- 1Central Potato Research Institute, ICAR, Shimla, HP, India
- 2Indian Institute of Vegetable Research, ICAR, Varanasi, UP, India
- 3Central Potato Research Institute, ICAR, Kufri-Unit, HP, India
- 4Central Potato Regional Station, ICAR, Meerut, UP, India
- 5Central Potato Research Station, ICAR, Gwalior, MP, India
- 6Central Potato Research Station, ICAR, Shillong, ML, India
- 7National Research Centre for Grapes, ICAR, Pune, MH, India
India is the second largest producer of potatoes in the world. Seed is the single most important input in potato cultivation. High seed rate (2.5–3.0 tons/ha), low rate of multiplication, progressive viral degeneration, storage, and transportation are major issues of potato seed production in the country. Potato seed alone accounts for 40%-50% of the total potato production cost, and huge quantities of potentially edible food is put back into the soil as potato seed. The delayed penetration of new improved potato/seed varieties into farmers’ fields due to the slow multiplication rate and frequent seed replacement because of degeneration are associated issues. To circumvent these issues, continuous efforts are being made by potato researchers to develop innovative technologies for quick multiplication of initial healthy breeder’s seed of the released varieties in sufficient quantities to meet the demand in our country. A paradigm shift in potato seed production methods has taken place globally since the early 1900s. Major potato producers of the world have shifted from conventional to hi-tech seed production systems to improve the seed quality and enhance seed multiplication rate. New innovations can overcome many of the problems associated with potato seed production, particularly in tropical and sub-tropical countries. Recent advances in potato seed production systems in India and challenges ahead for seed production are described here.
Introduction
Potato (Solanum tuberosum L) is emerging as one of the important food crops in India accounting for 11.3% of the total global potato area and contributing 12.5% to the global potato production. In 2019-2020, India harvested more than 51.3 million tons of potatoes from 2.16 million hectares of cropped area with an average production of 23.77 tons/ha (Food and Agriculture Organization (FAO), 2022). Supported by technological innovations and the development of improved potato varieties suitable for growing under sub-tropical conditions, and driven by the growing demands, potato productivity and production in India have increased 3.28- and 18.87-fold, respectively during the period of 1961–2020. Nonetheless, potato production in India is still constrained by scarcity of varieties having diverse economic attributes that give better options to the potato growers; inadequacy of quality seed, and shortage of storage infrastructure. Most important among these is the inadequacy of quality planting material, as it has a direct bearing on crop productivity (Singh and Sharma, 2018). Being bulky and having high seed rate and slow rate of multiplication (five to six times) pose a unique challenge to seed potato production and its supply chain (Young, 1990). Since the crop is multiplied vegetatively using the tuber as seed, it gets degenerated very fast, necessitating the replacement of the seed every year (ideally, or after every 2 years) (Singh et al., 2018). Furthermore, the seed is either short in supply or out of reach of farmers owing to high price. The seed-related issues are further aggravated because of the restricted availability of aphid-free, seed-producing regions in the country. It has been demonstrated that quality seeds alone can increase productivity by 15%–20% (Shaheb et al., 2016).
There is a huge gap between the requirement and supply of certified seed potatoes in India [National Academy of Agricultural Sciences (NAAS), 2021]. The Central Potato Research Institute (CPRI) of the Indian Council of Agricultural Research (ICAR) produces about 2,400 tons of breeder’s seed (basic seed) every year and supplies 80% of it to the states and other agencies for its multiplication ((ICAR-CPRI Annual Report, 2020). If this stock has to multiply in three stages, i.e., foundation 1, foundation 2, and certified grades (as per norms), we can produce only about 0.5 million tons of certified seed. On the assumption of a 100% seed replacement rate (SRR), it meets only 10% of the total seed requirement, leaving a deficit of about 4.9 million tons (Buckseth et al., 2020). It is virtually impossible to produce such a huge quantity of certified seed using traditional methods. The traditional system of seed potato production in India consists of producing breeders’ seed (basic seed) after four field multiplications of nucleus seed on research farms of ICAR-CPRI followed by three more field multiplications as foundation 1, foundation 2, and certified seed by respective states of the country (Venkataslam et al., 2017; Singh et al., 2019a). In addition to the low rate of field multiplications, the traditional system suffers from several other constraints like the requirement of a huge number of disease-free propagules in the initial stage and progressive accumulation of degenerative viral diseases with each field exposure (Venkataslam et al., 2017; Naik and Buckseth, 2018). The situation is further aggravated by the fact that the breeder’s seed supplied by ICAR-CPRI is seldom multiplied in three recommended generations by the states. About 1.2 million tons of correctly labeled potato seed are sold by private seed producers, especially those from Punjab, western Uttar Pradesh, West Bengal, and Haryana, without any mechanisms and infrastructure for monitoring the seed quality [National Academy of Agricultural Sciences (NAAS), 2021]. It is, therefore, imperative to evolve a seed production system, encompassing innovative techniques to improve the quality of seed and reduce field exposures, along with a robust system of certification and quality assurance of the seed produced and supplied by the private seed growers. This mini-review highlights the recent innovations in seed potato production in India.
Current innovations in seed potato production
Tissue culture-based seed potato production
The use of micropropagation for commercial seed production has moved potatoes from the test tube to the field (Wang and Hu, 1982). The first establishment of tissue culture from potato tubers was attempted as early as 1951 (Steward and Caplin, 1951), and since then, a variety of tissues from different plant organs, such as leaves, petioles, internode segments, ovaries, anthers, stems, roots, and shoot tips have been successfully demonstrated (reviewed in Wang and Hu, 1985; Bajaj, 1987; Wenzel, 1994; Naik et al., 2000; Naik and Sarkar, 2001). Seed potato production involving micropropagation techniques can overcome many of the problems associated with the traditional seed production system (Singh et al., 2019b). Figure 1 illustrates tissue culture-based commercial seed production systems.

Figure 1 Recent innovations in Tissue Culture based seed potato system. (A) Healthy microplants. (B) Minitubers directly produced from microplants. (C) minitubers developed in aeroponic chamber. (D) Harvested minitubers. (E) Apical rooted cuttings ready for transplanting (F) Apical rooted cuttings raised tubers. And (G) Minituber crop in the field.
Meristem culture for virus elimination. The meristem culture procedure is combined with thermotherapy and/or chemotherapy to increase the likelihood of obtaining virus-free plants (Sarkar et al., 2011). Even after taking all precautions for virus elimination, only a few virus-free mericlones are obtained. It is, therefore, essential that each mericlone is tested for viruses using enzyme-linked immunosorbent assay (ELISA), immunosorbent electron microscopy (ISEM), and polymerase chain reaction (PCR) techniques before it is used as a source plant in a large-scale micropropagation program (Naik and Buckseth, 2018). The virus-indexed and pathogen-free mericlones are subjected to a rapid tissue culture method to generate abundant quantities of pathogen-free cultures (Naik and Karihaloo, 2007).
Micropropagation. Single-node cuttings of virus-free potato mericlones are grown in semi-solid or liquid culture media under aseptic conditions to obtain new microplants (Naik et al., 2000). Hormone-free Murashige and Skoog’s (MS) medium supplemented with 2.0 mg L-1 D-calcium pantothenate and 30 g L-1 sucrose is ideally suited for large-scale in vitro micropropagation of potatoes (Buckseth et al., 2016). Cultures are incubated under a 16-h photoperiod (60 µmol m-2 s-1 PFD) at 20 ± 2°C. Usually, three single-node cuttings with one or two leaves are planted into each culture tube (25 × 150 mm). Within 3 weeks, the axillary/apical buds of these cuttings develop into full plants. These microplants are further sub-cultured up to 10 times on fresh culture media at an interval of 3 weeks. Therefore, it has the potential to produce about 59,049 microplants from a single culture tube within 7–8 months under controlled conditions [National Academy of Agricultural Sciences (NAAS), 2021]. Micropropagation now underpins many seed potato production systems and specifically provides the nuclear stock material, in the form of microplants or microtubers (in vitro produced tubers) for their subsequent use in potato seed production channel (Millam and Sharma, 2007; Chindi et al., 2014).
Microtuber production from microplants. Microtubers are miniature tubers produced in vitro under tuber-inducing conditions (Naik and Karihaloo, 2007). These small dormant tubers are particularly convenient for handling, storage, transportation, and conserving germplasm (Gopal et al., 2004). In general, 15-20 microtubers with an average size of about 100-200 mg, are obtained from each tissue culture flask. Microtubers are usually not used for raising commercial crops but utilized for the production of minitubers in greenhouses (Naik and Sarkar, 2000). The microtubers are planted in nursery beds under aphid-proof net houses (50 microtubers m2) to produce minitubers. This technology, however, has not become very popular because of the poor survival of microtubers (60-65%) and the poor crop emergence (50%-60%) in nursery beds (Venkataslam et al., 2017). Bioreactors have also been tried for the mass production of microtubers (Ankita and Takayama, 1994; Piao et al., 2003); however, bioreactors are expensive and add to the cost of microtuber production (Mamiya et al., 2020).
Minituber production in soil. Minitubers are small seed potato tubers that can be produced in glasshouses or aphid-proof nethouses from in vitro propagated plantlets planted at a high density (Buckseth et al., 2020). Minitubers can be produced using either microplants as planting material or microtubers (Naik, 2005). In this system, the hardened virus-free microplants or microtubers are transplanted inside a nethouse for the production of minitubers. The minitubers so obtained are multiplied usually in two subsequent field generations (generations 1 and 2) before supplying to farmers as seeds, thereby reducing the number of field exposures of initial disease-free material.
Soil-less aeroponic system. Aeroponics is a more recent technology that has made inroads into the potato sector in the last few decades. This technology has been developed for the production of minitubers by utilizing healthy in vitro plants (Otazu, 2010; Singh et al., 2012; Pandey and Singh, 2014; Buckseth et al., 2016). This system facilitates year-round production and adoption of phytosanitary standards (Singh et al., 2012; Tierno et al., 2014; Singh et al., 2016). In this system, microplants are planted on top of the growth chamber, and the developing root zone inside the chamber is fogged with nutrient solution (Tierno et al., 2014; Buckseth et al., 2016). The chambers are installed inside an insect-proof nethouse. The aeroponic chamber has a removable opening at the top with holes for holding the potato plants. The front of the aeroponic chamber is set with pivots and can be opened to harvest minitubers of ideal size at different time intervals. Picking of tubers begins after 45–50 days of planting when they attain a size of 3-10g. Picking of minitubers is done every week, and around 10–12 harvests are taken during the whole crop season of 4-5 months (Tiwari et al., 2019). Normally, 40-50 minitubers can be harvested from a single in vitro plant depending upon the variety as against 8–10 minitubers under the nethouse in nursery beds (Buckseth et al., 2020). The harvested minitubers are stored at 2°C–4°C to be utilized for planting in the next crop season. This system requires a high level of specialization in planning, operational expenses, and standardization of genotype-responsive nutrient solutions, but it offers quality seeds and has revolutionized seed potato production in India (Buckseth et al., 2022). Variation for root morphology and yield traits of all Indian varieties under aeroponics has also been identified (Tiwari et al., 2022).
Apical rooted cuttings technology. Rapidly growing young vegetative part of the potato plant can be cut and rooted in a number of ways (Bryan et al., 1981). Apical rooted cuttings have long been used in Southeast Asia (Vander Zaag and Escobar, 1990), particularly in Vietnam (Tran et al., 1990). The approach could be duplicated elsewhere in the developing world. On the same note, this technology has been successfully integrated into the potato breeders’ seed production program in India (Buckseth et al., 2019b). Apical rooted cuttings (ARCs) is simple, effective, easy to implement, and has a small production cycle; this has an edge over aeroponic technology, wherein the large capital investment and long production cycle required are limiting factors (Buckseth et al., 2019a). In ARC, the healthy buffer stock of microplants acts as initial planting material on nursery beds (400 microplants m2). The first round of cutting starts after 15-20 days of planting of the mother stock. The apical portion (1.5-2.0 cm) of the growing microplant is cut and planted in pro-trays containing cocopeat/other soilless media. Once the apical dominance in the mother plant is removed, the growth of lateral buds is promoted, and these buds grow into new branches, which are further used to increase the multiplication rate of potato plants. Sequential cuttings of laterals from mother plants are followed at an interval of 7-10 days, and it continues depending on the growth of new shoots in the variety for over 35-45 days. To follow the seed plot technique (i.e., growing potato seed crop under an aphid-free period of the season) and to fit into the seed production window, only three to four cuttings are recommended in the northwestern central plains of India. Thus, one microplant can produce six to eight rooted cuttings (Buckseth et al., 2022). Around 7-10-day-old rooted cuttings are planted under insect-proof nethouse for tuber production. Batch-wise pedigree of the cuttings is maintained so as even if any plant out of a cutting is found infected with a virus during testing, all the counterparts of the cutting/sister counterpart tubers can be rejected. Based on the available data, 7-10 tubers weighing 10-70 g can be harvested from a single cutting, thereby achieving a multiplication rate of more than 40 depending upon the variety. This technology has immense potential if it can be fitted in the seed production window following standard operating procedures to ensure seed health.
Botanical true potato seed
The idea of true potato seed (TPS) was conceived by Dr. S. Ramanujam as early as 1949. Like all other botanical seeds, TPS has the potential to grow into a full plant, but every plant is genetically different, thus making TPS population heterogeneous (Gaur and Pandey, 1993). Efforts were made at ICAR-CPRI to develop uniform TPS populations to get uniform crop stand, disease resistance, and tuber yields. TPS has many advantages over potato seed tubers. The major ones are disease- and pest-free planting material, easy storage and transportation, and a highly reduced seed rate (about 150 g/ha as against 2.5–3.0 t/ha of seed tubers). Three TPS populations, namely, HPS-I/13, TPS-C-3, and 92-PT-27, were developed by ICAR-CPRI during the 1980s. Quality control parameters for TPS production have been well prescribed in the Indian Minimum Seed Certification Standards (Indian Minimum Seed Certification Standards, 2013). However, TPS technology remains to be seen as a ready-to-use technology with the economic comparison of TPS versus seed tubers in the dynamics of the cropping system for potato production (Tiwari et al., 2017). The use of TPS appears to be the technology to solve the problem of a shortage of good quality potato seed tubers in developing countries (Muthoni et al., 2019). The obstacles that have prevented the adoption and widespread use of TPS are the late maturity of a TPS crop, unreliable germination, and non-uniformity of the produce. However, there has been a renewed enthusiasm for TPS technology since the advent of diploid F1 hybrid breeding in 2008 (which was declared International Potato Year by the UN).
Diploid hybrid TPS
Being an autotetraploid and highly heterozygous crop, the selfing of potatoes results in severe inbreeding depression. Bringing down the ploidy to a diploid level and selfing thereafter through the incorporation of a self-compatibility gene can result in homozygosity at a much faster rate than at the tetraploid level. This approach has been demonstrated by Lindhout et al. (2011). Since the offspring of a sexual cross would be a pristine true seed, which would be completely free of diseases and therefore make excellent seed material for potato growers around the world (Sood et al., 2021). Diploid potatoes can be converted from an outbreeding species, in which self-pollination is prevented by a gametophytic self-incompatibility system, into one where self-pollination is possible, either through a dominant self-incompatibility inhibitor gene (Sli) or knockout mutations in the incompatibility locus. As a result, diploid F1 hybrid breeding can be used to produce genetically uniform potato cultivars for propagation from true potato seeds by crossing two near-homozygous inbred lines, derived from a number of generations of self-pollination despite inbreeding depression (Bradshaw, 2022). Later, the developed F1 hybrid planting material can be delivered to farmers as true seeds or young plants and minitubers derived from true seeds. Many public and private organizations, including ICAR-CPRI, are working on the development of diploid inbred lines and exploiting heterosis in potatoes at the diploid level. Although the methodology of F1 diploid hybrid TPS-based potato breeding has several advantages over the conventional tetraploid tuber-based approach, a number of challenges need to be overcome to bring this technology to the forefront in the farmer’s field (Sood et al., 2021).
Concluding remarks
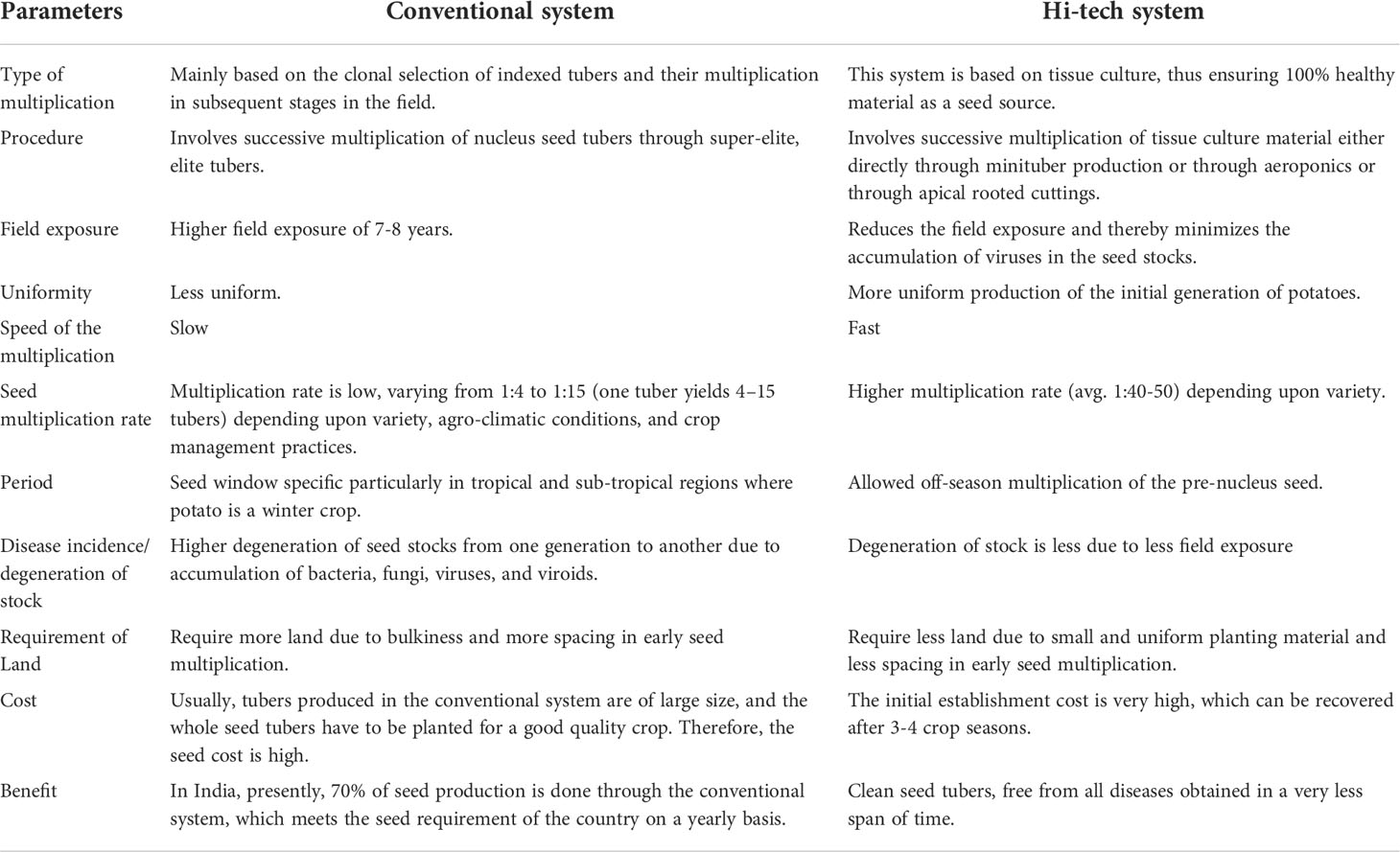
In mitigating the scarcity of good quality seeds, strategies to produce good quality potato seeds using tissue culture in conjunction with aeroponic systems and apical rooted cuttings have been tried. Currently, about 30% of potato breeders’ seed (basic seed) in India is being produced using these hi-tech methods. The merits and demerits of conventional and hi-tech seed potato production systems are highlighted in Table 1. These technologies need to be given serious thought and should be promoted in developing countries so as to increase potato productivity. In areas having high disease pressure, the hi-tech system of seed potato production has the advantage of better health status of seed stocks due to the reduced number of field multiplications over the conventional (clonal multiplication) system. In terms of the need for greater efficiency of seed potato production and for reduced energy input, research on soil-free techniques will continue to be the subject of focus in both established and developing potato-producing areas in the near and distant future. Advances in engineering technology will also assist in the development of more automated and controlled seed propagation systems. However, there are also options for simplifying the seed potato production systems for adaptation to low-cost technology situations for resource-poor potato growers, which has greater scope and relevance for the expansion of potato production in developing countries.
Author contributions
TB conceived the idea and wrote the manuscript. JT, AS, DD, VB, SS, MS, CC, and SN performed research and literature collection. VK, RS, MK, and NP edited the manuscript. All authors approved the manuscript.
Funding
This study was funded by the ICAR-Central Potato Research Institute, Shimla.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ankita M., Takayama S. (1994). Stimulation of potato (Solanum tuberosum l.) tuberization by semicontinuous liquid medium surface level control. Plant Cell Rep. 13, 184–187. doi: 10.1007/BF00239889
Bajaj Y. P. S. (1987). “Biotechnology and 21st century potato,” in Biotechnology in agriculture and forestry: Potato, vol. 3. Ed. Bajaj Y. P. S. (Berlin, Germany: Springer-Verlag), 3–22.
Bradshaw J. E. (2022). Breeding diploid f 1 hybrid potatoes for propagation from botanical seed (TPS): Comparisons with theory and other crops. Plants 11 (9), 1121. doi: 10.3390/plants11091121
Bryan J., Jackson M., Meléndez N. (1981). Rapid multiplication techniques for potatoes (Lima: International Potato Center (CIP).
Buckseth T., Sharma A. K., Pandey K. K., Singh B. P., Muthuraj R. (2016). Methods of pre-basic seed potato production with special reference to aeroponics-a review. Sci. Horti. 204, 79–87. doi: 10.1016/j.scienta.2016.03.041
Buckseth T., Singh R. K., Challam C., Tiwari J. K., Chakrabarti S. K. (2019a). Apical root cutting: a novel technique for the production of quality seed potato. Indian Hort 65 (6), 63–64.
Buckseth T., Singh R. K., Sharma A. K., Tiwari J. K., Chakrabarti S. K. (2019b). Hi-Tech seed potato production. Indian Horti. 64 (6), 18–20.
Buckseth T., Singh R. K., Tiwari J. K., Sharma S., Gautam A., Sharma A. K., et al. (2022). Influence of microplant hardening methods on aeroponic potato minituber production. Potato. Res 65, 335–348. doi: 10.1007/s11540-021-09530-z
Buckseth T., Singh R. K., Tiwari J. K., Sharma A. K., Singh S., Chakrabarti S. K. (2020). A novel sustainable aeroponic system for healthy seed production in India-an update. Indian J. Agric. Sci. 90 (2), 243–248.
Chindi A., Giorgi G. W., Atsede S., Lemma T., Negash K. (2014). Rapid multiplication techniques (RMTs): A tool for the production of quality seed potato (Solanum tuberosum l.). Asia. J. Crop Sci 6 (3), 176–185. doi: 10.3923/ajcs
Food and Agriculture Organization (FAO) (2022) FAOSTAT database. Available at: http://www.fao.org/faostat/en/#data/QC (Accessed 19 February 2022).
Gaur P. C., Pandey S. K. (1993). “True potato seed (TPS) technology,” in Advances in horticulture, vol. 7. Eds. Chadha K. L., Grewal J. S. (New Delhi, India: Malhotra Publishing House), 85–111.
Gopal J., Chamail A., Sarkar D. (2004). In vitro production of microtubers for conservation of potato germplasm: Effect of genotype, abscisic acid, and sucrose. In Vitro Cell. Dev. Biol. Plant 40, 485–490. doi: 10.1079/IVP2004540
ICAR-CPRI Annual Report (2020) Annual Report. Indian Council of Agricultural Research - Central Potato Research Institute, Shimla, Himachal Pradesh, India. Available at: https://cpri.icar.gov.in//11e44298b-584d-4d5c-89ad-c37862d3f9eb.pdf.
Indian Minimum Seed Certification Standards (2013). The central seed certification board.Department of Agriculture and Cooperation, Ministry of Agriculture and Farmers Welfare, Government of India.p 605p.
Lindhout P., Meijer D., Schotte T., Hutton R. C. B., Visser R. G. F., VanEck H. J. (2011). Towards F1 hybrid seed potato breeding. Potato. Res. 54, 301–312. doi: 10.1007/s11540-011-9196-z
Mamiya K., Tanabe K., Onishi N. (2020). Production of potato (Solanum tuberosum l.) microtubers using plastic culture bags. Plant Biotechnol. 37 (2), 233–238. doi: 10.5511/plantbiotechnology.20.0312a
Millam S., Sharma S. K. (2007). “Soil-free techniques,” in Potato biology and biotechnology advances and perspective. Ed. Vreugdenhil D. (Amsterdam: Elsevier), 822.
Muthoni J., Hussein S., Rob M. (2019). Production of hybrid potatoes: Are heterozygosity and ploidy levels important? Australlian. J. Crop Sci. 13 (05), 687–694. doi: 10.21475/ajcs.19.13.05.p1280
Naik P. S. (2005). “Achieving self sufficiency in quality seed production in roots and tuber crops with special reference to potato,” in Crop improvement and production technology of horticultural crops, vol. I. Eds. Chadha K. L., Ahloowalia B. S., Prasad K. V., Singh S. K. (New Delhi: The Horticultural Society of India), 243–255.
Naik P. S., Buckseth T. (2018). “Recent advances in virus elimination and tissue culture for quality potato seed production,” in Biotechnologies of crop improvement, vol. Volume 1 . Ed. Gosal S. S. (Cham Switzerland: Springer International Publishing), 131–158.
Naik P. S., Chakrabarti S. K., Sarkar D., Birman R. K. (2000). “Potato biotechnology: Indian perspective,” in Proceedings of potato: Global research and development. Eds. Khurana S. M. P., Shekhawat G. S., Singh B. P., Pandey S. K., 194–211. New Delhi, India
Naik P. S., Karihaloo J. L. (2007). Micropropagation for production of quality potato seed in Asia-pacific (New Delhi, India: Asia-Pacific Consortium on Agricultural Biotechnology).
Naik P. S., Sarkar D. (2000). “In vitro approaches to propagation and conservation of genetic resources in potato,” in Biotechnology in horticultural and plantation crops. Eds. Chadha K. L., Ravindran P. N., Sahijram L. (New Delhi, India: Malhotra Publishing House), 369–406.
Naik P. S., Sarkar D. (2001). “Microtubers: innovative propagules for virus-free potato seed production,” in Role of resistance in intensive agriculture. Eds. Nagarajan S., Singh D. P. (New Delhi, India: Kalyani Publishers), 12–28.
National Academy of Agricultural Sciences (NAAS) (2021). Innovations in potato seed production. strategy paper No.14 (New Delhi: National Academy of Agricultural Sciences).
Otazu V. (2010). Manual on quality seed potato production using aeroponics (Lima, Peru: International Potato Centre (CIP).
Pandey K. K., Singh B. P. (2014). “Effect of bower system on minituber yield ofdifferent varieties in aeroponic system,” in Abstract in national seminar on emerging problems of potato (Shimla: Indian Potato Association), 193–194.
Piao X. C., Chakrabarty D., Hahn E. J., Paek K. Y. (2003). A simple method for mass production of potato microtubers using a bioreactor system. Curr. Sci. 84, 1129–1132. Available at: https://www.jstor.org/stable/24107678.
Sarkar D., Pandey S. K., Sharma S., Chandel P. (2011). “Potato,” in Advance of horticulture biotechnology -regeneration systems -vegetables, ornamentals and tuber crops (Volume II). Eds. Singh H. P., Parthasarathy V. A., Nirmal B. K. (New Delhi: Westville Publishing House), 319–345.
Shaheb M. R. S., Begum M. M., Ahmed K. U., Nazrul M. I., Wiersema S. G. (2016). Challenges of seed potato (Solanum tuberosum l.) production and supply system in Bangladesh–a review. Agricult. 13 (1), 173–188.
Singh R. K., Buckseth T., Tiwari J. K., Sharma A. K., Chakrabarti S. K. (2019b). Recent advances in production of healthy planting material for disease management in potato. Biotech. Today.: Int. J. @ Bio. Sci. 9 (1), 7–15. doi: 10.5958/2322-0996.2019.00001.2
Singh R. K., Buckseth T., Tiwari J. K., Sharma A. K., Singh V., Kumar D., et al. (2019a). Seed potato (Solanum tuberosum l.) production systems in India: A chronological outlook. Indian J. Agri. Sci. 89 (4), 578–587.
Singh R. K., Dubey S. K., Rakesh K., Singh S. N., Buckseth T., Singh A. K. (2016). Farmer participatory seed potato production through krishi vigyan kendra networking: an action research for enhanced availability of seed potatoes in India. Potato. J. 43 (2), 193–199.
Singh A. K., Jankiram T., Chakrabarti S. K., Vinay B., Tiwari J. K. (2018). Indian Potato varieties (Shimla: ICAR-Central Potato Research Institute), 178p.
Singh V., Pandey K. K., Singh S., Dhruv K., Singh B. P. (2012). “Aeroponics: An innovative method of potato seed production,” in Abstract in national consultation on potato research and development: Way forward (Bhubaneswar: OAU&T), 45–49.
Singh B. P., Sharma S. (2018). Potato seed production systems: then and now. Potato. J. 45 (1), 1–16.
Sood S., Sundaresha S., Bhardwaj V., Kardile H., Chourasia K. N. (2021). Reinventing potato breeding through true potato seed based technology. Indian Horti. 66, 41–42.
Steward F. C., Caplin S. M. (1951). Tissue culture from potato tuber: the synergistic action of 2,4-d and of coconut milk. Science 111, 518–520. doi: 10.1126/science.113.2940.518
Tierno R., Carrasco A., Ritter E., Ignacio J., de Galarreta R. (2014). Differential growth response and minituber production of three potato cultivars under aeroponics and greenhouse bed culture. Am. J. Potato. Res. 91, 346–353. doi: 10.1007/s12230-013-9354-8
Tiwari J. K., Buckseth T., Singh R. K., Zinta R., Thakur K., Bhardwaj V., et al. (2022). Aeroponic evaluation identifies variation in Indian potato varieties for root morphology, nitrogen use efficiency parameters and yield traits. J. Plant Nutr. doi: 10.1080/01904167.2022.2046080
Tiwari J. K., Luthra S. K., Vinod K., Bhardwaj V., Singh R. K., Sridhar J., et al. (2017). “Genomics in true potato seeed technology: engineering cloning through seed,” in The potato genome. Eds. Ckakrabrti S. K., Conghua X., Tiwari J. K. (Switzerland: Springer Cham), 297–305.
Tiwari J. K., Singh R. K., Buckseth T., Chakrabarti S. K. (2019). Aeroponics: A novel precision system to study nutrient biology in potato. Indian Horti. 64 (5), 33–34.
Tran V. M., Nguyen V. U., Vander Zaag P. (1990). Rapid multiplication of potatoes: influence of environment and management on growth of juvenile apical cuttings. Am. Potato. J. 67, 789–797. doi: 10.1007/BF03044530
Vander Zaag P., Escobar V. (1990). Rapid multiplication of potatoes in the warm tropics: rooting and establishment of cuttings. Potato. Res. 33, 13–21. doi: 10.1007/BF02358126
Venkataslam E. P., Singh R. K., Sharma A. K., Singh V., Buckseth T., Chakrabarti S. K. (2017). Seed potato production techniques: Principles and applications (Shimla: ICAR-Central Potato Research Institute), 101p.
Wang P. J., Hu C. Y. (1982). In vitro mass tuberization and virus-free seed potato production in Taiwan. Am. J. Potato. Res. 59, 33–37. doi: 10.1007/BF02854881
Wang P. J., Hu C. Y. (1985). “Potato tissue culture and its application in agriculture,” in Potato physiology. Ed. Li P. H. (London, UK: Academic Press), 503–577.
Wenzel G. (1994). “Tissue culture,” in Potato genetics. Eds. Bradshaw J. E., Mackay G. R. (Wallingford, UK: CAB International), 173–195.
Keywords: solanum tuberosum, micropropagation, aeroponics, ARC, TPS, potato diploids
Citation: Buckseth T, Tiwari JK, Singh RK, Kumar V, Sharma AK, Dalamu D, Bhardwaj V, Sood S, Kumar M, Sadawarti M, Challam C, Naik S and Pandey NK (2022) Advances in innovative seed potato production systems in India. Front. Agron. 4:956667. doi: 10.3389/fagro.2022.956667
Received: 30 May 2022; Accepted: 01 August 2022;
Published: 22 August 2022.
Edited by:
Paul Christiaan Struik, Wageningen University and Research, NetherlandsReviewed by:
Niels Peter Louwaars, Plantum NL, NetherlandsCopyright © 2022 Buckseth, Tiwari, Singh, Kumar, Sharma, Dalamu, Bhardwaj, Sood, Kumar, Sadawarti, Challam, Naik and Pandey. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tanuja Buckseth, dGFudWphZ2JwdWF0QGdtYWlsLmNvbQ==; VGFudWphLkJ1Y2tzZXRoQGljYXIuZ292Lmlu
 Tanuja Buckseth
Tanuja Buckseth Jagesh K. Tiwari
Jagesh K. Tiwari Rajesh K. Singh
Rajesh K. Singh Vinod Kumar1
Vinod Kumar1 Vinay Bhardwaj
Vinay Bhardwaj Salej Sood
Salej Sood Manoj Kumar
Manoj Kumar Clarissa Challam
Clarissa Challam