing (81) which is usually time -limited, not progressing to asthma.
Neonates colonized in the hypopharyngeal region with S. pneumoniae, H. influenzae, or M. catarrhalis, or with a combination of these organisms, are at increased risk for recurrent wheeze and asthma early in life (124).
Acute wheezy episodes in young children were significantly associated with bacterial infections, similar to, but independent of, the association with virus infections (125).
The use of antibiotics in young children does not appear to be protective against asthma, but is rather associated with an increase in wheezing and allergies.
A cross-sectional pre-schooler study in Shanghai, China involved 13,335 questionnaires (response rate: 85.3%). A quarter of the children (N = 3,049; 24.1%) had antibiotic exposure in their first year of life. In multivariate logistic regression analyses this had significant associations with higher odds of wheeze (1.44, 1.30–1.60), asthma (1.38, 1.19–1.61), food allergy (1.29, 1.13–1.46), and allergic rhinitis (1.23, 1.07–1.41). These associations differed in children with different individual characteristics (age, sex, family history of atopy, and district) and environments (breastfeeding, home decoration, pet-keeping, and environmental tobacco smoke) (126). The mechanism may relate to effects on the gut microbiome (127). Data in adults also supports an association between macrolide use and asthma in 20–40 years and 40–60 years age groups. For individuals over 60 years old, quinolones were significantly associated with asthma (128).
The Bacille Calmette-Guérin (BCG) vaccine against tuberculosis also protects children from RSV hospitalisation and mortality as seen in vaccinated vs. non- vaccinated populations (129).
A recent meta-analysis involving twenty studies from 1950 to 2021 with a total of 222,928 participants concludes that M. bovis BCG in early life also protects against asthma development (OR 0.77, 95% CI 0.63–0.93), indicating a protective efficacy of 23% against asthma development among vaccinated children. However, early administration of tuberculosis vaccine did not significantly reduce the risk of developing eczema (OR 0.94, 95% CI 0.76–1.16) and rhinitis (OR 0.99, 95% CI 0.81–1.21). The effect of M. bovis BCG on asthma prevalence was significant especially in developed countries (OR 0.73, 95% CI 0.58–0.92) (130).
A small study tried SLIT as a means for primary prevention of allergy in the first year of life. In a blinded pilot study 111 infants less than 12 months, at high risk of atopy based on heredity (>2 first-degree family members with allergic diseases [asthma, allergic rhinoconjunctivitis, eczema or food allergy] but negative SPT responses to HDM, grass pollen, cat, peanut, milk and egg, were given HDM SLIT for 12 months. There was a 16.0% (range 1.7–30.4%) reduction in developing any sensitisation to common allergens. There was no statistical difference in the other primary outcome of HDM sensitisation, nor in allergic disease development. Follow up and future studies are needed to better evaluate this as an intervention (131).
Early AD is associated with asthma at school age. The broken skin of AD may represent a site for further allergen sensitization and disease progression. However, wheezing occurs in many of these children before or with the onset of AD and a marked loss in lung function is often noted, suggesting a distinct phenotype rather than a progression from AD to asthma (132).
However, prevention of AD is a worthwhile goal and any effect upon later asthma could be noted. Studies using emollients to prevent AD were initially encouraging, with a 50% reduction in atopic dermatitis development (133). However, two large, randomized trials (134, 135) have failed to confirm this observation and routine emollient use is not generally recommended. Avoidance of harsh detergent soaps and allergenic materials on the skin of infants is advised.
A meta-analysis of the database on dupilumab in AD from 12 clinical trials in AD showed a 37% overall reduction of the risk of development of new allergies, this rate was 54% when IgE was included. The best response was reached in patients under 18 years with white ethnicity background, early onset of AD (at age <2 years), severe AD, and asthma at baseline. Further data is needed to decide whether asthma incidence can be reduced by dupilumab use in severe AD (93).
Both allergic and late onset asthma have a high prevalence of upper airway disease. Over three quarters of teenage asthma subjects also suffer from rhinitis (136). A European longitudinal study showed that both AR and NAR carried an increased risk of asthma, with that of AR being over threefold (136).
This has been confirmed in younger children where AR until age 5 years predicted wheezing development between the ages of 5 and 13 years, with an adjusted relative risk of 3.82 (P < .001). This association was not related to sensitization type or severity, nor to AD during the first 2 years of life. Among the 1,314 children studied, 41.5% of all new cases of wheezing occurred among those with preceding AR (137).
This may offer an opportunity for prevention. Although allergic rhinitis requires effective treatment on its own merits, it could be helpful to identify those children most likely to progress to asthma and ensure that they are well—controlled. Those with early onset, severe and persistent allergic rhinitis symptoms may be at a higher risk of developing asthma, especially if other allergic conditions, such as food allergy and atopic dermatitis are present. A Korean study suggests that it is those with a high atopic burden and impaired lung function who are at higher risk of asthma (138, 139). Nasal challenge results in eosinophilic inflammation to the bronchi and vice versa (140). AR causes bronchial hyperreactivity (141), so perhaps reducing allergen exposure and controlling nasal inflammation well could reduce asthma development?
Pharmacotherapy for AR can improve asthma control (142) but there is no good evidence for its preventing asthma development.
Recent cohort data shows that approx. 75% of children with pollen-AR at 4 or 8 years had persistent disease up to 24 years, and 30% developed asthma (143). Further investigation to determine any identifiable pre- asthma characteristics in this cohort would be helpful.
There is considerable retrospective, observational data to suggest that allergen immunotherapy given for allergic rhinitis can reduce the incidence of new-onset asthma, as well as reducing the burden of asthma medication in those with established disease. Studies of prescription databases in France and Germany have demonstrated this for grass and birch pollen immunotherapy (144–146).
A study of a national health insurance database in Germany provided similar results for a broader range of both seasonal and perennial allergens (147), with similar results for both seasonal and perennial allergens. The overall incidence of asthma in these cohorts was in the region of 1%–2% over the course of the timeframe studied, with a relative risk of 0.6–0.7 in individuals receiving immunotherapy compared to those not. Treatment for at least 3 years appeared to give a greater effect (136). Of note, a further study using German national health insurance data found a slight increase in the chance of new onset asthma in the group receiving immunotherapy, despite seeing reductions in asthma medication use and frequency of asthma exacerbations in the immunotherapy group (148).
A number of prospective, randomised controlled trials have also investigated the effect of immunotherapy on asthma prevention. A European Academy of Allergy and Clinical Immunology (EAACI) systemic review and meta-analysis reviewed these and concluded there was evidence for reduction of risk of new onset asthma in the short term (up to 2 years after completion of treatment) (149). This was evident only for both sublingual and subcutaneous immunotherapy, but only in individuals younger than 18 and only with pollen immunotherapies. They were unable to conclude that the effect persisted in the longer term (beyond 2 years). A study has suggested an effect up to 10 years, though with high risk of bias (150–152). The Grazax Asthma Prevention (GAP) study looked at the preventative effect of 3 years of grass pollen sublingual immunotherapy tablets vs. placebo on new incidence of asthma in children aged 5–12 (153). Whilst the primary outcome—symptomatic asthma with demonstrable beta-agonist airflow reversibility—was not significantly different in the active arm, secondary outcomes were significant and included fewer asthma symptoms and less asthma medication use in the active group. The effect was greatest in younger children.
Overall, data for a protective effect on asthma development is most robust for pollen allergens and in children and for 2 years after completion of treatment. The effects may persist for longer, be applicable to perennial allergens, and hold true in adults (154), but high-quality confirmatory data is required as well as identification of those individual most likely to benefit.
Neither prophylactic use of inhaled corticosteroids nor of the antihistamine cetirizine showed any protective effect against asthma development in clinical trials. Non- interventional longitudinal studies do not suggest a role for allergen avoidance in early life (155, 156).
The ten- year Finnish allergy programme was associated with a levelling off of the prevalence of allergic asthma and rhinitis. This suggests that their primary causes are environmental and that both can be prevented by nature- relatedness, active mobility, and sustainable diet (157).
Currently costs of asthma are high to individuals, healthcare systems and to society, often involving lifelong treatment, once initiated. In the United Kingdom (UK), 1.1 million children (1 in 11) are currently receiving treatment for asthma and most adult asthma begins in childhood. The National Health Service (NHS) spends around £1 billion a year treating and caring for people with asthma (158). Reduction in incidence would improve quality of life and would likely be cost effective. Primary prevention measures have been suggested previously. Have we advanced any further than the following recommendations given in 2015?
…“ public health efforts should remain focused on measures with the potential to improve lung and general health, such as: reducing tobacco smoking and environmental tobacco smoke exposure; reducing indoor and outdoor air pollution and occupational exposures; reducing childhood obesity and encouraging a diet high in vegetables and fruit; improving feto-maternal health; encouraging breastfeeding; promoting childhood vaccinations; and reducing social inequalities.”
The answer is yes. The better understanding of the role of ultra processed foods in causing obesity would suggest that avoidance of these should be included, together with increased outdoor time in nature, allowing exposure to archaeo-organisms such as mycobacteria (91). These are sensible at a population level, as are warnings against unnecessary use of antibiotics and acid- suppressants in pregnancy. Reduction of the currently high rate of caesarean sections is another target which could decrease asthma incidence.
Other areas worth investigation relate to micro- organisms. Some observations in a previous EUFOREA paper (159) were that the one allergen still to be avoided was latex in children with spina bifida and that there were anti- wheezing effects of bacterial mucosal vaccines in recurrently wheezing pre-school children (160). Although not all early wheezing is asthma and the children in this study were non- atopic, it does show an effect of bacterial molecules upon innate immunity. Detailed analysis, possible with UK health records, into BCG and asthma occurrence might reveal an optimal timing for this to be given, either to the population or to those most at risk. The long-term results of RSV vaccination once available may show a reduction in asthma incidence. Prevention of rhinovirus infections has long been a goal which still appears unreachable. Farm dust may provide therapeutic molecules.
Supplementation with Beta Holo Lactoglobulin could be a promising intervention but needs more research.
Targeted therapy for those most at risk, i.e., those with pre-asthma, needs to be more specific. For pre-diabetes, levels of hyperglycaemia and HbA1c are used (161), though there is no universally agreed definition. Identification of likely pre-asthmatics could be as suggested earlier via family history, existing allergy to food, atopic dermatitis or upper airway disease, supplemented by tests for early lower airway involvement. No further test has yet been applied, but possible tests could include: lung function testing (including reversibility, impulse oscillometry and bronchial hyperresponsiveness), FENO, eosinophil counts in nasal secretions or sputum. Inclusion of these markers in future clinical trials would be helpful in reaching a definitive pre-asthma diagnosis. In children who already wheeze both FENO and specific IgE measured at 4 years were associated with wheezing and asthma at 8 years. Both tests also remained significant predictors after mutual adjustment and adjustment for clinical history: OR on wheezing at 8 years for FE(NO) (9) log-scale, per IQR) 1.6 (95% CI 1.1–2.2) and for specific IgE 2.8 (95% CI 1.9–4.1) (162). It may be that different definitions are needed for different therapeutic approaches: for example, severe AD carries a 60% risk, AR a threefold one. Severe sufferers with either of these could be regarded as likely to be pre-asthmatic and treated accordingly with careful characterisation, follow up and evaluation for more definitive identification of factors leading to progression. On a population level the new tool of AI could be used to trawl large healthcare databases for markers in subjects who developed asthma.
Allergen immunotherapy (AIT) for AR shows promise in asthma reduction, even in real life studies. Some allergens, such as those from animals and HDM are more highly associated with asthma than are pollens. In the European Community Respiratory Health Survey the overall attributable fraction (AF) of asthma symptoms caused by atopy was 45% with a physician diagnosis of asthma. The AF for atopy was significantly correlated with sensitization to house dust mite (r = 0.64). The percentage of asthma attributable to HDM sensitization was 18.2% in the overall population and 12%–48% in various study centres in France (163). Sublingual is significantly safer and more convenient than subcutaneous immunotherapy (164). The new HDM SLIT tablet is safe and well tolerated in adults (165). A study on safety and effectiveness in AR in children, where it is most likely to decrease subsequent asthma, has just completed (166). Studies are needed to see if its use reduces progression to asthma and, if so, on identifying those likely to respond to such treatment. Recent evidence suggests that AIT may to some extent repair epithelial barrier function (167). Upper airway disease needs to be taken more seriously with accurate diagnosis of AR, since it is likely that, if effectively treated early, some asthma may be prevented. EUFOREA is active in this regard with both paediatric and adult guidelines for AR management (168, 169) and suggestions for increased use of AIT in the recent summit paper (170).
Similarly, the use of dupilumab in atopic eczema needs to be closely monitored for any anti- asthma effect in real life. Employing artificial intelligence to healthcare databases and those from clinical trials might provide useful information using big data.
Identification of subjects most at risk of asthma (pre- asthma) followed by therapeutic intervention is becoming a possibility, but requires further research.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
GKS: Conceptualization, Formal Analysis, Methodology, Supervision, Visualization, Writing – original draft, Writing – review & editing, Resources. MM: Writing – review & editing, Formal Analysis, Investigation, Validation. VB: Conceptualization, Formal Analysis, Supervision, Visualization, Writing – review & editing. GS: Conceptualization, Investigation, Visualization, Writing – review & editing. MB-S: Conceptualization, Formal Analysis, Visualization, Writing – review & editing. DC: Supervision, Visualization, Writing – review & editing. ED: Conceptualization, Visualization, Writing – review & editing. ZD: Supervision, Visualization, Writing – review & editing. CG: Conceptualization, Validation, Visualization, Writing – review & editing. CH: Conceptualization, Resources, Visualization, Writing – review & editing. MJ: Conceptualization, Validation, Visualization, Writing – review & editing. PJ: Conceptualization, Formal Analysis, Visualization, Writing – review & editing. JK: Conceptualization, Resources, Visualization, Writing – review & editing. JM: Conceptualization, Resources, Visualization, Writing – review & editing. DP: Conceptualization, Data curation, Visualization, Writing – review & editing. SQ: Conceptualization, Formal Analysis, Visualization, Writing – review & editing. SR: Conceptualization, Data curation, Visualization, Writing – review & editing. SS: Conceptualization, Resources, Visualization, Writing – review & editing. BS: Conceptualization, Data curation, Visualization, Writing – review & editing. JT: Conceptualization, Data curation, Supervision, Writing – review & editing. UW: Conceptualization, Data curation, Validation, Visualization, Writing – review & editing. PH: Conceptualization, Formal Analysis, Supervision, Visualization, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
GKS: Honoraria for articles, speaker and advisory boards: ALK, Bayer, GlaxoSmithKline, Haleon, Noucor, Sanofi- Regeneron, and Viatris. Chair of BSACI rhinitis guidelines, Scientific Chief Editor, Rhinology Section of Frontiers in Allergy, Board member and AR lead for EUFOREA, and Chair/ member Data Monitoring Committees on SLIT for ALK. MM: Honoraria for speaker and advisory boards: Sanofi, Glenmark, P&G, Immunotek, Cipla, Thermo-Scientific, Lancet laboratories. EUFOREA expert panel member. VB: Is recipient of consultancy/llecture fees or unrestricted research grants from Sanofi/Regeneron, Novartis, GSK, AZ, ALK Abello and BORK-npc. GS: GWS has received speakers fees from ALK-Abello, Meda and Glenpharm, and received honoraria for participation in an advisory group from ALK-Abello. MB-S: Speaker honorarium from GSK Spain, Olympus Europe, Viatris Spain, Sanofi Spain. Editor-in-Chief Eur.Arch.ORL-HNS. ED: Received fee for consultation, speaker activity, advisory board by Sanofi, Regeneron, GSK, Novartis and Astrazeneca. ZD: In the past 3 years, ZD received speaker or consultant honoraria and/or served on advisory boards at: Antabio, Foresee Pharmaceuticals, GlaxoSmithKline, Hippo-Dx, QPS-Netherlands, Sanofi-Genzyme-Regeneron, and Springer Media all outside the submitted work. From 2012 to 2020 she acted as Director Respiratory & Allergy Research at QPS-Netherlands; in 2019-ongoing, QPS-Netherlands received a European grant from ERA4TB and funding Foresee Pharmaceuticals for early respiratory studies. CH: Ad boards - GSK, Sanofi Regeneron, Lilly. MJ: Has received consulting fees (ALK-Abello, Stallergenes-Greer, Takeda, Zentiva); honoraria for lectures, presentations (ALK-Abello, Stallergenes-Greer, Takeda, Zentiva, Mundipharma, AstraZeneca, SOBI, Chiesi, CSL Behring, Novartis, Benela, Pfizer, Viatris); support for attending meetings and/or travel (ALK-Abello, Stallergenes-Greer, Takeda, Novartis, Sanofi Pasteur) and honoraria for participation on Advisory Boards (ALK-Abello, Stallergenes-Greer, Chiesi, Novartis, SOBI, Pfizer, Sanofi Genzyme/Pasteur). JK: Jasper Kappen reports grants and/or personal fees from ALK, Chiesi, GSK, Novartis, AstraZeneca, Sanofi, Boerhinger, Teva, Viatris, Stallergen, Abbot, all outside the submitted work. JM: Joaquim Mullol is or has been member of national and international scientific advisory boards, consulting, received fees for lectures, and grants for research projects or clinical trials from AstraZeneca, Genentech-Roche, GSK, LETI, Lilly, Menarini, MSD, Mitsubishi-Tanabe, NOUCOR/Uriach Group, Novartis, OPTINOSE, Proctor & Gamble, Regeneron Pharmaceuticals Inc., Sanofi-Genzyme, UCB Pharma, and Viatris/MEDA Pharma. DP: David Price has advisory board membership with AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, Viatris, Teva Pharmaceuticals; consultancy agreements with AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, Viatris, Teva Pharmaceuticals; grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd) from AstraZeneca, Chiesi, Viatris, Novartis, Regeneron Pharmaceuticals, Sanofi Genzyme, and UK National Health Service; payment for lectures/speaking engagements from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Viatris, Novartis, Regeneron Pharmaceuticals and Sanofi Genzyme, Teva Pharmaceuticals; payment for travel/accommodation/meeting expenses from AstraZeneca, Boehringer Ingelheim, Novartis, Teva Pharmaceuticals; stock/stock options from AKL Research and Development Ltd which produces phytopharmaceuticals; owns 74% of the social enterprise Optimum Patient Care Ltd (Australia and UK) and 92.61% of Observational and Pragmatic Research Institute Pte Ltd (Singapore); 5% shareholding in Timestamp which develops adherence monitoring technology; is peer reviewer for grant committees of the UK Efficacy and Mechanism Evaluation programme, and Health Technology Assessment; and was an expert witness for GlaxoSmithKline. SQ: Speaking, lecture and consulting fees from Allergy Therapeutics, ALK, AstraZeneca, Chiesi, GSK, Mundipharma, Novartis, Sanofi-Genzyme, and Teva. SR: Has acted as a consultant and/or advisory board member for Sanofi, GSK, and Novartis. The department of Otorhinolaryngology and Head/Neck Surgery of the Amsterdam UMC has received research funding from Sanofi, GSK, and Novartis. SS: Reports consultancies for ALK-Abelló, AstraZeneca, ERT, GSK, Novartis, Sanofi, and Roche Products outside the submitted work, as well as grant of GSK outside the submitted work. BS: Lyra Therapeutics: consultant, Stryker: Consultant, Neurent: consultant, MCSP: consultant and American Rhinologic Society: VP of Development and Strategy. JT: Jacob Thyssen previously as an advisor for AbbVie, Almirall, Arena Pharmaceuticals, Coloplast, OM Pharma, Aslan Pharmaceuticals, Union Therapeutics, Eli Lilly & Co, Pfizer, Regeneron, and Sanofi-Genzyme; a speaker for AbbVie, Almirall, Eli Lilly & Co, Pfizer, Regeneron, and Sanofi Genzyme; and received research grants from Pfizer, Regeneron, and Sanofi Genzyme. He currently holds a shared position between the university and LEO Pharma where he receives a salary and holds stock options. UW: Consulting for VIATRIS, lecture fees from ALK Germany. PH: Is recipient of consultancy/llecture fees or unrestricted research grants from Sanofi/Regeneron, Novartis, GSK, Medtronic and Viatris.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Davie CA. A review of Parkinson’s disease. Br Med Bull. (2008) 86:109–27. doi: 10.1093/bmb/ldn013
2. Aisen PS, Cummings J, Jack CR Jr, Morris JC, Sperling R, Frölich L, et al. On the path to 2025: understanding the Alzheimer’s disease continuum. Alzheimers Res Ther. (2017) 9(1):60. doi: 10.1186/s13195-017-0283-5
3. Gao E, Hercun J, Heller T, Vilarinho S. Undiagnosed liver diseases. Transl Gastroenterol Hepatol. (2021) 6:28. doi: 10.21037/tgh.2020.04.04
4. Ejerblad E, Fored CM, Lindblad P, Fryzek J, McLaughlin JK, Nyrén O. Obesity and risk for chronic renal failure. J Am Soc Nephrol. (2006) 17(6):1695–702. doi: 10.1681/ASN.2005060638
5. DeTure MA, Dickson DW. The neuropathological diagnosis of Alzheimer’s disease. Mol Neurodegener. (2019) 14(1):32. doi: 10.1186/s13024-019-0333-5
6. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. (2002) 346(6):393–403. doi: 10.1056/NEJMoa012512
7. Hamman RF, Wing RR, Edelstein SL, Lachin JM, Bray GA, Delahanty L, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. (2006) 29(9):2102–7. doi: 10.2337/dc06-0560
8. International Diabetes Federation IDF Diabetes Atlas. 8th ed Brussels, Belgium: International Diabetes Federation (2017). Available online at: http://www.diabetesatlas.org
9. Glechner A, Keuchel L, Affengruber L, Titscher V, Sommer I, Matyas N, et al. Effects of lifestyle changes on adults with prediabetes: a systematic review and meta-analysis. Prim Care Diabetes. (2018) 12(5):393–408. doi: 10.1016/j.pcd.2018.07.003
10. Shea D, Colasurdo E, Smith A, Paschall C, Jayadev S, Keene CD, et al. SOBA: development and testing of a soluble oligomer binding assay for detection of amyloidogenic toxic oligomers. Proc Natl Acad Sci U S A. (2022) 119(50):e2213157119. doi: 10.1073/pnas.2213157119
11. Alalayah KM, Senan EM, Atlam HF, Ahmed IA, Shatnawi HSA. Automatic and early detection of Parkinson’s disease by analyzing acoustic signals using classification algorithms based on recursive feature elimination method. Diagnostics (Basel). (2023) 13(11):1924. doi: 10.3390/diagnostics13111924
12. Mortimer K, Reddel HK, Pitrez PM, Bateman ED. Asthma management in low and middle income countries: case for change. Eur Respir J. (2022) 60(3):2103179. doi: 10.1183/13993003.03179-2021
13. Asher MI, Rutter CE, Bissell K, Chiang CY, El Sony A, Ellwood E, et al. Worldwide trends in the burden of asthma symptoms in school-aged children: Global Asthma Network Phase I cross-sectional study. Lancet. (2021) 398(10311):1569–80. doi: 10.1016/S0140-6736(21)01450-1
14. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. (2023). Updated May, 2023. Available online at: www.ginasthma.org
15. Colicino S, Munblit D, Minelli C, Custovic A, Cullinan P. Validation of childhood asthma predictive tools: a systematic review. Clin Exp Allergy. (2019) 49(4):410–18. doi: 10.1111/cea.13336
16. Available online at: https://www.who.int/news-room/fact-sheets/detail/asthma#
17. Tliba O, Panettieri RA Jr. Paucigranulocytic asthma: uncoupling of airway obstruction from inflammation. J Allergy Clin Immunol. (2019) 143(4):1287–94. doi: 10.1016/j.jaci.2018.06.008
18. Arshad SH, Kurukulaaratchy RJ, Fenn M, Matthews S. Early life risk factors for current wheeze, asthma, and bronchial hyperresponsiveness at 10 years of age. Chest. (2005) 127(2):502–8. doi: 10.1378/chest.127.2.502
19. ten Brinke A. Risk factors associated with irreversible airflow limitation in asthma. Curr Opin Allergy Clin Immunol. (2008) 8(1):63–9. doi: 10.1097/ACI.0b013e3282f3b5b5
20. Kurowski M, Seys S, Bonini M, Del Giacco S, Delgado L, Diamant Z, et al. Physical exercise, immune response, and susceptibility to infections-current knowledge and growing research areas. Allergy. (2022) 77(9):2653–64. doi: 10.1111/all.15328
21. Dharmage SC, Perret JL, Custovic A. Epidemiology of asthma in children and adults. Front Pediatr. (2019) 7:246. doi: 10.3389/fped.2019.00246
22. Akdis CA. The epithelial barrier hypothesis proposes a comprehensive understanding of the origins of allergic and other chronic noncommunicable diseases. J Allergy Clin Immunol. (2022) 149(1):41–4. doi: 10.1016/j.jaci.2021.11.010
23. Clatworthy J, Price D, Ryan D, Haughney J, Horne R. The value of self-report assessment of adherence, rhinitis and smoking in relation to asthma control. Prim Care Respir J. (2009) 18(4):300–5. doi: 10.4104/pcrj.2009.00037
24. Chaudhuri R, Livingston E, McMahon AD. Cigarette smoking impairs the therapeutic response to oral corticosteroids in chronic asthma. Am J Respir Crit Care Med. (2003) 168(11):1308–11. doi: 10.1164/rccm.200304-503OC
25. Westergaard CG, Munck C, Helby J, Porsbjerg C, Hansen LH, Backer V. Predictors of neutrophilic airway inflammation in young smokers with asthma. J Asthma. (2014) 51(4):341–7. doi: 10.3109/02770903.2014.880718
26. Harmsen L, Gottlieb V, Makowska Rasmussen L, Backer V. Asthma patients who smoke have signs of chronic airflow limitation before age 45. J Asthma. (2010) 47(4):362–6. doi: 10.3109/02770901003692819
27. Eguiluz-Gracia I, Mathioudakis AG, Bartel S, Vijverberg SJH, Fuertes E, Comberiati P, et al. The need for clean air: The way air pollution and climate change affect allergic rhinitis and asthma. Allergy. (2020) 75(9):2170–84. doi: 10.1111/all.14177
28. Ciaccio CE, Gentile D. Effects of tobacco smoke exposure in childhood on atopic diseases. Curr Allergy Asthma Rep. (2013) 13(6):687–92. doi: 10.1007/s11882-013-0389-1
29. Rasmussen F, Siersted HC, Lambrechtsen J, Hansen HS, Hansen NC. Impact of airway lability, atopy, and tobacco smoking on the development of asthma-like symptoms in asymptomatic teenagers. Chest. (2000) 117(5):1330–5. doi: 10.1378/chest.117.5.1330
30. Bjerg A, Hedman L, Perzanowski M, Lundbäck B, Rönmark E. A strong synergism of low birth weight and prenatal smoking on asthma in schoolchildren. Pediatrics. (2011) 127(4):e905–12. doi: 10.1542/peds.2010-2850
31. Ulrik CS, Lange P. Cigarette smoking and asthma. Monaldi Arch Chest Dis. (2001) 56(4):349–53.11770219
32. Tiotiu A, Ioan I, Wirth N, Romero-Fernandez R, González-Barcala FJ. The Impact of Tobacco Smoking on Adult Asthma Outcomes. Int J Environ Res Public Health. (2021) 18(3):992. doi: 10.3390/ijerph18030992
33. Tomlinson JE, McMahon AD, Chaudhuri R, Thompson JM, Wood SF, Thomson NC. Efficacy of low and high dose inhaled corticosteroid in smokers versus non-smokers with mild asthma. Thorax. (2005) 60(4):282–7. doi: 10.1136/thx.2004.033688
34. Zheng X, Guan W, Zheng J, Ye P, Liu S, Zhou J, et al. Smoking influences response to inhaled corticosteroids in patients with asthma: a meta-analysis. Curr Med Res Opin. (2012) 28(11):1791–8. doi: 10.1185/03007995.2012.722991
35. Toppila-Salmi S, Luukkainen AT, Xu B, Lampi J, Auvinen J, Dhaygude K, et al. Maternal smoking during pregnancy affects adult onset of asthma in offspring: a follow up from birth to age 46 years. Eur Respir J. (2020) 55(6):1901857. doi: 10.1183/13993003.01857-2019
36. Hauck AS, Buchwald I, Watz H, Trinkmann F, Söling C, Rabenstein A, et al. Impact of chewing bags, E-cigarettes, and combustible cigarettes on arterial stiffness and small airway function in healthy students. Toxics. (2023) 11(1):77. doi: 10.3390/toxics11010077
37. Gudnadóttir AÝ, Ólafsdóttir IS, Middelveld R, Ekerljung L, Forsberg B, Franklin K, et al. An investigation on the use of snus and its association with respiratory and sleep-related symptoms: a cross-sectional population study. BMJ Open. (2017) 7(5):e015486. doi: 10.1136/bmjopen-2016-015486
38. Lin W, Brunekreef B, Gehring U. Meta-analysis of the effects of indoor nitrogen dioxide and gas cooking on asthma and wheeze in children. Int J Epidemiol. (2013) 42(6):1724–37. doi: 10.1093/ije/dyt150
39. Asher MI, Montefort S, Björkstén B, Lai CK, Strachan DP, Weiland SK, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC phases one and three repeat multicountry cross-sectional surveys. Lancet. (2006) 368(9537):733–43. doi: 10.1016/S0140-6736(06)69283-0. Erratum in: Lancet. (2007) 370(9593):1128.16935684
40. Abrams EM. Cleaning products and asthma risk: a potentially important public health concern. CMAJ. (2020) 192(7):E164–5. doi: 10.1503/cmaj.200025
41. Folletti I, Siracusa A, Paolocci G. Update on asthma and cleaning agents. Curr Opin Allergy Clin Immunol. (2017) 17(2):90–5. doi: 10.1097/ACI.0000000000000349
42. Kabata H, Artis D. Neuro-immune crosstalk and allergic inflammation. J Clin Invest. (2019) 129(4):1475–82. doi: 10.1172/JCI124609
43. Sivapalan P, Diamant Z, Ulrik CS. Obesity and asthma: current knowledge and future needs. Curr Opin Pulm Med. (2015) 21(1):80–5. doi: 10.1097/MCP.0000000000000119
44. Wood LG, Garg ML, Gibson PG. A high-fat challenge increases airway inflammation and impairs bronchodilator recovery in asthma. J Allergy Clin Immunol. (2011) 127(5):1133–40. doi: 10.1016/j.jaci.2011.01.036
45. Bantulà M, Arismendi E, Tubita V, Roca-Ferrer J, Mullol J, de Hollanda A, et al. Effect of obesity on the expression of genes associated with severe asthma-A pilot study. J Clin Med. (2023) 12(13):4398. doi: 10.3390/jcm12134398
46. Chen E, Miller GE. Stress and inflammation in exacerbations of asthma. Brain Behav Immun. (2007) 21(8):993–9. doi: 10.1016/j.bbi.2007.03.009
47. Sharma V, Ricketts HC, McCombie L, Brosnahan N, Crawford L, Slaughter L, et al. A total diet replacement weight management program for difficult-to-treat asthma associated with obesity: a randomized controlled feasibility trial. Chest. (2023) 163(5):1026–37. doi: 10.1016/j.chest.2023.01.015
48. Johnson O, Gerald LB, Harvey J, Roy G, Hazucha H, Large C, et al. An online weight loss intervention for people with obesity and poorly controlled asthma. J Allergy Clin Immunol Pract. (2022) 10(6):1577–86.e3. doi: 10.1016/j.jaip.2022.02.040
49. Scott HA, Gibson PG, Garg ML, Pretto JJ, Morgan PJ, Callister R, et al. Determinants of weight loss success utilizing a meal replacement plan and/or exercise, in overweight and obese adults with asthma. Respirology. (2015) 20(2):243–50. doi: 10.1111/resp.12423
50. Busse W, Bartels C, Rosenkranz M. Brain-airway interactions in asthma. Adv Exp Med Biol. (2023) 1426:185–214. doi: 10.1007/978-3-031-32259-4_9
51. Mikkelsen H, Landt EM, Benn M, Nordestgaard BG, Dahl M. Causal risk factors for asthma in Mendelian randomization studies: a systematic review and meta-analysis. Clin Transl Allergy. (2022) 12(11):e12207. doi: 10.1002/clt2.12207
52. Ferreira MA, Vonk JM, Baurecht H, Marenholz I, Tian C, Hoffman JD, et al. Shared genetic origin of asthma, hay fever and eczema elucidates allergic disease biology. Nat Genet. (2017) 49(12):1752–57. doi: 10.1038/ng.3985
53. Marenholz I, Esparza-Gordillo J, Rüschendorf F, Bauerfeind A, Strachan DP, Spycher BD, et al. Meta-analysis identifies seven susceptibility loci involved in the atopic march. Nat Commun. (2015) 6:8804. doi: 10.1038/ncomms9804
54. Ravn NH, Halling AS, Berkowitz AG, Rinnov MR, Silverberg JI, Egeberg A, et al. How does parental history of atopic disease predict the risk of atopic dermatitis in a child? A systematic review and meta-analysis. J Allergy Clin Immunol. (2020) 145(4):1182–93. doi: 10.1016/j.jaci.2019.12.899
55. Hernandez-Pacheco N, Kere M, Melén E. Gene-environment interactions in childhood asthma revisited; expanding the interaction concept. Pediatr Allergy Immunol. (2022) 33(5):e13780. doi: 10.1111/pai.13780
56. Ravnborg N, Ambikaibalan D, Agnihotri G, Price S, Rastogi S, Patel KR, et al. Prevalence of asthma in patients with atopic dermatitis: a systematic review and meta-analysis. J Am Acad Dermatol. (2021) 84(2):471–8. doi: 10.1016/j.jaad.2020.02.055
57. Halling AS, Rinnov MR, Ruge IF, Gerner T, Ravn NH, Knudgaard MH, et al. Skin TARC/CCL17 increase precedes the development of childhood atopic dermatitis. J Allergy Clin Immunol. (2023) 151(6):1550–57.e6. doi: 10.1016/j.jaci.2022.11.023
58. Scadding GK. Further marches: allergic and non-allergic. Clin Exp Allergy. (2007) 37(4):485–7. doi: 10.1111/j.1365-2222.2007.02675.x
59. Antó JM, Sunyer J, Basagaña X, Garcia-Esteban R, Cerveri I, de Marco R, et al. Risk factors of new-onset asthma in adults: a population-based international cohort study. Allergy. (2010) 65(8):1021–30. doi: 10.1111/j.1398-9995.2009.02301.x
60. Cullinan P, Muñoz X, Suojalehto H, Agius R, Jindal S, Sigsgaard T, et al. Occupational lung diseases: from old and novel exposures to effective preventive strategies. Lancet Respir Med. (2017) 5(5):445–55. doi: 10.1016/S2213-2600(16)30424-6. Erratum in: Lancet Respir Med. (2017) 5(6):e22.28089118
61. Kousha A, Mahdavi Gorabi A, Forouzesh M, Hosseini M, Alexander M, Imani D, et al. Interleukin 4 gene polymorphism (-589C/T) and the risk of asthma: a meta-analysis and met-regression based on 55 studies. BMC Immunol. (2020) 21(1):55. doi: 10.1186/s12865-020-00384-7
62. Ober C, Tsalenko A, Parry R. Genetics of asthma and allergic disease. In Middleton’s Allergy Principles and Practice. 6th ed. Mosby (2000). pp. 719–27.
63. Zhang JH, Zhang M, Wang YN, Zhang XY. Correlation between IL-4 and IL-13 gene polymorphisms and asthma in Uygur children in Xinjiang. Exp Ther Med. (2019) 17(2):1374–82. doi: 10.3892/etm.2018.7096
64. Liao M, Shi D, Wang Y, Zhang K, Chen X, Gao Y, et al. Genome-wide scan on total serum IgE levels identifies no common variants in a healthy Chinese male population. Immunogenetics. (2013) 65(8):561–8. doi: 10.1007/s00251-013-0706-9
65. Holgate ST. The epidemic of allergy and asthma. Nature. (1999) 402(6760):B2–4. doi: 10.1038/35037000
66. Kabesch M, Hoefler C, Carr D, Leupold W, Weiland SK, von Mutius E. Glutathione S transferase deficiency and passive smoking increase childhood asthma. Thorax. (2004) 59(7):569–73. doi: 10.1136/thx.2003.016667
67. Kormann MS, Depner M, Hartl D, Klopp N, Illig T, Adamski J, et al. Toll-like receptor heterodimer variants protect from childhood asthma. J Allergy Clin Immunol. (2008) 122(1):86–92; 92.e1-8. doi: 10.1016/j.jaci.2008.04.039
68. Bu X, Zhao H, Wang Y, Wu D, Chen Q. ORM1 Gene polymorphisms and childhood asthma susceptibility. Allergy. (2017) 72(6):879–84.
69. Almomani BA, Al-Obeidi A, Al-Sarayreh SA, Al-Qutob M, Shudiefat R. Association between ORM1 gene polymorphism and tobacco smoke exposure in the risk of asthma in Jordanian children. Eur Respir J. (2017) 50(suppl 61), PA3988.
70. Kormann MS, Carr D, Klopp N, Illig T, Leupold W, Fritzsch C, et al. G-Protein-coupled receptor polymorphisms are associated with asthma in a large German population. Am J Respir Crit Care Med. (2005) 171(12):1358–62. doi: 10.1164/rccm.200410-1312OC
71. Gaertner VD, Michel S, Curtin JA, Pulkkinen V, Acevedo N, Söderhäll C, et al. Nocturnal asthma is affected by genetic interactions between RORA and NPSR1. Pediatr Pulmonol. (2019) 54(6):847–57. doi: 10.1002/ppul.24292
72. Moffatt MF, Gut IG, Demenais F, Strachan DP, Bouzigon E, Heath S, et al. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. (2010) 363(13):1211–21. doi: 10.1056/NEJMoa0906312
73. Rice NE, Patel BD, Lang IA, Kumari M, Frayling TM, Murray A, et al. Filaggrin gene mutations are associated with asthma and eczema in later life. J Allergy Clin Immunol. (2008) 122(4):834–6. doi: 10.1016/j.jaci.2008.07.027
74. Reyman M, van Houten MA, van Baarle D, Bosch AATM, Man WH, Chu MLJN, et al. Impact of delivery mode-associated gut microbiota dynamics on health in the first year of life. Nat Commun. (2019) 10(1):4997. doi: 10.1038/s41467-019-13014-7. Erratum in: Nat Commun. (2019) 10(1):5352.31676793
75. Thompson AL. Caesarean delivery, immune function and inflammation in early life among Ecuadorian infants and young children. J Dev Orig Health Dis. (2019) 10(5):555–62. doi: 10.1017/S2040174419000047
76. Rusconi F, Zugna D, Annesi-Maesano I, et al. Mode of delivery and asthma at school age in 9 European birth cohorts. Am J Epidemiol. (2017) 185(6):465–73. doi: 10.1093/aje/kwx021
77. Davidson R, Roberts SE, Wotton CJ, et al. Influence of maternal and perinatal factors on subsequent hospitalisation for asthma in children: evidence from the Oxford record linkage study. BMC Pulm Med. (2010) 10(14). doi: 10.1186/1471-2466-10-14
78. Roduit C, Scholtens S, de Jongste JC, Wijga AH, Gerritsen J, Postma DS, et al. Asthma at 8 years of age in children born by caesarean section. Thorax. (2009) 64(2):107–13. doi: 10.1136/thx.2008.100875
79. McEvoy CT, Le Souef PN, Martinez FD. The role of lung function in determining which children develop asthma. J Allergy Clin Immunol Pract. (2023) 11(3):677–83. doi: 10.1016/j.jaip.2023.01.014
80. Fernandez-Rodriguez B, Gomez AR, Jimenez Moreno BS, de Alba C, Galindo A, Villalain C, et al. Smoking influence on early and late fetal growth. J Perinat Med. (2021) 50(2):200–206. doi: 10.1515/jpm-2021-0226
81. Jartti T, Gern JE. Role of viral infections in the development and exacerbation of asthma in children. J Allergy Clin Immunol. (2017) 140(4):895–906. doi: 10.1016/j.jaci.2017.08.003
82. Rosas-Salazar C, Chirkova T, Gebretsadik T, Chappell JD, Peebles RS Jr, Dupont WD, et al. Respiratory syncytial virus infection during infancy and asthma during childhood in the USA (INSPIRE): a population-based, prospective birth cohort study. Lancet. (2023) 401(10389):1669–80. doi: 10.1016/S0140-6736(23)00811-5
83. Shan J, Britton PN, King CL, Booy R. The immunogenicity and safety of respiratory syncytial virus vaccines in development: a systematic review. Influenza Other Respir Viruses. (2021) 15(4):539–51. doi: 10.1111/irv.12850
84. Do LAH, Toh ZQ, Licciardi PV, Mulholland EK. Can early measles vaccination control both measles and respiratory syncytial virus infections? Lancet Glob Health. (2022) 10(2):e288–92. doi: 10.1016/S2214-109X(21)00464-2
85. Mina MJ. Measles, immune suppression and vaccination: direct and indirect nonspecific vaccine benefits. J Infect. (2017) 74(Suppl 1):S10–7. doi: 10.1016/S0163-4453(17)30185-8
86. Di Cicco M, D'Elios S, Peroni DG, Comberiati P. The role of atopy in asthma development and persistence. Curr Opin Allergy Clin Immunol. (2020) 20(2):131–7. doi: 10.1097/ACI.0000000000000627
87. Zhong C, Guo J, Tan T, Wang H, Lin L, Gao D, et al. Increased food diversity in the first year of life is inversely associated with allergic outcomes in the second year. Pediatr Allergy Immunol. (2022) 33(1):e13707. doi: 10.1111/pai.13707
88. Stampfli M, Frei R, Divaret-Chauveau A, Schmausser-Hechfellner E, Karvonen AM, Pekkanen J, et al. Inverse associations between food diversity in the second year of life and allergic diseases. Ann Allergy Asthma Immunol. (2022) 128(1):39–45. doi: 10.1016/j.anai.2021.10.005
89. Du Toit G, Roberts G, Sayre PH, Bahnson HT, Radulovic S, Santos AF, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. (2015) 372(9):803–13. doi: 10.1056/NEJMoa1414850. Erratum in: N Engl J Med. (2016) 375(4):398.25705822
90. Perkin MR, Logan K, Bahnson HT, Marrs T, Radulovic S, Craven J, et al. Efficacy of the enquiring about tolerance (EAT) study among infants at high risk of developing food allergy. J Allergy Clin Immunol. (2019) 144(6):1606–14.e2. doi: 10.1016/j.jaci.2019.06.045
91. Rook GA, Lowry CA, Raison CL. Microbial ‘old friends’, immunoregulation and stress resilience. Evol Med Public Health. (2013) 2013(1):46–64. doi: 10.1093/emph/eot004
92. Dogaru CM, Nyffenegger D, Pescatore AM, Spycher BD, Kuehni CE. Breastfeeding and childhood asthma: systematic review and meta-analysis. Am J Epidemiol. (2014) 179(10):1153–67. doi: 10.1093/aje/kwu072
93. Geba GP, Li D, Xu M, Mohammadi K, Attre R, Ardeleanu M, et al. Attenuating the atopic march: meta-analysis of the dupilumab atopic dermatitis database for incident allergic events. J Allergy Clin Immunol. (2023) 151(3):756–66. doi: 10.1016/j.jaci.2022.08.026
94. Stikker BS, Hendriks RW, Stadhouders R. Decoding the genetic and epigenetic basis of asthma. Allergy. (2023) 78(4):940–56. doi: 10.1111/all.15666
95. Bieli C, Eder W, Frei R, Braun-Fahrländer C, Klimecki W, Waser M, et al. A polymorphism in CD14 modifies the effect of farm milk consumption on allergic diseases and CD14 gene expression. J Allergy Clin Immunol. (2007) 120(6):1308–15. doi: 10.1016/j.jaci.2007.07.034
96. Kothalawala DM, Kadalayil L, Curtin JA, Murray CS, Simpson A, Custovic A, et al. Integration of genomic risk scores to improve the prediction of childhood asthma diagnosis. J Pers Med. (2022) 12(1):75. doi: 10.3390/jpm12010075
97. Kothalawala DM, Murray CS, Simpson A, Custovic A, Tapper WJ, Arshad SH, et al. Development of childhood asthma prediction models using machine learning approaches. Clin Transl Allergy. (2021) 11(9):e12076. doi: 10.1002/clt2.12076
98. Lee HJ, Lee NR, Jung M, Kim DH, Choi EH. Atopic March from atopic dermatitis to asthma-like lesions in NC/Nga mice is accelerated or aggravated by neutralization of stratum corneum but partially inhibited by acidification. J Invest Dermatol. (2015) 135(12):3025–33. doi: 10.1038/jid.2015.333
99. Forno E, Young OM, Kumar R, Simhan H, Celedón JC. Maternal obesity in pregnancy, gestational weight gain, and risk of childhood asthma. Pediatrics. (2014) 134(2):e535–46. doi: 10.1542/peds.2014-0439
100. Peroni DG, Nuzzi G, Trambusti I, Di Cicco ME, Comberiati P. Microbiome composition and its impact on the development of allergic diseases. Front Immunol. (2020) 11:700. doi: 10.3389/fimmu.2020.00700
101. Gao Y, Stokholm J, O'Hely M, Ponsonby AL, Tang MLK, Ranganathan S, et al. Gut microbiota maturity mediates the protective effect of siblings on food allergy. J Allergy Clin Immunol. (2023) 152(3):667–75. doi: 10.1016/j.jaci.2023.02.034
102. Julia V, Macia L, Dombrowicz D. The impact of diet on asthma and allergic diseases. Nat Rev Immunol. (2015) 15(5):308–22. doi: 10.1038/nri3830
103. Venter C, Agostoni C, Arshad SH, Ben-Abdallah M, Du Toit G, Fleischer DM, et al. Dietary factors during pregnancy and atopic outcomes in childhood: a systematic review from the European academy of allergy and clinical immunology. Pediatr Allergy Immunol. (2020) 31(8):889–912. doi: 10.1111/pai.13303
104. Venter C, Palumbo MP, Glueck DH, Sauder KA, O'Mahony L, Fleischer DM, et al. The maternal diet index in pregnancy is associated with offspring allergic diseases: the healthy start study. Allergy. (2022) 77(1):162–172. doi: 10.1111/all.14949
105. Dehlink E, Yen E, Leichtner AM, Hait EJ, Fiebiger E. First evidence of a possible association between gastric acid suppression during pregnancy and childhood asthma: a population-based register study. Clin Exp Allergy. (2009) 39(2):246–53. doi: 10.1111/j.1365-2222.2008.03125.x
106. Loewen K, Monchka B, Mahmud SM, ‘t Jong G, Azad MB. Prenatal antibiotic exposure and childhood asthma: a population-based study. Eur Respir J. (2018) 52(1):1702070. doi: 10.1183/13993003.02070-2017
107. Momen NC, Liu X. Maternal antibiotic use during pregnancy and asthma in children: population-based cohort study and sibling design. Eur Respir J. (2021) 57(1):2000937. doi: 10.1183/13993003.00937-2020
108. Lodge CJ, Tan DJ, Lau MX, Dai X, Tham R, Lowe AJ, et al. Breastfeeding and asthma and allergies: a systematic review and meta-analysis. Acta Paediatr. (2015) 104(467):38–53. doi: 10.1111/apa.13132
109. Boudry G, Charton E, Le Huerou-Luron I, Ferret-Bernard S, Le Gall S, Even S, et al. The relationship between breast milk components and the infant gut microbiota. Front Nutr. (2021) 8:629740. doi: 10.3389/fnut.2021.629740
110. Trambusti I, Nuzzi G, Costagliola G, Verduci E, D'Auria E, Peroni DG, et al. Dietary interventions and nutritional factors in the prevention of pediatric asthma. Front Pediatr. (2020) 8:480. doi: 10.3389/fped.2020.00480
111. Shimizu M, Kato T, Adachi Y, Wada T, Murakami S, Ito Y, et al. Maternal dietary vitamin D intake during pregnancy is associated with allergic disease symptoms in children at 3 years old: the Japan environment and children’s study. Int Arch Allergy Immunol. (2023) 184(11):1106–15. doi: 10.1159/000531970
112. Talaei M, Sdona E, Calder PC, Jones LR, Emmett PM, Granell R, et al. Intake of n-3 polyunsaturated fatty acids in childhood, FADS genotype and incident asthma. Eur Respir J. (2021) 58(3):2003633. doi: 10.1183/13993003.03633-2020
113. Rose MA, Stieglitz F, Köksal A, Schubert R, Schulze J, Zielen S. Efficacy of probiotic lactobacillus GG on allergic sensitization and asthma in infants at risk. Clin Exp Allergy. (2010) 40(9):1398–405. doi: 10.1111/j.1365-2222.2010.03560.x
114. Wawryk-Gawda E, Markut-Miotła E, Emeryk A. Postnatal probiotics administration does not prevent asthma in children, but using prebiotics or synbiotics may be the effective potential strategies to decrease the frequency of asthma in high-risk children—a meta-analysis of clinical trials. Allergol Immunopathol (Madr). (2021) 49(4):4–14. doi: 10.15586/aei.v49i4.69
115. Afify SM, Regner A, Pacios LF, et al. Micronutritional supplementation with a holoBLG-based FSMP (food for special medical purposes)-lozenge alleviates allergic symptoms in BALB/c mice: imitating the protective farm effect. Clin Exp Allergy. (2022) 52:426–41. doi: 10.1111/cea.14050
116. Afify SM, Pali-Schöll I, Hufnagl K, Hofstetter G, El-Bassuoni MA, Roth-Walter F, et al. Bovine holo-beta-lactoglobulin cross-protects against pollen allergies in an innate manner in BALB/c mice: potential model for the farm effect. Front Immunol. (2021) 12:611474. doi: 10.3389/fimmu.2021.611474
117. Roth-Walter F, Afify SM, Pacios LF, et al. Cow’s milk protein β-lactoglobulin confers resilience against allergy by targeting complexed iron into immune cells. J Allergy Clin Immunol. (2021) 147(1):321–334.e4. doi: 10.1016/j.jaci.2020.05.023
118. Bergmann KC, Raab J, Graessel A, Zwingers T, Becker S, Kugler S, et al. The holo beta-lactoglobulin lozenge reduces symptoms in cat allergy-evaluation in an allergen exposure chamber and by titrated nasal allergen challenge. Clin Transl Allergy. (2023) 13(7):e12274. doi: 10.1002/clt2.12274
119. Illi S, Depner M, Genuneit J, Horak E, Loss G, Strunz-Lehner C, et al. Protection from childhood asthma and allergy in alpine farm environments-the GABRIEL advanced studies. J Allergy Clin Immunol. (2012) 129(6):1470–7.e6. doi: 10.1016/j.jaci.2012.03.013
120. Riedler J, Braun-Fahrländer C, Eder W, Schreuer M, Waser M, Maisch S, et al. Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. Lancet. (2001) 358(9288):1129–33. doi: 10.1016/S0140-6736(01)06252-3
121. Roduit C, Wohlgensinger J, Frei R, Bitter S, Bieli C, Loeliger S, et al. Prenatal animal contact and gene expression of innate immunity receptors at birth are associated with atopic dermatitis. J Allergy Clin Immunol. (2011) 127(1):179–85; 185.e1. doi: 10.1016/j.jaci.2010.10.010
122. Braun-Fahrländer C, Riedler J, Herz U, Eder W, Waser M, Grize L, et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med. (2002) 347(12):869–77. doi: 10.1056/NEJMoa020057
123. Stein MM, Hrusch CL, Gozdz J, Igartua C, Pivniouk V, Murray SE, et al. Innate immunity and asthma risk in amish and hutterite farm children. N Engl J Med. (2016) 375(5):411–21. doi: 10.1056/NEJMoa1508749
124. Bisgaard H, Hermansen MN, Buchvald F, et al. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med. (2007) 357(15):1487–95. doi: 10.1056/NEJMoa052632
125. Bisgaard H, Hermansen MN, Bønnelykke K, Stokholm J, Baty F, Skytt NL, et al. Association of bacteria and viruses with wheezy episodes in young children: prospective birth cohort study. BMJ. (2010) 341:c4978. doi: 10.1136/bmj.c4978
126. Zou Z, Liu W, Huang C, Sun C, Zhang J. First-year antibiotics exposure in relation to childhood asthma, allergies, and airway illnesses. Int J Environ Res Public Health. (2020) 17(16):5700. doi: 10.3390/ijerph17165700
127. Huang YJ, Porsche C, Kozik AJ, Lynch SV. Microbiome-Immune interactions in allergy and asthma. J Allergy Clin Immunol Pract. (2022) 10(9):2244–51. doi: 10.1016/j.jaip.2022.05.038
128. Li S, Chen F, Huang C, Huang G, Cheng Y, Li T, et al. Relationships between antibiotic exposure and asthma in adults in the United States: results of the national health and nutrition examination survey between 1999 and 2018. Front Public Health. (2023) 11:1123555. doi: 10.3389/fpubh.2023.1123555
129. Moulson AJ, Av-Gay Y. BCG Immunomodulation: from the ‘hygiene hypothesis’ to COVID-19. Immunobiology. (2021) 226(1):152052. doi: 10.1016/j.imbio.2020.152052
130. Zhao K, Miles P, Jiang X, Zhou Q, Cao C, Lin W, et al. BCG vaccination in early childhood and risk of atopic disease: a systematic review and meta-analysis. Can Respir J. (2021) 2021:5434315. doi: 10.1155/2021/5434315
131. Zolkipli Z, Roberts G, Cornelius V, Clayton B, Pearson S, Michaelis L, et al. Randomized controlled trial of primary prevention of atopy using house dust mite allergen oral immunotherapy in early childhood. J Allergy Clin Immunol. (2015) 136(6):1541–47.e11. doi: 10.1016/j.jaci.2015.04.045
132. Illi S, von Mutius E, Lau S, Nickel R, Grüber C, Niggemann B, et al. The natural course of atopic dermatitis from birth to age 7 years and the association with asthma. J Allergy Clin Immunol. (2004) 113(5):925–31. doi: 10.1016/j.jaci.2004.01.778
133. Simpson EL, Chalmers JR, Hanifin JM, Thomas KS, Cork MJ, McLean WH, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol. (2014) 134(4):818–23. doi: 10.1016/j.jaci.2014.08.005
134. Chalmers JR, Haines RH, Bradshaw LE, Montgomery AA, Thomas KS, Brown SJ, et al. Daily emollient during infancy for prevention of eczema: the BEEP randomised controlled trial. Lancet. (2020) 395(10228):962–72. doi: 10.1016/S0140-6736(19)32984-8
135. Haspeslagh E, Vanheerswynghels M, Deswarte K, Van Moorleghem J, Jacquet A, Lambrecht BN, et al. Prophylactic allergen immunotherapy with Der p 2 prevents murine asthma by regulating lung GM-CSF. J Allergy Clin Immunol. (2019) 143(6):2307–11.e5. doi: 10.1016/j.jaci.2019.02.007. Erratum in: J Allergy Clin Immunol. (2019) 144(4):1142.30796980
136. Shaaban R, Zureik M, Soussan D, Neukirch C, Heinrich J, Sunyer J, et al. Rhinitis and onset of asthma: a longitudinal population-based study. Lancet. (2008) 372(9643):1049–57. doi: 10.1016/S0140-6736(08)61446-4
137. Rochat MK, Illi S, Ege MJ, Lau S, Keil T, Wahn U, et al. Allergic rhinitis as a predictor for wheezing onset in school-aged children. J Allergy Clin Immunol. (2010) 126(6):1170–5.e2. doi: 10.1016/j.jaci.2010.09.008
138. Lee E, Lee SH, Kwon JW, et al. A rhinitis phenotype associated with increased development of bronchial hyperresponsiveness and asthma in children. Ann Allergy Asthma Immunol. (2016) 117(1):21–28.e1. doi: 10.1016/j.anai.2016.04.016.E
139. Olonisakin TF, Moore JA, Barel S, Uribe B, Parker DM, Bowers EMR, et al. Fractional exhaled nitric oxide as a marker of mucosal inflammation in chronic rhinosinusitis. Am J Rhinol Allergy. (2022) 36(4):465–72. doi: 10.1177/19458924221080260
140. Braunstahl GJ, Overbeek SE, Kleinjan A, Prins JB, Hoogsteden HC, Fokkens WJ. Nasal allergen provocation induces adhesion molecule expression and tissue eosinophilia in upper and lower airways. J Allergy Clin Immunol. (2001) 107(3):469–76. doi: 10.1067/mai.2001.113046
141. Corren J. The connection between allergic rhinitis and bronchial asthma. Curr Opin Pulm Med. (2007) 13(1):13–8. doi: 10.1097/MCP.0b013e328010d0db
142. Crystal-Peters J, Neslusan C, Crown WH, Torres A. Treating allergic rhinitis in patients with comorbid asthma: the risk of asthma-related hospitalizations and emergency department visits. J Allergy Clin Immunol. (2002) 109(1):57–62. doi: 10.1067/mai.2002.120554
143. Lindqvist M, Leth-Møller KB, Linneberg A, Kull I, Bergström A, Georgellis A, et al. Natural course of pollen-induced allergic rhinitis from childhood to adulthood: A 20-year follow up. Allergy. (2023). doi: 10.1111/all.15927
144. Devillier P, Molimard M, Ansolabehere X, Bardoulat I, Coulombel N, Maurel F, et al. Immunotherapy with grass pollen tablets reduces medication dispensing for allergic rhinitis and asthma: a retrospective database study in France. Allergy. (2019) 74(7):1317–26. doi: 10.1111/all.13705
145. Wahn U, Bachert C, Heinrich J, Richter H, Zielen S. Real-world benefits of allergen immunotherapy for birch pollen-associated allergic rhinitis and asthma. Allergy. (2019) 74(3):594–604. doi: 10.1111/all.13598
146. Zielen S, Devillier P, Heinrich J, Richter H, Wahn U. Sublingual immunotherapy provides long-term relief in allergic rhinitis and reduces the risk of asthma: a retrospective, real-world database analysis. Allergy. (2018) 73(1):165–77. doi: 10.1111/all.13213
147. Schmitt J, Schwarz K, Stadler E, Wüstenberg EG. Allergy immunotherapy for allergic rhinitis effectively prevents asthma: results from a large retrospective cohort study. J Allergy Clin Immunol. (2015) 136(6):1511–6. doi: 10.1016/j.jaci.2015.07.038
148. Fritzsching B, Contoli M, Porsbjerg C, Buchs S, Larsen JR, Elliott L, et al. Long-term real-world effectiveness of allergy immunotherapy in patients with allergic rhinitis and asthma: Results from the REACT study, a retrospective cohort study. Lancet Reg Health Eur. (2021) 13:100275. doi: 10.1016/j.lanepe.2021.100275
149. Kristiansen M, Dhami S, Netuveli G, Halken S, Muraro A, Roberts G, et al. Allergen immunotherapy for the prevention of allergy: a systematic review and meta-analysis. Pediatr Allergy Immunol. (2017) 28(1):18–29. doi: 10.1111/pai.12661
150. Möller C, Dreborg S, Ferdousi HA, Halken S, Høst A, Jacobsen L, et al. Pollen immunotherapy reduces the development of asthma in children with seasonal rhinoconjunctivitis (the PAT-study). J Allergy Clin Immunol. (2002) 109(2):251–6. doi: 10.1067/mai.2002.121317
151. Niggemann B, Jacobsen L, Dreborg S, Ferdousi HA, Halken S, Høst A, et al. Five-year follow-up on the PAT study: specific immunotherapy and long-term prevention of asthma in children. Allergy. (2006) 61(7):855–9. doi: 10.1111/j.1398-9995.2006.01068.x
152. Jacobsen L, Niggemann B, Dreborg S, Ferdousi HA, Halken S, Høst A, et al. Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT study. Allergy. (2007) 62(8):943–8. doi: 10.1111/j.1398-9995.2007.01451.x
153. Valovirta E, Petersen TH, Piotrowska T, Laursen MK, Andersen JS, Sørensen HF, et al. Results from the 5-year SQ grass sublingual immunotherapy tablet asthma prevention (GAP) trial in children with grass pollen allergy. J Allergy Clin Immunol. (2018) 141(2):529–38.e13. doi: 10.1016/j.jaci.2017.06.014
154. Diamant Z, van Maaren M, Muraro A, Jesenak M, Striz I. Allergen immunotherapy for allergic asthma: the future seems bright. Respir Med. (2023) 210:107125. doi: 10.1016/j.rmed.2023.107125
155. Martinez FD. New insights into the natural history of asthma: primary prevention on the horizon. J Allergy Clin Immunol. (2011) 128(5):939–45. doi: 10.1016/j.jaci.2011.09.020
156. Van Mason J, Portnoy JM. Immunologic strategies for prevention of asthma. J Allergy Clin Immunol Pract. (2020) 8(3):834–47. doi: 10.1016/j.jaip.2019.11.029
157. Haahtela T, Valovirta E, Saarinen K, Jantunen J, Lindström I, Kauppi P, et al. The finnish allergy program 2008–2018: society-wide proactive program for change of management to mitigate allergy burden. J Allergy Clin Immunol. (2021) 148(2):319–26.e4. doi: 10.1016/j.jaci.2021.03.037
158. Beasley R, Semprini A, Mitchell EA. Risk factors for asthma: is prevention possible? Lancet. (2015) 386(9998):1075–85. doi: 10.1016/S0140-6736(15)00156-7
159. Wahn U, Lau S, Eigenmann P, Melen E, Krauss-Etschmann S, Lex C, et al. Early priming of asthma and respiratory allergies: future aspects of prevention: a statement by the European forum for education and research in allergy and airway disease (EUFOREA) and the EAACI-Clemens von pirquet foundation. Pediatr Allergy Immunol. (2022) 33(4):e13773. doi: 10.1111/pai.13773
160. Nieto A, Mazón A, Nieto M, Calderón R, Calaforra S, Selva B, et al. Bacterial mucosal immunotherapy with MV130 prevents recurrent wheezing in children: a randomized, double-blind, placebo-controlled clinical trial. Am J Respir Crit Care Med. (2021) 204(4):462–72. doi: 10.1164/rccm.202003-0520OC
161. Twohig H, Hodges V, Mitchell C. Pre-diabetes: opportunity or overdiagnosis? Br J Gen Pract. (2018) 68(669):172–3. doi: 10.3399/bjgp18X695369
162. Caudri D, Wijga AH, Hoekstra MO, Kerkhof M, Koppelman GH, Brunekreef B, et al. Prediction of asthma in symptomatic preschool children using exhaled nitric oxide, Rint and specific IgE. Thorax. (2010) 65(9):801–7. doi: 10.1136/thx.2009.126912
163. Sunyer J, Jarvis D, Pekkanen J, Chinn S, Janson C, Leynaert B, et al. Geographic variations in the effect of atopy on asthma in the European community respiratory health study. J Allergy Clin Immunol. (2004) 114(5):1033–9. doi: 10.1016/j.jaci.2004.05.072
164. Durham SR, Penagos M. Sublingual or subcutaneous immunotherapy for allergic rhinitis? J Allergy Clin Immunol. (2016) 137(2):339–349.e10. doi: 10.1016/j.jaci.2015.12.1298
165. Demoly P, Leroyer C, Serrano E, Le Maux A, Magnier G, Chartier A. The SQ HDM SLIT-tablet is safe and well tolerated in patients with house dust mite allergic rhinitis with or without asthma: a “real-life” French study. Clin Transl Allergy. (2022) 12(3):e12129. doi: 10.1002/clt2.12129
166. House Dust Mite Allergy Trial In Children (MATIC) clinicaltrials.gov. Available online at: Available at: https://clinicaltrials.gov › show › NCT04145219.
167. Pfaar O, Fritzsching B, Wolf H, Woehlk C, Wüstenberg E. How does allergen immunotherapy-induced tolerance improve the airway epithelial barrier function: a mechanistical-driven hypothesis. Allergy. (2023) 78(10):2577–80. doi: 10.1111/all.15835
168. Scadding GK, Smith PK, Blaiss M, Roberts G, Hellings PW, Gevaert P, et al. Allergic rhinitis in childhood and the new EUFOREA algorithm. Front Allergy. (2021) 2:706589. doi: 10.3389/falgy.2021.706589
169. Mohebbi A, Dehghani Firouzabadi F, Dehghani Firouzabadi M, Roomiani M. Effect of the tongue-in-groove technique on the smile form. Rhinology. (2020) 58(6):626–28. doi: 10.4193/Rhin20.246
Keywords: pre-asthma, asthma, quality of life, asthma natural history, predisposition, risk factors
Citation: Scadding GK, McDonald M, Backer V, Scadding G, Bernal-Sprekelsen M, Conti DM, De Corso E, Diamant Z, Gray C, Hopkins C, Jesenak M, Johansen P, Kappen J, Mullol J, Price D, Quirce S, Reitsma S, Salmi S, Senior B, Thyssen JP, Wahn U and Hellings PW (2024) Pre-asthma: a useful concept for prevention and disease-modification? A EUFOREA paper. Part 1—allergic asthma. Front. Allergy 4:1291185. doi: 10.3389/falgy.2023.1291185
Received: 11 September 2023; Accepted: 26 December 2023;
Published: 30 January 2024.
Edited by:
Abdelilah Soussi Gounni, University of Manitoba, CanadaReviewed by:
Gavriela Feketea, Karamandaneio Prefecture Children Hospital of Patras, Greece© 2024 Scadding, McDonald, Backer, Scadding, Bernal-Sprekelsen, Conti, De Corso, Diamant, Gray, Hopkins, Jesenak, Johansen, Kappen, Mullol, Price, Quirce, Reitsma, Salmi, Senior, Thyssen, Wahn and Hellings. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: D. M. Conti Y29udGkuZGllZ29tYXJjZWxvQGdtYWlsLmNvbQ==
Abbreviations AD, atopic dermatitis; AIT, allergen immunotherapy; AR, allergic rhinitis; BCG, Bacille Calmette-Guérin; BSA, bovine serum albumin; BTS/SIGN, British Thoracic Society/Scottish Intercollegiate Guidelines Network; CS, caesarean section; DC, dendritic cell; EAACI, European Academy of Allergy and Clinical Immunology; EUFOREA, European Forum for Research and Education in Allergy and Airway Diseases; FADS, fatty acid desaturase; FSMP, food for special medical purposes; GINA, Global Initiative for Asthma; GAP, Grazax Asthma Prevention; HDM SLIT, House Dust Mite Sublingual Immunotherapy; NHS, National Health Service; OCS, oral corticosteroid; QoL, quality of life; RCT, randomized controlled trial; RSV, respiratory syncytial virus; RV, rhinovirus; SCIT, subcutaneous immunotherapy; SLIT, sublingual immunotherapy; TSLP, thymic stromal lymphopoietin; UK, United Kingdom; WHO, World Health Organization.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.