- UOC Medicina Generale, ASST Fatebenefratelli Sacco, Ospedale Luigi Sacco-Università degli Studi di Milano, Milan, Italy
Hereditary angioedema due to C1-inhibitor deficiency (C1-INH-HAE) is characterized by swelling attacks that may be even life-threatening. To reduce the frequency of attacks, some patients need a long-term prophylaxis (LTP). In addition to the intravenous administration, plasma-derived C1-inhibitor (pdC1-INH) has been proved effective also if administered subcutaneously at the dose of 120 IU/kg/week. In this case series, we collected from clinical records data about 5 patients with poorly controlled C1-INH-HAE with the registered LTPs or with difficult venous access, referred to the angioedema center in Milano (Italy), who received it at lower doses, i.e., 42.86–65.22 IU/kg/week. All the patients experienced a reduction in the attack rate, ranging from 29.67% to 96.53% compared with a control period with a different LTP or with no LTP. For one patient, the comparison was made with a period when he received s.c. pdC1-INH 2 (with poor outcomes) instead of 3 times a week, which made the patient experience a decrease in the attack rate from 5.26 to 1.12 attacks/month. Observation periods varied between 2.6 and 47.97 months. Two patients reported adverse events, which were localized at the infusion site and mild in severity. In conclusion, subcutaneous pdC1-INH represents an alternative therapeutic choice according to the physician's judgment for selected patients with HAE poorly controlled with registered LTPs. In patients with difficult venous access, in countries where pdC1-INH is not approved for subcutaneous administration, about half the recommended dose may be beneficial, although suboptimal results may be obtained.
Introduction
Hereditary angioedema due to C1-inhibitor deficiency (C1-INH-HAE) is characterized by recurrent attacks of swelling that may be life-threatening in cases of laryngeal involvement. Treatment strategies for HAE include on-demand therapy to rapidly resolve angioedema symptoms, short-term prophylaxis (STP), to prevent attacks when a patient is exposed to known triggers, and long-term prophylaxis (LTP), to decrease the frequency and severity of attacks (1). Treatment must be individualized to provide optimal care and normalize quality of life. Whereas on-demand therapy is required for all patients, LTP is used as needed for individual patients.
Among the products for LTP, plasma-derived C1-inhibitor concentrate (pdC1-INH) administered intravenously was shown to reduce the frequency of acute attacks by 50% compared with placebo in a study published in 2010 (2). Despite the LTP with pdC1-INH, the patients continued to experience, on average, 6.26 attacks in 12 weeks (2), which were treated with additional rescue infusions of pdC1-INH.
Subcutaneous (s.c.) administration of Berinert® (CSL Behring) has been studied in Phases 2 and 3, and open label extension COMPACT trials and shown to reduce the frequency of attacks compared with placebo (3–5). Unlike intravenous administration, s.c. administration maintained the plasma C1-INH activity levels continuously above ~40% of normal (3), which is the threshold known to have a clinically meaningful effect on preventing HAE attacks (6). In Europe, s.c. Berinert® is licensed as LTP at a dose of 60 international units (IU) per kg of body weight by s.c. injection two times weekly (7, 8).
In Italy, Berinert® for s.c. use has not yet been registered. Patients with severe disease and frequent attacks are prescribed other LTPs, such as intravenous pdC1-INH, androgens, and tranexamic acid. When data were collected, neither lanadelumab nor berotralstat was available in Italy. For patients experiencing frequent breakthrough attacks despite LTP or having poor venous access, an unmet need for prophylaxis is present (3).
Method
Patients with poorly controlled C1-INH-HAE with the registered LTPs or with difficult venous access, referred to the angioedema center in Milano (Italy), were treated with plasma-derived C1-inhibitor (Berinert®) registered for on-demand treatment at the dose of 20 IU/kg, administered subcutaneously as LTP. The dose of s.c. pdC1-INH injection was lower than the recommended dose for subcutaneous use (which is not approved in Italy). We collected relevant data and report the results.
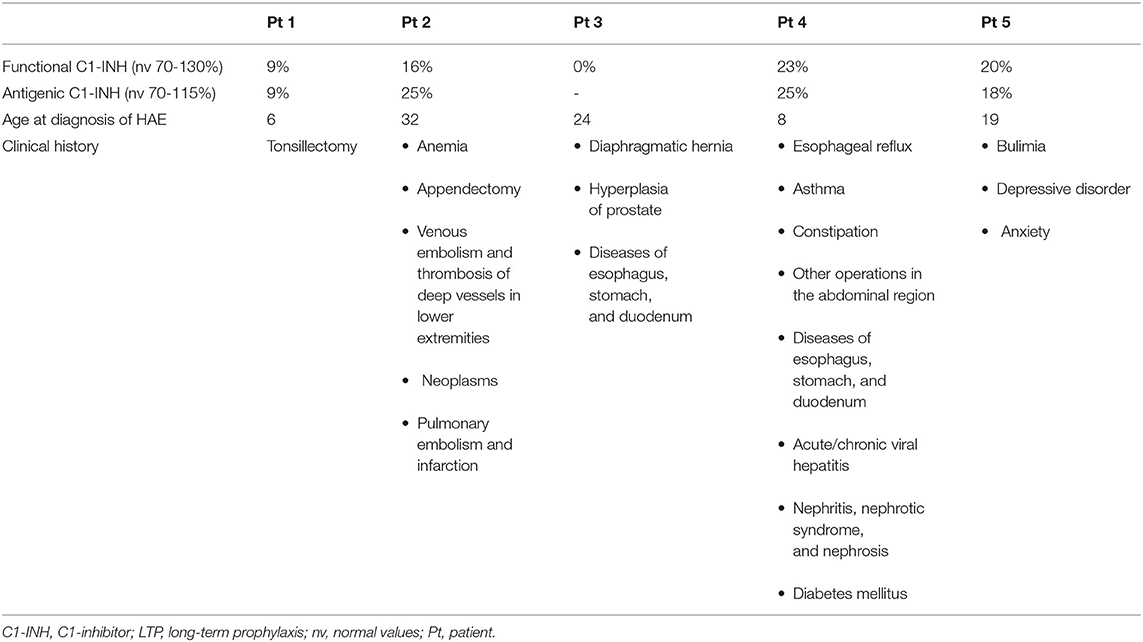
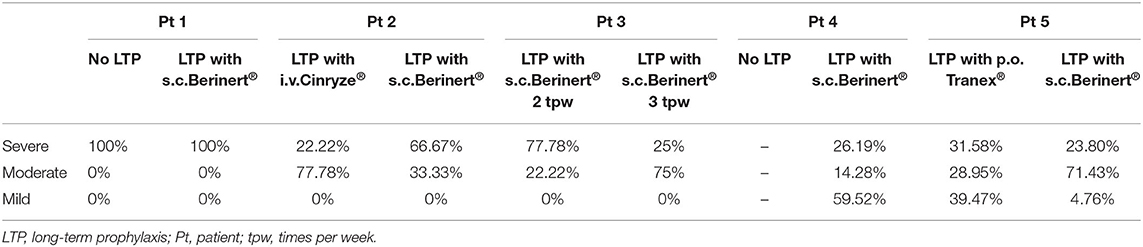
Demographic data and clinical history (frequency and treatment for attacks) were retrieved from the clinical records. The severity of attacks was categorized as mild if no interference with activities of daily living was experienced, moderate in case of partial interference, and severe for complete incapacity. The patients were tested for functional and antigenic C1-INH. C1-INH-HAE type 1 was diagnosed when both functional and antigenic C1-INH were ≤ 50% of normal (9).
Although the dose/weight ratio per administration is generally used in clinical studies, we chose to calculate the dose/weight ratio per week in order to allow comparisons among patients receiving a different number of administrations per week.
Results
In this analysis, 5 patients with C1-INH-HAE type 1 were observed.
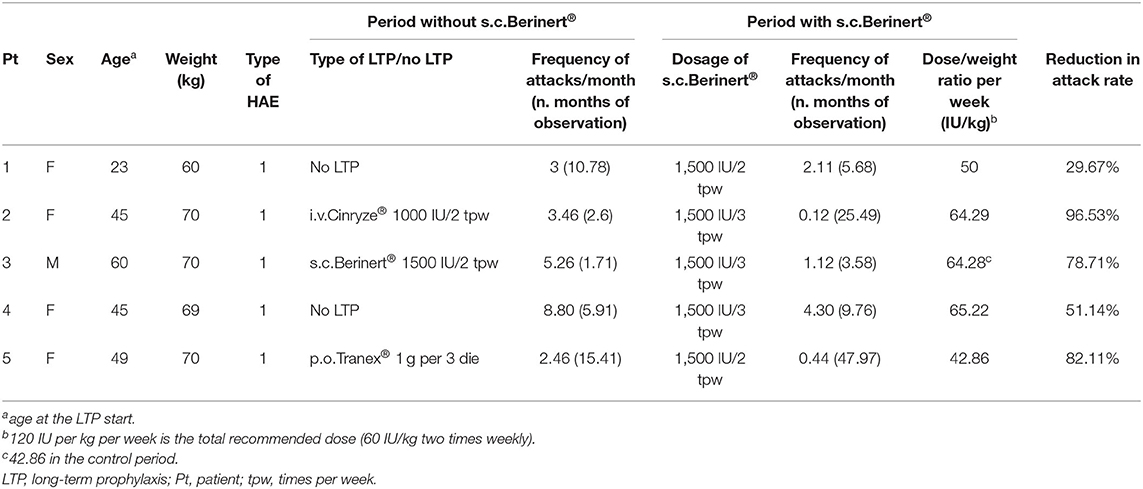
The dose/weight ratio of s.c. infusions weekly ranged from 42.86 to 65.22 IU/kg (Table 1).
The frequency of attacks/month during LTP decreased in three patients (2, 4, and 5) and remained substantially stable in one patient (1) compared to the period when they were not on LTP with s.c. C1-INH. Patient 3 did not initially experience an improvement in the frequency of attacks during LTP with s.c. C1-INH administered 2 times/week (the dose weight ratio = 42.86 IU/kg per week). Therefore, the frequency of administration was increased to 3 times/week (the weekly dose/weight ratio = 64.28 IU/kg per week) and the frequency of attacks decreased from 5.26 to 1.12 attacks/month. No patient was attack free.
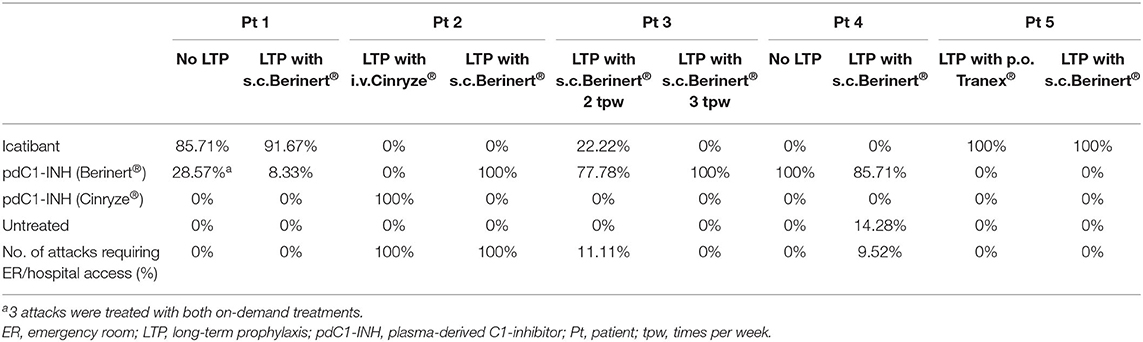
Additional data about the patients, laboratory findings, and attack characteristics are shown in Tables 2, 3, respectively.
Our cohort previously received attenuated androgens, but they were discontinued because of a lack of effectiveness or adverse events.
Adverse events during s.c. administration of C1-INH were reported by 2 patients, which included erythema at the injection site and mild itching. In both cases, the adverse events were not severe and resolved spontaneously.
During LTP with s.c. C1-INH, breakthrough attacks were mostly treated at home by patients with s.c. icatibant or intravenous (i.v) C1-INH as rescue therapy. In one patient, breakthrough attacks were treated with i.v. C1-INH by health care professionals in the hospital (Table 4).
Discussion
The patients showing a reduced frequency of attacks received a mean of ~60 IU/kg per week, which is lower than the recommended weekly dose of 120 IU per kg (8). It should be noted that, in the COMPACT study (4), even the dosage of 40 IU/kg two times weekly, i.e., 80 IU/kg per week, significantly reduced the frequency of attacks compared to placebo.
The patients included in this analysis had severe disease and required LTP. Androgens were previously prescribed in our patient cohort and were discontinued because of a lack of effectiveness or adverse events. One patient switched from LTP with tranexamic acid, which was ineffective. Another patient switched from prophylaxis with i.v. C1-INH (Cinryze®), discontinued because of breakthrough attacks and poor venous access.
Therefore, s.c. C1-INH was prescribed to this patient cohort. The dose that proved effective in reducing the number of attacks was ~60 IU/kg per week.
The adverse events reported in this study are similar to those reported in the clinical trials COMPACT (4) and COMPACT-OLE (5), i.e., local site reactions.
The data presented in this paper are affected by some limitations. Control periods without s.c. C1-INH were usually shorter than those with s.c. C1-INH. As the attack frequency might vary during patients' lives because of unpredictable factors [i.e., stress (10)], longer observation periods could be useful to better evaluate disease severity. A wash-out period from one LTP period to another was not considered. This is a case series reporting findings obtained in 5 patients, without a control group, as we never meant to conduct neither a clinical trial nor an observation study. The therapeutic results were suboptimal, as expected, and the number of treated patients was too small to draw definite conclusions. A multicenter study with more patients would be more informative.
Far from willing to recommend a lower dosage of s.c. C1-INH, we aimed to report our real-life experience. In conclusion, even half the recommended dose of subcutaneous C1-INH may be beneficial, although suboptimal results may be obtained. These results may be useful for physicians facing patients with difficult venous access in countries where pdC1-INH is not registered for subcutaneous administration or in the absence of availability of other new drugs for long-term prophylaxis such as lanadelumab and berotralstat.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
CSL Behring funded the publishing support and journal styling services but had no role in the conduct of the research, preparation of the article, in study design, in the collection, analysis and interpretation of data, in the writing of the report, and in the decision to submit the article for publication.
Conflict of Interest
AZ received speaker/consultancy fees from BioCryst, CSL Behring, Pharming, and Takeda. All the other authors declare that they have no financial competing interests about the topic of this article, except for the publishing support and journal styling services, which were provided by SEEd Medical Publishers and funded by CSL Behring, Italy.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We acknowledge SEEd Medical Publishers, which provided publishing support and journal styling services.
References
1. Caballero T. Treatment of hereditary angioedema. J Investig Allergol Clin Immunol. (2021) 31:1–16. doi: 10.18176/jiaci.0653
2. Zuraw BL, Busse PJ, White M, Jacobs J, Lumry W, Baker J, et al. Nanofiltered C1 inhibitor concentrate for treatment of hereditary angioedema. N Engl J Med. (2010) 363:513–22. doi: 10.1056/NEJMoa0805538
3. Zuraw BL, Cicardi M, Longhurst HJ, Bernstein JA, Li HH, Magerl M, et al. Phase II study results of a replacement therapy for hereditary angioedema with subcutaneous C1-inhibitor concentrate. Allergy. (2015) 70:1319–28. doi: 10.1111/all.12658
4. Longhurst H, Cicardi M, Craig T, Bork K, Grattan C, Baker J, et al. Prevention of hereditary angioedema attacks with a subcutaneous C1 inhibitor. N Engl J Med. (2017) 376:1131–40. doi: 10.1056/NEJMoa1613627
5. Craig T, Zuraw B, Longhurst H, Cicardi M, Bork K, Grattan C, et al. Long-term outcomes with subcutaneous C1-inhibitor replacement therapy for prevention of hereditary angioedema attacks. J Allergy Clin Immunol Pract. (2019) 7:1793–1802. doi: 10.1016/j.jaip.2019.01.054
6. Späth PJ, Wüthrich B, Bütler R. Quantification of C1-inhibitor functional activities by immunodiffusion assay in plasma of patients with hereditary angioedema–evidence of a functionally critical level of C1-inhibitor concentration. Complement. (1984) 1:147–59. doi: 10.1159/000467830
7. AIFA. Commento. Autorizzazione all'immissione in commercio del medicinale per uso umano ≪Berinert≫. Gazzetta ufficiale della Repubblica Italiana. 5-5-2018. Serie Generale n. 103:18A03038. Available online at: https://www.gazzettaufficiale.it/eli/id/2018/05/05/18A03069/sg (accessed March 28, 2022).
8. Berinert®. Riassunto delle caratteristiche del prodotto (Summary of product characteristics). Available online at: https://farmaci.agenziafarmaco.gov.it/bancadatifarmaci/farmaco?farmaco=039056 (accessed March 28, 2022).
9. Zanichelli A, Arcoleo F, Barca MP, Borrelli P, Bova M, Cancian M, et al. A nationwide survey of hereditary angioedema due to C1 inhibitor deficiency in Italy. Orphanet J Rare Dis. (2015) 10:11. doi: 10.1186/s13023-015-0233-x
Keywords: plasma-derived C1-inhibitor, hereditary angioedema, subcutaneous use, attack frequency, dose/weight ratio
Citation: Zanichelli A, Suffritti C, Popescu Janu V, Merlo A and Cogliati C (2022) Real-Life Experience With Subcutaneous Plasma-Derived C1-Inhibitor for Long-Term Prophylaxis in Patients With Hereditary Angioedema: A Case Series. Front. Allergy 3:818741. doi: 10.3389/falgy.2022.818741
Received: 19 November 2021; Accepted: 14 March 2022;
Published: 11 April 2022.
Edited by:
Henriette Farkas, Semmelweis University, HungaryReviewed by:
Bulent Enis Sekerel, Hacettepe University, TurkeyEmel Aygören—Pürsün, University Hospital Frankfurt, Germany
Copyright © 2022 Zanichelli, Suffritti, Popescu Janu, Merlo and Cogliati. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrea Zanichelli, YW5kcmVhLnphbmljaGVsbGlAdW5pbWkuaXQ=
 Andrea Zanichelli
Andrea Zanichelli Chiara Suffritti
Chiara Suffritti Valentina Popescu Janu
Valentina Popescu Janu Andrea Merlo
Andrea Merlo Chiara Cogliati
Chiara Cogliati