
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Allergy , 07 July 2022
Sec. Skin Allergy
Volume 3 - 2022 | https://doi.org/10.3389/falgy.2022.892673
This article is part of the Research Topic The Complexity of Urticaria View all 10 articles
 Emek Kocatürk1*†
Emek Kocatürk1*† Indrashis Podder2†
Indrashis Podder2† Ana C. Zenclussen3†
Ana C. Zenclussen3† Alicja Kasperska Zajac4,5†
Alicja Kasperska Zajac4,5† Daniel Elieh-Ali-Komi6,7†
Daniel Elieh-Ali-Komi6,7† Martin K. Church7†
Martin K. Church7† Marcus Maurer6,7†
Marcus Maurer6,7†Chronic urticaria (CU) is a mast cell-driven chronic inflammatory disease with a female predominance. Since CU affects mostly females in reproductive age, pregnancy is an important aspect to consider in the context of this disease. Sex hormones affect mast cell (MC) biology, and the hormonal changes that come with pregnancy can modulate the course of chronic inflammatory conditions, and they often do. Also, pregnancy-associated changes in the immune system, including local adaptation of innate and adaptive immune responses and skewing of adaptive immunity toward a Th2/Treg profile have been linked to changes in the course of inflammatory diseases. As of now, little is known about the effects of pregnancy on CU and the outcomes of pregnancy in CU patients. Also, there are no real-life studies to show the safety of urticaria medications during pregnancy. The recent PREG-CU study provided the first insights on this and showed that CU improves during pregnancy in half of the patients, whereas it worsens in one-third; and two of five CU patients experience flare-ups of their CU during pregnancy. The international EAACI/GA2LEN/EuroGuiDerm/APAAACI guideline for urticaria recommends adopting the same management strategy in pregnant and lactating CU patients; starting treatment with standard doses of second-generation (non-sedative) H1 antihistamines, to increase the dose up to 4-folds in case of no response, and to add omalizumab in antihistamine-refractory patients; but also emphasizes the lack of evidence-based information on the safety and efficacy of urticaria treatments during pregnancy. The PREG-CU study assessed treatments and their outcomes during pregnancy. Here, we review the reported effects of sex hormones and pregnancy-specific immunological changes on urticaria, we discuss the impact of pregnancy on urticaria, and we provide information and guidance on the management of urticaria during pregnancy and lactation.
Chronic urticaria (CU) is a chronic inflammatory disorder, which presents with the sudden and unpredictable appearance of wheals, angioedema, or both for longer than 6 weeks (1). CU is a female dominant disease with a higher diagnosed incidence (0.18 vs. 0.11%) and prevalence (0.62 vs. 0.37%) of females vs. males (2). Recently a meta-analysis showed that chronic spontaneous urticaria (CSU) has a point prevalence of 1.3 and 0.8% in women vs. men and chronic inducible urticaria (CIndU) shows a female: male ratio of 2:1 to 3:1 (3). From the results of the recent AWARE study, which focused on worldwide management patterns of antihistamine-refractory CU, it is also clear that rates of female CU are higher than those of males, i.e., 72% for CSU and 69.8% for CIndU (4). CU is not only more common in females but also more severe, with higher rates of high disease activity, angioedema, poor prognosis, refractoriness to treatment, and longer disease course (5–9). The lack of female predominance in children younger than 15 years (3) suggests a disease-modifying role for female hormones in CU. Female sex hormones can influence inflammatory diseases including autoimmune conditions in many different aspects. They are considered risk factors of disease onset and are held to contribute to the activity and progression of autoimmune diseases (10, 11).
From the clinical experience, change in hormone levels, for example across menstrual cycle or during pregnancy, with the onset of menopause, or as a result of using hormonal contraceptives or hormone replacement therapy, some changes in disease activity might be observed. Further, because CU affects mostly women in reproductive age, it is important to understand the consequences of hormonal changes within the menstrual cycle, because of hormonal contraception and during pregnancy for CU disease course and severity. Robust data on this is scarce, but a recent multicenter study revealed that CU tends to improve during pregnancy in half of the patients and worsen in one third of them (Figure 1). Worsening of urticaria was associated with having a mild disease before pregnancy and not being on treatment before pregnancy (12). These findings stress the importance of proper clinical and laboratory diagnosis as well as treatment of CU for patients willing to get pregnant and also a personalized follow-up during pregnancy. Therefore, optimal management of urticaria during pregnancy is vital to ensure the best outcome for the mother and the baby, however, medications' potential risks must be balanced against the consequences of untreated disease.
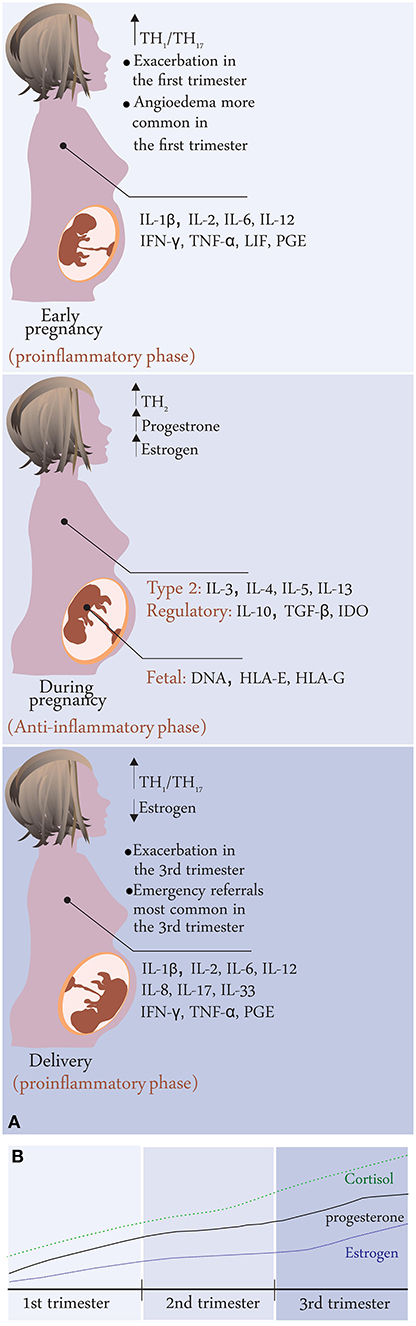
Figure 1. (A) Disease activity changes during pregnancy in CU patients; summary of results from the PREG-CU study with possible mechanisms related to immunological changes in pregnancy. (B) Change in hormone levels during pregnancy.
In this review, we are going to focus on the effect of sex hormones on urticaria, disease activity changes during pregnancy, and management of urticaria during pregnancy and lactation.
A number of important hormonal changes that modulate the immunological milieu take place during pregnancy. Some of these changes may influence CU during this period as highlighted below.
In addition to the emergence of the pregnancy-specific hormone human chorionic gonadotrophin (hCG), several other hormones are upregulated during pregnancy such as progesterone (P4), estrogens, cortisol, prolactin, leptin, vitamin D, and alpha-fetoprotein (AFP).
HCG is a placental glycoprotein hormone, that appears first during pregnancy and serves as pregnancy confirmation, peaks during the 9th−12th week of pregnancy, followed by a gradual decline until delivery, even though hCG levels during pregnancy remain high. Its major function is to maintain P4 synthesis by the corpus luteum (13). HCG promotes maternal immune tolerance and helps to ensure fetal survival (14). The hormone is able to convert naïve T cells into regulatory T cells (Treg) (15); can modulate dendritic cells (DCs) into tolerogenic antigen-presenting cells (APCs) (16, 17) and stimulate IL-10 production by B cells (18). HCG application was shown to in vivo boost the number of Treg cells and prevent abortion in mice (14) and is also injected intrauterine in IVF protocols for women with a history or implantation failure (19).
Progesterone (P4), is a member of the steroid hormone family and plays a crucial role in maintaining pregnancy, in addition to HCG (14). During the initial stages, it is secreted by the corpus luteum and, later on, by the placenta. P4 modulates the immune system via intracellular P4 receptors (PR) expressed by epithelial cells, eosinophils, macrophages, lymphocytes MCs, and DCs (20), and by the upregulation of progesterone-induced blocking factor (PIBF) and glycodelin A (a cell-surface glycoprotein expressed in endometrium/decidua, amniotic fluid, and maternal serum, with immunosuppressive properties) (14). P4 contributes to gestational tolerance by suppressing innate immunity via different mechanisms such as blocking the cytolytic action of NK cells, inducing tolerogenic DCs, and promoting Th2 polarization by preferential apoptosis of Th1 subset and increasing Th2 cytokine production (20, 21). Furthermore, P4 stimulates Treg cells by inducing Fork Head Box Protein 3 (FoxP3) expression in naïve T-cells at the feto-maternal interface, in murine pregnancies (21). P4 plays a crucial role in maintaining gestational tolerance by suppressing innate immunity and promoting Th2 polarization (20). The maternal P4 level continues to rise until about 10–12 weeks of gestation (corpus luteum), then returns to its baseline level and again starts to rise around the 32nd week of pregnancy (2nd peak-secreted by the placenta) to be maintained until conception (22). P4 levels drop during lactation (23). Occasionally, CSU-like cutaneous eruption has been reported due to excess serum P4, called progesterone hypersensitivity. The reasons for increased serum P4 include pregnancy or exposure to exogenous progesterone or increased level during the menstrual cycle (luteal phase; 3–10 days before the onset of menstruation) (24).
Estrogens, also belonging to the steroid hormone family, are of three major types, estrone (E1), estradiol (E2), and estriol (E3). Among them, E2 constitutes the major fraction in reproductive females (both pregnant and non-pregnant) and accounts for most of the classic estrogenic-induced effects. In contrast, E3 is exclusively secreted in pregnant females, by the fetoplacental unit, and comprises almost 90% of the pregnancy estrogen (20). During pregnancy, estrogen levels rise steadily until delivery due to placental secretion. Estrogens act via estrogen receptors (ERs) expressed by B and T lymphocytes, macrophages, and DCs and affect both innate and acquired immunity (14). It is generally accepted that estrogens play a role in the development of adaptive immunity such that low levels of estrogen promote pathogenic Th1/Th17 pathway while high levels (as during pregnancy) promote Th2/Treg responses. E3 downregulates innate immunity by programming DCs to become tolerogenic and anti-inflammatory (23). Many researchers have depicted the harmful role of exogenous estrogen mimickers, called endocrine-disrupting chemicals (EDCs), which act by disrupting the endocrine milieu. These substances are present in several daily use products such as plastic water bottles or food containers, which may be systemically absorbed by ingestion. Recently, the negative impact of EDCs, particularly Bisphenol A and phthalates, is being recognized on human pregnancy and fetal development by interfering with the developing embryonic epigenome (24). Rarely, premenstrual urticarial eruption has been reported in women with estrogen hypersensitivity. In such cases, removal of the exogenous estrogen results in remission e.g., discontinuation of estrogen-containing oral contraceptives or use of estrogen antagonists (leuprolide or tamoxifen) (25).
Cortisol, synthesized in the adrenal cortex and released into circulation after various physical and psychological stimuli; has strong anti-inflammatory effects. During pregnancy, maternal cortisol levels rise continuously to facilitate fetal development, followed by an abrupt drop post-partum (26). Cortisol exerts its anti-inflammatory effect by multiple pathways such as reducing the circulating levels of pro-inflammatory cytokines such as IL-2, IL-3, IL-6, IFN-γ, and TNF-α, activating tolerogenic Treg cells by enhancing the expression of high-affinity IL-2 receptor (CD25), inducing the apoptosis of T cells, and reducing the number of B cells in spleen and lymph nodes, thereby reducing IgG production (23). These effects may explain, in part, the improvement of some immunological disorders during pregnancy.
Prolactin, a polypeptide hormone, is largely produced by the lactotrophic cells of the pituitary gland and several extra-pituitary sources like mammary epithelium, ovaries, and placenta, under the influence of dopamine. Prolactin levels increase slightly during gestation with exponential rise during delivery and lactation, contrasting the abrupt reduction of sex hormones (E2, E3, and P4) post-delivery (27). Prolactin stimulates the immune system and causes aberrant activation by aiding the maturation of naïve Th0 cells to effector CD4 and CD8 cells, impairing the clonal deletion of auto-reactive B cells, and reducing the threshold for activating anergic B cells. Thus, hyperprolactinemia has been associated with several autoimmune disorders and might explain disease flare or relapse during breastfeeding (28). The effects of prolactin on CU during pregnancy remain largely unexplored, but Sabry et al. (29) reported significantly higher serum prolactin levels in a subset of CU patients (positive autologous serum skin test, ASST) and its association with disease severity. In contrast, Soliman et al. (30) did not find any relationship between serum prolactin levels and urticaria activity.
Leptin, secreted by adipocytes, primarily regulates energy metabolism, but its impact on the immune system is increasingly being recognized. Leptin promotes inflammatory responses by activating the JAK-STAT, PI3K, and MAPK pathways as its receptor mimics the IL-6 receptor (31). A recent review has highlighted the cross-talk between mast cells and adipocytes in certain situations like obesity, where adipose tissue-resident MCs release pro-inflammatory cytokines like TNF-α, under the influence of leptin, and worsen the inflammatory state (32). During pregnancy, leptin levels rise to counter the hypermetabolic state and modulate the feto-maternal immune system. The recent findings that the placenta is a relevant source of leptin and its trophoblastic effects further strengthen this view (33). Several authors have reported higher serum levels of leptin in patients with CU (34, 35), but, as of yet, pregnant CU patients have not been studied.
Vitamin D, a steroid hormone, plays an important role in modulating the immune system during pregnancy. The placenta is one of the major sites of extra-renal vitamin D synthesis and produces considerable amounts during pregnancy. Vitamin D promotes antibacterial innate immune responses and suppresses inflammatory adaptive immunity via negative effects on NK cells, T, and B cells (36). The effect on T-cells include a shift from Th1 to Th2 phenotype, in vitro suppression of Th17 axis and IL-17 secretion, and inducing the conversion of naïve T-cells into tolerogenic Treg cells, while antibody production by B-cells is suppressed by inhibiting the differentiation of plasma cells and memory cells (23). Furthermore, placental vitamin D contributes to the development of localized fetal-maternal immune tolerance (37). Vitamin D deficiency may negatively affect several immune-mediated disorders, such as psoriasis, type 1 diabetes, multiple sclerosis, rheumatoid arthritis, tuberculosis, sepsis, and systemic lupus erythematosus (23, 38). A recent systematic review concluded that adult patients with CU are at a higher risk of developing Vitamin D deficiency, and its supplementation may provide therapeutic benefit in this subset (39).
Alpha-fetoprotein (AFP) is another pregnancy-specific glycoprotein hormone secreted by the yolk sac and fetal liver. This hormone peaks between weeks 12 and 16 of pregnancy, and gradually declines thereafter. AFP may have immune regulatory effects, but conclusive evidence is lacking (40).
The human immune system is designed to recognize and eradicate possibly harmful foreign, i.e., non-self antigens. During pregnancy, paternal antigens that are expressed by the fetus are recognized as foreign, but the maternal immune system protects the fetus through several immunological changes briefly discussed here.
Innate immunity refers to the inborn, non-specific, immediate host defense against any antigen, which does not require a previous sensitization. During pregnancy, the innate immune system and its effector cells change considerably, and this adaptation is important rather locally, primarily aimed at uterine vascular remodeling for fetal development. Among the various components of innate immunity, uterine NK cells (uNK) constitute the most important population. The important changes pertaining to innate immunity are briefly discussed below.
Dendritic cells (DCs) are vital APCs and act as a conduit between innate and adaptive immunity. During pregnancy, P4, E2, and hCG stimulate most of the uterine/decidual DCs to become tolerogenic and secrete the anti-inflammatory cytokine IL-10, thus creating a favorable local environment for the growing fetus (14). This is supported by a study by Segerer et al. (41) and Wan et al. (42), who reported significant up-regulation of IL-10 secretion by human DCs when stimulated in vitro by pregnancy hormones. Additionally, sex hormone-primed decidual DCs demonstrate impaired up-regulation of MHC-II and other co-stimulatory molecules, thereby reducing their ability to secrete proinflammatory cytokines (43). Interestingly, these effects are restricted to uterine DCs expressing sex-hormone receptors, whereas bone marrow or spleen-derived DCs are spared, which may possibly explain how the pregnant immune system tolerates a semi-allogenic fetus while protecting it from infections at the same time. The exact mechanism of this selective sparing remains unclear, but it reinforces the pleiotropic nature of DCs and their alluring ability to respond depending on the situation (44).
Monocytes or macrophages, also important APCs, contribute to immune responses by phagocytosis and the production of cytokines. Decidual CD14+ monocytes secrete anti-inflammatory cytokines such as IL-10 and TGF-β and become tolerogenic under the influence of galectin-1 and macrophage inhibitory protein-1 (45). Uterine decidual macrophages also demonstrate prominent anti-inflammatory polarization during pregnancy, under the influence of Th2 cytokines (IL-4, IL-5, IL-10, IL-13) and high glucocorticoid concentrations, with converting from an inflammatory M1 phenotype to a non-inflammatory M2 phenotype (46). P4 further inhibits toll-like receptor (TLR)-4 mediated activation of macrophages, thereby suppressing innate immune response to prevent fetal rejection during normal pregnancy (47).
The rising level of estrogen during pregnancy activates uterine mast cells (uMCs) via estradiol receptors, and they promote their degranulation to release histamine, which aids proper blastocyst implantation (by tissue remodeling) and placental development (48). The pro-secretory role of estrogen is further confirmed as specific ER antagonist tamoxifen inhibits MC degranulation both in vitro and in vivo (49). Elevated histamine levels also induce pregnant myometrial contractions in-vivo, and this may possibly explain the increased number of pre-term deliveries reported in females with systemic mastocytosis (50).
During pregnancy, the number of uMCs increases, and there is a shift from tryptase and chymase positive MCs (MCTC) to only tryptase positive (MCT) phenotype (48). These MC proteases (tryptase and chymase) activate matrix metalloproteinase (MMP)2 and MMP9 to mediate extracellular matrix degradation and facilitate delivery (51). Interestingly, the role of MCs in delivery is further corroborated by significant rise of pre-term deliveries in women with asthma, another MC-mediated disorder (52). Besides histamine and proteases, uMCs also release VEGF and galectin-1 (a glycan-binding protein), which support uterine neovascularization, fetal spinal artery (SA) remodeling; and placental development, fetal growth, respectively, (51, 53). uMCs collaborate with uNKs for SA remodeling, as evidenced by worsened SA remodeling in the simultaneous absence of both cell lines, compared to isolated deficiency (54). Recent evidence suggests that Mcpt5, secreted by uMCs and uNKs, is essential for proper SA remodeling in pregnant mice (55). Additionally, MCs secrete pro-inflammatory cytokines (IL-2, IL-12, TNF-α, and IFN-γ) in the early and late stages of pregnancy, and anti-inflammatory cytokines (IL-4, IL-10) during mid-pregnancy, to maintain the Th1 and Th2 dynamics during early/late and mid-pregnancy, respectively, necessary for a successful outcome (48). In addition to sex hormones, regulatory T-cells (Tregs) also promote IL-9 mediated proliferation of uMCs and angiogenesis at the murine feto-maternal interface to prevent early abortion (41, 56). Thus, there is a complex interplay between MCs, sex hormones, and immune cells during pregnancy, which may influence urticaria, as it is primarily a MC-mediated disorder.
NK cells, specifically the uterine variant (uNKs) constitute the major fraction of uterus lymphocytes in early pregnancy (~70%) and are responsible for maternal uterine vasculature remodeling and fetal survival (14). uNK cells differ from peripheral NK both structurally (differential expression of genes and receptor repertoire) and functionally (uNKs have lower cytotoxic activity compared to peripheral NKs) (14) Thus, the major function of uNKs is uterine vasculature remodeling and spinal artery formation, mediated primarily by the proangiogenic factor VEGF (57). Additionally, these cells secrete IFN-γ, a prominent anti-viral cytokine for fetal protection (58). However, there is confusion regarding the origin of uNK cells- whether they are recruited from peripheral NK cells into uterus, or they expand in-situ after pregnancy is established (14). Decidual NK cells increase in number under the influence of P4, IL-15, TGF-β, and stem-cell factor (SCF). P4 also promotes uNK cell recruitment in the pregnant uterus via secretion of osteopontin (59). Notably, a recent study has highlighted the role of decidual stromal cells in uNK proliferation in early pregnancy, by secreting IL-24, in an autocrine fashion (60).
Cytokines are polypeptides secreted by both innate and adaptive immune cells, which maintain a particular microenvironment, e.g., inflammatory or tolerogenic. The feto-maternal interface demonstrates a pro-inflammatory cytokine profile [IFN-γ, TNF-α, IL-1, IL-6, 1L-17, and the IL-6 family leukemia inhibitory factor (LIF)] during implantation and delivery, and an anti-inflammatory/tolerogenic profile (IL-10 and TGF-β) during the 2nd and 3rd trimester (61).
Adaptive immunity refers to the acquired and specific host defense system against previously exposed antigens, primarily involving T and B lymphocytes.
Normal pregnancy reflects a pro-inflammatory Th1/Th17 profile at its early and late stages, essential for fetal implantation and onset of labor, respectively. Major adaptation occurs mid-gestation, involving a shift from the pro-inflammatoryTh1/Th17 spectrum toward Th2 immunity, thus creating a tolerogenic environment to ensure the survival of the semi-allogenic fetus (14, 23). E2 plays a major role in skewing immunity toward Th2 at the fetal-maternal interface along with depressing the inflammatory Th1 axis (20).
Although a conspicuous Th2 immunological shift occurs during pregnancy, absolute dominance of Th2 cytokines does not occur as evidenced by successful pregnancies in mice deficient in Th2 cytokines such as IL-4,5,9, and 13 (62). Recently, researchers have demonstrated up-regulation of soluble receptor antagonists of pro-inflammatory cytokines such as soluble IL-6 Ra, TNFRA and IL-1Ra, and expansion of Treg cells, in addition to Th2 cytokines, in healthy human pregnancies (63).
Apart from conventional T cells, CD4+ Treg cells are also involved in creating an anti-inflammatory milieu by “regulating/depressing” the immune system via cytokines like IL-10 and TGF-β and inhibition of decidual effector T-cells by silencing their chemokine genes. The concentration of Treg cells fluctuates during pregnancy and reaches its peak in mid-gestation, under the influence of P4, E2, and fetal antigens, to suppress the maternal immune system and prevent fetal rejection. The important contribution of Treg cells (CD4+CD25+) is further corroborated by worse pregnancy outcomes in their absence (64). A healthy pregnant uterus demonstrates increased endometrial expression of Foxp3, the major transcription factor of Treg cells, and its reduced expression has been associated with infertility (65). In addition to Treg cells, γδ T-cells (a minor fraction accounting for <5% of circulating T-lymphocytes) also increase in the feto-maternal interface and contribute to the local anti-inflammatory state by secreting IL-10, TGF-β, and PIBF (63).
B lymphocytes are classically associated with antibody production; however, they also perform other roles such as antigen presentation and modulation of T-cell function. Notably, two types of antibodies (Abs) are produced- natural antibodies (autoreactive and cause autoimmune diseases- harmful for pregnancy) and asymmetric antibodies (AAbs) (needed for a successful pregnancy by reducing alloreactive responses). In normal pregnancy, natural Ab significantly reduces during the 3rd trimester to induce labor, while AAbs remain elevated during the entire pregnancy. Serum hCG regulates natural Ab production, while AAbs are controlled by P4 (66, 67).
Similar to Treg cells, Breg cells also increase during pregnancy, under the influence of hCG. These cells secrete IL-10 and inhibit Ab production by the B-cells, thus minimizing the chance of autoimmune disorders and graft (fetus) rejection (68).
Pregnancy-induced immunologic tolerance may increase maternal susceptibility toward various bacterial and viral infections. These infections might trigger inflammation and tissue destruction and stimulate auto-reactive T cells as a “bystander phenomenon”, and possibly worsen some autoimmune disorders (69).
Another interesting consequence of this altered immune status is the maternal gut microbiome remodeling, characterized by expansion of Enterobacteriaceae sp., which may facilitate metabolic and immunological adjustments for a successful pregnancy outcome (70). Although several authors have reported an association between chronic urticaria and gut microbial dysbiosis, studies are lacking in pregnant women (71, 72).
Recently, the concept of feto-maternal microchimerism has gained importance, which states that the maternal immune system acquires a state of immunological tolerance by means of transplacental feto-maternal cross-talk (transfer of genetically heterogeneous fetal material into maternal circulation) (63). Triche et al. (73) have shown HLA disparity between a mother and fetus is essential for a normal pregnancy, while feto-maternal HLA matching (class I and class II) has resulted in spontaneous abortion and pre-eclampsia.
The hormonal and immunological changes during pregnancy are summarized in Table 1 and Figures 2, 3.
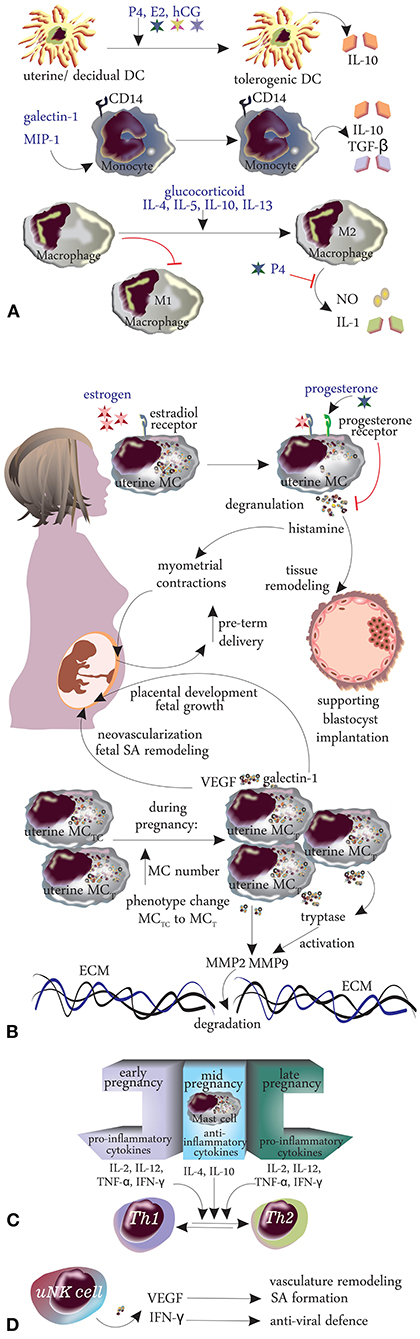
Figure 3. Changes in innate immunity during pregnancy. (A) Dendritic cells, monocytes, macrophages, mast cells. (B) Cytokines. (C) TH1/TH2 shift. (D) Natural killer cells.
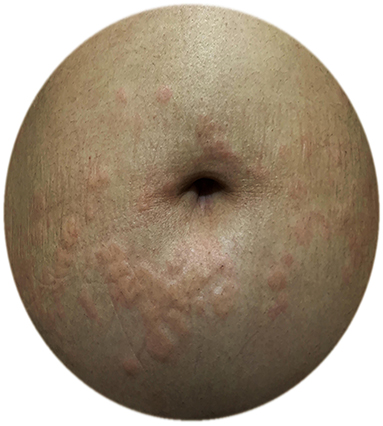
Figure 4. A pregnant CU patient with exacerbation of urticaria in the third trimester; urticarial plaques around the umbilicus.
During pregnancy, disease activity of chronic inflammatory disorders are subject to change due to the changes in immune responses such as decrease in Th1-type and Th17-type cytokines (that promote allograft rejection and may compromise pregnancy), increase in Th2-type cytokines (that inhibit the Th1 responses, promote allograft tolerance and therefore may improve pregnancy success) as well as an increase in T reg cells which dampen all the T helper responses and provides tolerance for fetal alloantigens and could induce fetoallograft tolerance through the production of IL-10 and TGF-β (75–77). As a result, Th2-type autoimmune disease get worsen and Th1/Th17-type autoimmune disease improve; i.e., rheumatoid arthritis (RA), multiple sclerosis, Graves' disease, and Hashimoto thyroiditis improve while systemic lupus erythematosus (SLE) and systemic sclerosis (SS) worsen during pregnancy (75). A favorable Treg–TH17 balance, the reduction in pro-inflammatory γδ T cells, and an increase in the soluble receptors that buffer the biological effects of TNF and IL-1 have been suggested to be the leading factors that contribute to pregnancy-related improvement of RA (63, 78, 79). Contrary to improvement in RA, lupus has been reported to deteriorate during pregnancy; however, the renal and skin lupus are differently affected by pregnancy; lupus nephritis deteriorates while skin lupus ameliorates during pregnancy. The worsened kidney function has been reported to be associated with renal inflammation and higher IFN-γ and IL-10 levels in the kidneys. IFN-γ by stimulating secretion of IgG antibodies and IL-10 by inducing B-cells to produce autoantibodies, which finally result in increased glomerular IgG deposition (80). In contrast, IL-10 was found to be increased and IFN-γ was decreased in the skin lesions of multiparous lupus-prone mice highlighting the role of IL-10 as a suppressor on skin lupus by possibly suppressing T-lymphocyte driven autoimmunity (81).
On the other hand, due to the increase in Th2 immune responses, an increased disease activity is anticipated in allergic disorders. That is, asthma exacerbations have been reported to range from 13 to 52% during pregnancy and most exacerbations occur in the second or beginning of the third trimester (82). Atopic dermatitis worsened during pregnancy in 52% in one study which also reported that most of the worsening occurred by 20 weeks of gestation (83) and in 61.0% in another (84).
Although CU is a very common and female-dominant disease that favors the reproductive age group, there is only one study that evaluates the effects of pregnancy on CU or the effects of CU on pregnancy outcomes, which is performed by the UCARE network (12, 85). In this study, 288 pregnant patients with CU from 21 centers/13 countries were asked to answer an a-47-item questionnaire which included questions on the exacerbations, angioedema attacks, emergency referrals, the overall course of CU during pregnancy, the course of urticaria after giving birth as well treatments before and during pregnancy, outcomes of pregnancy, and treatments given during breastfeeding. The study included both CSU and CIndU patients (CSU 66.9%, CIndU 12.8%, CSU + CIndU 20.3%) who experienced pregnancy within the last 3 years, and whose CU started before pregnancy (Figure 4). Disease activity before pregnancy was almost equally distributed among the patients (35.7% reported their disease activity as mild, 34.2% as moderate, and 29.7% as severe before pregnancy, respectively). Of 288 patients, 51% rated their CU as improved, 29% as worse, and 20% as unchanged during pregnancy. Two in five (43.5%) experienced acute CU exacerbations during pregnancy which most commonly occurred exclusively in the 3rd trimester (27.6%) or the 1st trimester (22.8%). Emergency referrals for CU were also most common in the 3rd trimester and angioedema occurrence was most common in the first trimester. The reason for the increase of disease activity during the first and third trimesters was explained by the predomination of Th1 immune responses and pro-inflammatory signals that promote MC activation in CU patients. These results were found similar to pregnant patients with mastocytosis who also showed exacerbation during the 1st or 3rd trimester (48).
The rate of emergency referrals (9.8%) and rate of angioedema (17.4%) during pregnancy were lower than the reported rates in non-pregnant CU patients (compared to 14.8 and 33.5%; and 40.3 and 45% in ASSURE and AWARE studies, respectively) (86, 87).
While the risk factors determined in the univariate analysis for CU worsening during pregnancy were having no angioedema and having mild disease activity before pregnancy, receiving no treatment before pregnancy, receiving treatment during pregnancy, having CIndU, worsening of CU during the previous pregnancy; after adjusting for cofounders, having mild disease before pregnancy and receiving treatment during pregnancy were left as the relevant risk factors for CU worsening during pregnancy.
After delivery, half of the patients (50%) whose urticaria improved during pregnancy reported worsening of CU after giving birth, while half (52%) with worsening of CU during pregnancy showed no change in their CU activity after giving birth. As an explanation to disease activity changes after birth, the authors proposed that the subsiding of Th2 skewing in the post-partum period results in the worsening of Th1 and Th17 autoimmune disorders and improvement in Th2-driven disorders (75) and concluded that CU patients with a dominant immune profile of Th1/Th17 might experience increased disease activity after birth, while CU patients with a Th2-linked autoallergic profile might show improvement.
As there were also patients who displayed disease activity increase during the second trimester and also patients whose urticaria had a worse course during pregnancy; the authors hypothesized that these patients might have the type 1 autoimmune (autoallergic) type of CSU.
The management of CU depends on four major steps; (1) Disease activity assessment and monitoring (2) Education of patients (3) Control of triggering factors such as physical factors, NSAIDs and stress (4) Pharmacotherapy
Even though CU is a common disease in the reproductive female population, there is a lack of information on the safe use of recommended treatments and outcomes of pregnancy in pregnant CU patients. In the recent, already mentioned PREG-CU study, which evaluated the treatment patterns during pregnancy and lactation as well as the outcomes of pregnancy in 288 pregnant CU patients. The study evaluated the treatments before and during pregnancy, pregnancy trimesters which the treatments were received, outcomes of pregnancy, and treatments given during breastfeeding with a questionnaire. The results of the study showed that 81.4% of CU patients continued to use their medication when they decided to become pregnant. During pregnancy 60% of the patients used regular medication for CU with half of them (48.8%) did so during the whole pregnancy. During pregnancy, standard-dose sg-AHs (35%), standard-dose first-generation AHs (fg-AH) (7.6%), higher than standard-dose sg-AHs (5.6%), and omalizumab (5.6%) were the most commonly used treatments, respectively. Most commonly used AHs were cetirizine (37.4%), loratadine (14.6%); and levocetirizine and fexofenadine (7.3%; each). The outcomes of pregnancy in patients with CU were similar to the normal population: a preterm birth rate of 10.2% and newborn medical problems rate of 7.9%. No risk factors were found to be associated with preterm birth and newborn medical problems (88). Eight of 10 CU patients breastfed their babies and 54.3% of them used medication for CU while breastfeeding. Of them, 63.4, 14.1, and 6% used a standard-dosed sg-AH, higher than standard-dosed sg-AH and omalizumab; respectively.
The EAACI/WAO/EDF International guideline for the management of urticaria recommends to start treatment with standard doses of second-generation (non-sedative) H1 antihistamines (sg-AH), to increase the dose up to 4-folds in case of no response to standard doses of sg-AHs and if there is no response in 2–4 weeks to add on omalizumab as the third step (1). The recommended treatment in omalizumab-resistant cases is cyclosporine-A (1). The guideline recommends adopting the same approach in the management of pregnant and lactating patients but also emphasizes the lack of evidence-based information on the safety of urticaria treatments.
For getting information on the safe use of medications during pregnancy, we have been using the letter category system of the Food and Drug Administration (FDA). Based on data derived from human and animal studies, this system has classified the reproductive safety of medications in five risk categories (A, B, C, D, and X). However, in 2015 a new system called “Pregnancy and Lactation Labeling Rule” or PLLR is implemented given to the oversimplified or sometimes misleading nature of the pregnancy risk category system (89). The new PPLR format summarizes data on pregnancy, lactation, and exposure registries and includes a new section for men and women with reproductive potential. With this new system, physicians will be able to evaluate benefits vs. risks while counseling pregnant and nursing patients who need to take medication. This new system required that FDA drug submissions on or after 30th June 2015 be in the new PLLR format. The drugs which were approved between 30th June 2007 and 29th June 2015 should have transitioned to the new PLLR format by 30th June 2019 (90). However, it is not known if the foreseen FDA drug labels have been transitioned to the new PLLR format.
The key elements of pharmacotherapy of CU are H1 antihistamines and antihistamines are among the most frequently prescribed medications during pregnancy. Approximately 15% of pregnant women use antihistamines during pregnancy, particularly during the first trimester (91). Despite associations of first- and second-generation H1AHs with birth defects have been reported in older reports, detailed analysis of the findings from these reports did not show a meaningful association between antihistamines and major congenital anomalies (92, 93).
Loratadine and cetirizine are the antihistamines of choice based on the data on their safety and the recommendations in the urticaria guidelines (1, 94–98). Compared with loratadine, the use of desloratadine during pregnancy did not increase the risk of adverse pregnancy outcomes (99) and a study from Denmark showed that use of fexofenadine during pregnancy did not increase the risk of major birth defects or spontaneous abortion compared with cetirizine (100).
The use of chlorpheniramine or diphenhydramine as first-generation H1 antihistamines is not recommended as the first-line treatment due to their various side effects. They have not been associated with adverse fetal outcomes in prospective cohort trials (97). The use of H1 antihistamines during the first trimester was not found to be associated with an increased risk of major malformations or other adverse pregnancy outcomes (91). Table 2 shows the pregnancy categories of H1-antihistamines. The safety of higher than approved doses of antihistamines has not been evaluated in pregnant patients, therefore potential risks and benefits have to be discussed with the patient before implementing it.
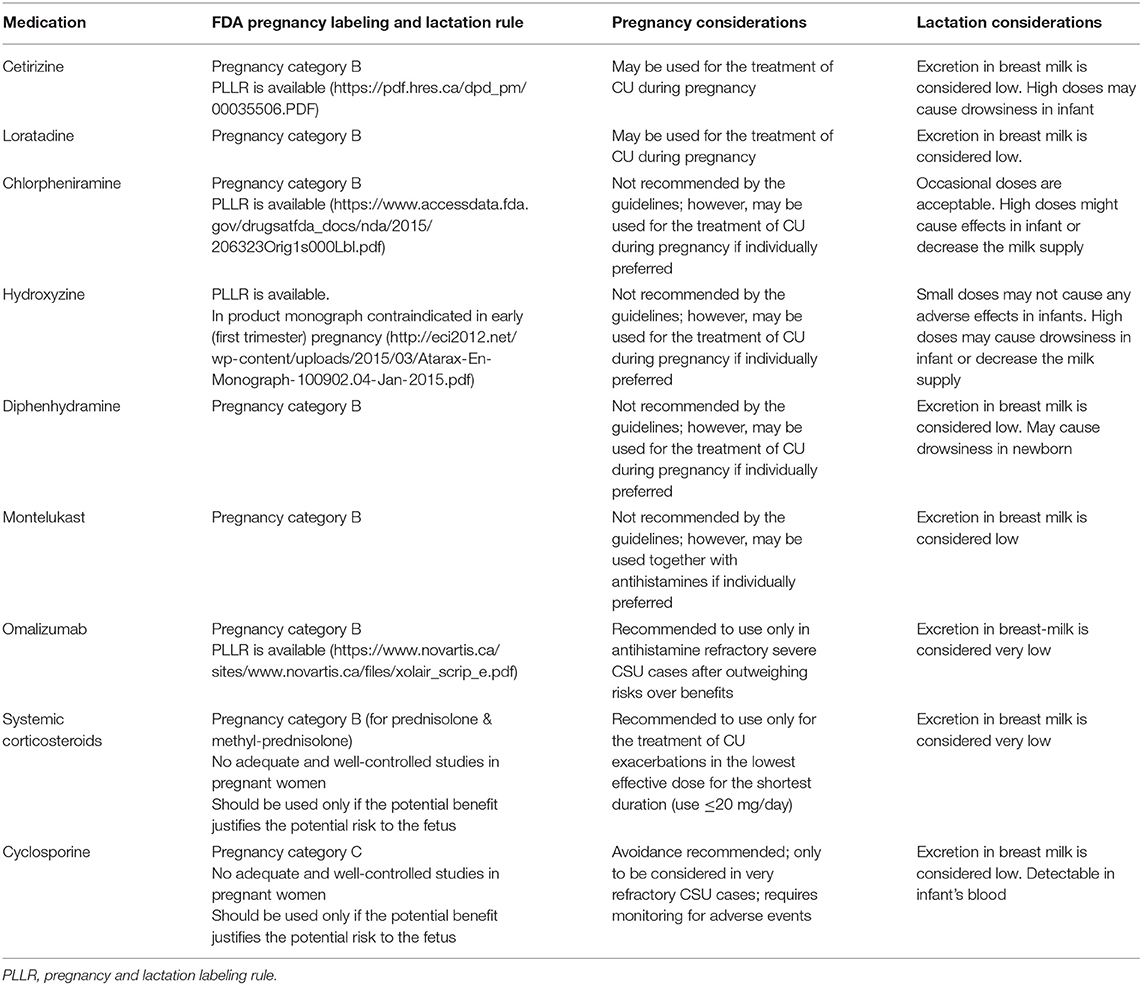
Table 2. Considerations for pregnancy and lactation for the medications used in the treatment of chronic urticarial.
Although the use of leukotriene antagonists for the treatment of CU is not recommended by the international guidelines due to insufficient level of evidence (1), in case of intention to use during pregnancy, it will be useful to know that montelukast has been assigned pregnancy category B and no increase in major malformations were reported with the use of this medication during pregnancy (101).
Omalizumab is a recombinant IgG1 anti-IgE monoclonal antibody which is recommended in the treatment of antihistamine resistant CSU (1). Animal data on omalizumab (reproduction studies in cynomolgus monkeys) showed no maternal toxicity when administered throughout late gestation, delivery and nursing, subcutaneously in doses up to 75 mg/kg (12-fold the maximum clinical dose) as well as no impaired male or female fertility, embryotoxicity or teratogenicity and no adverse effects on fetal or neonatal growth (102). The Xolair Pregnancy Registry (EXPECT) which was designed to compare the maternal and neonatal outcomes of asthma patients treated with omalizumab (n = 250) or conventional drugs but not omalizumab (n = 1,153) during pregnancy. The prevalence of major congenital anomalies (8.1 vs. 8.9%), live births (99.1 vs. 99.3%), premature birth (15.0 vs. 11.3%) was similar between omalizumab treated and the conventional treatment groups (103). Given that this study includes only asthmatic patients and is an observational study, it is difficult to draw definitive conclusions for the safety of omalizumab in pregnant CU patients, however, there are several case reports on the safe use of omalizumab during pregnancy in CU patients (104).
Of note, omalizumab has a very long life of elimination half-life (26 days), and omalizumab exposure of the neonate would persist for weeks after birth. This may also translate to the exposure of the fetus to omalizumab which has been given to the patient even she was not aware of her pregnancy (of note: elimination of a given drug totally from the body takes 4–5 half-lives; in case of omalizumab this would take 26 × 5 = 130 days).
Another point to remember is that although all IgG subtypes can cross the placenta, IgG1 has the greatest transplacental transfer. It is expected that the lowest omalizumab exposure occurs during the first trimester of pregnancy and the greatest during the third trimester (105).
In 2019, European Medicine Agency updated the European Public Assessment Report and stated that omalizumab might be considered for use in pregnancy (82). In antihistamine refractory, severe CU patients, omalizumab may be a reasonable choice of treatment; however, the benefit-risk ratio should be reconsidered in every pregnant case individually and should be discussed with the patient in detail.
Cyclosporine-A is the treatment recommended by the guidelines for CSU cases who do not respond to omalizumab treatment for 6 months. It is classified as category “C” in the FDA letter category system for pregnancy.
Bar Oz et al. reported in their meta-analysis which included 15 studies with 410 transplant patients that cyclosporine-A is not a major human teratogen but is associated with a trend toward increased risk of congenital malformations in the babies of transplant recipients and increased rates of prematurity (106).
Due to its side effects such as hypertension and nephrotoxicity which can potentiate gestational complications such as preeclampsia, cyclosporine-A is generally not recommended in pregnancy. It should be preserved for very severe cases only after other treatments have failed (107).
The use of systemic glucocorticosteroids (GCS) in CSU is limited only during exacerbations for short periods by the guidelines (1). GCS are generally not considered to be teratogenic but has been linked to growth retardation if fetal exposure (a median of 20 mg/day) happens during intrauterine development (108). Even though an increased risk of ~3-fold for oral clefts has been shown (109), the US National Birth Defects Prevention showed no increased risk of oral clefts with the 1st trimester use of GCS in a case-control study (110). It should be remembered that GCS use in pregnancy may lead to maternal side effects such as hypertension, gestational diabetes, and preeclampsia, and these can lead to poor pregnancy outcomes (i.e., intrauterine growth restriction, macrosomia, intrauterine fetal demise). Therefore, if possible, the use of GCS should be avoided in pregnancy, but if it must be used, it should be prescribed for severe cases at the lowest effective dose (≤ 20 mg/day prednisone) for a limited period (111, 112). Use of GCS during exacerbations of CU as short courses of 1–5 days with minimally effective dose is unlikely to cause pregnancy complications. With a short half-life and effective metabolization by 11-β-hydroxy-steroid present in the placenta, prednisone should be the steroid of choice (FDA category B). Fetal exposure is found ~10% of the maternal plasma level (113–115).
Hence the transfer rate to breast milk is minimal, second-generation antihistamines are safe to use during lactation (116). Cetirizine, loratadine, and fexofenadine are the best studied antihistamines (117, 118). Higher doses of terfenadine and loratadine showed very minimal transmission to the milk (114, 119). In refractory cases of CU who are nursing their babies, higher doses of second-generation antihistamines might be safely used since the transfer rate to breast milk is minimal (114). First-generation antihistamines might lead to infant irritability and drowsiness (120) and are better not used during lactation.
GCS are considered to be safe to use during lactation by The American Academy of Pediatrics. They recommend the use of minimal effective dose for the possible shortest duration and to prefer prednisone or prednisolone over other GCS options (121). Since low amounts of prednisolone can transfer to breast milk, delaying breastfeeding for 4 h after maternal GCS ingestion to avoid plasma level peaks occurring 1 h after ingestion is recommended (122, 123).
Montelukast is safe to use during breastfeeding given its very low levels in breastmilk. Since it is approved for use even in infants as young as 6 months of age, amounts ingested during breastfeeding by the infants are not expected to cause any adverse effects (124). A task force of respiratory experts reported that the use of these medications during breastfeeding is probably safe (125).
With a molecular weight of 145,058 Da, omalizumab is a large protein molecule and, likely, omalizumab transfer to the milk and, therefore the level in milk, is very low. It is partially destroyed in the gastrointestinal system of the infant and systemic absorption by the baby is probably minimal (126). Pregnant and nursing asthmatic patients have been followed in the EXPECT pregnancy registry for several years; 154 infants of these mothers were breastfed while their mothers were on omalizumab treatment. The results of this study showed that there is no difference in serious adverse events among the infants who received or did not receive omalizumab (102, 103). A case report of a CU patient who was treated with omalizumab during pregnancy and nursery showed that only 1/10,000 to 1/1,000 of omalizumab in the maternal serum is transferred into breast milk (127).
Cyclosporine-A transfers to the milk <1% of the mother's weight-adjusted dosage. It does not cause adverse effects on infant's growth, development, or kidney function. However, if cyclosporine-A is used during lactation, infants should be monitored for the serum levels of cyclosporine-A to rule out toxicity (128).
The considerations for pregnancy and lactation for CU medications are shown in Table 2.
Managing a pregnant patient with CU is often a challenge for treating physicians. Our review provides information on the hormonal and immunological changes across pregnancy and their potential relevance for CU, and we present what is known about the impact of pregnancy on CU. We also provide information on treatment options for pregnant patients with CU.
CU may improve, stay the same or worsen during pregnancy. This information as well as the fact that no treatment could end up with emergency referrals and worsening of the disease therefore requirement of more treatment should also be discussed with the patient.
For sure the ideal situation during pregnancy and lactation is “no pharmacologic therapy”, especially during the first trimester, however, it is almost impossible for a CU patient to have no disease activity during pregnancy. Therefore, treatment with the aim of zero or minimal disease activity with the least treatment should be commenced during pregnancy. The potential side effects of the medications should be balanced against the risks of inadequately treated disease for the mother and the fetus. These considerations should be discussed with the patient who is weighing the potential benefits of relief from the treated disease vs. the potential risks of the medication and an informed and shared decision should be made.
Currently, there is for sure, lack of sufficient information on the management of CU during pregnancy and questions remain to be answered are which treatments are safe to use during pregnancy, how CU manifests during pregnancy, which CU patients show amelioration or deterioration during pregnancy and are there biomarkers to show how CU will progress during pregnancy and lactation? To answer these questions, prospective studies with large patient populations which will determine patient characteristics both in the clinical level and in the molecular level are needed.
ENTIS (European Network of Teratology Information Service) https://www.entis-org.eu/ UK Teratology information service (UKTIS) https://medicinesinpregnancy.org
German: https://www.embryotox.de/;French: http://www.lecrat.fr/; Dutch: https://www.lareb.nl/ Organization of Teratology Information Specialists https://mothertobaby.org/
For pregnancy registry studies for the relevant drug (https://www.fda.gov/science-research/womens-health-research/list-pregnancy-exposure-registries).
For lactation database visit the Drugs and Lactation Database (LactMed) [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK501922/.
EK: concept. EK and MM: design. EK and IP: data collection or processing and writing. EK, IP, AZ, AK, DE-A-K, MC, and MM: analysis or interpretation and approval. EK, IP, AZ, and AK: literature search. DE-A-K: figures. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AAbs, Asymmetric antibodies; AFP, Alpha-fetoprotein; AH, Antihistamine; APCs, Antigen-presenting cells; ASST, Autologous serum skin test; Breg, Regulatory B-cells; CIndU, Chronic inducible urticaria; CSU, Chronic spontaneous urticaria; CU, Chronic urticaria; DCs, Dendritic cells; ERs, Estrogen receptors; FDA, Food and drug administration; Foxp3, Fork head box protein 3; GCs, Glucocorticosteroids; hCG, Human chorionic gonadotrophin; LIF, Leukemia inhibitory factor; MCs, Mast cells; NK cells, Natural killer cells; PIBF, Progesterone-induced blocking factor; PLLR, Pregnancy and lactation labeling rule; PRs, P4 receptors (progesterone receptors); TLR, Toll-like receptor; TPO, Thyroid peroxidase; Tregs, Regulatory T-cells; uMCs, Uterine mast cells; VEGF, Vascular endothelial-derived growth factor; MMP, Matrix metalloproteinase.
1. Zuberbier T, Abdul Latiff AH, Abuzakouk M, Aquilina S, Asero R, Baker D, et al. The international EAACI/GA2LEN/EuroGuiDerm/APAAACI guideline for the definition, classification, diagnosis, and management of urticaria. Allergy. (2022) 77:734–66. doi: 10.1111/all.15090
2. Weller K, Maurer M, Bauer A, Wedi B, Wagner N, Schliemann S, et al. Epidemiology, comorbidities, and healthcare utilization of patients with chronic urticaria in Germany. J Eur Acad Dermatol Venereol. (2022) 36:91–9. doi: 10.1111/jdv.17724
3. Fricke J, Ávila G, Keller T, Weller K, Lau S, Maurer M, et al. Prevalence of chronic urticaria in children and adults across the globe: systematic review with meta-analysis. Allergy. (2020) 75:423–32. doi: 10.1111/all.14037
4. Maurer M, Giménez-Arnau A, Ensina LF, Chu CY, Jaumont X, Tassinari P. Chronic urticarial treatment patterns and changes in quality of life: AWARE study 2-year results. World Allergy Organ J. (2020) 13:100460. doi: 10.1016/j.waojou.2020.100460
5. Maurer M, Ortonne J-P, Zuberbier T. Chronic urticaria: an internet survey on health behaviours, symptom patterns and treatment needs in European adult patients. Br J Dermatol. (2009) 160:633–41. doi: 10.1111/j.1365-2133.2008.08920.x
6. Ue AP, Souza PK, Rotta O, FurlaniWde J, Lima AR, Sabbag DS. Quality of life assessment in patients with chronic urticaria. An Bras Dermatol. (2011) 86:897–904. doi: 10.1590/S0365-05962011000500006
7. Gregoriou S, Rigopoulos D, Katsambas A, Katsarou A, Papaioannou D, Gkouvi A, et al. Etiologic aspects and prognostic factors of patients with chronic urticaria: nonrandomized, prospective, descriptive study. J Cutan Med Surg. (2009) 13:198–203. doi: 10.2310/7750.2008.08035
8. Sánchez Borges M, Tassinari S, Flores A. Epidemiologic features in patients with antihistamine-resistant chronic urticaria. Rev Aler México. (2015) 62:279–86. doi: 10.29262/ram.v62i4.130
9. Straesser MD, Oliver E, Palacios T, Kyin T, Patrie J, Borish L„ et al. Serum IgE as an immunological marker to predict response to omalizumab treatment in symptomatic chronic urticaria. J Allergy Clin Immunol Pract. (2018) 6:1386-8 e1. doi: 10.1016/j.jaip.2017.10.030
10. Pierdominici M, Maselli A, Colasanti T, Giammarioli AM, Delunardo F, Vacirca D, et al. Estrogen receptor profiles in human peripheral blood lymphocytes. Immunol Lett. (2010) 132:79–85. doi: 10.1016/j.imlet.2010.06.003
11. Moulton VR. Sex hormones in acquired immunity and autoimmune disease. Front Immunol. (2018) 9:2279. doi: 10.3389/fimmu.2018.02279
12. Kocatürk E, Al-Ahmad M, Krause K, Gimenez-Arnau A, Thomsen SF, Conlon N, et al. Effects of pregnancy on chronic urticaria: results of the PREG-CU UCARE study. Allergy. (2021) 76:3133–44. doi: 10.1111/all.14950
13. Braunstein GD, Rasor J, Danzer H, Adler D, Wade ME. Serum human chorionic gonadotropin levels throughout normal pregnancy. Am J Obstet Gynecol. (1976) 126:678–81. doi: 10.1016/0002-9378(76)90518-4
14. Schumacher A, Costa SD, Zenclussen AC. Endocrine factors modulating immune responses in pregnancy. Front Immunol. (2014) 5:196. doi: 10.3389/fimmu.2014.00196
15. Poloski E, Oettel A, Ehrentraut S, Luley L, Costa SD, Zenclussen AC, et al. JEG-3 trophoblast cells producing human chorionic gonadotropin promote conversion of human CD4+FOXP3- T cells into CD4+FOXP3+ regulatory T cells and foster T cell suppressive activity. Biol Reprod. (2016) 94:106. doi: 10.1095/biolreprod.115.135541
16. Dauven D, Ehrentraut S, Langwisch S, Zenclussen AC, Schumacher A. Immune modulatory effects of human chorionic gonadotropin on dendritic cells supporting fetal survival in murine pregnancy. Front Endocrinol. (2016) 7:146. doi: 10.3389/fendo.2016.00146
17. Sauss K, Ehrentraut S, Zenclussen AC, Schumacher A. The pregnancy hormone human chorionic gonadotropin differentially regulates plasmacytoid and myeloid blood dendritic cell subsets. Am J Reprod Immunol. (2018) 79:e12837. doi: 10.1111/aji.12837
18. Fettke F, Schumacher A, Canellada A, Toledo N, Bekeredjian-Ding I, Bondt A, et al. Maternal and fetal mechanisms of B cell regulation during pregnancy: human chorionic gonadotropin stimulates B cells to produce IL-10 while alpha-fetoprotein drives them into apoptosis. Front Immunol. (2016) 7:495. doi: 10.3389/fimmu.2016.00495
19. Mansour R, Tawab N, Kamal O, El-Faissal Y, Serour A, Aboulghar M, et al. Intrauterine injection of human chorionic gonadotropin before embryo transfer significantly improves the implantation and pregnancy rates in in vitro fertilization/intracytoplasmic sperm injection: a prospective randomized study. Fertil Steril. (2011) 96:1370–4.e1. doi: 10.1016/j.fertnstert.2011.09.044
20. Robinson DP, Klein SL. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm Behav. (2012) 62:263–71. doi: 10.1016/j.yhbeh.2012.02.023
21. Shah NM, Lai PF, Imami N, Johnson MR. Progesterone-related immune modulation of pregnancy and labor. Front Endocrinol. (2019) 10:198. doi: 10.3389/fendo.2019.00198
22. Kumar P, Magon N. Hormones in pregnancy. Niger Med J. (2012) 53:179–83. doi: 10.4103/0300-1652.107549
23. Borba VV, Zandman-Goddard G, Shoenfeld Y. Exacerbations of autoimmune diseases during pregnancy and postpartum. Best Pract Res Clin Endocrinol Metab. (2019) 33:101321. doi: 10.1016/j.beem.2019.101321
24. Rolfo A, Nuzzo AM, De Amicis R, Moretti L, Bertoli S, Leone A. Fetal-maternal exposure to endocrine disruptors: correlation with diet intake and pregnancy outcomes. Nutrients. (2020) 12:1744. doi: 10.3390/nu12061744
25. Bernstein JA, Bouillet L, Caballero T, Staevska M. Hormonal effects on urticaria and angioedema conditions. J Allergy Clin Immunol Pract. (2021) 9:2209–19. doi: 10.1016/j.jaip.2021.04.021
26. Conde A, Figueiredo B. 24-h urinary free cortisol from mid-pregnancy to 3-months postpartum: gender and parity differences and effects. Psychoneuroendocrinology. (2014) 50:264–73. doi: 10.1016/j.psyneuen.2014.08.013
27. Chuang E, Molitch ME. Prolactin and autoimmune diseases in humans. Acta Biomed. (2007) 78(Suppl. 1):255–61.
28. Shelly S, Boaz M, Orbach H. Prolactin and autoimmunity. Autoimmun Rev. (2012) 11:A465–70. doi: 10.1016/j.autrev.2011.11.009
29. Sabry MK, Farres MN, Melek NA, Arafa NA, Ohanessian AA. Prolactin and dehydroepiandrosterone sulfate: are they related to the severity of chronic urticaria? Arch Med Res. (2013) 44:21–6. doi: 10.1016/j.arcmed.2012.10.007
30. Soliman M, Khattab FM, Ebrahim HM, Nasr M. Serum prolactin level in chronic urticaria: Is bromocriptine inducing remission in chronic urticaria? J Dermatolog Treat. (2018) 29:826–30. doi: 10.1080/09546634.2018.1468062
31. Sanchez-Margalet V, Martin-Romero C. Human leptin signaling in human peripheral blood mononuclear cells: activation of the JAK-STAT pathway. Cell Immunol. (2001) 211:30–6. doi: 10.1006/cimm.2001.1815
32. Elieh Ali Komi D, Shafaghat F, Christian M. Crosstalk between mast cells and adipocytes in physiologic and pathologic conditions. Clin Rev Allergy Immunol. (2020) 58:388–400. doi: 10.1007/s12016-020-08785-7
33. Maymó JL, Pérez Pérez A, Gambino Y, Calvo JC, Sánchez-Margalet V, Varone CL. Review: Leptin gene expression in the placenta–regulation of a key hormone in trophoblast proliferation and survival. Placenta. (2011) 32:S146–53. doi: 10.1016/j.placenta.2011.01.004
34. Fang L, Egea E, Pereira-Sanandres N, Fernando R, Moreno-Woo S, Garavito-De Egea G. Serum levels of leptin, adiponectin and vitamin D in Colombian adults with chronic urticaria. J Aller Clin Immunol. (2018) 141:AB54. doi: 10.1016/j.jaci.2017.12.173
35. Farres MN, El khoderee MM, ELkady HM, Eissa NM. Serum Leptin in correlation to clinical severity in patients with chronic urticaria. QJM: An International Journal of Medicine. (2021) 114:hcab100-096. doi: 10.1093/qjmed/hcab100.096
36. Borella E, Nesher G, Israeli E, Shoenfeld Y. Vitamin D: a new anti-infective agent? Ann N Y Acad Sci. (2014) 1317:76–83. doi: 10.1111/nyas.12321
37. Oreshkova T, Dimitrov R, Mourdjeva M. A cross-talk of decidual stromal cells, trophoblast, and immune cells: a prerequisite for the success of pregnancy. Am J Reprod Immunol. (2012) 68:366–73. doi: 10.1111/j.1600-0897.2012.01165.x
38. Charoenngam N, Holick MF. Immunologic effects of vitamin D on human health and disease. Nutrients. (2020) 12:2097. doi: 10.3390/nu12072097
39. Li Y, Cao Z, Guo J, Li Q, Su J. Effects of serum vitamin D levels and vitamin D supplementation on urticaria: a systematic review and meta-analysis. Int J Environ Res Public Health. (2021) 18:4911. doi: 10.3390/ijerph18094911
40. Um SH, Mulhall C, Alisa A, Ives AR, Karani J, Williams R, et al. Alpha-fetoprotein impairs APC function and induces their apoptosis. J Immunol. (2004) 173:1772–8. doi: 10.4049/jimmunol.173.3.1772
41. Segerer SE, Müller N, van den Brandt J, Kapp M, Dietl J, Reichardt HM, et al. Impact of female sex hormones on the maturation and function of human dendritic cells. Am J Reprod Immunol. (2009) 62:165–73. doi: 10.1111/j.1600-0897.2009.00726.x
42. Wan H, Versnel MA, Leijten LM, van Helden-Meeuwsen CG, Fekkes D, Leenen PJ, et al. Chorionic gonadotropin induces dendritic cells to express a tolerogenic phenotype. J Leukoc Biol. (2008) 83:894–901. doi: 10.1189/jlb.0407258
43. Xu Y, He H, Li C, Shi Y, Wang Q, Li W, et al. Immunosuppressive effect of progesterone on dendritic cells in mice. J Reprod Immunol. (2011) 91:17–23. doi: 10.1016/j.jri.2011.06.101
44. Kovats S. Estrogen receptors regulate an inflammatory pathway of dendritic cell differentiation: mechanisms and implications for immunity. Horm Behav. (2012) 62:254–62. doi: 10.1016/j.yhbeh.2012.04.011
45. Arck PC, Hecher K. Fetomaternal immune cross-talk and its consequences for maternal and offspring's health. Nat Med. (2013) 19:548–56. doi: 10.1038/nm.3160
46. Nagamatsu T, Schust DJ. The contribution of macrophages to normal and pathological pregnancies. Am J Reprod Immunol. (2010) 63:460–71. doi: 10.1111/j.1600-0897.2010.00813.x
47. Su L, Sun Y, Ma F, Lü P, Huang H, Zhou J. Progesterone inhibits Toll-like receptor 4-mediated innate immune response in macrophages by suppressing NF-kappaB activation and enhancing SOCS1 expression. Immunol Lett. (2009) 125:151–5. doi: 10.1016/j.imlet.2009.07.003
48. Woidacki K, Zenclussen AC, Siebenhaar F. Mast cell-mediated and associated disorders in pregnancy: a risky game with an uncertain outcome? Front Immunol. (2014) 5:231. doi: 10.3389/fimmu.2014.00231
49. Zaitsu M, Narita S, Lambert KC, Grady JJ, Estes DM, Curran EM, et al. Estradiol activates mast cells via a non-genomic estrogen receptor-alpha and calcium influx. Mol Immunol. (2007) 44:1977–85. doi: 10.1016/j.molimm.2006.09.030
50. Madendag IC, Madendag Y, Tarhan I, Altinkaya SO, Danisman N. Mastocytosis in pregnancy. Taiwan J Obstet Gynecol. (2010) 49:192–6. doi: 10.1016/S1028-4559(10)60040-X
51. Elieh Ali Komi D, Shafaghat F, Haidl G. Significance of mast cells in spermatogenesis, implantation, pregnancy, and abortion: cross talk and molecular mechanisms. Am J Reprod Immunol. (2020) 83:e13228. doi: 10.1111/aji.13228
52. Vaezi A, Haghighi L, Beigmohammadi F, Nojomi M. Maternal asthma, pregnancy, delivery and birth outcomes: A retrospective cohort study. Iran J Allergy Asthma Immunol. (2017) 16:92–8.
53. Woidacki K, Meyer N, Schumacher A, Goldschmidt A, Maurer M, Zenclussen AC. Transfer of regulatory T cells into abortion-prone mice promotes the expansion of uterine mast cells and normalizes early pregnancy angiogenesis. Sci Rep. (2015) 5:13938. doi: 10.1038/srep13938
54. Meyer N, Schüler T, Zenclussen AC. Simultaneous ablation of uterine natural killer cells and uterine mast cells in mice leads to poor vascularization and abnormal doppler measurements that compromise fetal well-being. Front Immunol. (2018) 8:1913. doi: 10.3389/fimmu.2017.01913
55. Meyer N, Zenclussen AC. Mast cells-Good guys with a bad image? Am J Reprod Immunol. (2018) 80:e13002. doi: 10.1111/aji.13002
56. Lu LF, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K, et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. (2006) 442:997–1002. doi: 10.1038/nature05010
57. Wang C, Umesaki N, Nakamura H, Tanaka T, Nakatani K, Sakaguchi I, et al. Expression of vascular endothelial growth factor by granulated metrial gland cells in pregnant murine uteri. Cell Tissue Res. (2000) 300:285–93. doi: 10.1007/s004410000198
58. Ashkar AA, Croy BA. Functions of uterine natural killer cells are mediated by interferon gamma production during murine pregnancy. Semin Immunol. (2001) 13:235–41. doi: 10.1006/smim.2000.0319
59. Qu X, Yang M, Zhang W, Liang L, Yang Y, Zhang Y, et al. Osteopontin expression in human decidua is associated with decidual natural killer cells recruitment and regulated by progesterone. In Vivo. (2008) 22:55–61. doi: 10.1096/fasebj.22.1_supplement.665.5
60. Yang HL, Zhou WJ, Lu H, Lei ST, Ha SY, Lai ZZ, et al. Decidual stromal cells promote the differentiation of CD56bright CD16- NK cells by secreting IL-24 in early pregnancy. Am J Reprod Immunol. (2019) 81:e13110. doi: 10.1111/aji.13110
61. Yockey LJ, Iwasaki A. Interferons and proinflammatory cytokines in pregnancy and fetal development. Immunity. (2018) 49:397–412. doi: 10.1016/j.immuni.2018.07.017
62. Fallon PG, Jolin HE, Smith P, Emson CL, Townsend MJ, Fallon R, et al. IL-4 induces characteristic Th2 responses even in the combined absence of IL-5, IL-9, and IL-13. Immunity. (2002) 17:7–17. doi: 10.1016/S1074-7613(02)00332-1
63. Förger F, Villiger PM. Immunological adaptations in pregnancy that modulate rheumatoid arthritis disease activity. Nat Rev Rheumatol. (2020) 16:113–22. doi: 10.1038/s41584-019-0351-2
64. Sasaki Y, Sakai M, Miyazaki S, Higuma S, Shiozaki A, Saito S. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol Hum Reprod. (2004) 10:347–53. doi: 10.1093/molehr/gah044
65. Jasper MJ, Tremellen KP, Robertson SA. Primary unexplained infertility is associated with reduced expression of the T-regulatory cell transcription factor Foxp3 in endometrial tissue. Mol Hum Reprod. (2006) 12:301–8. doi: 10.1093/molehr/gal032
66. Duan B, Morel L. Role of B-1a cells in autoimmunity. Autoimmun Rev. (2006) 5:403–8. doi: 10.1016/j.autrev.2005.10.007
67. Gutierrez G, Gentile T, Miranda S, Margni RA. Asymmetric antibodies: a protective arm in pregnancy. Chem Immunol Allergy. (2005) 89:158–68. doi: 10.1159/000087964
68. Rolle L, Memarzadeh Tehran M, Morell-García A, Raeva Y, Schumacher A, Hartig R, et al. Cutting edge: IL-10-producing regulatory B cells in early human pregnancy. Am J Reprod Immunol. (2013) 70:448–53. doi: 10.1111/aji.12157
69. Jara LJ, Medina G, Saavedra MA. Autoimmune manifestations of infections. Curr Opin Rheumatol. (2018) 30:373–9. doi: 10.1097/BOR.0000000000000505
70. Konstantinov SR, van der Woude CJ, Peppelenbosch MP. Do pregnancy-related changes in the microbiome stimulate innate immunity? Trends Mol Med. (2013) 19:454–9. doi: 10.1016/j.molmed.2013.06.002
71. Nabizadeh E, Jazani NH, Bagheri M, Shahabi S. Association of altered gut microbiota composition with chronic urticaria. Ann Allergy Asthma Immunol. (2017) 119:48–53. doi: 10.1016/j.anai.2017.05.006
72. Lu T, Chen Y, Guo Y, Sun J, Shen W, Yuan M, et al. Altered gut microbiota diversity and composition in chronic urticaria. Dis Mark. (2019) 2019:6417471. doi: 10.1155/2019/6417471
73. Triche EW, Harland KK, Field EH, Rubenstein LM, Saftlas AF. Maternal-fetal HLA sharing and preeclampsia: variation in effects by seminal fluid exposure in a case-control study of nulliparous women in Iowa. J Reprod Immunol. (2014) 101–2:111–9. doi: 10.1016/j.jri.2013.06.004
74. Trombetta AC, Meroni M, Cutolo M. Steroids and autoimmunity. Front Horm Res. (2017) 48:121–32. doi: 10.1159/000452911
75. Piccinni MP, Lombardelli L, Logiodice F, Kullolli O, Parronchi P, Romagnani S. How pregnancy can affect autoimmune diseases progression? Clin Mol Allergy. (2016) 14:11. doi: 10.1186/s12948-016-0048-x
76. Graca L, Cobbolt SP, Waldmann H. Identification of regulatory T cells in tolerated allografts. J Exp Med. (2002) 195:1641–6. doi: 10.1084/jem.20012097
77. Zenclussen AC, Gerlof K, Zenclussen ML, Ritschel S. Zambon Bertoja A, Fest S, et al. Regulatory T cells induce a privileged tolerant microenvironment at the fetal-maternal interface. Eur J Immunol. (2006) 36:82–94. doi: 10.1002/eji.200535428
78. Tham M, Schlör GR, Yerly D, Mueller C, Surbek D, Villiger PM, et al. Reduced pro-inflammatory profile of γδT cells in pregnant patients with rheumatoid arthritis. Arthritis Res Ther. (2016) 18:26. doi: 10.1186/s13075-016-0925-1
79. Østensen M, Förger F, Nelson JL, Schuhmacher A, Hebisch G, Villiger PM. Pregnancy in patients with rheumatic disease: anti-inflammatory cytokines increase in pregnancy and decrease post partum. Ann Rheum Dis. (2005) 64:839–44. doi: 10.1136/ard.2004.029538
80. Zenclussen AC, Kökény G, Thimm O, Sollwedel A, Godo M, Casalis PA, et al. Mechanisms behind flare of renal lupus during murine pregnancy. Reprod Biomed Online. (2008) 17:114–26. doi: 10.1016/S1472-6483(10)60301-X
81. Kökény G, Godó M, Nagy E, Kardos M, Kotsch K, Casalis P, et al. Skin disease is prevented but nephritis is accelerated by multiple pregnancies in autoimmune MRL/LPR mice. Lupus. (2007) 16:465–77. doi: 10.1177/0961203307079456
82. Pfaller B, JoséYepes-Nuñez J, Agache I, Akdis CA, Alsalamah M, Bavbek S, et al. Biologicals in atopic disease in pregnancy: an EAACI position paper. Allergy. (2021) 76:71–89. doi: 10.1111/all.14282
83. Kemmett D, Tidman MJ. The influence of the menstrual cycle and pregnancy on atopic dermatitis. Br J Dermatol. (1991) 125:59–61. doi: 10.1111/j.1365-2133.1991.tb06041.x
84. Cho S, Kim HJ, Oh SH, Park CO, Jung JY, Lee KH. The influence of pregnancy and menstruation on the deterioration of atopic dermatitis symptoms. Ann Dermatol. (2010) 22:180–5. doi: 10.5021/ad.2010.22.2.180
85. Maurer M, Metz M, Bindslev-Jensen C, Bousquet J, Canonica GW, Church MK, et al., Definition, aims, and implementation of GA(2) LEN Urticaria Centers of Reference and Excellence. Allergy. (2016) 71:1210–8. doi: 10.1111/all.12901
86. Sussman G, Abuzakouk M, Bérard F, Canonica W, Oude Elberink H, Giménez-Arnau A, et al. Angioedema in chronic spontaneous urticaria is underdiagnosed and has a substantial impact: analyses from ASSURE-CSU. Allergy. (2018) 73:1724–34. doi: 10.1111/all.13430
87. Maurer M, Costa C, Gimenez Arnau A, Guillet G, Labrador-Horrillo M, Lapeere H, et al. Antihistamine-resistant chronic spontaneous urticaria remainsundertreated: 2-year data from the AWARE study. Clin Exp Allergy. (2020) 50:1166–75. doi: 10.1111/cea.13716
88. Lawlor F. Urticaria and angioedema in pregnancy and lactation. Immunol Allergy Clin North Am. (2014) 34:149–56. doi: 10.1016/j.iac.2013.09.006
89. Pernia S, DeMaagd G. The new pregnancy and lactation labeling rule. Pharm Ther. (2016) 41:713–5.
90. US Food Drug Administration. Two Years of PLLR Implementation. (2017). Available online at: https://www.fda.gov/media/111782/download (accessed March 18, 2020).
91. Etwel F, Faught LH, Rieder MJ, Koren G. The risk of adverse pregnancy outcome after first trimester exposure to H1 antihistamines: a systematic review and meta-analysis. Drug Saf. (2017) 40:121–32. doi: 10.1007/s40264-016-0479-9
92. Hansen C, Desrosiers TA, Wisniewski K, Strickland MJ, Werler MM, Gilboa SM. Use of antihistamine medications during early pregnancy and selected birth defects: The National Birth Defects Prevention Study, 1997-2011. Birth Defects Res. (2020) 112:1234–52. doi: 10.1002/bdr2.1749
93. Li Q, Mitchell AA, Werler MM, Yau WP, Hernández-Díaz S. Assessment of antihistamine use in early pregnancy and birth defects. J Allergy Clin Immunol Pract. (2013) 1:666–74.e1 doi: 10.1016/j.jaip.2013.07.008
94. Einarson A, Bailey B, Jung G, Spizzirri D, Baillie M, Koren G. Prospective controlled study of hydroxyzine and cetirizine in pregnancy. Ann Allergy Asthma Immunol. (1997) 78:183–6. doi: 10.1016/S1081-1206(10)63385-6
95. Kallen B. Use of antihistamine drugs in early pregnancy and delivery outcome. J Matern Fetal Neonatal Med. (2002) 11:146–52. doi: 10.1080/jmf.11.3.146.152
96. Moretti ME, Caprara D, Coutinho CJ, Bar-Oz B, Berkovitch M, Addis A, et al. Fetal safety of loratadine use in the first trimester of pregnancy: a multicenter study. J Allergy Clin Immunol. (2003) 111:479–83. doi: 10.1067/mai.2003.130
97. Diav-Citrin O, Shechtman S, Aharonovich A, Moerman L, Arnon J, Wajnberg R, et al. Pregnancy outcome after gestational exposure to loratadine or antihistamines: a prospective controlled cohort study. J Allergy Clin Immunol. (2003) 111:1239–43. doi: 10.1067/mai.2003.1499
98. Powell RJ, Leech SC, Till S, Huber PA, Nasser SM, Clark AT. British Society for Allergy and Clinical Immunology. BSACI guideline for the management of chronic urticaria and angioedema. Clin Exp Allergy. (2015) 45:547–65. doi: 10.1111/cea.12494
99. Andersson NW, Poulsen HE, Andersen JT. Desloratadine use during pregnancy and risk of adverse fetal outcomes: a Nationwide Cohort Study. J Allergy Clin Immunol Pract. (2020) 8:1598–605. doi: 10.1016/j.jaip.2020.02.017
100. Andersson NW, Torp-Pedersen C, Andersen JT. Association between fexofenadine use during pregnancy and fetal outcomes. JAMA Pediatr. (2020) 174:e201316. doi: 10.1001/jamapediatrics.2020.1316
101. Sarkar M, Koren G, Kalra S, Ying A, Smorlesi C, De Santis M, et al. Montelukast use during pregnancy: a multicentre, prospective, comparative study of infant outcomes. Eur J Clin Pharmacol. (2009) 65:1259–64. doi: 10.1007/s00228-009-0713-9
102. Available, online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/103976s5225lbl.pdf (accessed May 30, 2021).
103. Namazy JA, Blais L, Andrews EB, Scheuerle AE, Cabana MD, Thorp JM, et al. Pregnancy outcomes in the omalizumab pregnancy registry and a disease-matched comparator cohort. J Allergy Clin Immunol. (2020) 145:528–36.e1. doi: 10.1016/j.jaci.2019.05.019
104. Liao SL Yu M, Zhao ZT, Maurer M. Case report: omalizumab for chronic spontaneous urticaria in pregnancy. Front Immunol. (2021) 12:652973. doi: 10.3389/fimmu.2021.652973
105. Palmeira P, Quinello C, Silveira-Lessa AL, Zago CA, Carneiro-Sampaio M. IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol. (2012) 2012:985646. doi: 10.1155/2012/985646
106. Bar Oz B, Hackman R, Einarson T, Koren G. Pregnancy outcome after cyclosporine therapy during pregnancy: a meta-analysis. Transplantation. (2001) 71:1051–5. doi: 10.1097/00007890-200104270-00006
107. Babalola O, Strober BE. Treatment of atopic dermatitis in pregnancy. Dermatol Ther. (2013) 26:293–301. doi: 10.1111/dth.12074
108. Gur C, Diav-Citrin O, Shechtman S, Arnon J, Ornoy A. Pregnancy outcome after first trimester exposure to corticosteroids: a prospective controlled study. Reprod Toxicol. (2004) 18:93–101. doi: 10.1016/j.reprotox.2003.10.007
109. Park-Wyllie L, Mazzotta P, Pastuszak A, Moretti ME, Beique L, Hunnisett L, et al. Birth defects after maternal exposure to corticosteroids: prospective cohort study and meta-analysis of epidemiological studies. Teratology. (2000) 62:385–92. doi: 10.1002/1096-9926(200012)62:6<385::AID-TERA5>3.0.CO;2-Z
110. Skuladottir H, Wilcox AJ, Ma C, Lammer EJ, Rasmussen SA, Werler MM, et al. Corticosteroid use and risk of orofacial clefts. Birth Defects Res A Clin Mol Teratol. (2014) 100:499–506. doi: 10.1002/bdra.23248
111. Roth MM, Cristodor P, Kroumpouzos G. Prurigo, pruritic folliculitis, and atopic eruption of pregnancy: facts and controversies. Clin Dermatol. (2016) 34:392–400. doi: 10.1016/j.clindermatol.2016.02.012
112. Gupta R, High WA, Butler D, Murase JE. Medicolegal aspects of prescribing dermatological medications in pregnancy. Semin Cutan Med Surg. (2013) 32:209–16. doi: 10.12788/j.sder.0034
113. Makol A, Wright K, Amin S. Rheumatoid arthritis and pregnancy: safety considerations in pharmacological management. Drugs. (2011) 71:1973–87. doi: 10.2165/11596240-000000000-00000
114. Shyam RJ, David AK. Asthma, Allergic and Immunologic Diseases During Pregnancy. Cham: Springer (2018).
115. Chi CC, Kirtschig G, Aberer W, Gabbud JP, Lipozenčić J, Kárpáti S, et al. Evidence-based (S3) guideline on topical corticosteroids in pregnancy. Br J Dermatol. (2011) 165:943–52. doi: 10.1111/j.1365-2133.2011.10513.x
116. O'Brien TE. Excretion of drugs in human milk. Am J Hosp Pharm. (1974) 31:844–54. doi: 10.1093/ajhp/31.9.844
117. Hilbert J, Radwanski E, Affrime MB, Perentesis G, Symchowicz S, Zampaglione N. Excretion of loratadine in human breast milk. J Clin Pharmacol. (1988) 28:234–9. doi: 10.1002/j.1552-4604.1988.tb03138.x
118. So M, Bozzo P, Inoue M, Einarson A. Safety of antihistamines during pregnancy and lactation. Can Fam Phys. (2010) 56:427–9.
119. Lucas BD Jr, Purdy CY, Scarim SK, Benjamin S, Abel SR, Hilleman DE. Terfenadine pharmacokinetics in breast milk in lactating women. Clin Pharmacol Ther. (1995) 57:398–402. doi: 10.1016/0009-9236(95)90208-2
120. Moretti ME, Liau-Chu M, Taddio A, Ito S, Koren G. Adverse events in breastfed infants exposed to antihistamines in maternal milk. ReprodToxicol. (1995) 9:588. doi: 10.1016/0890-6238(95)02010-1
121. Committee on Drugs. American Academy of Pediatrics Committee on Drugs Transfer of drugs and other chemicals into human milk. Pediatrics. (2001) 108:776–89. doi: 10.1542/peds.108.3.776
122. Elliott AB. Chakravarty EF. Immunosuppressive medications during pregnancy and lactation in women with autoimmune diseases. Womens Health. (2010) 6:431–40. doi: 10.2217/WHE.10.24
123. Ost L, Wettrell G, Bjorkhem I, Rane A. Prednisolone excretion in human milk. J Pediatr. (1985) 106:1008–11. doi: 10.1016/S0022-3476(85)80259-6
124. Drugs and Lactation Database (LactMed). Montelukast. Bethesda, MD: National Library of Medicine (2006).
125. Middleton PG, Gade EJ, Aguilera C, MacKillop L, Button BM, Coleman C, et al. ERS/TSANZ Task Force Statement on the management of reproduction and pregnancy in women with airways diseases. Eur Respir J. (2020) 55:1901208. doi: 10.1183/13993003.01208-2019
126. Drugs and Lactation Database (LactMed). Bethesda, MD: National Library of Medicine; Omalizumab (2006). Available from: https://www.ncbi.nlm.nih.gov/books/NBK501801/ (accessed April 19, 2021).
127. Majou D, Moreira B, Martin C, Chhun S, Treluyer JM, Tsatsaris V, et al. Safety of omalizumab during pregnancy and breast-feeding with assessment of placental transfer: a case report. Allergy Asthma Immunol Res. (2021) 13:515–6. doi: 10.4168/aair.2021.13.3.515
Keywords: urticaria, pregnancy, lactation, treatment, autoimmunity, immunological changes, mast cells, hormones
Citation: Kocatürk E, Podder I, Zenclussen AC, Kasperska Zajac A, Elieh-Ali-Komi D, Church MK and Maurer M (2022) Urticaria in Pregnancy and Lactation. Front. Allergy 3:892673. doi: 10.3389/falgy.2022.892673
Received: 09 March 2022; Accepted: 09 June 2022;
Published: 07 July 2022.
Edited by:
Carla Pagliari, University of São Paulo, BrazilReviewed by:
Ignacio Jáuregui, Cruces University Hospital, SpainCopyright © 2022 Kocatürk, Podder, Zenclussen, Kasperska Zajac, Elieh-Ali-Komi, Church and Maurer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Emek Kocatürk, ZWtvY2F0dXJrQGt1LmVkdS50cg==
†ORCID: Emek Kocatürk orcid.org/0000-0003-2801-0959
Indrashis Podder orcid.org/0000-0002-9589-083X
Ana C. Zenclussen orcid.org/0000-0003-3544-4552
Alicja Kasperska Zajac orcid.org/0000-0002-2000-0070
Daniel Elieh-Ali-Komi orcid.org/0000-0003-0546-5280
Martin K. Church orcid.org/0000-0002-1639-9410
Marcus Maurer orcid.org/0000-0002-4121-481X
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.