
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
EDITORIAL article
Front. Allergy , 18 July 2023
Sec. Mechanisms in Allergy
Volume 4 - 2023 | https://doi.org/10.3389/falgy.2023.1248954
This article is part of the Research Topic Mast Cells in Allergic Diseases View all 5 articles
Editorial on the Research Topic
Mast cells in allergic diseases
Allergic disease poses a major public health concern that affects 10%–30% of the global population. The etiology of allergic diseases is complex and multifaceted. Although enormous strides have been made in the elucidation and treatment of allergic conditions, many questions remain. Mast cells are sentinel innate immune cells that play a key effector function in the allergic diathesis. Mast cells express the high affinity IgE receptor FcεRI, which upon IgE and antigen cross-linkage, triggers the release of mediators that are directly responsible for allergic symptomatology. As part of the ongoing effort to demystify allergic inflammation, many studies have focused on the mast cell since its discovery in 1879 by Paul Ehrlich, followed by renewed interest after the discovery of IgE in 1967 by Kimishige Ishikaka. This editorial will summarize recent efforts which have been published in the Research Topic Mast Cells in Allergic Diseases in the journal Frontiers in Allergy.
MacDonald et al. demonstrated that sodium butyrate suppresses the cell cycle progression of three malignant human mast cell lines. Butyrate is a short chain fatty acid microbial metabolite that functions as a class I histone deacetylase inhibitor (HDACi). The role of butyrate in promoting gastrointestinal health is well accepted, however its role in allergy remains controversial and unclear. Although multiple murine studies demonstrated that butyrate and butyrate-producing microbes attenuate allergic diseases, human association studies remain conflicting and uncertain, most likely due to the complex function of butyrate in multiple immune cell types (1, 2). Early studies have shown that sodium butyrate enhances mast cell maturation and increases granularity in cultured human and mouse cells, leading to enhanced mast cell degranulation (3–5). Conversely, a recent study demonstrated that butyrate suppressed primary human and murine mast cell IgE-mediated degranulation and IL-6 production (6). MacDonald et al. show that butyrate has an enhancing effect; however, these were “minimal” in the mast cell lines. Furthermore, they demonstrate that butyrate suppresses the expression of Kit (Figure 1) (6). This is potentially important as Kit is the receptor for the mast cell growth factor, SCF, and gain of function mutations in the gene encoding Kit are responsible for most mast cell malignancies. Although the role of sodium butyrate in mast cell mediated diseases remains unclear, these studies provide evidence that it may be an important regulator of mast cells.
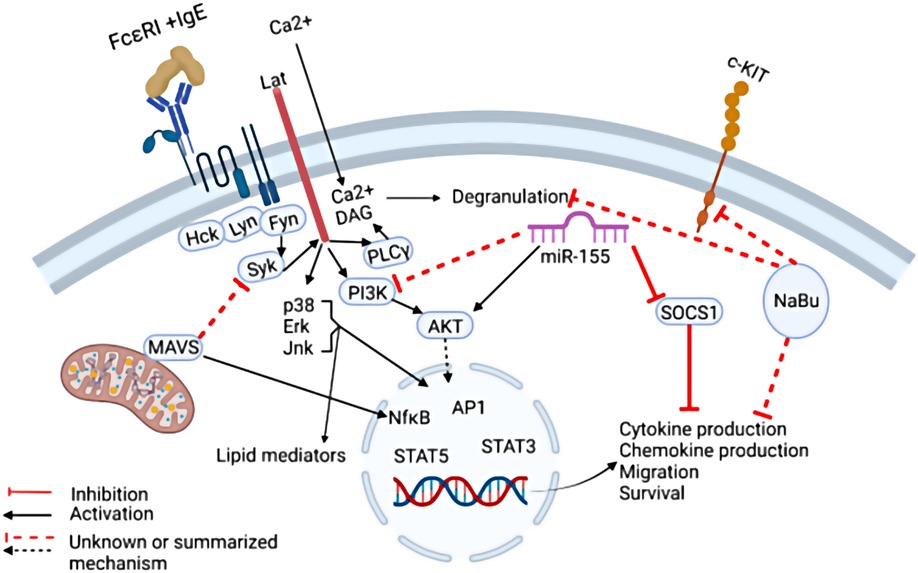
Figure 1. The regulation of IgE-mediated mast cell functions. IgE-mediated cytokine production can be suppressed by Mitochondrial antiviral signaling protein (MAVS) by targeting the signaling molecule SYK. In addition, Sodium Butyrate (NaBu) suppresses mast cell cKIT expression and cytokine production via mechanisms not completely understood. MiR-155 was shown to suppress SOCS1 expression which is a negative regulator of IgE-mediated cytokine production, thereby promoting IgE-mediated cytokine production. In addition, MiR-155 deficiency in mast cells led to increased IgE-mediated cytokine production via enhanced PI3Kγ expression in one study, however in another study, miR-155 deficiency in mast cells led to decreased cytokine production. Taken together, these findings highlight the need for further studies to fully elucidate the complex signals that govern mast cell IgE-mediated functions.
Toshiaki Kawakami et al. summarized the role of IgE in the rhinovirus exacerbation of asthma in a prospective review. It is well known that rhinoviruses are associated with asthma exacerbations, and this was recently reviewed by David Jackson and James Gern (7). Clinically, it is clear that IgE plays a role in this association since anti-IgE therapy was shown to reduce infection-associated asthma exacerbations in a randomized clinical trial (8). Mechanistically, multiple hypotheses are plausible as summarized in these two reviews, however uncertainties remain and a complete understanding of the relationship between viral and allergic inflammation requires further investigation. One step towards understanding the role of mast cells in viral immunity was provided by a brief research report by Yuko Kawakami et al. who demonstrated that the mitochondrial antiviral signaling protein (MAVS) plays a negative role in IgE-mediated cytokine production, possibly by altering SYK activation (Figure 1). The implication of this finding requires further in vivo studies utilizing viral infection and allergy models.
MicroRNAs (miR) are single stranded noncoding RNA molecules that have been shown to profoundly regulate various physiological processes through post-transcriptional gene regulation, including the immune system. In particular, the association between miR-155 and allergic diseases have been examined in multiple studies. Previously, miR-155 was shown to play both a negative and positive role in IgE-mediated mast cell functions by two independent groups. One group demonstrated that in vitro cultured mast cells deficient in miR-155 exhibited stronger IgE-mediated activation via enhanced PI3Kγ activity (9). Another group demonstrated that IL-10 induced miR-155 enhanced IgE-mediated mast cell functions by suppressing a negative regulator, suppressor of cytokine signaling 1 (SOCS1) (10).
Adding to these discoveries, Mohammed et al. demonstrated that IgE and antigen cross-linking induced miR-155 in human and murine mast cells in vitro. Furthermore, using murine bone marrow derived mast cells, the authors showed that miR-155 deficiency resulted in diminished Cyclooxygenase-2 expression as well as cytokine production (Figure 1). The conflicting results may be due to differences in culture conditions, animal sex, or animal facility microbiome. Further studies are needed to dissect the nuance of miR-155 regulation of mast cells and its relationship to allergic diseases.
Taken together, this Research Topic on mast cells in allergic diseases has led to a positive step towards understanding the complex regulation of mast cells via butyrates, anti-viral receptors, and miRs, as summarized in Figure 1.
SK provided proof reading and editing for this manuscript. TTH wrote, edited and performed literature searches for this manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Liu H, Wang J, He T, Becker S, Zhang G, Li D, et al. Butyrate: a double-edged sword for health? Adv Nutr. (2018) 9(21):21–9. doi: 10.1093/advances/nmx009
2. Yip W, Hughes MR, Li Y, Cait A, Hirst M, Mohn WW, et al. Butyrate shapes immune cell fate and function in allergic asthma. Front Immunol. (2021) 15(12):628453. doi: 10.3389/fimmu.2021.628453
3. Rydzynski K, Dalen H. Effect of sodium butyrate treatment on the granule morphology, histamine level and elemental content of the bone marrow-derived mast cell. Folia Histochem Cytobiol. (1994) 32:11–5. PMID: 8026597.8026597
4. DuBuske L, Austen KF, Czop J, Stevens RL. Granule-associated serine neutral proteases of the mouse bone marrow-derived mast cell that degrade fibronectin: their increase after sodium butyrate treatment of the cells. J Immunol. (1984) 133:1535–41. doi: 10.4049/jimmunol.133.3.1535
5. Dvorak AM, Hammel I, Galli SJ. Beige mouse mast cells generated in vitro: ultrastructural analysis of maturation induced by sodium butyrate and of IgE-mediated, antigen-dependent degranulation. Int Arch Allergy Appl Immunol. (1984) 82:261–8. doi: 10.1159/000234202
6. Folkerts J, Redegeld F, Folkerts G, Blokhuis B, van den Berg MPM, de Bruijn MJW, et al. Butyrate inhibits human mast cell activation via epigenetic regulation of FcεRI-mediated signaling. Allergy. (2020) 75(8):1966. doi: 10.1111/all.14254
7. Jackson DJ, Gern JE. Rhinovirus infections and their roles in asthma: etiology and exacerbations. J Allergy Clin Immunol Pract. (2022) 10(3):673–81. doi: 10.1016/j.jaip.2022.01.006
8. Bousquet J, Wenzel S, Holgate S, Lumry W, Freeman P, Fox H. Predicting response to omalizumab, an anti-IgE antibody, in patients with allergic asthma. Chest. (2004) 125(4):1378–86. doi: 10.1378/chest.125.4.1378
9. Biethahn K, Orinska Z, Vigorito E, Goyeneche-Patino DA, Mirghomizadeh F, Föger N, et al. miRNA-155 controls mast cell activation by regulating the PI3Kγ pathway and anaphylaxis in a mouse model. Allergy. (2014) 69(6):752–62. doi: 10.1111/all.12407
Keywords: allergy, mast cell (MC), IgE (Immunoglobulin E), atopic allergic conditions, FcER1
Citation: Kubala S and Haque TT (2023) Editorial: Mast cells in allergic diseases. Front. Allergy 4:1248954. doi: 10.3389/falgy.2023.1248954
Received: 27 June 2023; Accepted: 13 July 2023;
Published: 18 July 2023.
Edited by:
Rosemarie DeKruyff, Stanford University, United States© 2023 Kubala and Haque. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tamara T. Haque aGFxdWV0dEBuaWguZ292
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.