- Department of Otorhinolaryngology, Amsterdam University Medical Centres, University of Amsterdam, Amsterdam, Netherlands
Patients with non-steroidal anti-inflammatory drug (NSAID)-exacerbated respiratory disease (N-ERD) often suffer from chronic rhinosinusitis (CRS) with nasal polyps, a form of primary diffuse Type 2 CRS. Although this disease is also seen in NSAID-tolerant patients, CRS in N-ERD often is more severe and more treatment resistant; local nasal therapy (nasal corticosteroids) and endoscopic sinus surgery are employed like in NSAID-tolerant patients, but with limited and/or short-lived effects. This mini-review gives an overview of the current additional treatment options for CRS in N-ERD. As such diets, aspirin therapy after desensitization, antileukotriene therapy and biologicals are discussed based on the current body of literature. Selecting the right treatment strategy depends on shared-decision making, local availability and cooperation between ENT-surgeons, allergists, and pulmonologists.
Introduction
Chronic rhinosinusitis (CRS) is an inflammation of the nose and paranasal sinuses lasting for more than 12 weeks, and leading to nasal obstruction, rhinorrhea, loss of smell, and/or facial pain/pressure (1). This condition is common in most of the world, with a questionnaire-based prevalence ranging from 5 to 28% (2–4). Adding nasal endoscopy and/or imaging to confirm the diagnosis, reduces the prevalence to 3–6% (5–7). The societal burden of CRS is immense, and mostly dictated by indirect costs (absenteeism and presenteeism) (8, 9).
CRS symptoms are quite bothersome, leading to a significant reduction in health-related quality of life (10), especially when nasal polyps are present (CRSwNP), and even more in those suffering from non-steroidal anti-inflammatory drugs (NSAID)-exacerbated respiratory disease (N-ERD) (11, 12). The estimated prevalence of N-ERD related disease within the CRS population varies from 9.6 to 16%. The reported prevalence depends, amongst others on the population studied (general population vs. tertiary referral centers), differences between countries, and methodologies used (questionnaire-based vs. provocation-based) (13–15).
Patients with CRS and N-ERD are at risk for uncontrolled sinonasal disease, requiring more surgeries and/or more intense medical therapy, often with unsatisfactory results (15). This review aims to give an update of the current therapeutic options for the management of CRS in patients with N-ERD.
Pathophysiology and Classification of CRS in N-ERD Patients
The pathophysiology of N-ERD is incompletely understood. It appears that in hypersensitive patients, a baseline mast cell activation state is present. Upon exposure to NSAIDs, this is further triggered by decreased prostaglandin E2 synthesis through inhibition of cyclooxygenase-1, and subsequent activation and chemotaxis of inflammatory cells (such as basophils and eosinophils) through mast-cell derived prostaglandin D2 (16). As a result, nasal polyps show vast amounts of eosinophils and evidence of Type 2 inflammation, such as elevated levels of IL-5 and IL-13 in nasal secretion (17).
In the past decades, CRS was divided between “with polyps” (CRSwNP) or “without polyps” (CRSsNP) based on the endoscopic appearance of polyps. However, with the newest update of the European Position Paper on Rhinosinusitis and Nasal Polyps 2020 (EPOS2020), a more diverse classification is proposed (1). CRS is first classified as primary or secondary, based on the absence or presence of underlying pathology such as cystic fibrosis. Primary CRS is then divided between localized or diffuse disease, and endotyped roughly as either Type 2 or Non-type 2 disease. The CRS found in N-ERD patients thus classifies as primary diffuse Type 2 CRS. We will use this latter terminology in the rest of this review, although most literature is based on the former (simplified) diagnosis of CRS(wNP).
Appropriate Medical Therapy and Fess; Limitations in N-ERD
Following the management schemes of EPOS2020 the first line of treatment for primary diffuse CRS is appropriate medical therapy (AMT), consisting of, but not limited to local corticosteroids (either spray, drops, or rinses), saline rinses and/or oral corticosteroids. Those achieving disease controls with AMT should be advised to continue their medication without the need for further investigation or therapy. However, when patients indicate a poor disease control with AMT, it is advised to perform additional investigations in order to differentiate the disease as a Type 2 or Non-type 2 primary diffuse CRS. As described above, patients with CRSwNP and N-ERD fulfill the criteria for Type 2 disease.
Generally, the additional treatment options for primary diffuse Type 2 CRS are the addition of oral corticosteroids to AMT (if not tried before) or functional endoscopic sinus surgery (FESS). The long-term aim of performing FESS is to open up the paranasal sinuses in such a way that they are more accessible for local therapy, ideally leading to better disease control with AMT. Short-term goals include the removal of diseased mucosa and polyps, directly alleviating symptoms such as nasal obstruction and fullness. FESS in itself is an umbrella term and does not necessarily describe the extent of surgery. A FESS for diffuse Type 2 CRS can range from a simple polypectomy (removing polyps from the nasal cavity) to the opening of all the paranasal sinuses (maxillary, ethmoidal, sphenoidal and frontal approach) which is often termed “full house FESS.” Surgery can further be extended, for example by additional approaches to the maxillary sinus (such as a medial maxillectomy, removing the inferior nasal turbinate, and associated lateral nasal wall) or extended drilling procedures to the frontal sinuses (Draf III or “modified Lothrop” procedures). These extensive options challenge the “functional” approach in FESS and oftentimes authors refer to endoscopic sinus surgery, or ESS, to cover all possible (endonasal) approaches. Furthermore, ESS can be extended by aggressive removal of all sinus mucosa (so-called “reboot surgery”). As neatly summarized in EPOS2020, a debate is ongoing on the needed extent of surgery and as yet, no firm conclusions can be drawn on the added value of more aggressive approaches (18–20).
Studies show that especially patients with N-ERD show a limited effect of AMT and/or FESS, resulting in higher oral corticosteroid use, more frequent surgery and a lower quality of life (15, 21). Qualitative analyses show that patients experience great frustration as they often find themselves trapped between their allergist suggesting more oral corticosteroids and their ENT-surgeon advocating yet another surgery (22). As such, many N-ERD patients have to deal with a chronic, yet uncontrolled disease state of their CRS. It is therefore pivotal that treating physicians acquaint themselves with the additional treatment options listed below.
Drug Avoidance and Diet
N-ERD patients are advised to avoid NSAIDs, selective COX-1 inhibitors, and alcohol consumption (21) as these might trigger a sudden increase in symptoms from the upper and lower airways, asthma exacerbation, bronchospasm or even death. COX-2 inhibitors are generally well-tolerated. We are not aware of studies reporting on the success of such avoidance stratagems or how often patients are (unintentionally) exposed to these drugs or triggers.
Another possible option would be to consider a low-salicylate diet. A cross-over randomized-controlled trial performed in 30 patients (14 of which originating from a smaller trial), showed an improvement of all evaluated items including sinonasal disease-specific quality of life (22-item SinoNasal Outcome Test; SNOT-22), nasal complaints, nasal endoscopy scores, and asthma control (23). These results were confirmed in a questionnaire-based study with 30 N-ERD patients following a low-salicylate diet for 2 weeks. SNOT-22 scores decreased significantly and the difference was relevant (mean difference 12 points) (24). It should be noted, however, that there was no placebo group, numbers were small and a large portion of participants had mild CRS based on baseline SNOT-22 scores.
A different diet with high omega-3/low omega-6 fatty acids showed promising result in a small pilot trial with 10 adult patients suffering from primary diffuse Type 2 CRS and N-ERD (25). Along with biomarkers indicating a change in cellular fatty acid composition, SNOT-22 scores decreased 15.1 points on average (95% CI: −24.3; −6.0) after a 2-weeks high omega-3/low omega-6 fatty acids diet. This unblinded setup might contain placebo effects and the findings remain to be confirmed in larger studies.
Aspirin Therapy After Desensitization
Aspirin therapy after desensitization (ATAD) is based on a two-step treatment: first, patients are desensitized by exposure to increasing doses of oral aspirin; second, they continue using high doses of aspirin daily. Several studies have investigated this treatment option in primary diffuse Type 2 CRS in N-ERD patients.
The finding that N-ERD patients experience a short period (24–72 h) of symptom alleviation after a challenge with aspirin, and that they are refractory to additional aspirin challenges (26), has led to the development of several desensitization protocols. A widely used protocol aims at oral aspirin 625 mg twice daily after a startup phase (27). There have been several studies describing the effect of such protocols on CRS signs and symptoms, four of which are placebo-controlled trials.
A small study with 27 CRS patients undergoing ATAD (28), showed significant improvement of nasal symptoms, such as nasal congestion, discharge, and overall discomfort. The visible amount of polyps did not improve significantly. Moreover, only 12 of the original 27 patients were available for evaluation as 5 had ATAD treatment complications and 10 chose to discontinue ATAD. Similar findings were obtained in a small prospective trial with 12 patients of whom 8 were still on maintenance therapy after 6 months, with comparable endoscopic scores to baseline, but improved symptom scores as measured with the SNOT-22, decreasing from a mean of 30.0 at baseline to 18.5 after 6 months (29). A study with 30 post-operative CRS patients showed a sustained improvement of endoscopic scores compared to baseline, due to surgery, and further improving SNOT-22 scores during aspirin treatment after surgery up to 30 months of follow-up (30). Another retrospective study of post-operative ATAD showed similar results in 34 patients, of whom 2 could not complete the desensitization phase and 5 stopped in the maintenance phase due to gastrointestinal or respiratory side-effects (31). Apart from these (and other) small studies, four double-blind placebo-controlled trials have been published on the use of ATAD for CRS in N-ERD patients (32–35), three of which were used for a meta-analysis in EPOS2020 (1). ATAD was shown to have a significant effect over placebo in the reduction of SNOT-22 scores or other symptoms scores. However, this did not reach the threshold for a clinically relevant difference. The lung functions scores (FEV-1) were significantly better with ATAD. The meta-analyses were based on relatively small pooled patient groups, ranging between 70 and 85 patients.
The downsides of ATAD include side-effects. Some patients are not able to endure the desensitization phase, others experience respiratory or gastrointestinal problems. Other medical conditions that require elective surgery can also be a cause of discontinuation. Furthermore, the medication regime is strict and not a single day of aspirin can be missed in order for ATAD to be successful. Current evidence, in line with the studies described above, shows that for a relatively large portion of N-ERD patients, ATAD is not a long-term solution (36).
To conclude, ATAD is a relevant treatment option for primary diffuse Type 2 CRS in N-ERD patients. Patient selection, education and shared decision making are key for a successful application of this treatment.
Antileukotrienes
Leukotrienes are a class of breakdown products of arachidonic acid produced by eosinophils and mast cells. There might be a role for these leukotrienes in the pathophysiology of primary diffuse Type 2 CRS in general; especially in the context of N-ERD, there seems to be a more direct link. Nasal polyp biopsies from N-ERD patients show an increased number of leukocytes expressing leukotriene receptors as compared to non-N-ERD nasal polyps, with a reduction in these leukocytes after a desensitization protocol (37). Therefore, it seems logical to use antileukotriene drugs, such as montelukast or zileuton, in the treatment strategy of CRS in general, and specifically in N-ERD patients.
After reviewing the current evidence on antileukotrienes in CRS, the EPOS2020 steering committee concluded that the use of such drugs in CRS was not advised, as (limited) evidence shows no benefit over the use of nasal corticosteroid or when used in combination with nasal corticosteroids. Only in patients not tolerating nasal corticosteroids, one might consider antileukotrienes although there a no supporting studies (1).
Apart from the evidence reviewed by EPOS2020, two studies have been performed to address the effect of antileukotrienes for primary diffuse Type 2 CRS in the specific context of N-ERD. One study evaluating montelukast contained 33 N-ERD patients who were allocated to use either intranasal corticosteroids, montelukast, or both, directly after FESS. After a follow-up of 12 months, no differences were found in nasal endoscopy scores of the polyps, or patient-reported outcome measures (visual analog scale (VAS) and SNOT-22) (38). The other study retrospectively analyzed post-operative patients using zileuton (n = 18) vs. those without (n = 27), showing no differences in clinical outcomes over an average follow-up of 2.8 and 2.4 years, respectively (39). Of note, the zileuton group used this drug for 77 days on average (range 6–300 days). Although these two studies are small and from their setup give limited evidence, it seems that also for N-ERD patients specifically, antileukotrienes are not to be recommended as an additional treatment strategy for their CRS.
It is important to recognize, however, that the European Academy for Allergy and Clininal Immunology (EAACI) position paper on the diagnosis and management of N-ERD does describe the use of antileukotrienes in N-ERD patients as add-on therapy for their asthma (21). As such, a N-ERD patient with both CRS and asthma might benefit from these drugs, but the indication should be derived from the lower airway disease status.
Biologicals
With the advent of biological therapy, the management of primary diffuse Type 2 CRS has been tremendously revolutionized. Pivotal trials and the first real-life data show rapid disease control and improvement of the quality of life, often beyond the effect sizes that treatment strategies hitherto could offer (40–42). However, these trials target patients with primary diffuse Type 2 CRS per se, either in the context of N-ERD or in NSAID-tolerant patients. No randomized-controlled trials with N-ERD patients only have been performed.
Omalizumab
Omalizumab is a monoclonal antibody directed against immunoglobulin E. It has been approved as add-on therapy for CRSwNP in 2020. Previously, it has already been used for over a decade by pulmonologists for the treatment of asthma. Therefore, many (small) reports on the effects of omalizumab for N-ERD focus on the lower airways, with a SNOT-22 as marker for the upper airways at best (43). One open study with 16 N-ERD patients receiving omalizumab vs. 16 N-ERD patients with ATAD, describes improvement of CRS signs and symptoms in 14 of the 16 omalizumab patients. Together with a decrease in patient-reported scores, there was a reduction of nasal polyp scores on a scale from 0 to 8: baseline mean score 3; after 9 months the mean score was 0, indicating no visible polyps left. In the ATAD group, nasal polyp scores remained unchanged (mean score 3 at baseline and 2.9 at 9 months) (44).
Dupilumab
Dupilumab is a monoclonal antibody directed against the alpha unit of the IL4/IL13 receptor. It was approved late 2019 as add-on therapy for CRSwNP. In a post-hoc analysis from a previously published phase 2a trial (45), the N-ERD patients (n=19) and NSAID-tolerant patients (n = 41) were compared. Both groups showed significant improvements in disease control after 16 weeks of treatment, as assessed by imaging (Lund-Mackay score), patient-reported outcome measures (SNOT-22 total score, SNOT-22 sense of smell/taste score), and smell test score (University of Pennsylvania Smell Identification Test). Eight N-ERD patients were in the dupilumab-treated group. Their nasal polyp scores were significantly reduced by a mean of 2.51, whereas the NSAID-tolerant patients had a non-significant reduction of 0.72.
Mepolizumab
Mepolizumab is an antibody directed against IL-5. It is currently not registered for use in CRSwNP patients, but for asthma only. In a retrospective study including 17 N-ERD patients receiving mepolizumab for severe asthma, SNOT-22 scores were observed to decrease significantly and relevantly by 17.7 points (n = 11). Specific SNOT-22 questions on nasal congestion and smell/taste also showed an improvement with three or more doses of mepolizumab (46).
General Comments
Although there are hardly any specific studies on the effect of biologicals for CRS in the context of N-ERD, it is highly likely that these patients will follow the observations seen in NSAID-tolerant Type 2 CRS patients. In a recent meta-analysis on biologicals for CRS, all three biologicals with at least phase 2a data were shown to be effective. Direct comparisons were hard to make due to differences in study setup, patient population, treatment duration, etc. No clear advice could be given on which biological to prefer (47).
In practice, the use of biologicals in CRS, and therefore in N-ERD patients, is a matter of shared-decision making, depending on the local situation/availability, physician experience, and patient preference.
Future Needs
In order to better understand and compare treatment effects, and to facilitate meta-analyses, we would advise authors of future studies on primary diffuse Type 2 CRS to report effects in N-ERD patients separately. It remains to be elucidated whether the presence of N-ERD represents a clinically relevant subgroup when it comes to treatment outcomes, or that most patients with primary diffuse Type 2 CRS in the context of N-ERD respond largely similar to NSAID-tolerant patients.
Discussion
This mini-review describes the possible therapeutic options for primary diffuse Type 2 CRS in N-ERD patients. Apart from the customary appropriate medical therapy and surgery, ATAD and especially biologicals hold most promise for the future.
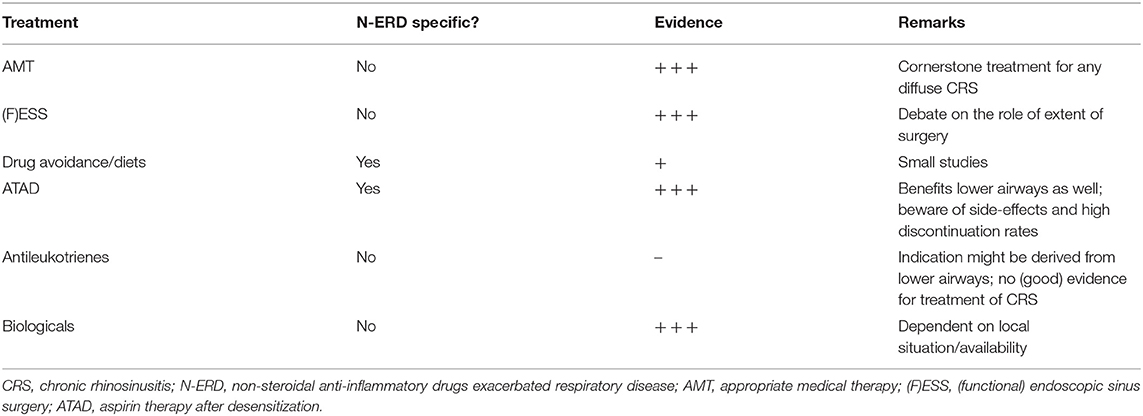
The treatment options are summarized in Table 1. For most of the therapeutic options described, patient counseling and shared-decision making are key to success. Whether one discusses diets, ATAD, or biologicals, all of these represent a long-term therapy, needing long-term treatment adherence. Treating physicians should be keen to inquire for patient preferences, past experiences and future hopes. As such, clear communication with the patient is essential, with an important role for managing expectations. Furthermore, a patient-tailored approach includes treatment plans for the upper airway (as discussed here), and for the lower airways as well. Most of these treatments are therefore best employed in close collaboration between allergists, pulmonologists and ENT-surgeons.
Unfortunately, the evidence for most treatment options relies on relatively small studies. Therefore, studies targeting N-ERD patients specifically are needed, and studies including both NSAID-tolerant and intolerant patients should report on the subgroup of N-ERD subjects separately. Especially biologicals deserve attention in the coming years, not only with a focus on treatment outcomes, but also on costs. It might well be that in general, biologicals will prove not to be cost-effective in a broad group of primary diffuse Type 2 CRS patients, but only in the more severe cases; N-ERD patients typically fall in the latter group.
Author Contributions
SR performed the literature search and wrote the initial manuscript. RL and WF reviewed and updated the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
RL, WF, and SR received research grants by Sanofi and Novartis. WF and SR have been employed as consultant for Sanofi, Novartis, and GSK.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Fokkens WJ, Lund VJ, Hopkins C, Hellings PW, Kern R, Reitsma S, et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. (2020) 58(Suppl. S29):1–464. doi: 10.4193/Rhin20.601
2. Hastan D, Fokkens WJ, Bachert C, Newson RB, Bislimovska J, Bockelbrink A, et al. Chronic rhinosinusitis in Europe–an underestimated disease. A GA(2)LEN study. Allergy. (2011) 66:1216–23. doi: 10.1111/j.1398-9995.2011.02646.x
3. Hirsch AG, Stewart WF, Sundaresan AS, Young AJ, Kennedy TL, Scott Greene J, et al. Nasal and sinus symptoms and chronic rhinosinusitis in a population-based sample. Allergy. (2017) 72:274–81. doi: 10.1111/all.13042
4. Ostovar A, Fokkens WJ, Vahdat K, Raeisi A, Mallahzadeh A, Farrokhi S. Epidemiology of chronic rhinosinusitis in Bushehr, southwestern region of Iran: a GA2LEN study. Rhinology. (2019) 57:43–8. doi: 10.4193/Rhin18.061
5. Dietz de Loos D, Lourijsen ES, Wildeman MAM, Freling NJM, Wolvers MDJ, Reitsma S, et al. Prevalence of chronic rhinosinusitis in the general population based on sinus radiology and symptomatology. J Allergy Clin Immunol. (2019) 143:1207–14. doi: 10.1016/j.jaci.2018.12.986
6. Hirsch AG, Nordberg C, Bandeen-Roche K, Tan BK, Schleimer RP, Kern RC, et al. Radiologic sinus inflammation and symptoms of chronic rhinosinusitis in a population-based sample. Allergy. (2020) 75:911–20. doi: 10.1111/all.14106
7. Tomassen P, Newson RB, Hoffmans R, Lotvall J, Cardell LO, Gunnbjornsdottir M, et al. Reliability of EP3OS symptom criteria and nasal endoscopy in the assessment of chronic rhinosinusitis–a GA(2) LEN study. Allergy. (2011) 66:556–61. doi: 10.1111/j.1398-9995.2010.02503.x
8. Lourijsen ES, Fokkens WJ, Reitsma S. Direct and indirect costs of adult patients with chronic rhinosinusitis with nasal polyps. Rhinology. (2020) 58:213–7. doi: 10.4193/RHINOL/20.092
9. Goetzel RZ, Hawkins K, Ozminkowski RJ, Wang S. The health and productivity cost burden of the “top 10” physical and mental health conditions affecting six large U.S. employers in 1999. J Occup Environ Med. (2003) 45:5–14. doi: 10.1097/00043764-200301000-00007
10. Klonaris D, Doulaptsi M, Karatzanis A, Velegrakis S, Milioni A, Prokopakis E. Assessing quality of life and burden of disease in chronic rhinosinusitis: a review. Rhinol Online. (2019) 2:6–13. doi: 10.4193/RHINOL/18.067
11. Khan A, Huynh TMT, Vandeplas G, Joish VN, Mannent LP, Tomassen P, et al. The GALEN rhinosinusitis cohort: chronic rhinosinusitis with nasal polyps affects health-related quality of life. Rhinology. (2019) 57:343–51. doi: 10.4193/Rhin19.158
12. Lange BM, Mortz CG, Bindslev-Jensen C, Kjeldsen AD. Nasal symptoms in patients with NSAID hypersensitivity. Rhinol Online. (2019) 2:91–6. doi: 10.4193/RHINOL/19.009
13. Philpott CM, Erskine S, Hopkins C, Kumar N, Anari S, Kara N, et al. Prevalence of asthma, aspirin sensitivity and allergy in chronic rhinosinusitis: data from the UK National Chronic Rhinosinusitis Epidemiology Study. Respir Res. (2018) 19:129. doi: 10.1186/s12931-018-0823-y
14. Makowska JS, Burney P, Jarvis D, Keil T, Tomassen P, Bislimovska J, et al. Respiratory hypersensitivity reactions to NSAIDs in Europe: the global allergy and asthma network (GA(2) LEN) survey. Allergy. (2016) 71:1603–11. doi: 10.1111/all.12941
15. Stevens WW, Peters AT, Hirsch AG, Nordberg CM, Schwartz BS, Mercer DG, et al. Clinical characteristics of patients with chronic rhinosinusitis with nasal polyps, asthma, and aspirin-exacerbated respiratory disease. J Allergy Clin Immunol Pract. (2017) 5:1061–70.e3. doi: 10.1016/j.jaip.2016.12.027
16. Cahill KN, Boyce JA. Aspirin-exacerbated respiratory disease: mediators and mechanisms of a clinical disease. J Allergy Clin Immunol. (2017) 139:764–6. doi: 10.1016/j.jaci.2016.09.025
17. Steiner UC, Bischoff S, Valaperti A, Ikenberg K, Starzyk J, Bucher S, et al. Endotypes of chronic rhinosinusitis with nasal polyps with and without NSAID - intolerance. Rhinology. (2020) 58:544–9. doi: 10.4193/Rhin19.423
18. Reitsma S, Adriaensen G, Cornet ME, van Haastert RM, Raftopulos MH, Fokkens WJ. The Amsterdam Classification of Completeness of Endoscopic Sinus Surgery (ACCESS): a new CT-based scoring system grading the extent of surgery. Rhinology. (2020) 58:538–43. doi: 10.4193/Rhin20.165
19. Zhang L, Zhang Y, Gao Y, Wang K, Lou H, Meng Y, et al. Long-term outcomes of different endoscopic sinus surgery in recurrent chronic rhinosinusitis with nasal polyps and asthma. Rhinology. (2020) 58:126–35. doi: 10.4193/Rhin19.184
20. Delarestaghi MM, Rajaeih S, Firouzabadi FD, Jamali M, Roomiani M, Firouzabadi MD, et al. Evaluation of the effect of endoscopic partial middleturbinectomy surgery on the quality of life of patients with chronic rhinosinusitis and nasal polyps. Rhinology. (2020) 58:208–12. doi: 10.4193/Rhin19.258
21. Kowalski ML, Agache I, Bavbek S, Bakirtas A, Blanca M, Bochenek G, et al. Diagnosis and management of NSAID-Exacerbated Respiratory Disease (N-ERD)-a EAACI position paper. Allergy. (2019) 74:28–39. doi: 10.1111/all.13599
22. Alanin MC, Laidlaw T, Society TS, Hopkins C. The burden of non-steroidal anti-inflammatory exacerbated respiratory disease from the patient's perspective - a qualitative analysis of posts from the Samter's Society. Rhinology. (2020) 58:333–40. doi: 10.4193/Rhin19.430
23. Sommer DD, Rotenberg BW, Sowerby LJ, Lee JM, Janjua A, Witterick IJ, et al. A novel treatment adjunct for aspirin exacerbated respiratory disease: the low-salicylate diet: a multicenter randomized control crossover trial. Int Forum Allergy Rhinol. (2016) 6:385–91. doi: 10.1002/alr.21678
24. Keszycka PK, Lange E, Gajewska D. Effectiveness of personalized low salicylate diet in the management of salicylates hypersensitive patients: interventional study. Nutrients. (2021) 13:991. doi: 10.3390/nu13030991
25. Schneider TR, Johns CB, Palumbo ML, Murphy KC, Cahill KN, Laidlaw TM. Dietary fatty acid modification for the treatment of aspirin-exacerbated respiratory disease: a prospective pilot trial. J Allergy Clin Immunol Pract. (2018) 6:825–31. doi: 10.1016/j.jaip.2017.10.011
26. Pleskow WW, Stevenson DD, Mathison DA, Simon RA, Schatz M, Zeiger RS. Aspirin desensitization in aspirin-sensitive asthmatic patients: clinical manifestations and characterization of the refractory period. J Allergy Clin Immunol. (1982) 69(1 Pt 1):11–9. doi: 10.1016/0091-6749(82)90081-1
27. White AA, Stevenson DD. Aspirin desensitization in aspirin-exacerbated respiratory disease. Immunol Allergy Clin North Am. (2013) 33:211–22. doi: 10.1016/j.iac.2012.10.013
28. Forer B, Kivity S, Sade J, Landsberg R. Aspirin desensitization for ASA triad patients–prospective study of the rhinologist‘s perspective. Rhinology. (2011) 49:95–9. doi: 10.4193/Rhino09.113
29. Cooper T, Greig SR, Zhang H, Seemann R, Wright ED, Vliagoftis H, et al. Objective and subjective sinonasal and pulmonary outcomes in aspirin desensitization therapy: a prospective cohort study. Auris Nasus Larynx. (2019) 46:526–32. doi: 10.1016/j.anl.2018.12.002
30. Cho KS, Soudry E, Psaltis AJ, Nadeau KC, McGhee SA, Nayak JV, et al. Long-term sinonasal outcomes of aspirin desensitization in aspirin exacerbated respiratory disease. Otolaryngol Head Neck Surg. (2014) 151:575–81. doi: 10.1177/0194599814545750
31. Adappa ND, Ranasinghe VJ, Trope M, Brooks SG, Glicksman JT, Parasher AK, et al. Outcomes after complete endoscopic sinus surgery and aspirin desensitization in aspirin-exacerbated respiratory disease. Int Forum Allergy Rhinol. (2018) 8:49–53. doi: 10.1002/alr.22036
32. Mortazavi N, Esmaeilzadeh H, Abbasinazari M, Babaie D, Alyasin S, Nabavizadeh H, et al. Clinical and immunological efficacy of aspirin desensitization in nasal polyp patients with aspirin-exacerbated respiratory disease. Iran J Pharm Res. (2017) 16:1639–47.
33. Esmaeilzadeh H, Nabavi M, Aryan Z, Arshi S, Bemanian MH, Fallahpour M, et al. Aspirin desensitization for patients with aspirin-exacerbated respiratory disease: a randomized double-blind placebo-controlled trial. Clin Immunol. (2015) 160:349–57. doi: 10.1016/j.clim.2015.05.012
34. Swierczynska-Krepa M, Sanak M, Bochenek G, Strek P, Cmiel A, Gielicz A, et al. Aspirin desensitization in patients with aspirin-induced and aspirin-tolerant asthma: a double-blind study. J Allergy Clin Immunol. (2014) 134:883–90. doi: 10.1016/j.jaci.2014.02.041
35. Fruth K, Pogorzelski B, Schmidtmann I, Springer J, Fennan N, Fraessdorf N, et al. Low-dose aspirin desensitization in individuals with aspirin-exacerbated respiratory disease. Allergy. (2013) 68:659–65. doi: 10.1111/all.12131
36. Laulajainen-Hongisto A, Turpeinen H, Vento SI, Numminen J, Sahlman J, Kauppi P, et al. High discontinuation rates of peroral ASA treatment for CRSwNP: a real-world multicenter study of 171 N-ERD patients. J Allergy Clin Immunol Pract. (2020) 8:3565–74. doi: 10.1016/j.jaip.2020.06.063
37. Sousa AR, Parikh A, Scadding G, Corrigan CJ, Lee TH. Leukotriene-receptor expression on nasal mucosal inflammatory cells in aspirin-sensitive rhinosinusitis. N Engl J Med. (2002) 347:1493–9. doi: 10.1056/NEJMoa013508
38. Stryjewska-Makuch G, Humeniuk-Arasiewicz M, Jura-Szoltys E, Gluck J. The effect of antileukotrienes on the results of postoperative treatment of paranasal sinuses in patients with non-steroidal anti-inflammatory drug-exacerbated respiratory disease. Int Arch Allergy Immunol. (2019) 179:281–9. doi: 10.1159/000499134
39. Lee SE, Farquhar DR, Adams KN, Masood MM, Senior BA, Thorp BD, et al. Effect of Zileuton treatment on sinonasal quality of life in patients with aspirin-exacerbated respiratory disease. Am J Rhinol Allergy. (2019) 33:791–5. doi: 10.1177/1945892419873211
40. Bachert C, Han JK, Desrosiers M, Hellings PW, Amin N, Lee SE, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet. (2019) 394:1638–50. doi: 10.1016/S0140-6736(19)31881-1
41. Gevaert P, Omachi TA, Corren J, Mullol J, Han J, Lee SE, et al. Efficacy and safety of omalizumab in nasal polyposis: 2 randomized phase 3 trials. J Allergy Clin Immunol. (2020) 146:595–605. doi: 10.1016/j.jaci.2020.05.032
42. Bachert C, Sousa AR, Lund VJ, Scadding GK, Gevaert P, Nasser S, et al. Reduced need for surgery in severe nasal polyposis with mepolizumab: randomized trial. J Allergy Clin Immunol. (2017) 140:1024–31.e14. doi: 10.1016/j.jaci.2017.05.044
43. Cameli P, Perruzza M, Salvini M, Fui A, Cekorja B, Refini RM, et al. Omalizumab treatment in Samter's triad: case series and review of the literature. Eur Rev Med Pharmacol Sci. (2019) 23:8124–9. doi: 10.26355/eurrev_201909_19031
44. Forster-Ruhrmann U, Stergioudi D, Pierchalla G, Fluhr JW, Bergmann KC, Olze H. Omalizumab in patients with NSAIDs-exacerbated respiratory disease. Rhinology. (2020) 58:226–32. doi: 10.4193/Rhin19.318
45. Bachert C, Mannent L, Naclerio RM, Mullol J, Ferguson BJ, Gevaert P, et al. Effect of subcutaneous dupilumab on nasal polyp burden in patients with chronic sinusitis and nasal polyposis: a randomized clinical trial. JAMA. (2016) 315:469–79. doi: 10.1001/jama.2015.19330
46. Tuttle KL, Buchheit KM, Laidlaw TM, Cahill KN. A retrospective analysis of mepolizumab in subjects with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol Pract. (2018) 6:1045–7. doi: 10.1016/j.jaip.2018.01.038
Keywords: NSAID (non-steroidal anti-inflammatory drug), N-ERD, chronic rhinosinusitis, aspirin desensitization, aspirin therapy, biological
Citation: van der Lans RJL, Fokkens WJ and Reitsma S (2021) Therapeutic Options for Chronic Rhinosinusitis in N-ERD Patients. Front. Allergy 2:734000. doi: 10.3389/falgy.2021.734000
Received: 30 June 2021; Accepted: 06 August 2021;
Published: 24 August 2021.
Edited by:
Sanna Katriina Toppila-Salmi, University of Helsinki, FinlandReviewed by:
Aleksandar Peric, Military Medical Academy, SerbiaElena Cantone, University of Naples Federico II, Italy
Copyright © 2021 van der Lans, Fokkens and Reitsma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sietze Reitsma, cy5yZWl0c21hJiN4MDAwNDA7YW1zdGVyZGFtdW1jLm5s
 Rik J. L. van der Lans
Rik J. L. van der Lans Wytske J. Fokkens
Wytske J. Fokkens Sietze Reitsma
Sietze Reitsma