- 1Department of Pediatrics, School of Medicine, University of North Carolina, Chapel Hill, NC, United States
- 2UNC Food Allergy Initiative, School of Medicine, University of North Carolina, Chapel Hill, NC, United States
- 3Orlance, Inc., Seattle, WA, United States
Background: Shellfish and tree nut allergies are among the most prevalent food allergies, now affecting 2%–3% and 1% of the US population, respectively. Currently, there are no approved therapies for shellfish or tree nut allergies, with strict avoidance being the standard of care. However, oral immunotherapy for peanut allergy and subcutaneous immunotherapy for environmental allergens are efficacious and lead to the production of allergen-specific IgG, which causes suppression of allergen effector cell degranulation. Since allergen-specific IgG is a desired response to alleviate IgE-mediated allergies, we tested transcutaneously-delivered DNA vaccines targeting shellfish and tree nut allergens for their ability to induce antigen-specific IgG, which would have therapeutic potential for food allergies.
Methods: We assessed Gene Gun-delivered DNA vaccines targeting either crustacean shellfish or walnut/pecan allergens, with or without IL-12, in naïve mice. Three strains of mice, BALB/cJ, C3H/HeJ and CC027/GeniUnc, were evaluated for IgG production following vaccination. Vaccines were administered twice via Gene Gun, three weeks apart and then blood was collected three weeks following the final vaccination.
Results: Vaccination with shellfish allergen DNA led to increased shrimp-specific IgG in all three strains, with the highest production in C3H/HeJ from the vaccine alone, whereas the vaccine with IL-12 led to the highest IgG production in BALB/cJ and CC027/GeniUnc mice. Similar IgG production was also induced against lobster and crab allergens. For walnut/pecan vaccines, BALB/cJ and C3H/HeJ mice produced significantly higher walnut- and pecan-specific IgG with the vaccine alone compared to the vaccine with IL-12, while the CC027 mice made significantly higher IgG with the addition of IL-12. Notably, intramuscular administration of the vaccines did not lead to increased antigen-specific IgG production, indicating that Gene Gun administration is a superior delivery modality.
Conclusions: Overall, these data demonstrate the utility of DNA vaccines against two lifelong food allergies, shellfish and tree nuts, suggesting their potential as a food allergy therapy in the future.
Introduction
Food allergies now affect 10% of the US population, greatly impacting the quality of life of patients and their caregivers (1–3). Annual costs to the US healthcare system are estimated at $25 billion per year (4). While some food allergies naturally resolve in the first few years of life, shellfish and tree nut allergies are often lifelong (5, 6). Approximately 2%–3% of the US population is allergic to shellfish, with the most prevalent allergies being to crustaceans, such as shrimp, lobster, and crab (1, 7). Importantly, there is a high degree of homology among the allergens across crustaceans. For example, tropomyosin from shrimp is 93% homologous to that of lobster (8). Approximately 1% of the population is allergic to tree nuts, with seed storage proteins being the major allergens (9, 10). Walnut and pecan allergens are highly homologous with one study demonstrating that walnut- and pecan-specific IgE having a correlation of 0.96 (11). Targeting allergens that are highly homologous could have broad applicability for the treatment of multiple food allergies (12–14).
Despite the increasing prevalence of food allergies, the mainstay of therapy is limited to strict dietary avoidance of allergens and access to epinephrine in case of an accidental exposure causing a reaction. In 2020, the FDA approved the first ever desensitization therapy for peanut allergy after a successful Phase 3 trial of peanut oral immunotherapy (OIT) (15). While approval of peanut OIT is a breakthrough for peanut allergy, there are currently no therapies for shellfish, tree nuts, or any other food allergies. Additionally, OIT has limitations including gastrointestinal side effects, required daily dosing, and transient desensitization (16). Immunologically, OIT induces significant increases in allergen-specific IgG and IgG4, which has inhibitory effects on mast cells and basophils in vitro (17). Animal models of food allergy have been used to demonstrate the function of allergen-specific IgG in blocking effector cell degranulation through FcɣRIIb (18). Since IgG is therapeutic in the context of food allergy, we aimed to utilize DNA vaccines to produce allergen-specific IgG against crustacean and walnut/pecan allergens in naïve mice.
Historically, DNA vaccines have been tested by intramuscular (i.m.) delivery of naked plasmid DNA, which is effective in small animals (19), but not as immunogenic in humans. Enhanced delivery modalities have been developed to increase DNA uptake and expression by targeted tissue cells. For example, electroporation uses electrical pulses to enhance uptake and expression over 100-fold (20–23) and has demonstrated efficacy in several clinical studies (24–32). Gene Gun delivery, another enhanced DNA delivery technique, uses a pressurized helium or hydrogen gas to propel dried DNA-coated gold microbeads into the epidermis and upper dermis of the skin (33–39). There are important differences between electroporation and Gene Gun delivery, two of which could be important for DNA-based food allergy therapies. First, Gene Gun delivery efficiently transfects professional antigen presenting cells (APCs) that express class II MHC (40–42) whereas i.m. delivery with or without electroporation does not (43, 44). This could allow Gene Gun delivery to more effectively target allergen-specific CD4+ T cells and antibody producing B cells. Second, whereas i.m. delivery with or without electroporation mainly transfects muscle cells and therefore primarily elicits systemic immune responses, Gene Gun delivery transfects epidermal cells making it a transcutaneous delivery technique that elicits mucosal immune responses in several mucosal compartments including the intestines and lungs (45–47). This could be a therapeutic advantage for Gene Gun delivery since food allergies trigger mucosal anti-allergen immune responses that may be targetable by a transcutaneous therapy but not by systemically delivered therapies like i.m. delivered DNA therapies.
DNA vaccines for peanut and Japanese Red Cedar allergies have been developed and tested in human trials (48). That DNA vaccine platform used lysosomal targeting of plasmid-expressed allergens by fusing the allergens to the lysosomal-associated membrane protein-1 (LAMP-1) that is a resident protein of the lysosome (49, 50). The attachment of the LAMP-1-targeting sequences to proteins in DNA plasmids directs the processing away from the class I MHC pathway towards the class II pathway (51, 52), leading to significantly enhanced immunogenicity of target antigens when delivered by Gene Gun. The LAMP-targeted DNA therapies for peanut and Japanese Red Cedar allergies showed promise in small animal models (49, 50), but were found to be suboptimal in humans (48).
DNA vaccines naturally evoke TH1 biased immune responses (53–55) making them ideal for allergen-specific therapeutic approaches. The TH1-biasing nature of DNA vaccines can also be enhanced by co-expressing TH1 cytokines with the vaccine antigens. IL-12 is a strong TH1-skewing cytokine (56, 57) and has been demonstrated to be a potent DNA vaccine adjuvant in small and large animal models (54, 58–64) and to be safe and effective in humans (25, 28, 65). For these reasons, we hypothesized that DNA-based allergy therapies could be dramatically enhanced by co-delivering them with IL-12 by Gene Gun. Here, we assessed Gene Gun-delivered DNA vaccines targeting either crustacean shellfish or walnut/pecan allergens in naïve BALB/cJ, C3H/HeJ, and CC027/GeniUnc mice for allergen-specific IgG induction.
Materials and methods
Mice
Four-week old female BALB/cJ and C3H/HeJ mice were purchased from Jackson Laboratories (Bar Harbor, ME). Four-to-six week old female CC027/GeniUnc mice were purchased from the UNC Systems Genetics Core Facility (Chapel Hill, NC). Mice were housed in a facility with a 12:12 light:dark cycle and kept on standard chow free of shellfish, tree nut, and peanut allergens. All studies were conducted under UNC IACUC protocol #21–044.
Protein extracts
Lyophilized shrimp, lobster, and crab extracts were purchased from Greer Stallergenes (Lenoir, NC) and resuspended in PBS. Walnut and pecan extracts were prepared from flours (Holmquist Hazelnut Orchards, Lynden, WA) as previously described (66).
DNA vaccines
The plasmid backbone used to construct the allergen-expressing plasmids is a dual promoter plasmid that has been used in several small and large animal DNA vaccine studies (25, 67) and has been evaluated in human clinical trials (25, 67). This plasmid has a human CMV promoter in the sense strand and a macaque CMV promoter in the opposite orientation in the opposing strand. The human and macaque CMV promoters express transgenes at similar levels. These different promoters were chosen to prevent the possibility of recombination events that could occur if two of the same promoters were incorporated into a single plasmid. The amino acid sequences of the walnut, pecan, and crustacean shellfish were obtained from the WHO/IUIS Allergen Nomenclature Database (allergen.org). The listed amino acid sequences for the walnut and pecan allergens were used, but for crustacean allergens, consensus sequences were derived using the consensus tool from the Influenza Research Database using input sequences from shrimp, prawn, lobster, crabs, and crayfish. Endogenous signal peptides were identified using SignalP and were replaced with human CD5 signal peptides to enhance secretion in human cells. The allergen amino acid sequences, including the CD5 signal peptides, were then human DNA codon-optimized by GeneWiz Inc. (South Plainfield, NJ). Those optimized DNA sequences were then synthesized and subcloned into the dual promoter plasmid backbone under the human or macaque CMV promoters as shown in Figures 1A,B by GeneWiz Inc. GeneWiz then verified the proper sequence of the allergens and their proper insertion into the plasmids by Sanger sequencing. Endotoxin-free plasmid maxipreps were made for vaccine use by Puresyn Inc. (Malvern, PA).
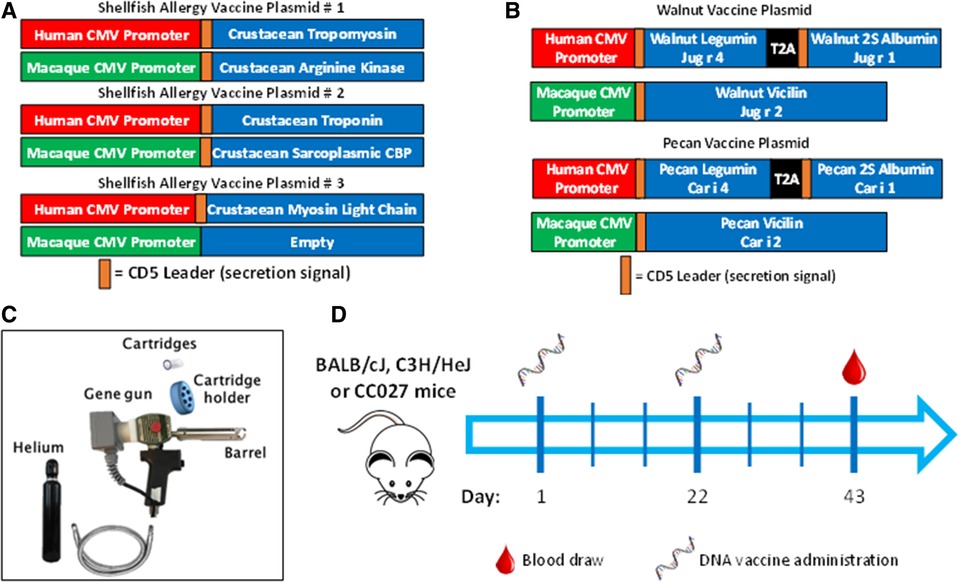
Figure 1. Vaccine formulation, delivery and schedule. DNA plasmid designs for (A) shellfish vaccine and (B) walnut/pecan vaccine. (C) Components of Gene Gun for transcutaneous vaccine administration. (D) Experimental scheme for vaccination.
Protein expression by mass spectrometry (MS)
For protein expression analysis, we used cell supernatants from allergen plasmid-transfected Expi293 cells. Briefly, the complete Expi293 Expression System was purchased from Thermo Fisher Scientific (Waltham, MA). The Expi293 cells were expanded in the supplied serum-free medium and then seeded into 6-well tissue culture plates. Individual wells of cells were then transfected with 2 μg of each plasmid (walnut, pecan, or all three shellfish plasmids combined) using the supplied transfection reagent according to the Manufacturer's instructions. Forty-eight hours after transfection, the supernatants were removed from the transfected Expi293 cells, pooled within transfections and then centrifuged to remove cell debris. The clarified supernatants were then frozen at −20 °C for shipment to MS Bioworks (Ann Arbor, MI) on dry ice for analysis using their Protein-Works Protein Profiling platform.
Vaccination with Gene Gun
Mice were vaccinated in the abdominal skin using a PowderJect XR DNA vaccine delivery system (referred to as the Gene Gun, PowderJect Vaccines, Inc., Madison, WI) as previously described (Figure 1C) (68). Briefly, mice were anesthetized with isoflurane, and abdominal fur was shaved with clippers prior to vaccination. Each DNA vaccination consisted of two tandem deliveries to non-overlapping areas of the abdominal epidermis. Each delivery consisted of 1 mg of 1–3-µm-diameter gold particles and 1–2 µg of total DNA. DNA vaccines were administered at a helium pressure of 400 lb/in2. Mice were administered vaccines on days 1 and 22, then bled via the submandibular vein on day 43 for antibody quantification (Figure 1D).
Vaccination via intramuscular injection
Mice were vaccinated via intramuscular injection with electroporation on days 1, 15, and 29 for a total of three vaccinations and bled on day 43. Mice were injected in the hind quadricep muscle and the inoculations were immediately followed by in vivo electroporation using a BTX 2 needle array and a BTX ECM 830 Electroporation Generator (Holliston, MA) with the following parameters: six 100 V pulses with 50 ms duration and 200 ms between pulses.
Sensitization to shrimp or walnut
Female BALB/cJ, C3H/HeJ, and CC027 mice were sensitized with shrimp or walnut extracts mixed with cholera toxin on days 1, 8, 15, and 22, followed by blood collection on day 36. Sensitizing doses were given by oral gavage with 2 mg food extract plus 10 µg cholera toxin.
Immunoglobulin quantification
Shrimp-, lobster-, crab-, walnut-, and pecan-specific IgE, IgG, IgG1, IgG2a were quantified by ELISA, as previously reported (69). Briefly, plates were coated with 20 µg/ml food extracts (for samples) or 20 µg/ml HSA-DNP (for standard curves). After blocking with 2% BSA in PBS-0.05% Tween, serum samples were diluted 1:100 for IgE, 1:5,000 for IgG, 1:20,000 for IgG1 and 1:1,000 for IgG2a. Standard curves of mouse IgE anti-DNP, IgG1 anti-DNP or IgG2a anti-DNP (Accurate Chemicals, Westbury, NY) were generated ranging from 0.002–2 µg/ml. For IgE plates, the following detection antibodies were used in succession: 0.5 µg/ml sheep IgG anti-mouse IgE (The Binding Site, Birmingham, UK), 0.5 µg/ml biotinylated donkey IgG anti-sheep IgG (Accurate Chemicals), and 0.5 µg/ml NeutrAvidin-HRP (Pierce Biotechnology, Rockford, IL). For IgG, IgG1, and IgG2a plates, HRP goat anti-mouse IgG (Invitrogen, Waltham, MA), anti-mouse IgG1-HRP (Southern Biotech, Birmingham, AL), or anti-mouse IgG2a-HRP (Southern Biotech) were used, respectively. All plates were developed with TMB (SeraCare, Milford, MA), stopped with 1% HCl (SeraCare), and read on a plate spectrophotometer (BioTek, Winooski, VT) at 450 nm. Antigen-specific IgE, IgG1, and IgG2a concentrations were calculated based on the standard curve and dilution factor. Antigen-specific IgG is presented as O.D. values.
Western blots
Shrimp, walnut and pecan extracts were separated on NuPage 4–12% Bis-Tris gels and transferred to nitrocellulose membranes before blocking with 2% BSA in PBS-0.05% Tween for 2 h at room temperature. Blots were incubated with pooled mouse serum (diluted 1:5,000 or 1:500 in 2% BSA PBS-0.05% Tween) overnight at 4°C with agitation. HRP goat anti-mouse IgG (Invitrogen) was diluted 1:5,000 in 2% BSA PBS-0.05% Tween and incubated with blots for 1 h at room temperature with agitation. Blots were developed with SuperSignal West Pico PLUS Chemiluminescent Substrate (Thermo Fisher Scientific), and imaged using an iBright imager (Invitrogen).
Results
Protein expression from DNA plasmids
Allergen protein expression from shellfish, walnut and pecan DNA plasmids were determined by mass spectrometry of secreted proteins from transfected Expi-293 cells. The major shellfish allergens, sarcoplasmic calcium-binding protein, troponin, tropomyosin, arginine kinase, and myosin light chain, encoded in the three DNA plasmids (Figure 1A) were all found to be highly expressed (Table 1). The walnut and pecan 11S legumin seed storage protein allergens Jug r 4 and Car i 4 were also highly expressed from their respective DNA plasmids (Figure 1B). By contrast, the 2S albumin seed storage protein allergens Jug r 1 and Car i 1 and the vicilin seed storage protein allergens Jug r 2 and Car i 2 were expressed at lower levels (Table 1).
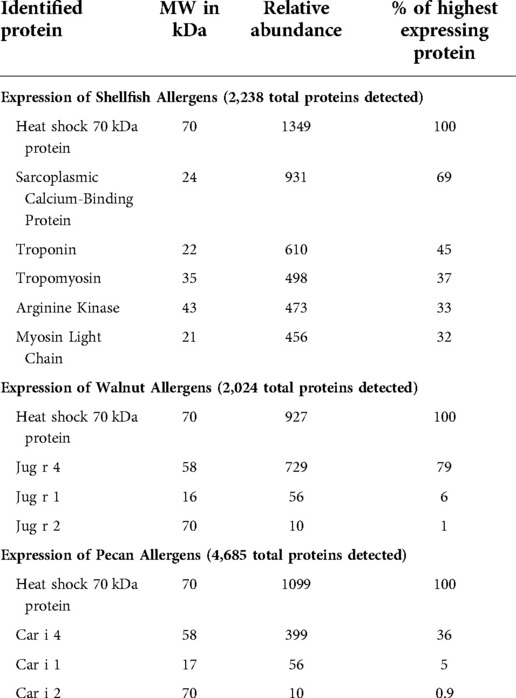
Table 1. Expression of shellfish, walnut, and pecan allergens from DNA plasmids as determined by mass spectrometry.
Shrimp-specific immunoglobulin responses following vaccination
Shellfish DNA vaccines were formulated onto gold microparticles with shellfish DNA plasmids alone or with mouse IL-12. Vaccines were administered transcutaneously via a PowderJect XR-1 Gene Gun (Figure 1C), which uses pressurized helium gas to propel dried DNA-coated gold microparticles into the epidermis. Naïve BALB/cJ, C3H/HeJ, or CC027 mice were vaccinated on days 1 and 22 and bled on day 43 (Figure 1D) to assess in vivo immunoglobulin production. Shrimp-specific IgG and IgG1 were significantly elevated in BALB/cJ and C3H/HeJ mice receiving either vaccine alone or vaccine with IL-12 compared to unvaccinated, naïve mice of the same strain (Figures 2A,B). CC027 mice produced increased quantities of shrimp-specific IgG and IgG1 following administration of vaccine with IL-12 as an adjuvant, but not when receiving the vaccine alone. Shrimp-specific IgG2a was produced in significantly higher quantities in BALB/cJ and C3H/HeJ mice that received the vaccine with IL-12 compared to naïve mice (Figure 2C). CC027 mice followed the same trend for shrimp-specific IgG2a. To determine which shrimp proteins the vaccine-induced IgG recognized, we used Western blotting. Importantly, the major shrimp allergen, tropomyosin (∼38 kD), was recognized by IgG induced in BALB/cJ, C3H/HeJ, and CC027, but not in unvaccinated naïve mouse sera (Figure 2D). In contrast, mice that received the vaccine alone or with IL-12 by intramuscular injection followed by electroporation did not make detectable levels of shrimp-specific IgG (Supplementary Figure S1).
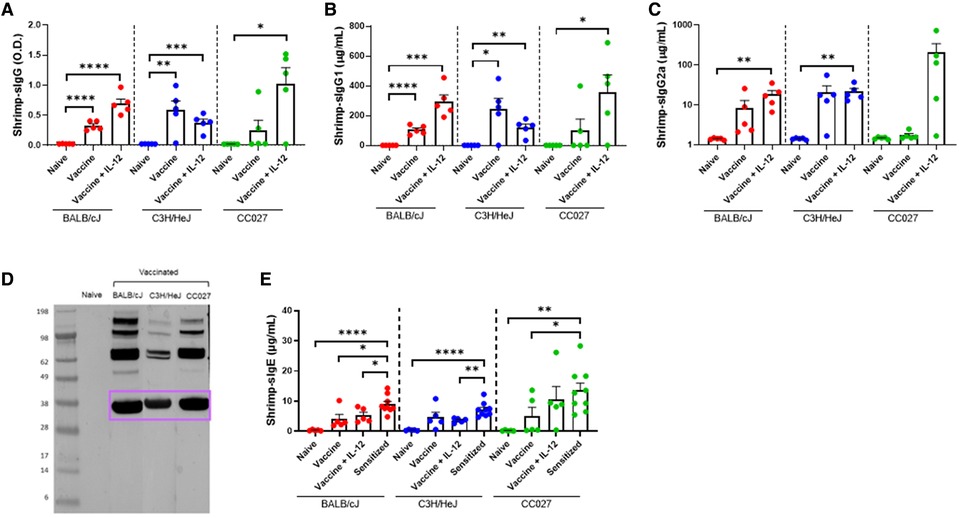
Figure 2. Shrimp-specific immunoglobulin responses following DNA vaccination with Gene Gun. Shrimp-specific (A) IgG, (B) IgG1, and (C) IgG2a in naïve and vaccinated BALB/cJ, C3H/HeJ and CC027 mice. (D) Western blot showing Gene Gun-vaccinated mice make shrimp-specific IgG against the major shellfish allergen tropomyosin (purple box). (E) Shrimp-specific IgE in naïve, vaccinated and sensitized BALB/cJ, C3H/HeJ, and CC027 mice. Statistical comparisons were made using unpaired t tests; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
To ensure that DNA-vaccinated mice produced limited quantities of shrimp-specific IgE, we quantified IgE from Gene Gun-vaccinated mice and compared these data to mice sensitized with shrimp plus cholera toxin. Vaccinated BALB/cJ mice made significantly less shrimp-specific IgE compared to sensitized mice (Figure 2E). On average, the vaccinated C3H/HeJ and CC027 mice produced less shrimp-specific IgE than their sensitized counterparts.
IgG responses to additional crustaceans following shellfish vaccination
Since there is a high degree of homology among crustacean allergens, we quantified IgG responses to lobster and crab from sera of mice vaccinated with the shellfish DNA vaccines (Figures 3A,B). Across all three strains of mice, the amounts of IgG produced were similar for shrimp, lobster, and crab within treatment groups (Supplementary Figures S2A–C). The correlation between crab- and shrimp-specific IgG was exceptionably high with an R2 of 0.98 (Figure 3C). Correlations between lobster- and shrimp-specific IgG and lobster- and crab-specific IgG also have R2 > 0.9, indicating the high degree of cross-reactivity between the IgG produced by the shellfish DNA vaccine (Figures 3D,E).
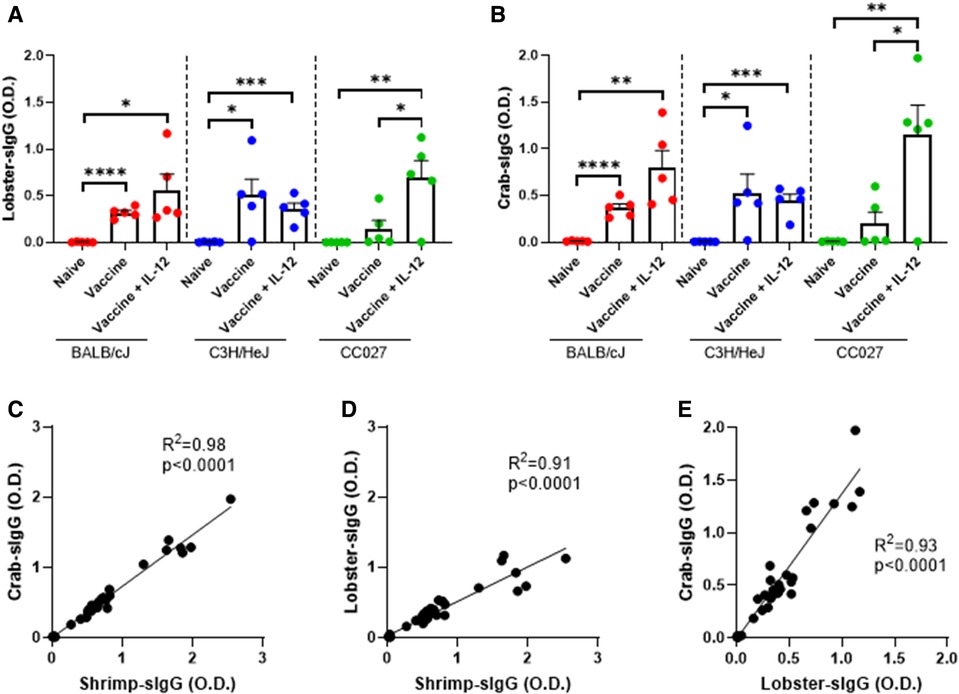
Figure 3. Lobster- and crab-specific IgG responses following DNA vaccination with Gene Gun. (A) Lobster-specific IgG and (B) Crab-specific IgG in naïve and vaccinated BALB/cJ, C3H/HeJ and CC027 mice. Correlations between (C) shrimp- and crab-specific IgG responses, (D) shrimp- and lobster-specific IgG responses, and (E) lobster- and crab-specific IgG responses in BALB/cJ, C3H/HeJ, and CC027 mice. Statistical comparisons were made using unpaired t tests; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Linear regression analyses were performed on the correlation plots.
Walnut-specific immunoglobulin responses following vaccination
To investigate the broad applicability of this DNA vaccination platform, we sought to apply our approach to walnut and pecan allergies, as an example for tree nut allergens. BALB/cJ, C3H/HeJ and CC027 mice were vaccinated via Gene Gun with walnut and pecan DNA plasmids following the same schedule as used for the shellfish vaccines (Figure 1D). Mice that were administered the walnut/pecan vaccine alone produced significantly higher levels of walnut-specific IgG compared to the respective naïve mice in all three strains (Figure 4A). BALB/cJ and CC027 mice that received the vaccine plus IL-12 also produced elevated levels of walnut-specific IgG compared to naïve mice, but this was not true for C3H/HeJ mice. Walnut-specific IgG1 production followed a similar trend, with C3H/HeJ and CC027 mice that received the vaccine alone having elevated levels compared to naïve mice (Figure 4B). CC027 mice that received the vaccine plus IL-12 also had significantly higher levels of walnut-specific IgG1 compared to naïve mice. Walnut-specific IgG2a production was most pronounced in the CC027 mice that received the vaccine plus IL-12, whereas the vaccine groups for the other strains made relatively low quantities of IgG2a (Figure 4C). IgG-binding proteins were identified by Western blot against walnut and pecan. We identified bands at ∼75, ∼33, and ∼12 kD corresponding to the vicilin (Jug r 2 and Car i 2), legumin (Jug r 4 and Car i 4), and 2S albumin (Car i 1) (Figure 4D), respectively.
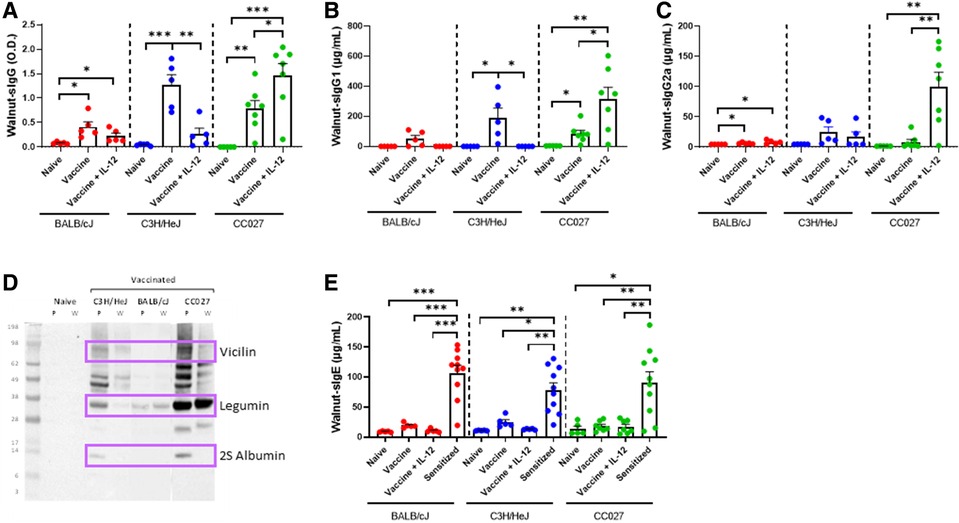
Figure 4. Walnut-specific immunoglobulin responses following DNA vaccination with Gene Gun. Walnut-specific (A) IgG, (B) IgG1, and (C) IgG2a in naïve and vaccinated BALB/cJ, C3H/HeJ and CC027 mice. (D) Western blot showing Gene Gun-vaccinated mice make walnut (W)- and pecan (P)-specific IgG against the major allergens (purple boxes): vicilin (Jug r 2 and Car i 2), legumin (Jug r 4 and Car i 4), and 2S albumin (Car i 1). (E) Walnut-specific IgE in naïve, vaccinated and sensitized BALB/cJ, C3H/HeJ, and CC027 mice. Statistical comparisons were made using unpaired t tests; *p < 0.05, **p < 0.01, ***p < 0.001.
Walnut-specific IgE was quantified in vaccinated mice and compared to mice that were sensitized with walnut plus cholera toxin. In all three strains, the sensitized mice produced significantly higher quantities of walnut-specific IgE compared to both vaccinated groups and the naïve groups (Figure 4E).
Pecan-specific IgG responses following vaccination
Since the walnut/pecan vaccine contained both walnut and pecan DNA plasmids, we next investigated the quantity of pecan-specific IgG produced by vaccinated BALB/cJ, C3H/HeJ and CC027 mice. In all three strains, mice that received the vaccine alone had significantly higher pecan-specific IgG compared to naïve mice (Figure 5A). Including IL-12 in the formulation only elevated IgG production in the CC027 mice compared to the vaccine alone. Overall, pecan-specific IgG production was comparable to walnut-specific IgG across all three strains (Supplementary Figures S3A–C). Indeed, there was high correlation between walnut- and pecan-specific IgG (R2 = 0.75) amongst all mice (Figure 5B).
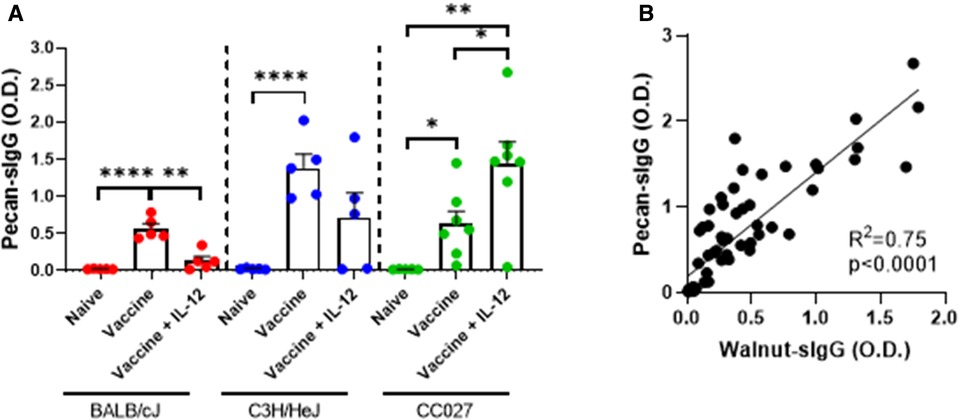
Figure 5. Pecan-specific IgG responses following DNA vaccination with Gene Gun. (A) Pecan-specific IgG quantities in naïve and vaccinated BALB/cJ, C3H/HeJ, and CC027 mice. (B) Correlation between walnut- and pecan-specific IgG responses. Statistical comparisons were made using unpaired t tests; *p < 0.05, **p < 0.01, ****p < 0.0001. Linear regression analysis was performed on the correlation plot.
Discussion
Mechanistic studies from food allergen immunotherapy trials have demonstrated the importance of allergen-specific IgG in positive clinical outcomes (17, 70). IgG plays dual roles by either intercepting allergen before binding to cell surface bound-allergen-specific IgE or binding to inhibitory receptors, including FcɣRIIb, on the surface of effector cells. Studies have demonstrated that post-OIT and -SLIT plasma, containing high quantities of peanut-specific IgG, inhibits peanut IgE-mediated reactions in vitro (18, 71, 72). Therapeutically, IgG directed against defined allergen epitopes may abrogate, or greatly reduce, IgE-mediated reactions. This was recently demonstrated in a small clinical study that tested administration of two monoclonal IgG4 antibodies against distinct epitopes of the major cat allergen, Fel d 1 (73). A single injection of these antibodies reduced symptoms following nasal allergen challenge, demonstrating the utility of IgG directed against allergens as a therapeutic approach.
LAMP-targeted DNA therapies for peanut and Japanese Red Cedar allergies were suboptimal in humans, although the exact reasons are unknown. One potential shortcoming is that the LAMP-targeted DNA platform was based on simple naked DNA inoculation resembling the early naked DNA vaccines that also failed in human trials. Second, the therapy did not use any immunomodulators to tolerize the allergic immune responses or to change the nature of the responses from IgE-dominated TH2 responses to IgG-dominated TH1 responses. Third, i.m. delivery does not generate mucosal immune responses and likely does not have a profound impact on existing mucosal anti-allergen immune responses. The DNA vaccines tested in our work are not naked DNA, rather DNA plasmids coated on gold microparticles. We also utilized IL-12, a TH1 skewing adjuvant, and delivered the vaccines transcutaneously via Gene Gun, which allows for efficient transfection of skin APCs (36, 74–77). Overall, our approach has addressed each of these potential shortcomings of the LAMP-targeted therapy.
We utilized DNA vaccines to induce allergen-specific IgG production by targeting major allergens that have high homology across species. For shellfish, the DNA plasmids encoded consensus sequences of the crustacean allergens: tropomyosin, arginine kinase, troponin, sarcoplasmic calcium-binding protein, and myosin light chain. For the walnut/pecan vaccine, the DNA plasmids encoded specific allergens for walnut and pecan: Jug r 1, Jug r 2, Jug r 4, Car i 1, Car i 2, and Car i 4. There was high expression of Jug r 4 and Car i 4, but lower expression of Jug r 1, Car i 1, Jug r 2 and Car i 2 in Expi293 transfected cell supernatants. Expression of shellfish, walnut, and pecan antigens confirms that the selected DNA plasmids could serve as a potential vaccine, although increasing the production of Jug r 1, Car i 1, Jug r 2 and Car i 2 from the vaccine may be beneficial.
In mice, vaccines were transcutaneously administered by Gene Gun twice, three weeks apart, to prime and then boost the immune response. DNA plasmids were administered alone, or in combination with a mouse IL-12 plasmid to investigate the ability of a TH1-skewing adjuvant to enhance IgG responses. Interestingly, IL-12 did not have a universal effect, but appeared to further increase IgG responses in the CC027 strain, which are deficient in IL-12 production (78). Inclusion of IL-12 in the vaccine likely provided the necessary cytokine required to promote IgG production in CC027 mice, although there was variability in the IgG responses, possibly due to varying endogenous IL-12 production in each mouse. Overall, these vaccines administered via Gene Gun were successful at inducing allergen-specific IgG against the target foods, however, future studies may assess additional adjuvants and delivery regimens to enhance IgG production.
The data presented here are encouraging for the potential therapeutic use of DNA vaccines in food allergy. One major advantage of DNA vaccines is that any antigen with a known DNA sequence can be readily made into a vaccine. This is especially useful for allergens that have high sequence homology, since a vaccine directed against one highly conserved sequence can potentially be protective against allergens from multiple species. Using DNA vaccines applied to the skin may also lead to less severe side effects compared to therapies like OIT that are applied to mucosal surfaces like the gastrointestinal tract. Another advantage is that immunoglobulin responses are observed after only two vaccinations, compared to the daily dosing that is required of oral and sublingual immunotherapy. Potential limitations of DNA vaccines include the induction of IgE and side effects once the allergens are expressed in vivo; however, these are limitations with any allergen-specific immunotherapy. Overall, DNA vaccines are attractive potential therapeutics that warrant further investigation in food allergy.
In conclusion, DNA vaccines targeting shellfish, walnut, and pecan allergens induced antigen-specific IgG in three distinct genetic backgrounds of mice. These vaccines will next be investigated in mice sensitized to shellfish or tree nuts to test their potential therapeutic efficacy. Importantly, transcutaneous administration with Gene Gun was superior to i.m. administration with electroporation, which demonstrates the potential for this new route of administration of allergy therapies in future clinical trials. Successful DNA vaccines with strong safety and efficacy profiles would alter the treatment landscape for food allergy.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was reviewed and approved by UNC IACUC.
Author contributions
JMS and MDK performed mouse experiments. KCB and HF formulated gold microparticle vaccines. JRK performed ELISAs and Western blots. JMS, KCB and MDK wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by NIH NIAID R03AI140161 and R43AI134225, and the American Research Foundation for Nut Allergies. The Systems Genetics Core Facility is supported in part by the P30 CA016086 Cancer Center Core Support Grant to the UNC Lineberger Comprehensive Cancer Center. JMS was supported by a T32 Allergy/Immunology Training Grant (AI007062) through Duke University and University of North Carolina at Chapel Hill.
Conflict of interest
Hannah Frizzell and Kenneth Bagley are employees of Orlance Inc. with stock options. Orlance Inc. is seeking to bring DNA vaccines, including those for food allergies, to market. All other authors declare that research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/falgy.2022.969337/full#supplementary-material.
References
1. Gupta RS, Warren CM, Smith BM, Jiang J, Blumenstock JA, Davis MM, et al. Prevalence and severity of food allergies among US adults. JAMA Netw Open. (2019) 2(1):e185630. doi: 10.1001/jamanetworkopen.2018.5630
2. DunnGalvin A, Dubois AE, Flokstra-de Blok BM, Hourihane JO. The effects of food allergy on quality of life. Chem Immunol Allergy. (2015) 101:235–52. doi: 10.1159/000375106
3. DunnGalvin A, Koman E, Raver E, Frome H, Adams M, Keena A, et al. An examination of the food allergy quality of life questionnaire performance in a countrywide American sample of children: cross-cultural differences in age and impact in the United States and Europe. J Allergy Clin Immunol Pract. (2017) 5(2):363–8 e2. doi: 10.1016/j.jaip.2016.09.049
4. Gupta R, Holdford D, Bilaver L, Dyer A, Holl JL, Meltzer D. The economic impact of childhood food allergy in the United States. JAMA Pediatr. (2013) 167(11):1026–31. doi: 10.1001/jamapediatrics.2013.2376
5. Fleischer DM, Conover-Walker MK, Matsui EC, Wood RA. The natural history of tree nut allergy. J Allergy Clin Immunol. (2005) 116(5):1087–93. doi: 10.1016/j.jaci.2005.09.002
6. Gupta RS, Lau CH, Sita EE, Smith B, Greenhawt MJ. Factors associated with reported food allergy tolerance among US children. Ann Allergy Asthma Immunol. (2013) 111(3):194–8 e4. doi: 10.1016/j.anai.2013.06.026
7. Wang HT, Warren CM, Gupta RS, Davis CM. Prevalence and characteristics of shellfish allergy in the pediatric population of the United States. J Allergy Clin Immunol Pract. (2020) 8(4):1359–70.e2. doi: 10.1016/j.jaip.2019.12.027
8. Faber MA, Pascal M, El Kharbouchi O, Sabato V, Hagendorens MM, Decuyper II, et al. Shellfish allergens: tropomyosin and beyond. Allergy. (2017) 72(6):842–8. doi: 10.1111/all.13115
9. Sicherer SH, Munoz-Furlong A, Godbold JH, Sampson HA. US Prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J Allergy Clin Immunol. (2010) 125(6):1322–6. doi: 10.1016/j.jaci.2010.03.029
10. Smeekens JM, Bagley K, Kulis M. Tree nut allergies: allergen homology, cross-reactivity, and implications for therapy. Clin Exp Allergy. (2018) 48(7):762–72. doi: 10.1111/cea.13163
11. Maloney JM, Rudengren M, Ahlstedt S, Bock SA, Sampson HA. The use of serum-specific IgE measurements for the diagnosis of peanut, tree nut, and seed allergy. J Allergy Clin Immunol. (2008) 122(1):145–51. doi: 10.1016/j.jaci.2008.04.014
12. Kulis M, Li Y, Lane H, Pons L, Burks W. Single-tree nut immunotherapy attenuates allergic reactions in mice with hypersensitivity to multiple tree nuts. J Allergy Clin Immunol. (2011) 127(1):81–8. doi: 10.1016/j.jaci.2010.09.014
13. Elizur A, Appel MY, Nachshon L, Levy MB, Epstein-Rigbi N, Pontoppidan B, et al. Walnut oral immunotherapy for desensitisation of walnut and additional tree nut allergies (Nut CRACKER): a single-centre, prospective cohort study. Lancet Child Adolesc Health. (2019) 3(5):312–21. doi: 10.1016/S2352-4642(19)30029-X
14. Elizur A, Appel MY, Nachshon L, Levy MB, Epstein-Rigbi N, Koren Y, et al. Cashew oral immunotherapy for desensitizing cashew-pistachio allergy (NUT CRACKER study). Allergy. (2022) 77(6):1863–72. doi: 10.1111/all.15212
15. Investigators P, Vickery BP, Vereda A, Casale TB, Beyer K, du Toit G, et al. AR101 oral immunotherapy for peanut allergy. N Engl J Med. (2018) 379(21):1991–2001. doi: 10.1056/NEJMoa1812856
16. Chu DK, Wood RA, French S, Fiocchi A, Jordana M, Waserman S, et al. Oral immunotherapy for peanut allergy (PACE): a systematic review and meta-analysis of efficacy and safety. Lancet. (2019) 393(10187):2222–32. doi: 10.1016/S0140-6736(19)30420-9
17. Smeekens JM, Kulis MD. Evolution of immune responses in food immunotherapy. Immunol Allergy Clin North Am. (2020) 40(1):87–95. doi: 10.1016/j.iac.2019.09.006
18. Burton OT, Logsdon SL, Zhou JS, Medina-Tamayo J, Abdel-Gadir A, Noval Rivas M, et al. Oral immunotherapy induces IgG antibodies that act through FcgammaRIIb to suppress IgE-mediated hypersensitivity. J Allergy Clin Immunol. (2014) 134(6):1310–7 e6. doi: 10.1016/j.jaci.2014.05.042
19. Wang B, Ugen KE, Srikantan V, Agadjanyan MG, Dang K, Refaeli Y, et al. Gene inoculation generates immune responses against human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. (1993) 90(9):4156–60. doi: 10.1073/pnas.90.9.4156
20. Aihara H, Miyazaki J. Gene transfer into muscle by electroporation in vivo. Nat Biotechnol. (1998) 16(9):867–70. doi: 10.1038/nbt0998-867
21. Mathiesen I. Electropermeabilization of skeletal muscle enhances gene transfer in vivo. Gene Ther. (1999) 6(4):508–14. doi: 10.1038/sj.gt.3300847
22. Rizzuto G, Cappelletti M, Maione D, Savino R, Lazzaro D, Costa P, et al. Efficient and regulated erythropoietin production by naked DNA injection and muscle electroporation. Proc Natl Acad Sci U S A. (1999) 96(11):6417–22. doi: 10.1073/pnas.96.11.6417
23. Widera G, Austin M, Rabussay D, Goldbeck C, Barnett SW, Chen M, et al. Increased DNA vaccine delivery and immunogenicity by electroporation in vivo. J Immunol. (2000) 164(9):4635–40. doi: 10.4049/jimmunol.164.9.4635
24. Mpendo J, Mutua G, Nyombayire J, Ingabire R, Nanvubya A, Anzala O, et al. A phase I double blind, placebo-controlled, randomized study of the safety and immunogenicity of electroporated HIV DNA with or without interleukin 12 in prime-boost combinations with an Ad35 HIV vaccine in healthy HIV-seronegative African adults. PLoS One. (2015) 10(8):e0134287. doi: 10.1371/journal.pone.0134287
25. Jacobson JM, Zheng L, Wilson CC, Tebas P, Matining RM, Egan MA, et al. The safety and immunogenicity of an interleukin-12-enhanced multiantigen DNA vaccine delivered by electroporation for the treatment of HIV-1 infection. J Acquir Immune Defic Syndr. (2016) 71(2):163–71. doi: 10.1097/QAI.0000000000000830
26. Li SS, Kochar NK, Elizaga M, Hay CM, Wilson GJ, Cohen KW, et al. DNA priming increases frequency of T-cell responses to a vesicular stomatitis virus HIV vaccine with specific enhancement of CD8(+) T-cell responses by interleukin-12 plasmid DNA. Clin Vaccine Immunol. (2017) 24(11):e00263-17. doi: 10.1128/CVI.00263-17
27. Sneller MC, Justement JS, Gittens KR, Petrone ME, Clarridge KE, Proschan MA, et al. A randomized controlled safety/efficacy trial of therapeutic vaccination in HIV-infected individuals who initiated antiretroviral therapy early in infection. Sci Transl Med. (2017) 9(419):eaan8848. doi: 10.1126/scitranslmed.aan8848
28. Elizaga ML, Li SS, Kochar NK, Wilson GJ, Allen MA, Tieu HVN, et al. Safety and tolerability of HIV-1 multiantigen pDNA vaccine given with IL-12 plasmid DNA via electroporation, boosted with a recombinant vesicular stomatitis virus HIV Gag vaccine in healthy volunteers in a randomized, controlled clinical trial. PLoS One. (2018) 13(9):e0202753. doi: 10.1371/journal.pone.0202753
29. Hu X, Valentin A, Dayton F, Kulkarni V, Alicea C, Rosati M, et al. DNA prime-boost vaccine regimen to increase breadth, magnitude, and cytotoxicity of the cellular immune responses to subdominant Gag epitopes of simian immunodeficiency virus and HIV. J Immunol. (2016) 197(10):3999–4013. doi: 10.4049/jimmunol.1600697
30. Hooper JW, Moon JE, Paolino KM, Newcomer R, McLain DE, Josleyn M, et al. A phase 1 clinical trial of Hantaan virus and Puumala virus M-segment DNA vaccines for haemorrhagic fever with renal syndrome delivered by intramuscular electroporation. Clin Microbiol Infect. (2014) 20(Suppl 5):110–7. doi: 10.1111/1469-0691.12553
31. Hannaman D, Dupuy LC, Ellefsen B, Schmaljohn CS. A phase 1 clinical trial of a DNA vaccine for Venezuelan equine encephalitis delivered by intramuscular or intradermal electroporation. Vaccine. (2016) 34(31):3607–12. doi: 10.1016/j.vaccine.2016.04.077
32. Spearman P, Mulligan M, Anderson EJ, Shane AL, Stephens K, Gibson T, et al. A phase 1, randomized, controlled dose-escalation study of EP-1300 polyepitope DNA vaccine against plasmodium falciparum malaria administered via electroporation. Vaccine. (2016) 34(46):5571–8. doi: 10.1016/j.vaccine.2016.09.041
33. Weiss R, Scheiblhofer S, Freund J, Ferreira F, Livey I, Thalhamer J. Gene gun bombardment with gold particles displays a particular Th2-promoting signal that over-rules the Th1-inducing effect of immunostimulatory CpG motifs in DNA vaccines. Vaccine. (2002) 20(25-26):3148–54. doi: 10.1016/S0264-410X(02)00250-5
34. Fuller DH, Loudon P, Schmaljohn C. Preclinical and clinical progress of particle-mediated DNA vaccines for infectious diseases. Methods. (2006) 40(1):86–97. doi: 10.1016/j.ymeth.2006.05.022
35. Yager EJ, Dean HJ, Fuller DH. Prospects for developing an effective particle-mediated DNA vaccine against influenza. Expert Rev Vaccines. (2009) 8(9):1205–20. doi: 10.1586/erv.09.82
36. Haynes JR, McCabe DE, Swain WF, Widera G, Fuller JT. Particle-mediated nucleic acid immunization. J Biotechnol. (1996) 44(1-3):37–42. doi: 10.1016/0168-1656(96)80298-7
37. Qiu P, Ziegelhoffer P, Sun J, Yang NS. Gene gun delivery of mRNA in situ results in efficient transgene expression and genetic immunization. Gene Ther. (1996) 3(3):262–8.8646558
38. Rakhmilevich AL, Turner J, Ford MJ, McCabe D, Sun WH, Sondel PM, et al. Gene gun-mediated skin transfection with interleukin 12 gene results in regression of established primary and metastatic murine tumors. Proc Natl Acad Sci U S A. (1996) 93(13):6291–6. doi: 10.1073/pnas.93.13.6291
39. Yang NS, Burkholder J, Roberts B, Martinell B, McCabe D. In vivo and in vitro gene transfer to mammalian somatic cells by particle bombardment. Proc Natl Acad Sci U S A. (1990) 87(24):9568–72. doi: 10.1073/pnas.87.24.9568
40. Condon C, Watkins SC, Celluzzi CM, Thompson K, Falo LD Jr. DNA-based immunization by in vivo transfection of dendritic cells. Nat Med. (1996) 2(10):1122–8. doi: 10.1038/nm1096-1122
41. Eisenbraun MD, Fuller DH, Haynes JR. Examination of parameters affecting the elicitation of humoral immune responses by particle bombardment-mediated genetic immunization. DNA Cell Biol. (1993) 12(9):791–7. doi: 10.1089/dna.1993.12.791
42. Falo LD J. Targeting the skin for genetic immunization. Proc Assoc Am Physicians. (1999) 111(3):211–9. doi: 10.1046/j.1525-1381.1999.99227.x
43. Dupuis M, Denis-Mize K, Woo C, Goldbeck C, Selby MJ, Chen M, et al. Distribution of DNA vaccines determines their immunogenicity after intramuscular injection in mice. J Immunol. (2000) 165(5):2850–8. doi: 10.4049/jimmunol.165.5.2850
44. Liu J, Kjeken R, Mathiesen I, Barouch DH. Recruitment of antigen-presenting cells to the site of inoculation and augmentation of human immunodeficiency virus type 1 DNA vaccine immunogenicity by in vivo electroporation. J Virol. (2008) 82(11):5643–9. doi: 10.1128/JVI.02564-07
45. Koday MT, Leonard JA, Munson P, Forero A, Koday M, Bratt DL, et al. Multigenic DNA vaccine induces protective cross-reactive T cell responses against heterologous influenza virus in nonhuman primates. PLoS One. (2017) 12(12):e0189780. doi: 10.1371/journal.pone.0189780
46. Fuller DH, Rajakumar P, Che JW, Narendran A, Nyaundi J, Michael H, et al. Therapeutic DNA vaccine induces broad T cell responses in the gut and sustained protection from viral rebound and AIDS in SIV-infected rhesus macaques. PLoS One. (2012) 7(3):e33715. doi: 10.1371/journal.pone.0033715
47. Fuller DH, Rajakumar PA, Wilson LA, Trichel AM, Fuller JT, Shipley T, et al. Induction of mucosal protection against primary, heterologous simian immunodeficiency virus by a DNA vaccine. J Virol. (2002) 76(7):3309–17. doi: 10.1128/JVI.76.7.3309-3317.2002
48. Su Y, Romeu-Bonilla E, Anagnostou A, Fitz-Patrick D, Hearl W, Heiland T. Safety and long-term immunological effects of CryJ2-LAMP plasmid vaccine in Japanese red cedar atopic subjects: a phase I study. Hum Vaccin Immunother. (2017) 13(12):2804–13. doi: 10.1080/21645515.2017.1329070
49. Li X-M, Song Y, Su Y, Heiland T, Sampson H. Immunization with ARA h1,2,3-lamp-vax peanut vaccine blocked IgE mediated-anaphylaxis in a peanut allergic murine model. J Allergy Clin Immunol. (2015) 135(2):AB167. doi: 10.1016/j.jaci.2014.12.1482
50. Su Y, Connolly M, Marketon A, Heiland T. CryJ-LAMP DNA vaccines for Japanese red cedar allergy induce robust Th1-type immune responses in murine model. J Immunol Res. (2016) 2016:4857869. doi: 10.1155/2016/4857869
51. Wu TC, Guarnieri FG, Staveley-O'Carroll KF, Viscidi RP, Levitsky HI, Hedrick L, et al. Engineering an intracellular pathway for major histocompatibility complex class II presentation of antigens. Proc Natl Acad Sci U S A. (1995) 92(25):11671–5. doi: 10.1073/pnas.92.25.11671
52. Ji H, Wang TL, Chen CH, Pai SI, Hung CF, Lin KY, et al. Targeting human papillomavirus type 16 E7 to the endosomal/lysosomal compartment enhances the antitumor immunity of DNA vaccines against murine human papillomavirus type 16 E7-expressing tumors. Hum Gene Ther. (1999) 10(17):2727–40. doi: 10.1089/10430349950016474
53. Sin JI, Bagarazzi M, Pachuk C, Weiner DB. DNA priming-protein boosting enhances both antigen-specific antibody and Th1-type cellular immune responses in a murine herpes simplex virus-2 gD vaccine model. DNA Cell Biol. (1999) 18(10):771–9. doi: 10.1089/104454999314917
54. Fouts TR, Bagley K, Prado IJ, Bobb KL, Schwartz JA, Xu R, et al. Balance of cellular and humoral immunity determines the level of protection by HIV vaccines in rhesus macaque models of HIV infection. Proc Natl Acad Sci U S A. (2015) 112(9):E992-9. doi: 10.1073/pnas.1423669112
55. Rush CM, Mitchell TJ, Garside P. A detailed characterisation of the distribution and presentation of DNA vaccine encoded antigen. Vaccine. (2010) 28(6):1620–34. doi: 10.1016/j.vaccine.2009.11.014
56. Macatonia SE, Hosken NA, Litton M, Vieira P, Hsieh CS, Culpepper JA, et al. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol. (1995) 154(10):5071–9.7730613
57. Kalinski P, Hilkens CM, Snijders A, Snijdewint FG, Kapsenberg ML. IL-12-deficient dendritic cells, generated in the presence of prostaglandin E2, promote type 2 cytokine production in maturing human naive T helper cells. J Immunol. (1997) 159(1):28–35.9200435
58. Kim JJ, Ayyavoo V, Bagarazzi ML, Chattergoon MA, Dang K, Wang B, et al. In vivo engineering of a cellular immune response by coadministration of IL-12 expression vector with a DNA immunogen. J Immunol. (1997) 158(2):816–26.8992999
59. Sin JI, Kim JJ, Arnold RL, Shroff KE, McCallus D, Pachuk C, et al. IL-12 gene as a DNA vaccine adjuvant in a herpes mouse model: iL-12 enhances Th1-type CD4+ T cell-mediated protective immunity against herpes simplex virus-2 challenge. J Immunol. (1999) 162(5):2912–21.10072541
60. Chattergoon MA, Saulino V, Shames JP, Stein J, Montaner LJ, Weiner DB. Co-immunization with plasmid IL-12 generates a strong T-cell memory response in mice. Vaccine. (2004) 22(13–14):1744–50. doi: 10.1016/j.vaccine.2004.01.036
61. Boyer JD, Robinson TM, Kutzler MA, Parkinson R, Calarota SA, Sidhu MK, et al. SIV DNA vaccine co-administered with IL-12 expression plasmid enhances CD8 SIV cellular immune responses in cynomolgus macaques. J Med Primatol. (2005) 34(5–6):262–70. doi: 10.1111/j.1600-0684.2005.00124.x
62. Chong SY, Egan MA, Kutzler MA, Megati S, Masood A, Roopchard V, et al. Comparative ability of plasmid IL-12 and IL-15 to enhance cellular and humoral immune responses elicited by a SIVgag plasmid DNA vaccine and alter disease progression following SHIV(89.6P) challenge in rhesus macaques. Vaccine. (2007) 25(26):4967–82. doi: 10.1016/j.vaccine.2006.11.070
63. Bagley KC, Schwartz JA, Andersen H, Eldridge JH, Xu R, Ota-Setlik A, et al. An interleukin 12 adjuvanted herpes simplex virus 2 DNA vaccine is more protective than a glycoprotein D subunit vaccine in a high-dose murine challenge model. Viral Immunol. (2017) 30(3):178–95. doi: 10.1089/vim.2016.0136
64. Tunggal HC, Munson PV, O'Connor MA, Hajari N, Dross SE, Bratt D, et al. Effects of therapeutic vaccination on the control of SIV in rhesus macaques with variable responsiveness to antiretroviral drugs. PLoS One. (2021) 16(6):e0253265. doi: 10.1371/journal.pone.0253265
65. Kalams SA, Parker SD, Elizaga M, Metch B, Edupuganti S, Hural J, et al. Safety and comparative immunogenicity of an HIV-1 DNA vaccine in combination with plasmid interleukin 12 and impact of intramuscular electroporation for delivery. J Infect Dis. (2013) 208(5):818–29. doi: 10.1093/infdis/jit236
66. Smeekens JM, Orgel KA, Kesselring J, Bagley K, Kulis MD. Model of walnut allergy in CC027/GeniUnc mice recapitulates key features of human disease. Yale J Biol Med. (2020) 93(5):669–73.33380927
67. Omosa-Manyonyi G, Mpendo J, Ruzagira E, Kilembe W, Chomba E, Roman F, et al. A phase I double blind, placebo-controlled, randomized study of the safety and immunogenicity of an adjuvanted HIV-1 Gag-Pol-Nef fusion protein and adenovirus 35 Gag-RT-Int-Nef vaccine in healthy HIV-uninfected African adults. PLoS One. (2015) 10(5):e0125954. doi: 10.1371/journal.pone.0125954
68. Pertmer TM, Roberts TR, Haynes JR. Influenza virus nucleoprotein-specific immunoglobulin G subclass and cytokine responses elicited by DNA vaccination are dependent on the route of vector DNA delivery. J Virol. (1996) 70(9):6119–25. doi: 10.1128/jvi.70.9.6119-6125.1996
69. Bednar KJ, Hardy L, Smeekens J, Raghuwanshi D, Duan S, Kulis MD, et al. Antigenic liposomes for generation of disease-specific antibodies. J Vis Exp: JoVE. (2018) 140:e58285. doi: 10.3791/58285
70. Hardy LC, Smeekens JM, Kulis MD. Biomarkers in food allergy immunotherapy. Curr Allergy Asthma Rep. (2019) 19(12):61. doi: 10.1007/s11882-019-0894-y
71. Orgel K, Burk C, Smeekens J, Suber J, Hardy L, Guo R, et al. Blocking antibodies induced by peanut oral and sublingual immunotherapy suppress basophil activation and are associated with sustained unresponsiveness. Clin Exp Allergy. (2019) 49(4):461–70. doi: 10.1111/cea.13305
72. Santos AF, James LK, Bahnson HT, Shamji MH, Couto-Francisco NC, Islam S, et al. Igg4 inhibits peanut-induced basophil and mast cell activation in peanut-tolerant children sensitized to peanut major allergens. J Allergy Clin Immunol. (2015) 135(5):1249–56. doi: 10.1016/j.jaci.2015.01.012
73. Shamji MH, Singh I, Layhadi JA, Ito C, Karamani A, Kouser L, et al. Passive prophylactic administration with a single dose of Anti-Fel d 1 monoclonal antibodies REGN1908-1909 in cat allergen-induced allergic rhinitis: a randomized, double-blind, placebo-controlled clinical trial. Am J Respir Crit Care Med. (2021) 204(1):23–33. doi: 10.1164/rccm.202011-4107OC
74. Fynan EF, Webster RG, Fuller DH, Haynes JR, Santoro JC, Robinson HL. DNA Vaccines: protective immunizations by parenteral, mucosal, and gene-gun inoculations. Proc Natl Acad Sci U S A. (1993) 90(24):11478–82. doi: 10.1073/pnas.90.24.11478
75. Pertmer TM, Eisenbraun MD, McCabe D, Prayaga SK, Fuller DH, Haynes JR. Gene gun-based nucleic acid immunization: elicitation of humoral and cytotoxic T lymphocyte responses following epidermal delivery of nanogram quantities of DNA. Vaccine. (1995) 13(15):1427–30. doi: 10.1016/0264-410X(95)00069-D
76. Roy MJ, Wu MS, Barr LJ, Fuller JT, Tussey LG, Speller S, et al. Induction of antigen-specific CD8+ T cells, T helper cells, and protective levels of antibody in humans by particle-mediated administration of a hepatitis B virus DNA vaccine. Vaccine. (2000) 19(7–8):764–78. doi: 10.1016/S0264-410X(00)00302-9
77. Arrington J, Braun RP, Dong L, Fuller DH, Macklin MD, Umlauf SW, et al. Plasmid vectors encoding cholera toxin or the heat-labile enterotoxin from Escherichia coli are strong adjuvants for DNA vaccines. J Virol. (2002) 76(9):4536–46. doi: 10.1128/JVI.76.9.4536-4546.2002
Keywords: food allergy, shrimp allergy, walnut allergy, CC027, C3H/HeJ, BALB/cJ, DNA vaccine, Gene Gun, IgG
Citation: Smeekens JM, Kesselring JR, Frizzell H, Bagley KC and Kulis MD (2022) Induction of food-specific IgG by Gene Gun-delivered DNA vaccines. Front. Allergy 3:969337. doi: 10.3389/falgy.2022.969337
Received: 14 June 2022; Accepted: 30 August 2022;
Published: 19 September 2022.
Edited by:
Richard L. Wasserman, Medical City Children's Hospital, United StatesReviewed by:
Daniel Adelman, University of California, United StatesSayantani B. Sindher, Stanford University, United States
© 2022 Smeekens, Kesselring, Frizzell, Bagley and Kulis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Johanna M. Smeekens c21lZWtlbjNAZW1haWwudW5jLmVkdQ==
Specialty Section: This article was submitted to Food Allergy, a section of the journal Frontiers in Allergy
 Johanna M. Smeekens
Johanna M. Smeekens Janelle R. Kesselring1,2
Janelle R. Kesselring1,2 Kenneth C. Bagley
Kenneth C. Bagley Michael D. Kulis
Michael D. Kulis