- 1Department of Translational Medical Science, University of Naples Federico II, Naples, Italy
- 2ImmunonutritionLab at the CEINGE Advanced Biotechnologies Research Center, University of Naples Federico II, Naples, Italy
- 3European Laboratory for the Investigation of Food-Induced Diseases, University of Naples Federico II, Naples, Italy
- 4Task Force for Microbiome Studies, University of Naples Federico II, Naples, Italy
Cow's milk allergy (CMA) is one of the most common food allergies and one of the main causes of food-induced anaphylaxis in the pediatric age. Moreover, up to 45% of CMA children develop other atopic manifestations later in life, a phenomenon commonly named atopic march. Thus, CMA imposes a significant cost to health care systems as well as to families, and has emerged as one of the most expensive allergic diseases. The immunonutrition strategy builds its foundation on the ability of selected dietary factors to modulate immune system development and function. Recent studies highlighted the potential of immunonutrition in the management of CMA. This review is focused on the mechanisms and long-term clinical outcomes of the immunonutrition approach in children with CMA.
Introduction
Much has changed during the recent decades regarding prevalence, persistence, and severity of clinical features and socio-economic burden of food allergy (FA) that currently affects up to 10% of children living in Western countries (1). Based on the immune mechanisms, FA may be classified as IgE-mediated, non-IgE-mediated, or a combination of both pathways (2). In addition, children presenting with FA in early life are at increased risk of developing other allergic manifestations later in life, such as allergic asthma and rhinitis, a phenomenon commonly named atopic march (AM) (3). With an estimated prevalence of up to 3%, cow's milk allergy (CMA) is one of the most common FA and one of the main causes of food-induced anaphylaxis in the pediatric age (4). This condition imposes a significant cost to health care systems as well as to families, and has emerged as one of the most expensive allergic diseases (4). Furthermore, early life CMA could be the first step of AM, which affects up to 45% of CMA children, also after the acquisition of immune tolerance to cow's milk proteins (CMP) (5–8). Both CMA and AM derive from a negative interaction between genetic and environmental factors (3) resulting in alteration in the gut microbiome (GM) and in immune system dysfunction. These modulatory effects are at least in part mediated by epigenetic mechanisms, and are now emerging as potential targets of intervention to facilitate the immune tolerance acquisition and to limit the occurrence of AM in CMA patients (5, 9–11).
The traditional dietary management of CMA has greatly changed in the last few years, moving from a passive approach based on the strict elimination diet of CMP-containing foods, to a proactive one, able to change the CMA course (12).
The discovery of the pivotal role of selected dietary factors in influencing immune system development and function has introduced the immunonutrition concept. The application of the immunonutrition approach in the management of CMA is paving the way to “active diet therapy,” an integrated dietary strategy able to facilitate the acquisition of immune tolerance and to prevent the occurrence of AM (5, 9, 13–15).
The modern dietary management in CMA pediatric patients is focused on three targets:
• Dietary education (allergen avoidance and healthy diet)
• Ensure adequate intake of macro and micro-nutrients (stimulation of optimal body growth and development)
• Active diet therapy (stimulation of immune tolerance and protection against AM occurrence).
The immunonutrition approach could be promoted for all three of these targets. This review is focused on the objectives and long-term clinical outcomes of the immunonutrition approach in children affected by CMA.
Dietary Education: From Allergen Avoidance to Healthy Diet Promotion
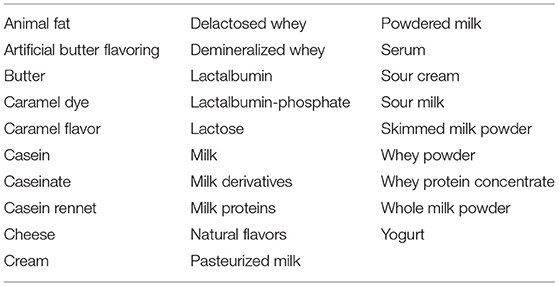
Soon after diagnosis, important information should be provided to the families for a successful elimination diet. This information includes label terminologies (including non-standard terms such as “casein” or “whey”) and possible cross-reacting foods. In addition, clear information regarding food preparation at home should be provided with the aim to limit the risk of cross-contamination (16). Table 1 displays the foods that should be avoided in designing a correct elimination diet in children affected by CMA.
Considering the emerging evidence on the role of dietary factors facilitating the occurrence of FA, the modern dietary education of CMA patients should also promote a healthy diet.
Diet plays a pivotal role in influencing the composition and metabolic features of the GM, which in turn is able to directly or indirectly modulate immune system development and function introducing the concept of the “diet-GM-immune system axis” (17). The Western-type diet is made up of highly processed and synthetic foods and sugars, rich in advanced glycation end products (AGEs), and food additives (emulsifiers, sweeteners, and preservatives). The consumption of this dietary pattern has been shown to affect negatively both the GM and the immune system (18, 19). Although to date no evidence has shown a direct relation between FA and AGEs in humans, preclinical data suggest an implication of AGEs in the mechanistic onset of FA (20, 21).
On the other hand, the promotion of a healthy dietary pattern that provides a high amount of fiber, a balanced ratio of ω-6/ω-3- polyunsaturated fatty acids (PUFAs), and polyphenols able to positively modulate the GM, should be one of the nutritional strategies to beneficially impact the GM-immune system axis (22–27).
The beneficial effect of this healthy dietary pattern on the GM is due to the synergistic and interactive combinations of nutrients, with a pivotal role exerted by fiber. Microbial conversions of dietary fiber leads to the production of some immune-modulatory metabolites such as SCFAs. Our research team has recently demonstrated that the GM of children who acquired tolerance to CMP is characterized by an enrichment of bacteria producing SCFAs, including butyric acid, which can have multiple effects on the immune response through the differentiation of Treg cells and the regulation of epithelial integrity, inducing an immune tolerance status (4).
Ensuring Adequate Body Growth and Development
Cow's milk is an important source of nutrients such as calcium, phosphorus, vitamin B2 (riboflavin), vitamin B5 (pantothenic acid), vitamin B12 (cobalamin), vitamin D, proteins, and lipids that can be deficient if cow's milk is completely excluded from the diet (28). In non-breastfed infants, the use of substitute formula is necessary until the development of immune tolerance to limit the risk of nutritional deficiencies. Commercially available substitutive formulas are commonly tested regarding allergenicity and effect on body growth pattern and are the preferred choice for optimal dietary management of CMA infants (29). The use of plant-based products, not designed for infant nutrition, should be discouraged for the high risk of nutritional deficiencies.
In the first semester of life, any calcium supplementation must be evaluated from time to time based on the composition of the substitutive formula. In the second semester, with the introduction of solid foods, the consumption of formula progressively decreases and calcium supplementation could be required when the formula intake is <500 ml/day. Moreover, in CMA children, who do not reach immune tolerance within the first year, calcium supplementation should be considered for the entire duration of the exclusion diet, based on the child's eating habits. Total calcium supplementation can vary from 600 mg/day in the early years of life to 1,000 mg/day during childhood and adolescence, according to the recommended intake for age (30). Calcium administration should always be associated with vitamin D assessment, considering a reduction from 30–40 to 10–15% in calcium absorption when vitamin D deficiency is concomitant.
Attention should be paid to the vitamin D content in substitutive formula. Vitamin D3 is more effective in raising serum 25(OH)D concentration than equimolar vitamin D2 (31), and vitamin D deficiency has been reported in CMA subjects (32, 33).
According to the international guidelines, vitamin D supplementation should be given in all CMA infants over 6 months old who receive breast milk as their main feed. In non-breastfed CMA infants, vitamin D supplementation should be evaluated based on the child's eating habits and should be continued throughout the exclusion diet period according to the recommended intakes for age, if needed (34–36). In addition to the pivotal role in calcium metabolism, vitamin D has been suggested to play a role in influencing the immune tolerance network, through a wide range of immune and non-immune mechanisms (37–39). Thus, optimal vitamin D supplementation should also be provided considering the recent evidence demonstrating an increased risk of FA development in children exposed to higher than recommended supplementation doses (40, 41).
Besides vitamin D and calcium, ω-3-PUFAs could also be relevant in the dietary management of CMA children. These micronutrients exert a wide range of beneficial immune-modulatory actions (42, 43). Deficiency of ω-3 PUFAs have been demonstrated in children affected by FA including CMA (44, 45). The origin of deficiency in these patients is still largely obscure, suggesting the importance of an individualized ω-3 PUFA supplementation in CMA children.
Active Diet Therapy: From Substitutive Formula to Oral Immunotherapy
The concept of active diet therapy is focused on the role of varying dietary factors to treat the underlying disease, changing the disease course, and impacting symptom relief. The CMA dietary approach, which is able to facilitate immune tolerance acquisition and prevent AM occurrence in pediatric patients, perfectly matches with this concept. In this paragraph, we focus on the role of different active dietary strategies, starting from substitutive formulas, pro-, pre-, and synbiotics, to baked milk products and oral immunotherapy.
Driving Immune System Function Through Substitutive Formulas
Whatever the clinical pattern of CMA, the mainstay of treatment is the elimination of CMP from the patient's diet. If breastfeeding is not available, the infant must be fed with a substitutive formula adapted to CMA dietary management. This formula should be adequate in terms of nutritional compounds and allergenic safety. The most used are the following: extensively hydrolyzed whey (EHWF), casein formula (EHCF), soy formula (SF), hydrolyzed rice formula (HRF), or amino acid-based formula (AAF) (46). It has been estimated that it is up to six times more expensive to feed a child with CMA, and substitutive formulas have emerged as a primary cost driver for the management of pediatric patients with CMA (46, 47). Thus, options to stimulate immune tolerance acquisition would be very welcomed by affected families and health care systems. The forefront of the immunonutrition approach is to move from a passive approach, consisting of an elimination diet to relieve symptoms, to a “pro-active” one, meaning the possibility to actively modulate the immune system toward immune tolerance.
We have recently demonstrated a different modulation on tolerogenic mechanisms elicited by the protein fraction derived from the substitutive formulas commonly used for CMA management in human cells (4). In particular, we found that only an EHCF-derived protein fraction could activate several tolerogenic mechanisms through, at least in part, an epigenetic regulation of gene expression. Other studied formulas (EHWF, HRF, SF, and AAF) were unable to modulate such mechanisms (4). Clinical trials demonstrated that EHCF could accelerate immune tolerance acquisition in CMA children if compared to other dietary strategies through, at least in part, an epigenetic modulation of the FoxP3 gene (8, 48). CD4+/CD25+/FoxP3+ cells are central in the maintenance of immune homeostasis and tolerance. We found that only an EHCF-derived protein fraction elicited a significant activation of CD4+/CD25+/FoxP3+ Tregs, through DNA demethylation of the FoxP3 transcription factor (4).
Driving Immune System Function Through Pro-, Pre-, or Synbiotics
The exposure to factors that positively influence GM composition and function, such as pro- and prebiotics, could lead to a positive modulation of the immune system with final beneficial effects in children with CMA.
Probiotics are live microorganisms that confer benefits to host health when administered in adequate amounts (49). The administration of probiotics in CMA infants could be helpful in improving gastrointestinal symptoms, as demonstrated in an open randomized trial investigating the effect of Bifidobacterium lactis BB-12 (1 × 109 CFU daily dose) and Streptococcus thermophilus TH-4 (1 × 108 CFU daily dose) in association with a CMP elimination diet (50). More recently, a randomized, double-blind, placebo-controlled clinical trial conducted on 100 CMA infants showed the efficacy of L. rhamnosus (LGG) together with a CMP-free diet in improving symptoms such as bloody and mucous stool, vomiting, diarrhea, restiveness, and abdominal distension (51). The role of the probiotic LGG in hastening the development of immune tolerance in IgE-mediated CMA infants has been also demonstrated. We have shown that the administration of EHCF supplemented with LGG induced higher tolerance rate acquisition after 6 and 12 months compared with EHCF alone or with other substitutive formulas (5, 8). Moreover, at the 3-year follow-up of 220 infants with CMA, those treated with EHCF+LGG showed a greater rate of immune tolerance acquisition and a lower incidence of AM onset compared with CMA children treated with EHCF alone (6). In addition, we have shown that CMA infants treated with EHCF+LGG presented an increased number of bacteria strains able to produce the SCFA butyrate, a pivotal tolerogenic metabolite (7). Figure 1 depicts the mechanisms of action of EHCF+LGG, these immunomodulatory effects could be responsible for the beneficial action of this formula in reducing the occurrence of AM as demonstrated in a large study involving CMA children (52). These data are well in line with those of a retrospective study revealing that the first-line management of newly diagnosed CMA infants treated with EHCF+LGG may slow down AM if compared with infants treated with EHWF (53). In another double-blind controlled trial, the prenatal administration of LGG to mothers with a family history of atopic diseases, and after birth to their infants for the first 6 months, was able to protect the offspring from atopic diseases at the age of 2 (54). In line with these results, EHWF supplementation with LGG in infants with atopic eczema and CMA resulted in the affective reduction of atopic eczema and allergic symptoms. However, GM structure was unchanged in these subjects (55). Altogether, these data highlight the role of EHCF+LGG in improving clinical outcomes, in freeing up healthcare resources for alternative use, in reducing the cost of patient management, and thereby affords a cost-effective dietetic strategy in the management of CMA infants for the health care system (56, 57).
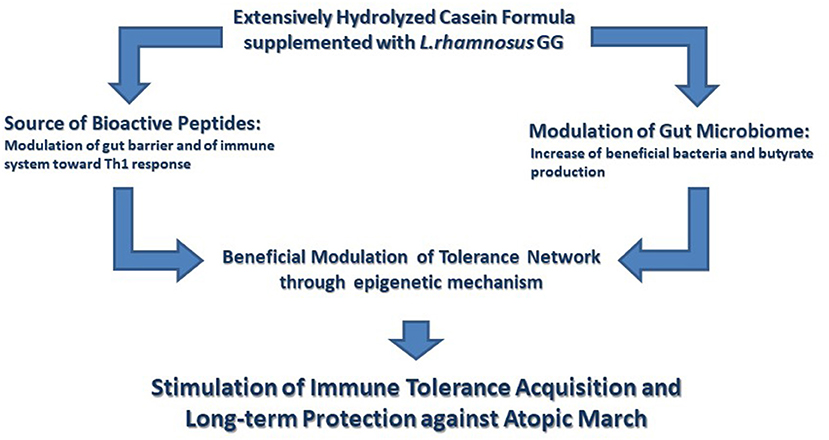
Figure 1. The immunomodulatory effects elicited by the extensively hydrolyzed casein formula supplemented with the probiotic Lactobacillus rhamnosus GG. Extensively hydrolyzed casein formula supplemented with the probiotic Lactobacillus rhamnosus GG (EHCF+LGG) activates several tolerogenic mechanisms. These tolerogenic mechanisms are activated by the synergist action of immunomodulatory peptides, deriving from casein hydrolysis, and by the beneficial action of LGG on gut microbiome structure and function leading to an increased production of butyrate. Many of these effects involve an epigenetic regulation of gene expression with a central role in the maintenance of immune homeostasis. Altogether these immunomodulatory effects are able to facilitate the development of immune tolerance and to reduce the occurrence of atopic march.
Besides the probiotics supplementation, the role of prebiotics in the GM and immune system modulation has been also suggested. Prebiotic carbohydrates are the major substrate for GM beneficial microbes' growth and/or activity. Bifidobacterium species can multiply in the GM of breastfed infants due to the bifidogenic effect of human milk (HM), a rich source of HM-oligosaccharides (HMOs) and of certain prebiotics (i.e., fructo- and galacto-oligosaccharides) (58). To positively modulate GM composition, it has been shown that the addition of lactose to EHWF in CMA infants is able to increase the total fecal counts of Lactobacillus/ Bifidobacteria. The elicited positive effect is completed by the increase of the median concentration of SCFAs, especially butyric acid, as shown by GM metabolomic analysis (59). Recently, it has been demonstrated that the co-administration of HMOs with partially hydrolyzed whey protein, can induce immunological tolerance in sensitized mice with whey and/or cholera toxin (60). In an experimental animal study, administration of a prebiotic mixture reduced the allergic skin response, whey-IgG levels, and increased the number of Foxp3+ cells in the proximal small intestine of whey- compared to sham-sensitized mice. Moreover, the mixture of galacto and fructo-oligosaccharides prevented the increased expression of Th2 and Th17 mRNA markers in the small intestine of the mice (61). Despite that, the ESPGHAN Committee on Nutrition concluded that there was insufficient evidence to recommend the use of prebiotics in infant formula to prevent atopic disease (62).
The definition of a synbiotic is “a mixture comprising live microorganisms and substrate(s) selectively utilized by host microorganisms that confers a health benefit to the host” (63). Recently, a double-blind, randomized, controlled multicenter trial was conducted in infants with non-IgE-mediated CMA managed with AAF containing fructo-oligosaccharides and Bifdobacterium breve M-16V or control product (AAF). The treatment with the synbiotic led to beneficial effects on GM composition (higher fecal percentages of Bifidobacteria and lower Eubacterium rectale/Clostridium coccoides) and a significantly lower use of agents for dermatological purposes and a lower incidence of ear infections in the test group compared with the control group, suggesting possible systemic immunomodulating effects elicited by this synbiotic (64). The treatment with AAF supplemented with the same synbiotic also resulted in the effective modulation of the GM of infants with CMA, bringing it close to a healthy breastfed profile (65). Despite findings that show that the use of AAF-Syn results in an improvement of GM dysbiosis, no data about an effect on immune tolerance induction has been reported. Another trial is on-going to explore the possible effect on immune tolerance in children with IgE-mediated CMA treated with AAF with or without synbiotics (scFOS/lcFOS/B breve M-16 V) (66).
The GM has a pivotal role in both innate and adaptive immune development and may have long-term effects both on the susceptibility and the persistence of allergic diseases. In ascertaining the beneficial role of AAF+synbiotics in modulating the GM and its metabolic activity, this management strategy could help in the long-term outcomes of allergic infants, such as CMP tolerance acquisition and prevention of the development of other allergic manifestations.
Driving Immune System Function Through Baked Milk Products and Milk Ladder
When solid food introduction starts, other dietary strategies can be applied in formula- and breast-fed CMA children. The possibility to introduce processed foods containing CMP to stimulate the immune tolerance acquisition represents a novel therapeutic approach, moving from the strict elimination diet to individualized avoidance in selected patients (67).
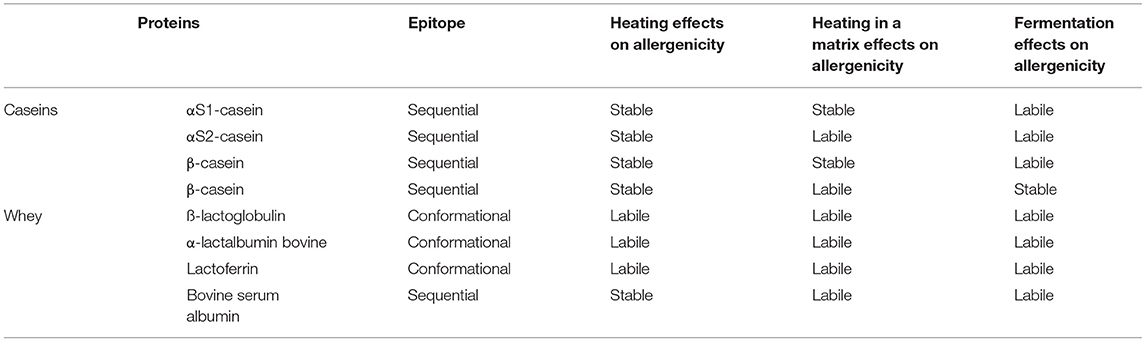
Cow's milk contains ~3–3.5 g of proteins per 100 mL characterized by different molecular structures and thermal and digestive characteristics, resulting in different allergenic properties (68, 69). Based on their solubility at pH 4.6 and 20°C, CMP are classified into two different categories: Caseins (αS1-casein, αS2-casein, β-casein, and β-casein) are precipitating proteins and represent 80% of CMP; while the group that remain soluble are known as serum or whey proteins: ß-lactoglobulin (ß-LG), α-lactalbumin (ALA), lactoferrin (LF), bovine serum albumin (BSA), and bovine immunoglobulins (Ig), and correspond to 20% of the total CMP content (68). Caseins, ß-LG, and ALA are considered the major allergens; while LF, BSA, and Ig, although present at lower quantities, could also have a role in inducing CMA (70). Table 2 summarizes the main characteristics of CMP, based on the current studies.
CMP are three-dimensional molecules held together by an electrostatic charge, and IgE binding sites, or epitopes, may be sequential or conformational. The sequential epitopes, such as αS1-casein, αS2-casein, β-casein, β-casein, and BSA, are characterized by several amino acids in a row, while the conformational epitopes, such as are ß-LG, ALA, and LF, made up of amino acids that are physically joined by the three-dimensional folding of proteins (68).
Allergenic characteristics of proteins can change during food processing such as heat treatment and lactic fermentation (71); for instance, the IgE-binding capacity can be affected by changes in the structure of proteins (aggregation, unfolding, glycation, and the occurrence of a Maillard reaction product) with a reduction of protein allergenicity (68, 70).
Moreover, CMP exposed to high temperatures in culinary recipes associated with a matrix (e.g., a muffin) showed a final reduced immunoreactivity compared with heated milk (180° C for 10 min), not included in a matrix (68, 69).
Numerous clinical studies have indicated that a large subset of children who react to unheated milk can tolerate extensively heated or fermented forms of these foods. These studies showed that among CMA children, 60–80% can eat extensively baked milk products (muffins, waffles, cakes, and breads), 60–70% can ingest fermented milk (yogurt) without presenting any adverse reactions, and about 60% can tolerate parmesan aged for 36 months (71–76).
Identifying children who tolerate baked milk products or other forms of processed milk is extremely important not only to increase the variety of diet and to improve quality of life, but also to speed up immune tolerance development (34).
Indeed, some research groups have demonstrated the role of baked milk product introduction in hastening immune tolerance acquisition to fresh milk (77, 78).
Some studies have tried to define specific IgE or skin prick test (SPT) cut-off levels to predict baked milk product reactions. For instance, Knol and colleagues proposed a specific IgE of cow's milk ≥15 KUA/L and a fresh milk SPT mean wheal diameter ≥8 mm as cut-off levels to identify a positive OFC for baked milk products in children aged ≤2 years (79). However, at present, there are no diagnostic screening tests available to identify CMA patients that are tolerant to baked products. Considering the positive effects of the introduction of these products and that more than 60% of CMA subjects tolerate baked milk products, the OFC with these processed foods should be considered in CMA children (67, 80).
In this way, a new approach has been designed by the British Society for Allergy and Clinical Immunology. They have proposed a “milk ladder” approach in CMA subjects to stimulate and speed up immune tolerance acquisition (34). This approach includes a step-by-step consumption of CMP, starting from fewer (baked) to more (fresh) allergenic forms of milk gradually increased at home (Figure 2). It is well known that the first home introduction of baked milk products in some CMA children could evocate an adverse reaction, and for this reason it is recommended to perform a home approach only in CMA children with a mild reaction to milk (e.g., mild rash, gastrointestinal symptoms); whereas children experiencing more severe symptoms may need undergo OFC under hospital supervision (34).
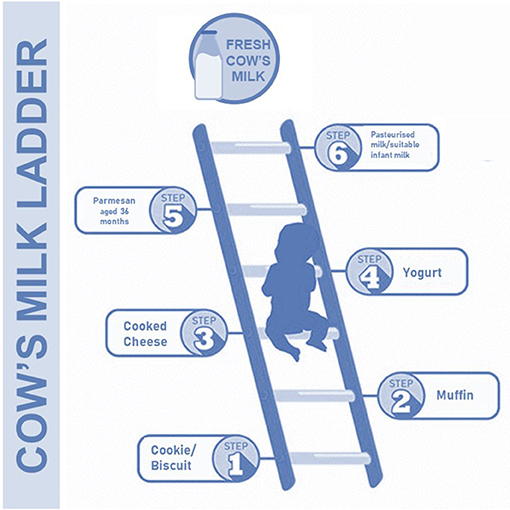
Figure 2. The cow's milk ladder approach. This step-by-step approach from fewer to more allergenic milk forms could speed up the immune tolerance acquisition in children with CMA.
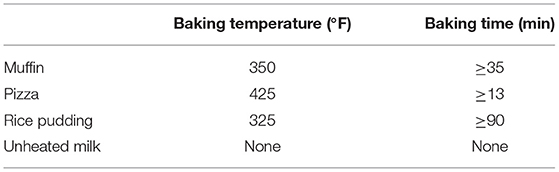
Table 3 summarizes baking temperatures and time of milk products, used in the “milk ladder” approach.
Although the milk ladder was initially proposed for children with non-IgE-mediated CMA (34), several studies have shown excellent results when used in children with IgE CMA (81–83).
To explore the effects of the milk ladder approach on immune tolerance acquisition, Nowak-Wegrzyn and colleagues evaluated the effect of introducing higher doses of more allergenic forms of milk (from muffins, pizzas, rice pudding to fresh milk) vs. rigorous elimination of CMP in IgE CMA children. They found that among children who added baked milk products to their diet, 48.2% developed tolerance to fresh milk at the age of 36 months; while none of the children in the comparison arm became tolerant to baked products or fresh milk in the same time frame (82).
Similarly, Efron and colleagues showed that gradual incorporation of milk in various forms (from extensively heated and cooked milk to fresh milk) significantly reduced the time and age of IgE CMA resolution. The median age at resolution was 36 ± 3.1 months in the intervention group compared with 98± 12.3 months in the controls (CMP-free diet children) (83).
Based on these studies a safer milk ladder approach could be proposed. In detail, the first exposure of any new more allergenic food containing CMP should be performed under medical supervision and could represent a more safety strategy compared to initial home introduction (82, 83).
Driving Immune System Function Through Oral Immunotherapy
In children with persistent IgE-mediated CMA, in which avoidance strategies are ineffective or severely limit quality of life, oral immunotherapy (OIT) could be considered as a potential active treatment. Due to the limited capacity of young children to report early allergic symptoms, and the natural tolerance acquisition in the majority of FA patients, the recommended age to start OIT is 4–5 years of age (84). OIT plays an immunological role by modulation of humoral and cell immunity. Humoral changes caused by OIT include a decrease in IgE levels and a rise in IgG levels, especially IgG4, which have a protective role on allergic reactions by blocking IgE-mediated basophil and mast cell activation. T cell response modifications include a reduction of the Th2 cell line and related cytokine expression (85, 86). These immunological changes seem to be transient, indeed OIT is also called desensitization therapy, defined as an increase in reactivity threshold to a specific allergen, that allows the patient to ingest increased amounts of a food without reaction, while continuing regular doses. The ability of OIT to induce longer-term tolerance has not yet been established (87). There are no standardized OIT protocols, but current OIT regimens typically involve the daily consumption of cow's milk, starting from a low dose, followed by incremental doses over several hours during the rush phase, and periodically (usually every 2 weeks) during the build-up phase, until the target maintenance dose is achieved. This maintenance dose is then continued daily for months to years or on-going. The main impediment to applying this approach in clinical practice is related to the high percentage of patients who cannot tolerate OIT, to the risk of severe reaction in patients with a history of anaphylaxis and to the lack of data about the ability to induce immune tolerance (87). To improve both safety and efficacy, adjunctive therapies to OIT have been evaluated in CMA children. One of the most studied adjunctive treatment is omalizumab, a humanized monoclonal anti-IgE, that seems to improve safety rather than efficacy in CMA children who underwent an OIT protocol with non-fat dry powdered milk (88). Another promising therapy is dupilumab, a monoclonal antibody directed against the alpha subunit of the IL-4 receptor (IL4R), that is able to block the IL-4 and IL-13 signaling pathways. This antibody, already approved in Italy for treatment of moderate to severe atopic dermatitis and asthma in patients aged ≥12 years of age, is now under investigation in a phase II trial (NCT04148352) as an OIT adjunctive treatment for CMA children. Data from preclinical studies on CMA mice models showed that OIT supplementation with plant-derived fructo-oligosaccharides (FOS) enhanced OIT efficacy (89) due to an increase of butyrate production, which in turn increased Treg differentiation (90). The possibility to improve OIT safety, quality of life, and the variety of diet in CMA children are the main abilities of biological therapies but data about higher efficacy compared with OIT alone require further studies.
Discussion
Considering that CMA is one of the most prevalent and expensive allergic diseases in the pediatric age, innovative strategies to limit the disease burden are highly advocated. The immunonutrition approach could provide the answer impacting long-term outcomes, changing the CMA disease course, and may be considered the new strategy for CMA treatment in the twenty first century.
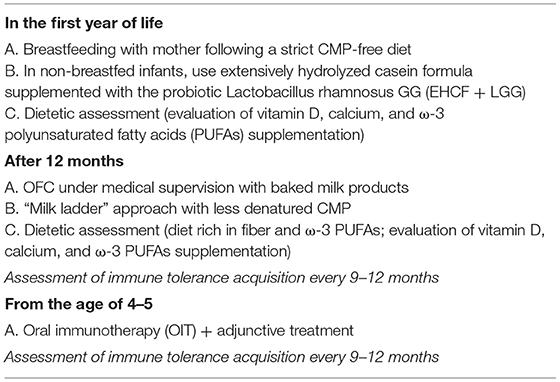
The immunonutrition strategy could be an effective approach to limit disease duration and the occurrence of AM (47). This strategy consists in an integrated multi-step approach, as illustrated in Figure 3. The first step occurs when the initial CMA diagnosis is made. Continuation of breastfeeding is highly recommended, but it is important to modulate the maternal diet avoiding CMP-containing foods and supporting an optimal adherence to a healthy dietary pattern (rich in fiber and ω-3 PUFAs). When breast milk is unavailable, the choice of a substitute formula should be based not only on allergenicity and nutritional features but also on the potential to modulate the natural disease history. Vitamin D, calcium, and ω-3 PUFAs supplementation should be considered and regularly evaluated based on the formula choice, age, and total formula daily intake. Actually, EHCF+LGG is the only substitutive formula able to stimulate immune tolerance acquisition and to prevent AM. A diet rich in fiber and ω-3 PUFAs should also be promoted after weaning together with a strict elimination of all CMP-containing foods. Then, if the child is unable to acquire immune tolerance to CMP after 12 months, baked milk products should be considered under medical supervision. If the child could tolerate baked milk products, the “milk ladder” approach could be proposed to promote both the immune tolerance acquisition and to increase the variety of the diet. In all cases, assessment of immune tolerance acquisitions every 9–12 months, depending on age and clinical history is highly recommended (91). The last but not least stage of the immunonutrition approach is OIT which could be considered in children with persistent CMA beyond the age of 4–5 years. One of the main limiting steps of OIT is the high percentage of patients who cannot tolerate it, so the adjunctive use of biologics, such as omalizumab, and improving OIT safety could be another promising strategy (84). Table 4 summarizes the key messages of the immunonutrition approach.
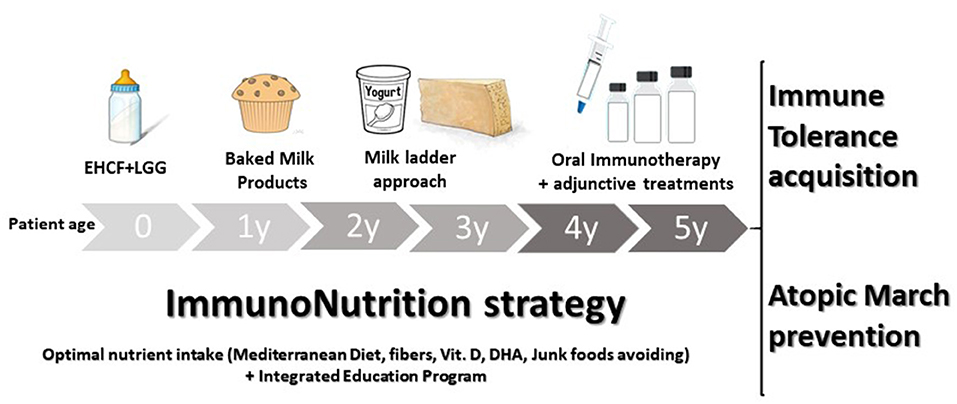
Figure 3. The immunonutrition strategy in cow's milk allergy (CMA) children. The active diet approach could start, in non-breastfed infants, with extensively hydrolyzed casein formula (EHCF) supplemented with the probiotic Lactobacillus rhamnosus GG (LGG). Then, if immune tolerance is not achieved in the first years of the disease, all the depicted steps should be considered to stimulate immune tolerance acquisition and to prevent the occurrence of other allergic manifestations. During the CMA course, the promotion of the Mediterranean diet, as well as vitamin D, calcium, and docosahexaenoic acid (DHA) supplementation should be evaluated based on the formula choice, age, and total formula daily intake. Lastly, an integrated education program aiming to limit any potential negative environmental factor exposure should be provided.
Author Contributions
LC analyzed the literature and wrote and read the manuscript. RBC designed and structured the review and wrote and read the manuscript. SC, AL, LV, VG, LP, and RN analyzed the literature and read the manuscript. All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Renz H, Allen KJ, Sicherer SH, Sampson HA, Lack G, Beyer K, et al. Food allergy. Nat Rev Dis Primers. (2018) 4:17098. doi: 10.1038/nrdp.2017.98
2. Sicherer SH, Sampson HA. Food allergy: a review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J Allergy Clin Immunol. (2018) 141:41–58. doi: 10.1016/j.jaci.2017.11.003
3. Aw M, Penn J, Gauvreau GM, Lima H, Sehmi R. Atopic march: collegium internationale allergologicum update 2020. Int Arch Allergy Immunol. (2020) 181:1–10. doi: 10.1159/000502958
4. Paparo L, Picariello G, Bruno C, Pisapia L, Canale V, Sarracino A, et al. Tolerogenic effect elicited by protein fraction derived from different formulas for dietary treatment of cow's milk allergy in human cells. Front Immunol. (2021) 1:3910. doi: 10.3389/fimmu.2020.604075
5. Berni Canani R, Nocerino R, Terrin G, Coruzzo A, Cosenza L, Leone L, et al. Effect of Lactobacillus GG on tolerance acquisition in infants with cow's milk allergy: a randomized trial. J Allergy Clin Immunol. (2012) 129:580–2:582.e1-5. doi: 10.1016/j.jaci.2011.10.004
6. Berni Canani R, Di Costanzo M, Bedogni G, Amoroso A, Cosenza L, Di Scala C, et al. Extensively hydrolyzed casein formula containing Lactobacillus rhamnosus GG reduces the occurrence of other allergic manifestation sin children with cow's milk allergy: 3–year randomized controlled trial. J Allergy Clin Immunol. (2017) 39:1906–13. doi: 10.1016/j.jaci.2016.10.050
7. Berni Canani R, Sangwan N, Stefka AT, Nocerino R, Paparo L, Aitoro R, et al. Lactobacillus rhamnosus GG supplemented formula expands butyrate producing bacterial strains in food allergic infants. ISME J. (2016) 10:742–50. doi: 10.1038/ismej.2015.151
8. Berni Canani R, Nocerino R, Terrin G, Frediani T, Lucarelli S, Cosenza L, et al. Formula selection for management of children with cow's milk allergy influences the rate of acquisition of tolerance: a prospective multicenter study. J Pediatr. (2013) 163:771–7.el. doi: 10.1016/j.jpeds.2013.03.008
9. Berni Canani R, Paparo L, Nocerino R, Di Scala C, Della Gatta G, Maddalena Y, et al. Gut microbiome as target for Innovative strategies against food allergy. Front Immunol. (2019) 10:191. doi: 10.3389/fimmu.2019.00191
10. Paparo L, Nocerino R, Bruno C, Di Scala C, Cosenza L, Bedogni G, et al. Randomized controlled trial on the influence of dietary intervention on epigenetic mechanisms in children with cow's milk allergy: the EPICMA study. Sci Rep. (2019) 9:2828. doi: 10.1038/s41598-019-38738-w
11. Carucci L, Nocerino R, Paparo L, Di Scala C, Berni Canani R. Dietary prevention of atopic march in pediatric subjects with cow's milk allergy. Front Pediatr. (2020) 8:440. doi: 10.3389/fped.2020.00440
12. Muraro A, Werfel T, Hoffmann-Sommergruber K, Roberts G, Beyer K, Bindslev-Jensen C, et al. EAACI food allergy and anaphylaxis guidelines group. EAACI food allergy and anaphylaxis guidelines: diagnosis and management of food allergy. Allergy. (2014) 69:1008–25. doi: 10.1111/all.12429
13. Berni Canani R, Di Costanzo M, Pezzella V, Cosenza L, Granata V, Terrin G, et al. The potential therapeutic efficacy of lactobacillus gG in children with food allergies. Pharmaceuticals. (2012) 5:655–64. doi: 10.3390/ph5060655
14. Venter C, O'Mahony L. Immunonutrition: the importance of a new European academy of allergy and clinical immunology working group addressing a significant burden and unmet need. Allergy. (2021) 76:2303–05. doi: 10.1111/all.14781
15. D'Auria E, Salvatore S, Pozzi E, Mantegazza C, Sartorio MUA, Pensabene L, et al. Cow's milk allergy: immunomodulation by dietary intervention. Nutrients. (2019) 11:1399. doi: 10.3390/nu11061399
16. Versluis A, Knulst AC, Kruizinga AG, Michelsen A, Houben GF, Baumert JL, et al. Frequency, severity and causes of unexpected allergic reactions to food: a systematic literature review. Clin Exp Allergy. (2015) 45:347–67. doi: 10.1111/cea.12328
17. Aitoro R, Paparo L, Amoroso A, Di Costanzo M, Cosenza L, Granata V, et al. Gut microbiota as a target for preventive and therapeutic intervention against food allergy. Nutrients. (2017) 9:672. doi: 10.3390/nu9070672
18. Smith PK, Masilamani M, Li XM, Sampson HA. The false alarm hypothesis: food allergy is associated with high dietary advanced glycation end-products and proglycating dietary sugars that mimic alarmins. J Allergy Clin Immunol. (2017) 139:429–437. doi: 10.1016/j.jaci.2016.05.040
19. West CE, Renz H, Jenmalm MC, Kozyrskyj AL, Allen KJ, Vuillermin P, et al. The gut microbiota and inflammatory noncommunicable diseases: associations and potentials for gut microbiota therapies. J Allergy Clin Immunol. (2015) 135:3–13; quiz 14. doi: 10.1016/j.jaci.2014.11.012
20. Zhang Q, Wang Y, Fu L. Dietary advanced glycation end-products: perspectives linking food processing with health implications. Compr Rev Food Sci Food Saf. (2020) 19:2559–87. doi: 10.1111/1541-4337.12593
21. Hilmenyuk T, Bellinghausen I, Heydenreich B, Ilchmann A, Toda M, Grabbe S, et al. Effects of glycation of the model food allergen ovalbumin on antigen uptake and presentation by human dendritic cells. Immunology. (2010) 129:437–45. doi: 10.1111/j.1365-2567.2009.03199.x
22. Ellwood P, Asher MI, Bjorksten B, Burr M, Pearce N, Robertson CF. Diet and asthma, allergic rhinoconjunctivitis and atopic eczema symptom prevalence: an ecological analysis of the international study of asthma and allergies in childhood (ISAAC) data. ISAAC phase one study group. Eur Respir J. (2001) 17:436–43. doi: 10.1183/09031936.01.17304360
23. Nagel G, Weinmayr G, Kleiner A, Garcia-Marcos L, Strachan DP. Effect of diet on asthma and allergic sensitisation in the international study on allergies and asthma in childhood (ISAAC) phase two. Thorax. (2010) 65:516–22. doi: 10.1136/thx.2009.128256
24. Ellwood P, Asher MI, Garcia-Marcos L, Williams H, Keil U, Robertson C, et al. Do fast foods cause asthma, rhinoconjunctivitis and eczema? Global findings from the International study of asthma and allergies in childhood (ISAAC) phase three. Thorax. (2013) 68:351–60. doi: 10.1136/thoraxjnl-2012-202285
25. Rinninella E, Cintoni M, Raoul P, Lopetuso LR, Scaldaferri F, Pulcini G, et al. Food components and dietary habits: keys for a healthy gut microbiota composition. Nutrients. (2019) 11:2393. doi: 10.3390/nu11102393
26. Venter C, Greenhawt M, Meyer RW, Agostoni C, Reese I, du Toit G, et al. EAACI position paper on diet diversity in pregnancy, infancy and childhood: novel concepts and implications for studies in allergy and asthma. Allergy. (2020) 75:497–523. doi: 10.1111/all.14051
27. De Filippis F, Pellegrini N, Vannini L, Jeffery IB, La Storia A, Laghi L, et al. High-level adherence to a mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut. (2016) 65:1812–21. doi: 10.1136/gutjnl-2015-309957
28. Kim J, Kwon J, Noh G, Lee SS. The effects of elimination diet on nutritional status in subjects with atopic dermatitis. Nutr Res Pract. (2013) 7:488–94. doi: 10.4162/nrp.2013.7.6.488
29. American Academy of Pediatrics. Committee on Nutrition. Hypoallergenic infant formulas. Pediatrics. (2000) 106:346–9. doi: 10.1542/peds.106.2.346
30. Straub DA. Calcium supplementation in clinical practice: a review of forms, doses, and indications. Nutr Clin Pract. (2007) 22:286–96. doi: 10.1177/0115426507022003286
31. Ramasamy I. Vitamin d metabolism and guidelines for vitamin D supplementation. Clin Biochem Rev. (2020) 41:103–26. doi: 10.33176/AACB-20-00006
32. Boaventura RM, Mendonça RB, Fonseca FA, Mallozi M, Souza FS, Sarni ROS. Nutritional status and food intake of children with cow's milk allergy. Allergol Immunopathol. (2019) 47:544–50. doi: 10.1016/j.aller.2019.03.003
33. Silva CM, Silva SAD, Antunes MMC, Silva GAPD, Sarinho ESC, Brandt KG. Do infants with cow's milk protein allergy have inadequate levels of vitamin d? J Pediatr. (2017) 93:632–8. doi: 10.1016/j.jpedp.2017.06.006
34. Luyt D, Ball H, Makwana N, Green MR, Bravin K, Nasser SM, et al. BSACI guideline for the diagnosis and management of cow's milk allergy. Clin Exp Allergy. (2014) 44:642–72. doi: 10.1111/cea.12302
35. Braegger C, Campoy C, Colomb V, Decsi T, Domellof M, Fewtrell M, et al. Vitamin D in the healthy European paediatric population. J Pediatr Gastroenterol Nutr. (2013) 56:692–701. doi: 10.1097/MPG.0b013e31828f3c05
36. Koletzko S, Niggemann B, Arato A, Dias JA, Heuschkel R, Husby S, et al. Diagnostic approach and management of cow's-milk protein allergy in infants and children: ESPGHAN gI committee practical guidelines. J Pediatr Gastroenterol Nutr. (2012) 55:221–9. doi: 10.1097/MPG.0b013e31825c9482
37. Poole A, Song Y, Brown H, Hart PH, Zhang GB. Cellular and molecular mechanisms of vitamin D in food allergy. J Cell Mol Med. (2018) 22:3270–7. doi: 10.1111/jcmm.13607
38. Fujimura KE, Lynch SV. Microbiota in allergy and asthma and the emerging relationship with the gut microbiome. Cell Host Microbe. (2015) 17:592–602. doi: 10.1016/j.chom.2015.04.007
39. Mailhot G, White JH. Vitamin d and immunity in infants and children. Nutrients. (2020) 12:1233. doi: 10.3390/nu12051233
40. Junge KM, Bauer T, Geissler S, Hirche F, Thürmann L, Bauer M, et al. Increased vitamin D levels at birth and in early infancy increase offspring allergy risk-evidence for involvement of epigenetic mechanisms. J Allergy Clin Immunol. (2016) 137:610–3. doi: 10.1016/j.jaci.2015.06.040
41. Grant WB, Karras SN, Bischoff-Ferrari HA, Annweiler C, Boucher BJ, Juzeniene A, et al. Do studies reporting 'U'-shaped serum 25-hydroxyvitamin d-health outcome relationships reflect adverse effects? Dermatoendocrinol. (2016) 8:e1187349. doi: 10.1080/19381980.2016.1187349
42. Venter C, Meyer RW, Nwaru BI, Roduit C, Untersmayr E, Adel-Patient K, et al. EAACI position paper: influence of dietary fatty acids on asthma, food allergy, and atopic dermatitis. Allergy. (2019) 74:1429–44. doi: 10.1111/all.13764
43. Stratakis N, Gielen M, Margetaki K, De Groot RHM, Apostolaki M, Chalkiadaki G, et al. PUFA status at birth and allergy-related phenotypes in childhood: a pooled analysis of the Maastricht essential fatty acid birth (MEFAB) and RHEA birth cohorts. Br J Nutr. (2018) 119:202–10. doi: 10.1017/S0007114517003348
44. Berni Canani R, Leone L, D'Auria E, Riva E, Nocerino R, Ruotolo S, et al. The effects of dietary counseling on children with food allergy: a prospective, multicenter intervention study. J Acad Nutr Diet. (2014) 114:1432–9. doi: 10.1016/j.jand.2014.03.018
45. Aldámiz-Echevarría L, Bilbao A, Andrade F, Elorz J, Prieto JA, Rodríguez-Soriano J. Fatty acid deficiency profile in children with food allergy managed with elimination diets. Acta Paediatr. (2008) 97:1572–6. doi: 10.1111/j.1651-2227.2008.00963.x
46. Fiocchi A, Schunemann H, Ansotegui I, Assa'ad A, Bahna S, Canani RB, et al. The global impact of the DRACMA guidelinescow'smilkallergyclinicalpractice. World AllergyOrgan J. (2018) 11:2. doi: 10.1186/s40413-017-0179-7
47. Bilaver LA, Chadha AS, Doshi P, O'Dwyer L, Gupta RS. Economic burden of food allergy: a systematic review. Ann Allergy Asthma Immunol. (2019) 122:373–80.e1. doi: 10.1016/j.anai.2019.01.014
48. Paparo L, Nocerino R, Cosenza L, Aitoro R, D'Argenio V, Del Monaco V, et al. Epigenetic features of FoxP3 in children with cow's milk allergy. Clin Epigenetics. (2016) 8:86. doi: 10.1186/s13148-016-0252-z
49. Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. Expert consensus document. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. (2014) 11:506–14. doi: 10.1038/nrgastro.2014.66
50. Ivakhnenko ES, Nian'kovskii SL. Effect of probiotics on the dynamics of gastrointestinal symptoms of food allergy to cow's milk protein in infants. Georgian Med News. (2013) 219:46–52.
51. Basturk A, Isik I, Atalay A, Yilmaz A. Investigation of the efficacy of lactobacillus rhamnosus gG in infants with cow's milk protein allergy: a randomised double-blind placebo-controlled trial. Probiotics Antimicrob Proteins. (2020) 12:138–43. doi: 10.1007/s12602-019-9516-1
52. Nocerino R, Bedogni G, Carucci L, Cosenza L, Cozzolino T, Paparo L, et al. The impact of formula choice for the management of pediatric cow's milk allergy on the occurrence of other allergic manifestations: the atopic march cohort study. J Pediatr. (2021) 232:183–91.e3. doi: 10.1016/j.jpeds.2021.01.059
53. Guest JF, Fuller GW. Effectiveness of using an extensively hydrolyzed casein formula supplemented with lactobacillus rhamnosus gG compared with an extensively hydrolysed whey formula in managing cow's milk protein allergic infants. J Comp Eff Res. (2019) 8:1317–26. doi: 10.2217/cer-2019-0088
54. Kalliomäki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet. (2001) 357:1076–9. doi: 10.1016/S0140-6736(00)04259-8
55. Kirjavainen PV, Salminen SJ, Isolauri E. Probiotic bacteria in the management of atopic disease: underscoring the importance of viability. J Pediatr Gastroenterol Nutr. (2003) 36:223–7. doi: 10.1097/00005176-200302000-00012
56. Guest JF, Singh H. Cost-effectiveness of using an extensively hydrolyzed casein formula supplemented with lactobacillus rhamnosus gG in managing igE-mediated cow's milk protein allergy in the UK. Curr Med Res Opin. (2019) 35:1677–85. doi: 10.1080/03007995.2019.1612339
57. Guest JF, Weidlich D, MascuñanDíaz JI, Díaz JJ, Ojeda PM, Ferrer-González JP, et al. Relative cost effectiveness of using an extensively hydrolyzed casein formula containing the probiotic Lactobacillus rhamnosus GG in managing infants with cow's milk allergy in Spain. Clinicoecon Outcomes Res. (2015) 7:583–91. doi: 10.2147/CEOR.S89347
58. Alderete TL, Autran C, Brekke BE, Knight R, Bode L, Goran MI, et al. Associations between human milk oligosaccharides infant body composition in the first 6 mo of life. Am J Clin Nutr. (2015) 102:1381–8. doi: 10.3945/ajcn.115.115451
59. Francavilla R, Calasso M, Calace L, Siragusa S, Ndagijimana M, Vernocchi P, et al. Effect of lactose on gut microbiota and metabolome of infants with cow's milk allergy. Pediatr Allergy Immunol. (2012) 23:420–7. doi: 10.1111/j.1399-3038.2012.01286.x
60. Kleinjans L, Veening-Griffioen DH, Wehkamp T, van Bergenhenegouwen J, Knol J, Garssen J, et al. Mice co-administrated with partially hydrolysed whey proteins and prebiotic fibre mixtures show allergen-specific tolerance and a modulated gut microbiota. Benef Microbes. (2019) 10:165–78. doi: 10.3920/BM2018.0001
61. Kerperien J, Jeurink PV, Wehkamp T, van der Veer A, van de Kant HJ, Hofman GA, et al. Non-digestible oligosaccharides modulate intestinal immune activation and suppress cow's milk allergic symptoms. Pediatr Allergy Immunol. (2014) 25:747–54. doi: 10.1111/pai.12311
62. Braegger C, Chmielewska A, Decsi T, Kolacek S, Mihatsch W, Moreno L, et al. Supplementation of infant formula with probiotics and/or prebiotics: a systematic review and comment by the ESPGHAN committee on nutrition. J Pediatr Gastroenterol Nutr. (2011) 52:238–50. doi: 10.1097/MPG.0b013e3181fb9e80
63. Swanson KS, Gibson GR, Hutkins R, Reimer RA, Reid G, Verbeke K, et al. The international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat Rev Gastroenterol Hepatol. (2020) 17:687–701. doi: 10.1038/s41575-020-0344-2
64. Fox AT, Wopereis H, Van Ampting MTJ, Oude Nijhuis MM, Butt AM, Peroni DG, et al. A specific synbiotic-containing amino acid-based formula in dietary management of cow's milk allergy: a randomized controlled trial. Clin Transl Allergy. (2019) 9:5. doi: 10.1186/s13601-019-0241-3
65. Wopereis H, van Ampting MTJ, Cetinyurek-Yavuz A, Slump R, Candy DCA, Butt AM, et al. A specific synbiotic-containing amino acid-based formula restores gut microbiota in non-IgE mediated cow's milk allergic infants: a randomized controlled trial. Clin Transl Allergy. (2019) 9:27. doi: 10.1186/s13601-019-0267-6
66. Netherlands Trial Register Trial info. A Prospective Double Blind Randomised Controlled Study to Evaluate the Immunological Benefits and Clinical Effects of an Elimination Diet Using an Amino Acid Formula (AAF) With an Added Pre-probiotic Blend in Infants With Cow's Milk Allergy (CMA). Available online at: http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=3725 (accessed May 2021).
67. Dantzer JA, Dunlop JH, Wood RA. Standard testing fails to identify patients who tolerate baked milk. J Allergy Clin Immunol. (2020) 146:1434–7.e2. doi: 10.1016/j.jaci.2020.03.030
68. Bavaro SL, De Angelis E, Barni S, Pilolli R, Mori F, Novembre EM, et al. Modulation of milk allergenicity by baking milk in foods: a proteomic investigation. Nutrients. (2019) 11:1536. doi: 10.3390/nu11071536
69. Vilar LK, Araújo FA, Santos TP, Menezes TT, Cheik MF, Segundo GRS. Baked tolerance in cow's milk allergy: quite frequent, hard to predict! Int Arch Allergy Immunol. (2020) 182:319–23. doi: 10.1159/000511148
70. Villa C, Costa J, Oliveira MBPP, Mafra I. Bovine milk allergens: a comprehensive review. Compr Rev Food Sci Food Saf. (2018) 17:137–64. doi: 10.1111/1541-4337.12318
71. Küçükosmanoglu E, Özen E, Eltan SB, Özkars MY, Keskin Ö. Most children who are allergic to cow's milk tolerate yogurt. J Int Med Res. (2018) 46:5099–106. doi: 10.1177/0300060518790430
72. Monaco S, Russo G, Romano A, Liotti L, Verga MC, Miceli Sopo S. Yogurt is tolerated by the majority of children with IgE-mediated cow's milk allergy. Allergol Immunopathol. (2019) 47:322–7. doi: 10.1016/j.aller.2018.10.005
73. Weinbrand-Goichberg J, Benor S, Rottem M, Shacham N, Mandelberg A, Levine A, et al. Long-term outcomes following baked milk-containing diet for IgE-mediated milk allergy. J Allergy Clin Immunol Pract. (2017) 5:1776–78.e1. doi: 10.1016/j.jaip.2017.04.018
74. Sackesen C, Suárez-Fariñas M, Silva R, Lin J, Schmidt S, Getts R, et al. A new luminex-based peptide assay to identify reactivity to baked, fermented, and whole milk. Allergy. (2019) 74:327–36. doi: 10.1111/all.13581
75. Sirin Kose S, Asilsoy S, Uzuner N, Karaman O, Anal O. Outcomes of baked milk and egg challenge in cow's milk and hen's egg allergy: can tolerance be predicted with allergen-specific IgE and prick-to-prick test? Int Arch Allergy Immunol. (2019) 180:264–73. doi: 10.1159/000502957
76. Alessandri C, Sforza S, Palazzo P, Lambertini F, Paolella S, Zennaro D, et al. Tolerability of a fully maturated cheese in cow's milk allergic children: biochemical, immunochemical, and clinical aspects. PLoS ONE. (2012) 7:e40945. doi: 10.1371/journal.pone.0040945
77. Kim JS, Nowak-Wegrzyn A, Sicherer SH, Noone S, Moshier EL, Sampson HA. Dietary baked milk accelerates the resolution of cow's milk allergy in children. J Allergy Clin Immunol. (2011) 128:125–31. doi: 10.1016/j.jaci.2011.04.036
78. Esmaeilzadeh H, Alyasin S, Haghighat M, Nabavizadeh H, Esmaeilzadeh E, Mosavat F. The effect of baked milk on accelerating unheated cow's milk tolerance: a control randomized clinical trial. Pediatr Allergy Immunol. (2018) 29:747–53. doi: 10.1111/pai.12958
79. Knol EF, de Jong NW, Ulfman LH, Tiemessen MM. Management of cow's milk allergy from an immunological perspective: what are the options? Nutrients. (2019) 11:2734. doi: 10.3390/nu11112734
80. Robinson ML, Lanser BJ. The role of baked egg and milk in the diets of allergic children. Immunol Allergy Clin North Am. (2018) 38:65–76. doi: 10.1016/j.iac.2017.09.007
81. Ball HB, Luyt D. Home-based cow's milk reintroduction using a milk ladder in children less than 3 years old with IgE-mediated cow's milk allergy. Clin Exp Allergy. (2019) 49:911–20. doi: 10.1111/cea.13366
82. Nowak-Wegrzyn A, Lawson K, Masilamani M, Kattan J, Bahnson HT, Sampson HA. Increased tolerance to less extensively heat-Denatured (Baked) milk products in milk-allergic children. J Allergy Clin Immunol Pract. (2018) 6:486–495.e5. doi: 10.1016/j.jaip.2017.10.021
83. Efron A, Zeldin Y, Gotesdyner L, Stauber T, Maoz Segal R, Binson I, et al. A structured gradual exposure protocol to baked and heated milk in the treatment of milk allergy. J Pediatr. (2018) 203:204–9.e2. doi: 10.1016/j.jpeds.2018.07.091
84. Pajno GB, Fernandez-Rivas M, Arasi S, Roberts G, Akdis CA, Alvaro-Lozano M, et al. EAACI guidelines on allergen immunotherapy: IgE-mediated food allergy. Allergy. (2018) 73:799–815. doi: 10.1111/all.13319
85. Tordesillas L, Berin MC, Sampson HA. Immunology of food allergy. Immunity. (2017) 47:32–50. doi: 10.1016/j.immuni.2017.07.004
86. Upton J, Nowak-Wegrzyn A. The impact of baked egg and baked milk diets on igE- and non-IgE-Mediated allergy. Clin Rev Allergy Immunol. (2018) 55:118–38. doi: 10.1007/s12016-018-8669-0
87. Wood RA. Oral immunotherapy for food allergy. J Investig Allergol Clin Immunol. (2017) 27:151–9. doi: 10.18176/jiaci.0143
88. Wood RA, Kim JS, Lindblad R, Nadeau K, Henning AK, Dawson P, et al. A randomized, double-blind, placebo-controlled study of omalizumab combined with oral immunotherapy for the treatment of cow's milk allergy. J Allergy Clin Immunol. (2016) 137:1103–10.e11. doi: 10.1016/j.jaci.2015.10.005
89. Vonk MM, Diks MAP, Wagenaar L, Smit JJ, Pieters RHH, Garssen J, et al. Improved efficacy of oral immunotherapy using non-digestible oligosaccharides in a murine cow's milk allergy model: a potential role for foxp3+ regulatory T cells. Front Immunol. (2017) 8:1230. doi: 10.3389/fimmu.2017.01230
90. Vonk MM, Blokhuis BRJ, Diks MAP, Wagenaar L, Smit JJ, Pieters RHH, et al. Butyrate enhances desensitization induced by oral immunotherapy in cow's milk allergic mice. Mediators Inflamm. (2019) 2019:9062537. doi: 10.1155/2019/9062537
91. Fiocchi A, Brozek J, Schünemann H, Bahna SL, von Berg A, Beyer K, et al. World allergy organization (WAO) special committee on food allergy. World allergy organization (WAO) diagnosis and rationale for action against cow's milk allergy (DRACMA) guidelines. Pediatr Allergy Immunol. (2010) 21(Suppl. 21):1–125. doi: 10.1111/j.1399-3038.2010.01068.x
Keywords: food allergy, gut microbiome, dietary peptides, immune tolerance, atopic march
Citation: Carucci L, Coppola S, Luzzetti A, Voto L, Giglio V, Paparo L, Nocerino R and Berni Canani R (2021) Immunonutrition for Pediatric Patients With Cow's Milk Allergy: How Early Interventions Could Impact Long-Term Outcomes. Front. Allergy 2:676200. doi: 10.3389/falgy.2021.676200
Received: 04 March 2021; Accepted: 18 May 2021;
Published: 09 July 2021.
Edited by:
Rosan Meyer, Imperial College London, United KingdomReviewed by:
Carina Venter, University of Colorado, United StatesSarah Comstock, Michigan State University, United States
Copyright © 2021 Carucci, Coppola, Luzzetti, Voto, Giglio, Paparo, Nocerino and Berni Canani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laura Carucci, bGF1cmEuY2FydWNjaUB1bmluYS5pdA==
 Laura Carucci
Laura Carucci Serena Coppola
Serena Coppola Anna Luzzetti1,2
Anna Luzzetti1,2 Lorella Paparo
Lorella Paparo Rita Nocerino
Rita Nocerino Roberto Berni Canani
Roberto Berni Canani