
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Allergy , 15 April 2022
Sec. Skin Allergy
Volume 3 - 2022 | https://doi.org/10.3389/falgy.2022.840999
This article is part of the Research Topic The Complexity of Urticaria View all 10 articles
Acute urticaria is a common condition that presents with wheals and/or angioedema. However, these symptoms are also frequent in anaphylaxis, a life-threatening reaction that should be immediately diagnosed and treated. In both, mast cells play a central role in the physiopathology. Causes and triggers of acute urticaria and anaphylaxis are similar in general, but some peculiarities can be observed. The diagnostic approach may differ, accordingly to the condition, suspicious causes, age groups and regions. Adrenaline is the first-line treatment for anaphylaxis, but not for acute urticaria, where H1-antihistamines are the first choice. In this paper, we review the main aspects, similarities and differences regarding definitions, mechanisms, causes, diagnosis and treatment of acute urticaria and anaphylaxis.
Urticaria is a condition with a lifetime prevalence rate of up to 20% and characterized by the development of wheals, angioedema, or both (Table 1) (1, 2). Acute urticaria, which is defined by the occurrence of symptoms for up to 6 weeks, can be the only manifestation of a hypersensitivity reaction but can also be associated with other systemic symptoms, indicating an anaphylactic reaction (1, 3). This paper aims to review the mechanisms, triggers, diagnosis, and treatment of acute urticaria and anaphylaxis, highlighting the differences in managing both conditions.
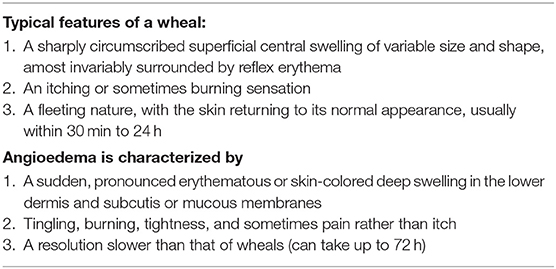
Table 1. Clinical characteristics of urticaria [adapted from (1)].
Urticaria and anaphylaxis are often but not always related to mast cell activation from multiple triggers, including IgE-mediated and non–IgE-mediated mechanisms. Mast cell plays a broad critical role in the innate and acquired immune response because they express multiple receptors responding to specific antigens, as well as circulating complement components and fragments, immune complexes binding IgG and IgM, cytokines, changes in blood pressure, and immunologic activation (4, 5). Therefore, mast cell activation in patients with urticaria and anaphylaxis is more likely to occur through multiple pathways in addition to IgE.
Mature mast cells are primarily found in tissues where external pathogens enter the body, including the skin, gastrointestinal tract, and airway. Immunological staining of tissues has revealed two types of human mast cells characterized by their neutral protease content: mast cells which are tryptase-positive but chymase-negative (MCT), and mast cells which are both tryptase- and chymase-positive (MCTC) (6). MCT are found typically at mucosal tissues, such as the intestine, lung and nose, are T-lymphocyte dependent and are increased in number in allergic disease (7). In contrast, the development of MCTC is independent of lymphocytes, and they are located primarily in the skin and gastrointestinal submucosa. MCTC account for more than 99% of the mast cells in the dermis of both lesional skin and non-lesional skin of patients with chronic spontaneous urticaria (CSU) (8). Immunoglobulin E (IgE)-dependent stimulation leads to degranulation of both subtypes, but MCTC can also be activated by IgE-independent mechanisms.
Activation of mast cells occurs when allergen-specific IgE is bound by allergen and interacts with high-affinity IgE receptor (FcεRI) on their surfaces (9). In addition to FcεRI, human mast cells express receptors for IgG (FcγRII/III), complement (C3a/C5a), drugs [Mas-related G protein-coupled receptor X2 (MRGPRX2)], opioids, neuropeptides, nerve growth factor, stem cell factor and cytokines, ligation of which modify mast cell function-survival, maturation, differentiation, growth, apoptosis, and degranulation (5, 10). Mast cells can be activated through newly identified MRGPRX2 by fluoroquinolones such as ciprofloxacin, icatibant and general anesthetics such as atracurium, rocuronium, tubocurarine, independent of the IgE-FcεRI pathway (11, 12). In this pathway, binding of these drugs and drugs expressing the THIQ (tetrahydroisoquinoline) motif directly to MRGPRX2 results in protein kinase A and phosphoinositide 3-kinase pathway activation, calcium release and degranulation (5, 11, 12). Also, activation of mast cells through MRGPRX2 may contribute to neurogenic inflammation, pain, itch, and pruritic skin diseases, including CSU (13). Increased MRGPRX2 protein expression has been reported in the skin of patients with CSU (14).
Common causes or triggers of acute urticaria include infections (viruses, bacteria, and parasites), food and medicines and less frequently latex, Hymenoptera venom, vaccines, physical stimuli, which a detailed history should identify. The prevalence of different etiologies varies among different age groups. In half of the cases, it is not possible to identify a specific cause for acute urticaria, being classified as idiopathic (15–17).
Respiratory infections, mainly of viral etiology, are considered the most related trigger to acute urticaria in all age groups (about 40% in adults and 60% in children) in different populations (16, 18–23). Gastrointestinal and urinary tract infections are also associated. In a study with children, infection was the most frequently documented cause for acute urticaria (48.6%), followed by drugs (5.4%), and food allergies (2.7%) (24). In the pediatric age, herpes virus (especially cytomegalovirus, Epstein-Barr virus, and herpes virus type 6) was the principal agent responsible for acute and recurrent flares of urticaria. Other viruses, including adenovirus, rotavirus, parvovirus B19, respiratory syncytial virus, and recently SARS-Cov2, have also been described as potential triggers (25). Mycoplasma pneumoniae and Streptococcus spp are frequent, while Chlamydia is less reported as an acute cause. Parasites may also induce acute urticaria with eosinophilia (15). In adults, hepatitis viruses (A, B and C) are most frequently implicated in acute urticaria (25).
However, the prevalence of infectious causes tends to reduce with age, and drug therapy with antibiotics (beta-lactams) and non-steroidal anti-inflammatory drugs (NSAIDs) often trigger urticaria in infants and children. At the same time, NSAIDs, angiotensin-converting enzyme inhibitors and neuromuscular blockers are more implicated as potential triggers of acute urticaria in adults (26).
Food allergies are minor causes of acute urticaria (16, 24, 27). The most implicated food allergens are cow milk, eggs, peanuts, tree nuts, wheat, and seafood (16, 18–23). Certain foods such as some types of fish (tuna, sardines, anchovies), cheeses (Emmental and gouda), salami, sausage, fruits (strawberry), vegetables (especially tomatoes) and beverages (wine and beer) have been described as triggers of recurrent urticarias, especially in patients intolerant to histamine or with deficiency of the enzyme diamine oxidase, responsible for histamine degradation. However, predicting the benefit of low histamine diets is practically impossible due to different dietary habits worldwide, and more studies on the subject are needed (28, 29).
Urticaria caused by latex, Hymenoptera venom and vaccines are less frequent. However, hypersensitivity to insect bites in Latin America countries is described as the main inducer of urticaria in children (30). Physical stimuli (dermographism, increased body temperature and cold) rarely cause acute urticaria, especially in children (15).
Similar to urticaria, the profile of anaphylaxis triggers depends on age and different geographic areas. Moreover, in up to 35% of anaphylaxis cases, a specific trigger may not be identified during the acute event or in subsequent evaluations, characterizing an idiopathic picture (31–39).
Worldwide, food, insect venom and drugs are the most frequent triggers (40–44). Food is the most common trigger for severe anaphylactic reactions in children, while drugs and insect venom are common triggers in adults (40, 42, 44–46).
In young children, due to the greater need for hospitalization, anaphylaxis from food and drugs is notably greater. In infants and young children, food, especially cow milk, eggs, peanuts, tree nuts, sesame and wheat, are the most common causes of anaphylaxis (41–44, 46, 47). Nuts, cashews, and hazelnuts are also causes of anaphylaxis in school children. Food dyes are not a common cause of food allergy (18).
Food-induced anaphylaxis in adults varies by region and food exposure. In North America and Australia, peanuts and nuts are the main triggers for anaphylaxis, while shellfish are most often associated in Asia. In central Europe, the foods most associated with anaphylaxis are peanuts, tree nuts, sesame, wheat, and shellfish. However, in southern Europe, it is lipid transfer proteins (pan-allergens responsible for cross-reactivity between fruits, vegetables, and pollens) associated with cofactors that are the most frequent food allergens. Sesame seed and buckwheat are common causes of anaphylaxis in the Middle East and Korea, respectively (3, 41–47).
Less common allergens that can trigger late anaphylaxis reactions such as alpha-gal should also be investigated (3).
Medications are also a cause of anaphylaxis, and reactions usually appear in school-age children and adolescents. They are found to be the most common cause of anaphylaxis-related deaths both in adults and in children in different countries, but this may vary depending on the method of the study and database searched (18, 31, 48–50).
Antibiotics, particularly beta-lactams, are described as the main triggers of drug-induced anaphylaxis in childhood, with few reports of anaphylaxis to other non-beta-lactam antibiotics, such as macrolides. In adults, penicillin, cephalosporins, and sulfonamides are the most implicated antibiotics (48, 51–58).
Non-steroidal anti-inflammatory drugs (NSAIDs) are the second leading cause of drug-induced anaphylaxis in children worldwide. However, in Latin America, NSAIDs are the first cause in both children and adults (56, 59). In addition to antibiotics and NSAIDs, neuromuscular blockers, anesthetics, opioids, hypnotics, ethylene oxide, plasma expanders, and dyes (patent blue and methylene blue) have been frequently involved in perioperative anaphylaxis (3, 22). In some countries latex allergens remain a significant trigger of perioperative anaphylaxis (60, 61). But the incidence of latex allergy has decreased in many places due to primary prevention measures such as wearing powder-free latex gloves and latex-free surgical material in the operating room (62–64). Reactions to radiographic contrast media have occurred less frequently with the use of non-ionic and low osmolality contrasts rather than with monomeric ionic (65).
New triggers have been identified as a cause of anaphylaxis and include immunobiological drugs, chemotherapeutics, chlorhexidine, polyethylene glycol, and methylcellulose. In general, medications are the leading cause of fatal anaphylaxis in adults and children (3).
In the United States, antibiotics, NSAIDs, immunomodulators and biologic agents are the most implicated agents in drug-induced anaphylaxis, whereas, in the United Kingdom, general anesthetics are frequently associated with fatal drug-induced anaphylaxis (32).
Insect venom-induced anaphylaxis also exhibits regional patterns. Bee venom is the most frequent trigger in South Korea, and it is also more frequent in children. While in central Europe, the wasp is the insect that induces the most anaphylaxis. In other regions, such as America, Asia and parts of Australia, and venom is an important trigger of anaphylaxis. Fatal cases of anaphylaxis from insect venom are most associated with adults (3).
Exercise-induced anaphylaxis and anaphylaxis induced by food-dependent exercise are two rare but significant entities. Various activities such as yard work, walking and running can trigger an exercise-induced anaphylaxis condition. Symptoms can occur during or after physical activity, but it is usually challenging to predict crises. In induced anaphylaxis by food-dependent exercise, symptoms occur when the causative food, such as seafood, dairy products, and wheat, is consumed minutes to several hours before exercise. In these cases, patients should avoid eating these foods 4–6 h before exercise (18).
Some external cofactors or associated conditions play an important role in the development of allergic reactions, including anaphylaxis. In the presence of cofactors such as physical exercise, drugs (e.g., nonsteroidal anti-inflammatory drugs, proton pump inhibitors), acute infections, alcohol and menstruation, allergic reactions may be elicited at lower doses or there may be more severe or life-threatening clinical reactions (40). There are associated conditions that work as cofactors jeopardizing patients, or increasing mortality (e.g., unstable asthma, mast cell disorders, cardiovascular diseases). However, the mechanism of action of such cofactors have not been fully identified yet, but increased bioavailability of allergen due to increased intestinal permeability and intestinal allergen absortion, decreased activation threshold on the cellular level and transient plasma hyperosmolality, are among the potential mechanisms proposed (40, 66, 67).
Supposedly, cofactors play a role in approximately 14–30% of anaphylactic reactions. Therefore, in a given patient these cofactors should always be considered in the clinical history and eliminated when possible, to reduce the risk of a future severe reaction (66, 68).
Anaphylaxis is a serious allergic reaction that is rapid in onset and can be fatal. Skin and mucosal manifestations are frequent but not always present (69). Anaphylaxis is highly likely when any one of three criteria are fulfilled (Table 2) (3, 32, 70, 71).

Table 2. Clinical criteria for diagnosing anaphylaxis [adapted from (67)].
Therefore, anaphylaxis may occur without skin involvement, resulting in delays in recognition of anaphylaxis. Cutaneous findings of urticaria and angioedema are the most frequent manifestations (about 80–90% of anaphylaxis cases) and usually last for <24 h (72). It is important to note that urticaria is not directly related to anaphylaxis severity. Severe anaphylaxis can present without urticaria, as in some cases reports of fatal anaphylaxis (73).
Anaphylaxis, urticaria, and angioedema have similar pathogenic mechanisms, including vasodilation and increased capillary permeability. Anaphylaxis symptoms may differ according to age group. For example, children younger than 6 years are more likely to experience vomiting and cough, while older children are more likely to experience chest tightness, dizziness, hypotension, and cardiovascular collapse (18).
Different elicitors can cause distinct clinical manifestations. In perioperative anaphylaxis, cutaneous signs may not be easily seen. Urticaria and angioedema may only become apparent when the perfusion is restored, or the surgical drapes are removed (74). A study on perioperative anaphylaxis reviewed 266 reports of Grades 3–5 anaphylaxis over 1 year from all NHS hospitals in the UK. They found that the most typical presenting features were hypotension (46%), bronchospasm (18%), tachycardia (9.8%), oxygen desaturation (4.7%), bradycardia (3%), and reduced/absent capnography trace (2.3%) (75).
Current guidelines recommend that acute urticaria usually does not require a diagnostic workup because it is usually self-limiting (1, 76). Although viral or other infectious illnesses cause many cases of acute urticaria, extensive evaluation for specific viral pathogens or antiviral therapy is not indicated unless suggested by the clinical history.
The recent international European Academy of Allergology and Clinical Immunology (EAACI)/Global Allergy and Asthma European Network (GA2LEN)/European Dermatology Forum (EuroGuiDerm)/Asia Pacific Association of Allergy, Asthma and Clinical Immunology (APAAACI) guideline state that the only exception is the suspicion of acute urticaria due to a type I food allergy in sensitized patients or the existence of other eliciting factors such as non-steroidal anti-inflammatory drugs (NSAIDs) (1).
An allergic cause is possible if the clinical history suggests a specific trigger to which the patient was exposed shortly before the onset of symptoms (usually within 1–2 h after exposure). If the history does suggest a possible allergy, skin testing, serum tests for allergen-specific immunoglobulin E (IgE) antibodies are appropriate. However, the interpretation of allergy tests can require some expertise. A positive result is suggestive, although not diagnostic of allergy, and a negative result does not exclude allergy. Allergy tests and educating the patients may be helpful to allow patients to avoid re-exposure to relevant causative factors. Occasionally, it is essential to confirm a diagnosis of allergy in acute urticaria with confirmatory tests to avoid mislabeling patients as allergic. Although skin biopsy is not indicated in most cases of acute urticaria, it might occasionally help differentiate this condition from other inflammatory disorders (76).
Tryptase is a marker of mast cell activation. It is a serine protease expressed in mast cells, and to a lesser degree, in basophils. There are four isoforms, but only α and β are considered biologically important (77).
During anaphylaxis, tryptase can be detected in serum 30 min after the onset of symptoms, peaks within 60 to 90 min, begins to decline after 2 h, and returns to normal levels within 24 to 48 h. Therefore, blood samples must be collected within 1–4 h of the reaction. Immunoassays allow detection of both total (baseline release) and mature (released only at the time of activation) tryptase. Another blood sample to measure the basal level of tryptase is needed 24 to 48 h after anaphylaxis (69, 78). In general, baseline tryptase levels >8 ng/ml are considered elevated, but this is not always a sign of mast cell activation. It is difficult to establish a cut-off point for the diagnosis. There are no special levels to confirm mast cell activation (hence anaphylactic episodes) as it must be calculated according to individual baseline tryptase levels with the formula: 1.2 x baseline + 2 ng/ml (79).
Tryptase levels are typically higher and more persistently elevated in anaphylactic reactions to intravenous drugs and insect venom than oral triggers such as food. Furthermore, elevations correlate with hypotension (69).
However, normal tryptase levels do not rule out anaphylaxis because the sensitivity is not optimal. This is explained by the fact that about 27% of the population does not have α-tryptase genes, affecting serum tryptase levels. Several studies use an equation with tryptase increasing at least 20% above baseline plus two ng/ml within 4 h of the allergic reaction (79–82). Tryptase levels ≥2 ng / mL + 1.2 × baseline is significantly increased for patients with low baseline tryptase (72, 79, 81).
Unfortunately, tryptase is not available everywhere. In a study by Jares et al. whose aim was to investigate the clinical features and management of drug-induced anaphylaxis (DIA) in Latin America, only 8 of 264 patients (3%) had tryptase levels accessed (56). In an online survey promoted by the Latin American Society of Allergy and Immunology to assess the current resources available in Latin American countries for the diagnosis and treatment of anaphylaxis, they found that the determination of serum tryptase was possible only in some health centers, often private, in five of the ten countries surveyed (83).
Differential diagnoses of elevated total tryptase levels include patients with systemic mastocytosis (SM), acute myelocytic leukemia, myelodysplastic syndromes, immunologic disorders (hypereosinophilic syndrome), severe renal failure, or familial tryptasemia – a disease associated with cutaneous flushing and pruritus, dysautonomia, functional gastrointestinal symptoms, chronic pain, and connective tissue abnormalities, due to the expression of more than two α-tryptase genes (69, 78).
The diagnoses of anaphylaxis should be based on relevant clinical history and a combination of available tests, i.e., skin tests, in vitro tests (serum tryptase, specific IgE serum levels, basophil activation test or histamine release tests) and/or provocation tests (3). However, the investigation of precipitating agents can become challenging given the complex variability in clinical presentation, multiple concurrent exposures, and many differential diagnoses, such as in the context of perioperative anaphylaxis (84).
Acute serum tryptase levels is an important tool during the diagnostic evaluation of anaphylaxis but it is not worldwide available. Moreover, it has high specificity, but low sensitivity and results should be carefully interpreted (84).
IgE-mediated anaphylactic reactions can be assessed by in vivo (skin tests to foods, venom, drugs, latex) and/or in vitro tests (serum specific IgE to foods, venom, and some drugs) (3). It is worth noting that, in the context of anaphylaxis, their detection facilitates guidance as to the allergen to be used in provocation tests. However, a positive skin test or elevated specific IgE are useful to confirm the etiology of an allergic reaction only when the clinical history is suggestive, otherwise they just reveal sensitization (84).
Most skin tests are considered safe and rapid but not free of systemic reactions. The perfect timing for performing skin tests may vary among different allergens. In general, a period of at least 4 weeks after the anaphylactic episode is suggested but could be longer for drug-induced anaphylaxis and each patient should be individually assessed. Comorbidities (e.g., asthma) must be controlled, and medications (antihistamines, high-dose corticosteroids, antidepressants, and antipsychotics with an antihistamine effect) paused prior to testing (84).
An obstacle faced when performing skin tests and determining serum specific IgE levels is to obtain cut-off values that could confirm the diagnosis and avoid provocation tests. In addition, specificity and sensitivity vary according to the trigger involved in the reaction, not being possible to accurately determine universal values of specific IgE (84).
The determination of molecular biology-based components has enabled advances in precision medicine by conferring greater specificity to diagnosis, allowing the identification of discriminative co-sensitization vs. cross-sensitization phenomena, stratifying the clinical risk associated with a specific sensitization pattern, and a better indication to the provocation test in cases of anaphylaxis by food (85–87). Molecular allergy diagnostics yielded best results in peanut and tree nut allergies (88, 89).
Despite all the scientific advancement in recent years, the provocation test is still considered the gold standard in diagnosing hypersensitivity to foods and drugs, regardless of the pathophysiological mechanism involved (85, 90). They are used to confirm, exclude, or prove tolerance to a particular food or drug and test a safe alternative (84). A significant disadvantage is a risk of inducing anaphylaxis, making provocation a high-risk procedure. The decision on its execution is influenced by clinical history, age, type of symptom, time of the last reaction, results of skin testing and/or serum levels of specific IgE, and the joint decision between physician and patient, carefully evaluating risk vs. benefit. Those with a convincing history of anaphylaxis from a specific allergen and proven evidence of specific IgE sensitization should not undergo provocation tests (87, 90).
Complementary tests, such as basophil activation test (BAT) with food, drugs, Hymenoptera venoms and latex, reflect tissue mast cell sensitization and activation. Due to the lack of standardized kits for most allergens is employed mainly in clinical research. However, BAT should be considered a diagnostic tool in selected patients, especially those with severe and high-risk anaphylaxis related to drugs (3, 90).
Further elucidation of the underlying mechanisms of anaphylaxis is needed to better characterize the phenotypes and endotypes of anaphylaxis and decrease the number of cases labeled as idiopathic anaphylaxis (3, 78).
Epinephrine (adrenaline) is the first-line drug recommended by the American, European and World Allergy Organization guidelines for treating anaphylaxis, although its use remains suboptimal. The recommended dose is 0.01 mg/kg, maximum 0.5 mg, given intramuscularly in the mid-anterolateral region of the thigh, which can be repeated every 5–15 min as needed (3, 32, 71).
The vasodilatory effect on skeletal muscles facilitates the rapid absorption of adrenaline into the central circulation, in contrast to its vasoconstrictor effect when injected into the subcutaneous tissue, delaying its absorption and onset of action. Intravenous administration is also not recommended for initial treatment, as potentially fatal arrhythmias can occur within bolus administration of epinephrine (3, 32). However, in special circumstances such as severe hypotension, intravenous administration appears to be more effective and should be used with caution (71).
There is no absolute contraindication to the administration of epinephrine, and delays in its administration are associated with progression to severe anaphylaxis and potential death (3, 32).
As it is a non-selective agonist of all adrenergic receptors present in all organ systems affected by anaphylaxis, it exerts effects on α1 receptors causing peripheral vasoconstriction, reversing hypotension and mucosal edema; on β1 receptors increasing cardiac output, thus reversing hypotension; and on β2 receptors reversing bronchoconstriction and inhibiting the additional release of histamine and other mediators by mast cells and basophils, also preventing worsening of symptoms (3, 32, 71).
A self-injectable adrenaline device is highly recommended among experts for patients at risk of anaphylaxis (71). But despite its critical role, the self-injectable form of adrenaline is not available in most countries, being limited to only 32% of all 195 countries in the world, mainly in developed countries. The high cost is one of the main limiting factors (3).
In Brazil, for example, there is neither the manufacture nor the marketing of these devices, requiring their importation. This fact dramatically hinders the management, implementation of the “action plan,” and self-management of anaphylactic reactions outside the hospital environment (71).
Another issue that is also relevant is the expired validity of the injectors. Because they remain unused for long periods, there is a high probability that patients carry this medication with its expiration date (71). All these aspects, notably the high cost, unavailability and expired validity are barriers to the use of adrenaline autoinjectors (91).
Second-line interventions include removing the trigger when possible, calling for help, correct positioning of the patient, offering high flow oxygen, administration of intravenous fluids (crystalloids) associated with the first dose of adrenaline in patients with cardiovascular involvement and severe pictures of anaphylaxis, should also be considered. However, no robust evidence is available (3, 71).
Additionally, in cases of bronchial obstruction, inhaled short-acting beta-2 adrenergic agonists (e.g., salbutamol) can be administered. When laryngeal/pharyngeal edema has been suspected, inhaled adrenaline administration by nebulizer, as a supplement to intramuscular adrenaline, and oxygen are recommended (3, 71).
Several other drugs can be used in the additional treatment of anaphylaxis, but never in isolation since they do not have a global effect capable of reversing the systemic symptoms of anaphylaxis. The need for any additional medication should be individualized and depend on the adrenaline response (3).
Systemic antihistamines have only been shown to relieve cutaneous symptoms, and a possible effect on non-cutaneous symptoms remains unconfirmed (71). It is noteworthy that antihistamines are now a third-line treatment in some guidelines due to concerns that their administration may delay more urgent measures, such as repeated administration of adrenaline (3, 32).
Glucocorticoids are commonly used in anaphylaxis, as they are believed to prevent prolonged symptoms and possibly biphasic reactions, but there is limited evidence of their efficacy, and they may be deleterious in children; their routine use is becoming controversial (3, 71).
Parenteral administration of glucagon may be helpful in the treatment of patients with anaphylaxis refractory to adrenaline use, particularly those on beta-blocker therapy, although evidence is very limited. The dose for adults is 1–5 mg in a slow bolus, intravenously, followed by a titrated infusion of 5–15 mcg/min (3, 71).
Patients with anaphylaxis are at risk of prolonged reactions and developing biphasic reactions, although the likelihood is low. In these cases, there is a recurrence of symptoms 8 to 10 h after the initial reaction, without a new exposure to the triggering antigen, and should be treated as any anaphylaxis. Thus, more prolonged monitoring should be considered in patients with asthma, those with a history of severe anaphylaxis, biphasic reactions, and/or a need for multiple doses of adrenaline (3, 32, 71).
Education and management of anaphylaxis should be customized according to the patient's clinical history and presentation, considering their age, concomitant diseases, concomitant medications, and triggering factors (3).
Initial treatment of acute urticaria should focus on the short-term alleviation of pruritus and reduction of wheals. The literature on the management of acute urticaria is rare, probably because the condition is too often self-limited. Current guidelines recommend modern second-generation H1-antihistamines (such as bilastine, cetirizine, desloratadine, ebastine, fexofenadine, levocetirizine, loratadine, and rupatadine) as a first-line symptomatic treatment for acute urticaria (1, 76). The newer, second-generation H1-antihistamines are minimally or non-sedating and free of anticholinergic effects that can complicate the use of first-generation agents (92). These medications have been mostly evaluated in treating chronic urticaria (CU), and in some cases, their use in acute urticaria is extrapolated from those researches. In current guidelines, there is no recommendation on which to choose for the treatment of acute urticaria, although a few studies in patients with CU suggest that cetirizine and levocetirizine may be modestly more effective than other agents (93). Some patients require higher than standard doses to control urticaria and may experience drowsiness at those higher doses. The higher doses may have better efficacy in some adults, although this has not been conclusively demonstrated in patients with acute urticaria. Current guidelines have no specific recommendation on how long H1-antihistamines should be used in acute urticaria, but it might be required until complete symptoms are controlled (1).
First-generation antihistamines available in parenteral presentation, such as diphenhydramine and promethazine, are rapidly acting and effective in emergency units for acute urticaria treatment. However, they can be associated with sedation and impaired motor skills because of their ability to cross the blood-brain barrier in both pediatric and adult patients besides other frequent prominent anticholinergic effects, including dryness of the mouth and eyes, constipation, inhibition of micturition, and potential provocation of narrow-angle glaucoma. Thus, its use should be limited, and non-sedating 2nd generation oral antihistamines preferred as first-line treatment for most patients, especially for those with mild and moderate disease (94).
In patients with poor response to antihistamines, a brief course of oral corticosteroids might also be required while attempting to eliminate suspected triggers and develop an effective treatment plan (76). The recent international guideline recommends that for acute urticaria and acute exacerbations of CSU, a short course of oral corticosteroids limited to 10 days might be necessary to some patients (1, 23, 95). H1-antihistamine therapy should be continued during and after the course of glucocorticoids because some patients experience an exacerbation as the glucocorticoids are tapered or discontinued. If symptoms do not recur for several days after stopping glucocorticoids, then antihistamines could also be discontinued.
Prevention of anaphylaxis includes education based on the known trigger of anaphylaxis. Thus, current management relies on allergen avoidance and treatment of severe reactions with epinephrine (3). In cases of anaphylaxis by stinging insects, this can be very difficult. Also, for food allergy, avoidance of the trigger is currently the only approved therapy, and while effective, diets can be difficult to carry out (96). For drug allergies, the most common situation is to avoid the drug that caused the reaction. Food allergy and insect venom allergy present a high risk of anaphylaxis, which is unpredictable in occurrence and severity. The unpredictable nature of these allergies can affect the patient and family's psychosocial functioning and quality of life (73).
In patients with a history of Hymenoptera sting, anaphylaxis and positive skin or in vitro tests (serum specific IgE) to Hymenoptera venom, venom immunotherapy (VIT) should be considered, especially in those patients with mastocytosis (97, 98).
To select VIT, it is essential to take a good clinical history. Initially, collect information about the stinging insect (i.e., number of stings, previous and subsequent re-stings, nest, extraction of the sting, death of offending insect). It is important to take information on occupational or activities linked to a higher likelihood of sting (e.g., farmers, beekeepers, outdoor sports). Furthermore, discriminate if the reaction was local or systemic. Local large reactions (LLR) are edema exceeding 10 cm, increasing within 24/48 h, and lasting longer than 72 h. Although worrisome for some patients, they have a low risk of evolution into systemic reactions (99). VIT indications are enumerated in Table 3.

Table 3. Indications of venom immunotherapy [adapted from (92)].
For patients with proven or highly suspected drug hypersensitivity reaction (DHR), drug desensitization (DS) is a procedure designed to safely reintroduce drugs into patients who have had IgE/non-IgE Type I reactions (100–102).
DS is defined as the induction of a temporary state of tolerance of a drug for a hypersensitivity reaction. It is indicated in some specific situations, as shown in Table 4 (101). It is performed by administering increasing doses of the medication over a short period (from several hours to a few days) until the total cumulative therapeutic dose is achieved and tolerated (100, 102). It is a procedure that helps to prevent anaphylaxis, keeping patients in the first-line treatment and, therefore, representing an important advance in their prognosis (69). Although several protocols have been proposed to desensitize patients to different drugs, the 12-steps rapid desensitization protocol has been demonstrated to be safe and efficient and can be adapted to be used with any parenteral drug (103, 104).

Table 4. Drug desensitization indications [adapted from (51)].
Immunotherapy has several routes of administration and has been performed subcutaneously, sublingually, epicutaneously and orally. The subcutaneous approach was abandoned many years ago due to safety concerns. The sublingual and epicutaneous approaches have both been shown to be safe, but efficacy is limited by a restricted dose capacity, that is the amount that can be absorbed through the skin or under the tongue. Oral immunotherapy (OIT) is more effective than the other routes, in part because much larger doses can be administered (105).
Cow's milk, hen's egg, wheat, soy, peanut, tree nut, fish, and shellfish are most often associated with food allergies. Oral immunotherapy (OIT) is an option for individuals who do not naturally tolerate these foods by late childhood or adulthood (96).
OIT for foods involves introducing an allergenic food mixed with a vehicle in gradually increasing doses. OIT protocols include an initial escalation phase, followed by a dose build-up phase and maintenance phases. The efficacy of the OIT depends on the chosen outcomes, including the ability to tolerate the treatment, induction of a state of desensitization, and/or the development of a more durable state of clinical tolerance, what is often referred to as lack of sustained response. Adverse reactions during OIT are common. Reactions are usually mild, with local symptoms such as oral itching. However, moderate and even severe reactions may also occur, and patients may require treatment with epinephrine, especially during dose escalation (96). Recently, a death from baked milf OIT was reported in Canada, as well as an exercise-induced anaphylaxis to wheat OIT in Japan (106, 107). Eosinophilic esophagitis occurs in some patients undergoing OIT, and it is not clear how often the disease was already present before the start of OIT and could complicate the procedure (108).
In cases of idiopathic anaphylaxis, when the trigger is not known, the anti-IgE monoclonal antibody omalizumab demonstrated to be a successful treatment, effectively reducing the number of episodes, and improving quality of life (109). In addition, omalizumab has been shown to be effective as an adjunct to treatment in patients who experience episodes of anaphylaxis during immunotherapy with food (OIT) or with Hymenoptera venom (VIT). Some studies showed more safety using omalizumab in groups of patients with OIT (milk and peanut). These patients were able to tolerate a higher amount of protein with fewer reactions (110).
Anaphylaxis is a life-threatening reaction that requires immediate diagnosis and treatment. Anaphylactic reactions can present with a variety of symptoms, and hives and angioedema are often observed (3). On the other hand, acute urticaria is limited to the skin and mucosa and, although not potentially lethal, may impact patients' quality of life (1).
Mast cells have a central role in the pathophysiology of the two conditions, and their activation can be triggered by allergic and non-allergic mechanisms (4, 5). Many of these triggers can cause both acute urticaria and anaphylaxis, but some are more frequent in a determined region, age group or type of reaction - NSAIDs, for example, is the leading cause of drug-induced anaphylaxis in Latin America but not in the United States, and viral infections are an important cause of acute urticaria in children but not anaphylaxis (28, 56). So, it is of extreme importance to understand the potential triggers for each condition and perform an adequate investigation when recommended. In general, an extensive investigation is not necessary for acute urticaria but mandatory to search for a cause in anaphylaxis, especially to prevent future and more severe reactions (1, 3).
Acute urticaria and anaphylaxis are treated differently, at least regarding first-line therapy. Whereas H1-antihistamines are the preferred therapy in acute urticaria, their effect in anaphylaxis is limited to skin symptoms. In addition, parenteral use of antihistamines may cause hypotension as a potential side effect (111). On the other hand, adrenaline is the first drug to be administered during an anaphylactic reaction, but its use in acute urticaria should be limited for patients with moderate to severe laryngeal angioedema (1, 3).
Finally, avoiding the trigger responsible for the reaction is the best way to prevent further episodes of anaphylaxis or acute urticaria. Of course, anaphylaxis prevention is mandatory because of the risk of a severe reaction. Desensitization to drugs and foods can be an option in selected patients, as well as venom immunotherapy. In acute urticaria, preventive measures are not always possible, mainly when it is caused by virus infections or in those cases where no specific trigger can be identified.
In conclusion, reactions presenting with hives and/or angioedema must be carefully assessed to distinguish between acute urticaria or anaphylaxis, as the diagnostic investigation, treatment and preventive measures are different and can directly impact in patient's survival and quality of life.
All authors contributed to manuscript conception, design, revision, read, and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Zuberbier T, Abdul Latiff AH, Abuzakouk M, Aquilina S, Asero R, Baker D, et al. The international EAACI/GA2LEN/EuroGuiDerm/APAAACI guideline for the definition, classification, diagnosis, and management of urticaria. Allergy. (2022) 77:734–66. doi: 10.26416/Aler.6.4.2021.5815
2. Zuberbier T, Balke M, Worm M, Edenharter G, Maurer M. Epidemiology of urticaria: a representative cross-sectional population survey. Clin Exp Dermatol. (2010) 35:869–73. doi: 10.1111/j.1365-2230.2010.03840.x
3. Cardona V, Ansotegui IJ, Ebisawa M, El-Gamal Y, Fernandez Rivas M, Fineman S, et al. World allergy organization anaphylaxis guidance 2020. World Allergy Organ J. (2020) 13:100472. doi: 10.1016/j.waojou.2020.100472
4. Church MK, Kolkhir P, Metz M, Maurer M. The role and relevance of mast cells in urticaria. Immunol Rev. (2018) 282:232–47. doi: 10.1111/imr.12632
5. Nguyen SMT, Rupprecht CP, Haque A, Pattanaik D, Yusin J. and Krishnaswamy G. Mechanisms Governing Anaphylaxis: Inflammatory Cells, Mediators, Endothelial Gap Junctions and Beyond. Int J Mol Sci. (2021) 22:7785. doi: 10.3390/ijms22157785
6. Irani AM, Schwartz LB. Mast cell heterogeneity. Clin Exp Allergy. (1989) 19:143–55. doi: 10.1111/j.1365-2222.1989.tb02357.x
7. Otsuka H, Inaba M, Fujikura T, Kunitomo M. Histochemical and functional characteristics of metachromatic cells in the nasal epithelium in allergic rhinitis: studies of nasal scrapings and their dispersed cells. J Allergy Clin Immunol. (1995) 96:528–36. doi: 10.1016/S0091-6749(95)70297-0
8. Smith CH, Kepley C, Schwartz LB, Lee TH. Mast cell number and phenotype in chronic idiopathic urticaria. J Allergy Clin Immunol. (1995) 96:360–4. doi: 10.1016/S0091-6749(95)70055-2
9. Peavy RD, Metcalfe DD. Understanding the mechanisms of anaphylaxis. Curr Opin Allergy Clin Immunol. (2008) 8:310–5. doi: 10.1097/ACI.0b013e3283036a90
10. Elieh Ali Komi D, Wöhrl S, Bielory L. Mast cell biology at molecular level: a comprehensive review. Clin Rev Allergy Immunol. (2020) 58:342–65. doi: 10.1007/s12016-019-08769-2
11. McNeil BD, Pundir P, Meeker S, Han L, Undem BJ, Kulka M, et al. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature. (2015) 519:237–41. doi: 10.1038/nature14022
12. Muñoz-Cano R, Picado C, Valero A, Bartra J. Mechanisms of Anaphylaxis Beyond IgE. J Investig Allergol Clin Immunol. (2016) 26:73–82. doi: 10.18176/jiaci.0046
13. Subramanian H, Gupta K, Ali H. Roles of Mas-related G protein-coupled receptor X2 on mast cell-mediated host defense, pseudoallergic drug reactions, and chronic inflammatory diseases. J Allergy Clin Immunol. (2016) 138:700–10. doi: 10.1016/j.jaci.2016.04.051
14. Fujisawa D, Kashiwakura J, Kita H, Kikukawa Y, Fujitani Y, Sasaki-Sakamoto T, et al. Expression of Mas-related gene X2 on mast cells is upregulated in the skin of patients with severe chronic urticaria. J Allergy Clin Immunol. (2014) 134:622–33. doi: 10.1016/j.jaci.2014.05.004
15. Minasi D, Manti S, Chiera F, Licari A, Marseglia GL. Acute urticaria in the infant. Pediatr Allergy Immunol. (2020) 31 Suppl 26:49–51. doi: 10.1111/pai.13350
16. Kulthanan K, Chiawsirikajorn Y, Jiamton S. Acute urticaria: etiologies, clinical course and quality of life. Asian Pac J Allergy Immunol. (2008) 26:1–9.
17. Losappio L, Heffler E, Bussolino C, Cannito CD, Carpentiere R, Raie A, et al. Acute urticaria presenting in the emergency room of a general hospital. Eur J Intern Med. (2014) 25:147–50. doi: 10.1016/j.ejim.2013.11.003
18. Pier J, Bingemann TA. Urticaria, Angioedema, and Anaphylaxis. Pediatr Rev. (2020) 41:283–92. doi: 10.1542/pir.2019-0056
19. Humphreys F, Hunter JA. The characteristics of urticaria in 390 patients. Br J Dermatol. (1998) 138:635–8. doi: 10.1046/j.1365-2133.1998.02175.x
20. Aoki T, Kojima M, Horiko T. Acute urticaria: history and natural course of 50 cases. J Dermatol. (1994) 21:73–7. doi: 10.1111/j.1346-8138.1994.tb01417.x
21. Ricci G, Giannetti A, Belotti T, Dondi A, Bendandi B, Cipriani F, et al. Allergy is not the main trigger of urticaria in children referred to the emergency room. J Eur Acad Dermatol Venereol. (2010) 24:1347–8. doi: 10.1111/j.1468-3083.2010.03634.x
22. Konstantinou GN, Papadopoulos NG, Tavladaki T, Tsekoura T, Tsilimigaki A, Grattan CE. Childhood acute urticaria in northern and southern Europe shows a similar epidemiological pattern and significant meteorological influences. Pediatr Allergy Immunol. (2011) 22:36–42. doi: 10.1111/j.1399-3038.2010.01093.x
23. Zuberbier T, Ifflander J, Semmler C, Henz BM. Acute urticaria: clinical aspects and therapeutic responsiveness. Acta Derm Venereol. (1996) 76:295–7. doi: 10.2340/0001555576295297
24. Sackesen C, Sekerel BE, Orhan F, Kocabas CN, Tuncer A, Adalioglu G. The etiology of different forms of urticaria in childhood. Pediatr Dermatol. (2004) 21:102–8. doi: 10.1111/j.0736-8046.2004.21202.x
25. Imbalzano E, Casciaro M, Quartuccio S, Minciullo PL, Cascio A, Calapai G, et al. Association between urticaria and virus infections: a systematic review. Allergy Asthma Proc. (2016) 37:18–22. doi: 10.2500/aap.2016.37.3915
26. Nettis E, Foti C, Ambrifi M, Baiardini I, Bianchi L, Borghi A, et al. Urticaria: recommendations from the Italian Society of Allergology, Asthma and Clinical Immunology and the Italian Society of Allergological, Occupational and Environmental Dermatology. Clin Mol Allergy. (2020) 18:8. doi: 10.1186/s12948-020-00123-8
27. Sicherer SH, Sampson HA. Food allergy. J Allergy Clin Immunol. (2010) 125 (2 Suppl 2):S116–25. doi: 10.1016/j.jaci.2009.08.028
28. Talarico V, Marseglia GL, Lanari M, Esposito S, Masi S, De Filippo M, et al. Pediatric urticaria in the Emergency Department: epidemiological characteristics and predictive factors for its persistence in children. Eur Ann Allergy Clin Immunol. (2021) 53:80–5. doi: 10.23822/EurAnnACI.1764-1489.148
29. Kudryavtseva AV, Neskorodova KA, Staubach P. Urticaria in children and adolescents: An updated review of the pathogenesis and management. Pediatr Allergy Immunol. (2019) 30:17–24. doi: 10.1111/pai.12967
30. Sánchez-Borges M, Capriles-Hulett A, Caballero-Fonseca F. Demographic and clinical profiles in patients with acute urticaria. Allergol Immunopathol (Madr). (2015) 43:409–15. doi: 10.1016/j.aller.2014.04.010
31. Jerschow E, Lin RY, Scaperotti MM, McGinn AP. Fatal anaphylaxis in the United States, 1999-2010: temporal patterns and demographic associations. J Allergy Clin Immunol. (2014). 134:1318–28.e7. doi: 10.1016/j.jaci.2014.08.018
32. Shaker MS, Wallace DV, Golden DBK, Oppenheimer J, Bernstein JA, Campbell RL, et al. Anaphylaxis - a 2020 practice parameter update, systematic review, and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) analysis. J Allergy Clin Immunol. (2020) 145:1082–123. doi: 10.1016/j.jaci.2020.01.017
33. Decker WW, Campbell RL, Manivannan V, Luke A, St Sauver JL, Weaver A, et al. The etiology and incidence of anaphylaxis in Rochester, Minnesota: a report from the Rochester Epidemiology Project. J Allergy Clin Immunol. (2008) 122:1161–5. doi: 10.1016/j.jaci.2008.09.043
34. Ross MP, Ferguson M, Street D, Klontz K, Schroeder T, Luccioli S. Analysis of food-allergic and anaphylactic events in the National Electronic Injury Surveillance System. J Allergy Clin Immunol. (2008) 121:166–71. doi: 10.1016/j.jaci.2007.10.012
35. Allen KJ, Koplin JJ. The epidemiology of IgE-mediated food allergy and anaphylaxis. Immunol Allergy Clin North Am. (2012) 32:35–50. doi: 10.1016/j.iac.2011.11.008
36. Clark S, Bock SA, Gaeta TJ, Brenner BE, Cydulka RK, Camargo CA. Multicenter study of emergency department visits for food allergies. J Allergy Clin Immunol. (2004) 113:347–52. doi: 10.1016/j.jaci.2003.10.053
37. Rudders SA, Banerji A, Vassallo MF, Clark S, Camargo CA Jr. Trends in pediatric emergency department visits for food-induced anaphylaxis. J Allergy Clin Immunol. (2010) 126:385-8. doi: 10.1016/j.jaci.2010.05.018
38. Clark S, Camargo CA Jr. Epidemiology of anaphylaxis. Immunol Allergy Clin North Am. (2007) 27:145–63. doi: 10.1016/j.iac.2007.03.002
39. Harduar-Morano L, Simon MR, Watkins S, Blackmore C. A population-based epidemiologic study of emergency department visits for anaphylaxis in Florida. J Allergy Clin Immunol. (2011) 128:594–600.e1. doi: 10.1016/j.jaci.2011.04.049
40. Worm M, Francuzik W, Renaudin JM, Bilo MB, Cardona V, Scherer Hofmeier K, et al. Factors increasing the risk for a severe reaction in anaphylaxis: An analysis of data from The European Anaphylaxis Registry. Allergy. (2018) 73:1322–30. doi: 10.1111/all.13380
41. Jeon YH, Lee S, Ahn K, Lee SY, Kim KW, Kim HH, et al. Infantile anaphylaxis in Korea: a multicenter retrospective case study. J Kor Med Sci. (2019) 34: e106. doi: 10.3346/jkms.2019.34.e106
42. Wood RA, Camargo CA Jr, Lieberman P, Sampson HA, Schwartz LB, Zitt M, et al. Anaphylaxis in America: the prevalence and characteristics of anaphylaxis in the United States. J Allergy Clin Immunol. (2014) 133:461–7. doi: 10.1016/j.jaci.2013.08.016
43. Liew WK, Chiang WC, Goh AE, Lim HH, Chay OM, Chang S, et al. Paediatric anaphylaxis in a Singaporean children cohort: changing food allergy triggers over time. Asia Pac Allergy. (2013) 3:29–34. doi: 10.5415/apallergy.2013.3.1.29
44. Nabavi M, Lavavpour M, Arshi S, Bemanian MH, Esmaeilzadeh H, Molatefi R, et al. Characteristics, etiology and treatment of pediatric and adult anaphylaxis in Iran. Iran J Allergy Asthma Immunol. (2017) 16:480–7.
45. Ring J, Beyer K, Biedermann T, Bircher A, Fischer M, Fuchs T, et al. Guideline (S2k) on acute therapy and management of anaphylaxis: 2021 update: S2k-Guideline of the German Society for Allergology and Clinical Immunology (DGAKI), the Medical Association of German Allergologists (AeDA), the Society of Pediatric Allergology and Environmental Medicine (GPA), the German Academy of Allergology and Environmental Medicine (DAAU), the German Professional Association of Pediatricians (BVKJ), the Society for Neonatology and Pediatric Intensive Care (GNPI), the German Society of Dermatology (DDG), the Austrian Society for Allergology and Immunology (ÖGAI), the Swiss Society for Allergy and Immunology (SGAI), the German Society of Anaesthesiology and Intensive Care Medicine (DGAI), the German Society of Pharmacology (DGP), the German Respiratory Society (DGP), the patient organization German Allergy and Asthma Association (DAAB), the German Working Group of Anaphylaxis Training and Education (AGATE). Allergo J Int. (2021) 30:1–25. doi: 10.1007/s40629-020-00158-y
46. Worm M, Moneret-Vautrin A, Scherer K, Lang R, Fernandez-Riva M, Cardona V, et al. First European data from the network of severe allergic reactions (NORA). Allergy. (2014) 69:1397–404. doi: 10.1111/all.12475
47. Kahveci M, Akarsu A, Koken G, Sahiner UM, Soyer O, Sekerel BE. Food-induced anaphylaxis in infants, as compared to toddlers and preschool children in Turkey. Pediatr Allergy Immunol. (2020) 31:954–61. doi: 10.1111/pai.13320
48. Atanaskovic-Markovic M, Gomes E, Cernadas JR, du Toit G, Kidon M, Kuyucu S, et al. Diagnosis and management of drug-induced anaphylaxis in children: An EAACI position paper. Pediatr Allergy Immunol. (2019) 30:269–76. doi: 10.1111/pai.13034
49. Bilò MB, Corsi A, Martini M, Penza E, Grippo F, Bignardi D. Fatal anaphylaxis in Italy: Analysis of cause-of-death national data, 2004-2016. Allergy. (2020) 75:2644–52. doi: 10.1111/all.14352
50. Tejedor-Alonso MA, Martínez-Fernandez P, Vallejo-de-Torres G, Navarro-Escayola E, Moro-Moro M, Alberti-Masgrau N. Clinical and demographic characteristics of fatal anaphylaxis in Spain (1998-2011): A comparison between a series from the hospital system and a national forensic series. Clin Exp Allergy. (2019)49:82–91. doi: 10.1111/cea.13272
51. Grabenhenrich LB, Dölle S, Moneret-Vautrin A, Köhli A, Lange L, Spindler T, et al. Anaphylaxis in children and adolescents: the European Anaphylaxis Registry. J Allergy Clin Immunol. (2016) 137:1128–37.e1. doi: 10.1016/j.jaci.2015.11.015
52. Solé D, Ivancevich JC, Borges MS, Coelho MA, Rosário NA, Ardusso L, et al. Anaphylaxis in Latin American children and adolescents: the Online Latin American Survey on Anaphylaxis (OLASA). Allergol Immunopathol (Madr). (2012) 40:331–5. doi: 10.1016/j.aller.2011.09.008
53. Gaspar Â, Santos N, Piedade S, Santa-Marta C, Pires G, Sampaio G, et al. One-year survey of paediatric anaphylaxis in an allergy department. Eur Ann Allergy Clin Immunol. (2015) 47:197–205.
54. Silva R, Gomes E, Cunha L, Falcao H. Anaphylaxis in children: a nine years retrospective study (2001–2009). Allergol Immunopathol (Madr). (2012) 40:31–6. doi: 10.1016/j.aller.2010.12.012
55. Liew WK, Williamson E, Tang ML. Anaphylaxis fatalities and admissions in Australia. J Allergy Clin Immunol. (2009) 123:434–42. doi: 10.1016/j.jaci.2008.10.049
56. Jares EJ, Baena-Cagnani CE, Sánchez-Borges M, Ensina LF, Arias-Cruz A, Gómez M, et al. Drug-Induced Anaphylaxis in Latin American Countries. J Allergy Clin Immunol Pract. (2015) 3:780–8. doi: 10.1016/j.jaip.2015.05.012
57. Sousa-Pinto B, Fonseca JA, Gomes ER. Frequency of self-reported drug allergy: a systematic review and meta-analysis with meta-regression. Ann Allergy Asthma Immunol. (2017) 119:362–73.e2. doi: 10.1016/j.anai.2017.07.009
58. Faria E, Rodrigues-Cernadas J, Gaspar A, Botelho C, Castro E, Lopes A, et al. Drug-induced anaphylaxis survey in Portuguese allergy departments. J Investig Allergol Clin Immunol. (2014) 24:40–8.
59. Ensina LF, Lacerda AE de, Andrade DM de, Machado L, Camelo-Nunes I, Solé D. Drug-induced anaphylaxis in children: Nonsteroidal anti-inflammatory drugs and drug provocation test. J Allergy Clin Immunol Pract. (2014) 2:825. doi: 10.1016/j.jaip.2014.08.016
60. Tacquard C, Collange O, Gomis P, Malinovsky JM, Petitpain N, Demoly P, et al. Anaesthetic hypersensitivity reactions in France between 2011 and 2012: the 10th GERAP epidemiologic survey. Acta Anaesthesiol Scand. (2017) 61:290–9. doi: 10.1111/aas.12855
61. Banerji A, Bhattacharya G, Huebner E, Fu X, Camargo CA Jr, Guyer A, et al. Perioperative Allergic Reactions: Allergy Assessment and Subsequent Anesthesia. J Allergy Clin Immunol Pract. (2021) 9:1980–91. doi: 10.1016/j.jaip.2020.11.025
62. Blaabjerg MS, Andersen KE, Bindslev-Jensen C, Mortz CG. Decrease in the rate of sensitization and clinical allergy to natural rubber latex. Contact Dermatitis. (2015) 73:21–8. doi: 10.1111/cod.12386
63. Kelly KJ, Sussman G. Latex Allergy: Where Are We Now and How Did We Get There? J Allergy Clin Immunol Pract. (2017) 5:1212–6. doi: 10.1016/j.jaip.2017.05.029
64. Mertes PM, Ebo DG, Garcez T, Rose M, Sabato V, Takazawa T, et al. Comparative epidemiology of suspected perioperative hypersensitivity reactions. Br J Anaesth. (2019) 123:e16–28. doi: 10.1016/j.bja.2019.01.027
65. Li X, Liu H, Zhao L, Liu J, Cai L, Liu L, et al. Clinical observation of adverse drug reactions to non-ionic iodinated contrast media in population with underlying diseases and risk factors. Br J Radiol. (2017) 90:20160729. doi: 10.1259/bjr.20160729
66. Niggemann B, Beyer K. Factors augmenting allergic reactions. Allergy. (2014) 69:1582–7. doi: 10.1111/all.12532
67. Gülen T, Akin C. Anaphylaxis and Mast Cell Disorders. Immunol Allergy Clin North Am. (2022) 42:45–63. doi: 10.1016/j.iac.2021.09.007
68. Shin M. Food allergies and food-induced anaphylaxis: role of cofactors. Clin Exp Pediatr. (2021) 64:393–9. doi: 10.3345/cep.2020.01088
69. Jimenez-Rodriguez TW, Garcia-Neuer M, Alenazy LA, Castells M. Anaphylaxis in the 21st century: phenotypes, endotypes, and biomarkers. J Asthma Allergy. (2018) 11:121–42. doi: 10.2147/JAA.S159411
70. Sampson HA, Muñoz-Furlong A, Campbell RL, Adkinson NF Jr, Bock SA, Branum A, et al. Second symposium on the definition and management of anaphylaxis: summary report–Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. (2006) 117:391–7. doi: 10.1016/j.jaci.2005.12.1303
71. Muraro A, Worm M, Alviani C, Cardona V, DunnGalvin A, Garvey LH, et al. EAACI guideline: Anaphylaxis (2021 update). Allergy. (2022) 77:357–77. doi: 10.1111/all.15032
72. Simons FE, Ardusso LR, Bilò MB, El-Gamal YM, Ledford DK, Ring J, et al. World allergy organization guidelines for the assessment and management of anaphylaxis. World Allergy Organ J. (2011) 4:13–37. doi: 10.1097/WOX.0b013e318211496c
73. Bartnikas LM, Sicherer SH. Fatal Anaphylaxis: Searching for Lessons from Tragedy. J Allergy Clin Immunol Pract. (2020) 8:334–5. doi: 10.1016/j.jaip.2019.11.005
74. Savic LC, Garvey LH. Perioperative anaphylaxis: diagnostic challenges and management. Curr Opin Anaesthesiol. (2020) 33:448–53. doi: 10.1097/ACO.0000000000000857
75. Harper NJN, Cook TM, Garcez T, Farmer L, Floss K, Marinho S, et al. Anaesthesia, surgery, and life-threatening allergic reactions: epidemiology and clinical features of perioperative anaphylaxis in the 6th National Audit Project (NAP6). Br J Anaesth. (2018) 121:159–71. doi: 10.1016/j.bja.2018.04.014
76. Bernstein JA, Lang DM, Khan DA, Craig T, Dreyfus D, Hsieh F, et al. The diagnosis and management of acute and chronic urticaria: 2014 update. J Allergy Clin Immunol. (2014) 133:1270–7. doi: 10.1016/j.jaci.2014.02.036
77. Bonadonna P, Scaffidi L, Boni E. Tryptase values in anaphylaxis and insect allergy. Curr Opin Allergy Clin Immunol. (2019) 19:462–7. doi: 10.1097/ACI.0000000000000569
78. Gulen T, Akin C. Idiopathic Anaphylaxis: a Perplexing Diagnostic Challenge for Allergists. Curr Allergy Asthma Rep. (2021) 21:11. doi: 10.1007/s11882-021-00988-y
79. Gülen T, Akin C, Bonadonna P, Siebenhaar F, Broesby-Olsen S, Brockow K, et al. Selecting the Right Criteria and Proper Classification to Diagnose Mast Cell Activation Syndromes: a critical review. J Allergy Clin Immunol Pract. (2021) 9:3918–28. doi: 10.1016/j.jaip.2021.06.011
80. Sala-Cunill A, Guilarte M, Cardona V. Phenotypes, endotypes and biomarkers in anaphylaxis: current insights. Curr Opin Allergy Clin Immunol. (2018) 18:370–6. doi: 10.1097/ACI.0000000000000472
81. Caughey GH. Tryptase genetics and anaphylaxis. J Allergy Clin Immunol. (2006) 117:1411–4. doi: 10.1016/j.jaci.2006.02.026
82. Akin C, Soto D, Brittain E, Chhabra A, Schwartz LB, Caughey GH, et al. Tryptase haplotype in mastocytosis: relationship to disease variant and diagnostic utility of total tryptase levels. Clin Immunol. (2007) 123:268–71. doi: 10.1016/j.clim.2007.02.007
83. Cardona V, Álvarez-Perea A, Ansotegui IJ, Arias-Cruz A, González-Díaz SN, Latour-Staffeld P, et al. Manejo de la anafilaxia en América Latina: situación actual [Management of anaphylaxis in Latin America: current situation]. Rev Alerg Mex/ (2017) 64:171–7. doi: 10.29262/ram.v64i2.250
84. Castells M. Diagnosis and management of anaphylaxis in precision medicine. J Allergy Clin Immunol. (2017) 140:321–33. doi: 10.1016/j.jaci.2017.06.012
85. Sicherer SH, Sampson HA. Food allergy: A review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J Allergy Clin Immunol. (2018) 141:41–58. doi: 10.1016/j.jaci.2017.11.003
86. Sturm GJ, Arzt-Gradwohl L, Varga EM. Medical Algorithms: Diagnosis and treatment of Hymenoptera venom allergy. Allergy. (2019) 74:2016–8. doi: 10.1111/all.13817
87. Parisi CAS, Kelly KJ, Ansotegui IJ, Gonzalez-Díaz SN, Bilò MB, Cardona V, et al. Update on latex allergy: New insights into an old problem. World Allergy Organ J. (2021) 14:100569. doi: 10.1016/j.waojou.2021.100569
88. Hemmings O, Niazi U, Kwok M, Radulovic S, Du Toit G, Lack G, et al. Combining Allergen Components Improves the Accuracy of Peanut Allergy Diagnosis. J Allergy Clin Immunol Pract. (2022) 10:189–99. doi: 10.1016/j.jaip.2021.08.029
89. Akarsu A, Ocak M, Sahiner UM, Soyer O, Sekerel BE. Multiplex component-based allergen macroarray test is useful to predict clinical reactivity to tree nuts in children. Allergol Int. (2022) 71:236–47. doi: 10.1016/j.alit.2021.10.001
90. Garvey LH, Ebo DG, Mertes PM, Dewachter P, Garcez T, Kopac P, et al. An EAACI position paper on the investigation of perioperative immediate hypersensitivity reactions. Allergy. (2019) 74:1872–84. doi: 10.1111/all.13820
91. Esenboga S, Ocak M, Cetinkaya PG, Sahiner UM, Soyer O, Buyuktiryaki B, et al. Physicians prescribe adrenaline autoinjectors, do parents use them when needed? Allergol Immunopathol (Madr). (2020) 48:3–7. doi: 10.1016/j.aller.2019.07.009
92. Kubo N, Senda M, Ohsumi Y, Sakamoto S, Matsumoto K, Tashiro M, et al. Brain histamine H1 receptor occupancy of loratadine measured by positron emission topography: comparison of H1 receptor occupancy and proportional impairment ratio. Hum Psychopharmacol. (2011) 26:133–9. doi: 10.1002/hup.1184
93. Antia C, Baquerizo K, Korman A, Alikhan A, Bernstein JA. Urticaria: A comprehensive review: Treatment of chronic urticaria, special populations, and disease outcomes. J Am Acad Dermatol. (2018) 79:617–33. doi: 10.1016/j.jaad.2018.01.023
94. Adelsberg BR. Sedation and performance issues in the treatment of allergic conditions. Arch Intern Med. (1997) 157:494–500. doi: 10.1001/archinte.1997.00440260028006
95. Asero R, Tedeschi A. Usefulness of a short course of oral prednisone in antihistamine-resistant chronic urticaria: a retrospective analysis. J Investig Allergol Clin Immunol. (2010) 20:386–90.
96. Wood RA. Oral Immunotherapy for Food Allergy. J Investig Allergol Clin Immunol. (2017) 27:151–9. doi: 10.18176/jiaci.0143
97. Bonadonna P., Gonzalez-de-Olano D, Zanotti R, Riccio A, De Ferrari L, Lombardo C, et al. Venom immunotherapy in patients with clonal mast cell disorders: efficacy, safety, and practical considerations J Allergy Clin Immunol Pract. (2013) 1:474–8. doi: 10.1016/j.jaip.2013.06.014
98. Jarkvist J, Salehi C, Akin C, Gülen T. Venom immunotherapy in patients with clonal mast cell disorders: IgG4 correlates with protection. Allergy. (2020) 75:169–77. doi: 10.1111/all.13980
99. Bilò MB, Tontini C, Martini M, Corsi A, Agolini S, Antonicelli L. Clinical aspects of hymenoptera venom allergy and venom immunotherapy. Eur Ann Allergy Clin Immunol. (2019) 51:244–58. doi: 10.23822/EurAnnACI.1764-1489.113
100. de Las Vecillas Sánchez L, Alenazy LA, Garcia-Neuer M, Castells MC. Drug Hypersensitivity and Desensitizations: Mechanisms and New Approaches. Int J Mol Sci. (2017) 18:1316. doi: 10.3390/ijms18061316
101. Cernadas JR, Brockow K, Romano A, Aberer W, Torres MJ, Bircher A, et al. General considerations on rapid desensitization for drug hypersensitivity - a consensus statement. Allergy. (2010) 65:1357–66. doi: 10.1111/j.1398-9995.2010.02441.x
102. Sloane D, Govindarajulu U, Harrow-Mortelliti J, Barry W, Hsu FI, Hong D, et al. Safety, Costs, and Efficacy of Rapid Drug Desensitizations to Chemotherapy and Monoclonal Antibodies. J Allergy Clin Immunol Pract. (2016) 4:497–504. doi: 10.1016/j.jaip.2015.12.019
103. Castells MC, Tennant NM, Sloane DE, Hsu FI, Barrett NA, Hong DI, et al. Hypersensitivity reactions to chemotherapy: Outcomes and safety of rapid desensitization in 413 cases. J Allergy Clin Immunol. (2008) 122:574. doi: 10.1016/j.jaci.2008.02.044
104. Ensina LF, Aranda CS, de Lacerda AE, Camelo-Nunes I, Sole D, Martins AM, et al. Laronidase hypersensitivity and desensitization in type I mucopolysaccharidosis: a case report. Pediatr Allergy Immunol. (2014) 25:498–9. doi: 10.1111/pai.12209
105. Costa C, Coimbra A, Vítor A, Aguiar R, Ferreira AL, Todo-Bom A. Food allergy-From food avoidance to active treatment. Scand J Immunol. (2020) 91:e12824. doi: 10.1111/sji.12824
106. Allergic Living. Girl With Milk Allergy Dies of Severe Reaction Related to Desensitization. (2021). Available online at: https://www.allergicliving.com/2021/12/20/girl-with-milk-allergy-dies-of-severe-reaction-related-to-desensitization (accessed March 12, 2022).
107. Leeds S, Liu EG, Nowak-Wegrzyn A. Wheat oral immunotherapy. Curr Opin Allergy Clin Immunol. (2021) 21:269–77. doi: 10.1097/ACI.0000000000000743
108. Sánchez-García S, Rodríguez Del Río P, Escudero C, Martínez-Gómez MJ, Ibáñez MD. Possible eosinophilic esophagitis induced by milk oral immunotherapy. J Allergy Clin Immunol. (2012) 129:1155–7. doi: 10.1016/j.jaci.2011.11.042
109. Warrier P, Casale TB. Omalizumab in idiopathic anaphylaxis. Ann Allergy Asthma Immunol. (2009) 102:257–8. doi: 10.1016/S1081-1206(10)60091-9
110. Wood RA, Kim JS, Lindblad R, Nadeau K, Henning AK., Dawson P, et al. A randomized, double-blind, placebo-controlled study of omalizumab combined with oral immunotherapy for the treatment of cow's milk allergy. J Allergy Clin Immunol. (2016) 137:1103–10. doi: 10.1016/j.jaci.2015.10.005
Keywords: urticaria, angioedema, anaphylaxis, diagnosis, treatment
Citation: Ensina LF, Min TK, Félix MMR, de Alcântara CT and Costa C (2022) Acute Urticaria and Anaphylaxis: Differences and Similarities in Clinical Management. Front. Allergy 3:840999. doi: 10.3389/falgy.2022.840999
Received: 21 December 2021; Accepted: 25 March 2022;
Published: 15 April 2022.
Edited by:
Cem Akin, University of Michigan, United StatesReviewed by:
Theo Gulen, Karolinska Institutet (KI), SwedenCopyright © 2022 Ensina, Min, Félix, de Alcântara and Costa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luis Felipe Ensina, MTAwYWxlcmdpYUBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.