- 1Department of Respiratory and Sleep Medicine, Royal Prince Alfred Hospital, Camperdown, NSW, Australia
- 2Faculty of Medicine and Health, University of Sydney, Camperdown, NSW, Australia
- 3Woolcock Institute of Medical Research, Glebe, NSW, Australia
- 4Department of Respiratory and Sleep Medicine, Westmead Hospital, Westmead, NSW, Australia
- 5Unit for Medicine and Clinical Research, Pulmonary Division, Kuopio University Hospital, Kuopio, Finland
- 6Faculty of Health Sciences, School of Medicine, Institute of Clinical Sciences, University of Eastern Finland, Kuopio, Finland
- 7Department of Respiratory and Sleep Medicine, John Hunter Hospital, New Lambton, NSW, Australia
- 8School of Medicine and Public Health, University of Newcastle, Callaghan, NSW, Australia
Background: Airway hyperresponsiveness (AHR) is a key pathophysiological feature of asthma and causes exercise-induced bronchoconstriction (EIB). Indirect bronchial provocation tests (BPTs) (e.g., exercise, mannitol) aid to diagnose asthma and identify EIB. Daily inhaled corticosteroids (ICS) can abolish AHR caused by indirect stimuli. Where strenuous physical exertion is integral to an occupation, identification of those at risk of EIB is important and documentation of inhibition of AHR with ICS is required before recruitment.
Methods/Objectives: A retrospective analysis was performed on 155 potential recruits with AHR to mannitol who underwent follow-up assessment after daily ICS treatment to determine the proportion that can abolish AHR using ICS and to determine any predictors of the persistence of AHR.
Results: Airway hyperresponsiveness was abolished in the majority (84%, n = 130) over the treatment period (mean ± SD 143 ± 72days), and it was defined as the provoking dose of mannitol to cause a 15% fall in FEV1 (cumulative inhaled dose of mannitol to cause 15% fall in FEV1, PD15) improved from (GeoMean) 183 to 521 mg. Compared with recruits in whom AHR was abolished with daily ICS (i.e., no 15% fall in FEV1 to the maximum cumulative dose of mannitol of 635 mg), in those where AHR remained (16%, n = 25), baseline AHR was more severe (PD15: 85 mg vs. 213 mg, P < 0.001), baseline FEV1% was lower (89 vs. 96%; 95%CI:2–12, P=0.004), and they had a longer follow-up duration (180 vs. 136 days; 13–74, P = 0.006). Baseline FEV1% (adjusted odds ratio 0.85; 95%CI:0.77–0.93), FEV1/FVC (0.78; 0.67–0.90), FEF25−75% (1.15; 1.06–1.25), and airway reactivity to mannitol (%Fall/cumulative dose of mannitol multiplied by 100) (1.07; 1.03–1.11) predicted AHR remaining after daily ICS.
Conclusion: Airway hyperresponsiveness to mannitol can be abolished after 20 weeks of daily treatment with ICS. Inhibition of AHR is likely due to attenuation of airway inflammation in response to ICS treatment. Increased airway reactivity and lower spirometry variables predicted the persistence of AHR. Thus, those with a slower response to daily ICS on AHR can potentially be identified at the commencement of monitoring ICS using inhaled mannitol.
Plain Language Summary
Airway hyperresponsiveness (AHR) is a key feature of asthma. In certain occupational settings, such as the military, AHR to mannitol is used to assess asthma and the risk of exercise induced-bronchoconstriction (EIB). Reduced airway calibre in the form of lower normal spirometry and more significant AHR (moderate to severe AHR to mannitol) predict a slower response to ICS treatment if the goal is to abolish AHR. Therefore, assessing AHR to mannitol along with spirometry prior to treatment may be useful to help predict the speed one can benefit from using daily ICS therapy, and the likelihood to abolish AHR.
Introduction
Airway hyperresponsiveness (AHR) is a key pathophysiological feature of asthma and is responsible for exercise-induced bronchoconstriction (EIB) (1). EIB commonly occurs in those where asthma is active and is characterised by bronchospasm following vigorous exercise. Indirect bronchial provocation tests (BPT) (e.g., exercise, eucapnic voluntary hyperpnoea, inhaled mannitol) can be performed to diagnose asthma and to identify EIB (2). Indirect BPT provokes bronchoconstriction by causing a release of inflammatory mediators from mast cells and eosinophils in the airways (2). Daily treatment with inhaled corticosteroids (ICS) decreases the number of inflammatory cells in the airway (3). AHR to mannitol can be attenuated using daily ICS treatment over 6–9 weeks of therapy (4). A reduction in the number of inflammatory cells may be reflected by attenuation of AHR, therefore AHR may be useful to monitor therapeutic response to daily ICS (5). Loss of AHR may also be a potential surrogate marker for EIB inhibition following daily treatment with ICS. The optimal goal of treatment when using indirect BPT to monitor the therapeutic response of using ICS treatment is to abolish AHR (6). This is particularly relevant for people with asthma or those with EIB who may work in occupations that involve vigorous exercise, thus EIB needs to be inhibited (7).
Strenuous physical exertion is integral in occupations such as in the military, police, and firefighters. Screening of individuals prior to recruitment into such professions using indirect BPT has been shown to be useful to identify those with AHR and at risk of EIB (8, 9). AHR may prevent optimal exercise performance, require the need for acute rescue therapy, and in very rare cases induce a fatal attack of asthma (10). Due to these risks associated with AHR, the objective identification of AHR using indirect BPT is required by both the Australian Defence Force (ADF) and the New South Wales Police Force (NSWPF). Candidates or “recruits” applying for positions in the ADF and NSWPF with a history of asthma are referred to pulmonary function laboratories (PFL) as part of a medical assessment for investigation of possible AHR using the mannitol BPT. Where significant AHR is demonstrated, ICS naïve recruits are prescribed ICS either alone or in combination with a long-acting beta-agonist (LABA). Any recruits already taking ICS have their dose of ICS either maintained or adjusted. Recruits with asthma may be accepted into the ADF and NSWPF if they can demonstrate resolution of AHR to mannitol following daily treatment with ICS. However, while AHR can be attenuated in some individuals, AHR can persist despite therapy. It is not known if there are characteristics that predict the persistence of AHR to mannitol.
We performed a retrospective analysis of occupational recruits having AHR to mannitol monitored in the presence of daily ICS. We also determined the proportions of recruits in whom AHR to mannitol was abolished and if baseline characteristics might determine persistence of AHR.
Methods
Participants
Data were only included from individuals seeking employment (i.e., recruits) in the ADF or NSWPF. Data were included in the analysis where an initial mannitol BPT was positive and where reassessment of mannitol BPT occurred on one or more occasions within a 12-month period while using daily treatment with ICS. The majority of participants were talking low dose ICS which was a part of the ADF recruitment policy. This is not the policy of the NSWPF where standard doses of ICS in combination with a LABA can be used. Where more than one reassessment occurred within a 12-month period, the follow-up data were reported from the visit with the longest duration. Data were excluded where the reassessment period was >12-months or ICS treatment was not used.
Study Design
A retrospective review of the patient databases of the PFL at Westmead and Royal Prince Alfred Hospitals from 2005 to 2018 was performed. A review of each database was approved by the Western Sydney and Sydney Local Health District Human Research Ethics Committees, respectively.
Spirometry and Mannitol Bronchial Provocation Test
Spirometry was performed immediately before the mannitol BPT according to American Thoracic Society and European Respiratory Society criteria (11), and predicted values were determined by comparison to reference values (12). The mannitol BPT (Aridol®/Osmohale® Pharmaxis Ltd, Frenchs Forest, NSW, Australia) was performed according to the standardised test protocol (13).
The provoking dose of mannitol causing a 15% fall in FEV1 (PD15) is a measure of airway sensitivity to mannitol. The response is classified as severe if the PD15 is ≤35 mg, moderate if >35 mg to ≤155 mg, and mild if >155 mg to 635 mg. If the fall in FEV1 is <15% (no PD15), then the test is negative, indicating the absence of AHR to mannitol.
Airway sensitivity to mannitol can also be determined using the dose that provokes a fall in FEV1 of 10% (PD10). The PD10 is associated with mild EIB and therefore the PD10 was also calculated for those that had a final fall in FEV1 of between 10 and 15% (14, 15).
The degree of airway reactivity to mannitol (percent fall in FEV1 divided by the final cumulative dose of mannitol), known as the response dose ratio (RDR), was also assessed. To simplify interpretation, the RDR was multiplied by 100 (RDR100). Complete resolution of AHR would be considered to have been achieved following effective ICS treatment if the RDR100 < 1, as this threshold represents the upper limit of reactivity and a flat dose response curve that has been observed in healthy non-asthmatic subjects (16).
A post hoc subgroup analysis was performed to assess the response to ICS treatment in recruits with moderate to severe AHR to mannitol at baseline (PD15 ≤155 mg), as this degree of AHR has been shown to be associated with a greater degree of airway inflammation (17, 18). We did this in order to determine if the degree of AHR to mannitol influences response to ICS treatment.
Statistics
Descriptive summaries and spirometry data are reported as mean and SD or number and percentage of the group. PD15, PD10, and RDR100 results were log transformed (base 2) for analysis and presented as the geometric mean (Gmean) and 95% CI. Where a 0% fall in FEV1 occurred, a fall of 0.1% was imputed to enable the calculation of RDR100. A PD15 of 635 mg was imputed when a PD15 did not occur on follow-up (interpreted as the resolution of AHR). Groups were generated based on follow-up PD15 (AHR abolished, noPD15 vs. persistent AHR, PD15 < 635 mg), follow-up PD10 (noPD10 vs. PD10), and RDR100 at follow up (<1 vs. >1). Independent samples t-tests were used to assess differences between groups. Paired samples t-tests were used for within group change from baseline to the follow-up visit. Spearman's correlation was used to assess relationships between variables at baseline and at follow-up and to assess relationships between the improvements in variables at the follow-up visit. Chi-squared and Fishers exact tests were used to compare proportions within and between groups.
Receiver operating characteristic (ROC) curves were created for baseline variables (PD15, RDR100, FEV1 %predicted, FEV1/FVC%, and FEF25−75% %predicted) to evaluate potential predictors of persistent AHR at follow up ICS treatment visit. The area under the curve (AUC) was calculated and “optimal” thresholds were reported, corresponding to the point on the ROC curve closest to the upper left corner. To generate a prediction model for AHR remaining on follow-up, the first univariate binary logistic regression was performed on baseline variables to determine potential predictors. Variables suggestive of an association (P < 0.1) with persistent AHR were considered for multivariate logistic regression analysis, performed on these variables with a backward stepwise process.
SPSS statistics version 26 was used for statistical analysis (IBM, Chicago, IL, USA).
Results
A total of 155 recruits with at least two visits over an average of 143 days [20.4 weeks] were analysed (Table 1). The majority were male [83%], in early adulthood [22 years ± 5], and were seeking employment in the ADF [72%] as opposed to applying for the NSWPF. Only one quarter [24%] were taking ICS prior to their baseline visit (Table 1). During the follow-up period, the majority were taking ICS alone [86%] with the remainder taking ICS with LABA in combination. A sub-analysis of those taking LABA in combination with ICS vs ICS alone found there were no differences in demographics or lung function characteristics between the groups and this also included no differences in AHR. However the numbers taking combination LABA/ICS (24%) were lower than those taking ICS alone (86%) (Table 1). At the baseline visit, group mean values for spirometry [FEV1 95% predicted ± 11] were within normal reference limits and the geometric mean (Gmean) PD15 represented mild AHR [Gmean (95%CI) PD15 184 mg (159 to 212)] (Table 1).
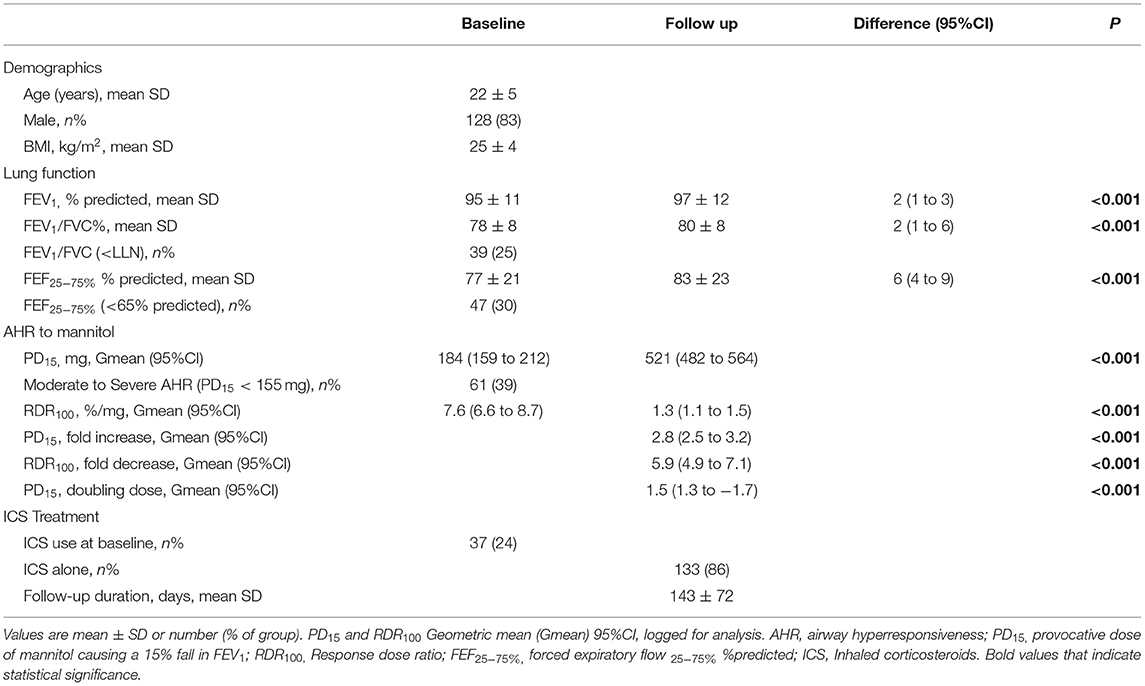
Table 1. Total cohort (n = 155): Baseline and ICS follow up: Demographics, lung function, AHR severity, ICS therapy at baseline visit, ICS type and duration of follow up period.
Resolution of AHR to Mannitol
Resolution of AHR to mannitol (i.e., noPD15) was achieved in most recruits [n = 130/155 (84%)] with a mean follow-up period of 136 days (Table 2; Figure 1). For the total group, with daily treatment with ICS, there was a 3.0-fold increase in PD15 (95%CI 2.6 to 3.4) equating to an increase of 1.6 doubling doses (DD) (95%CI: 1.4 to 1.8) (Table 1). However, nearly one-third [n = 42/130 (32%)] of those that abolished their PD15 had a PD10 at the follow-up visit (Table 3). Over one-third [n = 56 (36%)] of all recruits showed complete suppression of the airway response to mannitol or a flat dose response curve, defined by an RDR100 <1 at the end of the follow-up period (i.e., these subjects neither had a PD10 nor PD15) (Table 4).
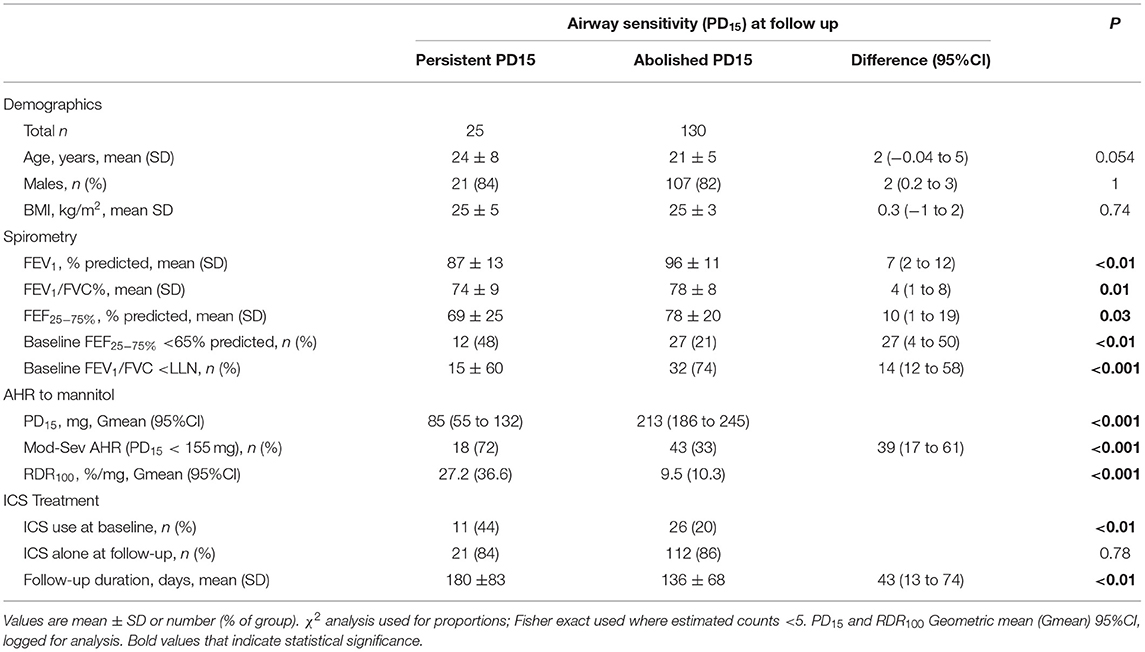
Table 2. Baseline demographics, spirometry, airway sensitivity and reactivity ICS therapy at baseline visit, ICS type and duration of follow up period; Grouped based on airway sensitivity (PD15) on final ICS treatment visit.
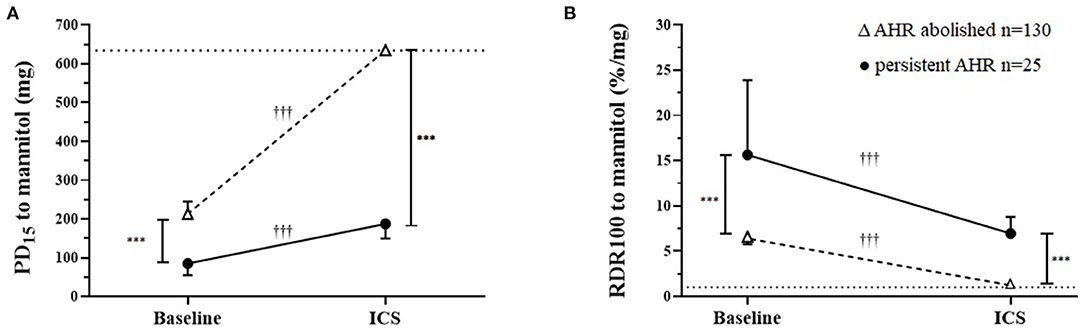
Figure 1. Airway sensitivity and reactivity to mannitol at baseline and after a mean of 20 weeks daily inhaled corticosteroids (ICS) in those where airway hyperresponsiveness (AHR) was either abolished or was persistent. (A) Cumulative inhaled provoking dose of mannitol to cause 15% fall in FEV1 (PD15) baseline and follow-up ICS treatment geometric mean and 95% CI. The dotted horizontal line represents the total cumulative dose (635 mg) the upper limit indicating the mildest AHR. (B) Baseline and follow-up ICS treatment for response dose ratio (RDR100) geometric mean and 95% CI. The dashed line represents the mean response for healthy non-asthmatics who do not have AHR to mannitol. †Within/ *Between group differences P-value: <0.05* †, <0.1** ††, <0.001*** †††.
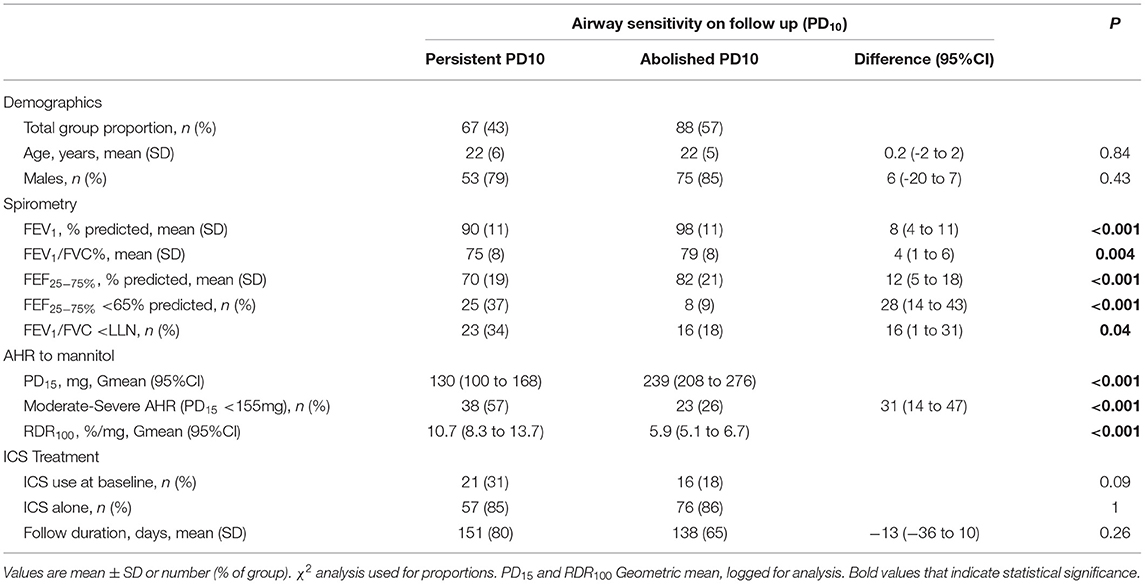
Table 3. Recruits baseline demographics, spirometry, AHR and ICS treatment; Grouped based on airway sensitivity (PD10) at follow-up ICS treatment visit.
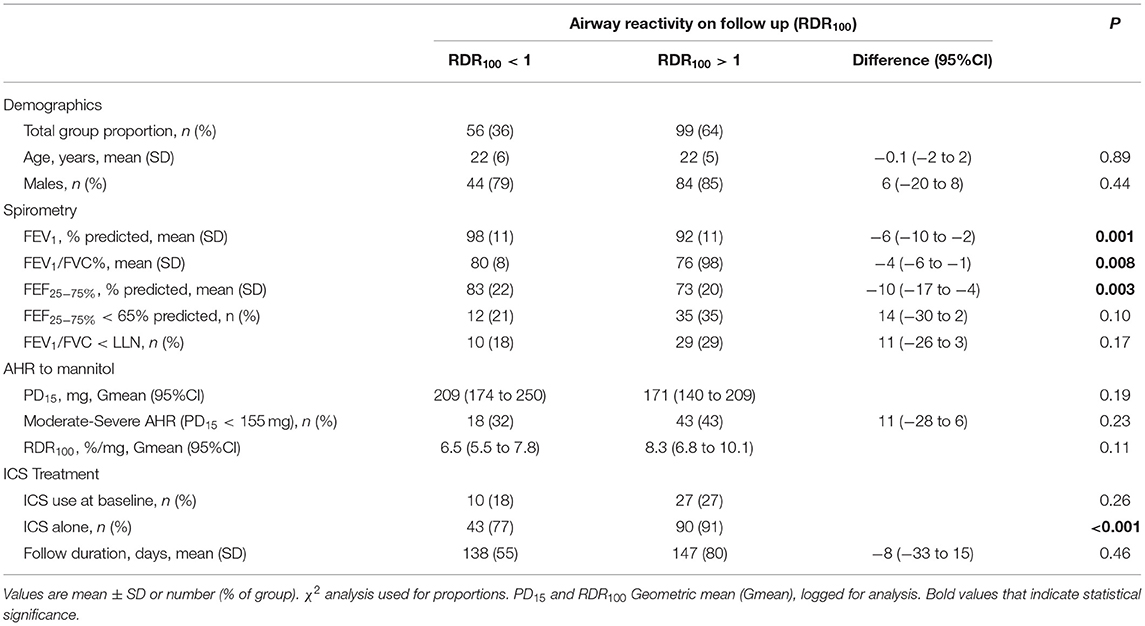
Table 4. Recruits baseline demographics, spirometry, AHR and ICS treatment; Grouped based on airway reactivity (RDR100) at follow-up ICS treatment visit.
Persistent AHR to Mannitol
In the group for whom AHR persisted in the presence of daily ICS, the mean baseline AHR severity was in the moderate range [PD15 Gmean (95% CI) 85 mg (55 to 132)], and these subjects still showed modest but statistically significant improvements in AHR [PD15 2.2-fold increase, P < 0.001] with ICS treatment (Table 2; Figure 2). These recruits were also more likely to be taking ICS prior to occupational assessment and were on ICS therapy for a mean of 43 days longer during the assessment compared with recruits in whom AHR was abolished (Table 2). In the group where AHR persisted, there was a greater proportion of individuals with an abnormal FEF25−75%, FEV1/FVC below the lower limit of normal (LLN), and moderate to severe AHR (PD15 <155 mg) at the baseline visit (Table 2).
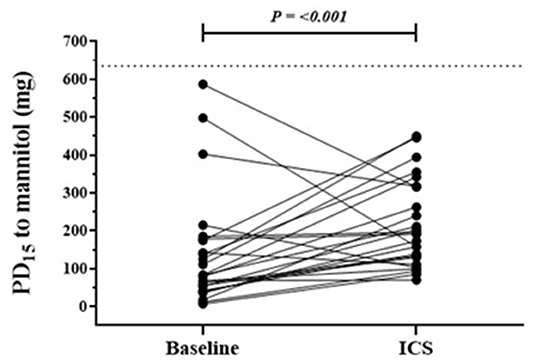
Figure 2. Individual response in those with persistent AHR to mannitol following a mean of 20 weeks of daily ICS. Baseline and follow-up individual PD15 to mannitol results in the group where AHR remained at ICS follow-up visit. n = 25, follow up mean duration 180 days. The dotted horizontal line represents the total cumulative dose (635 mg) of inhaled mannitol during bronchial provocation and the upper limit indicates the mildest AHR.
Response to Daily Treatment With ICS–Spirometry
There were improvements in spirometry with significant changes in FEV1, FEV1/FVC, and FEF25−75% following daily treatment with ICS over the assessment period for the total group (Table 1). Although the mean baseline spirometry values for both groups (PD15 and noPD15) were within normal reference limits, statistically significant improvements were observed in all spirometry variables in the group in which AHR was abolished (Figure 3). In contrast, there were no improvements in any spirometry variables in the group whose AHR persisted.
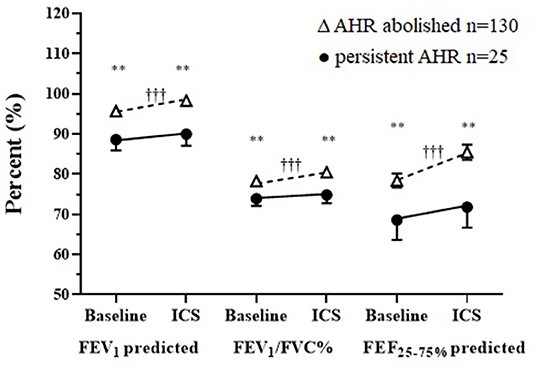
Figure 3. Baseline and follow-up treatment spirometry results following a mean of 20 weeks daily ICS in those where AHR was either abolished or was persistent for FEV1 and FEF25−75% % predicted, FEV1/FVC absolute % mean ± SD. †Within/*Between group differences at each visit. P-value: <0.05* †, <0.1** ††, <0.001*** †††.
Association Between Spirometry Variables and AHR to Mannitol
There was a very weak but statistically significant relationship between baseline PD15 and FEV1 and with FEF25−75% (Table 5). There was a weak but significant relationship between the improvement in RDR100 with the improvement in FEV1, FEV1/FVC, and FEF25−75% (Table 5).
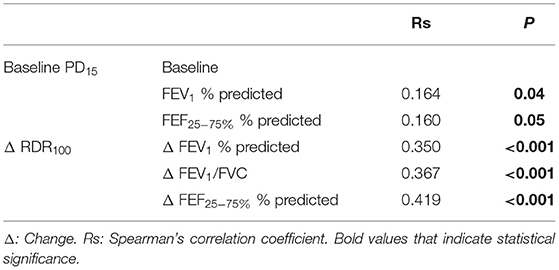
Table 5. Spearman's correlation between change in spirometry and change in RDR100 from baseline to follow-up ICS treatment visit and between baseline PD15 and baseline spirometry variables.
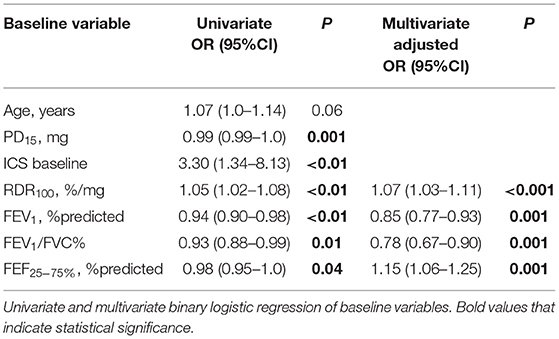
Predictors of Persistent AHR to Mannitol
Receiver operating characteristic analysis was used to evaluate the baseline lung function variables as predictors of persistent PD15 with ICS treatment. The AUC for baseline measurements of FEV1, FEV1/FVC, FEF25−75%, PD15, and RDR100 were 0.68, 0.66, 0.67, 0.75 and 0.76, respectively. The optimal thresholds that predicted AHR persisting in this population were <91% for FEV1, <75% for FEV1/FVC, <64% for FEF25−75%, <142 mg for PD15, and >8.6%/mg for RDR100.
Univariate logistic regression analysis of baseline variables identified age, ICS treatment at baseline, FEV1, FEV1/FVC, FEF25−75%, PD15, and RDR100 as possible predictors (p < 0.1) of persistent AHR on follow-up (Table 6). Increased baseline RDR100 and FEF25−75% and lower baseline FEV1 and FEV1/FVC remained significant independent predictors in the final multivariate model (Table 6) for which the AUC was 0.85 (Figure 4). The optimal threshold, corresponding to an estimated probability of 21%, yielded a sensitivity of 76%, specificity of 87%, a positive predictive value (PPV) of 53%, a negative predictive value (NPV) of 95%, and with a diagnostic odds ratio (DOR) of 21 to predict the likelihood of persistent AHR in a recruit after 20 weeks of daily ICS treatment. The estimated probability is calculated from baseline values of predictor variables from the logistic regression model as below:
where
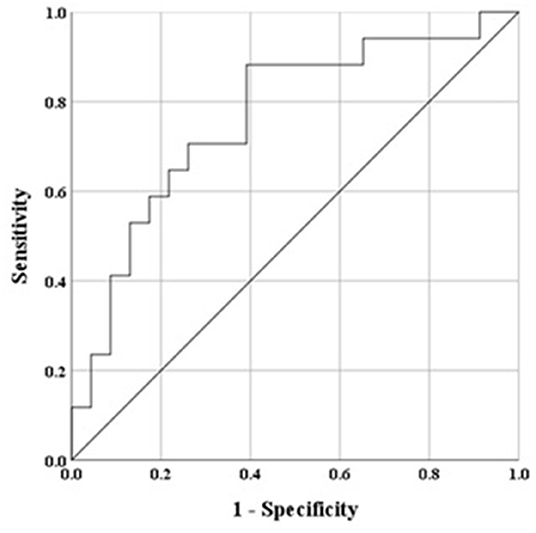
Figure 4. Receiver operator characteristic (ROC) based on baseline variables to predict persistent AHR to mannitol, ROC curve final multivariate model, area under the curve (AUC) for the final model was 0.851, whereby a value of 0.5 indicates chance model performance and 1.0 indicates perfect performance. The model determined lower FEV1% predicted and FEV1/FVC, and higher FEF25−75% % predicted and RDR100%/mg baseline variables as predictors of persistence of AHR to mannitol after 20 weeks daily treatment with ICS.
Treatment Response in Moderate-Severe AHR to Mannitol (PD15 < 155 mg)
The group of recruits with moderate-severe AHR at baseline [PD15 <155 mg, n = 61/155, 39%] made up 72% of those with persistent AHR. Their spirometry measurements were significantly lower than the group with mild AHR to mannitol. Within the moderate-severe group in those in whom AHR persisted, baseline AHR was more severe and spirometry variables were lower than in those where AHR was abolished (Table 7). In those where AHR persisted, a greater proportion had an abnormal FEF25−75% and an FEV1/FVC below the LLN. They were also more likely to be using ICS at the baseline visit and had a longer follow-up duration [50 days (95%CI 11–89), p < 0.05] (Table 7).
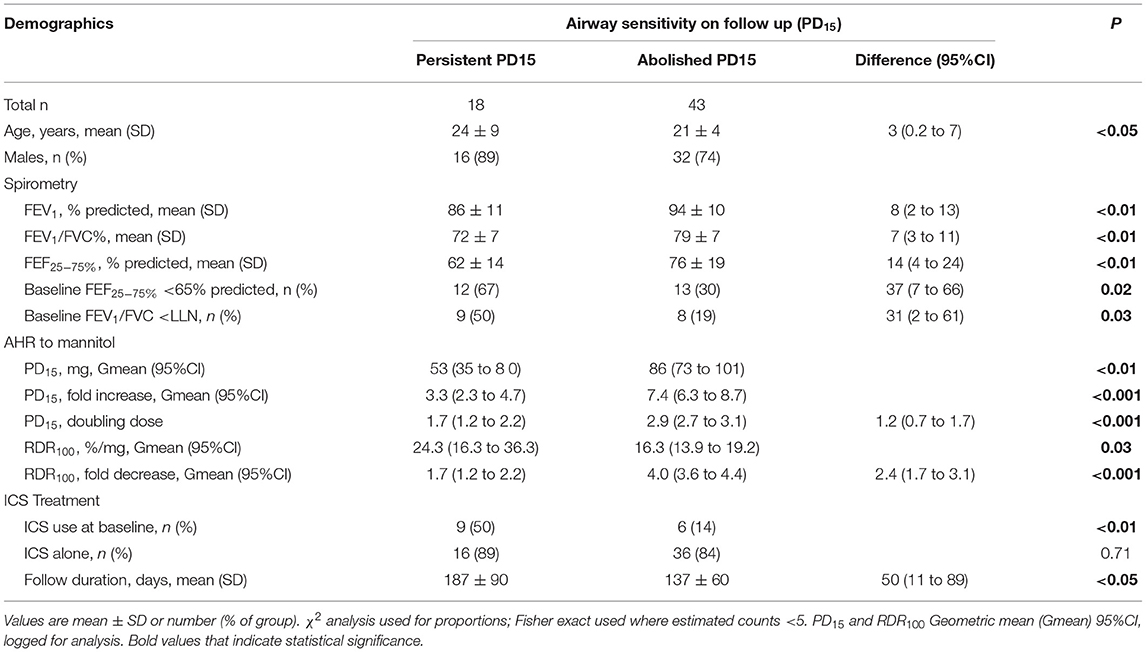
Table 7. Moderate to Severe AHR (PD15 < 155 mg) at baseline; demographics and baseline lung function, AHR and ICS treatment, type, and duration.
Discussion
In this population of occupational recruits with a past or current history of asthma and AHR to mannitol, AHR was abolished in the majority following approximately 4 months of daily treatment with ICS. Even in this population with overall normal lung function, small but significant improvements in spirometry were still observed. Improvements in spirometry were weakly associated with the attenuation of AHR to mannitol. In a small proportion of the study population, AHR persisted following daily ICS treatment. However, this group achieved a modest improvement in responsiveness to mannitol while still having significant AHR (PD15 <635 mg) suggesting active EIB would still be present. This group with persistent AHR had more severe airway sensitivity (lower PD15) and reactivity (higher RDR100) to mannitol at baseline than those in whom AHR was abolished at follow-up. They also had significantly lower lung function at baseline; however, spirometry parameters did not improve. Further, this group was more likely to already be using ICS at the baseline assessment and had a longer follow-up duration, hence a longer treatment period. With daily ICS treatment, approximately one-third of recruits achieved no AHR (i.e., a flat dose response curve) and this airway reactivity resolved similar to what is seen in normal non-asthmatic subjects who have no AHR. Those that achieved a flat dose response curve with ICS treatment had the mildest AHR severity and most normal lung function at baseline.
Airway hyperresponsiveness to mannitol has been shown to be a useful screening tool used in occupational assessments of those at risk of asthma (8, 9). It has been demonstrated in people with mild asthma symptoms but no clinical asthma diagnosis. AHR to mannitol was 1.4 times more likely to identify AHR compared to a laboratory exercise test (19). Underreporting of asthma symptoms is common in similar occupational cohorts (8, 9). Therefore, the use of a reliable objective marker is preferable to the use of other more accessible clinical indicators of disease activity, such as symptoms and spirometry. The identification and subsequent resolution of AHR with ICS treatment enable the enlistment of persons with a history of asthma, reducing the risks of EIB during their employment.
These results, in a population being assessed for asthma or EIB as part of an occupational assessment, have replicated findings in prior clinical trials that have assessed the effectiveness of ICS on AHR to mannitol in people with confirmed asthma (4, 6, 20). Furthermore, AHR to mannitol identifies those with active asthma that can be treated effectively with low daily doses of ICS (20, 21). Thus, the ADF policy requires recruits to be treated with ICS alone at a maximum dose equivalent to 200 μg/day of beclomethasone for a 3-month trial period before reassessment (22). We were not able to identify the actual dose of daily ICS for all patients. However, as the majority of our cohort were ADF recruits and on low doses of ICS, these data support the findings of the efficacy of low daily doses of ICS that are useful to attenuate AHR to mannitol (20, 21). Overall, the group studied were determined to have clinically mild or intermittent asthma during their medical assessments carried out by occupational physicians and their spirometry was normal to near normal. However, many still had demonstrable AHR to mannitol, a feature identifying those whose asthma was currently active who would benefit from treatment with ICS. While significant improvements in spirometry were seen, these changes alone would not necessarily represent a discernible clinical benefit due to the normality of the measurements at baseline. Consistent with previous studies, we found only a weak relationship between AHR to mannitol and spirometry prior to therapy, and between changes in airway reactivity and spirometry with ICS treatment (4, 6). These findings reinforce the limitations of using spirometry to monitor ICS. It is expected that over 90% of the overall improvement in airway calibre with the commencement of ICS occurs over the first 2 weeks of treatment in people with asthma who have AHR to an indirect test (23). However, these data also highlight that if the patient is identified as having AHR by an indirect test, they have active airway inflammation, and thus further and potentially meaningful improvements in airway calibre are possible following many weeks of daily treatment, even if baseline spirometry is normal.
While AHR to mannitol is useful at predicting clinical response to ICS, a small proportion of recruits were observed to have persistent AHR despite daily ICS treatment. We expected that adherence to ICS treatment would have been high in our cohort as they were motivated by the goal of recruitment into their desired occupation. However, poor adherence may still have been a factor for some of these recruits, and we could not objectively document adherence to ICS in this clinical setting. However, we investigated variables that may predict the likelihood of slower or reduced ICS responsiveness. The data suggests that a slower improvement in AHR to mannitol in response to daily ICS is more likely if there is evidence of more significant airway obstruction before treatment assessment, and AHR to mannitol is in the moderate to severe range. These findings suggest these patients may have more active airway inflammation and possibly established airway remodelling, considering the lack of observed improvement in airway calibre. In people with asthma who are not taking regular ICS, moderate to severe AHR to mannitol (≤155 mg) is associated with significant sputum eosinophilia (median 8%), compared to the absence of sputum eosinophils (median <2%) in those with mild AHR (>155 mg) (2, 17, 24). These findings suggests that those with moderate to severe AHR to mannitol have a greater inflammatory contribution to their airway pathology. This increased inflammation may contribute to the slow initial response to daily treatment with ICS observed in the recruits with more severe AHR.
The number of recruits with AHR (PD15) persisting in this group was small relative to those in whom AHR was abolished. These individuals may be less steroid responsive, as they had a longer treatment period and may potentially need more time on ICS to inhibit their AHR more effectively (6). However, compared to those in whom AHR was abolished, a larger proportion of those where AHR persisted were already taking ICS at the baseline visit, supporting the suggestion of a more established or more active disease. Considering this, they were also a group with a higher proportion with an abnormal FEF25−75% and FEV1/FVC at baseline. This may indicate the presence of a more fixed component of airway obstruction where it was also observed there was no improvement in spirometry, compared to those in whom AHR was abolished.
While most recruits abolished AHR to mannitol (noPD15), approximately one-third (32%) of the recruits still had a PD10 on follow-up. A PD10 has been found to be associated with the presence of EIB (14). Therefore, recruits with a PD10 alone following ICS may still be at risk of EIB, though it is likely to be mild. To further reduce the risk of EIB, resolving the PD10 to mannitol through extending the monitoring period may be desirable and achievable in occupational recruits. Flattening the dose response curve and suppressing airway reactivity (i.e., RDR100 <1) to a level of responsiveness seen in people without asthma was achieved in over a third (36%) of recruits (16). Demonstrating no AHR to mannitol is achievable with daily ICS treatment and maybe a more desirable goal of treatment, as the risk of EIB while performing occupational duties would be greatly reduced (25).
We performed a logistic regression model that predicted the liklihood of the persistence of AHR in this predominately young adult male population. For spirometry variables, lower measurements for FEV1 and FEV1/FVC increased the likelihood of AHR persisting. Greater airway reactivity (higher RDR100) also increased the likelihood of AHR persisting. However, in the final multivariate model, a lower FEF25−75% reduced the likelihood of AHR persisting. Importantly, the probability of this model predicting response would likely change depending on the pre-test probability of the population to be tested. This model should be investigated further to determine if it can be validated and extrapolated to other populations, such as those with active asthma. Such a predictive capacity may assist with choosing different treatment regimens to increase the success of resolving AHR in recruits.
The main limitation of this study is that the data were collected retrospectively. Retrospective studies can introduce bias and while like other uncontrolled studies investigating ICS on AHR to indirect stimuli (4, 6, 20, 21, 25) this remains a limitation. In addition, adherence to ICS was not monitored objectively and future studies could include more objective markers of daily ICS adherence using electronic adherence monitors. Furthermore we were not able to collect additional clinical information in these recruits such as asthma symptoms, asthma control, allergic status, disease duration or prior therapy. This would be important to obtain while considering that recruits may not be willing to fully admit symptom burden due to the desire to enter a chosen occupation (8, 9). However, this is a real-world study that has produced similar findings to previous clinical trials, validating findings in a large clinical population showing that AHR to mannitol can be attenuated and abolished with daily ICS treatment (4, 6, 21). Further studies are needed to investigate if routine monitoring of ICS using AHR to mannitol is useful to achieve sustained clinical benefits in asthma symptom control. Further, it would be useful to establish if the resolution of AHR to mannitol represents the attenuation of asthma pathophysiology. While the resolution of AHR to mannitol increases the likelihood of resolution of EIB, future studies may be useful to confirm this likelihood.
Airway hyperresponsiveness to mannitol can be abolished with 20 weeks of daily treatment with ICS, in recruits who are seeking employment in careers that require strenuous physical exertion. This was achieved in the majority with low daily doses of ICS alone without LABA. Reduced pre-treatment spirometry variables and increased airway reactivity to mannitol predict the likelihood of an incomplete or slower response to daily ICS. Assessing AHR to mannitol with daily ICS may provide a useful objective marker to identify clinically meaningful attenuation of airway inflammation.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Western Sydney and Sydney Local Health District Human Research Ethics Committees. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
CL contributed to trial design, data collection, preparation and analysis, manuscript preparation, and review. KW contributed to trial design, oversight of analysis, manuscript preparation, and review. CP contributed to data collection and manuscript review. HK contributed to trial design and manuscript review. JB contributed by supervising all aspects of the research, including the study concept and trial design, oversight of analysis, and manuscript preparation and review. All authors contributed to the article and approved the submitted version.
Conflict of Interest
JB receives a 10% share of royalties for the sale of Aridol after distribution to Royal Prince Alfred Hospital in countries outside Australia and owns shares in Pharmaxis Ltd. CP owns shares in Pharmaxis Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Dr. S. D. Anderson and Dr. K. Tonga for their assistance in proofreading the manuscript.
Abbreviations
ADF, Australian Defence Force; AHR, airway hyperresponsiveness; AUC, Area under the curve; BPT, Bronchial Provocation Test; EIB, Exercise induced bronchoconstriction; ICS, Inhaled Corticosteroids; LABA, Long-acting beta agonists; LLN, Lower limit of normal; NSWPF, New South Wales Police Force; PD10, Cumulative inhaled dose of mannitol to cause 10% fall in FEV1; PD15, Cumulative inhaled dose of mannitol to cause 15% fall in FEV1; PFL, Pulmonary Function Laboratories; ROC, Receiver operator characteristic; RDR, Response dose ratio.
References
1. Anderson SD, Daviskas E. The mechanism of exercise-induced asthma is. J Allergy Clin Immunol. (2000) 106:453–9. doi: 10.1067/mai.2000.109822
2. Brannan JD, Kippelen P. Bronchial provocation testing for the identification of exercise-induced bronchoconstriction. J Allergy Clin Immunol. (2020) 8:2156–64. doi: 10.1016/j.jaip.2020.03.034
3. Barnes PJ. Inhaled corticosteroids. Pharmaceuticals (Basel). (2010) 3:514–40. doi: 10.3390/ph3030514
4. Brannan JD, Koskela H, Anderson SD, Chan HK. Budesonide reduces sensitivity and reactivity to inhaled mannitol in asthmatic subjects. Respirology. (2002) 7:37–44. doi: 10.1046/j.1440-1843.2002.00357.x
5. Brannan JD. Bronchial hyperresponsiveness in the assessment of asthma control: Airway hyperresponsiveness in asthma: its measurement and clinical significance. Chest. (2010) 138:11S−7S. doi: 10.1378/chest.10-0231
6. Koskela HO, Hyvarinen L, Brannan JD, Chan HK, Anderson SD. Sensitivity and validity of three bronchial provocation tests to demonstrate the effect of inhaled corticosteroids in asthma. Chest. (2003) 124:1341–9. doi: 10.1378/chest.124.4.1341
7. Freed R. The role of bronchial provocation testing in the assessment of asthma for suitability for occupation, with particular reference to the Defence Forces (Master thesis). University of Technology, Sydney, NSW, Australia (1999).
8. Miedinger D, Chhajed PN, Tamm M, Stolz D, Surber C, Leuppi JD. Diagnostic tests for asthma in firefighters. Chest. (2007) 131:1760–7. doi: 10.1378/chest.06-2218
9. Miedinger D, Mosimann N, Meier R, Karli C, Florek P, Frey F, et al. Asthma tests in the assessment of military conscripts. Clin Exp Allergy. (2010) 40:224–31. doi: 10.1111/j.1365-2222.2009.03387.x
10. Becker JM, Rogers J, Rossini G, Mirchandani H, D'Alonzo GE. Jr. Asthma deaths during sports: report of a 7-year experience. J Allergy Clin Immunol. (2004) 113:264–7. doi: 10.1016/j.jaci.2003.10.052
11. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. (2005) 26:319–38. doi: 10.1183/09031936.05.00034805
12. Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Report working party standardization of lung function tests, european community for steel and coal official statement of the european respiratory society. Eur Respir J Suppl. (1993) 16:5–40. doi: 10.1183/09041950.005s1693
13. Brannan JD, Anderson SD, Perry CP, Freed-Martens R, Lassig AR, Charlton B, et al. The safety and efficacy of inhaled dry powder mannitol as a bronchial provocation test for airway hyperresponsiveness: a phase 3 comparison study with hypertonic (4. 5%) saline. Respir Res. (2005) 6:144. doi: 10.1186/1465-9921-6-144
14. Holzer K, Anderson SD, Chan HK, Douglass J. Mannitol as a challenge test to identify exercise-induced bronchoconstriction in elite athletes. Am J Respir Crit Care Med. (2003) 167:534–7. doi: 10.1164/rccm.200208-916OC
15. Anderson SD, Brannan JD, Koskela H, Freed-Martens R, Leuppi JD, Holzer K, et al. Provoking dose of mannitol to assess airway hyperresponsiveness in asthma: a retrospective analysis. J Allergy Clin Immunol. (2005) 115:S216–S7. doi: 10.1016/j.jaci.2004.12.874
16. Anderson SD, Brannan J, Spring J, Spalding N, Rodwell LT, Chan K, et al. A new method for bronchial-provocation testing in asthmatic subjects using a dry powder of mannitol. Am J Respir Crit Care Med. (1997) 156:758–65. doi: 10.1164/ajrccm.156.3.9701113
17. Porsbjerg C, Brannan JD, Anderson SD, Backer V. Relationship between airway responsiveness to mannitol and to methacholine and markers of airway inflammation, peak flow variability and quality of life in asthma patients. Clini Exp Allergy. (2008) 38:43–50. doi: 10.1111/j.1365-2222.2007.02878.x
18. Sverrild A, Bergqvist A, Baines KJ, Porsbjerg C, Andersson CK, Thomsen SF, et al. Airway responsiveness to mannitol in asthma is associated with chymase-positive mast cells and eosinophilic airway inflammation. Clin Exp Allergy. (2016) 46:288–97. doi: 10.1111/cea.12609
19. Anderson SD, Charlton B, Weiler JM, Nichols S, Spector SL, Pearlman DS, et al. Comparison of mannitol and methacholine to predict exercise-induced bronchoconstriction and a clinical diagnosis of asthma. Respir Res. (2009) 10:4. doi: 10.1186/1465-9921-10-4
20. Baraket M, Oliver BG, Burgess JK, Lim S, King GG, Black JL. Is low dose inhaled corticosteroid therapy as effective for inflammation and remodeling in asthma? A randomized, parallel group study. Respir Res. (2012) 13:11. doi: 10.1186/1465-9921-13-11
21. Anderson WJ, Short PM, Williamson PA, Lipworth BJ. Inhaled corticosteroid dose response using domiciliary exhaled nitric oxide in persistent asthma: The FENOtype trial. Chest. (2012) 142:1553–61. doi: 10.1378/chest.12-1310
22. Bailey J, Williams F. Asthma and eligibility for the Australian Defence Force. Aust Fam Physician. (2009) 38:897–900.
23. du Toit JI, Anderson SD, Jenkins CR, Woolcock AJ, Rodwell LT. Airway responsiveness in asthma: bronchial challenge with histamine and 4. 5% sodium chloride before and after budesonide. Allergy Asthma Proc. (1997) 18:7–14. doi: 10.2500/108854197778612817
24. Brannan JD, Bood J, Alkhabaz A, Balgoma D, Otis J, Delin I, et al. The effect of omega-3 fatty acids on bronchial hyperresponsiveness, sputum eosinophilia, and mast cell mediators in asthma. Chest. (2015) 147:397–405. doi: 10.1378/chest.14-1214
Keywords: airway hyperresponsiveness (AHR), asthma, inhaled corticosteroids, indirect bronchial challenge test, occupational assessment
Citation: Lake CD, Wong KKH, Perry CP, Koskela HO and Brannan JD (2022) Daily Inhaled Corticosteroids Treatment Abolishes Airway Hyperresponsiveness to Mannitol in Defence and Police Recruits. Front. Allergy 3:864890. doi: 10.3389/falgy.2022.864890
Received: 29 January 2022; Accepted: 11 April 2022;
Published: 31 May 2022.
Edited by:
James Martin, McGill University, CanadaReviewed by:
Parameswaran Nair, McMaster University, CanadaJorge Agustin Luna-Pech, Universidad de Guadalajara, Mexico
Copyright © 2022 Lake, Wong, Perry, Koskela and Brannan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: John D. Brannan, am9obi5icmFubmFuJiN4MDAwNDA7aGVhbHRoLm5zdy5nb3YuYXU=
 Clair D. Lake
Clair D. Lake Keith K. H. Wong
Keith K. H. Wong Clare P. Perry
Clare P. Perry Heikki O. Koskela5,6
Heikki O. Koskela5,6 John D. Brannan
John D. Brannan